Abstract
Introduction
The ventricular fibrillation (VF) waveform is dynamic and predicts defibrillation success. Quantitative waveform measures (QWM) quantify these changes. Coronary perfusion pressure (CPP), a surrogate for myocardial perfusion, also predicts defibrillation success. The relationship between QWM and CPP has been preliminarily explored. We sought to further delineate this relationship in our porcine model and to determine if it is different between animals with/without ROSC (return of spontaneous circulation).
Hypothesis
A relationship exists between QWM and CPP that is different between animals with/without ROSC.
Methods
Utilizing a prior experiment in our porcine model of prolonged out-of-hospital VF cardiac arrest, we calculated mean CPP, cumulative dose CPP, and percent recovery of three QWM during resuscitation before the first defibrillation: amplitude spectrum area (AMSA), median slope (MS), and logarithm of the absolute correlations (LAC). A random effects linear regression model with an interaction term CPP*ROSC investigated the association between CPP and percent recovery QWM and how this relationship changes with/without ROSC.
Results
For 12 animals, CPP and QWM measures (except LAC) improved during resuscitation. A linear relationship existed between CPP and percent recovery AMSA (coefficient 0.27; 95% CI 0.23, 0.31; p<0.001) and percent recovery MS (coefficient 0.80; 95% CI 0.70, 0.90; p<0.001). A linear relationship existed between cumulative dose CPP and percent recovery AMSA (coefficient 2.29; 95% CI 2.0, 2.56; p<0.001) and percent recovery MS (coefficient 6.68; 95% CI 6.09, 7.26; p<0.001). Animals with ROSC had a significantly “steeper” dose-response relationship.
Conclusions
There is a linear relationship between QWM and CPP during chest compressions in our porcine cardiac arrest model that is different between animals with/without ROSC.
Keywords: coronary perfusion pressure, ventricular fibrillation, waveform analysis, heart arrest, cardiopulmonary resuscitation
Introduction
Effective chest compressions are required for successful defibrillation in resuscitation during cardiac arrest (1–4). After circulatory arrest, myocardial energy stores rapidly deplete (5), metabolites such as lactate accumulate (6), and there is systemic hypoxemia and tissue stunning (7). CPR attenuates or reverses some of these effects (8), but the impact of CPR is dependent on the effectiveness of perfusion it provides (9). There is evidence that a defined amount of coronary perfusion pressure (CPP), a surrogate for myocardial perfusion, is required for defibrillation success. (10–20) Coronary perfusion can be conceptualized as a “threshold” value or a “dose”, both of which predict defibrillation success. (21, 22) Qualitative waveform measures (QWM) of the ventricular fibrillation (VF) electrocardiogram (ECG) predict shock success in animal and human studies (23–30) and have been correlated with depletion of myocardial energy stores during untreated VF. (30)
In other swine models of cardiac arrest, CPP has been correlated with basic electrocardiographic characteristics of the VF waveform, such as maximum amplitude, mean amplitude, and dominant frequency. (31) CPP has also been correlated with more sophisticated QWMs such as amplitude spectrum area (AMSA) and median slope MS). (24, 32) Seeking to further delineate this correlation between CPP and QWMs in our porcine model of prolonged out-of-hospital VF cardiac arrest, we retrospectively explored the relationship between CPP and QWM during the first five minutes of CPR before the first rescue shock in a randomized trial of manual, mechanical, and high-impulse chest compressions. (33) We also sought to determine if this relationship is different between animals with and without ROSC. We hypothesized that a quantifiable dose-response relationship exists between CPP and QWM during ongoing chest compressions in our porcine cardiac arrest model and that this relationship is different between animals with and without ROSC.
Materials and Methods
The care and handling of the animals were in accord with NIH guidelines and the study analyzed was approved by the University of Pittsburgh Institutional Animal Care and Use Committee. We conducted a retrospective analysis of a randomized trial of manual, mechanical, and high-impulse chest compressions performed in our lab using mixed-breed domestic swine of either sex. Animal preparation methods have been previously described in detail. (33)
In brief, anesthesia time was defined as the time from the initial bolus of alpha-chloralose until the time VF was induced. The interval of anesthesia was standardized by initiating VF in less than 40 minutes. We induced VF by delivering a three second, 60 Hz, 100mA AC current externally across the thorax and left it untreated for 8 minutes. Chest compressions were delivered manually, mechanically, or high-impulse. Manual compressions were delivered by one investigator (JJM) using the sound of the mechanical device as a metronome. Mechanical chest compressions were applied using an oxygen-driven resuscitation device (Thumper, Michigan Instruments, Grand Rapids, MI). High-impulse compressions were accomplished using a high-impulse Thumper (Model 1007, Michigan Instruments, Grand Rapids, MI). After 8 minutes of untreated VF, compressions were performed in an anterior-posterior direction at a rate of 100 per minute with a compression depth of 1.5 inches and a duty cycle of 50%. The compression to ventilation ratios was 5:1 for all groups, which was the standard at the time. Ventilation was performed with 100% FiO2 for all experiments at a tidal volume of approximately 400 cc.
All animals received drugs after 2 minutes of compressions (epinephrine 0.1 mg/kg, propanolol 1 mg, and vasopressin 40 IU) and defibrillation was attempted 5 minutes after the onset of CPR (13 minutes after the induction of VF). Thereafter, the animals may have received additional doses of epinephrine (0.015 mg/kg) every 3 minutes as the resuscitation continued and pulses were not restored. If pulses were restored, sodium bicarbonate was administered for acidemia as necessary. Dosing of sodium bicarbonate was based on an arterial blood gas (NaHCO3 = 0.3 mEq X weight in kg X base deficit).
We used an impedance-compensating, truncated exponential biphasic defibrillation waveform (LifePak 12, 3-D, Medtronic Physio-Control, Redmond WA) with a fixed energy dose of 150 J for all rescue shocks. All countershocks were delivered by one investigator (JJM) to eliminate inter-user variability.
ROSC was defined as an organized electrical rhythm with a systolic blood pressure of at least 80 mmHg for at least 1 minute continuously at any time during the resuscitation effort. We consider 1 minute of sustained ROSC to be an electrophysiologic success after defibrillation, while more prolonged maintenance of pulses can depend on many other variables. After a period of short-term survival (20–120 minutes), the animal was euthanized with 40 mEq of potassium chloride. Resuscitation efforts consisting of continued CPR, further rescue shocks, standard dose epinephrine (0.015 mg/kg), and sodium bicarbonate were continued for 20 minutes in those animals that did not experience ROSC. Twenty minutes of failed resuscitation indicated no ROSC.
Coronary Perfusion Pressure
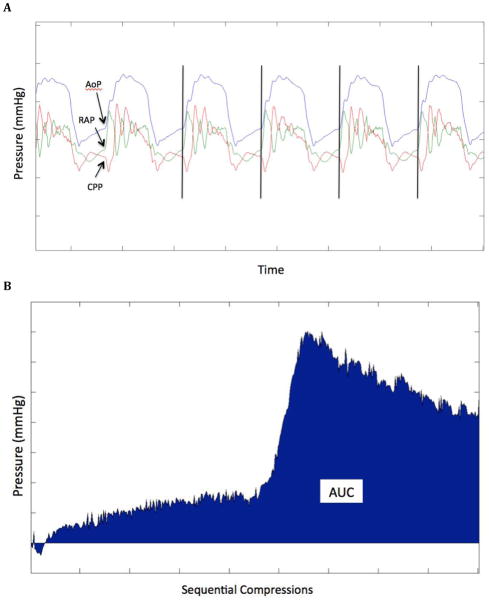
Coronary perfusion pressure (CPP) was defined as aortic diastolic pressure minus right atrial diastolic pressure and was determined at the time point immediately prior to the compression upsurge in pressure (ie: end-relaxation). Custom MatLab (Mathworks, Natick, Massachusetts) code was utilized to identify, isolate, and analyze segments of the coronary perfusion pressure tracing corresponding to individual chest compressions. Pressure tracings were also validated manually to confirm the accuracy in detection (JCR and DDS). Mean and standard deviation CPP were derived for successive 30-second epochs throughout the entire resuscitation. An area under the curve (AUC) was calculated for the set of raw CPP values contained within each 30-second epoch of CPR. (Figure 1) To reflect the state of myocardial perfusion, we also calculated the cumulative dose CPP throughout the resuscitation as a running total of the area under the CPP curve. We believe this is a more robust measurement than mean CPP for successive 30-second epochs. (21, 22)
Figure 1.
Calculation of the area under the curve coronary perfusion pressure. A: CPP is measured at the point just prior to compression upsurge in pressure (vertical black lines). B: AUC is calculated for the set of raw CPP values contained within each 30-second epoch and the cumulative AUC is calculated as a running total throughout the resuscitation. AoP: aortic pressure. RAP: right atrial pressure. CPP: coronary perfusion pressure. AUC: area under the curve.
Quantitative Electrocardiographic Waveform Measures
The ECG signal was collected from a standard lead II configuration of surface electrodes and connected to a commercial signal amplifier (DAM-50, WPI, Inc., Sarasota, FL). Methods for calculating AMSA, MS, and LAC have been described previously. (34) The particular QWM’s used in this study were chosen to account for three distinct, though related, signal properties underlying the derivation of QWMs: frequency, slope, and autocorrelation. Prior to calculation of each measure, ECG signal was down-sampled from 1000 Hz to 250 Hz. All calculations were made with original ECG voltages as recorded, which was not standardized, normalized, or otherwise filtered. The resulting signal was divided into sequential windows of 1250 samples (i.e. 5 seconds). The 3 QWMs were calculated for each of the windows to create a time series of QWM values. The mean QWM measure for the first 30 seconds of untreated VF was established as the “baseline” QWM value. To standardize QWM values across and within animals, we then calculated percentage recovery of each QWM value relative to its baseline value during the first 30 seconds of untreated VF.
We note that in prior demonstrations of correlation, QWM were measured during pauses in chest compressions. (24, 31, 32) We intentionally measured QWM during ongoing chest compressions to simulate the clinical use of these measures during continuous/minimally interrupted chest compressions, which are paramount to resuscitation success. (35) Waveform measures were computed from a specified frequency interval of 4 – 40 Hz, which did not include the frequency of chest compressions (100 compressions per minute = 1.7 Hz).
Statistical Analysis
Baseline physiologic data, including gender, weight, method of compressions, pre-resuscitation anesthesia duration, VF duration, and ROSC were abstracted from the experimental record. We used Microsoft Excel 2011 (Microsoft Corporation, Redmond, WA) and STATA 12.0 (StataCorp, College Station, TX) to record and analyze the data.
We hypothesized that there is a dose-response relationship between CPP and QWM. We analyzed repeated measures over time of both CPP and QWM for each animal. We used a random effects model of repeated measures to evaluate the relationship between CPP (measured by both raw CPP and cumulative dose CPP) and the percent recovery of the QWM throughout the resuscitation. We intentionally did not incorporate chest compression method into the model. The primary independent variable in question was CPP, regardless of how or how effectively CPP was generated. Interaction terms were generated to explore how the relationship between CPP and percent recovery of QWM is different between animals with and without ROSC. An alpha of 0.05 was used for predictive statistics.
Results
For n = 12 animals, 83.3% were male, the mean weight was 27.4 ± 2.4 kg, mean anesthesia time was 31.0 ± 4.8 minutes, and the rate of ROSC was 50.0%. All animals underwent 8 minutes of untreated VF and received CPR at a 5:1 compression to ventilation ratio.
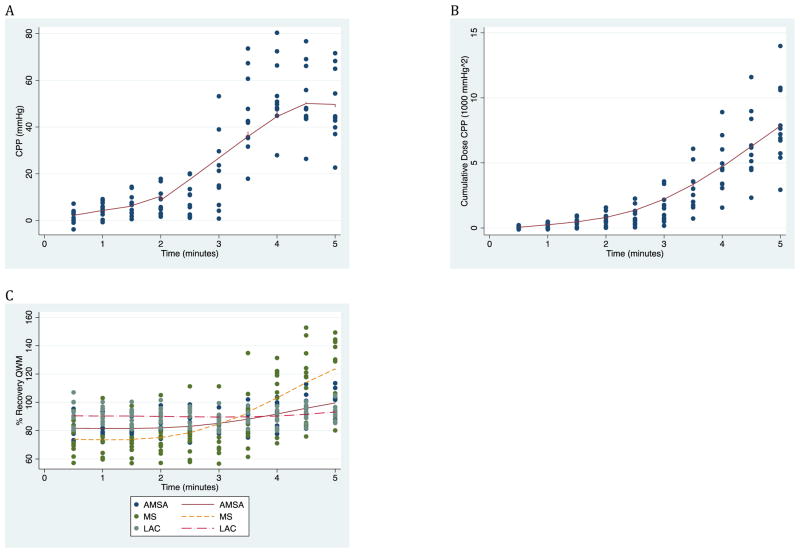
CPP, cumulative dose CPP, percent recovery of AMSA, and percent recovery of MS increased during chest compressions, while percent recovery of LAC appeared to stay relatively constant during chest compressions. (Figure 2)
Figure 2.
Increase in CPP (A), cumulative dose CPP (B), and QWMs (C) over time during the 5 minutes of CPR prior to first rescue shock with associated lowess curve. CPP: coronary perfusion pressure. QWM: qualitative waveform measure. AMSA: amplitude spectrum area. MS: median slope. LAC: logarithm of absolute correlations.
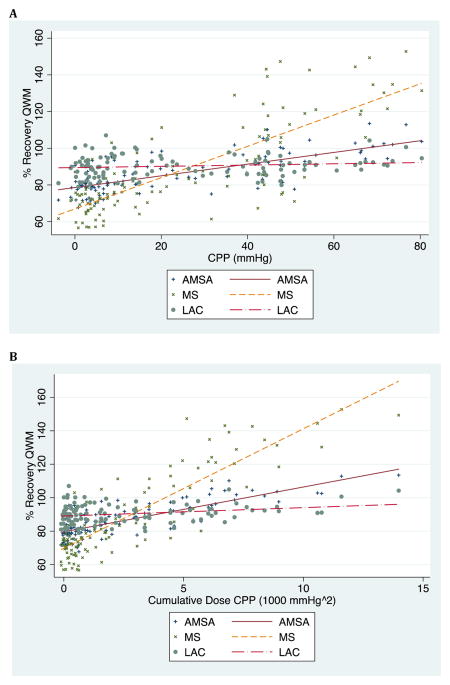
A significant linear relationship was present between CPP and QWM as measured by AMSA (coefficient 0.27; 95% CI 0.23, 0.31; p < 0.001) and MS (coefficient 0.80; 95% CI 0.70, 0.90; p < 0.001). The same relationship did not hold with CPP and LAC (coefficient 0.01; 95% CI --0.03, 0.05; p = 0.64). A significant linear relationship was present between cumulative dose CPP and QWM as measured by AMSA (coefficient 2.29; 95%CI 2.03, 2.56; p < 0.001) and MS (coefficient 6.68; 95%CI 6.09, 7.26; p < 0.001). The same relationship did not hold with cumulative dose CPP and LAC (coefficient 0.29; 95%CI −0.01, 0.59; p = 0.05). (Table 1 & Figure 3)
Table 1.
Linear regression analyses of the mean CPP value and the cumulative dose CPP.
| QWM | Coefficient | 95% CI | p-value | |
|---|---|---|---|---|
| CPP (mmHg) | AMSA | 0.27 | 0.23, 0.31 | < 0.001 |
| MS | 0.80 | 0.70, 0.90 | < 0.001 | |
| LAC | 0.01 | −0.03, 0.05 | 0.64 | |
|
| ||||
| Cumulative Dose CPP (1000 mmHg^2) | AMSA | 2.29 | 2.03, 2.56 | < 0.001 |
| MS | 6.68 | 6.09, 7.26 | < 0.001 | |
| LAC | 0.29 | −0.01, 0.59 | 0.05 | |
CPP: coronary perfusion pressure. QWM: % recovery of qualitative waveform measures. AMSA: amplitude spectrum area. MS: median slope. LAC: logarithm of absolute correlations. CI: confidence interval.
Figure 3.
Dose-response relationship between mean CPP (A) as well as cumulative dose CPP (B) and percent recovery of QWM (qualitative waveform measures) for the first 5 minutes of CPR before the first rescue shock. CPP: coronary perfusion pressure. AMSA: amplitude spectrum area. MS: median slope. LAC: logarithm of absolute correlations.
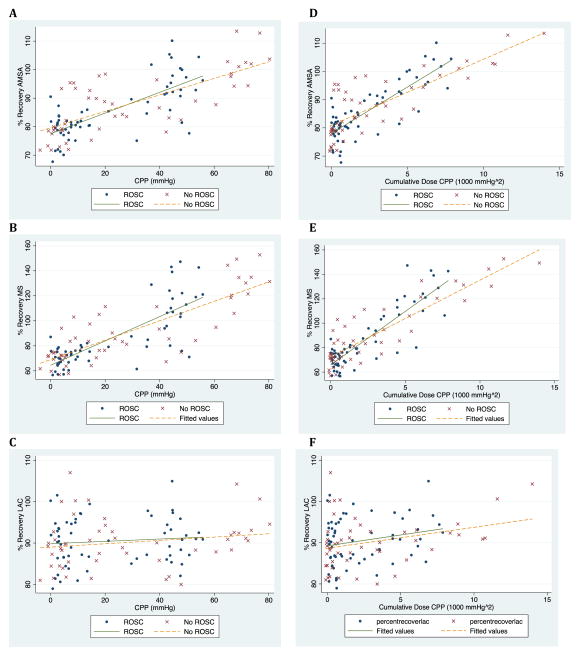
A significant interaction term was present between CPP and ROSC for AMSA (coefficient 0.14; 95%CI 0.06, 0.28; p < 0.001) and MS (coefficient 0.27; 95%CI 0.08, 0.46; p = 0.01). A significant interaction term was present between cumulative CPP and ROSC for AMSA (coefficient 1.42; 95%CI 0.97, 1.88; p < 0.001) and MS (coefficient 3.17; 95%CI 2.13, 4.20; p < 0.01). Interactions terms for LAC were not significant. (Table 2 & Figure 4)
Table 2.
Interaction terms between coronary perfusion pressure (CPP) and 8 minutes of untreated VF (ventricular fibrillation) in a linear regression analysis of CPP and percent recovery of the qualitative waveform measure (QWM).
| QWM | Interaction Term | Coefficient | 95% CI | p-value | |
|---|---|---|---|---|---|
| CPP (mmHg) | AMSA | Mean CPP x ROSC | 0.14 | 0.06, 0.22 | < 0.001 |
| MS | Mean CPP x ROSC | 0.27 | 0.08, 0.46 | 0.01 | |
| LAC | Mean CPP x ROSC | 0.03 | −0.05, 0.11 | 0.48 | |
|
| |||||
| Cumulative Dose CPP (1000 mmHg^2) | AMSA | Cumulative CPP x ROSC | 1.42 | 0.97, 1.88 | < 0.001 |
| MS | Cumulative CPP x ROSC | 3.17 | 2.13, 4.20 | < 0.001 | |
| LAC | Cumulative CPP x ROSC | 0.25 | −0.36, 0.86 | 0.42 | |
AMSA: amplitude spectrum area. MS: median slope. CI: confidence interval.
Figure 4.
Relationship between QWM recovery and mean CPP (A, B, C) and cumulative dose CPP (D, E, F) in animals with and without ROSC. QWM: qualitative waveform measures. CPP: coronary perfusion pressure. ROSC: return of spontaneous circulation. AMSA: amplitude spectrum area. MS: median slope. LAC: logarithm of absolute correlations.
Discussion
We demonstrated a linear correlational relationship between CPP and some of the QWMs that is different between animals with and without ROSC. Both CPP and percent recovery of QWM increased throughout resuscitation and their linear relationship may reflect the underlying myocardial responsiveness to perfusion. Mechanical energy expended by performing CPR generates a pressure gradient tempered by vascular resistance that results in myocardial blood flow. This flow delivers chemical energy substrates that the myocardium utilizes to generate electro-mechanical energy in the form of ECG organization coupled with fibrillating activity. We have previously demonstrated video evidence of the positive effect of chest compressions on ECG organization. (36)
For mean CPP and cumulative dose CPP in animals with and without ROSC, a significant linear relationship was present between CPP and QWM as measured by AMSA and MS, but the same relationship did not hold with CPP and LAC. (Figure 2) This could due to an inherent trait of LAC. LAC is a measure of the “longitudinal” self-similarity of the waveform over time (26), whereas AMSA measures the magnitude of the frequency spectrum between a defined range (37) and MS represents the average “steepness” of the slope of the ECG waveform. (38) Furthermore, Sherman, et al. demonstrated that the array of available QWMs has different test characteristics when predicting ROSC. (39) AMSA and MS were among those measures with the highest receiver operating characteristic AUC (0.72 & 0.73 respectively), compared with 0.65 for LAC. LAC had a higher sensitivity, but a lower specificity and positive predictive value than AMSA and MS. The negative predictive values were similar for all three measures. AMSA and MS may measure an electrophysiologic component that LAC does not, resulting in the discrepancy observed.
Significant interaction terms between CPP and ROSC indicate that animals with ROSC have a “steeper” dose-response relationship between CPP and QWM. (Table 2 & Figure 4) Even animals without ROSC achieved recovery of the QWM, yet required more CPP than animals with ROSC. The myocardia of animals with ROSC appeared to be more responsive to perfusion. We suspect this has to do with delivery and utilization of myocardial high-energy phosphate stores (29) and future inquiry could quantify these stores in animals with and without ROSC to physiologically explain the discrepancy in myocardial responsiveness to CPP. This has potential clinical utility of a measure that demonstrates myocardial responsiveness to ongoing resuscitation.
Our discussion of intra-resuscitation hemodynamics is limited to coronary perfusion pressure, which is a product of coronary blood flow and vascular resistance. We utilize young, presumably healthy swine in our model, which do not have any known coronary artery disease that would independently limit myocardial blood flow.
Prior work has explored the effects of myocardial ischemia on this relationship. (32) This could be validated in subsequent studies. Other directions to explore include the effect of therapeutic hypothermia on this relationship.
Limitations
There were several limitations to our study. First, we analyzed young healthy swine whose physiology may not reflect that of typical cardiac arrest patients. Second, there may be a species difference between swine and human cardiopulmonary physiologies. Third, we analyzed CPP, not myocardial blood flow directly. Typical cardiac arrest patients have a high burden of cardiovascular disease with increased coronary vascular resistance, adversely affecting the translation of pressure into flow. Finally, VF was electrically induced in all of these cases, not induced by ischemia.
Conclusion
In our porcine model of cardiac arrest, both CPP and recovery of QWM increase during chest compressions and there is a quantifiable linear relationship between these two variables. ROSC confers a positive effect on this relationship.
Acknowledgments
Support: Dr. Menegazzi receives support by contract RO1 HL080483, from the National Heart, Lung, and Blood Institute, National Institutes of Health.
Footnotes
Conflict of Interest Statement
Disclosures: Dr. Menegazzi is a co-inventor of a patented quantitative method of ECG analysis (the scaling exponent), which has been licensed to Medtronic Physio-Control, from which he receives royalties. To avoid potential conflict of interest, the scaling exponent was not utilized in data analysis for this manuscript. Neither of the other authors have anything to disclose.
Presented at the National Association of EMS Physicians (NAEMSP) Annual Meeting Tucson, AZ
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weisfeldt ML, Becker LB. Resuscitation after cardiac arrest: a 3-phase time-sensitive model. JAMA. 2002;288:3035–8. doi: 10.1001/jama.288.23.3035. [DOI] [PubMed] [Google Scholar]
- 2.Cobb LA, Fahrenbruch CE, Walsh TR, et al. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out-of-hospital ventricular fibrillation. JAMA. 1999;281:1182–8. doi: 10.1001/jama.281.13.1182. [DOI] [PubMed] [Google Scholar]
- 3.Wik L, Hansen TB, Fylling F, et al. Delaying defibrillation to give basic cardiopulmonary resuscitation to patients with out-of-hospital ventricular fibrillation: a randomized trial. JAMA. 2003;289:1389–95. doi: 10.1001/jama.289.11.1389. [DOI] [PubMed] [Google Scholar]
- 4.Berg RA, Hilwig RW, Ewy GA, Kern KB. Precoutnershock cardiopulmonary resuscitation improves initial response to defibrillation from prolonged ventricular fibrillation: a randomized, controlled swine study. Critical Care Medicine. 2004;32:1352–7. doi: 10.1097/01.ccm.0000127780.01362.e5. [DOI] [PubMed] [Google Scholar]
- 5.Neumar RW, Brown CG, Van Ligten P, Hoekstra J, Altschuld RA, Baker P. Estimation of myocardial ischemic injury during ventricular fibrillation with total circulatory arrest using high-energy phosphates and lactate as metabolic markers. Ann Emerg Med. 1991;20:222–9. doi: 10.1016/s0196-0644(05)80927-8. [DOI] [PubMed] [Google Scholar]
- 6.Carden DL, Martin GB, Nowak RM, Foreback CC, Tomlanovich MC. Lactic acidosis as a predictor of downtime during cardiopulmonary arrest in dogs. Am J Emerg Med. 1985;3:120–4. doi: 10.1016/0735-6757(85)90033-6. [DOI] [PubMed] [Google Scholar]
- 7.Kern KB, Hilwig RW, Rhee KG, Berg RA. Myocardial dysfunction after resuscitation from cardiac arrest: an example of global myocardial stunning. J Am Coll Cardiol. 1996;28:232–40. doi: 10.1016/0735-1097(96)00130-1. [DOI] [PubMed] [Google Scholar]
- 8.Angelos MG, Griffith RF, Beckley PD, Rath DP, Little CM. Myocardial metabolic changes during reperfusion of ventricular fibrillation: a 31p nuclear magnetic resonance study in swine. Crit Care Med. 1995;23:733–9. doi: 10.1097/00003246-199504000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Paradis NA, Martin GB, Rivers EP, et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263:1106–13. [PubMed] [Google Scholar]
- 10.Babbs CF. New versus old theories of blood flow during CPR. Critical Care Medicine. 1980;8:191–5. doi: 10.1097/00003246-198003000-00026. [DOI] [PubMed] [Google Scholar]
- 11.Kern KB, Ewy GA, Voorhees WD, Babbs CF, Tacker WA. Myocardial perfusion pressure: a predictor of 24-hour survival during prolonged cardiac arrest in dogs. Resuscitation. 1988;16:241–50. doi: 10.1016/0300-9572(88)90111-6. [DOI] [PubMed] [Google Scholar]
- 12.Redding JS, Pearson JW. Evaluation of drugs for cardiac resuscitation. Anesthesiology. 1963;24:203–7. doi: 10.1097/00000542-196303000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Ditchey RV, Winkler JV, Rhodes CA. Relative lack of coronary blood flow during closed-chest resuscitation in dogs. Circulation. 1982;66:297–302. doi: 10.1161/01.cir.66.2.297. [DOI] [PubMed] [Google Scholar]
- 14.Sanders AB, Ewy GA, Alferness CA, Taft T, Zimmerman M. Failure of one method of simultaneous chest compressions, ventilation, and abdominal binding during CPR. Critical Care medicine. 1982;10:509–13. doi: 10.1097/00003246-198208000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Michael JR, Guerci AD, Koehler RC, Shi AY, Tsitlik J, Chandra N, Niedermeyer E, Rogers MC, Traystmann RJ, Weisfeldt ML. Mechanisms by which epinephrine augments cerebral and myocardial perfusion during cardiopulmonary resuscitation in dogs. Circulation. 1984;69:822–35. doi: 10.1161/01.cir.69.4.822. [DOI] [PubMed] [Google Scholar]
- 16.Niemann JT, Rosborough JP, Niskanen RA, Alferness CA, Criley JM. Mechanical “cough” cardiopulmonary resuscitation during cardiac arrest in dogs. American Journal of Cardiology. 1985;55:199–204. doi: 10.1016/0002-9149(85)90328-5. [DOI] [PubMed] [Google Scholar]
- 17.Niemann JT, Criley JM, Rosborough JP, Niskanen RA, Alferness C. Predictive indices of successful cardiac resuscitation after prolonged arrest and experimental cardiopulmonary resuscitation. Annals of Emergency Medicine. 1985;14:521–8. doi: 10.1016/s0196-0644(85)80774-5. [DOI] [PubMed] [Google Scholar]
- 18.Sanders AB, Kern KB, Atlas M, Bragg S, Ewy GA. Importance of the duration of inadequate coronary perfusion pressure on resuscitation from cardiac arrest. Journal of the American College of Cardiology. 1985;6:113–8. doi: 10.1016/s0735-1097(85)80261-8. [DOI] [PubMed] [Google Scholar]
- 19.Niemann JT, Cruz B, Garner D, Lewis RJ. Immediate countershock versus cardiopulmonary resuscitation before countershock in a 5-minute swine model of ventricular fibrillation arrest. Annals of Emergency Medicine. 2000;36:543–6. doi: 10.1067/mem.2000.109441. [DOI] [PubMed] [Google Scholar]
- 20.Paradis NA, Martin GB, Rivers EP, et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263:1106–13. [PubMed] [Google Scholar]
- 21.Reynolds JC, Salcido DD, Menegazzi JJ. Coronary perfusion pressure and return of spontaneous circulation after prolonged cardiac arrest. Prehospital Emergency Care. 2010;14:78–84. doi: 10.3109/10903120903349796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynolds JC, Salcido DD, Menegazzi JJ. Conceptual models of coronary perfusion pressure and their relationship to defibrillation success in a porcine model of prolonged out-of-hospital cardiac arrest. Resuscitation. 2012 doi: 10.1016/j.resuscitation.2012.01.007. [Epub ahead of print] http://dx.doi.org/10.1016j.resuscitation.2012.01.007. [DOI] [PMC free article] [PubMed]
- 23.Weaver WD, Cobb LA, Dennis D, Ray R, Hallstrom AP, Copass MK. Amplitude of ventricular fibrillation waveform and outcome after cardiac arrest. Ann Intern Med. 1985;102:53–5. doi: 10.7326/0003-4819-102-1-53. [DOI] [PubMed] [Google Scholar]
- 24.Marn-Pernat A, Weil MH, Tang W, Pernat A, Bisera J. Optimizing timing of ventricular defibrillation. Crit Care Med. 2001;29:2360–5. doi: 10.1097/00003246-200112000-00019. [DOI] [PubMed] [Google Scholar]
- 25.Callaway CW, Sherman LD, Mosesso VN, Dietrich TJ, Holt E, Clarkson MC. Scaling exponent predicts defibrillation success for out-of-hospital ventricular fibrillation cardiac arrest. Circulation. 2001;103:1656–61. doi: 10.1161/01.cir.103.12.1656. [DOI] [PubMed] [Google Scholar]
- 26.Neurauter A, Eftestol T, Kramer-Johansen J, et al. Prediction of countershock success using single features from multiple ventricular fibrillation frequency bands and feature combinations using neural networks. Resuscitation. 2007;73:253–63. doi: 10.1016/j.resuscitation.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Sherman LD, Rea RD, Waters JD, Menegazzi JJ, Callaway CW. Logarithm of the absolute correlations of the ECG waveform estimates duration of ventricular fibrillation and predicts successful defibrillation. Resuscitation. 2008;78:346–54. doi: 10.1016/j.resuscitation.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin LY, Lo MT, Ko PC, et al. Detrended fluctuation analysis predicts successful defibrillation for out-of-hospital ventricular fibrillation cardiac arrest. Resuscitation. 2010;81:297–301. doi: 10.1016/j.resuscitation.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Povoas HP, Weil MH, Tang W, Bisera J, Klouche K, Barbatsis A. Predicting the success of defibrillation by electrocardiographic analysis. Resuscitation. 2002;53:77–82. doi: 10.1016/s0300-9572(01)00488-9. [DOI] [PubMed] [Google Scholar]
- 30.Salcido DD, Menegazzi JJ, Suffoletto BP, Logue ES, Sherman LD. Association of intramyocardial high energy phosphate concentrations with quantitative measures of the ventricular fibrillation electrocardiogram waveform. Resuscitation. 2009;80:946–50. doi: 10.1016/j.resuscitation.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Noc M, Weil MH, Tang W, Sun S, Pernat A, Bisera J. Electrocardiographic prediction of the success of cardiac resuscitation. Crit Care Med. 1999;27:708–14. doi: 10.1097/00003246-199904000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Indik JH, Allen D, Shanmugasundaram M, Zuercher M, Hilwig RW, Berg RA, Kern KB. Predictors of resuscitation in a swine model of ischemic and nonischemic ventricular fibrillation cardiac arrest: superiority of amplitude spectral area and slope to predict a return of spontaneous circulation when resuscitation efforts are prolonged. Crit Care Med. 2010;38:2352–7. doi: 10.1097/CCM.0b013e3181fa01ee. [DOI] [PubMed] [Google Scholar]
- 33.Betz AE, Menegazzi JJ, Logue ES, Callaway CW, Wang HE. A randomized comparison of manual, mechanical and high-impulse chest compression in a porcine model of prolonged ventricular fibrillation. Resuscitation. 2006;69:495–501. doi: 10.1016/j.resuscitation.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 34.Salcido DD, Kim YM, Sherman LD, Teng X, Logue ES, Menegazzi JJ. Quantitative waveform measures of the electrocardiogram as continuous physiologic feedback during resuscitation with cardiopulmonary bypass. Resusictation. 2011 doi: 10.1016/j.resuscitation.2011.09.018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sayre MR, Koster RW, Botha M, Cave DM, Cudnik MT, Handley AJ, Hatanaka T, Hazinski MF, Jacobs I, Monsiers K, Morley PT, Nolan JP, Travers AH. Part 5: Adult Basic Life Support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010;122:S298–S324. doi: 10.1161/CIRCULATIONAHA.110.970996. [DOI] [PubMed] [Google Scholar]
- 36.Salcido DD, Menegazzi JJ, Rittenberger JC. Electrophysiology and hemodynamics of open chest resuscitation from cardiac arrest in a swine. Acad Emerg Med. 2009;16:89–90. doi: 10.1111/j.1553-2712.2008.00297.x. [DOI] [PubMed] [Google Scholar]
- 37.Young C, Bisera J, Gehman S, et al. Amplitude spectrum area: Measuring the probability of successful defibrillation as applied to human data. Crit Care Med. 2004;32:S356–8. doi: 10.1097/01.ccm.0000134353.55378.88. [DOI] [PubMed] [Google Scholar]
- 38.Eilevstjønn J, Kramer-Johansen J, Sunde K. Shock outcome is related to prior rhythm and duration of ventricular fibrillation. Resuscitation. 2007;75:60–7. doi: 10.1016/j.resuscitation.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 39.Sherman LD, Rea TD, Fahrenbruch C, Phelps R. Abstract P76: Predictive characteristics of six ECG waveform measures in out of hospital ventricular fibrillation arrest. Circulation. 2009;120:S1457. [Google Scholar]