Abstract
Macromolecular contrast agents have the potential to assist magnetic resonance imaging (MRI) due to their high relaxivity, but are not clinically useful because of toxicity due to poor clearance. We have prepared a biodegradable ketal-based polymer contrast agent which is designed to degrade rapidly at physiological pH by hydrolysis, facilitating renal clearance. In vitro, the agent degraded more rapidly at lower pH, with complete fragmentation after 24 h at pH 7.4. In vitro relaxivity measurements showed a direct correlation between molecular weight and relaxivity. We compared our polymer contrast agent with commercially available Magnevist in vivo by MRI imaging, as well as measuring the Gd concentration in blood. Our results show that our polymer contrast agent gives a higher contrast and intensity in the same organs and areas as Magnevist and is cleared from the blood at a similar rate. We aim to improve our polymer contrast agent design to develop it for use as a MRI contrast agent, and explore its use as a platform for other imaging modalities.
Keywords: contrast agents, polyketal, extracellular degradation, Gd(III) complexes, bioresponsive
Introduction
Small molecule gadolinium (Gd) chelates offer enhanced contrast in magnetic resonance imaging (MRI) and are rapidly cleared by a healthy renal system 1, posing little risk of demetallation and thus Gd associated toxicity. Many Gd contrast agents are currently FDA approved and are used in over 25-30% of MRI imaging procedures 1. However, these small molecule agents do not offer strong MRI contrast because their relaxivity is low. Macromolecular Gd contrast agents, such as polymers 2-6 and dendrimers 7-11 offer higher molecular relaxivities and are thus promising Gd contrast agents. Their greater relaxivity results from their slower tumbling frequency and their ability to load a greater number of Gd atoms per macromolecule 12, 13.
Unfortunately, despite these promising properties of macromolecular Gd contrast agents, they have not been approved due to toxicity concerns 8, 14. These concerns stem from in vivo animal 15, 16 and human studies 17-19 that found their administration leads to accumulation of Gd in tissue, organs, and even bones. This accumulation results from slower clearance of macromolecular contrast agents, which allows more time for Gd to be transmetallated 20 and accumulate in the body 1, 12. However, longer circulation times can assist in the detection of vascular abnormalities associated with tumors or atherosclerosis, as well as allowing for higher resolution scans and improved signal-to-noise ratios14, 21. The challenge then is to create a macromolecular Gd contrast agent with a higher relaxivity that degrades rapidly to facilitate rapid renal clearance, and thus less Gd-associated toxicity.
An ideal macromolecular blood pool contrast agent would have a high relaxivity while simultaneously having a Gd-associated toxicity similar to that of a small molecule. If a macromolecular agent were able to degrade rapidly, the resulting small molecules would be cleared, reducing toxicity. However, there is a dearth of polymeric systems able to undergo rapid degradation at physiologically relevant pH values. One class of polymer that is rapidly degraded through hydrolysis are polyketals; however, they require mildly acidic conditions to do so 22-25. We hypothesized, based on literature reports 26, that we could tune the hydrolysis to degrade more rapidly at pH 7.4 by incorporating acidic moieties within the polymer in close proximity to the ketals. We envisioned the metal chelating groups would serve a two-fold purpose: one, to chelate Gd, and two, to provide an acidic moiety to catalyze ketal hydrolysis (Scheme 1).
Scheme 1.
Polymer synthesis, Gd chelation and degradation products.
This manuscript details the synthesis, characterization, relaxivity measurements, and in vivo imaging comparison of our pH-dependent degradable contrast agent with Magnevist, a commercially available contrast agent. We show that the polymer degrades rapidly, even at physiological pH values. Finally, we show that the in vivo contrast is enhanced and clearance of our degradable contrast agent is similar when compared to Magnevist at equal Gd concentrations.
Experimental Section
Materials and methods
Potassium hydrogen phosphate, potassium dihydrogen phosphate, and gadolinium trichloride hexahydrate (GdCl3•6H2O) (99.9%) were purchased from Alpha Aesar (Ward Hill, MA). Chelex 100 molecular biology grade resin was purchased from Bio-Rad (Hercules, CA). 1 μm automation compatible filter units were purchased from Millipore (Billerica, MA). 6000-8000 MWCO membranes were purchased from Spectrum Laboratories (Houston, TX). Gadopentetic acid (Gd-DTPA) was purchased from BioPal (Worcester, MA). Magnevist (gadopentetate dimeglumine) was purchased from Bayer Healthcare (Wayne, NJ). Diethylenetriaminepentaacetic (DTPA)-Bisanhydride, ethylenediamine and all other solvents and reagents were purchased from Sigma Aldrich (St. Louis, MO). All molecular weight measurements were performed using Agilent 1100 series HPLC with an ultrahydrogel 250 column with a VWD detector (254nm). The buffer used was 0.1M Sodium carbonate at 0.5ml/min flow rate. The molecular weights were based on polyethylene oxide standards.
Polymer synthesis (Scheme 1)
The pH-dependent degradable polymer was synthesized by first dissolving an acid-labile diamine 24 (0.38 g, 2.3 mmol) in 15 ml of DMSO containing 1.0 g (9.3 mmol) anhydrous sodium carbonate. DTPA-Bisanhydride (0.85 g, 2.3 mmol) was added portion-wise to the reaction mixture, capped with a Teflon cap, and purged with nitrogen. To this, 0.1 ml of triethylamine was added using a syringe and the contents were stirred for 24 h at room temperature. The polymerization was quenched by adding 10 ml of 1% sodium carbonate and the polymer was precipitated into 300 ml of acetone to give 3.00 g polymer with sodium carbonate. 0.5 g of this crude was re-dissolved in 10 ml of water, dialyzed against 0.1 M K2HPO4 (adjusted to pH 10 using KOH) using a 6000-8000 MWCO membrane for two days, and lyophilized.
MW =8900 ; PDI=1.88
Polymer degradation by gel permeation chromatography (GPC)
0.1 g of the lyophilized polymer with its buffer salts was dissolved in 5 ml of water. The pH of 1 ml aliquots of this solution were adjusted to either pH 10, 7.4, or 6.5. These solutions were then incubated at 37 °C and injected at various intervals into an aqueous GPC pH = 11 using an ultrahydrogel 250 column with an RI detector. The molecular weights were based on polyethylene oxide standards.
Polymer chelation to Gd for relaxivity experiments
400 mg of lyophilized polymer was added to a 40 ml glass vial along with an equal weight of GdCl3•6H2O and 20 ml of 100 mM K2HPO4 to give a final concentration of 20 mg/ml of polymer and GdCl3•6H2O, and the pH of the solution was adjusted to 10.2-10.4 with KOH. The mixture was stirred at room temperature for 3 days. The extended chelation time allowed appreciable Gd chelation to occur at higher pH values without polymer degradation. The solution was then centrifuged at 3,000 rpm for 5 min to remove insoluble Gd and the pellet was discarded. To remove free Gd from the supernatant, Chelex100 was added at 100 mg/ml. After stirring for an additional 90-120 min, the solution was centrifuged at 3,000 rpm for 5 min. The supernatant was filtered through a 1 μm syringe filter and its volume was recorded. After lyophilization and trituration, measuring the mass of powder allowed determination of the original concentration: weight post-lyophilization divided by the volume pre-lyophilization. The Gd content of the original concentration was determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES) to be 4.56 ± 0.06 mM Gd.
Polymer relaxivity measurements
To measure the relaxivity of the polymers, an inversion recovery spin echo experiment was performed in an Aspect M2 (1T field, Aspect Imaging, Shoham, Israel) with a 60 mm diameter whole body coil. Various concentrations of polymer were prepared at pH 10 to ensure that the pH-dependant degradable polymer would not degrade during the course of the experiment. Polymer was weighed out and diluted to the appropriate Gd concentration. Scan parameters: TR = 2800 ms, TE = 8.4 ms, and 12 TI values. The relaxivity was calculated through a custom Matlab program (Mathworks, Natick, MA, USA).
For phantom imaging of polymer degradation at room temperature, 500 μl solutions of polymer contrast agent were prepared at the desired pH values and a final Gd concentration of 0.5 mM. The solutions were placed into 700 μl microcentrifuge tubes arranged in a custom holder in the Aspect M2. The pH was adjusted to the desired pH values directly before experiment. The software was configured to take a T1-weighted image at regular intervals. Scan parameters: TR = 30 ms, TE = 1.7 ms, flip angle = 80°, number of excitations = 3. The images shown were adjusted for contrast/brightness in identical fashion.
For real-time relaxivity measurements, 200 μl of a polymer solution at pH 10 (at Gd conc. 2.28 mM), pH 7.4 (Gd Conc. 1.75 mM), and pH 6.5 (Gd conc. 1.50 mM) value was heated in a 37 °C bath for 5 min. The solution was then transferred to an NMR tube and placed in a Bruker Minispec mq60 contrast agent analyzer (1.4 T, Bruker, Karlsruhe, Germany). A T1-inversion recovery experiment was then performed continuously for 12 h. Relaxivity values (r1) were calculated by using the formula 1/T1,observed = 1/T1,baseline + r1•[Gd] where the baseline T1 time is buffer alone, and [Gd] is that previously determined by ICP-AES.
MTT assay
To evaluate cell viability in the presence of the polymer, an MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) assay was performed using an in vitro toxicology assay kit (Sigma, St. Louis, MO). RAW 264.7 murine macrophages were seeded in a 96-well plate at a density of 20,000 cells per well and incubated overnight. Solutions of 10 different Gd concentrations of the polymer contrast agent or Gd-DTPA were prepared into 50 mM K2HPO4. 5 μl of the various dilutions of the polymers were added to the cells (in 100 μl total volume). After 24 h, 10 μl of MTT reagent (5 mg/ml) was added to each well and incubated for 3 h. The resulting formazan crystals were dissolved with 100 μl acidified isopropanol, and the absorbance was measured at 570 nm with a FlexStation microplate reader (Molecular Devices, Sunnyvale, CA) (n=4).
In vivo imaging and Gd concentration
In vivo studies were performed using 8-week old female Swiss-Webster mice (Charles River Laboratories, Wilmington, MA; approximately 25 g each). All animal studies were approved by the UCSD Institutional Animal Care and Use Committee and conducted in accordance with the principles and procedures outlined in the NIH Guide for the Care and Use of Animals.
To reduce the buffer salt concentration, the polymer was first concentrated to 25 mM Gd filtered through a Micro Bio-Spin column P-6 (Bio-Rad; Hercules, CA) that had previously been exchanged 4 times with 1x PBS, and diluted in PBS. This step reduces the buffer salt concentration to a level appropriate for injection into mice. The resulting filtrate was injected intravenously via tail vein at 0.05 mmol Gd/kg; 200 μl at 6.25 mM. To compare our polymer contrast agent with Magnevist, a similar dose was injected intravenously via tail vein into the mice (0.05 mmol/kg; 200 μl at 6.25 mM).
For each MRI scan, Mice were anesthetized using isoflurane gas and placed on a dedicated bed inside the whole-body coil at the center of the magnet while their respiration was monitored. A Magnevist standard was placed adjacent to the mice for post-imaging data normalization. Intensities in the various regions of interest were normalized against the Magnevist standard, which is unchanging during the course of the experiment. A T1-weighted 3D Gradient Echo sequence was then run with the following parameters: coronal acquisition, field of view = 60mm, matrix size 256×256 (in-plane resolution of 0.23mm), 24 slices, 1.5mm slice thickness, TR = 30 ms, TE = 1.7 ms, flip angle = 50°, number of excitations = 2, approximately a 7 minute scan duration. A pre-injection image was first taken to record baseline intensities.
To measure the Gd concentration in mouse blood over time, mice were injected with either the polymer contrast agent or Magnevist at 0.01 mmol Gd/kg. Blood was extracted from the mice at regular time intervals (n = 3) via the tail artery. Blood was then placed in microcentrifuge tubes preloaded with 10 μl of 1000 U heparin-sodium and taken directly for ICP-AES measurements. Blood solutions were diluted into 1× PBS, milliQ water, and a final concentration of approximately 0.4% HNO3.
Results
Degradation at physiological pH
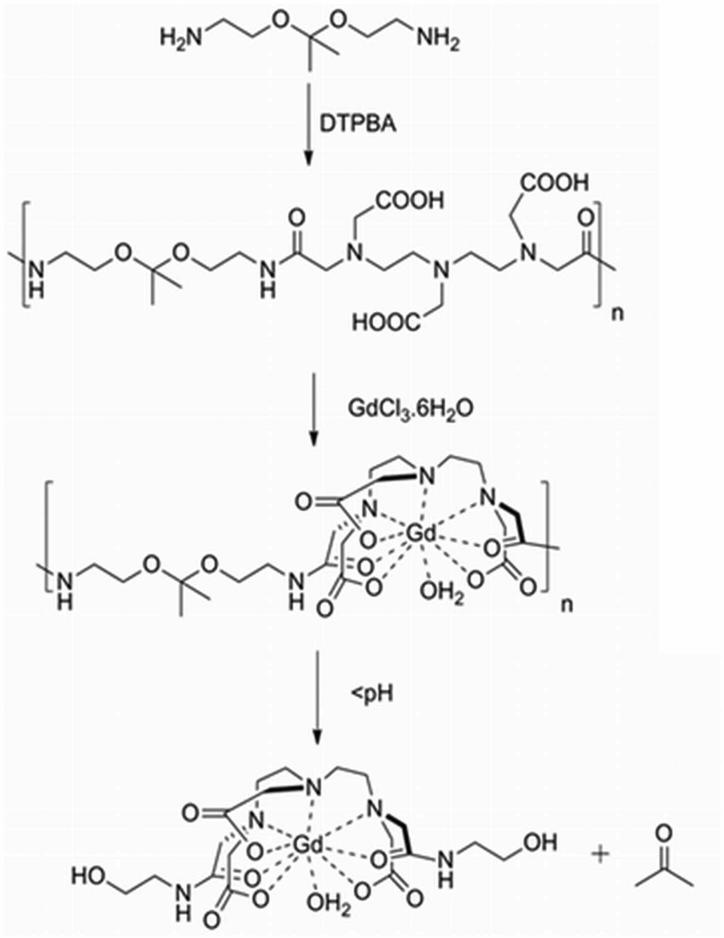
To assess the kinetics of degradation of our polymer contrast agent, we measured its molecular weight as a function of time at various pH values by GPC (Fig. 1A). At pH 10, the polymer shows no molecular weight change, indicating that it remains intact at and above pH 10. However, at pH 7.4, degradation of the polymer is clearly visible, with full degradation at 24 h. At pH 6.5, the degradation is even more rapid, reaching completion at approximately 8 h. Because ketal hydrolysis produces acetone as a byproduct (see Scheme 1), we simultaneously measured acetone release over time by UV spectroscopy (Fig. 1B). In agreement with the molecular weight measure, no acetone release is observed at pH 10, and lower pH values increase the rate of acetone release. Maximum acetone release at pH 6.5 is observed at 10 h, while at pH 7.4, the acetone release is 90% of maximum at 24 h.
Figure 1.
pH dependent degradation. (A) Calculated molecular weight change of the polymer versus time at three pH values measured by GPC. (B) Percent acetone release from the same samples measured by UV absorbance. In both figures, circles, squares and triangles represent pH 10, 7.4 and 6.5 respectively.
Polymeric agent relaxivity and cytotoxicity
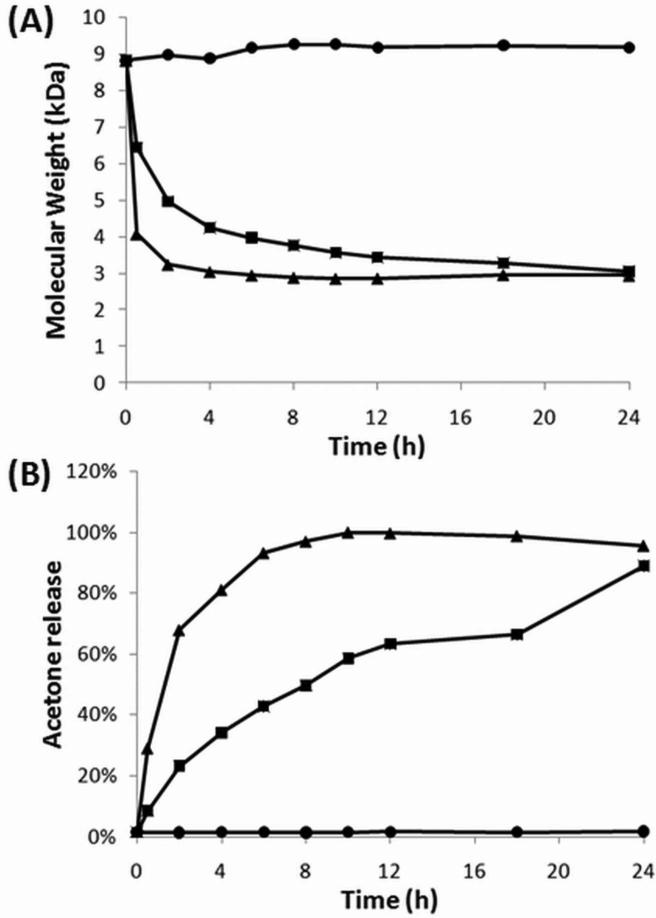
Because relaxivity is the slope of the relationship between inverse T1 time and Gd concentration (Fig. 2), we measured inverse T1 time of the polymer diluted to various Gd concentrations at pH 10 (to prevent degradation) and determined the polymer's ionic relaxivity was 8.16 ± 0.25 mM-1s-1. To determine the polymer's effect on cell viability, RAW 264.7 murine macrophages were treated with various Gd concentrations of polymer or Gd-DTPA and cytotoxicity was measured by MTT assay. The results of the assay indicate that our polymer exhibits slightly negative effects on cell viability at high Gd concentrations.
Figure 2.
Polymeric agent has high relaxivity and is tolerated by cells. (A) Relaxivity determination of the pH-dependent degradable polymer contrast agent. (B) MTT assay measuring metabolic activity. Gd-DTPA is shown in white and the pH-dependent degradable polymer contrast agent is shown in grey.
pH dependence of image intensity
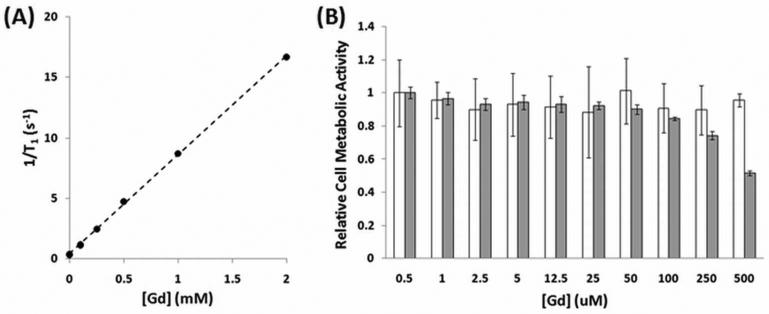
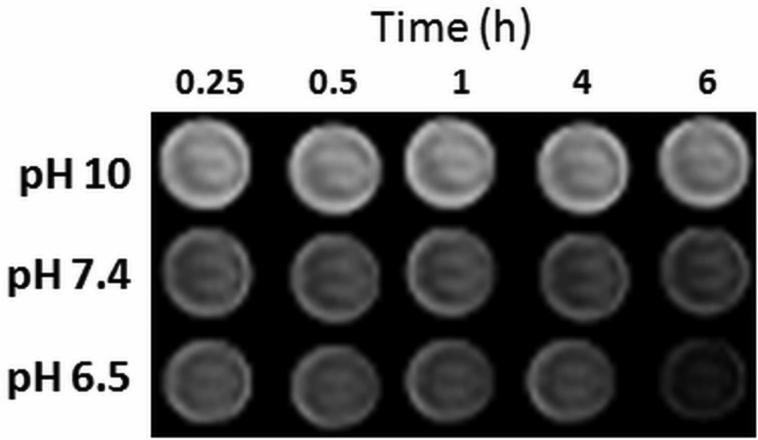
To determine whether polymer degradation causes a change in relaxivity, a phantom image experiment was performed over time. T1-weighted images were taken of tubes containing the polymer at 0.5 mM solutions of Gd at three pH values (Fig. 3). At zero time, the solution pH was adjusted and the tubes were placed inside the MRI scanner; due to this time delay as well as the scan duration, the first scan was not completed until 0.25 h. At constant Gd concentrations, the intensity in each tube is proportional to the relaxivity. The intensity of the polymer contrast agent decreased slightly over a 6 h period at pH 7.4 and more noticeably at pH 6.5, confirming that polymer degradation leads to a relaxivity change; the intensity of the solution at pH 10 did not change.
Figure 3.
Time and pH dependence of image intensity. Phantom images of 0.5 mM solutions of the polymer contrast agent at different pH values over 6 h.
Change in relaxivity over time
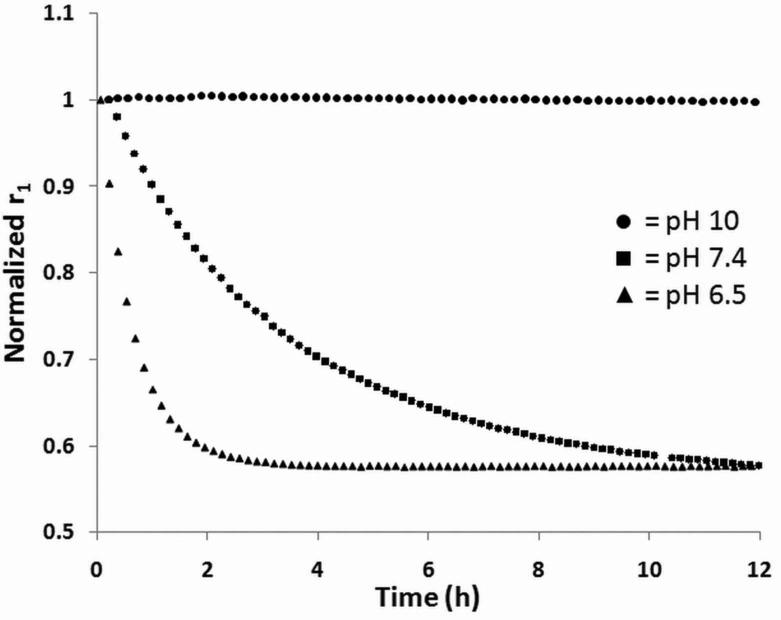
To investigate the rate of change in relaxivity over time at different pH values further, we took continuous T1 measurements using a bench top contrast agent analyzer, a Bruker minispec mq60. The mq60's short, sequential measurements allow real-time investigation of the T1 time over 24 h. The T1 values were converted to relaxivity values based on the relationship described in the Methods section and normalized by dividing by the initial relaxivity measurements (Fig. 4). Similar to the dynamic phantom experiment described earlier, the polymer contrast agent's relaxivity does not change at pH 10, while it decreases to below 60% of the initial relaxivity at both pH 7.4 and 6.5.
Figure 4.
Faster decrease in relaxivity at lower pH. Relaxivity versus time for different pH values of the polymer contrast agent.
In vivo contrast enhancement and clearance
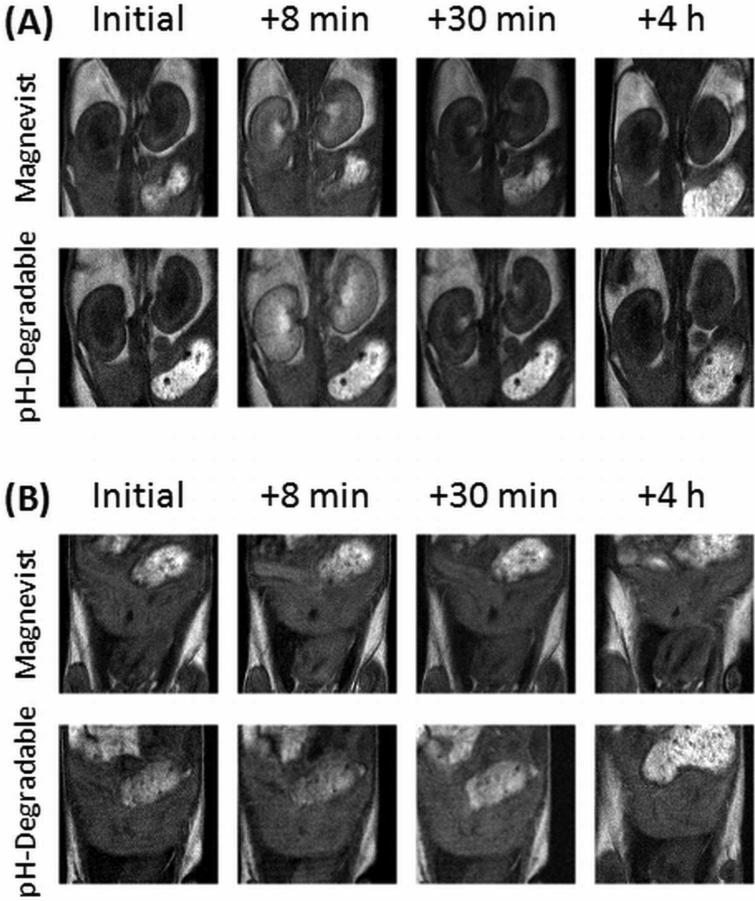
To compare the ability of our agent to enhance contrast to that of Magnevist, mice were injected with either contrast agent and imaged several times over the first hour and at 4 h post injection (Fig. 5). Immediately after injection, both agents enhance contrast of the blood and kidneys; this contrast decreases significantly 30 min post-injection. The polymer contrast agent shows a greater image enhancement in similar locations as Magnevist. For example, both enter the kidneys rapidly, but neither affects the intensity of the liver.
Figure 5.
pH-dependent degradable polymer contrast agent enhances MRI contrast similarly to Magnevist in vivo. Two different image slices are shown for each contrast agent initially, as well as three time points post injection, focusing on (A) the kidneys and (B) the liver. Images are representative of each triplicate. In (A), the solid arrow points to a kidney while the dashed arrow points to the caudal vena cava; in (B) the solid arrow points to the liver.
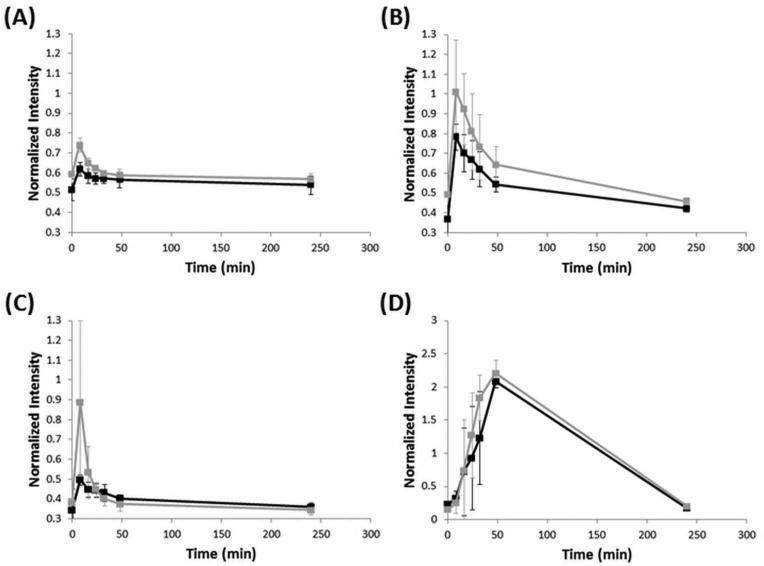
For quantitative analysis of the MRI images obtained, the intensity in various regions of interest was measured using ImageJ (NIH) or OsiriX and normalized to the Gd standard placed next to the animal during imaging (Fig. 6). The liver shows the smallest change in signal, while there is an almost doubling of the intensity in both the caudal vena cava and kidneys immediately after injection. An accumulation of intensity in the bladder post-injection indicates clearance from the blood. After 4 h, the intensity in all organs has almost returned to the original, pre-injection value. Finally, the polymer contrast agent increases contrast slightly more than Magnevist in the kidneys and caudal vena cava, and no different than Magnevist in the liver and bladder.
Figure 6.
pH-dependent degradable polymer contrast agent enhances MRI contrast more than Magnevist in blood vessels and kidney . Normalized intensity values (n = 3) for three regions of interest, the liver (A), the kidney (B), caudal vena cava (C), and bladder (D). Black corresponds to Magnevist, and grey corresponds to the polymer contrast agent.
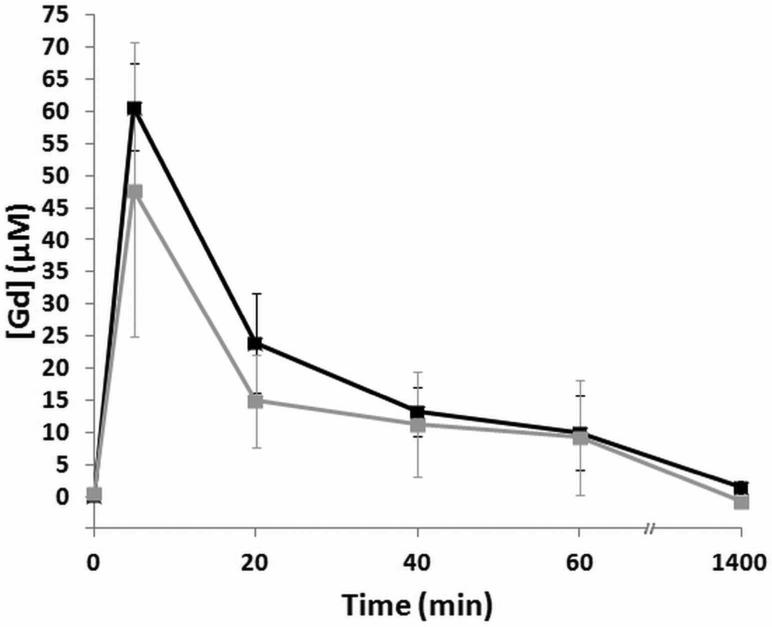
To investigate clearance rates, Gd concentration in the blood of mice injected with either our agent or Magnevist was measured by ICP-AES after dilution. The concentration profiles (Fig. 7) of both Magnevist and our polymer contrast agent show a spike in Gd immediately after injection, followed by a rapid drop and an eventual return to baseline values 24 h post-injection.
Figure 7.
Clearance of polymeric contrast agent is similar to that of Magnevist. Gd concentrations in mouse blood at various time intervals were measured by ICP-AES (n = 3). Black corresponds to Magnevist, and grey corresponds to the polymer contrast agent.
Discussion
Polymer degradation is pH-dependent, rapid, and correlates with changes in relaxivity
The pH dependence of polymer degradation was measured in two ways using GPC: molecular weight change over time, and acetone (degradation byproduct) release at different pH values. Though the acetone release results suggest that the molecular weight change graphs slightly underestimate the time for full degradation, both measures show that the polymer is degraded fully at pH 7.4 in one day, at pH 6.5 in eight hours, and is stable at pH 10.
Previous groups have designed degradable polymer contrast agents 14, 27-32, but have not shown a direct relationship between changes in the relaxivity during the degradation time. Real-time T1 measurements of our polymer solutions coupled with our GPC degradation showed a direct correlation between the change in relaxivity and the degradation rate. We were excited to observe that at pH 7.4 and pH 6.5, the polymer degrades into fragments which have the same relaxivity. This value (3.8 mM-1s-1) is slightly higher than the relaxivity for Gd-DTPA measured in our system, which was 3.64 ± 0.12 mM-1s-1, and slightly lower than that of Gd-DTPA-bisamide (Omniscan) relaxivity in plasma, at 37 °C and in a 1.5T field, 4.1 mM-1s-1 1. Interestingly, the time required for relaxivity to decrease to its minimal value is shorter for both pH 6.5 (3.7 h) and 7.4 (17h) than the time for the polymer to fully degrade by GPC. We speculate that this results from the similar relaxivity of small oligomers formed earlier in degradation to that of the small molecule. These ‘small fragments’ do not tumble slower than the small molecule contrast agent. Our polymer relaxivity measurements are similar to other polymeric contrast agents 2-5 and peptide-based contrast agents 31, 32.
Degradation-independent effects of pH on relaxivity
While we are confident that the effects of pH on relaxivity over time result from degradation, it should be noted that pH could have additional effects on relaxivity. First, high pH would lead to stronger intermolecular associations, which would lower the degrees of freedom of the system and increase relaxivity 13. This can be seen (Fig. 3) when at pH 10, the polymer contrast agent at time 0.25 h appears brighter than at pH 7.4, even though according to Fig. 4, the relaxivity has decreased by only a percent or two. . Similar to our system, relaxivity values for Gd-DTPA have been shown previously to be pH-dependent 33. Additionally, because each pH is achieved by combinations of different potassium phosphate buffers, each may have a different viscosity, which could affect relaxivity 33. However, these factors would not cause changes in relaxivity over time. While the starting relaxivity of our data might not be identical, we do observe a change in relaxivity over time, supporting our hypothesis that degradation decreases relaxivity proportionally to molecular weight.
In vivo contrast and clearance
Our polymer contrast agent shows an enhanced contrast and intensity over time when compared to Magnevist (Fig. 5 and 6). Because both our degradable polymer contrast agent and Magnevist are or become small in size, uptake in the liver should be quite limited 34 (Fig. 6A). Furthermore, both contrast agents appear to enhance similar areas in vivo; both contrast agents seem to prefer the bloodstream and kidneys versus the liver.
The change in contrast patterns over time suggest that our agent is cleared at a similar rate as Magnevist. With both agents, contrast in the bloodstream decreases quickly and that in the kidneys increases simultaneously, followed by a slower decrease towards the initial value after approximately 4 h. Further, contrast increases over the first hour in the bladder, which is associated with renal filtration 35. We measured Gd concentration in the blood over time (Fig. 7), which indicated that the contrast agent is removed quite rapidly from the bloodstream. We were not surprised that our polymer contrast agent would be cleared rapidly, but had hoped to observe slower clearance time than that of Magnevist. However, our polymer contrast agent could be improved significantly in this regard (see next section).
Limitations and Improvements
Though our MTT assay shows that the pH-dependent degradable polymer contrast agent negatively affects cell viability at high concentrations, we believe this would not likely translate in vivo. These poor-viability readings could result from the high concentrations of buffer needed to attain high Gd concentrations. Furthermore, in the assay, polymer solutions are incubated with cells for 24 h, significantly longer than a similar concentration would persist in vivo. Our polymer rapidly clears into the bladder, which would reduce not only the overall polymer concentration in the blood over time, but also the time window in which the polymer could enter cells in the body.
One area we could improve our polymer system would be to design different degradation rates. Different imaging applications require different circulation times, so a system with an adjustable clearance rate is advantageous. The degradation rate of this polymer could be slowed by incorporating hydrophobic components similar to the work of Paramonov et al. 36. Alternatively, changing the distance between the ketal and Gd chelating moiety could also slow degradation.
The pH-dependent degradable polymer contrast agent developed here was intended to be a proof-of-concept model, so we chose a straightforward synthesis rather than developing something applicable immediately for human use. Moreover, we believe our pH-dependent degradable polymer is the first step in development of a clinically-relevant blood pool contrast agent. The most critical improvement the polymer system would need is a longer blood pool retention time. This could be achieved in one of two ways. First, following the example of Mohs and co-workers, incorporating a short PEG chain would increase vascular retention, as they demonstrated for a polymer whose molecular weight was quite similar to ours prior to PEG incorporation 37-39. Second, generating polymers with a higher molecular weight by changing the synthesis route would show an improvement in not only blood retention time, but increased ionic relaxivity, as Karfeld-Sulzer and co-workers have shown32.
The second critical improvement is to replace the metal chelating unit with one that binds Gd more stably to further reduce risk of toxicity. DTPA-bisanhydride reacts with amines to form DTPA-bisamides, which are less stable than other Gd-binding FDA approved ligands 1; their thermodynamic stability constant (log K) is 16.84, versus (log K of 22.46) for Gd-DTPA 40 or over 23, up to 25.3 for DOTA and DOTA-modified chelators 41, 42. While incorporating a stable chelator such as DOTA would involve a more complicated synthetic route, such an agent would be much more feasible for a practical in vivo contrast agent.
Conclusion
In conclusion, we have shown the synthesis of a polymer contrast agent which can rapidly degrade at physiological pH. This polymer contains ketals in the backbone, allowing it to degrade by hydrolysis at pH 7.4, catalyzed by the close proximity of these ketals to the carboxylic acids present in the polymer. We have shown degradation using GPC to monitor both molecular weight changes and acetone release. Our polymer contrast agent shows an ionic relaxivity similar to other contrast agents found in the literature. The relaxivity change of the polymer contrast agent was monitored over time, and correlates well with the change in molecular weight. In vivo, our contrast agent is found in similar areas and organs as Magnevist. The intensity measured in these areas is higher than Magnevist at similar Gd concentrations. Imaging suggests that our polymer system is cleared from the bloodstream into the kidneys and subsequently into the bladder. Further studies are required to assess the toxicity of our system, including full pharmacokinetics and investigation into Gd distribution in the mice.
Finally, while we have described a MRI application of our pH-dependent degradable polymer, there are other applications in different biomedical imaging modalities43. Other Lanthanides could be incorporated for luminescent detection 44, 45, or radioisotopes 46, 47 could be chelated for radioactive decay detection. In each of these cases, the ligand used would need to be tailored to fit the requirements for proper chelation of the metal, such as coordination sites and binding affinity. In this regard, the pH-dependent degradable aspect of our polymer could be applied in different ways to meet the needs of three imaging modalities. We believe the versatility of imaging modalities, combined with the physiologically-relevant degradation of our polymer is a new and exciting addition to the imaging field.
Acknowledgment
The research described in this paper was sponsored in part by the King Abdulaziz City for Science and Technology (KACST) and the NIH New Innovator Award (1 DP2 OD006499-01). We are extremely grateful for the assistance of Jacqueline Corbeil, Dr. Olivier Girard, Salman Farshchi-Heydari, and Dr. Robert Mattrey for assistance with in vivo studies and phantom imaging, and Sean Duncan at the Scripps Institute of Oceanography for performing and assisting with ICP-AES measurements.
Footnotes
A pH-dependent degradable polymer MRI contrast agent.
References
- 1.Aime S, Caravan P. Biodistribution of Gadolinium-Based Contrast Agents, Including Gadolinium Deposition. J. Magn. Reson. Imaging. 2009;30(6):1259–1267. doi: 10.1002/jmri.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryson JM, Fichter KM, Chu WJ, Lee JH, Li J, Madsen LA, McLendon PM, Reineke TM. Polymer beacons for luminescence and magnetic resonance imaging of DNA delivery. Proc. Natl. Acad. Sci. U.S.A. 2009;106(40):16913–16918. doi: 10.1073/pnas.0904860106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucas RL, Benjamin M, Reineke TM. Comparison of a tartaric acid derived polymeric MRI contrast agent to a small molecule model chelate. Bioconjugate Chem. 2008;19(1):24–27. doi: 10.1021/bc700375m. [DOI] [PubMed] [Google Scholar]
- 4.Unger EC, Shen D, Wu G, Stewart L, Matsunaga TO, Trouard TP. Gadolinium-containing copolymeric chelates--a new potential MR contrast agent. MAGMA. 1999;8(3):154–62. doi: 10.1007/BF02594593. [DOI] [PubMed] [Google Scholar]
- 5.Vaidya A, Sun YG, Feng Y, Emerson L, Jeong EK, Lu ZR. Contrast-Enhanced MRI-Guided Photodynamic Cancer Therapy with a Pegylated Bifunctional Polymer Conjugate. Pharm. Res. 2008;25(9):2002–2011. doi: 10.1007/s11095-008-9608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu QL, Zhu LT, Yu MH, Feng FD, An LL, Xing CF, Wang S. Gadolinium(III) chelated conjugated polymer as a potential MRI contrast agent. Polymer. 2010;51(6):1336–1340. [Google Scholar]
- 7.Bryant LH, Brechbiel MW, Wu CC, Bulte JWM, Herynek V, Frank JA. Synthesis and relaxometry of high-generation (G = 5, 7, 9, and 10) PAMAM dendrimer-DOTA-gadolinium chelates. J. Magn. Reson. Imaging. 1999;9(2):348–352. doi: 10.1002/(sici)1522-2586(199902)9:2<348::aid-jmri30>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi H, Kawamoto S, Jo SK, Bryant HL, Brechbiel MW, Star RA. Macromolecular MRI contrast agents with small dendrimers: Pharmacokinetic differences between sizes and cores. Bioconjugate Chem. 2003;14(2):388–394. doi: 10.1021/bc025633c. [DOI] [PubMed] [Google Scholar]
- 9.Langereis S, de Lussanet QG, van Genderen MHP, Backes WH, Meijer EW. Multivalent contrast agents based on gadolinium-diethylenetriaminepentaacetic acid-terminated poly(propylene imine) dendrimers for magnetic resonance imaging. Macromolecules. 2004;37(9):3084–3091. [Google Scholar]
- 10.Rudovsky J, Hermann P, Botta M, Aime S, Lukes I. Dendrimeric Gd(III) complex of a monophosphinated DOTA analogue: optimizing relaxivity by reducing internal motion. Chem. Commun. 2005;(18):2390–2392. doi: 10.1039/b418712a. [DOI] [PubMed] [Google Scholar]
- 11.Wiener EC, Brechbiel MW, Brothers H, Magin RL, Gansow OA, Tomalia DA, Lauterbur PC. Dendrimer-Based Metal-Chelates - A New Class of Magnetic-Resonance-Imaging Contrast Agents. Magn. Reson. Med. 1994;31(1):1–8. doi: 10.1002/mrm.1910310102. [DOI] [PubMed] [Google Scholar]
- 12.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) chelates as MRI contrast agents: Structure, dynamics, and applications. Chem. Rev. 1999;99(9):2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 13.Querol M, Bogdanov A. Amplification strategies in MR imaging: Activation and accumulation of sensing contrast agents (SCAs). J. Magn. Reson. Imaging. 2006;24(5):971–982. doi: 10.1002/jmri.20724. [DOI] [PubMed] [Google Scholar]
- 14.Feng Y, Zong YD, Ke TY, Jeong EK, Parker DL, Lu ZR. Pharmacokinetics, biodistribution and contrast enhanced MR blood pool imaging of Gd-DTPA cystine copolymers and Gd-DTPA cystine diethyl ester copolymers in a rat model. Pharm. Res. 2006;23(8):1736–1742. doi: 10.1007/s11095-006-9028-z. [DOI] [PubMed] [Google Scholar]
- 15.Tweedle MF. Physiochemical Properties of Gadoteridol and Other Magnetic-Resonance Contrast Agents. Invest. Radiol. 1992;27:S2–S6. [PubMed] [Google Scholar]
- 16.Wedeking P, Tweedle M. Comparison of the biodistribution of 153Gd-labeled Gd(DTPA)2-, Gd(DOTA)-, and Gd(acetate)n in mice. Nucl. Med. Biol. 1988;15(4):395–402. doi: 10.1016/0883-2897(88)90009-8. [DOI] [PubMed] [Google Scholar]
- 17.Gibby WA, Gibby KA. Comparison of Gd DTPA-BMA (Omniscan) versus GdHP-DO3A (ProHance) retention in human bone tissue by inductively coupled plasma atomic emission spectroscopy. Invest. Radiol. 2004;39(3):138–142. doi: 10.1097/01.rli.0000112789.57341.01. [DOI] [PubMed] [Google Scholar]
- 18.Kay J, Bazari H, Avery LL, Koreishi AF, Cibotti-Granof N, Harris NL, Colvin RB. A woman with renal failure and stiffness of the joints and skin - Nephrogenic systemic fibrosis, involving the skin, heart, lungs, diaphragm and psoas muscles, dura, and possibly the kidney. End-stage renal disease with nephrocalcinosis. Acute and organizing bronchopneumonia. N. Engl. J. Med. 2008;358(8):827–838. [Google Scholar]
- 19.Thakral C, Alhariri J, Abraham JL. Long-term retention of gadolinium in tissues from nephrogenic systemic fibrosis patient after multiple gadolinium-enhanced MRI scans: case report and implications. Contrast Media Mol. Imaging. 2007;2:199–205. doi: 10.1002/cmmi.146. [DOI] [PubMed] [Google Scholar]
- 20.Idee JM, Port M, Raynal I, Schaefer M, Le Greneur S, Corot C. Clinical and biological consequences of transmetallation induced by contrast agents for magnetic resonance imaging: a review. Fundam. Clin. Pharmacol. 2006;20(6):563–576. doi: 10.1111/j.1472-8206.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 21.Geraldes CFGC, Lauren S. Classification and basic properties of contrast agents for magnetic resonance imaging. Contrast Media Mol. Imaging. 2008;4(1):1–23. doi: 10.1002/cmmi.265. [DOI] [PubMed] [Google Scholar]
- 22.Heffernan MJ, Murthy N. Polyketal nanoparticles: A new pH-sensitive biodegradable drug delivery vehicle. Bioconjugate Chem. 2005;16(6):1340–1342. doi: 10.1021/bc050176w. [DOI] [PubMed] [Google Scholar]
- 23.Lynn DM, Amiji MM, Langer R. pH-responsive polymer microspheres: Rapid release of encapsulated material within the range of intracellular pH. Angew. Chem. Int. Ed. 2001;40(9):1707–1710. [PubMed] [Google Scholar]
- 24.Sankaranarayanan J, Mahmoud EA, Kim G, Morachis JM, Almutairi A. Multiresponse Strategies To Modulate Burst Degradation and Release from Nanoparticles. ACS Nano. 2010;4(10):5930–5936. doi: 10.1021/nn100968e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang SC, Bhide M, Crispe IN, Pierce RH, Murthy N. Polyketal copolymers: A new acid-sensitive delivery vehicle for treating acute inflammatory diseases. Bioconjugate Chem. 2008;19(6):1164–1169. doi: 10.1021/bc700442g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruice TC, Piszkiew D. A search for carboxyl-group catalysis in ketal hydrolysis. J. Am. Chem. Soc. 1967;89(14):3568–3576. [Google Scholar]
- 27.Feng Y, Jeong E-K, Mohs AM, Emerson L, Lu Z-R. Characterization of Tumor Angiogenesis With Dynamic Contrast-Enhanced MRI and Biodegradable Macromolecular Contrast Agents in Mice. Magn. Reson. Med. 2008;60(6):1347–1352. doi: 10.1002/mrm.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu ZR, Wang XH, Parker DL, Goodrich KC, Buswell HR. Poly(L-glutamic acid) Gd(III)-DOTA conjugate with a degradable spacer for magnetic resonance imaging. Bioconjugate Chem. 2003;14(4):715–719. doi: 10.1021/bc0340464. [DOI] [PubMed] [Google Scholar]
- 29.Zong Y, Guo J, Ke T, Mohs AM, Parker DL, Lu ZR. Effect of size and charge on pharmacokinetics enhancement of biodegradable and in vivo MRI contrast polydisulfide Gd(III) complexes. J. Controlled Release. 2006;112(3):350–356. doi: 10.1016/j.jconrel.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Zong YD, Wang XL, Jeong EK, Parker DL, Lu ZR. Structural effect on degradability and in vivo contrast enhancement of polydisulfide Gd(III) complexes as biodegradable macromolecular MRI contrast agents. J. Magn. Reson. Imaging. 2009;27(4):503–511. doi: 10.1016/j.mri.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karfeld LS, Bull SR, Davis NE, Meade TJ, Barron AE. Use of a genetically engineered protein for the design of a multivalent MR1 contrast agent. Bioconjugate Chem. 2007;18(6):1697–1700. doi: 10.1021/bc700149u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karfeld-Sulzer LS, Waters EA, Davis NE, Meade TJ, Barron AE. Multivalent Protein Polymer MRI Contrast Agents: Controlling Relaxivity via Modulation of Amino Acid Sequence. Biomacromolecules. 2010;11(6):1429–1436. doi: 10.1021/bm901378a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang CA, Brittain HG, Telser J, Tweedle MF. pH-Dependence of Relaxivities and Hydration Numbers of Gadolinium(III) Complexes of Linear Amino Carboxylates. Inorg. Chem. 1990;29(22):4468–4473. [Google Scholar]
- 34.Kobayashi H, Brechbiel MW. Nano-sized MRI contrast agents with dendrimer cores. Adv. Drug Delivery Rev. 2005;57(15):2271–2286. doi: 10.1016/j.addr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 35.Lu ZR, Parker DL, Goodrich KC, Wang XH, Dalle JG, Buswell HR. Extracellular biodegradable macromolecular gadolinium(III) complexes for MRI. Magn. Reson. Med. 2004;51(1):27–34. doi: 10.1002/mrm.10656. [DOI] [PubMed] [Google Scholar]
- 36.Paramonov SE, Bachelder EM, Beaudette TT, Standley SM, Lee CC, Dashe J, Frechet JM. J. Fully acid-degradable biocompatible polyacetal microparticles for drug delivery. Bioconjugate Chem. 2008;19(4):911–919. doi: 10.1021/bc7004472. [DOI] [PubMed] [Google Scholar]
- 37.Mohs AM, Nguyen T, Jeong EK, Feng Y, Emerson L, Zong YD, Parker DL, Lu ZR. Modification of Gd-DTPA cystine copolymers with PEG-1000 optimizes pharmacokinetics and tissue retention for magnetic resonance angiography. Magn. Reson. Med. 2007;58(1):110–118. doi: 10.1002/mrm.21270. [DOI] [PubMed] [Google Scholar]
- 38.Mohs AM, Wang XH, Goodrich KC, Zong YD, Parker DL, Lu ZR. PEG-g-poly(GdDTPA-co-L-cystine): A biodegradable macromolecular blood pool contrast agent for MR imaging. Bioconjugate Chem. 2004;15(6):1424–1430. doi: 10.1021/bc049828r. [DOI] [PubMed] [Google Scholar]
- 39.Mohs AM, Zong YD, Guo JY, Parker DL, Lu ZR. PEG-g-poly(GdDTPA-co-L-cystine): Effect of PEG chain length on in vivo contrast enhancement in MRI. Biomacromolecules. 2005;6(4):2305–2311. doi: 10.1021/bm050194g. [DOI] [PubMed] [Google Scholar]
- 40.White DH, Delearie LA, Moore DA, Wallace RA, Dunn TJ, Cacheris WP, Imura H, Choppin GR. The Thermodynamics of Complexation of Lanthanide (III) DTPA-Bisamide Complexes and Their Implication for Stability and Solution Structure. Invest. Radiol. 1991;26:S226–S228. doi: 10.1097/00004424-199111001-00077. [DOI] [PubMed] [Google Scholar]
- 41.Kumar K, Chang CA, Francesconi LC, Dischino DD, Malley MF, Gougoutas JZ, Tweedle MF. Synthesis, Stability, and Structure of Gadolinium(III) and Yttrium(III) Macrocyclic Poly(amino carboxylates). Inorg. Chem. 1994;33(16):3567–3575. [Google Scholar]
- 42.Delgado R, Dasilva J. Metal-Complexes of Cyclic Tetra-Azatetra-Acetic Acids. Talanta. 1982;29(10):815–822. doi: 10.1016/0039-9140(82)80251-8. [DOI] [PubMed] [Google Scholar]
- 43.Mewis RE, Archibald SJ. Biomedical applications of macrocyclic ligand complexes. Coord. Chem. Rev. 2010;254(15-16):1686–1712. [Google Scholar]
- 44.Hanaoka K. Development of Responsive Lanthanide-Based Magnetic Resonance Imaging and Luminescent Probes for Biological Applications. Chem. Pharm. Bull. 2010;58(10):1283–1294. doi: 10.1248/cpb.58.1283. [DOI] [PubMed] [Google Scholar]
- 45.Thibon A, Pierre VC. Principles of responsive lanthanide-based luminescent probes for cellular imaging. Anal. Bioanal. Chem. 2009;394(1):107–120. doi: 10.1007/s00216-009-2683-2. [DOI] [PubMed] [Google Scholar]
- 46.Agdeppa ED, Spilker ME. A Review of Imaging Agent Development. The AAPS Journal. 2009;11(2):286–299. doi: 10.1208/s12248-009-9104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Nahhas A, Win Z, Szyszko T, Singh A, Nanni C, Fanti S, Rubello D. Gallium-68 PET: A New Frontier in Receptor Cancer Imaging. Anticancer Res. 2007;27(6B):4087–4094. [PubMed] [Google Scholar]