Abstract
A liquid chromatography in tandem with electro-spray ionization mass spectrometry method has been developed and validated for the quantitative determination of BUP and its major metabolites (hydroxybupropion, threo- and erythrohydrobupropion) in human umbilical cord plasma and placental tissue. The samples were acidified with trichloroacetic acid, and protein precipitated by adding acetonitrile. Chromatographic separation of drug and metabolites was achieved by using a Waters Symmetry C18 column, with an isocratic elution of 31% methanol and 69% formic acid (0.04%, v/v) aqueous solution at a flow rate of 1.0 mL/min. Detection was carried out by mass spectrometry using positive electro-spray ionization mode, and the compounds were monitored using multiple reactions monitoring method. Deuterium-labeled isotopes of the compounds were used as internal standards. Calibration curves were linear (r2 >0.99) in the tested ranges. The lower limit of quantification of analytes in umbilical cord plasma samples is < 0.72 ng/mL and 0.92 ng/g in placental tissue samples. The relative deviation of this method was < 15% for intra- and inter-day assays, and the accuracy ranged between 88 and 105%. The extraction recovery of the four analytes ranged between 89 and 96% in umbilical cord plasma, and 64 and 80% in placental tissue. No significant matrix effect was observed in the presented method.
Keywords: Bupropion, metabolites, umbilical cord plasma, placenta, LC-MS/MS
1. Introduction
Cigarette smoking is the largest modifiable risk factor for pregnancy-related morbidity and mortality in the U.S. [1]. Approximately 5%–10% of perinatal deaths, 20%–35% of low-birth-weight infants, and 8%–15% of preterm deliveries have been attributed to smoking [2,3]. Despite the known risks, some women continue to smoke during pregnancy, and is particularly true for those with less education and heavy smokers [4, 5]. Although behavioral interventions during pregnancy have consistent and beneficial effects on quitting due to greater motivation, a significant number of heavy smokers fail to achieve this goal [6] and thus the use of pharmacotherapy can be beneficial [7].
Bupropion hydrochloride (BUP, (±)-1-(3-chlorophenyl)-2-[(1,1-dimethylethyl)amino]-1propanone hydrochloride) is an antidepressant drug that has been used as an aid for smoking cessation in non-pregnant smokers [1]. However, BUP is classified as FDA Pregnancy Category C and is not approved for smoking cessation during pregnancy due to limited data on its safety and efficacy in this patient population. To fulfill this gap, a placebo-controlled trial of the safety and efficacy of bupropion sustained release for smoking cessation during pregnancy was initiated in 2011 by the OB/GYN department at the University of Texas Medical Branch, Galveston, Texas, according to a protocol approved by the Institutional Review Board.
In human’s BUP is extensively metabolized. The major and pharmacologically active metabolites of BUP in plasma are hydroxybupropion (OH-BUP), threohydrobupropion (TB), and erythrohydrobupropion (EB) [8]. Utilizing the ex vivo technique of dual perfusion of placental lobule it was demonstrated that BUP crosses human placenta [9]. In vitro, the placental enzyme 11β-hydroxysteroid dehydrogenases metabolizes bupropion to threo- and erythrohydro-bupropion [10]. These data, obtained from ex vivo and in vitro experiments, suggest that in vivo fetal exposure to BUP and its metabolites is plausible. However, the concentrations of BUP and its metabolites in the umbilical cord blood and placental tissue and, consequently, the risk of fetal exposure to them is yet to be determined.
Analytical methods using liquid chromatography for separation of the drug and metabolites and detection by UV (LC-UV) at 254 nm (for bupropion) and 214 nm (for its metabolites), mass spectrometry (LC-MS) or LC-MS/MS have been developed and validated for quantitative determination of BUP and its major metabolite OH-BUP in human plasma [11,12,13,14,15] and urine [14]. Simultaneous quantitative determination of BUP and all metabolites in plasma using LC-MS/MS method coupled with monolithic column [16] or LC-UV [17,18], and in whole blood using ultra-LC-MS/MS [19] have been also reported. However, incomplete chromatographic separation of TB and EB [16], the use of 1 ml of plasma for extraction [17,18], and lower extraction recoveries (58–63%) of BUP and its metabolites [19] limited our abilities to adopt one of these methods to analyze plasma samples (< 1 ml) obtained from umbilical arteries and veins with drug concentrations lower than in maternal plasma. In addition, to the best of our knowledge there were no reports on the quantitative analysis of BUP and its metabolites in human placenta.
Therefore, the goal of this investigation was to develop and fully validate an analytical method for the simultaneous quantitative determination of BUP and its metabolites in umbilical cord plasma and human placental tissue by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The method will be used to analyze umbilical cord plasma and placental tissue samples obtained from patients treated with bupropion during pregnancy in an ongoing clinical trial.
2. Experimental
2.1. Chemicals
Chemicals were purchased from the following companies: Bupropion hydrochloride, from Sigma Chemical Co. (St. Louis, MO); hydroxybupropion, erythro/threohydrobupropion, and the deuterium labeled internal standards d9-bupropion hydrochloride, d6-hydroxybupropion and d9-erythro/threohydrobupropion from Toronto Research Chemicals Inc. (North York, Canada); HPLC-grade methanol, acetonitrile, formic acid and trichloroacetic acid (TCA) from Fisher Scientific (Fair Lawn, NJ).
2.2. Instrumental and analytical conditions
The analysis of bupropion and its three metabolites was achieved by an Agilent HPLC 1200 series system coupled with an API 4000 triple quadrupole mass spectrometer (Applied Biosystems, Foster City, CA). The HPLC system consisted of a degasser (G1379B), binary pump delivery system (G1312A), Hip-ALS auto-sampler (G1376B) and column compartment (G1316A) controlled by Analyst™1.5 Software (MDS INC. and Applera Corporation, USA). Separation of analytes was achieved by a Waters Symmetry C18 column (150×4.6mm, 5µm) connected to a Phenomenex C18 guard (4×3.0mm) at 15° C. The mobile phase was made of methanol and 0.04% formic acid aqueous solution (v/v). Elution in 31% methanol was isocratic for 12 min at flow rate 1 mL/min. A T-connector was used to direct the post-column eluent to an integrated Valco valve (Valco Instrument Co., Inc., Houston, TX, USA) at a flow rate of 150 µL/min; this design was employed to remove impurities prior to the eluent’s flow to the MS detector.
The API 4000 triple quadrupole mass spectrometer is equipped with a Turbo (V) ion source (ESI) and was operated in positive mode. Multiple reactions monitoring (MRM) mode was applied for the quantification of analytes. The source/gas dependent MS parameters were as follows: IonSpray Voltage, 4000 V; Curtain Gas, 15 L/h; Ion Source Gas1, 40 L/h; Ion Source Gas2, 20 L/h; Temperature, 300°C; Collision Gas 5 L/h. The compound dependent parameters for each MRM transition of analytes and deuterium labeled internal standards are shown in Table 1.
Table 1.
The compound dependent parameters for the MRM transition of bupropion and its metabolites.
| Compound dependent parametersa | BUP | OH-BUP | EB | TB |
|---|---|---|---|---|
| Retention time of analyte (min) | 7.7 | 6.7 | 8.5 | 9.5 |
| Analyte MRM transition (m/z) | 240→184 | 256→238 | 242→168 | 242→168 |
| Retention time of internal standard (min) | 7.6 | 6.6 | 8.4 | 9.3 |
| Internal standard MRM transition(m/z)b | 249→185 | 262→244 | 251→169 | 251→169 |
| Declustering Potential (v) | 40 | 40 | 50 | 50 |
| Entrance Potential (v) | 6 | 6 | 6 | 6 |
| Collision energy (v) | 18 | 18 | 25 | 25 |
| Collision Cell Exit Potential (v) | 14 | 14 | 14 | 14 |
BUP=buproipion, OH-BUP=hydroxybupropion, EB=erythrohydrobupropion, TB=threohydrobupropion.
The deuterium labeled internal standards are d9-bupropion hydrochloride, d6-hydroxybupropion, d9-erythrohydrobupropion and d9-threohydrobupropion.
2.3. Preparation of stock and working standard solutions
Stock solutions for OH-BUP, EB, TB, BUP were dissolved in 30% methanol. For analysis of umbilical plasma the working standards solutions were prepared in the following ranges: BUP, 2.50-2.50×103 ng/mL; OH-BUP, 7.15-7.15×103 ng/mL; EB, 2.45-2.45×103 ng/mL ; and TB, 3.65-3.65×103 ng/mL. For analysis of placental tissue the working standards solutions were prepared in the following ranges: BUP, 6.25-5.00×103 ng/mL; OH-BUP, 17.9-14.3×103 ng/mL; EB, 6.10-4.88×103 ng/mL, and TB, 18.4-14.7×103 ng/mL. The stock solutions of internal standard (IS) were prepared at final concentrations of 80.0 ng/mL for d9-BUP, 67.0 ng/mL for d6-OH-BUP, 73.0 ng/mL for d9-EB and 67.0 ng/mL for d9-TB in 30% methanol aqueous solution. All the solutions were stored at 4°C.
2.4. Preparation of calibration standards and quality control (QC) samples
Calibration curves for standards were constructed by adding 10 µL of working solution into 100 µL blank umbilical cord plasma or to 0.2 g placental tissue. For analysis of umbilical plasma calibration standards were prepared in the following concentrations: BUP, 0.250-250 ng/mL; OH-BUP, 0.716-716 ng/mL; EB, 0.245-245 ng/mL, and TB 0.365-365 ng/mL of TB. For analysis of placental tissue calibration standards were prepared in the following concentrations: BUP, 0.312-250 ng/g; OH-BUP, 0.895-715 ng/g; EB, 0.305-244 ng/g, and TB 0.918-735 ng/g. To each calibration standard, 10µL of IS stock solution was added. Quality control (QC) samples were similarly prepared at high, medium, low concentration levels for each analytes, as well as for lower limit of quantification (LLOQ).
2.5. Preparation of human placental tissue and umbilical cord plasma samples
Placentas, umbilical venous and arterial blood were obtained immediately after delivery from the Labor and Delivery ward of John Sealy Hospital according to a protocol approved by the Institutional Review Board of the University of Texas Medical Branch at Galveston. The blood was collected into the BD Vacutainer® blood collection tubes contained with lithium heparin (87 USP Units). Plasma was separated by centrifugation and all biological were kept frozen at −80°C until analyzed.
Preparation of umbilical cord plasma samples was as follows: An IS solution (10 µL) was added to 100 µL umbilical cord plasma and vortexed for 30 sec. Then 50 µL of 2%, w/v of TCA and 800 µL acetonitrile were added to the samples. The solution was vortexed for 30 sec and centrifuged at 12000 × g for 15 min at 4°C. The supernatant was transferred to a tube and dried under a stream of air at 40°C.
Placental tissue samples were prepared as follows: tissue (0.2 ± 0.005 g) was blotted with the filter paper to remove excess of blood and weighted. IS stock solution 10 µL, 10 µL of TCA (10%, w/v) and 300 µL saline were added to the tissue, homogenized for 30 sec at 3000 rpm using an ULTRA-TURRAX® dispersers (IKA Works GmbH & Co. KG, German). Acetonitrile 1.2 mL was added to the homogenate, vortexed for 30 sec and centrifuged at 12000 × g for 15 min at 4°C. The supernatant was transferred to a tube and dried under a stream of air at 40°C.
All the above dried residues were reconstituted into 120 µL of the initial mobile phase and filtered using 0.45 µm syringe filter. Aliquot of 25 µL of each sample was analyzed by HPLC system.
2.6. Method validation
The method was fully validated for specificity, matrix effect, linearity, sensitivity, precision, accuracy and stability according to the US Food and Drug Administration guideline (FDA, 2001) [20]. The selectivity was evaluated by analyzing blank umbilical cord plasma and placental tissue samples obtained from six patients. The MRM chromatograms of these blank samples were compared with LLOQ samples. The peak area of endogenous substances co-eluting with the analytes was < 5% of the peak area of analytes at LLOQ concentration levels.
The series of calibration curves (n=9) were prepared in blank umbilical cord plasma and placental tissue samples as described above for preparation of the calibration standards. Samples for each calibration curve were determined in triplicate. The internal ratios (analyte peak area/IS peak area) were calculated for each point, and calibration curves were fitted using weighted least-squares linear regression of internal ratio versus concentration. A correlation >0.99 was desirable for all calibration curves. The limit of detection and quantification were determined by measuring the signal-to-noise (S/N) ratio for each analyte. S/N ratio was 3:1 for LOD and 10:1 for LLOQ. In addition, precision of analytes at LLOQ did not exceed 20% and accuracy was in the range of 80–120% [20].
The matrix effect of analytes and IS was measured quantitatively with Matrix Factor which was defined as the ratio of the analyte peak area of the post-extracted samples versus the analyte peak area of pure standards [21].The Matrix Factor of each analyte was investigated at low and high concentrations in umbilical cord plasma and placental tissue samples obtained from six patients. The variability of Matrix Factor, as measured by the relative standard deviation (RSD) was <15% [21].
The accuracy and precision of the method were evaluated by analyzing the QC samples of umbilical cord plasma and placental tissue at high, medium, low and LLOQ concentrations in six replicates, on 3 validation days. The relative standard deviation for each concentration level did not exceed 15%, except for LLOQ which was < 20%. Accuracy of the method was evaluated by comparing the calculated concentration using calibration curves to added concentrations. The value of the accuracy should be in the range of 85–115% of the nominal concentration, except for the LLOQ which should be in the range of 80–120% [20].
Recovery of extracted analytes in umbilical cord plasma and placental tissue samples were evaluated with the peak area of analytes in QC samples versus the peak area of analytes in post-extracted samples at high, medium and low concentration levels.
The stability of analytes in samples of human umbilical cord plasma and placental tissue were investigated by analyzing replicates (n=3) QC samples at the high and low concentrations, and were exposed to different time and temperature conditions. Short-term stability of bupropion and its metabolites in the QC samples were analyzed after being thawed and kept at room temperature (22–25°C) for 4 hours. Freeze-thaw stability was determined after three cycles between room temperature and −80°C. The long-term stability was determined after the QC samples were kept at −80°C for 30days. Thereafter, the samples were analyzed and the concentration was calculated using freshly prepared calibration curves. The analytes were considered stable if the RSD for each concentration did not exceed 15%, and the accuracy did not deviate by ±15% of the nominal concentration.
3. Results and Discussion
3.1 Mass spectrometric conditions
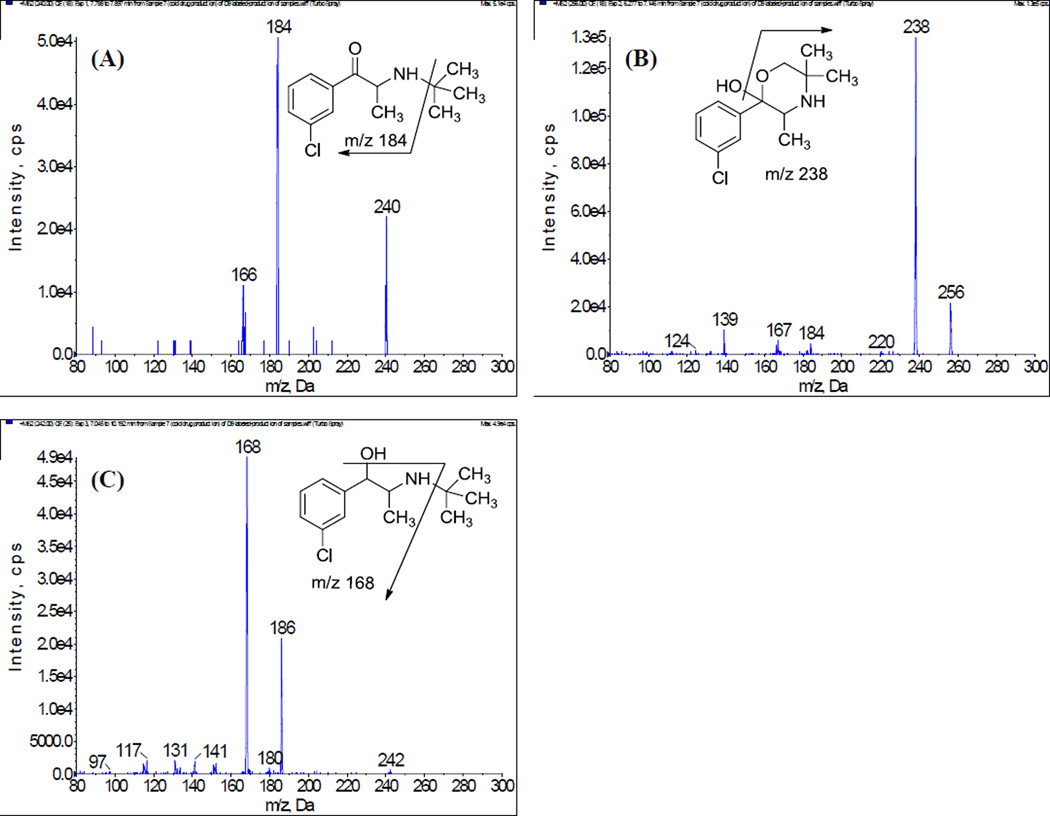
The responses of the precursor ions of bupropion and its metabolites were optimized by using infusion of the neat solution (in initial mobile phase) under both positive and negative modes of ESI. The analytes showed good responses and stability in the positive ionization mode for the protonated precursor [M+H]+ ions of BUP at m/z 240 and OH-BUP at m/z 256. The EB and TB have the same protonated precursor ions at m/z 242. The corresponding product ion mass spectra of the analytes are shown in Figure 1, where [M+H]+ for each compound was selected as the precursor ion. The most abundant and consistent product ions observed were as follows: at m/z 184 for BUP after losing one tert-butyl group (m/z 56); at m/z 238 for OH-BUP after loss of a water molecule; at m/z 168 for TB and EB after loss of a tert-butyl group and a water molecule. The deuterium-labeled compounds were chosen as internal standards (IS). Each of the internal standard compounds was labeled with deuterium in the tert-butyl group. The product ion spectra of IS (supplementary data, Figure 4) showed that the deuterium atom was transferred from the protonated precursor ion of IS to its corresponding fragment prior to loss of the tert-butyl group. Thus, the MRM transitions of IS were chosen as m/z 249→185 for d9-BUP, m/z 262→244 for d6-OH-BUP, and m/z251→169 for d9-TB and d9-EB (Table 1).
Figure 1.
Product ion spectra of [M+H]+ of (A) bupropion, (B) hydroxybuproipion, (C) threo/erythrohydrobupropion.
3.2 Liquid Chromatographic conditions
Theoretically, BUP and TB/EB do not require chromatographic separation because of their different MRM transitions (Table 1). However, the peak interference from 37Cl-BUP (m/z 242→168) was observed in the TB/EB MRM transitions [16] due to the loss of the tert-butyl group and water molecule from the protonated precursor ion 37Cl-BUP. Therefore, the BUP and TB/EB required chromatographic separation using LC. Moreover, TB and EB also required chromatographic separation because they have the same MRM transitions (Table 1). Accordingly, the chromatographic conditions including buffer composition and pH of mobile phase were modified and optimized for peak resolution, retention time and peak symmetry of the analytes.
The retention times and resolution of BUP and its metabolites were affected by the composition and pH values of the mobile phase. While acetonitrile and methanol were optimized as organic modifiers, methanol was chosen because it enabled good resolution between TB and EB as well as good peak symmetry of the four analytes. The mobile phase additives ammonium acetate and ammonium formate buffers were optimized at different pH values of 6.0, 5.5, 5.0 and 4.0. Although the use of 10 mM ammonium acetate (pH 6.0) resulted in good resolutions of the analytes, the elution gradient had to be adjusted because the retention time of BUP was much longer than that for the metabolites. Several mobile phases containing different percentages of formic acid or acetic acid (1.0%, 0.5%, 0.1% and 0.04% (v/v)) were tested. Both formic and acetic acids resulted in good resolution, as reflected by the retention times of the four analytes, at a concentration of 0.04% (v/v). However, 0.04% (v/v) acetic acid generated significant baseline noise at the MRM transition of OH-BUP (m/z 256→238) compared with 0.04% (v/v) formic acid. Finally, the mobile phase was chosen as 31% methanol and 69% formic acid (0.04%, v/v) aqueous solution with an isocratic elution flow rate 1.0 mL/min.
Several analytical columns were tested (Luna® C18, Synergi Hydro-RP C18, Synergi Fusion-RP C18 from Phenomenex, C18 Zorbax Eclipse XDB from Agilent and Symmetry® C18 from Waters). The column of Symmetry® C18 provided best resolution, retention time and peak symmetry of the analytes. Ideally, a deuterium-labeled internal standard should have the same retention time as that for its isotope to minimize the matrix effect of the samples. In this system, the column temperature was set to 15°C in order to minimize differences in the retention times of the analytes and their deuterated internal standards. For example, with the column oven set at 40°C, the differences in retention time could be as high as 0.4 minutes, but at 15°C, these differences were reduced to about 0.1 min.
3.3 Sample preparation
The extraction methods of BUP and its metabolites from biological matrix include liquid-liquid extraction (LLE) [16–18], solid phase extraction (SPE) [11, 14, 19] and protein precipitation (PP) [12,22]. In this investigation, LLE and PP methods were used. Plasma and tissue samples were acidified by 0.1 M HCl or alkalized by 0.5 M carbonate buffer and then extracted with isoamyl alcohol, n-heptane, ethyl acetate and dichloromethane. LLE provided clean extracts but the recovery was low (60–75%) and not quantitative at the LLOQ concentration levels. Protein precipitation with organic solvents, such as acetonitrile and methanol, was optimized on the basis of analyte recovery. Protein precipitation with methanol and acetonitrile resulted in good recovery for OH-BUP, TB, and EB, but the recovery of BUP was not as high (data not shown). Therefore, the combination of acetonitrile and TCA was used instead, which resulted in high recovery for all four analytes in plasma (~89–96%, see Table 5). In the previous reports [12, 22], 3–4% TCA (w/v) was used for rat plasma protein precipitation for the determination of BUP and OH-BUP. However, TCA acts as an ion-pairing reagent in the chromatographic method [23] and its final concentration in the samples had a significant effect on the retention time of BUP and its metabolites. In the present study, the final concentration of TCA in the samples was 0.31% and 0.62% (w/v) in the handling of tissue and plasma samples, respectively. This resulted in reproducible retention times for all analytes.
Table 5.
Extraction recovery of the bupropion and its three metabolites in umbilical cord plasma and placental tissue (n=3).
| Bio-matrix | Analytea | Added concentrationb | Mean recoveryc (%) | Precision (RSD, %) |
|---|---|---|---|---|
| Umbilical cord plasma | BUP | 0.625 62.5 188 |
92.4 89.2 91.3 |
6.8 4.8 2.9 |
| OH-BUP | 1.79 179 536 |
95.5 91.9 94.7 |
4.8 4.1 4.3 |
|
| EB | 0.612 61.2 184 |
95.9 88.6 93.0 |
3.0 5.8 1.4 |
|
| TB | 0.912 91.2 274 |
94.0 90.0 93.0 |
2.5 5.1 1.3 |
|
| Placental tissue | BUP | 0.625 62.5 188 |
64.3 66.1 67.4 |
10.8 18.6 15.2 |
| OH-BUP | 1.79 179 537 |
73.3 75.9 76.5 |
17.4 18.3 14.7 |
|
| EB | 0.610 61.0 183 |
69.6 73.0 76.0 |
18.3 18.4 15.7 |
|
| TB | 1.84 184 550 |
73.4 77.7 79.8 |
17.5 16.9 14.7 |
BUP=buproipion, OH-BUP=hydroxybupropion, EB=erythrohydrobupropion, TB=threohydrobupropion.
The unit of analyte in umbilical cord plasma is ng/mL; the unit of analyte in placental tissue is ng/g.
Recovery= (peak area of QC sample/peak area of post-extract sample)×100
3.4 Method validation
3.4.1 Selectivity and matrix effect
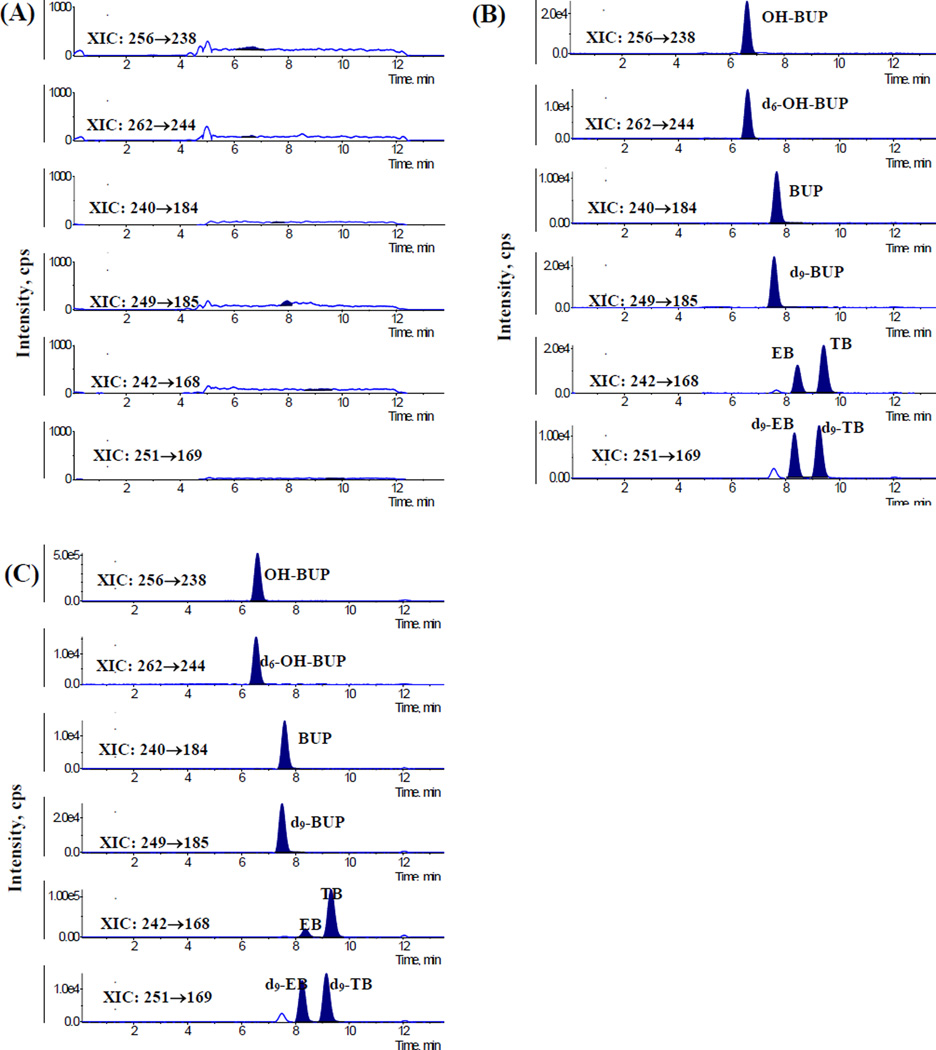
The selectivity of the method was achieved by comparing MRM chromatograms of six different blank (non exposed) samples of umbilical cord plasma and placental tissue. Figure 2 shows typical MRM chromatograms of blank samples of umbilical cord plasma, blank umbilical cord plasma spiked with BUP and its metabolites, and umbilical cord plasma obtained from patients treated with 150 mg/day dose of BUP. Endogenous metabolites in umbilical cord plasma and placental tissue samples did not interfere with the retention times of BUP, its metabolites and IS.
Figure 2.
The MRM chromatograms for the determination of bupropion and its three metabolites in umbilical cord plasma: (A) chromatogram of blank umbilical cord plasma; (B) chromatogram of blank umbilical cord plasma spiked with bupropion (BUP, 6.25 ng/mL), hydroxybupropion (OH-BUP, 17.9 ng/mL), erythrohydrobupropion (EB, 6.12 ng/mL), threohydrobupropion (TB, 9.12 ng/mL) and internal standards. (C) Umbilical cord plasma of pregnant patient prescribed 150mg/day of BUP dosage.
The Matrix Factor of the four analytes in six umbilical cord plasma and placental tissue samples at the low and high concentration levels ranged between 94% and 103%, with relative standard deviation < 8% (Table 2). These results indicate that the ion suppression or enhancement of the analytes in human umbilical cord plasma and placental tissue matrix were negligible.
Table 2.
Matrix effect factor of bupropion and its three metabolites in umbilical cord plasma (n=3)
| Bio-matrix | Analytea | Added concentrationb | Matrix factorc (%) | Precision (RSD, %) |
|---|---|---|---|---|
| Umbilical cord plasma | BUP | 0.625 188 |
94.9 100.1 |
7.6 1.4 |
| OH-BUP | 1.79 536.2 |
99.5 100.0 |
5.9 1.8 |
|
| EB | 0.612 184 |
98.9 99.5 |
4.0 1.5 |
|
| TB | 0.912 274 |
98.5 100.3 |
4.8 2.8 |
|
| Placental tissue | BUP | 0.625 188 |
100.5 99.2 |
2.6 0.9 |
| OH-BUP | 1.79 537 |
100.2 100.7 |
1.5 0.9 |
|
| EB | 0.610 183 |
100.0 101.1 |
2.8 0.9 |
|
| TB | 1.84 550 |
103.7 100.2 |
2.8 0.4 |
BUP=buproipion, OH-BUP=hydroxybupropion, EB=erythrohydrobupropion, TB=threohydrobupropion.
The unit of analyte in umbilical cord plasma is ng/mL; the unit of analyte in placental tissue is ng/g.
Matrix factor=(peak area of post-extract sample/peak area of pure standard)×100
3.4.2 Linearity, Lower limit of quantification (LLOQ) and limit of detection (LOD)
Calibration curves for the samples were constructed by the internal standard method and fit by using weighted (1/y2) least-squares linear regression analysis of internal ratio versus concentration (Table 3). The calibration curve exhibited good linear regressions (r2>0.99) within test range. The lack of fit test of the regression between the concentration and the internal ratio was not significant using F-test at α=0.05 levels [24]. LLOQ of the four analytes ranged between 0.250 ng/mL and 0.715 ng/mL for umbilical cord plasma samples, and between 0.312 ng/g and 0.918 ng/g for placental tissue samples. The intra- and inter-day accuracy of the four analytes at LLOQ concentration levels ranged between 88% and 102%, with the precision < 16% (Table 4). LOD for the four analytes ranged between 0.049 ng/mL and 0.073 ng/mL for umbilical cord plasma samples and between 0.122 ng/g and 0.184 ng/g for placental tissue samples.
Table 3.
Calibration curves of bupropion and its three metabolites in umbilical cord plasma and placental tissue (n=3).
| Bio-matrix | Analytea | Regression equationb, c | r2 |
F-testd |
Linear rangec | LODc | |
|---|---|---|---|---|---|---|---|
| F | p-Value | ||||||
| Umbilical cord plasma | BUP | y=0.007+0.074x | 0.995 | 0.79 | 0.614 | 0.250-250 | 0.050 |
| OH-BUP | y=0.010+0.089x | 0.996 | 1.02 | 0.434 | 0.715-715 | 0.072 | |
| EB | y=0.004+0.181x | 0.996 | 1.01 | 0.440 | 0.245-245 | 0.049 | |
| TB | y=0.022+0.183x | 0.995 | 1.00 | 0.447 | 0.365-365 | 0.073 | |
| Placental tissue | BUP | y=0.011+0.110x | 0.992 | 1.31 | 0.301 | 0.312-250 | 0.125 |
| OH-BUP | y=0.023+0.174x | 0.991 | 1.33 | 0.294 | 0.895-716 | 0.179 | |
| EB | y=0.005+0.199x | 0.991 | 1.09 | 0.411 | 0.305-244 | 0.122 | |
| TB | y=0.015+0.132x | 0.989 | 1.41 | 0.262 | 0.918-734 | 0.184 | |
BUP=buproipion, OH-BUP=hydroxybupropion, EB=erythrohydrobupropion, TB=threohydrobupropion.
In the regression equation y=b+ax, x refers to the concentration of the analyte in bio-matrix; y refers to the peak area ratio of the analyte vs. IS.
The unit of analyte in umbilical cord plasma is ng/mL; the unit of analyte in placental tissue is ng/g.
F-test for lack-of-fit.
Table 4.
Intra-day and inter-day precision and accuracy of the method for umbilical plasma and placental tissue.
| Bio-matrix | Analytea | Addedb concentration |
Intra-day (n=6) | Inter-day (n=3) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean concentration obtainedb |
Mean accuracyc (%) |
Precision (RSD, %) |
Mean concentration obtainedb |
Mean accuracyc (%) |
Precision (RSD, %) |
|||
| Umbilical cord plasma | BUP | 0.250 | 0.220 | 88.1 | 11.3 | 0.237 | 94.7 | 10.4 |
| 0.625 | 0.553 | 88.5 | 5.3 | 0.577 | 92.4 | 4.4 | ||
| 62.5 | 59.2 | 94.7 | 6.2 | 60.8 | 97.3 | 4.4 | ||
| 188 | 190 | 101.5 | 4.1 | 186.6 | 99.5 | 1.8 | ||
| OH-BUP | 0.715 | 0.702 | 98.2 | 5.7 | 0.680 | 95.0 | 7.4 | |
| 1.79 | 1.77 | 98.9 | 5.7 | 1.774 | 99.2 | 1.8 | ||
| 179 | 166 | 92.9 | 5.5 | 172.8 | 96.7 | 3.6 | ||
| 536 | 512 | 95.5 | 2.2 | 519.0 | 96.8 | 2.0 | ||
| EB | 0.245 | 0.239 | 97.7 | 5.3 | 0.234 | 95.7 | 6.4 | |
| 0.612 | 0.571 | 93.2 | 3.8 | 0.587 | 95.8 | 4.3 | ||
| 61.2 | 57.1 | 93.3 | 6.0 | 58.8 | 95.9 | 3.1 | ||
| 184 | 182 | 98.9 | 4.9 | 180.2 | 98.1 | 1.7 | ||
| TB | 0.365 | 0.347 | 95.0 | 8.3 | 0.344 | 94.3 | 2.3 | |
| 0.912 | 0.860 | 94.2 | 4.8 | 0.873 | 95.7 | 2.1 | ||
| 91.2 | 85.9 | 94.2 | 7.4 | 89.1 | 97.7 | 4.1 | ||
| 274 | 266 | 97.1 | 5.0 | 266.4 | 97.3 | 1.1 | ||
| Placental tissue | BUP | 0.312 | 0.320 | 102.4 | 9.6 | 0.301 | 96.4 | 9.3 |
| 0.625 | 0.605 | 96.8 | 8.8 | 0.605 | 96.8 | 7.7 | ||
| 62.5 | 64.7 | 103.6 | 4.9 | 64.2 | 102.7 | 5.6 | ||
| 188 | 185 | 98.5 | 8.6 | 185 | 98.8 | 7.4 | ||
| OH-BUP | 0.895 | 0.811 | 90.7 | 12.0 | 0.839 | 93.8 | 12.5 | |
| 1.79 | 1.78 | 99.4 | 5.1 | 1.76 | 98.3 | 6.1 | ||
| 179 | 188 | 105.1 | 4.1 | 182 | 101.8 | 6.4 | ||
| 537 | 522 | 97.3 | 8.8 | 522 | 97.2 | 7.2 | ||
| EB | 0.305 | 0.273 | 89.6 | 15.2 | 0.279 | 91.6 | 13.2 | |
| 0.610 | 0.597 | 97.9 | 8.8 | 0.587 | 96.3 | 8.3 | ||
| 61.0 | 63.2 | 103.6 | 3.8 | 61.8 | 101.3 | 5.7 | ||
| 183 | 181 | 98.7 | 8.4 | 180 | 98.5 | 7.4 | ||
| TB | 0.918 | 0.856 | 93.3 | 11.5 | 0.858 | 93.5 | 11.2 | |
| 1.84 | 1.85 | 100.6 | 6.5 | 1.82 | 99.1 | 6.5 | ||
| 184 | 193 | 105.3 | 4.1 | 190 | 103.5 | 5.4 | ||
| 550 | 524 | 95.3 | 7.7 | 526 | 95.5 | 9.6 | ||
BUP=buproipion, OH-BUP=hydroxybupropion, EB=erythrohydrobupropion, TB=threohydrobupropion.
The unit of analyte in umbilical cord plasma is ng/mL; the unit of analyte in placental tissue is ng/g.
accuracy=(obtained concentration/added concentration)×100
3.4.3 Accuracy and precision
The results of accuracy and precision for the QC samples at high, medium and low concentration levels are shown in Table 4. The intra-day accuracy for the four analytes in umbilical cord plasma and placental tissue samples ranged between 88% and 105% with precision < 8%, and the inter-day accuracy ranged between 92% and 104% with precision < 10%. These results indicate acceptable reproducibility of the method used.
3.4.4 Extraction recovery
As shown in Table 5, the extraction recovery of BUP and its metabolites from umbilical cord plasma ranged between 89% and 96% with variation < 7% at low, medium and high concentration levels. The extraction recovery for the four analytes from placental tissue ranged between 64% and 80% with a variation < 19%. The observed lower extraction recovery from the placental tissue as compared to plasma is attributed to the differences between solid and liquid biomatrices.
3.4.5 Stability of the samples
The stability of BUP is significantly affected by storage temperature and pH of the bio-matrix, and hence all biological samples should be frozen soon after collection [25]. At room temperature (22°C) BUP in plasma was degraded by 5% of its total amount within 4h, 10% in 8h, 26% in 24h and 46% in 48h [25]. In this study, short-term stability tests were performed for 4h at room temperature (22–25°C). The accuracy of short-term stability ranged between 91% and 103% with precision < 7% at the two QC levels (low and high), indicating that the four analytes were stable for 4h in umbilical cord plasma and placental tissue samples at room temperature (22–25°C, Table 6). The accuracy after three freeze-thaw cycles ranged between 89% and 105% with the precision < 11%, indicating that the four analytes are stable under these conditions. Furthermore, BUP and its metabolites were stable at −80°C for 30 days in human umbilical cord plasma and placental tissue samples.
Table 6.
Stability of bupropion and its three metabolites in umbilical cord plasma and placental tissue (n=3)
| Bio-matrix | Analytea | Added concentrationb |
Freeze and thaw stability (3cycles, −80°C) |
Short-term stability (4h, 22–25°C) |
Long-term stability (30days, −80°C) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean concentration obtainedb |
Mean accuracyc (%) |
Precision (RSD, %) |
Mean concentration obtainedb |
Mean accuracyc (%) |
Precision (RSD, %) |
Mean concentration obtainedb |
Mean accuracyc (%) |
Precision (RSD, %) |
|||
| Umbilical cord plasma | BUP | 0.625 | 0.606 | 96.9 | 4.5 | 0.601 | 96.2 | 6.2 | 0.629 | 100.6 | 11.8 |
| 188 | 172 | 91.9 | 6.9 | 172 | 92.0 | 3.1 | 203 | 108.3 | 7.1 | ||
| OH-BUP | 1.79 | 1.74 | 97.5 | 10.7 | 1.75 | 97.7 | 3.0 | 2.02 | 112.8 | 11.6 | |
| 536 | 494 | 92.1 | 5.0 | 492 | 91.8 | 2.3 | 553 | 103.1 | 7.0 | ||
| EB | 0.612 | 0.549 | 89.6 | 7.7 | 0.560 | 91.5 | 6.2 | 0.653 | 106.7 | 12.5 | |
| 184 | 166 | 90.5 | 3.1 | 166 | 90.7 | 2.7 | 193 | 104.8 | 7.8 | ||
| TB | 0.912 | 0.814 | 89.2 | 6.4 | 0.882 | 96.7 | 5.3 | 0.991 | 108.6 | 12.0 | |
| 274 | 249 | 91.1 | 3.3 | 254 | 93.0 | 2.9 | 259 | 104.4 | 7.7 | ||
| Placental tissue | BUP | 0.625 | 0.571 | 91.4 | 1.7 | 0.622 | 99.5 | 5.2 | 0.659 | 105.4 | 5.5 |
| 188 | 193 | 103.0 | 4.8 | 192 | 102.7 | 5.8 | 186 | 99.1 | 2.3 | ||
| OH-BUP | 1.79 | 1.65 | 91.7 | 3.8 | 1.78 | 99.5 | 2.3 | 1.73 | 97.0 | 5.2 | |
| 536 | 539 | 100.3 | 4.1 | 536 | 99.8 | 4.9 | 510 | 95.2 | 1.3 | ||
| EB | 0.610 | 0.555 | 91.0 | 4.8 | 0.586 | 96.1 | 7.1 | 0.617 | 100.7 | 5.7 | |
| 183 | 191 | 104.4 | 6.1 | 186 | 101.8 | 5.2 | 179 | 97.2 | 5.7 | ||
| TB | 1.84 | 1.74 | 95.0 | 4.0 | 1.81 | 99.1 | 2.9 | 1.73 | 94.5 | 5.2 | |
| 550 | 522 | 100.3 | 4.4 | 547 | 99.4 | 3.6 | 508 | 92.2 | 8.3 | ||
BUP=buproipion, OH-BUP=hydroxybupropion, EB=erythrohydrobupropion, TB=threohydrobupropion.
The unit of analyte in umbilical cord plasma is ng/mL; the unit of analyte in placental tissue is ng/g.
accuracy=(obtained concentration/added concentration)×100
3.4.6 Method application
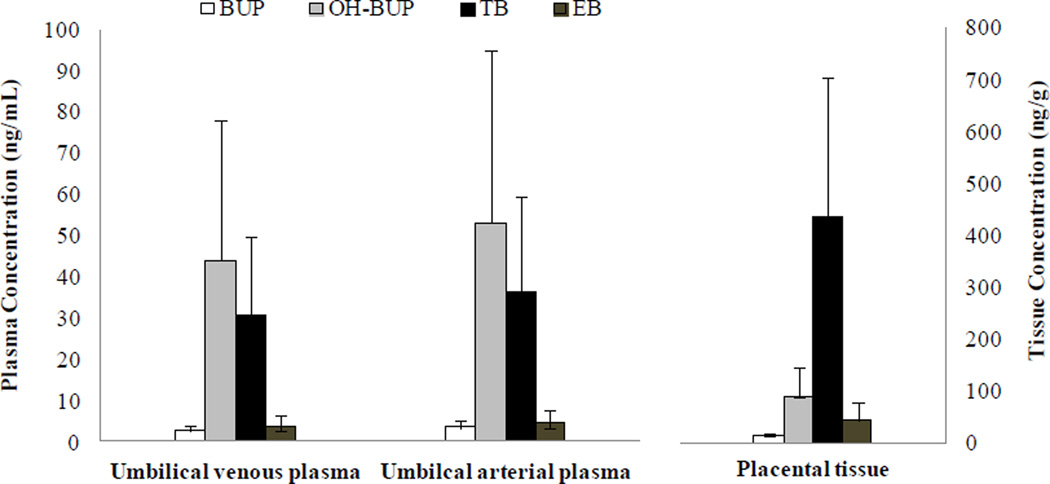
This validated analytical method was used to determine the concentrations of BUP and its metabolites in umbilical cord blood and placental tissue samples obtained from patients (n=3). Pregnant patients with depression received an oral dose of bupropion 150 mg per day according to care provider prescription. Preliminary data revealed that TB is the major metabolite of BUP in placental tissue (Figure 4) and is in agreement with the previous report on the biotransformation of BUP by placental microsomes [10]. The concentrations of OH-BUP and TB determined in the umbilical vein were significantly higher than concentrations of BUP and EB. These data suggest that in utero fetal exposure to OH-BUP and TB is likely but not to BUP and EB. The presence of BUP and its three metabolites in the umbilical artery in concentrations approximately similar to the umbilical vein suggest that their route of elimination by the fetus is by their transfer to the maternal circulation.
4. Conclusion
This is the first report, to the best of our knowledge, on an LC/MS/MS method for the simultaneous determination of BUP and its three major metabolites in human umbilical cord blood and placental tissue. The method reported here proved to be simple, rapid, accurate and reliable. The optimization of the chromatographic conditions and procedures for sample preparation resulted in a sensitive method with an LLOQ of < 0.72 ng/mL for umbilical cord plasma, and < 0.92 ng/g for placental tissue in a sample volume smaller than that previously reported [17–19]. This validated method will be used to determine the distribution of BUP and its metabolites between umbilical cord blood and placental tissue samples obtained from patients enrolled in a “clinical trial” for the safety and efficacy of bupropion sustained release use for smoking cessation during pregnancy.
Supplementary Material
Figure 3.
The concentration of BUP and its three metabolites in umbilical venous and arterial plasma, and placental tissue of pregnant patient prescribed 150mg/day of BUP dosage. The data was presented as mean±SE (n=3).
Highlights.
An LC/MS quantitative method for determining the concentrations of bupropion and its three major metabolites was developed and validated.
The lower limit of quantification for the four analytes is less than 0.8 ng/mL of human umbilical cord plasma and 1.0ng/g of placental tissue.
The method was applied to determine the concentration of BUP and its metabolites in human umbilical cord blood and placental tissue samples.
Acknowledgment
The authors appreciate the support of the physicians and nurses of the Labor & Delivery Ward of the John Sealy Hospital, the teaching hospital of the UTMB, Galveston, Texas. The authors also appreciate the assistance of the Perinatal Research Division and Publication, Grant, & Media Support Office of the Department of Obstetrics & Gynecology.
This work was supported by RO1 DA030998 to GH and TN.
A list of non-standard abbreviations
- BUP
bupropion
- OH-BUP
hydroxybupropion
- TB
Threohydrobupropion
- EB
erythrohydrobupropion
- TCA
trichloroacetic acid
- MS
mass spectrometry
- ESI
electro-spray ionization
- MRM
multiple reactions monitoring
- LOD
Limit of detection
- LLOQ
lower limit of quantification
- S/N
signal-to-noise ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- 1.Benowitz NL, Dempsey DA. Pharmacotherapy for smoking cessation during pregnancy. Nicotine and Tobacco. Res. 2004;6:S189–S202. doi: 10.1080/14622200410001669169. [DOI] [PubMed] [Google Scholar]
- 2.U.S. department of Health and Human Services. The Health Benefits of Smoking Cessation. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1990. [Google Scholar]
- 3.Fang WL, Goldstein AO, Butzen AY, Hartsock A, Hartmann KE, et al. Smoking cessation in pregnancy: A review of postpartum relapse prevention strategies. J. Am Board. Fam. Pract. 2004;17:264–275. doi: 10.3122/jabfm.17.4.264. [DOI] [PubMed] [Google Scholar]
- 4.Gilman SE, Breslau J, Subramanian SV, Hitsman B, Koenen KC. Social factors, psychopathology, and maternal smoking during pregnancy. Am. J Public. Health. 2008;98:448–453. doi: 10.2105/AJPH.2006.102772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider S, Maul H, Freerksen N, Pötschke-Langer M. Who smokes during pregnancy? An analysis of the German Perinatal Quality Survey 2005. Public. Health. 2008;122:1210–1216. doi: 10.1016/j.puhe.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Windsor R, Oncken C, Henningfield J, Hartmann K, Edwards N. Behavioral and pharmacological treatment methods for pregnant smokers: issues for clinical practice. J. Am Med. Womens. Assoc. 2000;55:304–310. [PubMed] [Google Scholar]
- 7.Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF, Froelicher ES, Goldstein MG, Healton CG, Henderson PN, Heyman RB, Koh HK, Kottke TE, Lando HA, Mecklenburg RE, Mermelstein RJ, Mullen PD, Orleans CT, Robinson L, Stitzer ML, Tommasello AC, Villejo L, Wewers ME, Murray EW, Bennett G, Heishman S, Husten C, Morgan G, Williams C, Christiansen BA, Piper ME, Hasselblad V, Fraser D, Theobald W, Connell M, Leitzke C. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2008. May, Treating tobacco use and dependence: 2008 update. 2008. [Google Scholar]
- 8.Schroeder DH. Metabolism and kinetics of bupropion. J. Clin. Psychiatry. 1981;44:79–81. [PubMed] [Google Scholar]
- 9.Earhart AD, Patrikeeva S, Wang X, Abdelrahman DR, Hankins GD, Ahmed MS, Nanovskaya T. Transplacental transfer and metabolism of bupropion. J. Matern. Fetal. Neonatal. Med. 2010;23:409–416. doi: 10.1080/14767050903168424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang XM, Abdelrahman DR, Zharikova OL, Patrikeeva SL, Hankins GD, Ahmed MS, Nanovskaya TN. Bupropion metabolism by human placenta. Biochem. Pharmacol. 2010;79:1684–1690. doi: 10.1016/j.bcp.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parekh JM, Sutariya DK, Vaghela RN, Sanyal M, Yadav M, Shrivastav PS. Sensitive, selective and rapid determination of bupropion and its major active metabolite, hydroxylbupropion, in human plasma by LC-MS/MS: application to a bioequivalence study in healthy Indian subjects. Biomed. Chromatogr. 2011;26:314–326. doi: 10.1002/bmc.1660. [DOI] [PubMed] [Google Scholar]
- 12.Yeniceli D, Dogrukol-AK An LC Method for the determination of bupropion and its main metabolite, hydroxybupropion in human plasma. Chromatographia. 2009;780:1703–1708. [Google Scholar]
- 13.Zhang D, Yuan B, Qiao M, Li F. HPLC determination and pharmacokinetics of sustained-release bupropion tablets in dogs. J. Pharm. Biomed. Anal. 2003;33:287–293. doi: 10.1016/s0731-7085(03)00314-5. [DOI] [PubMed] [Google Scholar]
- 14.Coles R, Kharasch E. Stereoselective analysis of bupropion and hydroxybupropion in human plasma and urine by LC/MS/MS. J. Chromatogr. B. 2007;857:67–75. doi: 10.1016/j.jchromb.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Xu H, Loboz K, Gross A, Mclachlan AJ. Stereoselective analysis of hydroxybupropion and application to drug interaction studies. Chirality. 2007;19:163–170. doi: 10.1002/chir.20356. [DOI] [PubMed] [Google Scholar]
- 16.Borges V, Yang E, Dunn J, Henion J. High-throughput liquid chromatography-tandem mass spectrometry determination of bupropion and its metabolites in human, mous and rat plasma using a monolithic column. J. Chromatogr. B. 2004;804:277–287. doi: 10.1016/j.jchromb.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Cooper TB, Suchow RF, Glassman A. Determination of bupropion and its major basic metabolites in plasma by liquid chromatography with dual-wavelength ultraviolet detection. J. Pharm. Sci. 1984;73:1104–1107. doi: 10.1002/jps.2600730820. [DOI] [PubMed] [Google Scholar]
- 18.Loboz KK, Gross AS, Ray J, McLachlan AJ. HPLC assay for bupropion and its metabolites in human plasma. J. Chromatogr. B. 2005;823:115–121. doi: 10.1016/j.jchromb.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Denooz O, Mercerolle M, Lachatre G, Charlier C. Ultra-performance liquid chromatography-tandem mass spectrometry method for the determination of bupropion and its main metabolites in human whole blood. J. Anal. Toxicol. 2010;34:280–286. doi: 10.1093/jat/34.5.280. [DOI] [PubMed] [Google Scholar]
- 20.FDA. Guidance for Industry, Bio-analytical Method Validation. Food and Drug Administration, Centre for Drug Evaluation and Research; 2011. May, http://www.fda.gov/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/ucm123635.htm. [Google Scholar]
- 21.Viswanathan CT, Bansal S, Booth B, DeStefano AJ, Rose MJ, Sailstad J, Shah VP, Skelly JP, Swann PG, Weiner R. Workshop/Conference Report-Quantitative bioanalytical methods validation and implementation: Best practices for chromatographic and ligand biding assays. AAPs J. 2007;9:E30–E42. doi: 10.1007/s11095-007-9291-7. [DOI] [PubMed] [Google Scholar]
- 22.Yeniceli D, Sener E, Korkmaz OT, Dogrukol-AK D, Tuncel N. A simple and sensitive LC-ESI-MS (ion-trap) method for the determination of bupropion and its metabolite, hydroxyburpopion in rat plasma and brain microdialysates. Talanta. 2011;84:19–26. doi: 10.1016/j.talanta.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 23.Cheng C, Liu S, Xiao D, Hanel S. The Application of Trichloroacetic Acid as an Ion Pairing Reagent in LC–MS–MS Method Development for Highly Polar Aminoglycoside Compounds. Chromatographia. 2010;72:133–139. [Google Scholar]
- 24.Loco JV, Elskens M, Croux C, Beernaert H. Linearity of calibration curves: use and misuse of the correlation coefficient. Accred. Qual. Assur. 2002;7:281–285. [Google Scholar]
- 25.Laizure SC, Vane CLD. Stability of bupropion and its major metabolites in human plasma. Ther Drug Monit. 1985;7:447–450. doi: 10.1097/00007691-198512000-00015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.