Abstract
Neurotoxic lesions of the nigrostriatal pathway model the deficits found in Parkinson’s disease. This study used stereology and a novel staining method to examine the effects of a partial unilateral striatal 6-hydroxydopamine (6-OHDA) lesion on substantia nigra pars compacta (SNpc) dopamine neuron number and morphology in rats. Adult male Long-Evans rats were subjected to unilateral lesion of the SNpc by intrastriatal microinjection of 6-OHDA (12.5 μg). Lesions were verified by d-amphetamine-stimulated rotation (2.5 mg/kg, sc) by force-plate rotometry 7 days post-surgery. Seven days after rotation testing, rats were euthanized, and brains were prepared for either histology (n = 12) or determination of striatal dopamine content by HPLC-EC (n =20). Brains prepared for histology were stained for tyrosine hydroxylase (TH) combined with a silver nucleolar stain (AgNOR) using a modified protocol developed for stereological assessment. The AgNOR counterstain allowed for precise definition of the nucleolus of the cells, facilitating both counting and qualitative morphometry of TH-positive neurons. Stereological quantitation determined a 54% decrease in TH-positive neuron number (P<0.01), and a 14% decrease in neuron volume (P<0.05) on the lesioned side. Striatal dopamine concentration was decreased by 92% (P<0.01), suggesting that striatal dopamine analysis may overestimate the numbers of SNpc neurons lost. These findings demonstrate that combined use of TH and AgNOR staining provides improved characterization of 6-OHDA-induced pathology. Furthermore, the data suggest that decreased neuronal volume as well as number contributes to the functional deficits observed after unilateral intrastriatal 6-OHDA lesion.
Keywords: silver nucleolar stain, 6-hydroxydopamine, stereology, substantia nigra pars compacta, neuronal volume, neuronal number
1. Introduction
Parkinson’s disease is a chronic neurodegenerative disorder marked by loss of nigrostriatal dopamine neurons, resulting in clinical signs when about 80% of striatal dopamine is depleted (Calne and Langston, 1983). Current treatments are largely palliative, emphasizing the urgent need for preventative and disease-modifying treatments. However, the development of such early-stage treatments is fraught with challenges. The relationships between the causative factors of Parkinson’s disease and the effects of neuroprotective treatments are still unclear (Ybot-Gorrin et al., 2011), and a better understanding of neuronal morphology is needed to elucidate the mechanisms of neurodegenerative disease (Przedborski et al., 2003). Thus the importance of early- to moderate-stage Parkinson’s disease models is vital to advancing the treatment of the disease.
The partial unilateral intrastriatal 6-hydroxydopamine (6-OHDA) model is a clinically-relevant model of early-stage Parkinson’s disease (Deumens et al., 2002; Kirik et al., 1998). This technique has been widely used as an investigational tool for both screening potential therapeutics and for understanding the underlying mechanisms of early Parkinson’s disease. Although rotational behavior and striatal dopamine concentration are routinely quantified after neurotoxic lesions to verify a functional dopaminergic deficit, advanced histological analysis is needed to assess the effects of the lesion and potential treatments on morphologic changes to the dopaminergic neurons. Histological analysis of partial 6-OHDA lesions in the substantia nigra pars compacta (SNpc) is challenging, however, because moderate degenerative changes to neuron morphology can be difficult to quantify. Stereological analysis using tyrosine hydroxylase (TH) staining of dopamine neurons has been used to study the morphological changes in several animal models of Parkinson’s disease, but the morphometric outcomes vary according to study. The variation in the results of Parkinson’s disease model morphology studies has been attributed to the varying lesion, staining and quantitation methods used (Stark and Pakkenberg, 2004); and while a certain amount of inter-study variation is unavoidable, a clear understanding of the morphology of dopaminergic neurons has undoubtedly been clouded by the inconsistent findings in the literature.
Darkly pigmented TH antibody immunostaining can mask morphological markers such as the nucleolus and individual neuronal body outlines in regions thickly populated with TH-positive neurons. With the goal of better understanding the effects of 6-OHDA on dopaminergic morphology, this study used stereological methods and a newly available commercial TH stain combined with a silver nucleolar stain (TH-AgNOR) to determine the effects of 6-OHDA partial unilateral intrastriatal lesions on cell number and morphology. AgNOR staining is a silver nitrate preparation that targets chromosomal proteins in the nucleolus known as nucleolar organizing regions (NOR). This stain, which has been extensively used by cancer pathologists to assess cell proliferation (Trerè, 2000), has recently been exploited for use as a co-stain in dopaminergic neurodegeneration studies (Switzer III et al., 2011). We will show that the combined use of TH and AgNOR staining provides improved characterization of 6-OHDA-induced pathology by facilitating the identification and quantification of neuronal structures used in stereology. In addition, our findings suggest that decreased neuronal volume as well as decreased neuronal number contributes to the functional deficits observed after unilateral 6-OHDA lesion.
2. Materials and methods
All experiments were performed in compliance with the NIH Guide for the Care and Use of Animals and were approved by the University of Kansas Medical Center Institutional Care and Use Committee.
2.1. Animals and husbandry
Male Long-Evans rats (90–100 days old) were bred in-house (breeding stock obtained from Harlan, Indianapolis, IN). Rats were housed in a temperature- and humidity-controlled animal facility with a 14:10-h light-dark cycle (on at 06:00 h) with ad libitum access to water and chow AIN-93G (Teklad, Indianapolis, IN). Rats were weighed and handled regularly. Litters were culled to 8 pups on postnatal day 1 and weaned on postnatal day 20. Rats were housed four per cage from weaning until lesion surgery, after which they were kept in single housing until euthanized to ensure undisturbed recovery.
2.2. Procedures
Rats were subjected to a partial unilateral intrastriatal 6-OHDA lesion. Amphetamine-stimulated rotation was assessed 7 days later. After an additional 7 days to ensure clearance of drug [>10 t1/2s, tissue t1/2 = 5–9 hrs for the elimination phase (Kuhn and Schanberg, 1978)], rats were euthanized for dopamine quantitation or histology. Separate groups of rats were used for the histological and neurochemical end points, but all rats were evaluated for amphetamine-stimulated rotation.
2.2.1. 6-OHDA lesions
Rats (n=36) were anesthetized with isoflurane (3%) and secured in a stereotaxic frame. After creating a burr hole in the skull over the right striatum (AP+ 1.0 mm, ML+ 2.5 mm vs. bregma), a 26-gauge dome-tipped needle attached to a microliter syringe with Teflon tubing was lowered into the striatum (5.0 mm from dural surface). 6-OHDA (12.5 μg administered in a volume of 5.0 μl, at 2.5 μg/μl, in 0.9% saline with 0.1% ascorbic acid) was infused at the rate of 0.5 μl/min based on previously published methods (Bethel-Brown et al., 2011; Bethel-Brown et al., 2010). The infusion needle was left in place for an additional 5 minutes and then slowly withdrawn. Bone wax was applied to the burr hole, and the scalp closed with wound clips. Buprenex (0.05 mg/kg body weight) and ketoprofen (5 mg/kg body weight) were given post-surgery, with follow-up doses of ketoprofen for two days, and animals were allowed to recover in single housing.
2.2.2. Amphetamine-stimulated rotations
D-amphetamine sulfate was injected (2.5 mg/kg in 0.9% saline, S.C.) and animals (n=36) were placed in a rotometer for 60 min. The rotometer was a force-sensing actometer with a 26.5 cm diameter cylindrical chamber (Bethel-Brown et al., 2010). Custom software was used to quantify the number of rotations.
2.2.3. Determination of striatal dopamine content
Rats (n = 20, from the 36 lesioned) were decapitated and brains rapidly removed and frozen on dry ice. Left and right caudate-putamen were isolated by freehand dissection on ice and stored at −80° C. Concentrations of dopamine were quantified using an isocratic HPLC-EC system (ESA Coulochem III, Chelmsford, MA) coupled to a Coulochem III dual-channel electrochemical array detector (E1 + 0.35 mV and E2 − 0.25 mV using a 5011 dual analytical cell ESA Model 5100A) as previously described (Levant et al., 2008). Tissues were extracted in 0.3 N perchloric acid. Analytes were separated using a C18 reverse phase column (ESA HR-80 C18, 4.6 mm × 80 mm, 3 μm) with a pH 4.0 citrate-acetate mobile phase containing 4.0% methanol and ~0.35 mM 1-octane-sulfonic acid at a flow rate of 1.8 ml/min. 3,4-dihydroxy-benzylamine was used as the internal standard. A standard curve was generated for each analyte. Protein concentrations of the extracted tissues were determined by the BCA method (Pierce, Rockford, IL). Striatal dopamine concentrations were expressed as ng/mg protein.
2.2.4. Histological analysis
2.2.4.1. TH-thionine and TH-AgNOR staining
Rats (n = 16, from the 36 lesioned) were deeply anesthetized with pentobarbital and perfused with 0.1 M phosphate buffered saline (PBS) (pH 7.2), followed by 4% paraformaldehyde in PBS, and decapitated. Skulls were post-fixed at 4° C for at least 7 days, and then transferred to PBS. Brains were extracted and coronal sections (50 μm) were prepared and stained by NeuroScience Associates (Knoxville, TN) as previously described (Switzer III et al., 2011). Brains were processed using MultiBrain™ Technology, which embeds 16 brains in a single gelatin block positioned for random and unbiased sampling of each of the brain sections on each slide. Free-floating sections were stained with a standard TH with thionine counterstain (TH-thionine). Another set of sections from the same animals was stained with a modified TH plus silver nucleolar stain (TH-AgNOR). To accommodate the unique requirements of stereology, sections stained for TH-AgNOR were incubated in HCl to enhance permeabilization, bleached to avoid non-specific silver staining, and incubated with a one-quarter strength concentration of TH antibody to provide optimal contrast with the AgNOR stain while retaining robust pigmentation of TH-positive structures (Switzer III et al., 2011). Sections were mounted on gelatinized (subbed) glass slides.
2.2.4.2. Stereology
Every sixth section containing SNpc was selected (Bregma −4.70 to −6.30 mm), and the SNpc was carefully outlined to exclude other subdivisions of the substantia nigra and the ventral tegmental area using an atlas (Fig. 1). TH-AgNOR-stained cells were quantified using the MicroBrightField Stereoinvestigator software package combined with a Nikon Eclipse TE2000-U microscope and a Heidenhein linear encoder unit. The microscope was coupled to a QImaging Retiga-2000R color digital video camera. Identification of the regions of interest was performed at 4X, and stereology was performed at 100X. The optical fractionator method was used for cell counts based on identification of the nucleus and/or nucleolus in each neuron, and sections were assessed using the nucleator tool to determine TH-positive neuronal body volumes. SNpc regional volume was determined by planimetry. The coefficient of error for all samples was 0.15 or less, and population estimates were based on the mean section thickness (measured at 35 ± 0.5 μm).
Fig. 1. Anatomical definition of regions of interest.
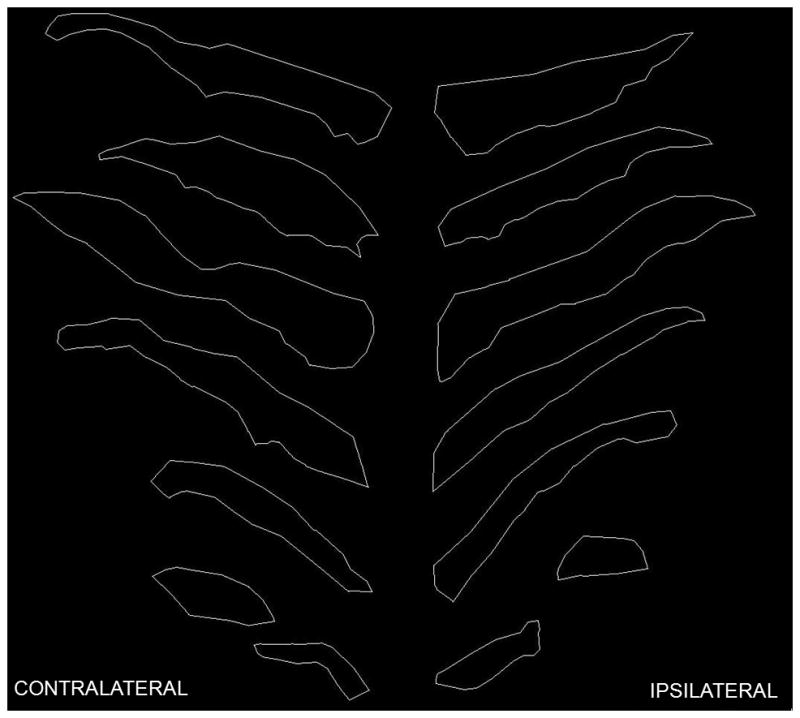
Representative photomicrographs (4X) of digital tracings of the boundaries of the SNpc in contralateral and ipsilateral brain through 6 coronal sections of a single animal. Tracings shown are rostral to caudal from the top to the bottom of the figure. TH-AgNOR-stained number and neuronal body volume were assessed within these boundaries. Tracings were produced using MicroBrightField’s Stereoinvestigator software, and sections were aligned to facilitate comparison.
2.3. Data analysis
Data are presented as the mean ± S.E.M. The effects of 6-OHDA between the ipsilateral and contralateral hemispheres were tested for statistical significance using paired t-tests. Within-subject changes in any parameter were expressed as a percentage relative to the contralateral side. One rat did not exhibit both amphetamine-stimulated rotation and a decrease in SNpc cell number or striatal dopamine concentration after lesion, and was excluded from analysis as a failed lesion. The rotometry apparatus failed to record rotations for one of the rats designated for dopamine analysis. Sections from 3 rats showed incomplete staining; consequently stereological quantification for these rats was not possible, and these rats were also excluded from further analysis. Differences with a P<0.05 were considered significant.
3. Results
3.1. Amphetamine-stimulated rotation
The mean number of amphetamine-stimulated rotations completed by lesioned rats during the 60-minute observation period was 145 ± 24 (n = 31), indicating a functional dopaminergic deficit in the ipsilateral striatum.
3.2. Striatal dopamine concentrations
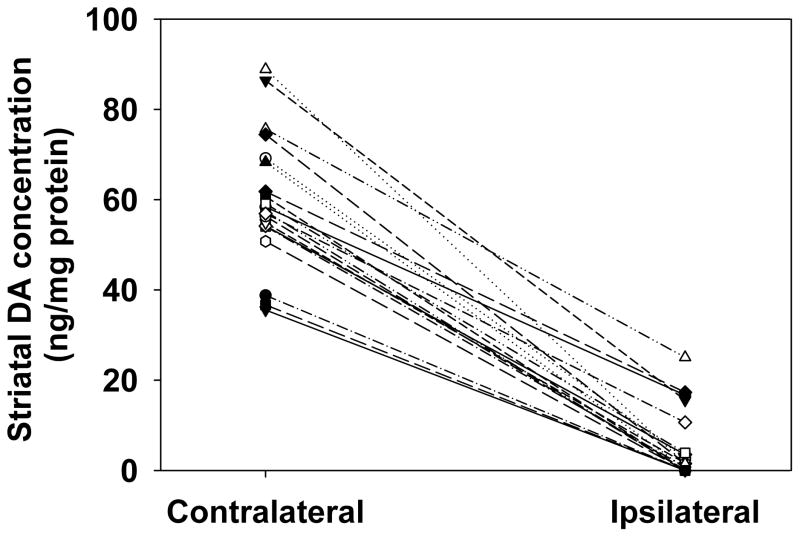
Dopamine concentration was decreased between the ipsilateral and contralateral striata of all rats subjected to 6-OHDA lesion, with a mean within-subject decrease in striatal dopamine of 92 ± 2.1% (P<0.01) (Fig. 2). The dopamine levels measured in the unlesioned hemispheres were comparable to those found in previous studies (Davis et al., 2010; Kilts et al., 1981; Levant et al., 2011; Widerlöv et al., 1982).
Fig. 2. Effects of unilateral intrastriatal 6-OHDA lesion on striatal dopamine.
All rats had decreased striatal dopamine content in the ipsilateral striatum compared to the contralateral striatum. The mean within-subject decrease in dopamine concentration between the ipsilateral and contralateral striata was 92 ± 2.1% (P<0.01 by paired t-test, n=12).
3.3. Stereological analysis of SNpc TH-AgNOR-stained neurons
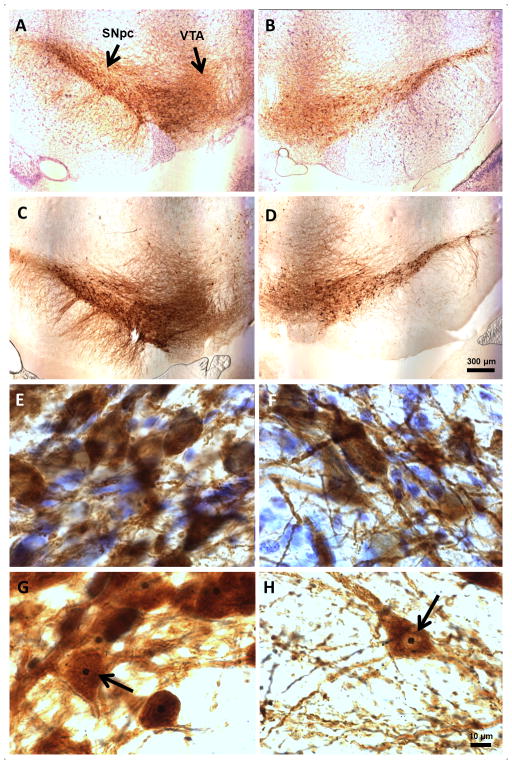
Low-and high-power photomicrographs demonstrate the effects of the lesion using TH-AgNOR staining or TH-thionine counterstain (Fig. 3). Neurodegeneration of the 6-OHDA-lesioned SNpc was evident at low magnification (Figs. 3A–3D). High-power magnification illuminates the advantages of using a combined modified TH and AgNOR staining protocol. The neuronal bodies were more clearly delineated in the AgNOR-stained sections than with the TH-thionine counterstain, allowing for more precise marking of the cell borders using the nucleator tool for volume calculations. The reduced TH antibody concentration used in the TH-AgNOR protocol results in reduced background darkening and staining of debris. In addition, the lack of thionine counterstain prevented further background clutter and facilitated a clear view of individual neuronal bodies and axons (Figs. 3G and 3H vs. 3E and 3F). Most importantly, the addition of the AgNOR stain resulted in a darkly pigmented and clearly distinguishable nucleolus (Figs. 3G and 3H). This selectivity in staining facilitated the use of the nucleolus within the nucleus as both a unique singly occurring cell landmark for counting with the optical fractionator, and as a center point for positioning crossed measurement rays using the nucleator software marking tool in Stereoinvestigator. In the few instances in which the nucleus of the cell, but not the nucleolus, was visible, the rays were placed centered on the nucleus.
Fig. 3. Effects of unilateral intrastriatal 6-OHDA lesion on TH-thionine- and TH-AgNOR-staining in the SNpc.
A. TH-thionine contralateral, 4X. B. TH-thionine ipsilateral, 4X. C. TH-AgNOR contralateral, 4X. D. TH-AgNOR ipsilateral, 4X. E. TH-thionine contralateral, 100X. F. TH-thionine ipsilateral, 100X. G. TH-AgNOR contralateral, 100X. H. TH-AgNOR ipsilateral, 100X. Representative images are shown and are all from the same animal. Scale bars are 300 μm and 10 μm for the 100X and 4X images, respectively.
3.3.1. TH-AgNOR-stained SNpc region volume
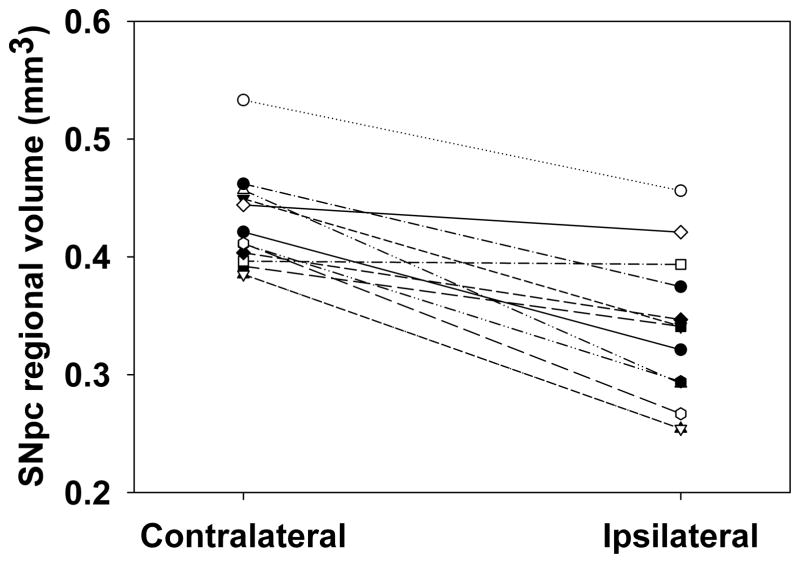
Eleven of 12 rats exhibited a smaller regional volume of the ipsilateral SNpc compared to the contralateral SNpc (Fig. 4). The mean within-subject decrease in volume of the SNpc region between the ipsilateral and contralateral SNpc was 20 ± 3.0% (P<0.05) as measured by planimetry.
Fig. 4. Effects of unilateral intrastriatal 6-OHDA lesion on SNpc regional volume.
Eleven of 12 rats exhibited a smaller regional volume in the ipsilateral SNpc compared to the contralateral SNpc assessed by planimetry. The mean within-subject decrease in regional volume between the ipsilateral and contralateral SNpc was 20 ± 3.0% (P<0.05 by paired t-test, n=12).
3.3.2. TH-AgNOR-stained SNpc neuron number
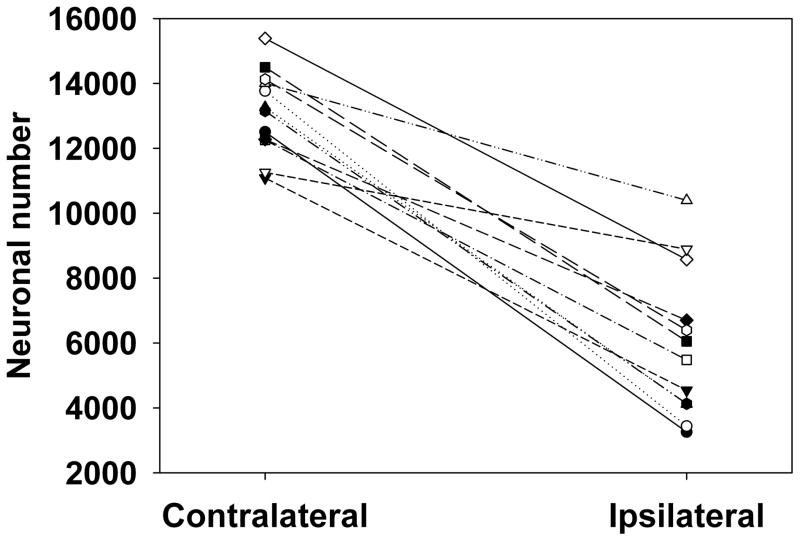
The mean number of SNpc TH-positive neurons on the intact side (13,133 ± 361) was comparable to the range of findings in previous stereological studies using traditional TH staining (Ahmad et al., 2008; Carvalho and Nikkhah, 2001; Debeir et al., 2005; Eriksen et al., 2009; Gomide et al., 2005; Kirik et al., 1998; Lindner et al., 1999). All rats showed a decrease in the number of TH-positive cells in the ipsilateral SNpc compared to the contralateral SNpc (Fig. 5). The mean within-subject decrease in TH-positive neuron number between the ipsilateral and contralateral SNpc was 54% ± 5.1% (P<0.01).
Fig. 5. Effects of unilateral intrastriatal 6-OHDA lesion on TH-AgNOR-stained neuron number in the SNpc.
All rats exhibited a decrease in neuronal number in the ipsilateral SNpc compared to the contralateral SNpc with a mean within-subject decrease in neuron number between the ipsilateral and contralateral SNpc of 54 ± 5.1% (P<0.01 by paired t-test, n=12).
3.3.3 TH-AgNOR-stained SNpc neuron volume
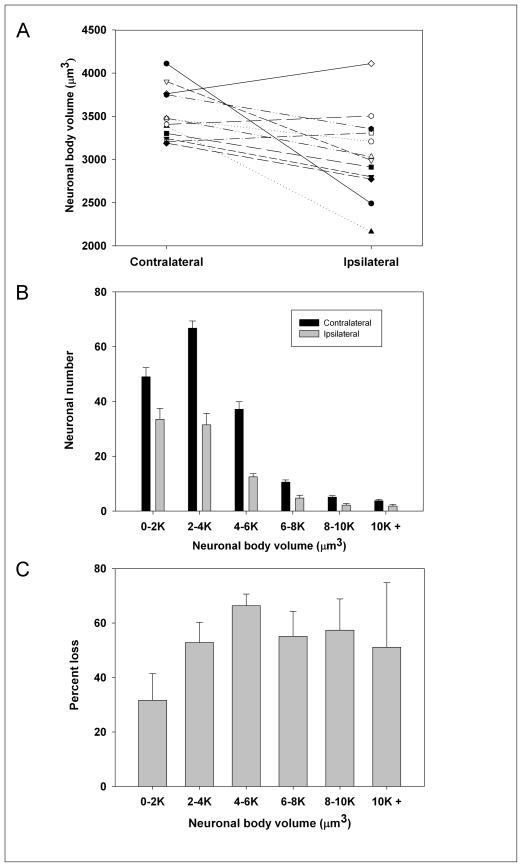
Nine of 12 rats exhibited a smaller average neuronal body volume in the ipsilateral SNpc compared to the contralateral SNpc with a mean within-subject decrease of 14 ± 3.8% (P<0.05) (Fig. 6A). Fig. 6B shows the change in the number of neurons in various size categories between ipsilateral and contralateral SNpc.
Fig. 6. Effects of unilateral intrastriatal 6-OHDA lesion on TH-AgNOR-stained neuronal body volume.
(A) Effects on average neuronal cell body volume. Nine of 12 rats exhibited a smaller average neuronal body volume in the ipsilateral SNpc compared to the contralateral SNpc. The mean within-subject decrease in average neuronal body volume between the ipsilateral and contralateral SNpc was 14 ± 3.8% (P<0.05 by paired t-test, n=12). (B) Effects on frequency distribution of neuronal volume (P<0.05 by paired t-test, n=12), (C) Effect on percent loss in the number of cells in each size category between the ipsilateral and contralateral SNpc.
4. Discussion
The 6-OHDA toxin, a structural analog of dopamine, is transported by the monoamine transporter into catecholaminergic cells, leading to their destruction by oxidative mechanisms (Glinka et al., 1997; Kostrzewa and Jacobowitz, 1974). Although lacking the Lewy bodies and the progressive course of clinical Parkinson’s disease, the 6-OHDA lesion nonetheless produces marked degeneration of targeted dopaminergic neurons, providing a functional Parkinson’s disease model quantifiable by characteristic behavioral changes, dopamine depletion and histological observations (Kirik et al., 1998; Ungerstedt and Arbuthnott, 1970).
The partial unilateral intrastriatal 6-OHDA model, with its relatively moderate loss of SNpc TH-positive neurons, is particularly appropriate as an early-stage Parkinson’s disease model (Bethel-Brown et al., 2010; Deumens et al., 2002; Kirik et al., 1998). The importance of early Parkinson’s disease models is underscored by our existing knowledge about the destruction of pigmented SN neurons long before onset of the clinical disease in humans. Estimates hold that patients with the earliest detectable clinical signs have already lost about 50% of their pigmented SN neurons (Marsden, 1990). Furthermore, pigmented SN neurons are also lost as a part of natural aging (Rudow et al., 2008), and although the degree of loss is still debated, this natural attrition adds another layer of complexity to the investigation of early Parkinson’s disease. Differences in the degree of dopaminergic degeneration among the existing studies of the partial unilateral intrastriatal 6-OHDA model have been attributed to the differences in lesion site, histology methods, toxin dose, and to biologic variability (Deumens et al., 2002; Kirik et al., 1998; Stark and Pakkenberg, 2004). Thus, the capacity for accurate, accessible morphometry of the neuronal populations at risk in this Parkinson’s model is important for the advancement of Parkinson’s research.
Stereological analysis, with its use of random sampling techniques and generation of unbiased estimates of 3D characteristics, is the optimal toolkit for assessing the morphological changes induced by neurotoxic lesions and therapeutic interventions (Gundersen et al., 1988; Schmitz and Hof, 2005). Stereology comes with its own set of challenges, however, especially pertaining to its requirement of staining thick tissue sections, which can be difficult for stains to penetrate. An additional challenge is the necessary definition of specific morphological landmarks like the nucleus and nucleolus, which can be easily obscured by improper staining. This is especially important in dense, mostly homogenous populations of a darkly-staining cell type, such as TH-positive SNpc neurons. In particular, the nucleus and nucleolus can be difficult to distinguish from other darkly stained structures within the cell. Since the nucleus and the nucleolus are unique anatomical markers useful in stereological quantitation, improved staining methods such as the TH-AgNOR protocol are needed to be able to specifically identify these structures in TH-positive tissues.
AgNOR staining offers a versatile tool in specific identification of the nucleolus in TH-stained preparations. The nucleolus is localized around chromosomal segments known as NORs, which contain ribosomal RNA-encoding genes. NORs also contain acidic AgNOR proteins. Because the quantity of AgNOR proteins and cell proliferation rate are related, AgNOR staining has been extensively used as a tool by cancer pathologists to characterize a variety of cancer types (Derenzini et al., 2004; Derenzini et al., 2000; Trerè, 2000). The highly specific nucleolar staining achieved with this method lends it to other applications in which visualization of the nucleolus is desirable, especially to stereological study of neural tissues.
The present findings demonstrate that the modified TH-AgNOR staining protocol offered a number of qualitative advantages observed during the histopathological analysis (Fig. 3). Enhanced stain penetration facilitated by the HCl permeabilization step in the modified TH-staining protocol was combined with less background staining of fibers and debris with the reduced concentration of TH antibody. This enabled better visualization and potentially resulted in more accurate counting of both the lesioned and unlesioned SNpc than has been possible in previous studies. The TH-AgNOR staining advantage may be more marked in lesioned tissue, where a higher degree of cell destruction and accumulation of debris makes identification of individual neurons especially challenging. Since technical variations are a likely explanation for the discrepancies between neuronal morphometry findings in the literature, this modified TH-AgNOR staining protocol should prove to be a beneficial tool in the modern characterization of classic dopaminergic neurotoxin models. It may be anticipated that its use will lead to a fuller understanding of the affected neurons, and importantly, the underlying functional changes related to morphology.
The data also clearly demonstrate that unilateral intrastriatal 6-OHDA lesions result in decreased SNpc regional volume and cell loss (Figs. 4 and 5) in addition to amphetamine-stimulated rotation and the depletion of striatal dopamine (Fig. 2)
Compared to the near-complete destruction of both SNc neurons and striatal dopamine in medial forebrain bundle or SNc lesions, the degree of neuron and dopamine loss is more variable in intrastriatal 6-OHDA lesions, dependent on the site and dose of the toxin administration (Kirik et al., 1998). Even so, we found a greater disparity between percent depletion in SNpc neurons (54%) and striatal dopamine (92%) than is typical for intrastriatal lesions in the literature (Deumens et al., 2002). Striatal dopamine concentration is rarely measured in histological 6-OHDA studies, because it requires a separate set of animals for dopamine analysis; however, other studies have found decreases in striatal dopamine content and nigral dopamine neuron number that were more similar that those observed in this study. Of note, a stereological study using a bilateral 6-OHDA infusion (12.5 μg) found a 79% dopaminergic SN neuron loss associated with a 77% striatal dopamine loss quantified by HPLC-EC (Lindner et al., 1999). Similarly, a non-stereological study of unilateral 6-OHDA lesions (12 uGu) that quantified a subsection of the SNpc, found a 47% TH-positive cell loss (SN and VTA) accompanied by a 54% dopamine content loss measured by radioenzymatic immunocytochemistry (Lee et al., 1996). In contrast to these studies, our lesion resulted in a larger decrease in striatal dopamine content relative to cell loss. Thus, our results suggest that even severe unilateral striatal dopamine depletion may reflect a relatively modest loss of SNpc dopamine neurons, underscoring the importance of using stereological techniques to assess the effects of the toxin on cell number and morphology.
Although only a few studies have examined both changes in dopamine cell number and striatal dopamine content, a number of studies have examined changes in SN dopaminergic cell number after 6-OHDA lesion using both non-stereologic and stereologic methods. Non-stereologic studies indicate a loss of SNpc neurons after 6-OHDA lesions (for review see: Deumens et al., 2002); however, 2D methods for counting cells have inherent bias that can result in inaccurate cell counts (Sterio, 1984; West, 1993). Stereological studies also demonstrate a loss of SN dopaminergic neurons after unilateral striatal 6-OHDA studies. We found a 20% decrease in SNpc regional volume and a 54% depletion of SNpc TH-positive neurons after a single-site moderate 6-OHDA dose of 12.5 μg (Figs. 4 and 5). This finding complements two recent stereological studies that found a 33% TH-positive cell loss in the SN with an 8.75 μg dose, and a 33% loss with an 8 μg dose (Debeir et al., 2005; Gomide et al., 2005). Taken together, these stereological results support a dose-response effect of 6-OHDA dose on the number of SNpc TH-positive neurons.
Even when using stereology to reduce bias, studies must be compared carefully. Differences in lesion sites and in region definition can produce varying results. For example, Kirik et al. (Kirik et al., 1998) used a total 20 μg dose produced a loss of neurons in the SN (including the pars reticulata and lateralis) of approximately 50%, although the percentage of loss varied depending on the lesion location within the striatum and the number of sites lesioned, with as much as 70% loss with a pre-terminal three-site injection of 7 μg per site. Increasing the total terminal dose to 28–30 μg resulted in a 60–76% loss, depending on the number of sites lesioned. Similarly, another study that used a total of 28 μg over 4 striatal injection sites found a 60% loss of SNpc neurons (Carvalho and Nikkhah, 2001). In the context of the available literature overall, we observed a large loss of neurons based on the total dose of 6-OHDA. Given the high striatal dopamine loss, however, the degree of SNpc loss is less surprising.
In addition to yielding enhanced data on changes in cell number after lesion, stereological methods can quantify subtle changes in cell morphology. In this study, the addition of AgNOR staining to the standard TH staining protocol resulted in clearly defined nucleolar bodies, which could be used as foci for using the nucleator tool to determine cell body volume. We found a 14% reduction in neuronal body volume in 6-OHDA lesioned rats, indicating that volume is a quantifiable morphological characteristic related to toxicity and subsequent neurodegeneration (Fig. 6A). Another study using stereology to quantify the effects of a unilateral striatal 6-OHDA lesion on cell volume found no difference between lesioned and intact SNpc TH-positive neurons (Gomide et al., 2005). While the time from lesion to sacrifice (14 days) was the same in both studies, the dose used by Gomide et al. (8 μg) was lower than that used in this study (12.5 μg), indicating that the observed difference in volume changes may be dose-related.
The dopaminergic cells in the rat SNpc are medium-sized neurons that are about 11 × 20 μm in size, although individual neurons vary (Paxinos, 1995). The observation of both decreased neuronal number and volume then raises the question: is the decreased volume truly due to atrophy, or is the shift in the mean volume a selective loss of the larger neurons? Both hypertrophy and atrophy of SNpc dopaminergic neurons have been observed in Parkinson’s disease brains (Rudow et al., 2008), and atrophy of the SNpc dopaminergic neurons has been observed after intrastriatal 6-OHDA injection (Lee et al., 1996; Sauer and Oertel, 1994). The frequency histogram of the distribution of the absolute number of TH-positive SNpc neurons by size category shows a clear post-lesion shift toward smaller neuronal volumes, and there was a lower percent loss in the smallest size category of neurons (Figs. 6B and 6C). Studies of the frequency distributions of the neurons in demonstrating atrophy are informative yet inconclusive, as they cannot rule out the preferential loss of large SNpc neurons. In fact, there is evidence that the subdivisions of the SNpc populated with mostly large neurons are also those most affected by neurotoxic lesions and by Parkinson’s disease (Rodríguez et al., 2001). However, another analysis of the size frequency distribution of absolute number of neurons when a range of doses were administered showed a small increase in the numbers of smaller cells with the smallest dose of toxin, suggestive of an atrophic response rather than a strictly selective loss of large neurons (Lee et al., 1996). It is also possible that a combination of atrophic development and preferential loss of large dopaminergic neurons occurs in neurotoxic-induced degeneration, as well as in the clinical disease. Although more research is needed to better characterize the effects of 6-OHDA lesions on dopaminergic neuronal volume, our study contributes to the current understanding of toxin-induced morphology changes and offers an improved method for further investigation. Overall, the addition of the AgNOR stain to a modified TH staining protocol resulted in facilitated identification of the nucleolus and better visualization of the neuronal bodies, and thus the likelihood of improved quantitation.
5. Conclusions
In conclusion, we find that TH-AgNOR staining facilitates stereological analysis of the effects of 6-OHDA lesions on the SNpc of rats when compared to standard TH-thionine staining. Using this staining protocol combined with stereological analysis to determine the effects of a unilateral intrastriatal 6-OHDA lesion revealed a moderate loss of SNpc TH-positive neurons despite severe striatal dopamine depletion. In addition, volumetric measurements confirmed SNpc TH-positive neuronal atrophy 14 days post-lesion, suggesting that 6-OHDA induces morphological changes to SN neurons relevant to those found in the Parkinson’s disease literature (Rudow et al., 2008). These findings confirm the utility of this staining protocol, and suggest the utility of this method in further investigation of Parkinson’s disease lesion models. Furthermore, these findings suggest that decreased neuronal volume as well as number contributes to the functional deficits observed after unilateral 6-OHDA lesion, and may also play a role in Parkinson’s disease.
Highlights.
Tyrosine hydroxylase-silver nucleolar (TH-AgNOR) stain was used in 6-OHDA lesioned rats
AgNOR staining improves visibility of the nucleolus for stereological analysis
Neuron number and volume was decreased in the SNpc of unilaterally-lesioned rats
The magnitude of SNpc cell loss was smaller than the decrease in striatal dopamine content
Acknowledgments
The authors thank Drs. Robert Switzer and Stanley Benkovic of NeuroScience Associates for preparing the AgNOR stained sections and facilitating our use of this staining technique in this study. Supported by NIH Grants NS067422 (BL), RR016475 (BL), HD02528 (BL JAS), the Institute for Advancing Medical Innovation (MH-S), and the Mabel A. Woodyard Fellowship in Neurodegenerative Disorders (MH-S).
Role of funding source:
The funding sources had no involvement in the study design, collection, analysis and interpretation of the data, the writing of the report, or the decision to submit the paper for publication.
Abbreviations
- HPLC-EC
high-performance liquid chromatography-electrochemical detection
- 6-OHDA
6-hydroxydopamine
- NOR
nucleolar organizing region
- AgNOR
silver nucleolar
- SNpc
substantia nigra pars compacta
- TH
tyrosine hydroxylase
- TH-AgNOR
tyrosine hydroxylase - silver nucleolar
Footnotes
Conflicts of interest:
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad S, Park J, Radel J, Levant B. Reduced numbers of dopamine neurons in the substantia nigra pars compacta and ventral tegmental area of rats fed an n-3 polyunsaturated fatty acid-deficient diet: a stereological study. Neurosci Lett. 2008;438:303–7. doi: 10.1016/j.neulet.2008.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethel-Brown CS, Morris JK, Stanford JA. Young and middle-aged rats exhibit isometric forelimb force control deficits in a model of early-stage Parkinson’s disease. Behav Brain Res. 2011;225:97–103. doi: 10.1016/j.bbr.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethel-Brown CS, Zhang H, Fowler SC, Chertoff ME, Watson GS, Stanford JA. Within-session analysis of amphetamine-elicited rotation behavior reveals differences between young adult and middle-aged F344/BN rats with partial unilateral striatal dopamine depletion. Pharmacol Biochem Behav. 2010;96:423–8. doi: 10.1016/j.pbb.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calne D, Langston J. Aetiology of Parkinson’s disease. Lancet. 1983;2:1457–9. doi: 10.1016/s0140-6736(83)90802-4. [DOI] [PubMed] [Google Scholar]
- Carvalho GA, Nikkhah G. Subthalamic nucleus lesions are neuroprotective against terminal 6-OHDA-induced striatal lesions and restore postural balancing reactions. Exp Neurol. 2001;171:405–17. doi: 10.1006/exnr.2001.7742. [DOI] [PubMed] [Google Scholar]
- Davis PF, Ozias MK, Carlson SE, Reed GA, Winter MK, McCarson KE, Levant B. Dopamine receptor alterations in female rats with diet-induced decreased brain docosahexaenoic acid (DHA): interactions with reproductive status. Nutr Neurosci. 2010;13:161–9. doi: 10.1179/147683010X12611460764282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeir T, Ginestet L, François C, Laurens S, Martel JC, Chopin P, Marien M, Colpaert F, Raisman-Vozari R. Effect of intrastriatal 6-OHDA lesion on dopaminergic innervation of the rat cortex and globus pallidus. Exp Neurol. 2005;193:444–54. doi: 10.1016/j.expneurol.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Derenzini M, Ceccarelli C, Santini D, Taffurelli M, Treré D. The prognostic value of the AgNOR parameter in human breast cancer depends on the pRb and p53 status. J Clin Pathol. 2004;57:755–61. doi: 10.1136/jcp.2003.015917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derenzini M, Trerè D, Pession A, Govoni M, Sirri V, Chieco P. Nucleolar size indicates the rapidity of cell proliferation in cancer tissues. J Pathol. 2000;191:181–6. doi: 10.1002/(SICI)1096-9896(200006)191:2<181::AID-PATH607>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Deumens R, Blokland A, Prickaerts J. Modeling Parkinson’s disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol. 2002;175:303–17. doi: 10.1006/exnr.2002.7891. [DOI] [PubMed] [Google Scholar]
- Eriksen N, Stark AK, Pakkenberg B. Age and Parkinson’s disease-related neuronal death in the substantia nigra pars compacta. J Neural Transm Suppl. 2009:203–13. doi: 10.1007/978-3-211-92660-4_16. [DOI] [PubMed] [Google Scholar]
- Glinka Y, Gassen M, Youdim MB. Mechanism of 6-hydroxydopamine neurotoxicity. J Neural Transm Suppl. 1997;50:55–66. doi: 10.1007/978-3-7091-6842-4_7. [DOI] [PubMed] [Google Scholar]
- Gomide V, Bibancos T, Chadi G. Dopamine cell morphology and glial cell hypertrophy and process branching in the nigrostriatal system after striatal 6-OHDA analyzed by specific sterological tools. Int J Neurosci. 2005;115:557–82. doi: 10.1080/00207450590521118. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96:857–81. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Breese GR, Mailman RB. Simultaneous quantification of dopamine, 5-hydroxytryptamine and four metabolically related compounds by means of reversed-phase high-performance liquid chromatography with electrochemical detection. J Chromatogr. 1981;225:347–57. doi: 10.1016/s0378-4347(00)80283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Björklund A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol. 1998;152:259–77. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- Kostrzewa RM, Jacobowitz DM. Pharmacological actions of 6-hydroxydopamine. Pharmacol Rev. 1974;26:199–288. [PubMed] [Google Scholar]
- Kuhn CM, Schanberg SM. Metabolism of amphetamine after acute and chronic administration to the rat. J Pharmacol Exp Ther. 1978;207:544–54. [PubMed] [Google Scholar]
- Lee CS, Sauer H, Bjorklund A. Dopaminergic neuronal degeneration and motor impairments following axon terminal lesion by instrastriatal 6-hydroxydopamine in the rat. Neuroscience. 1996;72:641–53. doi: 10.1016/0306-4522(95)00571-4. [DOI] [PubMed] [Google Scholar]
- Levant B, Ozias MK, Davis PF, Winter M, Russell KL, Carlson SE, Reed GA, McCarson KE. Decreased brain docosahexaenoic acid content produces neurobiological effects associated with depression: interactions with reproductive status in female rats. Psychoneuroendocrinology. 2008;33:1279–92. doi: 10.1016/j.psyneuen.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levant B, Zarcone TJ, Davis PF, Ozias MK, Fowler SC. Differences in methylphenidate dose response between periadolescent and adult rats in the familiar arena-novel alcove task. J Pharmacol Exp Ther. 2011;337:83–91. doi: 10.1124/jpet.110.174425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner MD, Cain CK, Plone MA, Frydel BR, Blaney TJ, Emerich DF, Hoane MR. Incomplete nigrostriatal dopaminergic cell loss and partial reductions in striatal dopamine produce akinesia, rigidity, tremor and cognitive deficits in middle-aged rats. Behav Brain Res. 1999;102:1–16. doi: 10.1016/s0166-4328(98)00160-0. [DOI] [PubMed] [Google Scholar]
- Marsden CD. Parkinson’s disease. Lancet. 1990;335:948–52. doi: 10.1016/0140-6736(90)91006-v. [DOI] [PubMed] [Google Scholar]
- Paxinos G. The rat nervous system. 2. Academic Press; San Diego: 1995. [Google Scholar]
- Przedborski S, Vila M, Jackson-Lewis V. Neurodegeneration: what is it and where are we? J Clin Invest. 2003;111:3–10. doi: 10.1172/JCI17522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez M, Barroso-Chinea P, Abdala P, Obeso J, González-Hernández T. Dopamine cell degeneration induced by intraventricular administration of 6-hydroxydopamine in the rat: similarities with cell loss in parkinson’s disease. Exp Neurol. 2001;169:163–81. doi: 10.1006/exnr.2000.7624. [DOI] [PubMed] [Google Scholar]
- Rudow G, O’Brien R, Savonenko AV, Resnick SM, Zonderman AB, Pletnikova O, Marsh L, Dawson TM, Crain BJ, West MJ, Troncoso JC. Morphometry of the human substantia nigra in ageing and Parkinson’s disease. Acta Neuropathol. 2008;115:461–70. doi: 10.1007/s00401-008-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer H, Oertel WH. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994;59:401–15. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Design-based stereology in neuroscience. Neuroscience. 2005;130:813–31. doi: 10.1016/j.neuroscience.2004.08.050. [DOI] [PubMed] [Google Scholar]
- Stark AK, Pakkenberg B. Histological changes of the dopaminergic nigrostriatal system in aging. Cell Tissue Res. 2004;318:81–92. doi: 10.1007/s00441-004-0972-9. [DOI] [PubMed] [Google Scholar]
- Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134:127–36. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Switzer RC, III, Baun J, Tipton B, Segovia C, Ahmad SO, Benkovic SA. Soc Neurosci Abst. Vol. 304.05. Washington, DC: Society for Neuroscience; 2011. Modification of AgNOR staining to reveal the nucleolus in thick sections specified for stereological assessment of dopaminergic neurotoxicity in substantia nigra, pars compacta; p. 2011. Abstract Viewer/Itinerary Planner. Online. [Google Scholar]
- Trerè D. AgNOR staining and quantification. Micron. 2000;31:127–31. doi: 10.1016/s0968-4328(99)00069-4. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U, Arbuthnott GW. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 1970;24:485–93. doi: 10.1016/0006-8993(70)90187-3. [DOI] [PubMed] [Google Scholar]
- West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993;14:275–85. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- Widerlöv E, Kilts CD, Mailman RB, Nemeroff CB, Mc Cown TJ, Prange AJ, Breese GR. Increase in dopamine metabolites in rat brain by neurotensin. J Pharmacol Exp Ther. 1982;223:1–6. [PubMed] [Google Scholar]
- Ybot-Gorrin I, Vivancos-Matellano F, Chacón-Peña JR, Alonso-Navarro H, Jiménez-Jiménez FJ. Assessment of Parkinson disease: what do we need to show neuroprotection? Neurologist. 2011;17:S21–9. doi: 10.1097/NRL.0b013e31823966c6. [DOI] [PubMed] [Google Scholar]