Abstract
Background
Few interventions have effectively improved health outcomes among youth with diabetes in chronic poor metabolic control.
Purpose
To determine whether Multisystemic Therapy (MST), an intensive, home-based, tailored family treatment, was superior to weekly telephone support for improving regimen adherence and metabolic control among adolescents with chronic poor metabolic control.
Methods
A randomized controlled trial was conducted with 146 adolescents with type 1 or 2 diabetes. Data were collected at baseline, seven months (treatment termination) and twelve months (six month follow-up).
Results
Adolescents receiving MST had significantly improved metabolic control at seven (1.01% decrease) and twelve months (0.74% decrease) compared to adolescents in telephone support. Parents of adolescents receiving MST reported significant improvements in adolescent adherence. However, adolescent-reported adherence was unchanged.
Conclusions
MST improved health outcomes among adolescents with chronic poor metabolic control when compared to telephone support. Home-based approaches may provide a viable means to improve access to behavioral interventions for such youth.
Keywords: Adherence, Youth, Diabetes, Family Treatment
The deterioration in regimen adherence and metabolic control associated with the adolescent developmental period is well-documented among adolescents with diabetes (1, 2). However, a subset of high-risk adolescents with diabetes demonstrates much more serious adherence problems, as evidenced by chronically poor metabolic control. Although sometimes regarded as related to adolescent development and therefore as transient, longitudinal research with youth with diabetes has shown that once established, chronic poor metabolic control tends to persist into adulthood (3). Therefore, adolescents with chronic poor metabolic control represent a group at high risk for both short and long term diabetes complications and are heavy users of medical resources and health care dollars (4, 5).
Multiple self-management interventions have been developed for use with adolescents with diabetes, with varying degrees of impact on regimen adherence and metabolic control. These include coping skills training (6), parent-adolescent teamwork interventions (7, 8), diabetes personal trainers (9), motivational interviewing (10) and case management approaches (11). However, very few intervention studies have targeted or enrolled only high-risk adolescents (12). The two primary exceptions are Behavioral Family Systems Therapy for Diabetes (BFST-D) and our own work using Multisystemic Therapy (MST). BFST-D is an office-based family therapy intervention focusing on improving communication and problem-solving skills related to diabetes care while incorporating cognitive restructuring and functional/structural family therapy approaches. A randomized controlled trial of BFST-D showed that although educational support groups and BFST-D had similar effects in improving metabolic control relative to standard care at treatment termination (13), only BFST-D had long-term effects in improving metabolic control at subsequent follow-up (14). Despite these promising results, recruitment rates for the study were low (i.e., only 27% of eligible participants enrolled). One explanation for the low recruitment rates may be that adolescents with chronic poor metabolic control could not easily be served through an office-based approach, especially as the broader intervention literature shows that high-risk adolescents and families are infrequent users of outpatient health services, both medical and behavioral (15, 16).
Our group has adapted MST, an intensive, home and community-based family intervention that was originally designed for the treatment of antisocial behavior (17), to treat serious regimen adherence difficulties in adolescents with chronic illness. The MST treatment approach is an excellent fit with the multi-determined etiology of severe adherence problems (18) because the scope of MST interventions encompasses the individual adolescent, the family system and the broader community systems within which the family operates (e.g. school, health care system). The home and community based approach of MST also allows barriers to service access for families of adolescents with chronic poor metabolic control to be more effectively managed. Our first randomized controlled trial of MST demonstrated that youth receiving MST had improvements in regimen adherence and metabolic control at treatment completion (19), as well as significant improvements in psychosocial domains such as secondary caregiver involvement in diabetes care and adolescent diabetes stress (20, 21). However, the initial trial compared MST to standard medical care rather than an active behavioral intervention. Findings favoring MST could therefore reflect the effects of increased attention or positive regard from therapists rather than intervention content specific to MST. Therefore, the purpose of the present study was to test the efficacy of MST as compared to an attention control, telephone support. We hypothesized that adolescents receiving MST would have greater improvements in regimen adherence and metabolic control than those receiving telephone support and that these improvements would be sustained six months after treatment completion.
Method
Participants
Adolescents with chronic poor metabolic control and their families were recruited from a university-affiliated pediatric endocrinology clinic within a tertiary care children’s hospital located in a major Midwestern metropolitan area between 2006 and 2010. Potential participants were initially approached in person by medical staff at the time of a regularly scheduled clinic visit or during an inpatient hospitalization. This was followed up by contacts from study research staff and home-based consent visits if families indicated an interest in participating. The research was approved by the Human Investigation Committee of the university affiliated with the hospital where the adolescents were seen for medical care. All participants provided informed consent or assent to participate. The trial was registered in ClinicalTrials.gov under registry number NCT00372814.
In order to be eligible, participants had to be between 10 and 18 years of age, have a diagnosis of type 1 or type 2 diabetes for at least one year that required management with insulin, have a current HbA1c of 8% or higher and a mean HbA1c of 8% or higher during the year before study entry, and be residing in a home setting (e.g., not in residential psychiatric treatment). No child psychiatric diagnoses were exclusionary with the exception of moderate or severe mental retardation and psychosis. Families were also excluded if they were not English speaking or could not complete study measures in English.
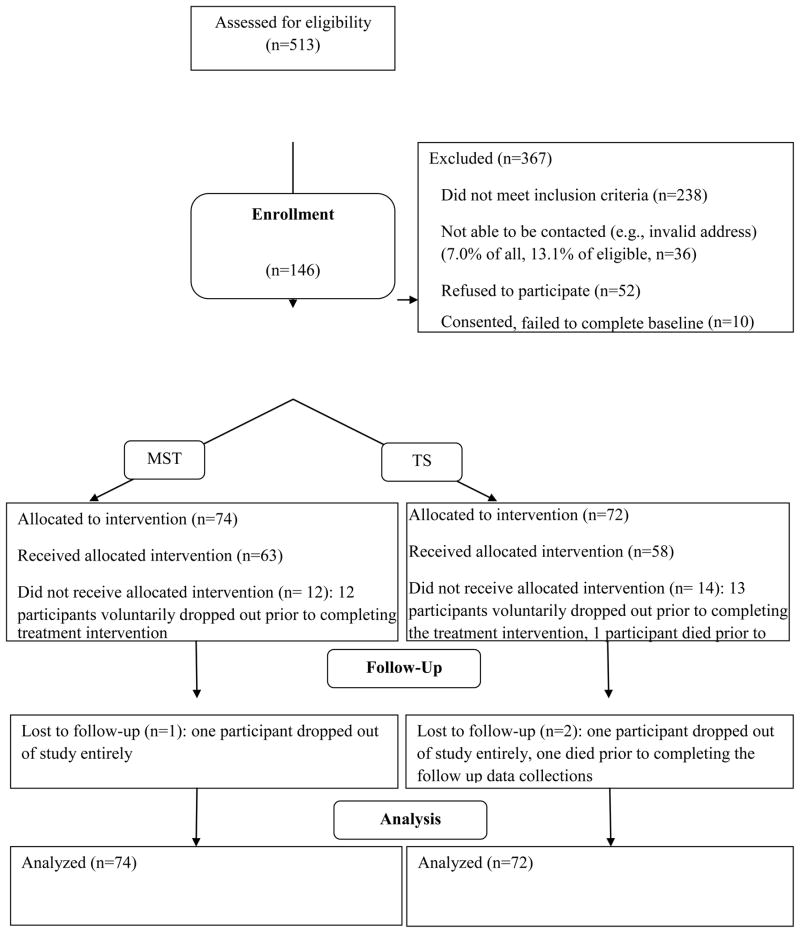
Of the 513 families screened for participation, 238 were ineligible and 36 could not be contacted (see Figure 1). Of the remaining 239, 52 refused to participate and 10 withdrew prior to randomization for a 74% participation rate. The final sample consisted of 146 adolescents and their families. Seventy-four were assigned to MST and 72 to telephone support. Twelve MST families (16%) and 14 TS families (19%) failed to complete a full course of treatment. Only two of the 74 MST families and one of the telephone support families refused to complete follow-up data collection subsequent to treatment drop-out (98% retention rate).
Figure 1.
CONSORT diagram showing flow of participants through the study
Procedures
The study was a randomized controlled trial with a repeated measures design. Data collection occurred at baseline and at seven and 12 months post-baseline (corresponding to treatment termination and six month follow-up). Participants were randomized in a 1:1 ratio to MST or telephone support. Randomization occurred immediately after baseline data collection using a permuted block algorithm to ensure equivalence across treatment condition. The project statistician generated the randomization sequence and participants were notified of their randomization status by the project manager. Randomization was stratified by baseline HbA1c (> 10.5% versus ≤ 10.5% based on the median HbA1c in our prior trial) and baseline weight status (overweight or not based on 85th BMI %tile). Adolescents randomized to MST received approximately six months of home-based family treatment plus standard medical care, while adolescents randomized to telephone support (attention control condition) received weekly supportive phone calls plus standard medical care. All measures were collected by a trained research assistant in the participants’ homes. The research assistant was blind to treatment assignment to the extent possible in a behavioral trial. Both the adolescent and the primary caregiver completed questionnaires. Families were provided $50 to compensate them for participating in each data collection session.
Standard Medical Care
All participants in the trial received standard medical care in addition to MST or TS. Standard care consisted of visits in a pediatric diabetes clinic every 3–4 months in accordance with American Diabetes Association guidelines (22). At each visit, participants were seen by a pediatric endocrinologist, nurse educator and a dietician. Social workers and clinical psychologists were also available to meet with families as needed. During a typical 30-minute visit, HbA1c was obtained, blood glucose records were reviewed, any illness management problems were identified and addressed, target goals for diabetes care were suggested and diabetes education was provided. The linear growth and weight gain of the child was also assessed at each visit and changes in insulin dosage made as necessary. Between visits, assistance with problem-solving and blood glucose management was provided by phone through a daily phone hour and additional diabetes education sessions were provided as needed.
Multisystemic Therapy (MST)
Adolescents assigned to the intervention condition received Multisystemic Therapy (MST). MST is an evidence-based, intensive, family-centered, community-based treatment originally designed for use with adolescents presenting with serious antisocial behavior (23). Our group has extensively adapted MST to the treatment of poor self-management in adolescents with chronic illnesses including diabetes (24), HIV infection (25) and asthma (26). MST includes several key features: (a) A comprehensive set of risk factors across multiple systems (e.g., individual, family, peer, school, and community) associated with the problem behavior is targeted through interventions that are individualized for each adolescent (b) Interventions integrate empirically-based clinical treatments (e.g., cognitive–behavioral therapy), which typically are used to focus on only a few aspects of the adolescent’s social ecology (only the individual adolescent or at most the adolescent and family), into a broad-based ecological framework that addresses relevant risk factors across family, school, and community contexts (c) Interventions focus on promoting behavioral changes in the adolescent’s natural ecology by empowering caregivers with parenting skills and resources and empowering adolescents to cope with family, school, and neighborhood problems (d) Services are delivered via a community-based model in home, school, and/or neighborhood settings at times convenient to the family, which facilitates high engagement and low dropout rate and (e) MST programs include an intensive quality assurance system that aims to optimize adolescent outcomes by supporting therapist fidelity to MST treatment protocols (27).
As adapted for the treatment of poorly controlled diabetes, MST family interventions focused on improving parental knowledge regarding diabetes care, developing parenting skills such as increasing support, supervision/monitoring and rules/discipline related to the adolescent’s diabetes care, improving family organizational routines related to diabetes care and teaching caregivers to communicate effectively with each other about the adolescent’s diabetes care needs. Peer interventions included enlisting the active support of peers regarding regimen adherence. School interventions included improving family-school communication about the adolescent’s diabetes care needs and adherence behaviors (e.g. having school personnel report blood glucose readings from school meter to parents, weekly) and working with school personnel to monitor and support the adolescent’s regimen completion (e.g. finding a private place to test blood glucose). At the community level, interventions included developing strategies to monitor and promote the youth’s diabetes care while in school or other settings (i.e. extracurricular activities, visiting extended family members). Interventions within the health care system included helping the family resolve barriers to keeping appointments and working with the family and health care providers to promote a positive working relationship. Therapists also routinely accompanied families to their medical appointments. Individual interventions with the youth included improving diabetes care skills and increasing motivation for diabetes care completion. Since MST sessions included a variety of types of contacts, length of sessions was also variable. Sessions including traditional one hour family treatment sessions, briefer contacts for skills practice (e.g. spending 15 minutes in the home to observe a caregiver implementing a reward or consequence as part of a behavior plan), attending school meetings to provide information to staff regarding diabetes care (e.g. one- two hour staff trainings) and attending clinic visits with families (two hours or more)
MST treatment was provided by five masters-level therapists with varied backgrounds (three psychologists, two social workers). Three therapists were African-American and two were White. In order to promote fidelity to the MST model, state-of-the-art quality assurance protocols were used that included diabetes-specific MST treatment manuals, an initial five-day training, weekly on-site clinical supervision from a PhD level supervisor with an extensive background with MST and its application to treatment of chronically ill children, weekly phone consultation with an MST expert experienced in the application of MST to diabetes, and follow-up booster training. The initial five-day training was augmented by the research team to include formal diabetes education for therapists as well as education regarding factors predictive of poor treatment adherence and metabolic control among adolescents with diabetes. Therapists were trained to have sufficient knowledge regarding diabetes to enable them to conduct diabetes adherence interventions with families (e.g., to reinforce how to count carbohydrates). Quality assurance protocols also included feedback on therapist and supervisor fidelity to MST procedures via standardized MST treatment fidelity questionnaires and coding of audiotaped treatment sessions (28).
In the current study, therapists were expected to meet with families and/or their related contacts (e.g. extended family, physicians, school personnel) a minimum of two times per week at the beginning of treatment with an option for reduced number of sessions at the end of treatment based on family progress toward treatment goals. Treatment was terminated when treatment goals were met rather than when a set number of sessions were completed. However, based on previous MST trials and our own prior experience, treatment was planned to last for approximately six months.
Telephone Support
Adolescents assigned to the telephone support condition received weekly phone calls focusing on support for diabetes care using a client-centered, non-directive counseling approach. Safer and Hugo (29) describe state-of-the-art procedures for designing a control condition for testing psychotherapy interventions. Control conditions should 1) include treatment processes shared in common by psychotherapy treatments such as support and encouragement from therapists; 2) avoid processes specific to the experimental intervention; 3) avoid processes specific to other existing interventions and 4) be credible enough to generate participant interest. Therefore, the primary goal of the telephone support intervention was to control for expectancies for improvement associated with treatment entry (placebo effects) and general warmth, support and encouragement provided by the MST therapists (requirements 1 & 2) while still delivering content that would be acceptable to adolescents and feasible to deliver (requirement 4). As a result, the possibility of matching the control condition with MST for dose of treatment (e.g. calling adolescents three times per week for phone sessions) was considered but discarded as lacking the potential for feasible delivery given busy adolescent schedules. The telephone support condition did not include elements specific to MST such as cognitive-behavioral intervention content or family interventions (requirement 3).
Therapists were master’s level therapists (two) or doctoral students (four) in clinical psychology or social work. Four were White and two were African American. All families in the telephone support condition received an initial home visit during which the therapist introduced themselves to the adolescent and primary caregiver in person and described TS program content. Subsequent phone calls were designed to last approximately 30 minutes and were audiorecorded for treatment fidelity purposes. Calls focused upon providing emotional support regarding the adolescent’s diabetes, assessing adherence to diabetes care during the prior week, including a review of readings in the blood glucose meter, and helping the adolescent identify solutions to any barriers they identified to completion of their diabetes care. Adolescents were directed to contact the diabetes clinic in the case of any new medical problems or for medical advice. Non-diabetes related problems such as peer, school or family relationship problems were also addressed during the call if desired by the adolescent. Therapists completed an initial training to competency that included training in intervention content from a written treatment manual that included scripts for phone calls, training in supportive listening skills and completion of role-plays of phone calls. Telephone support therapists completed the same formal diabetes education training completed by MST therapists. Treatment fidelity was maintained by weekly supervisory sessions with therapists and supervisor review of session audiotapes.
Measures
Metabolic control was calculated using hemoglobin A1c (HbA1c). Values were obtained using the Accubase A1c test kit, which is FDA approved. The test uses a capillary tube blood collection method instead of venipuncture and is therefore suitable for home-based data collection by non-phlebotomists. High performance liquid chromatography is used to analyze the blood sample. Body mass index (BMI) in kg/m2 was calculated and converted to BMI %tile using age and gender norms from the Centers for Disease Control and Prevention.
Regimen adherence was measured using the Diabetes Management Scale (30). The Diabetes Management Scale is a self-report questionnaire designed to measure a broad range of diabetes management behaviors, such as insulin management, dietary management, blood glucose monitoring, and symptom response. Adolescents are asked “What percent of the time do you (take your insulin)?” and answer on a 0–100% scale. Adequate reliability and validity have been reported (31, 32). Items are summed to obtain a total score reflecting overall management behavior. A parent report version is also available and was used in the present study to obtain parent ratings of adolescent adherence.
Statistical Analyses
The study was designed to have at least 80% power (two-tailed alpha = .05) to detect an intervention effect size of .42 on HbA1c based on findings from our previous MST trial (19). All analyses used an intent-to-treat approach. No more than 7% of the data was missing for any variable.
The effects of MST on regimen adherence and HbA1c were first evaluated using the following multiple linear regression models:
| (1) |
| (2) |
Y0 and Y1 represented the outcome variable (adherence or HbA1c) measured at baseline and follow up (7 or 12 months) respectively, α and βs’ were regression coefficients, and β2 indicated the net change in the outcome variable in response to the intervention received (MST or TS) after adjusting for baseline differences (model 1) or the baseline differences and the effects of covariates (model 2). Covariates included in model 2 were age, race, number of parents in the home and diabetes type (1 or 2). In these models, a β2 falling within a 95% confidence interval not including zero is equivalent to p <0.05 and therefore supports the effect of MST. For significant effects, the intervention effect size was calculated using Cohen’s d. Statistical analyses were conducted using the software SAS version 9.3 (SAS Institute Inc, Cary, NC).
Results
Sample characteristics are summarized in Table 1. The sample was comprised primarily of African American adolescents and single parent families. Mean HbA1c of the sample at baseline was 11.7%, suggesting very poor metabolic control overall among youth in the study. There were no statistically significant differences by randomization group at baseline on any demographic or clinical variable, including HbA1c, insulin dose, insulin regimen, or diabetes type. For telephone support participants who received any treatment (at least one session) (n=65/72), the mean number of phone calls was 14.0 (SD=6.3) over a mean of 4.9 months (SD=1.6). For MST participants who received any treatment (n=69/74), the mean number of sessions was 45.7 (SD=18.6) over a mean of 5.6 months (SD=1.2).
Table 1.
Baseline Characteristics of Adolescents and their Families by Randomization Group: Multisystemic Treatment (MST) and Telephone Support (TS).
| Total (n=146) | MST (n=74) | TS (n=72) | |
|---|---|---|---|
| Child Age, years | 14.2 (2.3) | 14.2 (2.2) | 14.1 (2.4) |
| Child Gender | |||
| Male | 64 (44%) | 32 (43%) | 32 (44%) |
| Female | 82 (56%) | 42 (57%) | 40 (56%) |
| Number of Parents in home | |||
| Two Parents | 60 (41%) | 31 (42%) | 29 (40%) |
| Single Parent | 86 (59%) | 43 (58%) | 43 (60%) |
| Child Race | |||
| African American | 113 (77%) | 60 (81%) | 53 (74%) |
| Whites | 29 (20%) | 13 (18%) | 16 (22%) |
| Other | 4 (3%) | 1 (1%) | 3 (4%) |
| Duration of diabetes, years | 4.7 (3.0) | 4.7 (3.2) | 4.6 (2.9) |
| Type of diabetes | |||
| 1 | 131 (90%) | 65 (88%) | 66 (92%) |
| 2 | 15 (10%) | 9 (12%) | 6 (8%) |
| HbA1c, % | 11.7 (2.5) | 11.6 (2.5) | 11.8 (2.6) |
| DMS-Parent | 67.6(16.7) | 68.2(16.1) | 67.0(17.4) |
| DMS-Teen | 67.6 (15.7) | 66.1(15.4) | 69.2(16.0) |
| Insulin Regimen | |||
| Conventional | 39 (27%) | 21 (28%) | 18 (25%) |
| BBT injections | 83 (57%) | 39 (53%) | 44 (61%) |
| Insulin pump | 19 (13%) | 10 (14%) | 9 (13%) |
| Long-acting insulin only | 5 (3%) | 4 (5%) | 1(1%) |
Note. DMS= Diabetes Management Scale. BBT= Basal-Bolus Therapy. Data are mean (SD), or n (%).
Demonstrating that MST treatment was implemented within multiple systems (e.g. family, school, community), for 61% of MST participants, an adult other than the primary caregiver attended sessions. For 34% of participants, siblings attended sessions. The therapist had contact with school personnel for 91% of participants and had contact with the diabetes clinic staff for 92% of participants. Finally, for 46% of MST participants, therapists had contact with a person within a community system other than the school or medical treatment team (e.g. a coach, daycare provider, neighbor, etc).
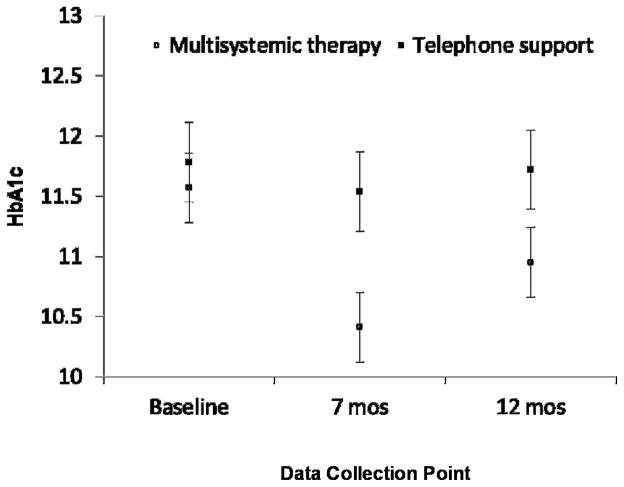
Results of regression analyses are shown in Table 2. All the models fitted the data well, and, as expected, inclusion of covariates resulted in increases in R2. At the 7 month post-test, youth receiving MST had a significantly greater reduction in HbA1c than youth receiving telephone support for both models 1 and 2. At 12 months, a significant effect of MST versus telephone support was found when covariates were included in the model (Model 2). For youth receiving MST, the adjusted net reduction in HbA1c was −1.01% (95% CI: −1.67%, −0.36%) and −0.74% (95% CI: −1.48%, −0.01%) at 7 month and 12 month post-test respectively, after controlling for baseline status and covariates. Reductions of 0.5% in HbA1c are considered clinically significant (33). The effect sizes associated with the reductions in HbA1c for youth receiving MST were 0.36 and 0.24 at 7 month and 12 month post-test, respectively. Figure 2 shows the trajectories of HbA1c for youth receiving MST and TS over the 12 month study period. During the study period, there were no significant differences in the rates of adverse events between the groups, including episodes of hypoglycemia [16 in MST vs 10 in telephone support, t (144)=.82, n.s.]
Table 2.
Effects of Multisystemic Therapy versus Telephone Support on Metabolic Control and Regimen Adherence
| Time and regression model | HbA1c | DMS-Parent | DMS-Youth |
|---|---|---|---|
| 7 Months post-test | |||
| Model 1 | |||
| Baseline status, β1 (95% CI) | 0.61 (0.49, 0.74) * | 0.53 (0.41, 0.66) * | 0.49 (0.36, 0.63) |
| Intervention condition, β2 (95% CI) | −0.98 (−1.63, −0.34) * | 5.70 (1.58, 9.82) * | 1.61 (−2.64, 5.86) |
| Model fit: R2 | 0.43 | 0.37 | 0.27 |
| Model 2 | |||
| Baseline measure, β1 (95% CI) | 0.58 (0.44,0.73) * | 0.47 (0.34, 0.60) * | 0.44 (0.30, 0.58) |
| Intervention condition, β2 (95% CI) | −1.01 (−1.67, −0.36) * | 5.72 (1.61, 9.83) * | 1.54 (−2.67, 5.74) |
| Age (in years), β3 (95% CI) | 0.05 (−0.10, 0.21) | −0.93 (−1.92, 0.05) | −0.97 (−1.94, 0.01) |
| Race, β4 (95% CI) | 0.39 (−0.45, 1.24) | 1.15 (−3.94, 6.23) | 1.92 (−3.22, 7.06) |
| Single parent, β4(95% CI) | −0.01 (−0.70, 0.67) | 2.21 (−2.12, 6.54) | 0.68 (−3.82, 5.17) |
| Type 1 vs. type 2, β5 (95% CI) | −0.32 (−1.41, 0.76) | −2.70 (−9.50, 4.10) | −5.86 (−12.81, 1.08) |
| Model fit: R2 | 0.44 | 0.40 | 0.32 |
| 12 Months post-test | |||
| Model 1 | |||
| Baseline measure, β1 (95% CI) | 0.68 (0.54, 0.83) * | 0.55 (0.43, 0.67) * | 0.66 (0.51, 0.80) |
| Intervention condition, β2 (95% CI) | −0.67(−1.40, 0.07) | 4.77 (0.88, 8.67) * | 1.37 (−3.08, 5.83) |
| Model fit: R2 | 0.41 | 0.41 | 0.37 |
| Model 2 | |||
| Baseline measure, β1 (95% CI) | 0.63 (0.47, 0.78)* | 0.50 (0.38, 0.63)* | 0.59 (0.43, 0.74) |
| Intervention condition, β2 (95% CI) | −0.74 (−1.48, −0.01)* | 5.10 (1.21, 8.99)* | 1.51 (−2.86, 5.88) |
| Age (in years), β3 (95% CI) | 0.07 (−0.11, 0.24) | −0.84 (−1.74, 0.07) | −1.36 (−2.35, −0.38) |
| Race, β4 (95% CI) | 0.39 (−0.59, 1.36) | −0.80 (−5.72, 4.12) | −0.91 (−6.48, 4.66) |
| Single parent, β4(95% CI) | 0.06 (−0.71, 0.83) | −1.53 (−5.60, 2.53) | 1.39 (−3.25, 6.03) |
| Type 1 vs. type 2, β5 (95% CI) | 1.14 (−0.11, 2.39) | −4.55(−11.11, 2.02) | −4.43 (−11.80, 2.95) |
| Model fit: R2 | 0.43 | 0.44 | 0.42 |
Note. DMS= Diabetes Management Scale.
p < .05.
Figure 2.
Changes in hemoglobin A1c (HbA1c) from baseline to seven months (treatment termination) and twelve months (six months post-treatment).
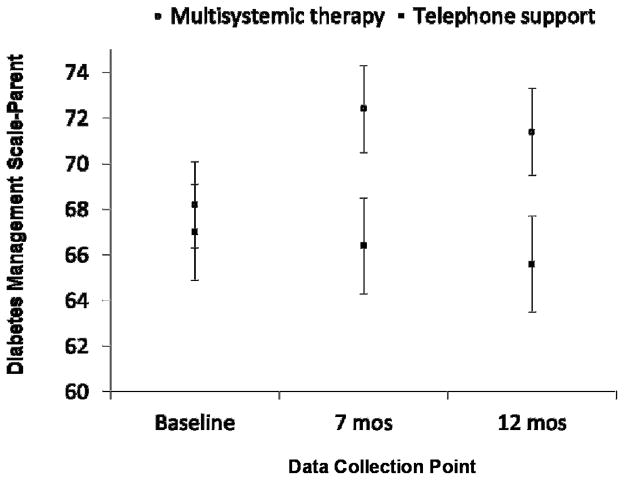
Similar results were found for parent-reported regimen adherence on the Diabetes Management Scale. As compared to parents of youth receiving telephone support, parents of youth receiving MST reported significantly improved regimen adherence at both 7 and 12 month post-test for models 1 and 2 (Table 2). The adjusted net improvement on the Diabetes Management Scale was 5.72 (95% CI: 1.61, 9.83) and 5.10 (95% CI: 1.21, 8.99) at 7 and 12 months, respectively. The associated effect sizes were 0.29 and 0.28. Figure 3 shows change in parent-reported adherence over the 12 month study period.
Figure 3.
Changes in parent-reported regimen adherence on the Diabetes Management Scale from baseline to seven months (treatment termination) and twelve months (six months post-treatment).
Mean scores on the Diabetes Management Scale reported by youth were higher for the MST group than for TS group at 7 months (1.54, 95% CI: −2.67, 5.74) and 12 months (1.51, 95% CI: −2.86, 5.88) post intervention respectively, but the differences were not statistically significant even after adjusting for baseline differences and the impact of covariates.
Discussion
Effective treatments for adolescents with chronic poor metabolic control have the potential to improve short-term quality of life, delay the onset of diabetes complications and reduce the costs of health care (34). Unfortunately, high-risk adolescents face multiple barriers to improved metabolic control and they are the least likely to access hospital-based health care, traditional diabetes education/supportive interventions or behavioral outpatient services such as psychotherapy (14–16). Therefore, alternative approaches to office or hospital-based interventions appear warranted.
In the present study, we were able to recruit and retain the majority of eligible youth with chronic poor metabolic control and their families into a home-based treatment for six months. Findings from the present study indicate that MST improved metabolic control among urban adolescents with chronic poor metabolic control when compared to a behavioral intervention (telephone support) that included weekly review of blood glucose meter readings with the youth as well as support and encouragement for improved diabetes management. Results are also consistent with prior findings from our group that showed higher fidelity by therapists to the MST treatment model (e.g. use of MST-specific content) resulted in greater improvements in regimen adherence and metabolic control (28). Other attempts to treat adolescents with chronic poor metabolic control via phone support have also shown that interventions targeting only the adolescent are insufficient to improve metabolic control (35) given the multi-determined nature of chronic poor metabolic control.
In addition, improvements in regimen adherence and metabolic control were sustained six months after completion of the intervention. The reduction in HbA1c six months after intervention completion was both clinically meaningful as well as statistically significant. For example, reductions in the range of 1.0% during the Diabetes Control and Complications Trial have been shown to reduce the incidence of neuropathy at 8 year follow-up by as much at 38% (36). MST is an intensive intervention and is costly to implement (37); prior work by our group suggests that costs to deliver the intervention are approximately $7,000 per youth. Therefore, the fact that the intervention had stable effects after therapists were no longer present in the home is important when determining whether MST may have the potential to be cost effective for youth with poorly controlled diabetes. Additional studies are needed to address intervention costs and costs savings.
Although parent report of adolescent regimen adherence paralleled the findings with regard to metabolic control and suggested that the MST intervention had the intended effect on improving diabetes management behaviors, adolescent-reported regimen adherence did not improve significantly within either the MST or control group. The fact that parent report of improved adherence was more consistent with improvement on objective measures of health status (i.e. HbA1c) was unexpected. Parents are not always present during their teen’s diabetes care completion (e.g. during school hours) and therefore, adolescents might be predicted to be more accurate reporters of their own diabetes management. However, it is possible that adolescents were more affected by social desirability biases than their parents when reporting about their diabetes care. As a result, they may have been more likely to over-report their adherence at baseline, minimizing the opportunity to find intervention effects at follow-up.
Limitations of the present study include the nature of the sample, which was predominantly low income and African American. Although minorities are over-represented among youth with chronic poor metabolic control, findings require replication in more diverse samples. In addition, the follow-up period in the current study was limited to six months after the conclusion of treatment. While still significant, reductions in average blood glucose levels were attenuated at six month follow-up. Longer-term follow-up is needed to better assess the sustainability of MST effects over time. MST and telephone support were not matched on dose (e.g. 2–3 contacts per week versus one contact per week). Therefore, the possibility that a higher intervention dose in the MST condition accounted for better health outcomes for youth receiving MST cannot be ruled out.
In summary, results of the current study suggest that an intensive home-based intervention targeting the multiple factors responsible for severe adherence difficulties successfully improved metabolic control among adolescents with chronic poor metabolic control. Additional studies are needed to further develop effective and cost-efficient treatments for high-risk adolescents with diabetes.
Acknowledgments
This project was supported by grant # R01 DK59067 from the National Institute of Diabetes, Digestive and Kidney Diseases. The authors would like to acknowledge Yuanjing Ren for her assistance with preparation of data for use in the analyses.
Footnotes
Conflict of Interest Statement
Three of the authors are board members of Evidence Based Services, which has a licensing agreement with MST Services, LLC, for dissemination of multisystemic therapy treatment technology. There are no other potential author conflicts of interest.
Contributor Information
Deborah A. Ellis, Carman and Ann Adams Department of Pediatrics, Wayne State University
Sylvie Naar-King, Carman and Ann Adams Department of Pediatrics, Wayne State University
Xinguang Chen, Carman and Ann Adams Department of Pediatrics, Wayne State University
Kathleen Moltz, Carman and Ann Adams Department of Pediatrics, Wayne State University
Phillippe B. Cunningham, Department of Psychiatry, Medical University of South Carolina
April Idalski-Carcone, Carman and Ann Adams Department of Pediatrics, Wayne State University
References
- 1.Burdick J, Chase HP, Slover RH, et al. Missed insulin meal boluses and elevated hemoglobin A1c levels in children receiving insulin pump therapy. Pediatrics. 2004;113:e221–224. doi: 10.1542/peds.113.3.e221. [DOI] [PubMed] [Google Scholar]
- 2.Helgeson VS, Siminerio L, Escobar O, Becker D. Predictors of metabolic control among adolescents with diabetes: A 4-Year longitudinal study. J Pediatr Psychol. 2009;34:254–270. doi: 10.1093/jpepsy/jsn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seiffge-Krenke I, Stemmler M. Coping with everyday stress and links to medical and psychosocial adaptation in diabetic adolescents. Journal of Adolescent Health. 2003;33:180–188. doi: 10.1016/s1054-139x(02)00707-3. [DOI] [PubMed] [Google Scholar]
- 4.Menzin J, Langley-Hawthorne C, Friedman M, Boulanger L, Cavanaugh R. Potential short-term economic benefits of improved glycemic control: A managed care perspective. Diabetes Care. 2001;24:51–55. doi: 10.2337/diacare.24.1.51. [DOI] [PubMed] [Google Scholar]
- 5.Menzin J, Korn JR, Cohen J, et al. Relationship between glycemic control and diabetes-related hospital costs in patients with type 1 and type 2 diabetes mellitus. Journal of Managed Care Pharmacy. 2010;16:264–275. doi: 10.18553/jmcp.2010.16.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grey M, Boland EA, Davidson M, Li J, Tamborlane WV. Coping skills training for youth with diabetes mellitus has long-lasting effects on metabolic control and quality of life. Journal of Pediatrics. 2000;137:107–113. doi: 10.1067/mpd.2000.106568. [DOI] [PubMed] [Google Scholar]
- 7.Anderson BJ, Brackett J, Ho J, Laffel LM. An office-based intervention to maintain parent-adolescent teamwork in diabetes management. Impact on parent involvement, family conflict, and subsequent glycemic control. Diabetes Care. 1999;22:713–721. doi: 10.2337/diacare.22.5.713. [DOI] [PubMed] [Google Scholar]
- 8.Laffel LMB, Vangsness L, Connell A, et al. Impact of ambulatory, family-focused teamwork intervention on glycemic control in youth with type 1 diabetes. Journal of Pediatrics. 2003;142:409–416. doi: 10.1067/mpd.2003.138. [DOI] [PubMed] [Google Scholar]
- 9.Nansel TR, Iannotti RJ, Simons-Morton BG, et al. Long-Term Maintenance of Treatment Outcomes: Diabetes Personal Trainer Intervention for Youth With Type 1 Diabetes. Diabetes Care. 2009;32:807–809. doi: 10.2337/dc08-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Channon SJ, Huws-Thomas MV, Rollnick S, et al. A multicenter randomized controlled trial of motivational interviewing in teenagers wtih diabetes. Diabetes Care. 2007;30:1390–1395. doi: 10.2337/dc06-2260. [DOI] [PubMed] [Google Scholar]
- 11.Svoren BM, Butler D, Levine BS, Anderson BJ, Laffel LM. Reducing acute adverse outcomes in youths with type 1 diabetes: A randomized, controlled trial. Pediatrics. 2003;112:914–922. doi: 10.1542/peds.112.4.914. [DOI] [PubMed] [Google Scholar]
- 12.Delamater AM, Jacobson AM, Anderson B, et al. Psychosocial therapies in diabetes: Report of the psychosocial therapies working group. Diabetes Care. 2001;24:1286–1292. doi: 10.2337/diacare.24.7.1286. [DOI] [PubMed] [Google Scholar]
- 13.Wysocki T, Harris MA, Buckloh LM, et al. Effects of behavioral family systems therapy for diabetes on adolescents’ family relationships, treatment adherence, and metabolic control. J Pediatr Psychol. 2006;31:928–938. doi: 10.1093/jpepsy/jsj098. [DOI] [PubMed] [Google Scholar]
- 14.Wysocki T, Harris MA, Buckloh LM, et al. Randomized trial of behavioral family systems therapy for diabetes: Maintenance of effects on diabetes outcomes in adolescents. Diabetes Care. 2007;30:555–560. doi: 10.2337/dc06-1613. [DOI] [PubMed] [Google Scholar]
- 15.Urbach SL, LaFranchi S, Lambert L, et al. Predictors of glucose control in children and adolescents with type 1 diabetes mellitus. Pediatric diabetes. 2005;6:69–74. doi: 10.1111/j.1399-543X.2005.00104.x. [DOI] [PubMed] [Google Scholar]
- 16.Tershakovec AM, Kuppler K. Ethnicity, insurance type, and follow-up in a pediatric weight management program. Obesity Research. 2003;11:17–20. doi: 10.1038/oby.2003.4. [DOI] [PubMed] [Google Scholar]
- 17.Henggeler SW. Efficacy studies to large-scale transport: the development and validation of multisystemic therapy programs. Annual Review of Clinical Psychology. 2011;7:351–81. doi: 10.1146/annurev-clinpsy-032210-104615. [DOI] [PubMed] [Google Scholar]
- 18.Naar-King S, Podolski CL, Ellis DA, Frey MA. Social ecological model of illness management in high risk youth with type 1 diabetes. Journal of Consulting and Clinical Psychology. 2006;74:785–789. doi: 10.1037/0022-006X.74.4.785. [DOI] [PubMed] [Google Scholar]
- 19.Ellis DA, Templin T, Naar-King S, et al. Multisystemic therapy for adolescents with poorly controlled type I diabetes: Stability of treatment effects in a randomized controlled trial. J Consult Clin Psychol. 2007;75:168–174. doi: 10.1037/0022-006X.75.1.168. [DOI] [PubMed] [Google Scholar]
- 20.Ellis DA, Frey MA, Naar-King S, et al. The effects of Multisystemic Therapy on diabetes stress in adolescents with chronically poorly controlled type I diabetes: Findings from a randomized controlled trial. Pediatrics. 2005;116:e826–e832. doi: 10.1542/peds.2005-0638. [DOI] [PubMed] [Google Scholar]
- 21.Ellis DA, Yopp J, Templin T, et al. Family mediators and moderators of treatment outcomes among youths with poorly controlled type 1 diabetes: Results from a randomized controlled trial. Journal of Pediatric Psychology. 2007;32:194–205. doi: 10.1093/jpepsy/jsj116. [DOI] [PubMed] [Google Scholar]
- 22.Silverstein J, Klingensmith G, Copeland K, et al. Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care. 2005;28:186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- 23.Henggeler SW, Schoenwald SK, Borduin CM. Multisystemic therapy for antisocial behavior in children and adolescents. 2. New York: The Guilford Press; 2009. [Google Scholar]
- 24.Ellis DA, Frey MA, Naar-King S, et al. Use of multisystemic therapy to improve regimen adherence among adolescents with type 1 diabetes in chronic poor metabolic control: A randomized controlled trial. Diabetes Care. 2005;28:1604–1610. doi: 10.2337/diacare.28.7.1604. [DOI] [PubMed] [Google Scholar]
- 25.Ellis DA, Naar-King S, Cunningham PB, Secord E. Use of multisystemic therapy to improve antiretroviral adherence and health outcomes in HIV-infected pediatric patients: evaluation of a pilot program. AIDS Patient Care & STDs. 2006;20:112–121. doi: 10.1089/apc.2006.20.112. [DOI] [PubMed] [Google Scholar]
- 26.Naar-King S, Ellis D, Kolmodin K, Cunningham P, Secord E. Feasibility of adapting multisystemic therapy to improve illness management behaviors and reduce asthma morbidity in high risk African American youth: A case series. Journal of Child and Family Studies. 2009;18:564–573. [Google Scholar]
- 27.Henggeler SW, Halliday-Boykins CA, Cunningham PB, et al. Juvenile drug court: Enhancing outcomes by integrating evidence-based treatments. Journal of Consulting and Clinical Psychology. 2006;74:42. doi: 10.1037/0022-006X.74.1.42. [DOI] [PubMed] [Google Scholar]
- 28.Ellis DA, Naar-King S, Templin T, Frey MA, Cunningham PB. Improving health outcomes among youth with poorly controlled type 1 diabetes: The role of treatment fidelity in a randomized clinical trial of multisystemic therapy. Journal of Family Psychology. 2007;21:363–371. doi: 10.1037/0893-3200.21.3.363. [DOI] [PubMed] [Google Scholar]
- 29.Safer DL, Hugo EM. Designing a control for a behavioral group therapy. Behavior Therapy. 2006;37:120–130. doi: 10.1016/j.beth.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frey MA, Ellis DA, Naar-King S, Greger N. Diabetes management of adolescents in poor metabolic control. The Diabetes Educator. 2004;30:647–657. doi: 10.1177/014572170403000416. [DOI] [PubMed] [Google Scholar]
- 31.Frey MA, Denyes MJ. Health and illness self-care in adolescents with IDDM: A test of Orem’s theory. Advances in Nursing Science. 1989;12:67–75. doi: 10.1097/00012272-198910000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Schilling LS, Grey M, Knafl K. A review of measures of self-management of type 1 diabetes by youth and their parents. Diabetes Educator. 2002;28:796–808. doi: 10.1177/014572170202800520. [DOI] [PubMed] [Google Scholar]
- 33.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. New England Journal of Medicine. 2000;342:381–389. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group. Beneficial effects of intensive therapy of diabetes during adolescence: Outcomes after the conclusion of the Diabetes Control and Complications Trial (DCCT) J Pediatr. 2001;139:804–812. doi: 10.1067/mpd.2001.118887. [DOI] [PubMed] [Google Scholar]
- 35.Nunn E, King B, Smart C, Anderson D. A randomized controlled trial of telephone calls to young patients with poorly controlled type 1 diabetes. Pediatric diabetes. 2006;7:254–259. doi: 10.1111/j.1399-5448.2006.00200.x. [DOI] [PubMed] [Google Scholar]
- 36.Martin CL, Albers J, Herman WH, et al. Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care. 2006;29:340–4. doi: 10.2337/diacare.29.02.06.dc05-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellis D, Naar-King S, Templin T, et al. Multisystemic therapy for adolescents with poorly controlled type 1 diabetes: Reduced diabetic ketoacidosis admissions and related costs over 24 months. Diabetes Care. 2008;31:1746–1747. doi: 10.2337/dc07-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]