Abstract
Human parainfluenza viruses (HPIV) are uncommon, yet high-risk pathogens after hematopoietic stem cell transplantation (HCT). We evaluated 5178 pediatric and adult patients undergoing HCT between 1974 and 2010 to determine the incidence, risk factors, response to treatment, and outcome of HPIV infection as well as any change in frequency or character of HPIV infection over time. HPIV was identified in 173 patients (3.3%); type 3 was most common (66%). HPIV involved the upper respiratory tract (URTI) (57%), lower respiratory tract (LRTI) (9%), and both (34%) areas of the respiratory tract, at a median of 62 days after transplant. In more recent years, HPIV has occurred later after HCT, while the proportion with nosocomial infection and mortality decreased. Over the last decade, HPIV was more common in older patients and in those receiving reduced intensity conditioning (RIC). RIC was a significant risk factor for later (beyond day +30) HPIV infections and this association was strongest in patients with URTI. HCT using a matched unrelated donor (MUD), mismatched related donor (MMRD), age 10-19 years, and graft-versus-host disease (GVHD) were all risk factors for HPIV infections. LRTI, early (<30 days) infections, age 10-19 years, MMRD, steroid use, and coinfection with other pathogens were risk factors for mortality. The survival of patients with LRTI, especially very early infections, was poor regardless of ribavirin treatment. HPIV incidence remains low, but may have delayed onset associated with RIC regimens and improving survival. Effective prophylaxis and treatment for HPIV are needed.
Keywords: Human parainfluenza virus, Infection, Respiratory Virus, HCT (hematopoietic cell transplantation), Reduced Intensity Conditioning
INTRODUCTION
Human parainfluenza viruses (HPIV), medium-sized enveloped single-stranded RNA viruses, belong to the Paramyxoviridae family. HPIVs are divided into 4 different types due to their genetic and antigenic characteristics, although most clinical infections are due to types 1, 2, and 3. HPIV can cause both upper and lower respiratory tract infections (RTIs), and less frequently, central nervous system (CNS) infections [1-3]. Clinical manifestations range widely, from croup, otitis media, and bronchitis to life-threatening pneumonitis.[4-6] The vast majority of HPIV infections occur in infants and children [2]. A second population vulnerable to HPIV infections is immunocompromised patients including hematopoietic stem cell transplant (HCT) recipients [1, 7-14]. The incidence of HPIV has been reported between 2 to 7% after HCT [1, 9, 13]. Beyond this variation in HPIV incidence after HCT, the preferred treatment and impact on mortality are uncertain [9, 13, 15, 16].
We evaluated HPIV incidence, risk factors, outcome, and response to treatment in pediatric and adult patients undergoing autologous or allogeneic HCT (autoHCT or alloHCT) at the University of Minnesota. We evaluated changes in the timing and character of HPIV infection over several decades, comparing the frequency of HPIV infection, type of HPIV, clusters or isolated infections, nosocomial or community-acquired infections, upper respiratory tract infections (URTI) or lower RTI (LRTI), and seasonal predominance.
PATIENTS AND METHODS
Patient population
This retrospective cohort study included 5178 consecutive pediatric and adult patients who received HCT at the University of Minnesota (median age 29; range, 0 to 74 years) between January, 1974, and December, 2010. Transplant types included 1717 (33%) autoHCT and 3461 (67%) alloHCT. 173 HPIV cases were identified, including seven diagnosed in the two weeks prior to HCT. Patients received conditioning for transplant, prophylaxis of graft-versus-host disease (GVHD) and infections per active institutional protocols which, of course, varied over the period of study [17-19]. The intensity of conditioning regimens was defined according to the Center for International Blood and Marrow Transplant Research (CIBMTR) guidelines [20]. For analysis, ex vivo and in vivo (e.g., anti-thymocyte globulin, ATG) T-cell depletion were combined as T-cell depletion. All transplant protocols were approved by the University of Minnesota Institutional Review Board. All patients or their legal guardians provided written informed consent for the transplantation procedure including collection of long-term prospective outcome data. The median follow-up time for all survivors and for patients with HPIV was 6.5 and 6.0 years, respectively.
For patients demonstrating acute onset of URTI symptoms, respiratory virus cultures of throat and/or nasopharyngeal swabs or washes were obtained. For any HCT patient undergoing bronchoalveolar lavage (BAL), lung biopsy, or autopsy, respiratory virus cultures were part of preplanned diagnostic protocols and obtained on all samples. Inpatients with HPIV infection were placed in droplet precautions, which involved using gown, glove, and mask procedures to prevent spread of infection to other patients or healthcare workers.
To evaluate any changing characteristics of HPIV over time, we examined patients in 3 time periods: 1974-1992, 1993-2001, and 2002-2010. The first period was composed of almost 2 decades because it had fewer patients and it reflected the data from an early transplantation era. Available data on 387 adult patients surviving greater than 80 days were also evaluated for late-onset noninfectious pulmonary complications (LONIPCs) between 2002 and 2007. Diffuse alveolar hemorrhage, idiopathic pneumonia syndrome, bronchiolitis obliterans, and bronchiolitis obliterans with organizing pneumonia were considered as LONIPCs.
Virology Procedures
Standard methods were used for isolation of HPIV from respiratory samples. HPIV was isolated in tube cultures containing a monolayer of primary rhesus-monkey kidney cells and detected by hemadsorption with guinea pig red cells at 3, 7, and 10 days. All negative viral cultures were finalized at 21 days of incubation. All BAL and lung tissue specimens were concurrently submitted for bacterial, mycobacterial, and fungal cultures. Sputum and autopsy samples were submitted for bacterial and fungal cultures.
Isolates recovered in culture were typed by immunofluorescence with commercially available antiserum. HPIV virus identification typically took 7-10 days overall: 7 days for types 1 and 3, and 8-9 days for type 2.
Since January of 2004, the method of conventional tube culture was supplemented with an R-Mix shell vial culture. Monolayer staining was checked at 2 days, using a pooled stain that can detect parainfluenza viruses 1, 2, and 3 in addition to other respiratory viruses.
Definitions
Infection Episode was defined as the cultured presence of HPIV in a sample from the pulmonary tree of a patient concomitantly displaying symptoms compatible with clinical infection. Patients with symptoms / signs of croup, nasopharyngitis, conjunctivitis, and otitis media were defined as URTI. URTI infection excluded dyspnea and/or newly appearing infiltrates on radiographic imaging methods (chest radiograph or CT scan). Patients were considered to have LRTI if they had symptoms / signs (e.g., fever, expiratory wheezing, tachypnea, dyspnea, retractions, rales, pulmonary infiltrates on chest X-ray or computed tomograpy) of tracheobronchitis or pneumonia. Respiratory failure is defined as mechanical ventilation occurring within 30 days of HPIV.
Nosocomial infection was defined as infection developing 5 or more days following hospital admission. All other infections were classified as community-acquired.
Cluster Infection was defined as 3 or more cases of nosocomial or community acquired infection apparent within a 30-day period.
Coinfection was a second respiratory pathogen occurring within a month of HPIV infection. Fever of unknown origin was not categorized as coinfection.
Treatment of HPIV Infection
Treatment of HPIV infections depended on the patient’s condition, URTI or LRTI, and was at the discretion of treating physician. Those patients who required treatment received aerosolized ribavirin. Aerosolized ribavirin was administered as nebulization through mask or endotracheal tube. From 1984 to 1995, the drug was generally administered as 6 grams in a continuous aerosol nebulization for 18 to 24 hours per day. From 1995 to 2005, some patients received aerosolized ribavirin using a schedule of 2 grams over 2 hours 3 times daily. The duration of therapy differed between patients. Usually, a 5-day course of treatment was delivered, unless continued symptoms and/or ongoing positive cultures indicated a clinical need for repeated or longer courses. Some patients received intravenous immunoglobulin (IVIG) along with ribavirin.
Data and Statistical analysis
Analysis of the University of Minnesota Blood and Marrow Transplant database was used for data extraction. The University of Minnesota HCT database contains prospectively collected data on all patients transplanted at our center. Clinical data in this computerized research database included details of antiviral medications used to treat HPIV infection episodes. Additional clinical information was collected from available medical records. For patients who never developed HPIV, their first transplant was included for analysis. For patients with HPIV, the most recent transplant relative to HPIV onset was used.
Patient characteristics were compared by the chi-square test. Cox regression was used to identify risk factors for HPIV, with time 0 set as the transplant date and time to HPIV as the outcome. A Fine and Gray competing risks model was also fit to identify risk factors for HPIV, accounting for death as a competing risk. These results (not reported) showed good agreement with the Cox model. Cox regression was used to identify risk factors for mortality within 100 days of HPIV, and for subgroups with LRTI. Survival time was taken as time from infection. No violations of proportional hazards were detected. Multivariate Cox models used a forward stepwise selection process with a p-value cutoff of 0.15 for inclusion in the final model. Acute and chronic GVHD (aGVHD and cGVHD) were considered as time-dependent covariates. Additionally, the association between HPIV and mortality was tested using a Cox model, with URTI and LRTI as time-dependent covariates and adjusting for other factors (diagnosis category, donor type, transplant year, age, conditioning intensity, and CMV) potentially associated with survival.
RESULTS
HPIV patient and infection characteristics
Transplantation characteristics for patients with HPIV infection and controls with no infection are shown in Table 1. HPIV infection was diagnosed in 173 patients (3.3%). The majority of HPIV infections were caused by HPIV type 3 (66%) (Figure 1). The observed rates of nosocomial (41%) or community acquired (59%) infection and of cluster (45%) or single case (55%) infection were similar. The median interval from transplantation to HPIV infection was 62 days (range -8 to +259 days). Most infections (83%) occurred during the first year after transplant and more than half of infections (58%) were diagnosed in the first 100 days; 34% in the first 30 days. HPIV infection affected only the upper respiratory tract (URTI) in 98 patients (57%), only lower respiratory tract (LRTI) in 15 patients (9%), and both in 60 patients (34%). Among the 75 patients with LRTI, 43 (57%) were culture positive in BAL, the rest of the patients were diagnosed via radiologic or physical exam findings supported by virologic diagnostic material from non-invasive (nasal or throat) sampling. Ribavirin with or without IVIG was administered to 51 patients (29%); 10 with URTI (10%); and 41 with LRTI (55%).
Table 1.
Comparison of HPIV patients with no HPIV patients over 3 different time eras
| Transplant Era | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1974-1992 | 1993-2001 | 2002-2010 | |||||||
| Controls n (%) |
HPIV Cases n (%) |
P- value |
Controls n (%) |
HPIV Cases n (%) |
P- value |
Controls n (%) |
HPIV Cases n (%) |
P- value |
|
| N | 1657 | 51 | 1737 | 57 | 1611 | 65 | |||
| Median age | 18.3 | 17.5 | 0.85 | 32.8 | 33.1 | 0.97 | 39.0 | 46.9 | 0.14 |
| Age by range | 0.80 | 0.10 | 0.03 | ||||||
| Age < 10 | 514 (31) | 18 (35) | 424 (24) | 14 (25) | 346 (21) | 8 (12) | |||
| Age 10-19 | 359 (22) | 10 (20) | 224 (13) | 2 (4) | 210 (13) | 4 (6) | |||
| Age ≥ 20 | 784 (47) | 23 (45) | 1089 (63) | 41 (72) | 1055 | 53 (82) | |||
| Male | 977 (59) | 33 (65) | 0.41 | 933 (54) | 36 (63) | 0.16 | 984 (61) | 35 (54) | 0.24 |
| Donor Sex Match | 0.57 | 0.23 | 0.60 | ||||||
| Match | 554 (51) | 16 (46) | 565 (52) | 29 (60) | 488 (43) | 20 (39) | |||
| Mismatch | 540 (49) | 19 (54) | 531 (48) | 19 (40) | 649 (57) | 31 (61) | |||
| Diagnosis category | 0.30 | 0.39 | 0.30 | ||||||
| Lymphoid-malignant | 227 (14) | 4 (8) | 306 (18) | 12 (21) | 401 (25) | 18 (28) | |||
| Myeloid-malignant | 1174 | 36 (71) | 1127 (65) | 32 (56) | 886 (55) | 39 (60) | |||
| Non-malignant | 256 (15) | 11 (22) | 304 (18) | 13 (23) | 324 (20) | 8 (12) | |||
| Autologous | 563 (34) | 16 (32) | 0.70 | 641 (37) | 9 (16) | < 0.01 | 474 (29) | 14 (22) | 0.17 |
| Allogeneic | < 0.01 | 0.76 | 0.32 | ||||||
| UCB | 1 (<1) | 0 (0) | 140 (8) | 7 (13) | 590 (37) | 26 (40) | |||
| Matched sibling | 824 (50) | 19 (37) | 445 (26) | 16 (28) | 359 (22) | 14 (22) | |||
| Other relatives | 67 (4) | 8 (16) | 49 (3) | 3 (5) | 49 (3) | 5 (8) | |||
| Unrelated | 202 (12) | 8 (16) | 462 (27) | 22 (39) | 139 (9) | 6 (9) | |||
| CMV serostatus | 0.30 | 0.84 | 0.61 | ||||||
| R+ | 893 (54) | 33 (65) | 813 (47) | 28 (49) | 859 (53) | 31 (48) | |||
| R−/D− | 608 (37) | 15 (30) | 754 (43) | 22 (39) | 676 (42) | 29 (45) | |||
| R−/D+ | 115 (7) | 2 (4) | 165 (10) | 5 (9) | 69 (4) | 4 (6) | |||
| Unknown | 41 (3) | 1 (2) | 5 (<1) | 2 (4) | 7 (<1) | 1 (2) | |||
| Conditioning Intensity | 0.20 | 0.11 | 0.01 | ||||||
| Myeloablative + TBI | 1266 | 35 (69) | 1225 (71) | 42 (74) | 631 (39) | 23 (35) | |||
| Myeloablative, no | 391 (24) | 16 (31) | 461 (27) | 11 (19) | 529 (33) | 13 (20) | |||
| RIC | 0 (0) | 0 (0) | 49 (3) | 4 (7) | 447 (28) | 29 (45) | |||
| T-depletion | 433 (26) | 20 (39) | 0.04 | 288 (18) | 19 (33) | < 0.01 | 404 (25) | 17 (26) | 0.85 |
P values represent chi square comparisons of characteristics of HPIV infected vs. control patients within each time era.
Abbreviations: CMV, Cytomegalovirus; R, recipient; D, donor; RIC, reduced intensity conditioning; TBI, total body irradiation; UCB, umbilical cord blood
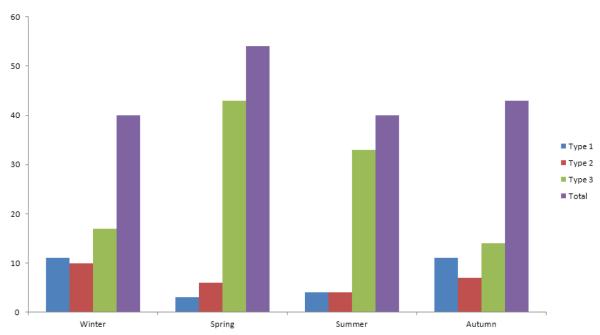
Figure 1.
Frequency of HPIV infections per season
Seasonal Prevalence of HPIV
HPIV infections occurred throughout the year with similar rates, ranging from 40 to 53 events over the 4 seasons (Figure 1). HPIV 3 was the most common isolate compared to other HPIV types throughout the year and was more common in warmer seasons (spring and summer, 76 events) compared to autumn and winter (31 events) (Figure 1).
Changes in HPIV over Time
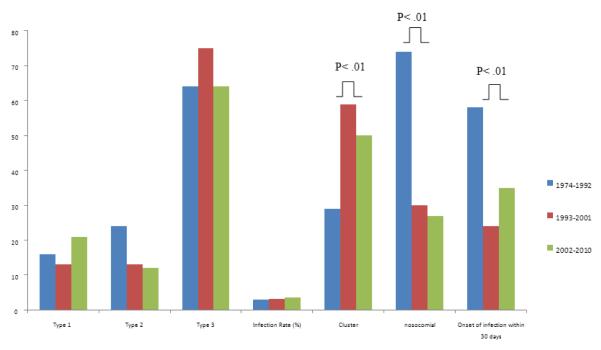
Over time (1973-1992 vs. 1993-2001 vs. 2002-2010), the type of HPIV and total HPIV infection rate did not change (3.0% vs. 3.2% vs. 3.9%, P= .31) (Figure 2). The proportion of nosocomial HPIV infections (74% vs. 30% vs. 27%, P< .01) and early HPIV infections (diagnosed within 30 days post HCT (58% vs. 24% vs. 35%, P< .01) decreased in the more recent eras. The median time to HPIV infection was later as well occurring at a median of 21 days (interquartile range (IQR) 6-79 days) from 1974-1992 compared to 135 days (IQR 45-395 days) from 1993-2001 and 75 days (IQR 18-210 days) from 2002-2010. The proportion of cluster HPIV cases increased (29% vs. 59% vs. 50%, P< .01) (Figure 2), though this may in part be due to more aggressive surveillance in the latter eras.
Figure 2.
Trends of infection characteristics over time
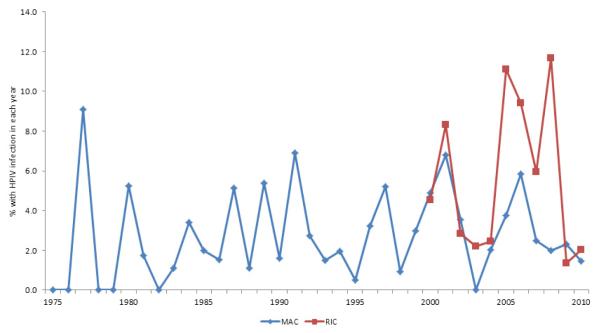
A comparison of demographic data between the patients with HPIV and the control group without HPIV infection in different transplantation eras is shown in Table 1. Over the last decade, HPIV occurred in more often in older patients and in patients who received RIC (Table 1, Figure 3). In earlier decades, this age and conditioning regimen disparity in HPIV infection rates was not apparent. In contrast, T-cell depletion was associated with more HPIV infections in earlier decades; however this difference was not noted over the last decade. Sibling alloHCT was associated with less HPIV in the earliest transplantation era compared to the more recent decades of study.
Figure 3.
Incidence of HPIV by year and conditioning intensity
Coinfections
Co-existing infection with other pathogens was detected in 68 HPIV patients (39%). Organisms identified concurrent with HPIV infections included bacteria (55 patients), viruses (37 patients), and fungi (19 patients, including 13 cases of Aspergillus). Polymicrobial infection occurred in 35 patients.
Association of HPIV with LONIPCs
Out of 387 patients (22 with HPIV and 365 with no HPIV), 65 developed LONIPCs. The rates of LONIPCs were 23% and 17% in HPIV and no HPIV patients (p=0.39), respectively.
Risk Factors for all-HPIV and LRT-HPIV Infections
Multivariate analysis showed that mismatched related donor (MMRD) and preceding cGVHD were independent risk factors for both all- and LRT-HPIV infections. AGHVD and a matched unrelated donor (MUD) were risk factors for HPIV infection. RIC regimens were risk factors for HPIV infections beyond 30 days after HCT (Table 2). This effect was strongest in patients with URTI, HR, 3.0; 95% CI, 1.4-6.1, P< .01. Male recipients had more frequent LRTI. Umbilical cord blood transplantation (UCBT) and MUD showed a trend for increased LRTI.
Table 2.
Multivariate analysis of risk factors for all and lower respiratory tract (LRT) HPIV Infections.
| All HPIV | LRTI | |||
|---|---|---|---|---|
| Factor | Risk ratio (95% CI) |
P-value | Risk ratio (95% CI) |
P-value |
| Donor type | ||||
| Autologous | Ref | Ref | ||
| UCB | 1.4 (0.8-2.5) | 0.31 | 2.4 (1.0-6.1) | 0.06 |
| Matched related | 0.9 (0.6-1.6) | 0.80 | 1.4 (0.6-3.2) | 0.41 |
| Matched unrelated | 2.0 (1.1-3.7) | 0.03 | 2.3 (0.8-6.0) | 0.11 |
| Mismatched related | 3.2 (1.6-6.3) | < 0.01 | 7.0 (2.7-18.3) | < 0.01 |
| Mismatched unrelated | 1.3 (0.6-2.7) | 0.55 | 0.9 (0.3-3.8) | 0.98 |
| Gender | ||||
| Male | Ref | Ref | ||
| Female | 0.9 (0.7-1.3) | 0.75 | 0.6 (0.3-1.0) | 0.04 |
| Diagnosis category | ||||
| Lymphoid/malignant | 1.2 (0.8-1.8) | 0.46 | 1.0 (0.5-1.9) | 0.89 |
| Myeloid/malignant | Ref | Ref | ||
| Non-malignant | 1.1 (0.7-1.8) | 0.66 | 0.9 (0.4-2.1) | 0.89 |
| CMV | ||||
| R+ | 1.0 (0.7-1.5) | 0.80 | 1.5 (0.9-2.5) | 0.15 |
| R−/D− | Ref | Ref | ||
| R−/D+ | 0.9 (0.4-1.7) | 0.66 | 1.2 (0.5-3.2) | 0.66 |
| Unknown | 2.1 (0.6-6.9) | 0.22 | 0.0 (0.0-0.0) | 0.98 |
| Age | ||||
| Age < 10 years | 0.8 (0.5-1.3) | 0.42 | 0.6 (0.3-1.3) | 0.18 |
| Age 10-19 | 0.5 (0.3-0.9) | 0.03 | 0.5 (0.2-1.1) | 0.07 |
| Age ≥ 20 | Ref | Ref | ||
| Transplant Era | ||||
| 1974-1992 | 0.8 (0.5-1.4) | 0.56 | 0.8 (0.4-1.6) | 0.47 |
| 1993-2001 | 0.7 (0.3-1.8) | 0.48 | 0.9 (0.5-1.7) | 0.71 |
| 2002-2010 | Ref | Ref | ||
| RIC (risk < 30 days) | 0.7 (0.3-1.8) | 0.48 | 0.5 (0.1-1.9) | 0.54 |
| RIC (risk ≥ 30 days) | 2.1 (1.2-3.5) | 0.01 | 1.4 (0.6-3.1) | 0.42 |
| Acute GVHD | 1.9 (1.1-3.4) | 0.03 | 2.1 (0.8-5.3) | 0.11 |
| Chronic GVHD | 6.2 (3.7-10.4) | < 0.01 | 7.1 (3.4-14.8) | < 0.01 |
Abbreviations: GVHD, graft-versus-host disease; Ref, reference group for each comparison category; RIC; reduced intensity conditioning; UCB, umbilical cord blood
Respiratory failure and Survival
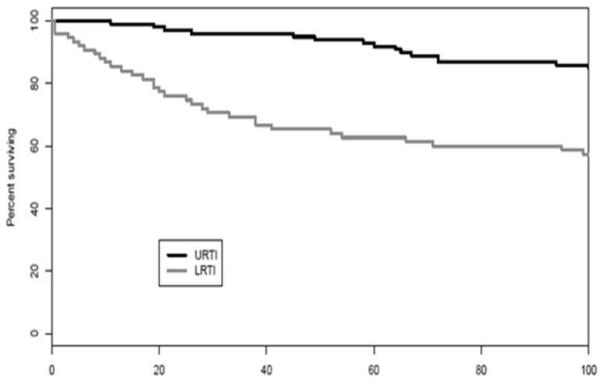
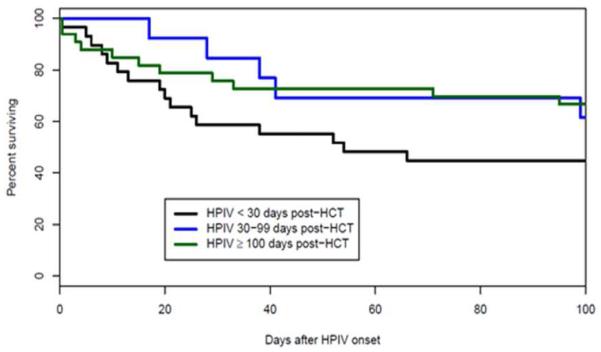
The need for mechanical ventilation within 30 days of HPIV infections and of LRT HPIV infections was 19% and 43%, respectively. The 100-day survival from the onset of mechanical ventilation was 23% in these patients. By comparison, 100-day survival from the onset of mechanical ventilation was similar in other, HPIV-uninfected patients, 22%. In multivariate analysis LRTI (HR, 2.6; 95% CI, 1.3-5.4, P< .01), but not URTI was associated with increased mortality (Figure 4A), and the mortality of LRTI patients at 100 days after infection decreased over time: 71% (15/21) from 1974-1992, 25% (6/24) from 1993-2001 and 37% (11/30) from 2002-2010.
Figure 4A.
Lower respiratory tract infection with HPIV is associated with higher mortality than upper tract infection.
In multivariate analysis LRTI, age 10-19 years (HR, 4.2; 95% CI, 1.8-9.9, P< .01), MMRD (HR, 3.8; 95% CI, 1.7-8.5, P< .01), early HPIV infection (HR, 2.3; 95% CI, 1.2-4.3, P< .01) (Figure 4B), the presence of coinfection (HR, 2.1; 95% CI, 1.0-4.2, P= .04), and steroid use (HR, 2.0; 95% CI, 1.0-3.9, P= .05) were also found be risk factors for mortality. Transplantation era and nosocomial acquisition of HPIV were not independent risk factors for mortality as they were significant only in univariate analysis.
Figure 4B.
Higher mortality with Early onset (< 30 days) of LRT HPIV
Anti-Viral Treatment and Outcome
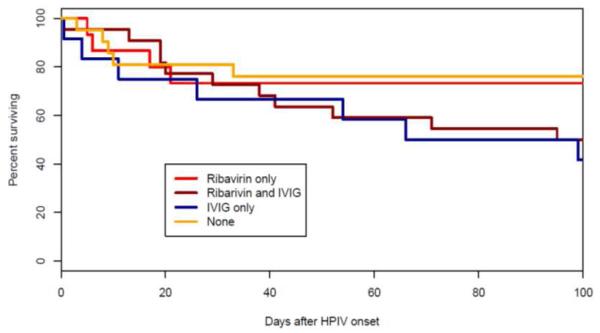
Overall, treated HPIV patients had higher mortality rates at 100-days after infection compared to those who were not treated (40% vs. 15%, P<.01) (Figure 4C), although clinical factors including the acuity at presentation may, of course, have directly influenced the decision to initiate specific therapy. However, even among those patients with LRTI, ribavirin-treatment led to similar mortality at 100-days after infection (46%) as no treatment with ribavirin (38%, P= .60).
Figure 4C.
Effect of antiviral treatment for LRT HPIV infection on survival.
DISCUSSION
This long term study has shown, for the first time, how RIC has changed HPIV infections over time. Over the 35 years of study, we observed a relatively stable incidence (approximately 3%) of HPIV infection in our large HCT population, similar to that shown by our and other earlier studies [1, 9, 13]. Notably, the age of HPIV patients has increased over the decades of study. HPIV 3 was the most common type we observed, which is consistent with data showing that HPIV 3 is the most common type in healthy community based pediatric populations [1, 2, 9, 13, 21]. Most HPIV 3 cases were diagnosed in warmer seasons among our HCT patients; also consistent with data showing that spring is its peak season in the general population [22]. Limitations of this retrospective cohort study include changes in the duration of follow-up of patients over time, as patients in the latter years of the study had only several years of long term follow-up.
Reduced intensity conditioning regimens were found to be a risk factor for human parainfluenza virus infection occurring later than the first 30 days posttransplantation. Most of these patients had upper respiratory tract infections. Although RIC was used mostly in older patients, older patients experienced similar HPIV compared to younger patients, and higher incidences of HPIV among RIC patients were present in all age groups. Moreover, while both acute and chronic GVHD increased HPIV infections, RIC remained as a risk factor in the multivariate analysis. Recent transplant patients might be followed longer at transplantation centers, live longer, or HPIV might be considered more often within time, which could have explained increased HPIV with RIC regimens. On the other hand, transplant era was not a significant factor for HPIV infections in the multivariate analysis. Therefore, RIC regimens seem to increase delayed HPIV infections.
Despite stability in the incidence of HPIV, there has been a change in the day of onset with more recent HPIV infections occurring later post-HCT. This later onset of infection was also associated with fewer nosocomial infections, more cluster infections, and slightly lower mortality. This delay may be due in part to a change in screening practices, as well as ongoing virologic surveillance at later time points after HCT. Our recognition of late infections has increased, because patients remain under review at transplantation centers longer, and patients survive longer, remaining at risk to develop infection later after HCT. Other as yet undefined changes in our clinical practice of HCT may influence this epidemiology as well.
In recent years, testing for respiratory viruses has come to include polymerase chain reaction (PCR) testing, together with or in place of culture or shell vial testing. Several studies have shown that reverse transcription-PCR and real-time PCR with specific primers and probes for each type of HPIV are more sensitive and specific than both viral culture and immunofluorescence-based detection methods [23-27]. The use of nucleic acid detection is so new that only future studies will be able to determine whether the incidence will change once most centers start exclusively using PCR testing. In setting of allogeneic HCT, these methods have detected viruses even in asymptomatic patients, with negative cultures [8, 27]. Taking into account these asymptomatic patients and low sensitivity of cultures, the current incidence of HPIV may be underestimated.
Higher risks for developing HPIV infection were observed with certain donor types (MUD and MMRD), and in patients with aGVHD and cGVHD. RIC was also a risk factor for later infection. RIC regimens are reported to be associated with increased delayed rate of viral infections, including CMV [28, 29]. Chakrabarti et al reported that median time to HPIV infection from RIC alloHCT was 72 days, which is very similar to our finding (75 days) over the last decade [21]. Alemtuzumab-containing RIC regimens were associated with increased HPIV infections, but not with high mortality rates [21].
Risks for mortality after HPIV infection included LRTI and early infections after HCT. Mortality from LRTI has improved over decades. However, respiratory failure requiring mechanical ventilation remained a poor prognostic factor for survival, which was similar to HPIV-uninfected patients in this study. Mechanical ventilation need was a significant risk factor for mortality in a report from Seattle as well [9]. Early HPIV infection associated with higher mortality was reported with 16% (10/61) mortality in HPIV patients overall, which was higher (21%) in patients with early (<100 days) HPIV infection versus only 5% for later infections [13]. We also observed higher mortality following matched URD and MMRD HCT, patients age 10-19, steroid use, and the presence of coinfections. Concurrent other infections have also been noted to increase risks of mortality in other studies [9, 13].
Ribavirin with or without IVIG was administered for some HPIV patients, but with no demonstrable improvement in survival, similar to some, but not all earlier reports [9, 30, 31]. However, differences in prognostic factors in those treated or not treated may preclude definitive conclusions. There is clinical bias in choosing who to treat with ribavirin, as they may appear sicker. Moreover, because of limited number of treated patients with URTI, the efficacy of ribavirin in patients with less severe infections is unknown. Sparrelid et al suggested that in patients with LRT viral infections, the level of nebulized ribavirin might be limited by bronchospasm or tissue inflammation blocking delivery of aerosolized drug to distal parts of lung [30]. This early study suggested use of intravenous ribavirin, but open label use of intravenous ribavirin associated with hemolysis [32].
Prophylaxis of HPIV infection may be more important given that no effective antiviral therapy is available. HPIV vaccines have been developed, but are not licensed or used for routine clinical practice [33, 34]. The non-seasonal nature of HPIV differs from respiratory syncytial virus, which is distinctly seasonal and can be recognized as a threat during community-associated outbreaks in children. Moreover, asymptomatic HPIV infection and shedding may occur in the HCT setting, making it difficult to know who is contagious [8]. The low rate of shedding also makes surveillance using PCR analysis cost-prohibitive and thereby impairs the prevention of HPIV infection by isolation for these contagious, yet asymptomatic HPIV patients. Aggressive screening for respiratory viral infection during any URI symptoms (year round) may be the most valuable method to limit HPIV infections and their associated morbidity and mortality following HCT. Once a HPIV vaccine is commercially available, systematic vaccination of donors, HCT staff, and close patient contacts would be an additional prophylactic measure to prevent HPIV infections.
This study shows that HPIV infection occurring in later phases of alloHCT (RIC is a risk factor) may be less serious than HPIV infections occurring very early posttransplantation, that can increase mortality. Treatment of HPIV in LRTI infections has not improved survival. Therefore, extreme caution should be taken to prevent infection from infected individuals to these hospitalized patients. Moreover, any patients with respiratory symptoms with imminent transplantation should be tested for HPIV, and transplantation should be postponed until the results are negative for HPIV.
ACKNOWLEDGMENTS
This work was supported in part by NIH P30 CA77598 utilizing the Biostatistics and Bioinformatics shared resource at the University of Minnesota Masonic Cancer Center.
Footnotes
All of the authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Wendt CH, Weisdorf DJ, Jordan MC, Balfour HH, Jr., Hertz MI. Parainfluenza virus respiratory infection after bone marrow transplantation. N Engl J Med. 1992;326:921–926. doi: 10.1056/NEJM199204023261404. [DOI] [PubMed] [Google Scholar]
- 2.Reed G, Jewett PH, Thompson J, Tollefson S, Wright PF. Epidemiology and clinical impact of parainfluenza virus infections in otherwise healthy infants and young children < 5 years old. J Infect Dis. 1997;175:807–813. doi: 10.1086/513975. [DOI] [PubMed] [Google Scholar]
- 3.Arisoy ES, Demmler GJ, Thakar S, Doerr C. Meningitis due to parainfluenza virus type 3: report of two cases and review. Clin Infect Dis. 1993;17:995–997. doi: 10.1093/clinids/17.6.995. [DOI] [PubMed] [Google Scholar]
- 4.Henderson FW. Pulmonary infections with respiratory syncytial virus and the parainfluenza viruses. Semin Respir Infect. 1987;2:112–121. [PubMed] [Google Scholar]
- 5.Henderson FW, Collier AM, Sanyal MA, et al. A longitudinal study of respiratory viruses and bacteria in the etiology of acute otitis media with effusion. N Engl J Med. 1982;306:1377–1383. doi: 10.1056/NEJM198206103062301. [DOI] [PubMed] [Google Scholar]
- 6.Cox DR. Regression models and life tables. J Royal Stat Soc Bulletin. 1972;34:187–220. [Google Scholar]
- 7.Apalsch AM, Green M, Ledesma-Medina J, Nour B, Wald ER. Parainfluenza and influenza virus infections in pediatric organ transplant recipients. Clin Infect Dis. 1995;20:394–399. doi: 10.1093/clinids/20.2.394. [DOI] [PubMed] [Google Scholar]
- 8.Peck AJ, Englund JA, Kuypers J, et al. Respiratory virus infection among hematopoietic cell transplant recipients: evidence for asymptomatic parainfluenza virus infection. Blood. 2007;110:1681–1688. doi: 10.1182/blood-2006-12-060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood. 2001;98:573–578. doi: 10.1182/blood.v98.3.573. [DOI] [PubMed] [Google Scholar]
- 10.Karp D, Willis J, Wilfert CM. Parainfluenza virus II and the immunocompromised host. Am J Dis Child. 1974;127:592–593. doi: 10.1001/archpedi.1974.02110230138025. [DOI] [PubMed] [Google Scholar]
- 11.Cortez KJ, Erdman DD, Peret TC, et al. Outbreak of human parainfluenza virus 3 infections in a hematopoietic stem cell transplant population. J Infect Dis. 2001;184:1093–1097. doi: 10.1086/322041. [DOI] [PubMed] [Google Scholar]
- 12.Martino R, Porras RP, Rabella N, et al. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant. 2005;11:781–796. doi: 10.1016/j.bbmt.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis VA, Champlin R, Englund J, et al. Respiratory disease due to parainfluenza virus in adult bone marrow transplant recipients. Clin Infect Dis. 1996;23:1033–1037. doi: 10.1093/clinids/23.5.1033. [DOI] [PubMed] [Google Scholar]
- 14.Raboni SM, Nogueira MB, Tsuchiya LR, et al. Respiratory tract viral infections in bone marrow transplant patients. Transplantation. 2003;76:142–146. doi: 10.1097/01.TP.0000072012.26176.58. [DOI] [PubMed] [Google Scholar]
- 15.Machado CM, Boas LS, Mendes AV, et al. Low mortality rates related to respiratory virus infections after bone marrow transplantation. Bone Marrow Transplant. 2003;31:695–700. doi: 10.1038/sj.bmt.1703900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakrabarti S, Collingham KE, Holder K, Oyaide S, Pillay D, Milligan DW. Parainfluenza virus type 3 infections in hematopoetic stem cell transplant recipients: response to ribavirin therapy. Clin Infect Dis. 2000;31:1516–1518. doi: 10.1086/317482. [DOI] [PubMed] [Google Scholar]
- 17.Wagner JE, Barker JN, DeFor TE, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 18.Warlick ED, Tomblyn M, Cao Q, et al. Reduced-intensity conditioning followed by related allografts in hematologic malignancies: long-term outcomes most successful in indolent and aggressive non-Hodgkin lymphomas. Biol Blood Marrow Transplant. 2011;17:1025–1032. doi: 10.1016/j.bbmt.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bachanova V, Brunstein CG, Burns LJ, et al. Fewer infections and lower infection-related mortality following non-myeloablative versus myeloablative conditioning for allotransplantation of patients with lymphoma. Bone Marrow Transplant. 2009;43:237–244. doi: 10.1038/bmt.2008.313. [DOI] [PubMed] [Google Scholar]
- 20.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chakrabarti S, Avivi I, Mackinnon S, et al. Respiratory virus infections in transplant recipients after reduced-intensity conditioning with Campath-1H: high incidence but low mortality. Br J Haematol. 2002;119:1125–1132. doi: 10.1046/j.1365-2141.2002.03992.x. [DOI] [PubMed] [Google Scholar]
- 22.Knott AM, Long CE, Hall CB. Parainfluenza viral infections in pediatric outpatients: seasonal patterns and clinical characteristics. Pediatr Infect Dis J. 1994;13:269–273. doi: 10.1097/00006454-199404000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Bellau-Pujol S, Vabret A, Legrand L, et al. Development of three multiplex RT-PCR assays for the detection of 12 respiratory RNA viruses. J Virol Methods. 2005;126:53–63. doi: 10.1016/j.jviromet.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aguilar JC, Perez-Brena MP, Garcia ML, Cruz N, Erdman DD, Echevarria JE. Detection and identification of human parainfluenza viruses 1, 2, 3, and 4 in clinical samples of pediatric patients by multiplex reverse transcription-PCR. J Clin Microbiol. 2000;38:1191–1195. doi: 10.1128/jcm.38.3.1191-1195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuypers J, Wright N, Ferrenberg J, et al. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol. 2006;44:2382–2388. doi: 10.1128/JCM.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Templeton KE, Scheltinga SA, Beersma MFC, Kroes ACM, Claas ECJ. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza A and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3, and 4. J Clin Microbiol. 2004;42:1564–1569. doi: 10.1128/JCM.42.4.1564-1569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Kraaij MGJ, van Elden LJR, van Loon AM, et al. Frequent detection of respiratory viruses in adult recipients of stem cell transplants with the use of real-time polymerase chain reaction, compared with viral culture. Clin Infect Dis. 2005;40:662–669. doi: 10.1086/427801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamae H, Kirby KA, Sandmaier BM, et al. Effect of conditioning regimen intensity on CMV infection in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15:694–703. doi: 10.1016/j.bbmt.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Safdar A, Rodriguez GH, Mihu CN, et al. Infections in non-myeloablative hematopoietic stem cell transplantation patients with lymphoid malignancies: spectrum of infections, predictors of outcome and proposed guidelines for fungal infection prevention. Bone Marrow Transplant. 2010;45:339–347. doi: 10.1038/bmt.2009.149. [DOI] [PubMed] [Google Scholar]
- 30.Sparrelid E, Ljungman P, Ekelof-Andstrom E, et al. Ribavirin therapy in bone marrow transplant recipients with viral respiratory tract infections. Bone Marrow Transplant. 1997;19:905–908. doi: 10.1038/sj.bmt.1700752. [DOI] [PubMed] [Google Scholar]
- 31.Crooks BN, Taylor CE, Turner AJ, et al. Respiratory viral infections in primary immune deficiencies: significance and relevance to clinical outcome in a single BMT unit. Bone Marrow Transplant. 2000;26:1097–1102. doi: 10.1038/sj.bmt.1702656. [DOI] [PubMed] [Google Scholar]
- 32.Lewinsohn DM, Bowden RA, Mattson D, Crawford SW. Phase I study of intravenous ribavirin treatment of respiratory syncytial virus pneumonia after marrow transplantation. Antimicrob Agents Chemother. 1996;40:2555–2557. doi: 10.1128/aac.40.11.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karron RA, Wright PF, Hall SL, et al. A Live Attenuated Bovine Parainfluenza Virus Type-3 Vaccine Is Safe, Infectious, Immunogenic, and Phenotypically Stable in Infants and Children. J Infect Dis. 1995;171:1107–1114. doi: 10.1093/infdis/171.5.1107. [DOI] [PubMed] [Google Scholar]
- 34.Karron RA, Wright PF, Newman FK, et al. A Live Human Parainfluenza Type-3 Virus-Vaccine Is Attenuated and Immunogenic in Healthy Infants and Children. J Infect Dis. 1995;172:1445–1450. doi: 10.1093/infdis/172.6.1445. [DOI] [PubMed] [Google Scholar]