Abstract
Hyalophora gloveri gloverin is a glycine-rich and heat stable antimicrobial protein with activity mainly against Escherichia coli. However, Spodoptera exigua gloverin is active against a Gram-positive bacterium but inactive against E. coli. In this study, we investigated expression profile, binding ability and antimicrobial activity of Manduca sexta gloverin (MsGlv). MsGlv transcript was detected in several tissues of naïve larvae with higher levels in the midgut and testis. Expression of MsGlv mRNA in larvae was up-regulated by active Spätzle-C108 and peptidoglycans (PGs) of E. coli and Staphylococcus aureus, and the activation was blocked by pre-injection of antibody to M. sexta Toll, suggesting that MsGlv expression is regulated by the Toll-Spätzle pathway. Recombinant MsGlv bound to the O-specific antigen and outer core carbohydrate of lipopolysaccharide (LPS), Gram-positive lipoteichoic acid (LTA) and PG, and laminarin, but not to E. coli PG or mannan. MsGlv was active against Bacillus cereus, Saccharomyces cerevisiae and Cryptococcus neoformans, but was almost inactive against E. coli and S. aureus. Our results suggest that gloverins are active against some bacteria and fungi.
Keywords: Gloverin, Toll-Spätzle pathway, lipopolysaccharide, lipoteichoic acid, peptidoglycan, antimicrobial activity
1. Introduction
Insects rely on the innate immune system to fight against microbial infections. Insect innate immune system shares similarities with the innate immune system of vertebrates and is also composed of humoral and cellular responses (Ferrandon et al., 2007; Lemaitre and Hoffmann, 2007; Muller et al., 2008). Insect cellular immune responses include hemocyte (blood cell)-mediated nodule formation, phagocytosis and encapsulation, while synthesis of antimicrobial peptides/proteins (AMPs) and activation of proteinase cascades are major components of humoral immune responses (Bulet and Stocklin, 2005; Bulet et al., 2004; Chae et al., 2012; Ferrandon et al., 2007; Jiang et al., 2010; Lemaitre and Hoffmann, 2007). Insects can synthesize a variety of AMPs with activities against bacteria, fungi, viruses and some parasites (Bulet and Stocklin, 2005; Bulet et al., 2004). Some AMPs, such as cecropins, attacins, and insect defensins, are common and present in most insect species, while some other AMPs like moricins, gloverins and lebocins have been identified only in lepidopteran insects so far.
Gloverin was first isolated from the hemolymph of immunized Hyalophora gloveri pupae (Axen et al., 1997). H. gloveri gloverin (HgGlv) is a basic, heat-stable and glycine-rich antibacterial protein (~14kDa) with activity against Escherichia coli (Axen et al., 1997). Homologous gloverin proteins or cDNAs have also been isolated in other lepidopteran species, including Helicoverpa armigera (Mackintosh et al., 1998), Trichoplusia ni (Lundstrom et al., 2002; Seitz et al., 2003), Galleria mellonella (Seitz et al., 2003), Bombyx mori (Cheng et al., 2006; Kaneko et al., 2007; Kawaoka et al., 2008; Mrinal and Nagaraju, 2008), Diatraea saccharialis (Silva et al., 2010), Plutella xylostella (Etebari et al., 2011), Spodoptera exigua (Hwang and Kim, 2011), and Manduca sexta (Abdel-latief and Hilker, 2008; Zhu et al., 2003). H. armigera gloverin is active against Gram-negative bacteria but inactive against Gram-positive bacteria and the fungus Candida albicans (Mackintosh et al., 1998). Recombinant T. ni pro-67 gloverin (containing the pro-segment) is active against E. coli, and its activity is comparable to that of mature HgGlv protein (Lundstrom et al., 2002). B. mori has four gloverin genes, and all four recombinant gloverins are active against E. coli (Kawaoka et al., 2008; Mrinal and Nagaraju, 2008). Gloverins have been reported to be active almost exclusively against Gram-negative bacteria, however, recombinant S. exigua gloverin (SeGlv) is active against a Gram-positive bacterium (Flavobacterium sp.) but inactive against E. coli, and knockdown expression of Seglv by RNA interference (RNAi) increases susceptibility of S. exigua larvae to Gram-positive Bacillus thuringiensis infection (Hwang and Kim, 2011). A recent report also shows that T. ni gloverins have anti-viral activity (Moreno-Habel et al., 2012).
HgGlv has a random-coil conformation in aqueous solution, but can convert to more α-helical structure in a hydrophobic membrane-like environment (Axen et al., 1997). HgGlv can inhibit synthesis of E. coli outer membrane proteins to increase the permeability of bacterial outer membrane (Axen et al., 1997). Pre-incubation of Rd mutant LPS with HgGlv can inhibit the activity against E. coli, suggesting that HgGlv may interact with the lipid A moiety of LPS, since lipid A is negatively charged, which may interact with basic gloverin through electrostatic interaction (Axen et al., 1997). But the isoelectric point (pI) of gloverins from different insect species varies from slightly acidic to neutral (e.g., pI 5.5-7 for four B. mori gloverins), basic (pI ~8.3) to highly basic (pI > 9.3). In addition, direct binding of gloverin to lipid A or LPS has not been demonstrated, and it is not known whether gloverin can also bind to other microbial components such as bacterial lipoteichoic acid (LTA) and peptidoglycan (PG), or fungal β-1, 3-glucan and mannan. In this study, we investigate expression profile of M. sexta gloverin (Msglv), binding of recombinant MsGlv to microbial components and antimicrobial activity of MsGlv against Gram-negative and Gram-positive bacteria as well as fungi.
2. Materials and methods
2.1 Insect rearing and Drosophila S2 cell line
M. sexta eggs were purchased from Carolina Biological Supplies (Burlington, NC, USA). Larvae were reared on an artificial diet at 25°C (Dunn and Drake, 1983), and the fifth instar larvae were used for the experiments. D. melanogaster Schneider S2 cells were purchased from American Type Culture Collection (ATCC).
2.2 Microorganisms
E. coli XL1-blue was from Stratagene (CA, USA), E. coli DH5α was from Invitrogen (CA, USA), Serratia marcescens and Bacillus thuringiensis were from American Type Culture Collection (ATCC). Staphylococcus aureus and B. cereus were kindly provided by Professor Brian Geisbrecht, Saccharomyces cerevisiae (BY4741) and Cryptococcus neoformans (alpha) were provided by Professor Alexander Idnurm, B. subtilis was provided by Professor Michael O'Connor, School of Biological Sciences at University of Missouri-Kansas City.
2.3 Sequence analysis
Sequence similarity search was carried out using blast biological software (http://www.ncbi.nlm.nih.gov/blastp). Multiple sequence alignments were performed using ClustalW (http://www.ch.embnet.org/software/ClustalW.html). A phylogenetic tree of the mature gloverin proteins from some insect species was constructed by MEGA 5.05 software (Tamura et al., 2011). Signal peptide sequences were predicted with SignalP 3.0 Server (http://www.cbs.dtu.dk/services/SignalP/) (Bendtsen et al., 2004). Figures were made with the GraphPad Prism software (GraphPad, CA, USA) with one representative set of data. Significance of difference was determined by an unpaired t-test or by one way ANOVA followed by a Tukey's multiple comparison test using the same software (GraphPad, CA, USA).
2.4 Tissue distribution and induced expression of M. sexta gloverin
To determine tissue distribution of M. sexta gloverin (Msglv) mRNA, day 2 fifth instar M. sexta naïve larvae were dissected, hemocytes, fat body, midgut, epidermis and testis were collected and washed 3 times in anti-coagulant (AC) saline (4 mM NaCl, 40 mM KCl, 8 mM EDTA, 9.5 mM citric acid-monohydrate, 27 mM sodium citrate, 5% sucrose, 0.1% polyvinylpyrollidone, 1.7 mM PIPES). Total RNAs from these tissues were extracted with TRIzol® Reagent (T9424, Sigma-Aldrich), and cDNA was prepared from 1μg total RNA in a 25-μl reaction using moloney murine leukemia virus (M-MLV) reverse transcriptase (M1701, Promega) with an anchor-oligo(dT)18 primer following the manufacturer's instructions.
To determine induced expression of Msglv, day 2 fifth instar naïve larvae were injected with H2O, heat-killed E. coli strain XL1-blue (5×107cells/larva), S. marcescens (5×107 cells/larva), S. aureus (5×107 cells/larva), B. subtilis (5×107 cells/larva) or C. neoformans (107 cells/larva). Twenty-four hours after injection, hemocytes, fat body and midgut were collected separately. Total RNA and cDNA were prepared as described above. Real-time PCR was performed in 20-μl reactions containing 10 μl 2×SYBR® GreenER™ qPCR SuperMix Universal (No. 204141, Qiagen), 4 μl H2O, 4 μl diluted (1:50) cDNA, and 1 μl each reverse and forward diluted primer (10 pmol/μl). For Msglv gene, primers MsGlv-F (5’-CCC GCA ATA CGC TCA GAT A-3’) and MsGlv-R (5’-TGC TGG AAG AGA CCT TGG A-3’) were used, for the control ribosomal protein S3 (rpS3) gene, primers RPS3-F (5’-GTT GCG AGG TGG TGG TTT C-3’) and RPS3-R (5’- CCG TTC TTG CCC TGT TGG TC-3’) were used. Real-time PCR program was 2 min at 50°C, 10 min at 95°C, followed by 40 cycles of 95°C for 15s, 60°C for 1 min and the dissociation curve analysis. Data from three replicates of each sample was analyzed with SDS software (ABI) using a comparative method (2-ΔΔCt) and these experiments were repeated with 3 different biological samples. Protein extracts were also prepared from fat body, hemocytes and midgut, and expression of MsGlv protein in these tissues was determined by Western blot analysis using polyclonal rabbit antiserum against recombinant pro-MsGlv.
2.5 Activation of M. sexta gloverin by Spätzle and peptidoglycans
Recombinant full-length M. sexta Spätzle-1A (MsSpz) and the C-terminal active domain of Spätzle-1A (MsSpz-C108) were expressed in Drosophila S2 cells and purified by affinity chromatography as described previously (Zhong et al., 2012). Day 1 fifth instar M. sexta larvae were injected with MsSpz (3 μg/larva), MsSpz-C108 (1 μg/larva), S. aureus peptidoglycan (PG-144 SA) or E. coli PG-K12 (1 μg/larva) (Zhong et al., 2012). Twenty hours later, fat body, hemocytes and midgut samples were collected for preparation of total RNA and cDNA, and expression of Msglv in these tissues was determined by real-time PCR as described above.
For antibody blocking assay, day 1 fifth instar M. sexta naïve larvae were pre-injected with purified IgG to the ecto-domain of M. sexta Toll (Toll Ab, 5 μg/larva) or IgG from pre-149 immune rabbit serum (Control Ab, 5 μg/larva). One hour later, these larvae were injected with water, MsSpz (3 μg/larva), MsSpz-C108 (1 μg/larva), S. aureus PG-SA (1 μg/larva), E. coli PG-151 K12 (1 μg/larva), or without second injection (control) as described previously (Zhong et al., 2012). Twenty hours later, fat body, hemocyte and midgut samples were collected for real-time PCR analysis. M. sexta rpS3 gene was used as an internal standard to normalize the amount of RNA template, and expression levels of Msglv were calculated by the 2–ΔΔCT method as described above.
2.6 Expression and purification of recombinant M. sexta pro-gloverin in bacteria and preparation of polyclonal rabbit antiserum
RT-PCR was performed to obtain cDNA sequence encoding M. sexta pro-gloverin (pro-MsGlv, residues 23-177) using primers pro-MsGlv-F (5’-GGA CCA TGG CCC CGC AAT ACG CTC AGA T-3’) and pro-MsGlv-R (5’-CCA CTC GAG CCA TCT ATG CTG GAA GAG ACC-3’). PCR fragment was purified by agarose gel electrophoresis, digested with Nco I and Xho I enzymes, ligated into the Nco I/Xho I sites of the expression vector pET-32a (+) (Novagen), and then transformed into competent E. coli BL21 (DE3) cells. A single positive bacterial colony, which was confirmed by restriction enzyme digestion and sequencing, was inoculated into LB medium containing ampicillin (100 μg/ml) and grown overnight. The overnight culture was diluted 1:100 in LB medium and grown at 37°C to OD600 = 0.8 and then isopropyl-D-167 thiogalactoside (IPTG) (0.5 mM) was added to induce protein expression. After 6 h incubation at 28°C, bacterial cells were harvested by centrifugation and lyzed with the lysis solution (50 mM Tris-HCl, pH 8.0, 50 mM NaCl, 0.5% Triton X-100, 2 mg/ml lysozyme). Protein purification was performed with a His-Bind® Buffer Kit (Novagen). The purified protein was further separated on 15% SDS-PAGE and the gel slice containing recombinant pro-MsGlv was used as an antigen to produce rabbit polyclonal antiserum at Cocalico Biologicals, Inc (Pennsylvania, USA).
2.7 Establishment of stable S2 cell lines expressing M. sexta mature gloverin (MsGlv) and green fluorescent protein (GFP)
The cDNA sequence encoding mature MsGlv (residues 46-177) was amplified by PCR using primers MsGlvM-F (5’-GCG AGA TCT GAC GTG ACC TGG GAC AAG CAA G-3’) and MsGlvMF-R (5’-GGA CTC GAG CCA TCT ATG CTG GAA GAG ACC T-3’). The forward primer contained a Bgl II site at the 5’ end and the reverse primer contained an Xho I site at the 3’ end. PCR product was recovered by agarose gel electrophoresis-Wizard® SV Gel and PCR Clean-Up System (A9285, Promega), subcloned into T-Easy vector (A1360, Promega), and recombinant plasm id was isolated. After digested with Bgl II/Xho I, cDNA fragment was recovered and inserted into the same restriction sites of the expression vector pMT/BiP/V5-His A (V413020, Invitrogen) using T4 DNA ligase (M0202L, NEB). Green Fluorescent Protein (GFP) in the expression vector pMT/Bip/V5-His/GFP (V413020, Invitrogen) was used as a control. Recombinant expression vectors were purified using PureYield™ Plasmid Miniprep System (A1222, Promega) according to the manufacturer's instruction and used to generate stable S2 cell lines after the insert sequences were confirmed by DNA sequencing.
Drosophila Schneider S2 cells were maintained at 27°C in Insect Cell Culture Media (SH30610.02, Hyclone) supplemented with 10% heat-inactivated fetal bovine serum (#10082063, Invitrogen) and 1% penicillin-streptomycin solution (G6784, Sigma-Aldrich). For transfection assay, S2 cells (in 6-well plates) were seeded overnight in serum-free medium (SH30278.01, Hyclone) and GenCarrier-1™ transfection reagent (#31-00110, Epoch Biolabs) was used for transient transfection based on the manufacturer's instructions. Culture dishes or plates were prepared to 70% confluence prior to transfection. DES®–Inducible/Secreted Kit with pCoBlast (K5130-01, Invitrogen) was used to construct stable S2 cell lines as described previously (Zhong et al., 2012). For Western blot analysis, copper sulfate (250μM) was added to stable S2 cells in a 6-well plate to induce protein expression for 48h as described previously (Zhong et al., 2012).
2.8 Purification of recombinant MsGlv and GFP from S2 cells
To purify recombinant MsGlv and GFP, copper sulfate (final concentration of 250μM) was added to stable S2 cells expressing MsGlv or GFP in 75-cm2 flasks to induce protein expression. Cell culture medium was collected starting at 24h after protein expression for 10 days by collecting culture medium every day and re-suspending the cells with fresh medium. Cell culture medium was combined, cell debris was removed by centrifugation, and cell-free medium was incubated overnight at 4°C with 500 μl of Anti-V5 agarose beads (A7345, Sigma-Aldrich) equilibrated with initial buffer (50 mM Tris-HCl, 150 mM NaCl, pH 7.4). Purification of MsGlv and GFP by Anti-V5-agarose beads was performed essentially the same as described previously for purification of M. sexta Spätzle proteins (Zhong et al., 2012). Recombinant MsGlv and GFP were sequentially eluted from the column with 1-ml aliquots of the elution buffer (0.1 M glycine-HCl, pH 3.5, 1% Triton X-100) into vials containing 100 μl of 1 M Tris-base, pH 8.0. Fractions were analyzed by 15% SDS-PAGE, and those containing recombinant MsGlv or GFP were combined and desalted using D-salt™ Excellulose™ GF-5 desalting column (#1851850, Pierce) pre-equilibrated with H2O. Recombinant MsGlv or GFP was eluted with water, and fractions containing MsGlv or GFP were pooled and concentrated for the following binding and activity assays.
2.9 Binding of MsGlv to microbial components
To test binding of MsGlv to microbial cell wall components, plate ELISA assays were performed using different microbial components. Smooth LPS from Salmonella enteric, S. marcescens, E. coli 055:B5, E. coli 026:B6 and E. coli 0111:B4, rough mutants of LPS from E. coli EH100 (Ra mutant), E. coli J5 (Rc mutant), E. coli F583 (Rd mutant) and S. enteric serotype minnesota Re 595 (Re mutant), lipid A monophosphoryl from E. coli F583 (Rd mutant) and lipid A diphosphoryl from E. coli F583 (Rd mutant), laminarin, mannan, and zymosan were from Sigma-Aldrich (MO, USA), ultrapure TLRgrade LPS and PG from E. coli K12 (LPS-K12 and PG-K12), lipoteichoic acid (LTA) and PG from B. subtilis (LTA-BS and PG-BS) and S. aureus (LTA-SA and PG-SA) were from Invivogen (CA, USA).
Briefly, wells of a flat bottom 96-well plate (Costar, Fisher) were coated with different microbial components (2 μg/well) as described previously (Yu and Kanost, 2000; Yu et al., 2005). The plates were placed overnight at room temperature until the water evaporated completely, heated to 60°C for 30 min, and then blocked with 200 μl/well of 1 mg/ml BSA in Tris buffer (TB) (50 mM Tris-HCl, 50 mM NaCl, pH 8.0) for 2 h at 37°C. Then, plates were rinsed four times with 200 μl/well of TB, and increasing concentrations or 120 nM of purified MsGlv or GFP (a control protein) diluted in TB containing 0.1 mg/ml BSA were added to the coated plates (50 μl/well), and binding was allowed to occur for 3 h at room temperature. The plates were rinsed four times with 200 μl/well of TB, and monoclonal anti-polyHistidine antibody (Sigma-Aldrich, USA) (1:2,000 in TB containing 0.1 mg/ml BSA) was added (100 μl/well) and incubated overnight at 4°C. The plates were rinsed four times with TB (200 μl/well), and alkaline phosphatase-conjugated goat anti-mouse-IgG (Sigma-Aldrich, USA) (1:3,000 in TB containing 0.1 mg/ml BSA) was added (100 μl /well) and incubated for 2 h at 37°C. The plates were rinsed, p-nitro-phenyl phosphate (1 mg/ml in 10 mM diethanolamine, 0.5 mM MgCl2) was added (50 μl/well), and absorbance at 405 nm of each well was determined every minute for 30 min using a microtiter plate reader (Bio-Tek Instrument, Inc.).
2.10 Antimicrobial activity assays
Antimicrobial activity of purified MsGlv was tested against six bacterial strains (B. cereus, E. coli DH5α, S. marcescens, B. subtilis, B. thuringiensis and S. aureus) and two fungal strains (S. cerevisiae (BY4741) and C. neoformans (alpha)). A broth microdilution assay was used to generate growth curves as described previously (Rao et al., 2012). Briefly, overnight bacterial cultures were sub-cultured in LB medium and fungal cultures were sub-cultured in YPD medium (1% yeast extract, 2% peptone and 2% dextrose) until mid-log phase. The bacterial and fungal cultures were centrifuged at 1,000 g for 10 min at 4°C and washed once with 10 mM Tris-HCl, pH 7.5, 0.1 mM EDTA. The bacterial and fungal cells were diluted to OD600 = 10-5 in LB and YPD media, respectively, and the diluted cell cultures (75 μl) were mixed with purified MsGlv (25 μl of ~85 μg/ml) (final concentration of 21 μg/ml or 1.5 μM) or water (Control) in 96-well plates. Bacteria were cultured at 37°C with 220 rpm shaking, while fungi were cultured at 30°C with 220 rpm shaking. OD600 was measured every hour by Powerwave XS plate reader (BioTek, VT, USA). Bacterial growth curves were generated using the Graphpad Prism version 4.0 for Windows (GraphPad Software, CA, USA).
3. Results and Discussion
3.1 Sequence analysis of M. sexta gloverin
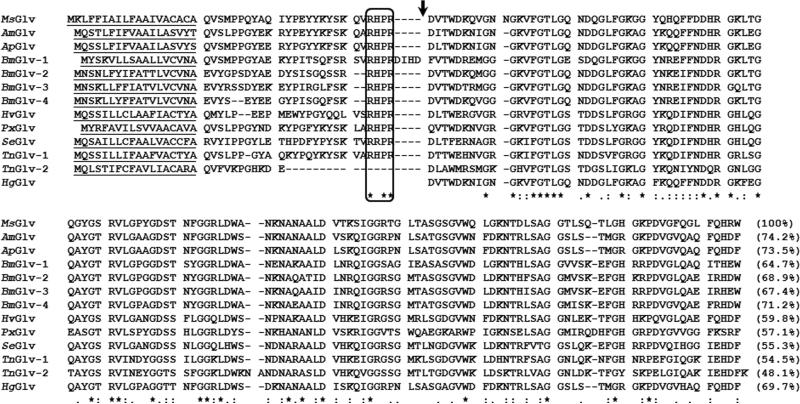
M. sexta gloverin (MsGlv) (Genbank accession number: CAL25129) is 177-residue long, with a predicted 19-residue signal peptide, 26-residue pro-segment, and 132-residue mature protein (Fig. 1). Mature MsGlv contains 28 glycines (21.2%) with theoretical mass of 13986 Da and pI of 9.35. MsGlv is most similar to gloverins from Antheraea mylitta (74.2% identity in the mature proteins, same for the followings), Antheraea pernyi (73.5%), B. mori (gloverin-4, 71.2%), H. gloveri (69.7%), and also has 48-69% identities to other mature gloverins (Fig. 1).
Fig. 1. Multiple sequence alignment of gloverin proteins from some lepidopteran species.
Gloverin protein sequences from M. sexta (CAL25129), A. mylitta (ABG72699), A. pernyi (ACB45565), B. mori (NP_001036930, NP_001037683, NP_001093312 and NP_001037684), Heliothis virescens (ACR78446), Plutella xylostella (ACM69342), S. exigua (ADL27731), T. ni (ABV68856 and AAG44367), and H. gloveri (Axen et al., 1997) were aligned by ClustalW and residues conserved in all 13 proteins are indicated by asterisks. Predicted signal peptide sequences are underlined, the box indicates a conserved endopeptidase cleavage site and the arrow indicates the beginning of mature gloverins. Identity between M. sexta mature gloverin and a mature gloverin from another species is indicated in the parenthesis.
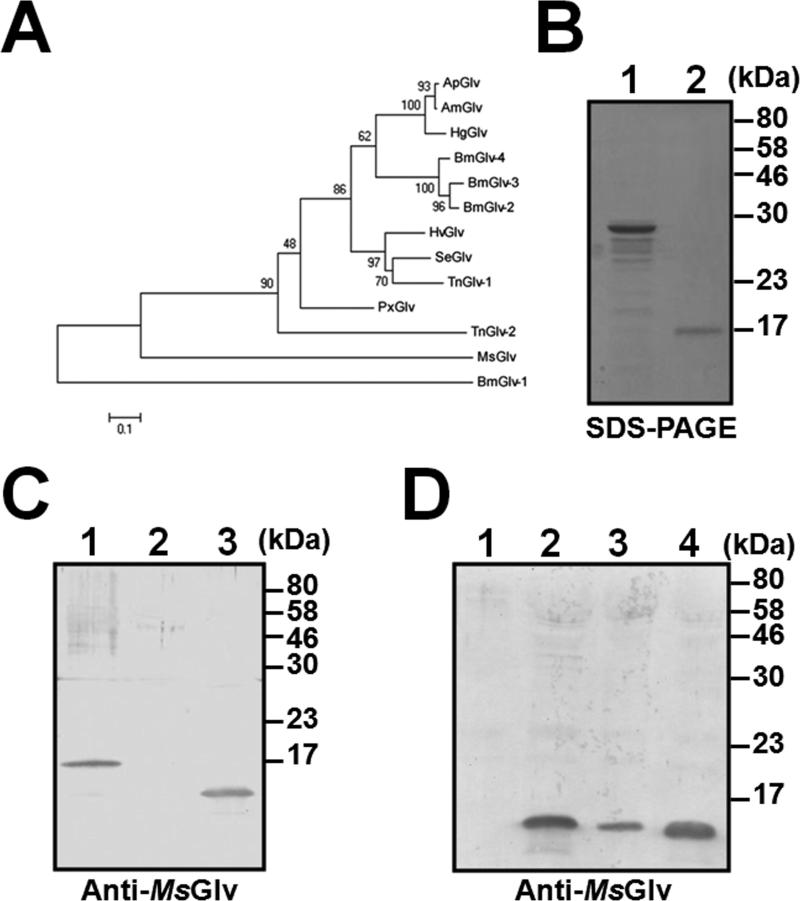
Among the four B. mori gloverin genes, Bmglv1 is the ancestral gene, whereas Bmglv2-4 genes are derived from duplication (Mrinal and Nagaraju, 2008). Phylogenetic analysis of mature gloverin protein sequences showed that A. mylitta, A. pernyi and H. gloveri gloverins clustered in one group, BmGlv2-4 clustered in one group, and H. virescens, S. exigua and T. ni (Glv-1) gloverins clustered in one group (Fig. 2A). These gloverins along with PxGlv and TnGlv2 may come from the same ancestral gene. However, MsGlv and BmGlv1 did not cluster with any of the three groups, thus Msglv may be an ancestral gene in M. sexta.
Fig. 2. Phylogenetic analysis of mature gloverin sequences and expression of M. sexta gloverin.
Thirteen mature gloverin protein sequences from some lepidopteran species from Figure 1 were used to construct the NJ tree by the MEGA5.05 software (A). Recombinant M. sexta pro-gloverin (pro-MsGlv) and mature gloverin (MsGlv) were purified from bacteria and Drosophila S2 cells, respectively, and analyzed by SDS-PAGE (B). Lane 1: pro-MsGlv (2 μg) and lane 2: MsGlv (0.5 μg). Western blot analysis of recombinant and hemolymph MsGlv (C). Purified recombinant MsGlv (lane 1, 0.2 μg), cell-free hemolymph (1 μl each) from naïve larvae (lane 2) and larvae immunized with E. coli (lane 3) were analyzed by Western blot using polyclonal rabbit antiserum against pro-MsGlv. Induced expression of MsGlv in hemolymph by Western blot (D). Cell-free hemolymph (1 μl each) from naïve larvae (lane 1) and larvae immunized with S. aureus (lane 2), E. coli (lane 3) and S. cerevisiae (lane 4) were analyzed by Western blot using polyclonal rabbit antiserum against pro-MsGlv.
3.2 Expression and purification of recombinant MsGlv
Pro-MsGlv (residues 23-177) was expressed in bacteria, purified by nickel affinity column (Fig. 2B, lane 1), and used as an antigen to generate polyclonal antibody in a rabbit. Mature MsGlv (residues 46-177) was expressed in Drosophila S2 cells and purified by affinity chromatography (Fig. 2B, lane 2) for binding and activity assays (see below). Polyclonal anti-pro-MsGlv antibody could recognize recombinant MsGlv (Fig. 2C, lane 1) and natural MsGlv in the cell-free hemolymph of larvae immunized with E. coli (Fig. 2C, lane 3). Western blot analysis showed that MsGlv protein was not detected in the cell-free hemolymph of naïve larvae (Fig. 2D, lane 1), but was present at relatively high levels in the hemolymph of larvae immunized with S. aureus, E. coli and S. cerevisiae (Fig. 2D, lanes 2-4).
3.3 Tissue distribution and induced expression of M. sexta gloverin
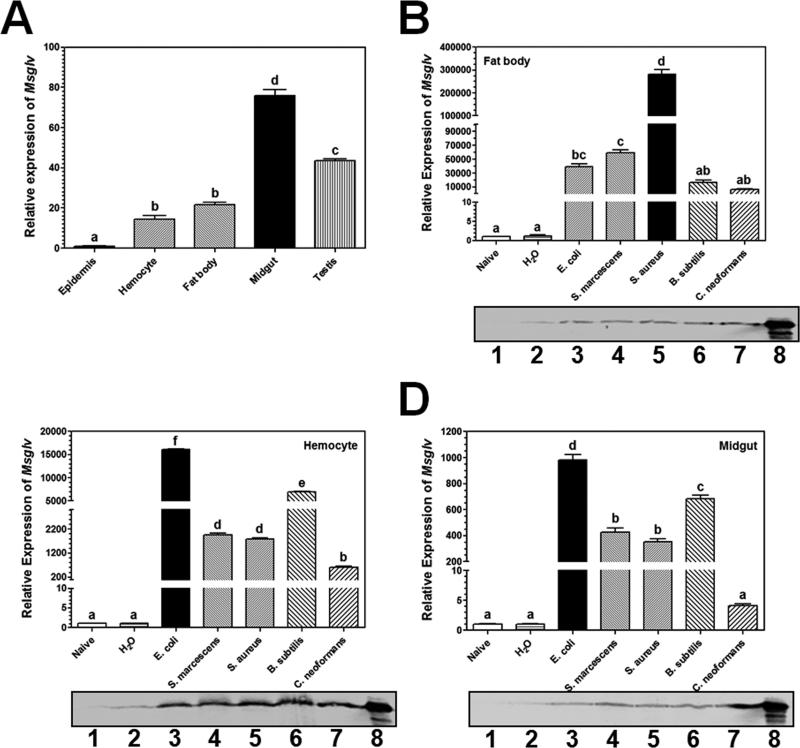
Real-time PCR analysis showed that Msglv mRNA was detected in the epidermis, hemocytes, fat body, midgut and testis of M. sexta naïve larvae, with significantly higher expression levels in the midgut and testis (Fig. 3A). Expression of Msglv transcript was induced to a significantly higher level in the fat body by Gram-positive S. aureus than other microorganisms tested (Gram-negative E. coli and S. marcescens, Gram-positive B. subtilis, and the fungus C. neoformans) (Fig. 3B, upper panel), and was induced to significantly higher levels in the hemocytes and midgut by Gram-negative E. coli than other microorganisms (Fig. 3C and D, upper panels), but Msglv mRNA was not induced by water injection (Fig. 3B-D, upper panels). Activation of Msglv gene by different microorganisms showed tissue-specific pattern, which is consistent with tissue-specific activation of some Drosophila AMP genes (Imler and Bullet, 2005).
Fig. 3. Tissue distribution and expression of Msglv in M. sexta larvae after microbial infection.
Day 2 fifth instar M. sexta naïve larvae were dissected, epidermis, hemocytes, fat body, midgut and testis were collected for preparation of total RNAs. Expression of Msglv mRNA in these tissues was determined by quantitative real-time PCR (A). Total RNAs were also prepared from fat body, hemocytes and midgut of fifth instar larvae immunized with different microorganisms at 24 h post-injection, and expression of Msglv mRNA was also determined by quantitative real-time PCR (B-D, upper panels). M. sexta ribosomal protein S3 (rpS3) gene was used as an internal control. The bars represent the mean of three individual measurements ± SEM. Relative expression of Msglv mRNA in epidermis (A), fat body (B), hemocytes (C) or midgut (D) of naïve larvae was set as 1. Comparing expression of Msglv mRNA in different tissues (A) or after microbial injections, identical letters among tissues or treatments indicate not significant difference (p>0.05), while different letters indicate significant difference (p<0.05) determined by one way ANOVA followed by a Tukey's multiple comparison test. Protein extracts from fat body, hemocytes and midgut (B-D, lower panels) of naïve larvae (lane 1) and larvae injected with water (lane 2), E. coli (lane 3), S. marcescens (lane 4), S. aureus (lane 5), B. subtilis (lane 6) and C. neoformans (lane 7) (60 μg total protein per lane), and cell-free hemolymph from larvae immunized with S. aureus (lane 8, 1 μl per lane) were also analyzed by SDS-PAGE, and MsGlv was detected by Western blot using polyclonal rabbit antiserum against pro-MsGlv.
MsGlv protein was almost not detected in fat body, hemocytes and midgut of naïve larvae (Fig. 3B-D, low panels, lanes 1) and was present at very low levels in these tissues when larvae were injected with water (Fig. 3B-D, low panels, lanes 2). MsGlv protein in fat body, hemocytes and midgut was induced by Gram-negative and Gram-positive bacteria as well as C. neoformans (Fig. 3B-D, low panels, lanes 3-7), a result consistent with the induction of Msglv mRNA by microorganisms in these tissues (Fig. 3B-D, upper panels). MsGlv protein in hemolymph was also induced after larvae were injected with S. aureus, E. coli and S. cerevisiae (Fig. 2D). Together, these results suggest that M. sexta gloverin gene expression can be induced by different microorganisms at both the transcriptional and protein levels.
In B. mori, Bmglv1 gene is expressed in larval but not adult gonads, while Bmglv2-4 genes are expressed in adult but not larval gonads (Mrinal and Nagaraju, 2008), suggesting that Bmglv genes may be developmentally regulated. Knockdown expression of Bmglv2 gene by RNAi in B. mori embryos reduces hatching (Mrinal and Nagaraju, 2008), and RNAi of Seglv gene in S. exigua larvae reduces pupation and prolongs larval period (Hwang and Kim, 2011). In the M. sexta eggs parasitized by Trichogramma evanescens, Msglv gene expression is suppressed at 2 days after parasitization, while expression of like-moricin (L-Mor), leureptin and attacin-2 genes do not change significantly (Abdel-latief and Hilker, 2008). We showed that Msglv gene was expressed at a higher level in the testis of M. sexta naïve larvae (Fig. 3A). Altogether, these results suggest that gloverin may play a role in development in addition to be an AMP.
3.4 Expression of M. sexta gloverin is regulated by the Toll pathway
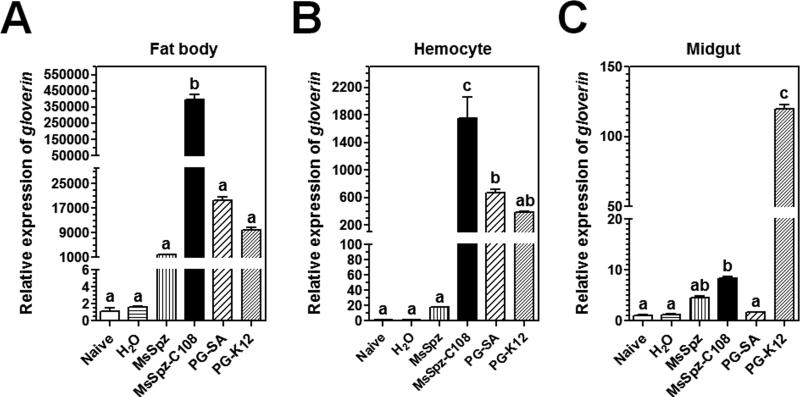
In D. melanogaster, expression of AMP genes is regulated by the Toll and Imd pathways. Drosophila Toll pathway is activated by Gram-positive Lys-type peptidoglycan (PG), while the Imd pathway is activated by Gram-negative meso-diaminopimelic acid (DAP)-type PG (Ganesan et al., 2011; Lemaitre and Hoffmann, 2007; Lemaitre et al., 1995; Lemaitre et al., 1996; Leulier et al., 2003; Valanne et al., 2011). In M. sexta, both Lys-type and DAP-type PGs can activate expression of M. sexta AMP genes (Rao and Yu, 2010). To determine whether expression of Msglv gene is regulated by the Toll and/or Imd pathways in M. sexta, naïve larvae were injected with purified recombinant active MsSpz-C108 (a Toll pathway ligand) (Zhong et al., 2012), S. aureus and E. coli peptidoglycans (PGs), and expression of Msglv mRNA was determined. Real-time PCR results showed that MsSpz-C108, S. aureus PG-SA (Lys-type) and E. coli PG-K12 (DAP-type) could all activate expression of Msglv mRNA to significantly higher levels in the fat body (Fig. 4A) and hemocytes (Fig. 4B) compared to the water injection control, and MsSpz-C108 and PG-K12, but not PG-SA, also activated Msglv mRNA in the midgut (Fig. 4C). The full-length MsSpz activated Msglv mRNA in the fat body to a significantly higher level compared to water injection (Fig. 4A), probably due to activation of some MsSpz by hemolymph proteinases. Comparing the three ligands, MsSpz-C108 was more potent than PG-SA and PG-K12 in activation of Msglv gene expression in fat body and hemocytes, while PG-SA and PG-K12 were equally potent in activation of Msglv gene (Fig. 4A and B). But PG-K12 was more potent than MsSpz-C108 in activation of Msglv gene expression in the midgut (Fig. 4C). These results suggest that expression of Msglv gene in the fat body and hemocytes is mainly regulated by the Toll-Spz pathway.
Fig. 4. Msglv expression in M. sexta larvae is activated by MsSpz-C108 and bacterial peptidoglycans.
Day 1 fifth instar M. sexta naïve larvae were injected with purified recombinant MsSpz (3 μg/larva), MsSpz-C108 (1 μg/larva), S. aureus PG (PG-SA) (1 μg/larva), E. coli PG (PG-K12) (1 μg/larva), or water (control), or left untreated (naïve), fat body, hemocytes and midgut were then collected at 20 h post-injection for preparation of total RNAs. Expression of Msglv mRNA was determined by real-time PCR. M. sexta rpS3 gene was used as an internal control. The bars represent the mean of three individual measurements ± SEM. Relative expression of Msglv in naïve larvae was set as 1. Comparing induced expression of Msglv mRNA after different treatments, identical letters indicate not significant difference (p>0.05), while different letters indicate significant difference (p<0.05) determined by one way ANOVA followed by a Tukey's multiple comparison test.
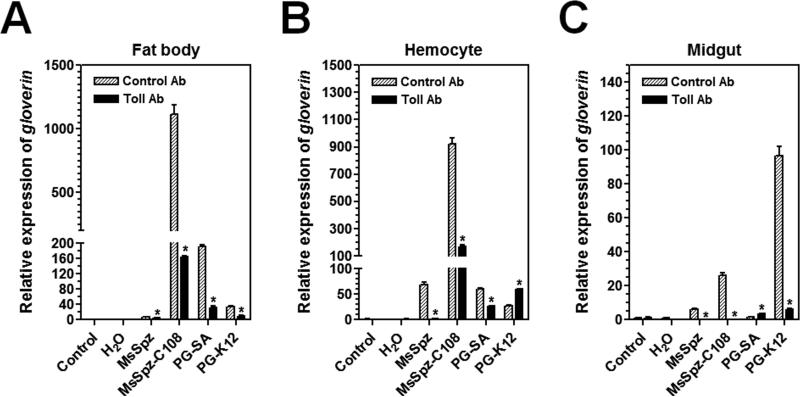
To confirm that expression of Msglv gene is regulated by the Toll pathway and to determine whether PG-K12 also activates the Toll or Imd pathway, an antibody blocking assay was performed. M. sexta naïve larvae were pre-injected with purified IgG to the ecto-domain of M. sexta Toll (MsToll) or IgG from pre-immune rabbit serum (Zhong et al., 2012), and then injected with MsSpz, MsSpz-C108, PG-SA or PG-K12. Expression of Msglv mRNA in fat body, hemocytes and midgut was determined by real-time PCR (Fig. 5). Our results showed that in the control IgG pre-injected larvae, MsSpz-C108 significantly up-regulated Msglv gene expression in the fat body and hemocytes (Fig. 5A and B), PG-SA significantly up-regulated Msglv gene expression in fat body (Fig. 5A), and PG-K12 significantly up-regulated Msglv gene expression in the midgut (Fig. 5C). However, in the MsToll IgG pre-injected larvae, up-regulations of Msglv gene by MsSpz-C108 in fat body and hemocytes, by PG-SA in fat body, and by PG-K12 in midgut were all significantly suppressed (Fig. 5). But activation of Msglv gene in hemocytes by PG-K12 was stimulated after pre-injection of MsToll antibody (Fig. 5B). These results suggest that pre-injection of larvae with IgG to MsToll blocks the Toll receptor from binding to MsSpz-C108, resulting in blocking the Toll pathway to activate Msglv gene expression.
Fig. 5. Activation of Msglv triggered by MsSpz-C108 and bacterial peptidoglycans is blocked by antibody to M. sexta Toll.
Day 1 fifth instar M. sexta naïve larvae were pre-injected with purified IgG to the ecto-domain of M. sexta Toll (Toll Ab, 5 μg/larva) or IgG from pre-immune rabbit serum (Control Ab, 5 μg/larva). One hour later, these larvae were injected with purified recombinant MsSpz (3 μg/larva), MsSpz-C108 (1 μg/larva), S. aureus PG (PG-SA) (1 μg/larva), E. coli PG (PG-K12) (1 μg/larva), or water, or without second injection (control), fat body, hemocytes and midgut were then collected at 20 h after second injection for preparation of total RNAs. Expression of Msglv mRNA was determined by real-time PCR. M. sexta rpS3 gene was used as an internal control. The bars represent the mean of three individual measurements ± SEM. Relative expression of Msglv mRNA after pre-injection of antibody but without second injection (control) was set as 1. Asterisks indicate significant difference (p<0.05) between Toll and Control antibody pre-injections for Msglv determined by an unpaired t-test.
We have previously shown that a Toll-Spätzle pathway regulates expression of cecropin, attacin, moricin and lebocin genes in M. sexta (Zhong et al., 2012). Thus, our results suggest that systematic expression of AMP genes in fat body, hemocytes and midgut of M. sexta larvae are mainly regulated by the Toll pathway. Interestingly, the Toll receptor is involved in activation of Msglv gene in midgut (Figs. 4C and 5C), moricin gene in hemocytes and lebocin-b/c genes in fat body (Zhong et al., 2012) of M. sexta larvae by PG-K12 (DAP-type PG), a result differs from D. melanogaster in that DAP-type PG activates the Imd pathway (Leulier et al., 2003). In addition, MsSpz-C108 was more potent than PG-SA and PG-K12 in activation of Msglv gene in fat body and hemocytes, but PG-K12 was more potent than MsSpz-C108 and PG-SA in activation of Msglv gene in midgut (Fig. 4), suggesting that even though all three ligands can activate the Toll pathway, the recognition process at the cell surface may differ, and tissue-specific co-activators/receptors may also be involved. Activation of Msglv gene in hemocytes by PG-K12 was not blocked but stimulated after pre-injection of MsToll antibody (Fig. 5B), which is similar to activation of lebocin-b/c in hemocytes by PG-K12 (Zhong et al., 2012). These results suggest that PG-K12-activated expression of Msglv and lebocin-b/c genes in hemocytes may not be Toll-dependent and may be regulated by other pathways such as the Imd pathway. Thus, our results also suggest tissue-specific regulation of insect AMP genes.
The promoter regions of insect AMP genes contain NF-κB binding sites for Rel/NF-κB factors such as Dorsal, Dif and Relish. In Drosophila, Dorsal and Dif regulate the Toll pathway, while Relish regulates the Imd pathway. Therefore, whether an AMP gene is regulated by the Toll, Imd or both pathways depends upon the NF-κB binding sites in the promoter regions. For example, D. melanogaster drosomycin gene is synergistically regulated by the Toll and Imd pathways, since drosomycin gene promoter contains NF-κB binding sites for both Dif/Dorsal and Relish (Ganesan et al., 2011; Tanji et al., 2007; Tanji et al., 2010). On the other hand, diptericin gene promoter contains NF-κB binding sites only for Relish and it is predominantly regulated by the Imd pathway (Ganesan et al., 2011). Some species/tissue-specific activators or suppressors may also contribute to regulation of AMP genes. M. sexta moricin gene promoter has high activity in Sf9 cells but has low or no activity in S2 cells, because the promoter region may contain a binding site for species-specific activator(s) (Rao et al., 2011).
3.5 MsGlv binds to microbial components and to the O-specific antigen and outer core carbohydrate of LPS
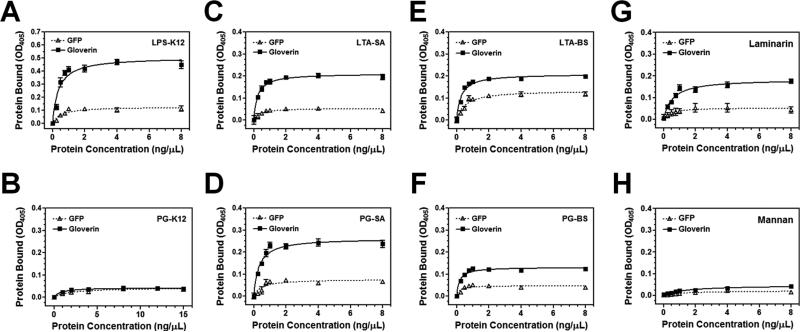
It was suggested that HgGlv can interact with LPS to inhibit synthesis of E. coli outer membrane proteins (Axen et al., 1997). To demonstrate direct binding of MsGlv to LPS and other microbial cell wall components, plate ELISA assays were performed using recombinant MsGlv purified from Drosophila S2 cells. The results showed that MsGlv bound to E. coli LPS, Gram-positive (S. aureus and B. subtilis) lipoteichoic acids (LTA-SA and LTA-BS) and peptidoglycans (PG-SA and PG-BS), and fungal laminarin (β-1, 3-glucan), but did not bind to E. coli PG (PG-K12) or fungal mannan (Fig. 6). More MsGlv protein bound to LPS (Fig. 6A) compared to other microbial components, and more MsGlv protein bound to S. aureus LTA-SA and PG-SA (Fig. 6C and D) than to B. subtilis LTA-BS and PG-BS (Fig. 6E and F). Our results confirm direct binding of gloverin to LPS and showed broad binding of MsGlv to other microbial components.
Fig. 6. Recombinant MsGlv binds to LPS, Gram-positive peptidoglycan (PG) and lipoteichoic acid (LTA), and laminarin.
Wells of 96-well fat-bottom microtiter plates were coated with E. coli LPS-K12 and PG-K12, B. subtilis LTA-BS and PG-BS, S. aureus LTA-SA and PG-SA, laminarin and mannan. Increasing concentrations of recombinant MsGlv and GFP purified from Drosophila S2 cells were added to the ligand-coated plates and the binding assay was performed as described in the Materials and Methods. Each point represents the mean of four individual measurements ± SEM, and the lines represent nonlinear regression calculation of one-site binding curve. The figures showed binding of recombinant MsGlv and GFP to LPS-K12 (A), PG-K12 (B), LTA-SA (C), PG-SA (D), LTA-BS (E), PG-BS (F), laminarin (G), and mannan (H).
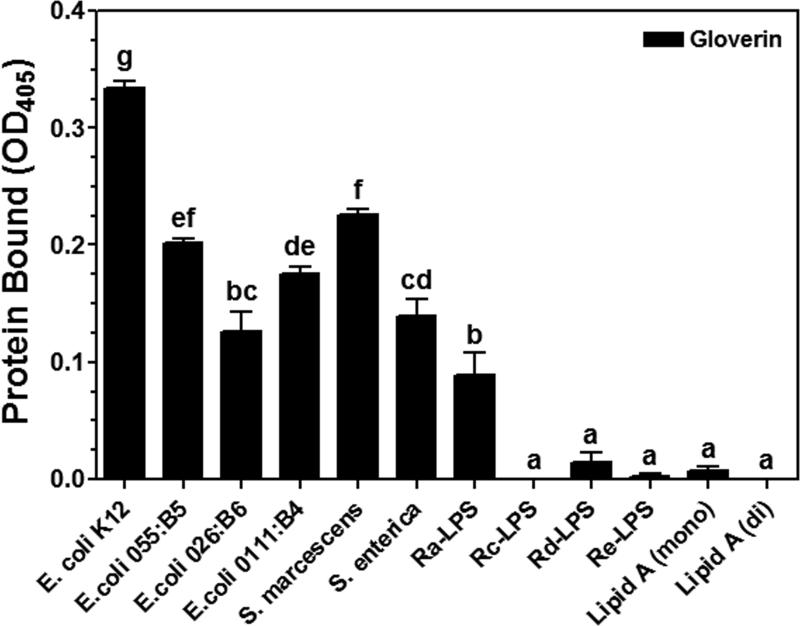
LPS is composed of three moieties: the O-specific antigen, the core (outer and inner core) carbohydrate, and the lipid A (Raetz, 1990; Yu and Kanost, 2002). It was suggested that HgGlv may bind to the lipid A moiety because pre-incubation of HgGlv with Rd-LPS inhibits the activity against E. coli (Axen et al., 1997). To test binding of MsGlv to different moieties of LPS, several smooth LPS containing different O-specific antigens, different rough mutants of LPS, and lipid A were used in the binding assay. Plate ELISA results showed that various amounts of MsGlv bound to all five smooth LPS and Ra-LPS with most MsGlv bound to LPS-K12 (ultrapure TLRgrade) (Fig. 7), indicating that binding of MsGlv to LPS is specific. Almost no binding of MsGlv to Rc-, Rd-, Re-LPS or lipid A was observed (Fig. 7), suggesting that MsGlv can bind to the O-specific antigen and the outer core carbohydrate moieties of LPS.
Fig. 7. Recombinant MsGlv binds to the O-specific antigen and outer core carbohydrate moieties of LPS.
Wells of 96-well fat-bottom microtiter plates were coated with smooth LPS from several bacteria, Ra-, Rc-, Rd- and Re-LPS, monophosphoryl and diphosphoryl lipid A. Recombinant MsGlv and GFP purified from Drosophila S2 cells were diluted to 120 nM and added to the ligand-coated plates, and the binding assay was performed as described in the Materials and Methods. The figure showed specific binding of recombinant MsGlv to LPS and lipid A after subtracting the total binding of the control GFP from the total binding of MsGlv. Each bar represent the mean of three individual measurements ± SEM. Comparing binding of recombinant MsGlv to different ligands, identical letters indicate not significant difference (p>0.05), while different letters indicate significant difference (p<0.05) determined by one way ANOVA followed by a Tukey's multiple comparison test.
HgGlv can interact with Rd-LPS and Re-LPS, but may not bind to Ra-LPS, since E. coli K12 D21 (with Ra-LPS) is less sensitive to HgGlv than E. coli K12 D21f2 (with Re-LPS) (Axen et al., 1997). Thus, MsGlv bound to both the O-specific antigen and the outer core carbohydrate of LPS (Fig. 7), while HgGlv may bind to the lipid A moiety of LPS. MsGlv has a pI of 9.35, while HgGlv has a pI of 8.23. The difference in the pI values of MsGlv and HgGlv may not completely account for the difference in binding to LPS. HgGlv has a random conformation in solution, but has a more defined structure in a membrane-like environment (Axen et al., 1997). Therefore, it is possible that differences in the defined structures of gloverins after contacting microbial components or microorganisms account for the differences in binding to microbial components or activities against different microorganisms. A 3-dimentional structure of gloverin may provide insight into the relationship between microbial binding and antimicrobial activity of gloverin. So far, no structure of glycine-rich antimicrobial proteins is available.
3.6 MsGlv is active against Gram-negative and Gram-positive bacteria and fungi
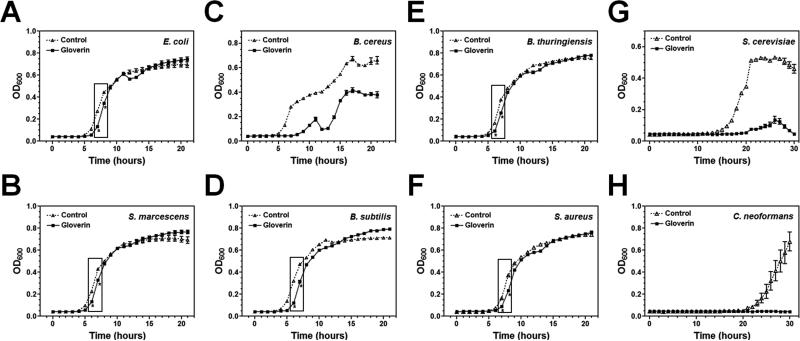
Gloverins are active almost exclusively against E. coli (Axen et al., 1997; Kawaoka et al., 2008; Lundstrom et al., 2002; Mackintosh et al., 1998). However, SeGlv is active against a Gram-positive bacterium (Flavobacterium sp.) but inactive against E. coli (Hwang and Kim, 2011). Our binding assays showed that MsGlv could bind to Gram-negative LPS, Gram-positive LTA and PG, and fungal laminarin (Figs. 6 and 7), suggesting that gloverin may be active against different microorganisms. To determine the activity of recombinant MsGlv against microorganisms, microbial growth curves were performed. Our results showed that MsGlv at low concentration (~1.5 μM or ~21 μg/ml) could inhibit the growth of Gram-positive B. cereus (Fig. 8C), and fungi S. cerevisiae (BY4741) (Fig. 8G) and C. neoformans (Fig. 8H) even after long incubation time (over 20 h for bacteria and 30 h for fungi). But MsGlv was almost inactive against Gram-negative E. coli (Fig. 8A) and S. marcescens (Fig. 8B), and Gram-positive B. subtilis (Fig. 8D), B. thuringiensis (Fig. 8E) and S. aureus (Fig. 8E), although inferior activity could be observed when the incubation time was short (less than 10 h). In addition, when the initial CFU of S. cerevisiae or C. neoformans was increased, MsGlv could not completely inhibit their growth and the growth curves showed a pattern similar to that of E. coli or S. aureus (data not shown). These results suggest that MsGlv is active against bacteria and fungi to some extents depending upon the strains of microorganisms.
Fig. 8. Antimicrobial activities of recombinant MsGlv.
Mid-log phase bacteria (Gram-negative E. coli and S. marcescens, Gram-positive B. cereus, B. subtilis, B. thuringiensis and S. aureus) and fungi (S. cerevisiae (BY4741) and C. neoformans (alpha)) were diluted to OD600 = 10-5 and incubated with recombinant MsGlv purified from S2 cells (final concentration of ~21 μg/ml or 1.5 μM) or water (Control) in 96-well plates with 220 rpm shaking at 37°C (for bacteria) or 30°C (for fungi). OD600 was recorded every hour up to 20 h (for bacteria) or 30 h (for fungi) after incubation. The points represent the mean of four individual measurements ± SEM. The asterisks in the boxes indicate inferior but significant activity (p<0.05) between MsGlv and the control at the indicated time points determined by an unpaired t-test.
MsGlv could bind to Gram-positive LTA and PG, as well as fungal laminarin (Fig. 6), which may account for its activity against B. cereus, S. cerevisiae and C. neoformans. MsGlv also bound to LPS (Figs. 6A and 7), but it was almost inactive against E. coli (Fig. 8A), while most gloverins from other insect species are active against E. coli but inactive against Gram-positive bacteria (Axen et al., 1997; Kawaoka et al., 2008; Lundstrom et al., 2002; Mackintosh et al., 1998). We think that in vitro assay to test whether a gloverin is active against Gram-negative, Gram-positive bacteria, and/or fungi may depend upon microbial strains, initial number (CFU) of microorganisms, incubation time, and the concentration of gloverin. For example, B. mori gloverins (BmGlvs) are active against E. coli in phosphate buffer within 6 h of incubation, but the activity is weak (Kawaoka et al., 2008). We observed that when the initial CFU of S. cerevisiae and C. neoformans was increased, MsGlv (at ~1.5 μM) was almost inactive against the two fungi with a growth curve similar to that of E. coli or S. aureus (data not shown). Thus, gloverin may play a role in defense against initial infection in insects when the number of microbes is low.
Msglv expression is up-regulated by Spätzle, Lys-type and DAP-type peptidoglycans.
Msglv activation by Spätzle and peptidoglycans (PGs) is regulated by the Toll pathway.
M. sexta gloverin (MsGlv) binds to LPS, Gram-positive LTA and PG, but not to E. coli PG.
MsGlv binds to the O-specific antigen and outer core carbohydrate moieties of LPS.
MsGlv is active against some Gram-positive and Gram-negative bacteria, as well as fungi.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-latief M, Hilker M. Innate immunity: eggs of Manduca sexta are able to respond to parasitism by Trichogramma evanescens. Insect Biochem Mol Biol. 2008;38:136–45. doi: 10.1016/j.ibmb.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Axen A, Carlsson A, Engstrom A, Bennich H. Gloverin, an antibacterial protein from the immune hemolymph of Hyalophora pupae. Eur J Biochem. 1997;247:614–9. doi: 10.1111/j.1432-1033.1997.00614.x. [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. Journal of Molecular Biology. 2004;340:783–95. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Bulet P, Stocklin R. Insect antimicrobial peptides: structures, properties and gene regulation. Protein Pept Lett. 2005;12:3–11. doi: 10.2174/0929866053406011. [DOI] [PubMed] [Google Scholar]
- Bulet P, Stocklin R, Menin L. Anti-microbial peptides: from invertebrates to vertebrates. Immunol Rev. 2004;198:169–84. doi: 10.1111/j.0105-2896.2004.0124.x. [DOI] [PubMed] [Google Scholar]
- Chae JH, Kurokawa K, So YI, Hwang HO, Kim MS, Park JW, Jo YH, Lee YS, Lee BL. Purification and characterization of tenecin 4, a new anti-Gram-negative bacterial peptide, from the beetle Tenebrio molitor. Dev Comp Immunol. 2012;36:540–6. doi: 10.1016/j.dci.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Cheng T, Zhao P, Liu C, Xu P, Gao Z, Xia Q, Xiang Z. Structures, regulatory regions, and inductive expression patterns of antimicrobial peptide genes in the silkworm Bombyx mori. Genomics. 2006;87:356–65. doi: 10.1016/j.ygeno.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Dunn P, Drake D. Fate of bacteria injected into naïve and immunized larvae of the tobacco hornworm, Manduca sexta. J.Invertebr. Pathol. 1983;41:77–85. [Google Scholar]
- Etebari K, Palfreyman RW, Schlipalius D, Nielsen LK, Glatz RV, Asgari S. Deep sequencing-based transcriptome analysis of Plutella xylostella larvae parasitized by Diadegma semiclausum. BMC Genomics. 2011;12:446. doi: 10.1186/1471-2164-12-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol. 2007;7:862–74. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- Ganesan S, Aggarwal K, Paquette N, Silverman N. NF-kappaB/Rel proteins and the humoral immune responses of Drosophila melanogaster. Curr Top Microbiol Immunol. 2011;349:25–60. doi: 10.1007/82_2010_107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J, Kim Y. RNA interference of an antimicrobial peptide, gloverin, of the beet armyworm, Spodoptera exigua, enhances susceptibility to Bacillus thuringiensis. J Invertebr Pathol. 2011;108:194–200. doi: 10.1016/j.jip.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Imler JL, Bulet P. Antimicrobial peptides in Drosophila: structures, activities and gene regulation. Chem Immunol Allergy. 2005;86:1–21. doi: 10.1159/000086648. [DOI] [PubMed] [Google Scholar]
- Jiang H, Vilcinskas A, Kanost MR. Immunity in lepidopteran insects. Adv Exp Med Biol. 2010;708:181–204. doi: 10.1007/978-1-4419-8059-5_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y, Furukawa S, Tanaka H, Yamakawa M. Expression of antimicrobial peptide genes encoding Enbocin and Gloverin isoforms in the silkworm, Bombyx mori. Biosci Biotechnol Biochem. 2007;71:2233–41. doi: 10.1271/bbb.70212. [DOI] [PubMed] [Google Scholar]
- Kawaoka S, Katsuma S, Daimon T, Isono R, Omuro N, Mita K, Shimada T. Functional analysis of four Gloverin-like genes in the silkworm, Bombyx mori. Arch Insect Biochem Physiol. 2008;67:87–96. doi: 10.1002/arch.20223. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Kromer-Metzger E, Michaut L, Nicolas E, Meister M, Georgel P, Reichhart JM, Hoffmann JA. A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense. Proc Natl Acad Sci U S A. 1995;92:9465–9. doi: 10.1073/pnas.92.21.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–83. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- Leulier F, Parquet C, Pili-Floury S, Ryu JH, Caroff M, Lee WJ, Mengin-Lecreulx D, Lemaitre B. The Drosophila immune system detects bacteria through specific peptidoglycan recognition. Nat Immunol. 2003;4:478–484. doi: 10.1038/ni922. [DOI] [PubMed] [Google Scholar]
- Lundstrom A, Liu G, Kang D, Berzins K, Steiner H. Trichoplusia ni gloverin, an inducible immune gene encoding an antibacterial insect protein. Insect Biochem Mol Biol. 2002;32:795–801. doi: 10.1016/s0965-1748(01)00162-x. [DOI] [PubMed] [Google Scholar]
- Mackintosh JA, Gooley AA, Karuso PH, Beattie AJ, Jardine DR, Veal DA. A gloverin-like antibacterial protein is synthesized in Helicoverpa armigera following bacterial challenge. Dev Comp Immunol. 1998;22:387–99. doi: 10.1016/s0145-305x(98)00025-1. [DOI] [PubMed] [Google Scholar]
- Moreno-Habel DA, Biglang-Awa IM, Dulce A, Luu DD, Garcia P, Weers PM, Haas-Stapleton EJ. Inactivation of the budded virus of Autographa californica M nucleopolyhedrovirus by gloverin. J Invertebr Pathol. 2012 doi: 10.1016/j.jip.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrinal N, Nagaraju J. Intron loss is associated with gain of function in the evolution of the gloverin family of antibacterial genes in Bombyx mori. J Biol Chem. 2008;283:23376–87. doi: 10.1074/jbc.M801080200. [DOI] [PubMed] [Google Scholar]
- Muller U, Vogel P, Alber G, Schaub GA. The innate immune system of mammals and insects. Contrib Microbiol. 2008;15:21–44. doi: 10.1159/000135684. [DOI] [PubMed] [Google Scholar]
- Raetz CR. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–70. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- Rao XJ, Xu XX, Yu XQ. Manduca sexta moricin promoter elements can increase promoter activities of Drosophila melanogaster antimicrobial peptide genes. Insect Biochem Mol Biol. 2011;41:982–92. doi: 10.1016/j.ibmb.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao XJ, Xu XX, Yu XQ. Functional analysis of two lebocin-related proteins from Manduca sexta. Insect Biochem Mol Biol. 2012;42:231–9. doi: 10.1016/j.ibmb.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao XJ, Yu XQ. Lipoteichoic acid and lipopolysaccharide can activate antimicrobial peptide expression in the tobacco hornworm Manduca sexta. Dev Comp Immunol. 2010;34:1119–1128. doi: 10.1016/j.dci.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz V, Clermont A, Wedde M, Hummel M, Vilcinskas A, Schlatterer K, Podsiadlowski L. Identification of immunorelevant genes from greater wax moth (Galleria mellonella) by a subtractive hybridization approach. Dev Comp Immunol. 2003;27:207–15. doi: 10.1016/s0145-305x(02)00097-6. [DOI] [PubMed] [Google Scholar]
- Silva JL, Barbosa JF, Bravo JP, Souza EM, Huergo LF, Pedrosa FO, Esteves E, Daffre S, Fernandez MA. Induction of a gloverin-like antimicrobial polypeptide in the sugarcane borer Diatraea saccharalis challenged by septic injury. Braz J Med Biol Res. 2010;43:431–6. doi: 10.1590/s0100-879x2010005000010. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Molecular Biology and Evolution. 2011 doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji T, Hu X, Weber AN, Ip YT. Toll and IMD pathways synergistically activate an innate immune response in Drosophila melanogaster. Mol Cell Biol. 2007;27:4578–88. doi: 10.1128/MCB.01814-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji T, Yun EY, Ip YT. Heterodimers of NF-kappaB transcription factors DIF and Relish regulate antimicrobial peptide genes in Drosophila. Proc Natl Acad Sci U S A. 2010;107:14715–20. doi: 10.1073/pnas.1009473107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valanne S, Wang JH, Ramet M. The Drosophila Toll signaling pathway. J Immunol. 2011;186:649–56. doi: 10.4049/jimmunol.1002302. [DOI] [PubMed] [Google Scholar]
- Yu XQ, Kanost MR. Immulectin-2, a lipopolysaccharide-specific lectin from an insect, Manduca sexta, is induced in response to gram-negative bacteria. J Biol Chem. 2000;275:37373–81. doi: 10.1074/jbc.M003021200. [DOI] [PubMed] [Google Scholar]
- Yu XQ, Kanost MR. Binding of hemolin to bacterial lipopolysaccharide and lipoteichoic acid. An immunoglobulin superfamily member from insects as a pattern-recognition receptor. Eur J Biochem. 2002;269:1827–34. doi: 10.1046/j.1432-1033.2002.02830.x. [DOI] [PubMed] [Google Scholar]
- Yu XQ, Tracy ME, Ling E, Scholz FR, Trenczek T. A novel C-type immulectin-3 from Manduca sexta is translocated from hemolymph into the cytoplasm of hemocytes. Insect Biochem Mol Biol. 2005;35:285–95. doi: 10.1016/j.ibmb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Zhong X, Xu XX, Yi HY, Lin C, Yu XQ. A Toll-Spätzle pathway in the tobacco hornworm, Manduca sexta. Insect Biochem Mol Biol. 2012 doi: 10.1016/j.ibmb.2012.03.009. http://dx.doi.org/10.1016/j.ibmb.2012.03.009. [DOI] [PMC free article] [PubMed]
- Zhu Y, Johnson TJ, Myers AA, Kanost MR. Identification by subtractive suppression hybridization of bacteria-induced genes expressed in Manduca sexta fat body. Insect Biochem Mol Biol. 2003;33:541–59. doi: 10.1016/s0965-1748(03)00028-6. [DOI] [PubMed] [Google Scholar]