Abstract
We recently demonstrated that cilnidipine, an L/N-type calcium channel blocker, elicits protective effects against glomerular podocyte injury, in particular, in obese hypertensive rats that express the N-type calcium channel (N-CC). Since the N-CC is known to be expressed in sympathetic nerve endings, we evaluated the reno-protective effects of cilnidipine in innervated and denervated spontaneously hypertensive rats (SHR). Male SHR were uninephrectomized and fed 4% high-salt diet (HS-UNX-SHR). Animals were divided into groups, as follows, and observed from 9 to 27 weeks of age: 1) vehicle (n = 14), 2) vehicle plus renal-denervation (n = 15), 3) cilnidipine (50 mg/kg per day, p.o.; n = 10), and 4) cilnidipine plus renal-denervation (n = 15). Renal denervation attenuated elevations in blood pressure, but failed to suppress urinary protein excretion and podocyte injury in HS-UNX-SHR. Cilnidipine in both innervated and denervated HS-UNX-SHR similarly induced significant antihypertensive effects, as well as suppressing the urinary protein excretion and podocyte injury, compared to vehicle-treated HS-UNX-SHR. These data indicate that renal nerves have a limited contribution to the cilnidipine-induced reno-protective effects in HS-UNX-SHR.
Keywords: cilnidipine, hypertension, N-type calcium channel, podocyte, renal sympathetic nerve
Introduction
Calcium-channel blockers (CCBs) are widely used in the treatment of hypertension and its associated complications. Cilnidipine is an L/N-type CCB that has been recently shown to suppress the development of urinary protein excretion in hypertensive patients (1 – 3). A previous study conducted by our group showed that cilnidipine prevents the development of proteinuria and glomerular podocyte injury in rats with metabolic syndrome (4). These reno-protective effects cannot be simply explained by the hypotensive effects of cilnidipine, as its reno-protective effects were greater than those of amlodipine, an L-type CCB, with similar levels of blood pressure control.
The reno-protective effects of cilnidipine may be induced via its inhibitory effects on N-type calcium channels (N-CCs). N-CCs are distributed throughout sympathetic nerve endings and are widely believed to be involved in controlling the release of neurotransmitters from the nerve terminals of sympathetic neurons. This N-CC–induced release of neurotransmitters has been implicated in the over-activation of sympathetic nerves (5, 6), which could also be involved in the pathogenesis of resistant hypertension (7) and chronic renal diseases (8, 9). We recently demonstrated the immunoreactivity of N-CCs in vascular walls, possibly in the nerves in adventitia, distal tubules, and renal podocytes and that N-CC in cultured podocytes was involved in the angiotensin II (Ang II)-induced reactive oxygen species production (4). Given that cilnidipine was previously found to attenuate podocyte injury and proteinuria in a rat model of obesity and hypertension (4), then protection of podocyte, cells that play an important role in the glomerular filtration barrier against plasma protein leakage into urine (4, 10), by N-CC inhibition may be the mechanism behind the cilnidipine-induced reno-protection. On the other hand, cilnidipine is reported to dilate efferent arteries and to suppress glomerular hypertension that could cause hyper-filtration and stimulate sclerotic changes (11 – 13). However, it is still unclear whether cilnidipine targets renal nerves or other cells to induce its reno-protective effects in hypertensive subjects.
Therefore, the present study was conducted to separately evaluate the reno-protective effects of cilnidipine, specifically its actions on renal sympathetic nerves and other cells such as podocytes. We performed renal nerve denervations in spontaneously hypertensive rats (SHR), a model for essential hypertension that has been shown to express N-CC in podocytes (14), and assessed the effects of cilnidipine in these animals compared to innervated rats.
Materials and Methods
Animals and experiment design
All experimental procedures were performed according to the guidelines for the care and use of animals, as established by the Kagawa University and the National Research Council. Male SHR and Wistar-Kyoto rats (WKY) were purchased from SLC (Shizuoka). Two weeks prior to the initiation of the study (at 7 weeks of age), the right kidney was removed (uninephrectomized: UNX) through a small flank incision under pentobarbital anesthesia (50 mg/kg, i.p.). Denervation of the left kidney was accomplished by cutting all of the visible nerves entering the renal hilus, stripping the renal artery and vein of adventitia, and applying 10% phenol in 70% ethanol to the vessels, as previously described (15). Denervation was confirmed by measuring renal norepinephrine (NE) content at 27 weeks of age.
After recovery, UNX animals were divided into five experimental groups as follows: 1) vehicle (n = 14), 2) vehicle plus renal denervation (n = 15), 3) cilnidipine (50 mg/kg per day, p.o. via gavage, n = 10), 4) cilnidipine plus renal denervation (50 mg/kg per day, p.o. via gavage, n = 15), and 5) WKY (vehicle-administered, n = 10). UNX-SHR and -WKY rats were fed a high-salt (HS: 4% NaCl) diet (HS-UNX-SHR and HS-UNX-WKY) throughout the experimental period to accelerate the progression of renal injury (16, 17).
Systolic blood pressure (SBP) was measured in conscious rats by tail-cuff plethysmography (BP-98A; Softron, Tokyo); and 24-h urine samples were collected at 9, 12, 15, 18, 21, 24, and 27 weeks of age. All animals underwent a 12-h acclimatization period in metabolic cages prior to urine collection. Blood and kidney samples were harvested from 27-week-old animals. Half of the kidney was dissected immediately after blood collection by decapitation and was snap-frozen in liquid nitrogen for measurement of renal Ang II content.
Renal NE content
Extraction of NE from renal cortical tissue and subsequent processing were performed as follows. Briefly, pieces of renal cortex were homogenized in a 5-fold volume of ice-cold saline containing 0.1 N HCl. After centrifugation (12,000 × g at 4°C) for 30 min, the supernatant was subjected to high-performance liquid chromatography with an amperometric detector (ECD-300; Eicom, Kyoto; detection limit: 10 pg/mL) for measurement of NE.
Immunohistochemistry for desmin and N-CC
Immunohistochemistry for desmin, a marker of podocyte injury, and N-CC (Cav2.2 subunit) were performed by using the Histofine Simple Stain MAX-PO MULTI (Nichirei Biosciences, Tokyo), as previously described (18, 19). Deparaffinized sections were incubated with 0.1% hydrogen peroxide for 10 min for desmin or 0.3% hydrogen peroxide in methanol for 30 min for N-CC to block endogenous enzymes. For antigen retrieval, N-CC sections were exposed to 0.1% Triton X for 30 min. After blocking, sections were incubated with primary antibodies (anti-Human Desmin Mouse monoclonal antibody, D33, 1:500, from DakoCytomation, Glostrup, Denmark; anti- Cav2.2 antibody, 1:100, from Alomone Labs, Jerusalem, Israel) for 10 min (desmin) or 1 h (N-CC) at room temperature. Antibodies were visualized with DAB substrate (DakoCytomation) and counterstained with hematoxylin (DakoCytomation). Sections that were incubated without primary antibodies were used as controls. Antibody-positive areas were calculated from 20 randomly selected microscope fields (× 200) in each section. Histological analyses were performed using a color image analyzing system (Image J) in a blinded manner.
Histological examination
Kidneys were fixed with 10% formalin (pH 7.4), embedded in paraffin, sectioned into 4-μm slices, and stained with periodic acid – Schiff (PAS) reagent for analyzing mesangial expansion and matrix protein accumulation. PAS staining was evaluated using light microscopy (× 200). Positive glomerular sclerotic areas were counted using a photo-imaging system (Image J).
Cell culture condition and real-time PCR
Conditionally immortalized mouse podocyte cell lines were used for the cell culture study (20, 21). Cells were maintained in RPMI 1640 (Sigma, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS) containing 100 μg/ml penicillin and 100 μg/ml streptomycin (without γ-interferon) at 37°C for 10 days. After that, cells that achieved 60% confluence were first cultured for 24 h in serum-free RPMI 1640 and then exposed to vehicle, Ang II (100 nM), cilnidipine (10 μM), or ω-conotoxin (1 μM) for 24 h. The drug concentrations selected were based on previous studies (22, 23).
N-CC (Cacna 1b gene) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression levels were analyzed via real-time polymerase chain reaction (PCR) using a LightCycler FastStart DNA Master SYBR Green I kit (Applied Biosystems, Foster City, CA, USA), as previously described (24). The primer sequences for Cacna 1b and transforming growth factor β1 (TGF-β1) were also previously described elsewhere (4, 25, 26)
Other analytical procedures
Urinary protein and albumin excretion were determined via commercially available assay kits, microTP-test (Wako, Osaka) and ELISA kit (Shibayagi, Shibukawa), respectively (14, 18).
Statistical analyses
Values were presented as the mean ± S.E.M. Statistical analysis was performed by using Prism 5 (GraphPad, La Jolla, CA, USA). Statistical differences between treatments and other parameters were determined via a one-way ANOVA followed by a Tukey’s post-hoc test. A P-value < 0.05 was considered statistically significant.
Results
Renal NE contents
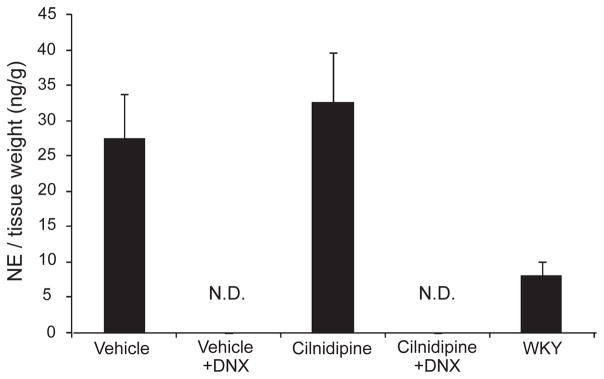
Renal NE content was measured to confirm the successful completion of the renal denervation procedure. Renal NE content was undetectable in all denervated HS-UNX-SHR rats. The renal NE content tended to be higher in vehicle-treated HS-UNX-SHR than WKY, but the difference was not statistically significant. Cilnidipine- treated innervated HS-UNX-SHR showed similar renal NE content as the vehicle group (Fig. 1).
Fig. 1.
Renal norepinephrine (NE) levels. Renal NE levels were undetectable in both cilnidipine-treated and untreated denervated SHR rats. The cilnidipine-treated groups demonstrated similar renal NE to tissue weight ratios compared with the vehicle group. All rats were uninephrectomized and fed the 4% NaCl–containing diet. WKY: Wistar Kyoto rat, DNX: denervation, N.D.: undetectable.
SBP and body weights
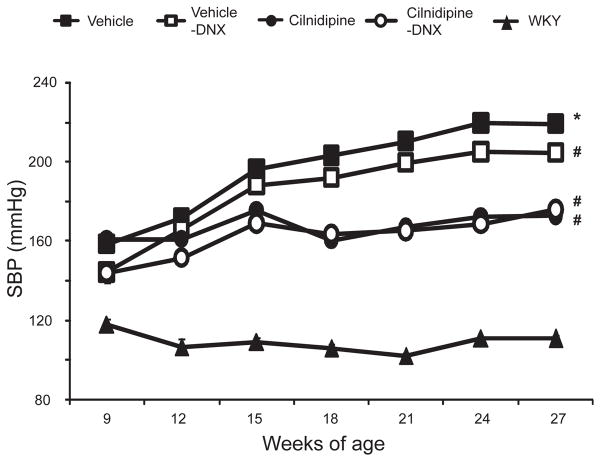
Vehicle-treated HS-UNX-SHR had significantly higher SBP compared to HS-UNX-WKY throughout the experimental period. Renal denervation resulted in a comparable reduction in SBP of HS-UNX-SHR (P < 0.05 at 9, 24, and 27 weeks of age). Treatment with cilnidipine significantly decreased SBP at 15, 18, 21, 24, and 27 weeks of age in both denervated and innervated HS-UNX- SHR, and there were no differences in SBP between these two groups except at 9 weeks of age (Fig. 2). Neither denervation nor cilnidipine affected the body weight of HS-UNX-SHR (vehicle, 377 ± 5 g; vehicle plus renal denervation, 384 ± 6 g; cilnidipine, 384 ± 4 g; cilnidipine plus renal denervation, 379 ± 4 g; and WKY, 470 ± 3 g).
Fig. 2.
Systolic blood pressure (SBP). Renal denervation induced a comparable reduction in SBP in SHR compared to the vehicle group. Cilnidipine treatment significantly decreased SBP compared to the vehicle group, whereas there were no differences between the denervated and innervated groups. All rats were uninephrectomized and fed the 4% NaCl–containing diet. *P < 0.05 vs. WKY, #P < 0.05 vs. the vehicle group. WKY: Wistar Kyoto rat, DNX: denervation.
Urinary protein and albumin excretion
Vehicle-treated HS-UNX-SHR demonstrated an age-dependent increase in urinary protein excretion, where values at 27 weeks of age were substantially higher than those of HS-UNX-WKY (Fig. 3A). A significant increase in urinary albumin excretion was also observed in vehicle- treated HS-UNX-SHR compared to HS-UNX-WKY at 27 weeks of age (Fig. 3B). Denervation tended to decrease the urinary excretion of protein and albumin in vehicle-treated denervated HS-UNX-SHR; however, the difference did not achieve statistical significance. In contrast, treatment with cilnidipine significantly suppressed urinary protein excretion at 27 weeks of age in both innervated and denervated HS-UNX-SHR. Cilnidipine also demonstrated a strong inhibitory effect on urinary albumin excretion at 27 weeks of age. Denervation did not further reduce the proteinuria and albuminuria in cilnidipine-treated rats.
Fig. 3.
Reno-protective effects of cilnidipine. Urinary protein excretion (A) and urinary albumin excretion (B). The vehicle group demonstrated substantial proteinuria and albuminuria compared with WKY. Cilnidipine significantly suppressed proteinuria and albuminuria in both the denervated and innervated groups. C) Representative images with desmin immunostaining (a – e, × 200) and PAS staining (f – j, × 200), as well as the quantitative comparisons between the groups. Podocyte injury revealed by desmin immunostaining was greater in the vehicle group (a) compared to WKY (e). Podocyte injury was significantly reduced with cilnidipine treatment in innervated (c) and denervated (d) rats. Denervation did not attenuate desmin staining in the glomeruli of the vehicle group (b). Glomerulosclerosis and extracellular matrix accumulation were revealed with PAS staining in the vehicle group (f) compared with WKY (j). Denervation attenuated the PAS staining (g). Cilnidipine treatment either in innervated (h) or denervated (i) animals prevented the increase in PAS staining. Denervation in cilnidipine-treated animals did not show additional attenuation. Scale bars: 100 μm. D) Transforming growth factor β1 (TGF-β1) mRNA expression. Expression of TGF-β1 mRNA was significantly higher in the vehicle group than in WKY. The increase in expression was significantly improved in both the cilnidipine-DNX and cilnidipine groups. All rats were uninephrectomized and fed the 4% NaCl–containing diet. *P < 0.05 vs. WKY, #P < 0.05 vs. the vehicle group. WKY: Wistar Kyoto rat, DNX: denervation.
Desmin staining
Given that cilnidipine suppressed albuminuria in both denervated and innervated HS-UNX-SHR, we evaluated glomerular podocyte damage with desmin staining (18). At 27 weeks of age, desmin-positive areas were markedly greater in vehicle-treated innervated and denervated groups versus HS-UNX-WKY (Fig. 3C). This increase in positive areas was significantly attenuated in cilnidipine- treated innervated and denervated HS-UNX-SHR. There was no statistical difference between innervated and denervated groups.
Renal histochemistry
PAS staining clearly revealed the presence of mesangial expansion and increases in matrix protein accumulation in vehicle-treated HS-UNX-SHR when compared to age-matched HS-UNX-WKY (Fig. 3C). These renal pathological changes were improved following renal denervation and/or cilnidipine treatment. There were no significant differences in PAS-stained areas between innervated and denervated cilnidipine-treated groups. Furthermore, renal TGF-β1 mRNA was highly expressed in the vehicle group compared to HS-UNX-WKY (Fig. 3D). Renal denervation tended to decrease TGF-β1 expression, but the difference failed to reach statistical significance. Cilnidipine strongly suppressed TGF-β1 mRNA in HS-UNX-SHR, and again there were no differences between the innervated and denervated groups.
Renal Ang II
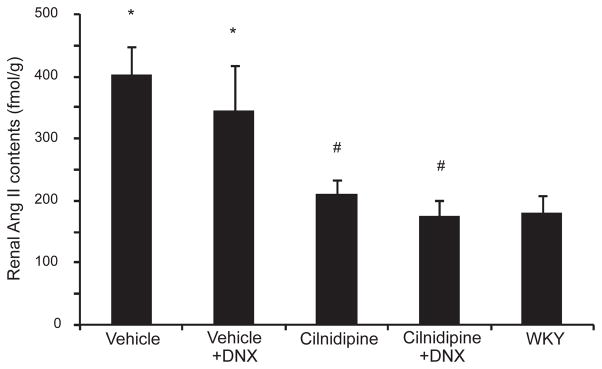
Renal Ang II content, which was evaluated to determine the renal renin–angiotensin system (RAS) activation (27), was greater in vehicle-treated HS-UNX-SHR than in WKY (Fig. 4). Denervation did not affect the increased renal Ang II level in HS-UNX-SHR. Cilnidipine treatment significantly decreased renal Ang II, regardless of the presence or absence of the renal nerves. There were no differences between the innervated and denervated groups.
Fig. 4.
Renal angiotensin II (Ang II) levels. The vehicle group had significantly higher renal Ang II levels compared to WKY. Cilnidipine treatment significantly reduced renal Ang II levels. However, there were no differences between the cilnidipine and cilnidipine- DNX groups. All rats were uninephrectomized and fed the 4% NaCl– containing diet. *P < 0.05 vs. WKY, #P < 0.05 vs. the vehicle group. WKY: Wistar Kyoto rat, DNX: denervation.
N-CC expression in podocytes
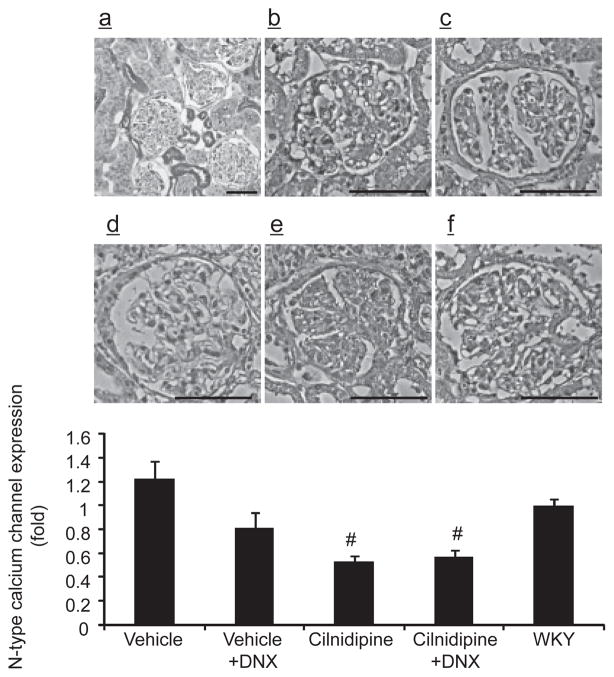
N-CC was previously found to be expressed in the vascular walls, possibly in the nerves in adventitia, distal tubules, and glomerular podocytes of the kidneys (4). Podocyte injury has been implicated in the development of proteinuria and albuminuria (28, 29); thus, we evaluated the expression of N-CC in glomerular podocytes. The N-CC–positive area in glomeruli in the denervated vehicle group tended to be lower than that of the innervated vehicle group (Fig. 5). In contrast, cilnidipine treatment significantly decreased the N-CC–positive areas compared to the vehicle group, and there were no differences in the immune-positive areas between the cilnidipine-treated innervated and denervated groups.
Fig. 5.
N-type calcium channel (N-CC) immunostaining (a: × 100, b – f: × 200). N-CC was strongly expressed in distal nephron and podocyte (a). The area positive for N-CC immunoreactivity in glomeruli was similar between WKY (f) and vehicle group (b). Cilnidipine significantly decreased N-CC immunostaining in the glomeruli of both the denervated (e) and innervated (d) groups compared to the vehicle group (b). Denervation did not attenuate N-CC immunostaining in the glomeruli of the cilnidipine-treated (e) and untreated (c) groups. All rats were uninephrectomized and fed the 4% NaCl– containing diet. #P < 0.05 vs. the vehicle group. WKY: Wistar Kyoto rat, DNX: denervation. Scale bars: 100 μm.
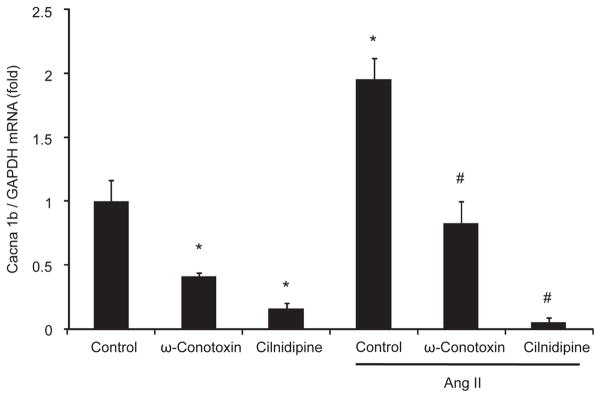
To further confirm the effects of cilnidipine on N-CC expression in vivo, we performed an in vitro study in cultured murine podocytes (20). We found both ω-conotoxin, a selective N-CC inhibitor, and cilnidipine significantly decreased Cacna 1b mRNA, which codes for N-CC, in podocytes. Exogenous Ang II resulted in an increased expression of N-CC mRNA in podocytes. However, this increase was significantly suppressed by pre-incubation with either ω-conotoxin or cilnidipine (Fig. 6). These results indicate that the inhibition of N-CC in podocytes results in a negative feedback on its expression.
Fig. 6.
N-CC mRNA expression in cultured murine podocytes. N-CC mRNA expression was strongly suppressed when cultured mice podocytes were treated with ω-conotoxin or cilnidipine. Ang II significantly increased N-CC mRNA expression in cells. This increase was prevented with a pre-incubation with either ω-conotoxin or cilnidipine. *P < 0.05 vs. control, #P < 0.05 vs. Ang II control (n = 6).
Discussion
In agreement with the findings of previous animal and clinical studies (4, 30, 31), cilnidipine, an N/L-type CCB, demonstrated strong anti-proteinuric and anti- albuminuric effects in HS-UNX-SHR, confirming its reno-protective effects in hypertensive renal damage. Results from the renal Ang II and glomerular desmin staining experiments also suggest that cilnidipine inhibits renal RAS and protects podocytes. Moreover, the major finding of the present study was that cilnidipine similarly attenuated renal injury in both denervated and innervated HS-UNX-SHR, suggesting that its reno-protective mechanisms are independent of renal sympathetic nerves (4).
Cilnidipine demonstrated greater renal protective effects compared to L-type calcium-channel blockers (e.g., amlodipine) in various animal models (4, 13, 32, 33), as well as hypertensive patients (2, 3, 34, 35). Based on these findings, more attention has been recently paid to elucidate the relationship between the reno-protective effects of cilnidipine and its N-CC inhibition within the kidney. Since N-CC is known to be expressed in renal nerves and is capable of controlling neurotransmitter release (e.g., NE), it was suggested that the reno-protective mechanisms of cilnidipine primarily involve the sympathetic nervous system (36, 37). Therefore, we investigated the involvement of renal sympathetic nerves in cilnidipine- induced reno-protection in denervated HS-UNXSHR. We found that renal denervation reduced renal NE content to undetectable levels, and suppressed elevations in blood pressure in HS-UNX-SHR, as previously reported (7, 38, 39). Furthermore, glomerulo-mesangial expansion was similarly attenuated, which was possibly due to the anti-hypertensive effects of cilnidipine. However, there were no statistically significant improvements in renal parameters, such as podocyte injury, albuminuria, and proteinuria, indicating that renal sympathetic nerves did not contribute to the development of the renal lesions in our model. Conversely, regardless of whether renal nerves were present or not, cilnidipine suppressed the development of proteinuria and normalized podocyte injury, despite that the blood pressures of the cilnidipine-treated animals were still much greater than those of the normotensive controls. These findings indicate that cilnidipine appears to have protective effects on podocytes, independent of its inhibitory effects on N-CCs in the renal sympathetic nerves of HS-UNX-SHR.
We previously demonstrated that glomerular podocytes, cells that play an important role in the glomerular filtration barrier, express N-CC (10). In the present study, cilnidipine prevented the development of renal injury in HS-UNX-SHR. The precise mechanism by which cilnidipine may protect podocytes is unclear, but its inhibitory effects on L-CCs are unlikely to participate in reno-protection, as it was previously reported that L-CCs do not affect the changes in the intracellular calcium levels of podocytes induced by Ang II (40), NE (41), and acetylcholine (42). Additionally, to the best of our knowledge, there are no reports showing that L-CCs are expressed in podocytes. Conversely, N-CCs are known to be involved in the generation of reactive oxygen species in response to Ang II in cultured podocytes (4), and thus, the inhibition of this response could be one of the mechanisms involved in cilnidipine-induced podocyte protection. Moreover, in the present study, we found that the protective effects against podocyte injury were accompanied by the suppression of N-CC expression in cilnidipine-treated HS-UNX-SHR. Furthermore, N-CC inhibition down-regulated the expression of N-CC in cultured podocytes. Therefore, cilnidipine may, in part, exert its protective effects on podocytes via either its pharmacological inhibition or down-regulation of N-CC in podocytes.
CCBs regulate glomerular pressure by changing the afferent and efferent arteriole tone (11 – 13). Zhou et al. (11) demonstrated that cilnidipine decreases the single nephron filtration fraction, glomerular capillary pressure, afferent and efferent arteriole resistance, and proteinuria in nitric oxide synthase-inhibited SHR, indicating that cilnidipine attenuates glomerular hypertension and, subsequently, prevents proteinuria. Similar arteriole responses were observed in dog kidneys (12) and hydro-nephrotic SHR (13), More importantly, Konno et al. (13) revealed that nifedipine, another L-CC blocker, dilated the afferent, but not the efferent arteriole, whereas cilnidipine dilated both the afferent and efferent arterioles. Their findings suggest that N-CC inhibition via cilnidipine may elicit efferent vasodilation. Therefore, it is possible that the effects of cilnidipine on glomerular hemodynamics are, in part, involved in its reno-protective effects in HS-UNX-SHR. However, it should be mentioned that we did not assess glomerular hemodynamics in the present study. Thus, further studies are warranted to address whether cilnidipine has any effects on glomerular hemodynamics.
Although cilnidipine was expected to both inhibit N-CCs in renal nerve endings and reduce neurotransmitter release (5, 6), it was found that cilnidipine did not reduce the increased renal NE levels in HS-UNX-SHR. This may be a result of a negative feedback response due to the blood pressure decreases induced by cilnidipine (37). That is, the suppressing effects of cilnidipine on renal NE content may be masked by the increase in NE release induced by its blood pressure reduction, as also suggested by other investigators (37).
Renal denervation in HS-UNX-SHR did not significantly reduce renal Ang II content. However, it is well known that renal sympathetic tone regulates renin release from the juxtaglomerular apparatus. This finding is consistent with those of previous studies, which demonstrated that plasma renin activity is not affected in high-salt–fed SHR following renal denervation (43, 44). Thus, the contribution of the sympathetic nervous system on renin release may be reduced on a high-salt diet.
Cilnidipine suppressed renal Ang II content. The cells that produce Ang II are unidentified in the present study due to the limitation in performing immunohistochemistry for this octapeptide in vivo. It has been shown that renal proximal tubular cells express all components that are necessary to produce Ang II and that Ang II in proximal tubular lumen can be retrieved into the cells through AT1 receptor–dependent endocytosis; this process accelerates de novo angiotensinogen synthesis and further Ang II accumulation (45). Moreover, Cao et al. (46) demonstrated that albumin stress stimulated RAS in proximal tubular cells. Therefore, the increase in renal Ang II in HS-UNX-SHR may have resulted from the increased albumin stress in proximal tubules as a consequence of podocyte injury, and it should be deemed reasonable to say that cilnidipine reduced the renal Ang II through the reduction of albumin leakage from glomeruli by podocyte protection.
We cannot exclude the possibility that reduced glomerular damage (e.g., desmin staining for podocyte damage and PAS staining for sclerotic change) result in less expression of NCC in the glomeruli of cilnidipine-treated HS-UNX-SHR. On the other hand, in our previous study, we have demonstrated that cilnidipine attenuated the glomerular damage in obese SHR model without the changes in NCC expression in podocytes (14). Thus, taken together, the relationship between NCC expression and glomerular damage does not seem to be simple and could be independent consequences of cilnidipine treatment.
In conclusion, cilnidipine suppressed proteinuria and albuminuria by attenuating podocyte injury in HS-UNX-SHR. It appears that the reno-protective effects of cilnidipine are induced via mechanisms that are independent of renal sympathetic nerve inhibition. Future studies need to clarify the possible involvement of N-CC on the development of podocyte injury.
Acknowledgments
We are grateful to Mochida Pharma Co. (Tokyo) for supplying cilnidipine. This work was supported, in part, by a grant-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to Akira Nishiyama (20590253).
References
- 1.Fujita T, Ando K, Nishimura H, Ideura T, Yasuda G, Isshiki M, et al. Antiproteinuric effect of the calcium channel blocker cilnidipine added to renin-angiotensin inhibition in hypertensive patients with chronic renal disease. Kidney Int. 2007;72:1543–1549. doi: 10.1038/sj.ki.5002623. [DOI] [PubMed] [Google Scholar]
- 2.Morimoto S, Yano Y, Maki K, Iwasaka T. Renal and vascular protective effects of cilnidipine in patients with essential hypertension. J Hypertens. 2007;25:2178–2183. doi: 10.1097/HJH.0b013e3282c2fa62. [DOI] [PubMed] [Google Scholar]
- 3.Konoshita T, Makino Y, Kimura T, Fujii M, Wakahara S, Arakawa K, et al. A new-generation N/L-type calcium channel blocker leads to less activation of the renin-angiotensin system compared with conventional L type calcium channel blocker. J Hypertens. 2010;28:2156–2160. doi: 10.1097/HJH.0b013e32833d01dd. [DOI] [PubMed] [Google Scholar]
- 4.Fan YY, Kohno M, Nakano D, Ohsaki H, Kobori H, Suwarni D, et al. Cilnidipine suppresses podocyte injury and proteinuria in metabolic syndrome rats: possible involvement of N-type calcium channel in podocyte. J Hypertens. 2010;28:1034–1043. doi: 10.1097/hjh.0b013e328336ade3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirning LD, Fox AP, McCleskey EW, Olivera BM, Thayer SA, Miller RJ, et al. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988;239:57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- 6.Takahara A, Dohmoto H, Hisa H, Satoh S, Yoshimoto R. Cilnidipine attenuates renal nerve stimulation-induced renal vasoconstriction and antinatriuresis in anesthetized dogs. Jpn J Pharmacol. 1997;75:27–32. doi: 10.1254/jjp.75.27. [DOI] [PubMed] [Google Scholar]
- 7.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment- resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 8.Klein IH, Ligtenberg G, Neumann J, Oey PL, Koomans HA, Blankestijn PJ. Sympathetic nerve activity is inappropriately increased in chronic renal disease. J Am Soc Nephrol. 2003;14:3239–3244. doi: 10.1097/01.asn.0000098687.01005.a5. [DOI] [PubMed] [Google Scholar]
- 9.Schlaich MP, Socratous F, Hennebry S, Eikelis N, Lambert EA, Straznicky N, et al. Sympathetic activation in chronic renal failure. J Am Soc Nephrol. 2009;20:933–939. doi: 10.1681/ASN.2008040402. [DOI] [PubMed] [Google Scholar]
- 10.Comper WD, Hilliard LM, Nikolic-Paterson DJ, Russo LM. Disease-dependent mechanisms of albuminuria. Am J Physiol Renal Physiol. 2008;295:F1589–F1600. doi: 10.1152/ajprenal.00142.2008. [DOI] [PubMed] [Google Scholar]
- 11.Zhou X, Ono H, Ono Y, Frohlich ED. N- and L-type calcium channel antagonist improves glomerular dynamics, reverses severe nephrosclerosis, and inhibits apoptosis and proliferation in an l-NAME/SHR model. J Hypertens. 2002;20:993–1000. doi: 10.1097/00004872-200205000-00035. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi K, Wakino S, Sugano N, Ozawa Y, Homma K, Saruta T. Ca2+ channel subtypes and pharmacology in the kidney. Circ Res. 2007;100:342–353. doi: 10.1161/01.RES.0000256155.31133.49. [DOI] [PubMed] [Google Scholar]
- 13.Konno Y, Kimura K. Vasodilatory effect of cilnidipine, an L-type and N-type calcium channel blocker, on rat kidney glomerular arterioles. Int Heart J. 2008;49:723–732. doi: 10.1536/ihj.49.723. [DOI] [PubMed] [Google Scholar]
- 14.Fan YY, Kohno M, Nakano D, Hitomi H, Nagai Y, Fujisawa Y, et al. Inhibitory effects of a dihydropyridine calcium channel blocker on renal injury in aldosterone-infused rats. J Hypertens. 2009;27:1855–1862. doi: 10.1097/HJH.0b013e32832dda6f. [DOI] [PubMed] [Google Scholar]
- 15.Fujii T, Kurata H, Takaoka M, Muraoka T, Fujisawa Y, Shokoji T, et al. The role of renal sympathetic nervous system in the pathogenesis of ischemic acute renal failure. Eur J Pharmacol. 2003;481:241–248. doi: 10.1016/j.ejphar.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 16.Zhou X, Liu JX, Shi R, Yang N, Song DL, Pang W, et al. Compound ion salt, a novel low-sodium salt substitute: from animal study to community-based population trial. Am J Hypertens. 2009;22:934–942. doi: 10.1038/ajh.2009.135. [DOI] [PubMed] [Google Scholar]
- 17.Susic D, Frohlich ED, Kobori H, Shao W, Seth D, Navar LG. Salt-induced renal injury in SHRs is mediated by AT1 receptor activation. J Hypertens. 2011;29:716–723. doi: 10.1097/HJH.0b013e3283440683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishiyama A, Nakagawa T, Kobori H, Nagai Y, Okada N, Konishi Y, et al. Strict angiotensin blockade prevents the augmentation of intrarenal angiotensin II and podocyte abnormalities in type 2 diabetic rats with microalbuminuria. J Hypertens. 2008;26:1849–1859. doi: 10.1097/HJH.0b013e3283060efa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pardo NE, Hajela RK, Atchison WD. Acetylcholine release at neuromuscular junctions of adult tottering mice is controlled by N-(cav2.2) and R-type (cav2.3) but not L-type (cav1.2) Ca2+ channels. J Pharmacol Exp Ther. 2006;319:1009–1020. doi: 10.1124/jpet.106.108670. [DOI] [PubMed] [Google Scholar]
- 20.Mundel P, Reiser J, Zuniga Mejia Borja A, Pavenstadt H, Davidson GR, Kriz W, et al. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- 21.Asanuma K, Yanagida-Asanuma E, Faul C, Tomino Y, Kim K, Mundel P. Synaptopodin orchestrates actin organization and cell motility via regulation of rhoa signalling. Nat Cell Biol. 2006;8:485–491. doi: 10.1038/ncb1400. [DOI] [PubMed] [Google Scholar]
- 22.Hu WY, Fukuda N, Su JZ, Kanmatsuse K. Effects of the L- and N-type calcium channel blocker cilnidipine on growth of vascular smooth muscle cells from spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2001;38:450–459. doi: 10.1097/00005344-200109000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Takahara A, Fujita S, Moki K, Ono Y, Koganei H, Iwayama S, et al. Neuronal Ca2+ channel blocking action of an antihypertensive drug, cilnidipine, in IMR-32 human neuroblastoma cells. Hypertens Res. 2003;26:743–747. doi: 10.1291/hypres.26.743. [DOI] [PubMed] [Google Scholar]
- 24.Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, et al. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension. 2004;43:841–848. doi: 10.1161/01.HYP.0000118519.66430.22. [DOI] [PubMed] [Google Scholar]
- 25.Fan YY, Baba R, Nagai Y, Miyatake A, Hosomi N, Kimura S, et al. Augmentation of intrarenal angiotensin II levels in uninephrectomized aldosterone/salt-treated hypertensive rats; renoprotective effects of an ultrahigh dose of olmesartan. Hypertens Res. 2006;29:169–178. doi: 10.1291/hypres.29.169. [DOI] [PubMed] [Google Scholar]
- 26.Lei B, Hitomi H, Mori T, Nagai Y, Deguchi K, Mori H, et al. Effect of efonidipine on TGF-β1-induced cardiac fibrosis through Smad2-dependent pathway in rat cardiac fibroblasts. J Pharmacol Sci. 2011;117:98–105. doi: 10.1254/jphs.11065fp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobori H, Urushihara M, Xu JH, Berenson GS, Navar LG. Urinary angiotensinogen is correlated with blood pressure in men (bogalusa heart study) J Hypertens. 2010;28:1422–1428. doi: 10.1097/HJH.0b013e3283392673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bu X, Zhou Y, Zhang H, Qiu W, Chen L, Cao H, et al. Systemic administration of naked plasmid encoding HGF attenuates puromycin aminonucleoside-induced damage of murine glomerular podocytes. Am J Physiol Renal Physiol. 2011;301:F784–F792. doi: 10.1152/ajprenal.00210.2011. [DOI] [PubMed] [Google Scholar]
- 29.Habibi J, Hayden MR, Sowers JR, Pulakat L, Tilmon RD, Manrique C, et al. Nebivolol attenuates redox-sensitive glomerular and tubular mediated proteinuria in obese rats. Endocrinology. 2011;152:659–668. doi: 10.1210/en.2010-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aritomi S, Koganei H, Wagatsuma H, Mitsui A, Ogawa T, Nitta K, et al. The N-type and L-type calcium channel blocker cilnidipine suppresses renal injury in Dahl rats fed a high-salt diet. Heart Vessels. 2010;25:549–555. doi: 10.1007/s00380-010-0005-4. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka M. The L/N-type calcium channel blocker, Cilnidipine, reduces heart rate and albuminuria in patients with type 2 diabetes. J Int Med Res. 2010;38:602–610. doi: 10.1177/147323001003800222. [DOI] [PubMed] [Google Scholar]
- 32.Toba H, Yoshida M, Tojo C, Nakano A, Oshima Y, Kojima Y, et al. L/N-type calcium channel blocker cilnidipine ameliorates proteinuria and inhibits the renal renin-angiotensin-aldosterone system in deoxycorticosterone acetate-salt hypertensive rats. Hypertens Res. 2011;34:521–529. doi: 10.1038/hr.2010.279. [DOI] [PubMed] [Google Scholar]
- 33.Konda T, Enomoto A, Matsushita J, Takahara A, Moriyama T. The N- and L-type calcium channel blocker cilnidipine suppresses renal injury in dahl rats fed a high-sucrose diet, an experimental model of metabolic syndrome. Nephron Physiol. 2005;101:p1–p13. doi: 10.1159/000085713. [DOI] [PubMed] [Google Scholar]
- 34.Masuda T, Ogura MN, Moriya T, Takahira N, Matsumoto T, Kutsuna T, et al. Beneficial effects of L- and N-type calcium channel blocker on glucose and lipid metabolism and renal function in patients with hypertension and type II diabetes mellitus. Cardiovasc Ther. 2011;29:46–53. doi: 10.1111/j.1755-5922.2009.00126.x. [DOI] [PubMed] [Google Scholar]
- 35.Katayama K, Nomura S, Ishikawa H, Murata T, Koyabu S, Nakano T. Comparison between valsartan and valsartan plus cilnidipine in type II diabetics with normo- and microalbuminuria. Kidney Int. 2006;70:151–156. doi: 10.1038/sj.ki.5000349. [DOI] [PubMed] [Google Scholar]
- 36.Konda T, Enomoto A, Takahara A, Yamamoto H. Effects of L/N-type calcium channel antagonist, cilnidipine on progressive renal injuries in dahl salt-sensitive rats. Biol Pharm Bull. 2006;29:933–937. doi: 10.1248/bpb.29.933. [DOI] [PubMed] [Google Scholar]
- 37.Konda T, Enomoto A, Aritomi S, Niinuma K, Koganei H, Ogawa T, et al. Different effects of L/N-type and L-type calcium channel Cilnidipine and Renal Sympathetic Nerves 9 blockers on the renin-angiotensin-aldosterone system in SHR/Izm. Am J Nephrol. 2009;30:155–161. doi: 10.1159/000210396. [DOI] [PubMed] [Google Scholar]
- 38.Iliescu R, Yanes LL, Bell W, Dwyer T, Baltatu OC, Reckelhoff JF. Role of the renal nerves in blood pressure in male and female SHR. Am J Physiol Regul Integr Comp Physiol. 2006;290:R341–R344. doi: 10.1152/ajpregu.00035.2005. [DOI] [PubMed] [Google Scholar]
- 39.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 40.Nitschke R, Henger A, Ricken S, Gloy J, Muller V, Greger R, et al. Angiotensin II increases the intracellular calcium activity in podocytes of the intact glomerulus. Kidney Int. 2000;57:41–49. doi: 10.1046/j.1523-1755.2000.00810.x. [DOI] [PubMed] [Google Scholar]
- 41.Huber TB, Gloy J, Henger A, Schollmeyer P, Greger R, Mundel P, et al. Catecholamines modulate podocyte function. J Am Soc Nephrol. 1998;9:335–345. doi: 10.1681/ASN.V93335. [DOI] [PubMed] [Google Scholar]
- 42.Nitschke R, Henger A, Ricken S, Muller V, Kottgen M, Bek M, et al. Acetylcholine increases the free intracellular calcium concentration in podocytes in intact rat glomeruli via muscarinic M(5) receptors. J Am Soc Nephrol. 2001;12:678–687. doi: 10.1681/ASN.V124678. [DOI] [PubMed] [Google Scholar]
- 43.Golin R, Pieruzzi F, Munforti C, Busca G, Di Blasio A, Zanchetti A. Role of the renal nerves in the control of renin synthesis during different sodium intakes in the rat. J Hypertens. 2001;19:1271–1277. doi: 10.1097/00004872-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 44.Pieruzzi F, Munforti C, Di Blasio A, Busca G, Dadone V, Zanchetti A, et al. Neuronal nitric oxide synthase and renin stimulation by sodium deprivation are dependent on the renal nerves. J Hypertens. 2002;20:2039–2045. doi: 10.1097/00004872-200210000-00024. [DOI] [PubMed] [Google Scholar]
- 45.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 46.Cao W, Zhou QG, Nie J, Wang GB, Liu Y, Zhou ZM, et al. Albumin overload activates intrarenal renin-angiotensin system through protein kinase C and NADPH oxidase-dependent pathway. J Hypertens. 2011;29:1411–1421. doi: 10.1097/HJH.0b013e32834786f0. [DOI] [PubMed] [Google Scholar]