Abstract
Newborn feeding, maternal, bonding, growth and wellbeing depend upon intact odor recognition in the early postnatal period. Antenatal stress may affect postnatal odor recognition. We investigated the exact role of a neurotransmitter, nitric oxide (NO), in newborn olfactory function. We hypothesized that olfactory neuron activity depended on NO generated by neuronal NO synthase (NOS). Utilizing in vivo functional manganese enhanced MRI (MEMRI) in a rabbit model of cerebral palsy we had shown previously that in utero hypoxia ischemia (H-I) at E22 (70% gestation) resulted in impaired postnatal response to odorants and poor feeding. With the same antenatal insult, we manipulated NO levels in the olfactory neuron in postnatal day 1 (P1) kits by administration of intranasal NO donors or a highly selective nNOS inhibitor. Olfactory function was quantitatively measured by the response to amyl acetate stimulation by MEMRI. The relevance of nNOS to normal olfactory development was confirmed by the increase of nNOS gene expression from fetal ages to P1 in olfactory epithelium and bulbs. In control kits, nNOS inhibition decreased NO production in the olfactory system and increased MEMRI slope enhancement. In H-I kits the MEMRI slope did not increase, implicating modification of endogenous NO-mediated olfactory function by the antenatal insult. NO donors as a source of exogenous NO did not significantly change function in either group. In conclusion, olfactory epithelium nNOS in newborn rabbits probably modulates olfactory signal transduction. Antenatal H-I injury remote from delivery may affect early functional development of the olfactory system by decreasing NO-dependent signal transduction.
Keywords: nitric oxide, nNOS, olfactory development, MEMRI, olfactory injury
INTRODUCTION
It is increasingly being recognized that enriched environment is needed for developing the sensory capabilities and preferences of newborns (Schaal, et al., 2004). Olfactory cues soothe newborns, and olfactory familiarity with the mother may help the newborn transition to extra-uterine life (Varendi, et al., 1998) and promote early mother–infant attachment (Varendi, et al., 1997, Winberg and Porter, 1998). Breast feeding, depends on olfactory cues (Varendi, et al., 1994), and the odor of breast milk increases non-nutritive sucking in tube fed premature infants (Bingham, et al., 2007). Early olfactory conditioning contributes to the development of preferences in the alimentary (including obesity) and social domains (Varendi, et al., 1994, Winberg and Porter, 1998) and has a developmentally sensitive period (Wilson and Sullivan, 1994). Improvements in neonatal care have increased premature newborn survival but the routine neonatal intensive care practices of nasal cannula and other respiratory devices, and early feeding with oro- or nasogastric gavage result in a chemo-sensory experience that is detrimental to normal development (Kuhn, et al., 2011). In the rabbit model of cerebral palsy (CP) (Derrick, et al., 2007), the motor responses of kits to odorants and MRI measures of olfactory function are impaired (Drobyshevsky, et al., 2006) implicating the importance of antenatal stress in the development of abnormal olfactory function.
Nitric oxide (NO) is assumed to play a key role in the development (Bredt and Snyder, 1994) and functioning (Breer and Shepherd, 1993, Lowe, et al., 2008, Wilson, et al., 2007) of the olfactory system. The neuronal nitric oxide synthase isoform (nNOS) is transiently over-expressed late in gestation and early postnatal period in the rodent olfactory epithelium (OE) and remains high in olfactory bulbs (OB) through adulthood (Bredt and Snyder, 1994, Chen, et al., 2004). Nitric oxide mediates formation of synaptic connections in developing and regenerating olfactory receptor neurons (ORN) (Roskams, et al., 1994) and is essential in shaping glomerular organization in the OB (Chen, et al., 2004).
The exact function of nNOS in the peripheral olfactory system remains controversial (Wilson, et al., 2007), especially whether nNOS participates in olfactory signal transduction in the OE, as previously suggested (Breer and Shepherd, 1993). Previous studies utilized NOS inhibitors with low nNOS specificity (Romero-Grimaldi, et al., 2008), which resulted in some doubt about the exact contribution of the isoform nNOS. New selective nNOS inhibitors now allow us to investigate specific involvement of endogenous nitric oxide in the function of a single central nervous system (CNS) neuron. We hypothesized, that during the perinatal period in which there is high expression of nNOS in the OE, ORN activity depends on NO generated by nNOS in response to olfactory stimuli. We further hypothesized that NO-regulated pathways are involved in postnatal sensory deficits following an antenatal insults. , Maternal administration of a selective nNOS inhibitor prior to antenatal hypoxia-ischemia (H-I) has a neuroprotective effect on postnatal motor deficits in rabbits (Yu, et al., 2011). Using a highly specific novel nNOS inhibitor and the ORN response to olfactory stimulation with functional manganese enhanced MRI (MEMRI), we show here that antenatal H-I injury at premature gestation affects NO-regulated pathways in olfactory signal transduction at term in postnatal rabbit kits.
METHODS
The Institutional Animal Care and Use Committee of NorthShore University HealthSystem Research Institute approved all experimental procedures with animals.
Antenatal hypoxia-ischemia
The surgical procedure has been previously described (Derrick, et al., 2004). In vivo global H-I of fetuses was induced by sustained 40-min uterine ischemia at 22 days gestation (70% term) in timed pregnant New Zealand white rabbits (Myrtle’s Rabbits, Thompson Station, TN). This procedure models acute placental insufficiency at a premature gestation. Briefly, dams were anesthetized with intravenous fentanyl (75 µg/kg/hr) and droperidol (3.75 mg/kg/hr), followed by spinal anesthesia using 0.75% bupivicaine. A balloon catheter was introduced into the left femoral artery and advanced into the descending aorta to above the uterine and below the renal arteries. The balloon was inflated for 40 minutes causing uterine ischemia and subsequent global fetal H-I. At the end of H-I, the balloon was deflated, resulting in uterine reperfusion and reperfusion-reoxygenation to the fetal brains. The catheter was removed, the femoral artery reconstructed, and the dam returned to her cage and allowed to deliver spontaneously.
On postnatal day 1 (P1) kits underwent neurobehavioral testing for the presence of sensory and motor deficits, including the test for aversive reaction to amyl acetate as described previously (Derrick, et al., 2004). Olfaction was tested by aversive response to a cotton swab soaked with odorant. The test was videotaped, and the results were scored from 0 (no reaction) to 4 (active avoidance), assessed by 2 observers who were blind to the experimental groups. In our previous study (Drobyshevsky, et al., 2006) MEMRI found deficits in olfactory function that were not detected by neurobehavioral tests. In the current study, we expanded the olfactory scoring range from 0 to 4 for the neurobehavioral tests.
The surviving kits were categorized as 1) Non-hypertonic kits with normal tone in all limbs and 2) Hypertonic kits with increased muscle tone. Hypertonia was defined as increased resistance to passive stretch in any limb. Immediately after neurobehavioral assessment the newborn P1 kits underwent MEMRI examination.
Experimental groups
Two groups of P1 kits were examined in the study: 1) kits after global antenatal 40 min H-I at E22. Only kits with muscle hypertonia and motor deficits were included in the H-I group of the study. The presence of hypertonia was used as indication of significant CNS brain injury in newborn kits. 2) Naïve newborn P1 kits served as a control group. In our experience, even with 30 min hypoxia-ischemia at E22, the postnatal kits at P1 were indistinguishable from naïve controls in the motor response to olfactory function. E22 fetuses well tolerate sham surgery and newborn rabbit kits born 10 days are exactly like naïve controls. Since our goal was to also to delineate the carefully the normal behavior, we chose to use naïve control kits.
The drug subgroups were as follows:
NO donors: Spermine NONOate (Sigma, St. Louis, MO) or DETA NONOate (Sigma, St. Louis, MO), were administered intranasally in a dose of 25 µl/nostril of 1000 µM solution.
To control for the effect of off-target effects, we elected to use inactive NO donors (instead of vehicle) to serve as controls for Drug Subgroup 1 above. NO donors were made inactive by keeping them at room temperature and exposed to light for 24 hours. These controls were better than saline as the vehicle, as they would have only one difference from the drug, which was the absence of donation of NO.
NOS inhibitor: JI-5 (cis-N1-[4’-6”-Amino-4”-methyl-pyridin-2”-ylmethyl)-pyrrolidin-3’-yl]-N2-2’-(3”fluoro-phenyl)-ethyl]-ethane-1,2-diamine) in two doses was used. JI-5 is a highly selective nNOS inhibitor with Ki for nNOS of 0.0053 µM (Delker, et al., 2010). The Ki of JI-5 for nNOS is 743 and 3830 times less than iNOS and eNOS respectively. JI-5 was administered intranasally 0.025 ml/nostril of either 1.59 µM or 3.08 µM. These concentrations were estimated to reach 300 Ki and 600 Ki, respectively, in the olfactory neuron, if there was perfect distribution across the entire kit. We have shown that the new class of nNOS inhibitors is far superior to 7-nitroindazole, the previous favorite nNOS inhibitor, in neuroprotection for behavioral endpoints as well as side effects (Yu, et al., 2011).
Control for the nNOS inhibitors (Subgroup 3) was equi-volume administration of the saline vehicle.
Time of Administration
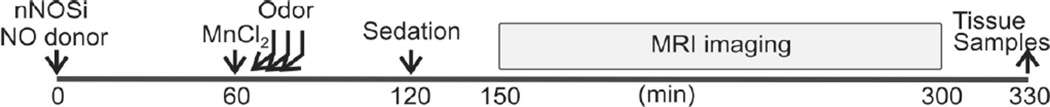
Timeline of MEMRI experiments is presented in Figure 1. NO donors and JI-5 were administered 60 min before imaging. We were targeting the effect of NO produced by nNOS only for 60 to 150 min on olfactory function. The JI-5 group would thus have an absence of NO production for 60 to 150 min but not necessarily get rid of the NO present which was produced before Time 0.
Figure 1.
Timeline of the MEMRI experiments. Note that the effect of NO manipulation on MEMRI begins at 150 min while tissue sampling for NOx is taken later at 330 min.
Manganese enhanced MRI imaging
Uptake of paramagnetic Mn2+ ion via Ca2+ voltage gate channels of olfactory neurons can be registered on T1-weighted images. Signal enhancement serves as a surrogate marker for olfactory neuronal activity (Pautler, et al., 1998). We have previously shown that the dynamic imaging of T1-weighted signal in OB reveals information about the rate of Mn2+ ion uptake, recorded as the slope of signal enhancement, as well as the speed of axonal transport in olfactory neurons, recorded as the onset of signal enhancement (Drobyshevsky, et al., 2006).
Manganese chloride was obtained from Sigma (St. Louis, MO) and dissolved in saline. Kits were administered MnCl2 intranasally by pipette 90 min before imaging in a dose of 10 mg/kg in 0.02 mL per nostril. The animals were placed in a chamber and were given odor stimulation by blowing 2 L/min air passing through a test tube containing 1 mL of amyl acetate (Sigma, St. Louis, MO), diluted 1:10 in mineral oil. Stimulation consisted of blocks of odorant (30 sec) or clear air (2 min) alternately for 15 min.
One hour after manganese chloride administration, rabbit kits were sedated with intramuscular injection of Ketamine (35 mg/kg), Xylazine (5 mg/kg), and Acepromazine (1.0 mg/kg). Imaging was performed in 4.7T Bruker BioSpec scanner with 93 mm birdcage coil. Series of T1-weighted spin-echo images (RARE-T1 sequence, RARE factor 4, TR/TE=842/10 ms, NEX=3, FOV 70 mm, matrix 256×128, slice thickness 1 mm, 1 min volume acquisition time, 150 volumes for 2 hours 30 min) were acquired. Simultaneous imaging of 8 kits by a custom made animal holder allowed us to remove biases in imaging at different times, scanner drift, and temperature variation and prevents different drug exposure times. To control for inter-experiment variables, kits were randomly assigned to a drug or corresponding control in each imaging session. Inter-session controls were obtained to account for scanner drift, change of body temperature and other possible factors. The gradient cooling system settings was adjusted to keep ambient air temperature inside the magnet bore at 28°C and kits body core temperature at ~35 °C. Body core temperature was monitored in one of the kits during imaging session using MRI compatible fiber optic probe (FOTS100, Biopac Inc, CA).
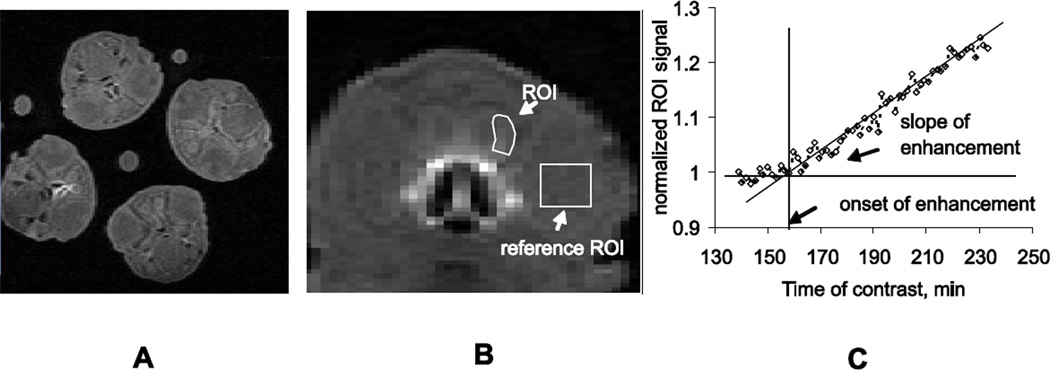
Rabbit kits were positioned in the cradle in groups of equal number of randomly assigned kits, facing each other toward the magnet isocenter. 8 coronal slices were placed in two slice groups (16 slices total) covering the head region from the nasal turbinates and OE to the beginning of piriform cortex. Regions of interest (ROI) were placed on the glomerular layer of OB (Figure 2). T1-weighted images were normalized by values from the ROI placed on kit’s eye in a corresponding slice, where there is no enhancement by Mn2+. Onset and slope of signal enhancement in the OB, corresponding to arrival and accumulation of Mn2+ were determined from the ROI signal time course as before (Drobyshevsky, et al., 2006) as outcome parameters.
Figure 2.
A. An equal number of drug and control kits were placed in a custom made animal holder and imaged simultaneously. B. The region of interest (ROI) was placed on the lateral portion of glomerular layer in the olfactory bulb of each kit and the ROI signal was normalized to a reference placed on one eye of the kit (reference ROI). C. The slope of enhancement was estimated from the rising portion of the normalized ROI signal, and the onset of enhancement was calculated from the intersection of the slope with the baseline before enhancement takes place.
To account for possible difference in kits sizes between litters, changes in ambient and body temperature (Smith, et al., 2007), scanner drift, olfactory exposure and other intersession experimental confounds, two levels of normalization procedures were applied. First, signal from OB was normalized to a region of interest in the kit’s eye, having the same temperature, but no manganese uptake. Second, kits from the same litter were randomly divided into saline and drug groups, and imaged simultaneously using the custom made animal holder. MRI derived parameters in the drug groups were normalized to the average values of control group littermates of the same day.
Determinations of Nitrogen Oxides (NOx)
NO production in the OE and OB was indirectly determined by measuring nitrogen oxides (NOx) consisting of NO2− and NO3− (Michelakis and Archer, 1998). The effect of nNOS inhibition on NO production was monitored starting 60 min prior to imaging. Immediately after MRI (4 hours after intranasal application of NOS inhibitors), samples of OE and OB were obtained. Fresh tissue was frozen in liquid nitrogen and then stored at −80°C. For analysis, the tissue was defrosted and homogenized with an ultrasonic homogenizer. The homogenate was centrifuged at 4200 rpm for 15 minutes at 4°C. The supernatant was kept on ice and used immediately for analysis. NOx was detected with a Nitric Oxide Chemiluminescent Analyzer, Sievers 280i NOA™ (Sievers Instruments, Boulder, CO). To measure nitrate, vanadium (III) chloride in hydrochloric acid at 90°C was used to convert nitrate to NO. To measure nitrite, sodium iodide in acetic acid converted nitrite to NO, and NO was detected by chemiluminescence. Samples were compared to a range of standards of sodium nitrate and nitrite. The difference of NOx measurements between nNOS inhibitors and vehicle controls gave an indirect estimate of the actual NO produced due to nNOS.
Gene expression
Gene expression of nNOS was determined in the OE and olfactory bulb at various gestational ages. Total RNA from different brain regions and gestational age fetuses (n=4/age) was extracted with RNeasy Protect Cell Mini Kit (Valencia, CA) and 2 µg of RNA was reverse transcribed to cDNA with High Capacity cDNA Reverse Transcription Kit (ABI, Foster City, CA). SYBR Green based real-time PCR primers were designed based on the full sequence of rabbit nNOS cDNA and rabbit 18s rRNA with Primer Express (ABI, Foster City, CA). Real-time PCR was conducted with SYBR Green Master mix, the signal collected by Rotor Gene system with 1 µl of RT reaction loaded into each 20 µl real time PCR reaction. The relative steady state levels of mRNA were calculated with ΔΔCT and rabbit 18s as an internal control. For rabbit nNOS: forward, 5'- CAG AGA CCA CTT TGA GAG CGC; reverse, 5'- ACG GAG AAC CTC ACA TTG GC.
Nasal epithelium blood flow
Micro-vascular blood flow change in OE was measured with a laser Doppler flow meter (PeriFlux System 5000, Perimed Inc, OH) and intranasal microprobe (probe 415-230). Relative blood flow in arbitrary units, calibrated to motility standard, was recorded from the anesthetized rabbit kit before and after intranasal administration of 50µL saline and 100µM of NaNO2, and NaNO3.
Statistical analysis
Data is presented as means ± SEM. Comparisons across groups were done with a t-test, ANOVA, or Wilcoxon signed rank test where appropriate. Differences with p-values less than 0.05 were considered significant. Correlations between ordinal neurobehavioral scores of smell with MEMRI were done with the Spearman correlation coefficient (rs). Developmental changes in gene expression were tested using repeated measures ANOVA.
RESULTS
Developmental profile of NOS gene expression in olfactory bulbs
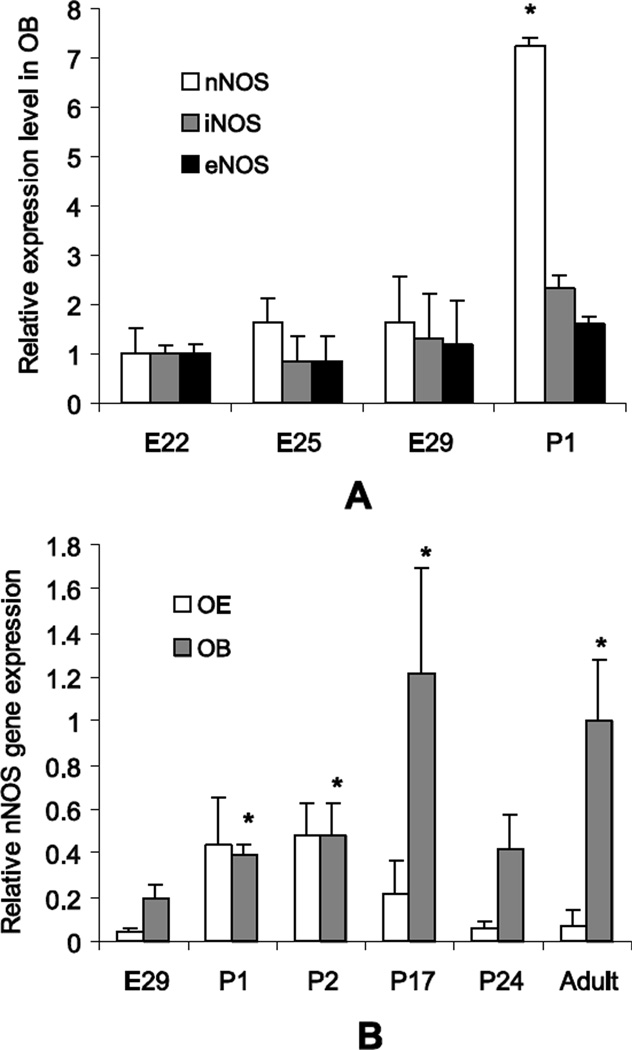
Olfactory bulb nNOS gene expression increased rapidly from late fetal to early postnatal period (Figure 3A). In contrast the other 2 isoforms of NOS (iNOS and eNOS) remained unchanged. Newborn rabbit kits do not respond to sound or light stimulation at P1 which makes olfactory stimulation by ambient odors, including that of the mother, mother’s milk and the nest important. The seven fold increase of nNOS in newborn rabbit kits likely indicates the importance of nNOS in smell recognition and learning after delivery. Gene expression of nNOS did not significantly change in OB of H-I rabbit kits at E29 (0.28±0.08 vs 0.03±0.01, NS), and P1 (0.33±0.05 vs 0.51±0.08, NS), as well as in OE at E29 (0.06±0.02 vs 0.01±0.00, NS), and P1 (0.44±0.21 vs 0.04±0.01, NS), compared to controls (data normalized to OB adult nNOS level).
Figure 3.
A. Gene expression of nNOS in OB of normal kits increased rapidly around birth, whereas gene expression of the other NOS isoforms remained the same from E22 to P1. Gene expression levels are normalized to corresponding average levels for each isoform at E22. * - p<0.05 difference from E22, repeated measures ANOVA.
B. Gene expression of nNOS in the OE was relatively high at P1 and decreased with maturation to low levels in adults. Expression of nNOS in OB peaked at P17. Gene expression levels are normalized to the average level of nNOS in adult olfactory bulb. 3–6 samples in each group. *-p<0.05 difference from E29, repeated measures ANOVA.
Gene expression of nNOS in the OE was significantly upregulated in newborn kits at P1-P2 and gradually decreased with maturation (Figure 3B). Gene expression of nNOS in OB progressively increased from fetal through postnatal period and peaked around 3rd postnatal week and then decreased and remained stable till adulthood (Figure 3B).
Suppression of nNOS activity in the olfactory system by selective inhibitors
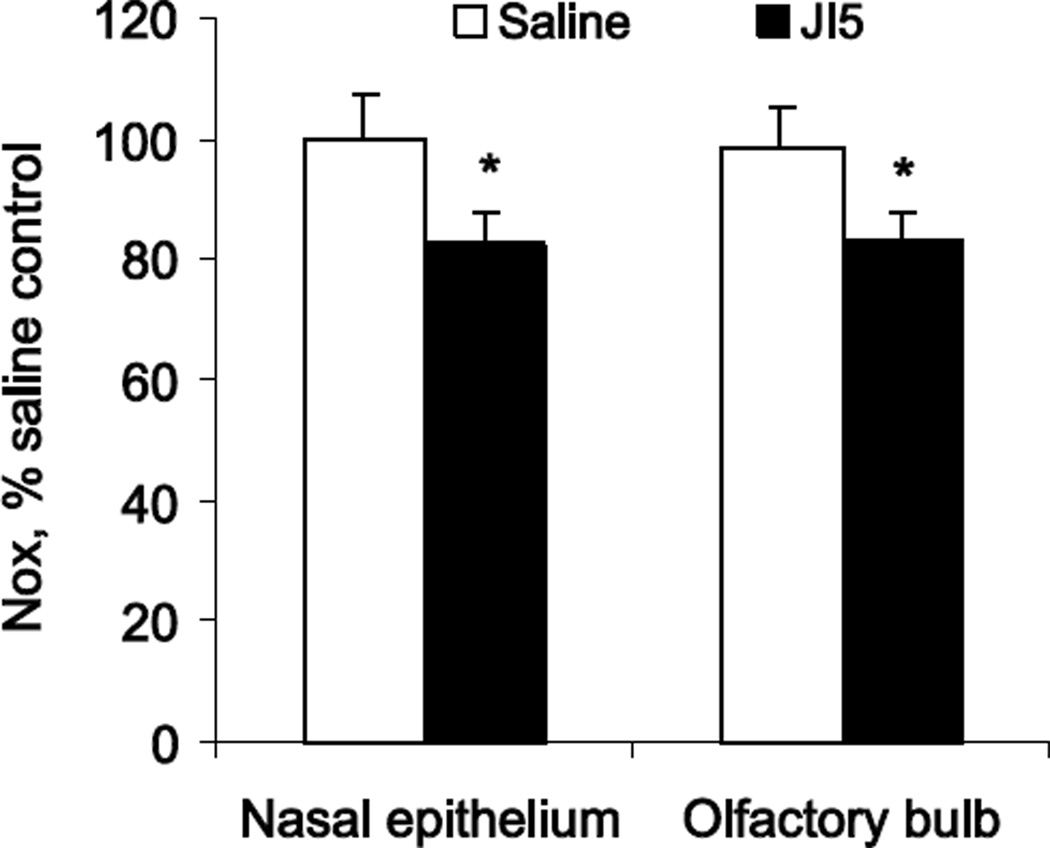
To examine the role of NO in perinatal smell function development we manipulated NO levels by either blocking nNOS activity with JI-5, a highly selective inhibitor of nNOS, or increasing NO levels with NO donors. NO production was determined by measuring NOx in tissues 4 hours after intranasal application of equal volumes of either saline or nNOS inhibitor. NO production in the saline group was significantly higher in OB, 6.01±0.59 mmol/mg protein, than in the nasal OE, 4.85±0.45 mmol/mg protein. To account for possible differences in litter sizes and kit weights, ambient temperature, olfactory exposure and other inter session experimental confounds, NOx tissue concentrations in OE or OB were normalized by the average NOx concentration in corresponding tissue from the same day saline group littermates. Intranasal application of nNOS inhibitor significantly decreased NO production in OE and OB (Figure 4). Relative suppression of NO production by nNOS inhibitor JI-5 was similar in OE (82.4%) and in OB (82.5 %).
Figure 4.
NO production was significantly suppressed in both OE and OB after intranasal application of nNOS inhibitor JI5 in theoretical concentration of 600 Ki (see methods). NO production was determined by measuring NOx in tissue 4 hours after application of nNOS inhibitor. NOx concentrations were normalized to values in corresponding tissue of littermate saline controls, obtained at the same time after identical odor stimulation protocol. Note that the decrease of NO production is large as it represents only 4 hours of inhibition of nNOS. Number of subjects was 15 in control and 19 in JI5 group.
* - p<0.05 compared to saline controls
Inhibition of nNOS increases olfactory neuronal activity
Next we evaluated whether decreasing NO in the olfactory system affects olfactory neuronal activity in response to odor stimulation using manganese enhanced MRI.
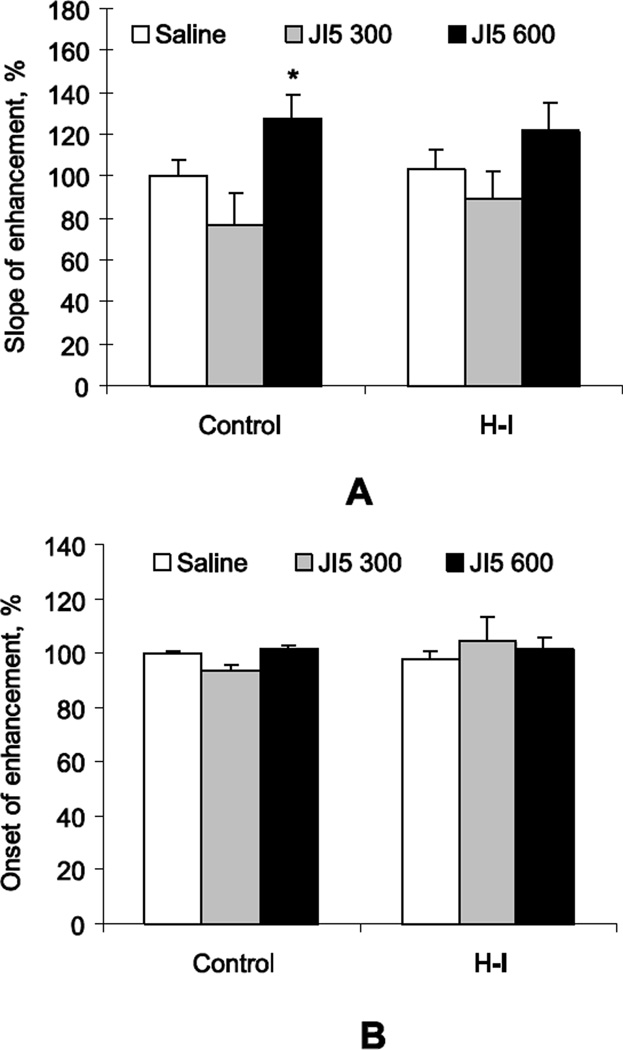
JI-5, the nNOS inhibitor, was administered in the newborn kits’ nostrils 1 hour prior to contrast administration and odor exposure. The slope of signal enhancement in control kits was significantly increased when the high dose of JI-5 (600Ki) was applied (Figure 5A). The slope of signal enhancement in H-I kits was not significantly increased when either dose of JI-5 was applied (Figure 5A). There was no difference in the other parameter of MEMRI, onset of enhancement, between saline and JI-5 in control or H-I groups (Figure 5B).
Figure 5.
A. Intranasal application of nNOS inhibitors JI-5 in normal kits significantly increased the slope of MEMRI signal enhancement in OB with odor stimulation when the inhibitor was applied in the higher theoretical dose of 600 Ki. This increase was not significant in the H-I group. The values in the JI-5 treated kits are adjusted to the same day vehicle kits, represented as 100%, in both Control and H-I groups. Because of the previous study comparing controls and H-I (Drobyshevsky, et al., 2006), we did not directly compare Control and H-I in the vehicle treated kits in this study. B. There was no significant effect of JI-5 on the onset of MEMRI enhancement. Number of kits: n=6 for Controls and n=12–24 for H-I.
* - p<0.05 compared to corresponding saline controls in each group
Supplementation of NO did not affect olfactory neuronal activity
The NO donor DETA NONOate, applied intranasally 1 hour prior to contrast administration and odor stimulation did not significantly change the slope of signal enhancement (normalized to average inactive form, 1.00±0.30 drug vs. 1.00±0.27 inactive form, NS) either in the contrast arrival time (1.00±0.02 drug vs. 0.90±0.02 inactive form, NS), in control P1 kits. We suspected that there was not enough incubation time for the compound to release sufficient NO, since the peak of NO production of this compound is 4–6 hours. For this reason the next experiment used the faster releasing NO donor, Spermine NONOate.
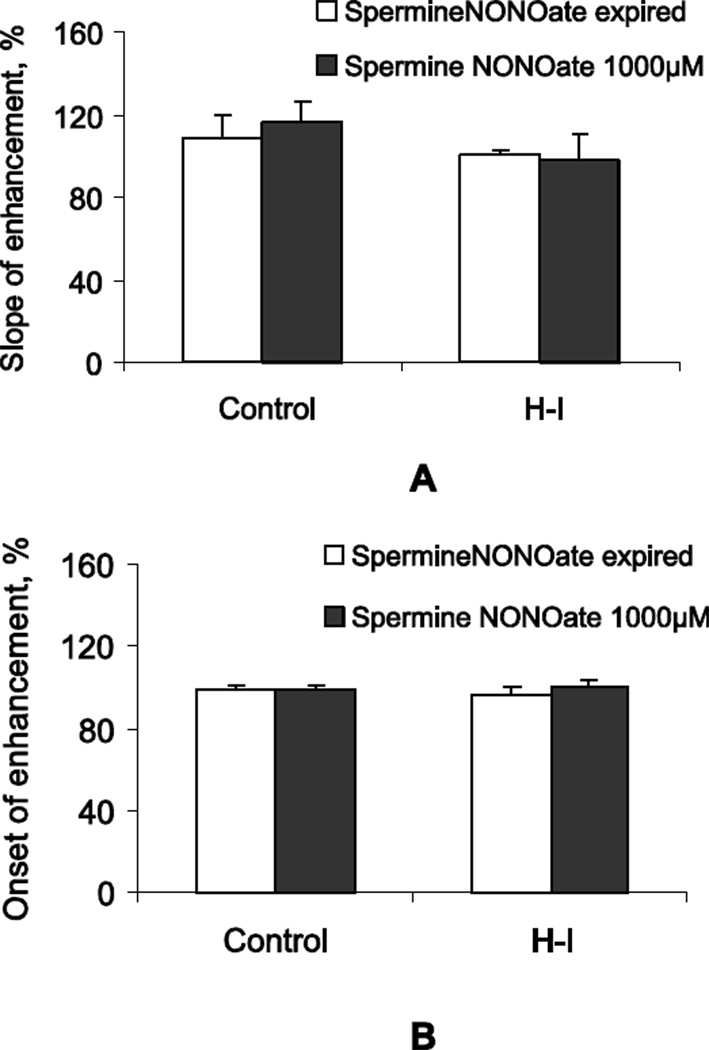
The NO donor Spermine NONOate, applied intranasally 1 hour prior to contrast administration and odor stimulation also did not significantly change the slope of signal enhancement either in controls or in the H-I group compared to the group that received the inactive form (Figure 6A). There was also no difference between groups in the contrast arrival time, suggesting that the speed of axonal transport in OE neurons is not affected by exogenous NO in both control and H-I groups (Figure 6B).
Figure 6.
A. Intranasal application of NO donor Spermine NONOate, 25 µl/nostril of 1000 µM, did not change the slope of MEMRI signal enhancement in OB with odor stimulation. The data are presented as a percentage of the same day inactive form control. B. There was no significant effect of Spermine NONOate on the onset of MEMRI enhancement. Number of kits: n=13–21 for Controls and n=21–22 for H-I.
Slope of contrast enhancement correlated with olfactory scores in control group
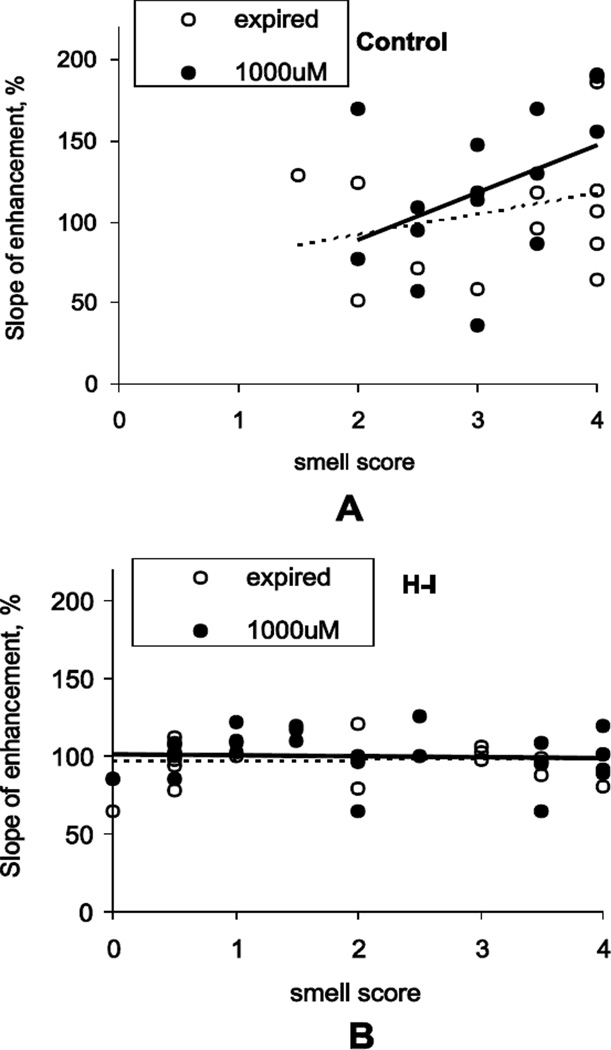
No significant correlation between MEMRI parameters and smell score was observed in vehicle control groups (rs = 0.31, p=0.66 for enhancement slope in expired Spermine NONOate group). However, with Spermine NONOate application, control kits with higher smell scores had larger enhancement in slope (Figure 7). The correlation between olfactory scores and slope of enhancement was significantly different from zero, rs =0.57, p=0.020. There were no significant correlations between MEMRI parameters and behavioral olfactory scores with JI-5 administration.
Figure 7.
A. Slope of contrast enhancement positively correlated with behavioral response scores, assessed by aversive reaction to noxious odor (swab with amyl acetate), in control kits with Spermine NONOate application in dose 1000 µM 25 µl/nostril. The increase of slope in Spermine NONOate group was noticeable in Control kits with higher olfactory scores. B. There was no correlation between olfactory scores and slope of enhancement in H-I group. Number of kits: n=13–14 for Controls and n=20–22 for H-I.
Effect of nitrites and nitrates on nasal epithelium blood flow
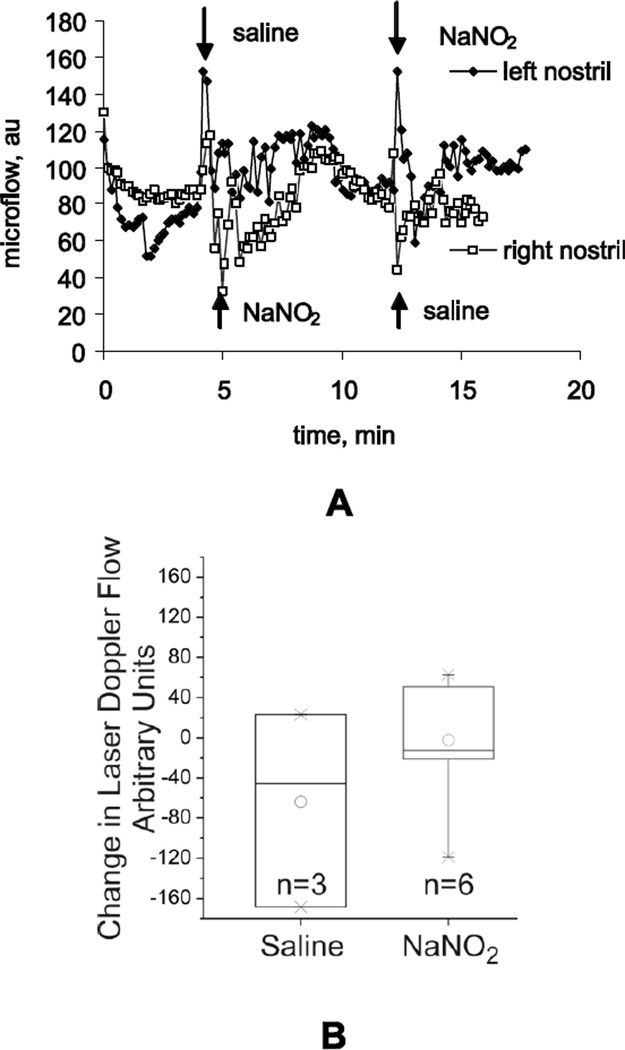
We confirmed that changes in olfactory receptor neuron responses on MEMRI could not be attributed to epithelial blood flow changes caused by metabolites of NO, since we did not observe significant differences in microvascular blood flow change in OE between saline and NO oxidation products (NaNO2 or NaNO3, 100µM, n = 3 kits). Microvascular blood flow was measured by laser Doppler flow meter and intranasal microprobe in anaesthetized P1 rabbit kits. After a short spike in response to the liquid administration, the blood flow returned to the baseline within few minutes (Figure 8A). The experiment demonstrated that there was no long term effect of intranasal nitrite and nitrate on microvascular blood flow (Figure 8B).
Figure 8.
Microvascular blood flow was measured in the nasal epithelium of P1 rabbit kits using laser Doppler probe (A) and was similar after intranasal administration of equal volumes saline or sodium nitrite at 21°C (B). The order of saline or sodium nitrite was switched in the left and right nostrils of the same animal and the effect was the same. The difference in the blood flow response magnitude between saline and sodium nitrate was not significant. The power of the t- test is 0.17. Ruling out a Type II error with power=0.80 would require 29 animals in each group.
DISCUSSION
Using genetic and functional methods, we provide evidence that nNOS is present in OE of newborn rabbit and modulates olfactory signal transduction. This function is altered by antenatal H-I injury. In control kits nNOS inhibition decreased NO production in the olfactory system and significantly increased MEMRI slope enhancement. In H-I kits the MEMRI slope did not increase with decreased NO. NO donors did not significantly change MEMRI slope or onset in either group. However, the correlation of MEMRI slope with behavioral score was affected in control but not in H-I kits. This reinforces the view of modification of endogenous NO-mediated olfactory function by the antenatal insult.
Previous studies have demonstrated that the role that nitric oxide plays depends upon its site of production, as nNOS expression is dynamic during development and during the postnatal period (Roskams, et al., 1994). In developing ORNs, nNOS is highly expressed during embryogenesis (Roskams, et al., 1994), similar to its expression in the developing cortex(Bredt and Snyder, 1994), but is down regulated shortly after birth (Roskams, et al., 1994). Using nNOS-deficient mice it has been shown that the role of nNOS in development depends on its localization: it promotes neurogenesis in early postnatal development in OE and glomerular organization in the OB in adult (Chen, et al., 2004). The dramatic decrease in proliferation of neuronal precursors seen in nNOS null mice at the first postnatal week can be explained if nNOS acts as an autocrine or paracrine neuroproliferative factor. While the pool of neuronal precursors and neurogenesis normalized in the nNOS null mouse by sixth postnatal week, there was an overgrowth of mitral or tufted cell dendrites and a decreased number of active synapses in the OB. Mice deficient in nNOS also have decreased levels of cyclic 3',5'-guanosine monophosphate (cGMP), suggesting that nNOS activity plays a role in olfactory system organization. Further evidence for the function of nNOS in OE as a modulator of neurogenesis comes from studies of OB removal, which results in retrograde neuronal degeneration and repopulation of the epithelium (Weruaga, et al., 2000) and is associated with up-regulation of nNOS expression. nNOS has been assumed to play a role in proliferation of ORNs but not in olfactory signal transduction as nNOS is down regulated by the second week of postnatal development and is absent from ORNs in adult rodents (Bredt and Snyder, 1994, Roskams, et al., 1994).
Based on NADPH diaphorase staining in ORNs and a NO- sensitive guanyl-cyclase, it has been proposed that NO may have several functions in ORN signal transduction (Breer and Shepherd, 1993). First, NO, can easily diffuse across cell membranes and may recruit adjacent ORNs to encode very intense stimuli by the cGMP pathway. At the same time such activation may promote functional and structural organization of ORN clusters responding to similar odorants (Moulton, 1976). Second, slow elevation of cGMP may play a role in sensory adaptation of ORNs to prolong and intense stimuli.
High levels of NOS and cGMP are found in the OB (Lowe, et al., 2008). There is an increase of NO during olfactory stimulation (Lowe, et al., 2008). nNOS is highly expressed in the periglomerular and granule cells in the OB in neonates and adults (Bredt and Snyder, 1994, Chen, et al., 2004, Roskams, et al., 1994).
There has yet been no conclusive evidence that nNOS is a component of normal signal transduction in the ORN (Roskams, et al., 1994). Some recent experimental data provide evidence that eNOS is present in adult OE (although not in ORN) (Brunert, et al., 2009). Using NO-sensitive micro electrodes, they showed that stimulation liberates NO from isolated wild-type ORNs, but not from ORNs of eNOS deficient mice. Integrated electrophysiological recordings from the OE of these mice show that NO facilitates adaptation for repetitive olfactory stimuli. However, the functional role of nNOS in stimulus dependent activity maturation during early postnatal development has not been previously recognized.
This study provides first time experimental evidence in support of the role of nNOS in normal olfactory signal transduction during the early postnatal period. Quantitative gene expression analysis revealed that, in rabbits, nNOS expression remains relatively high for at least three postnatal weeks (Figure 3B), similar to those in rodents (Bredt and Snyder, 1994, Roskams, et al., 1994), with the exception that epithelial nNOS did not completely disappear in mature rabbits. This suggests nNOS does more than just regulate neurogenesis in perinatal period. Using highly selective nNOS inhibitors we demonstrated that formation of NO due to nNOS activity is present in P1 rabbit kits, both in OE and OB (Figure 4), and production in 4 hours was equivalent to about 20% of cumulative NO production from all NOS isoforms at all proximate sites. Most importantly, we showed that ORN activity in response to olfactory stimulation is modulated by nNOS activity (Figure 5). In fact, ORN activity was increased when NO production was decreased with application of nNOS inhibitors and did not change with application of NO donors. This effect could not be attributed to the prolonged increase of OE blood flow, as indicated by microvascular blood flow response recordings (Figure 8). The absence of an effect of NO donors on ORN activity, despite possible supra-physiological NO levels, emphasizes the importance of localized NO production by nNOS in ORNs in response to olfactory stimulation. It is possible that NO behaves in a threshold stimulatory manner, and exogenous NO does not enhance the inhibitory effect on olfactory function.
Currently it is unclear what causes the increase of ORN activity after inhibition of NO production. We hypothesize that it may be related to the decrease of lateral inhibition by NO (Breer and Shepherd, 1993) and therefore decreased adaptation to the repetitive olfactory stimulus. In the current study, olfactory stimulation consisted of long blocks of odorant (30 sec) intermittent with clear air (2 min) alternately for 15 min. This stimulation protocol, typical for olfactory MEMRI studies (Pautler and Koretsky, 2002), is designed to increase total uptake of MRI contrast, which is proportional to ORN Ca2+ voltage gated channel activity, and provide better ability to detect signal change on MRI. However, ORN activity with such prolonged odor exposure may be influenced by adaptation (Breer and Shepherd, 1993, Chen, et al., 2004, Wilson and Sullivan, 1994). The effect of NO-mediated lateral inhibition may be abolished with decreased levels of NO after application of nNOS inhibitors, resulting in elevation of ORN activity. Habituation may also occur on higher olfactory processing levels, OB and piriform cortex, resulting in changing of respiration and sniffing pattern and decreasing exposure of OE to odorant (Kabir, et al., 2010, Scott, 2006). Further studies with several stimulation paradigms, accounting for the effect of olfactory adaptation, may test the hypothesis about the role of nNOS in lateral inhibition of ORN activity.
Regardless of the exact role of NO in olfactory signal transduction and memory, our study emphasizes the role of nNOS in the early postnatal period as an activity dependent modulator of olfactory system maturation in response to a plethora of novel olfactory stimuli in postnatal environment. Quick learning of important olfactory cues, such as odor of mother, milk, nest, and siblings, is critical for survival of neonates with otherwise limited sensory and motor capabilities. In our rabbit model of perinatal H-I brain injury, newborns exhibit deficits in behavioral responses to olfactory stimulation (Derrick, et al., 2004), confirmed by functional and structural abnormalities of OE (Drobyshevsky, et al., 2006). Injury of OE in rabbit kits after antenatal H-I resulted in decreased number of olfactory mature neurons in OE, total number of cells in OE and epithelial thickness, and decrease of functional activity of ONR in response to olfactory stimulation. We did not directly compare the controls and H-I in this study but only the effect of NO up- or downregulation. The effect of endogenous NO in H-I kits was impaired as shown by MEMRI (Figure 5A), suggesting decreased nNOS activity in injured kits. This may result in abnormalities in olfactory system development, in particular, in formation of OB glomerular structure (Chen, et al., 2004) and odor recognition. Olfactory memories formed in the early postnatal period may affect development of preferences in the alimentary (including obesity) and social domains (Varendi, et al., 1994, Winberg and Porter, 1998).
In hypoxic human infants, inhaled nitric oxide (NO) given as a gas is currently used as therapy for modulation of pulmonary vasculature (Barrington and Finer, 2006) . One clinical study (Marks and Schreiber, 2008) and some animal studies (Olivier, et al., 2010, Pansiot, et al., 2010) have suggested that inhaled nitric oxide may also have a neuroprotective effect, although this has not been borne out in subsequent clinical studies in premature infants (Cole, et al., 2011, Mercier, et al., 2010). Although the current study used short and reversible NO modulation in the olfactory system, the emerging role of peripheral olfactory nNOS in functional shaping of adult olfactory system warrants further study of prolonged NO modulation in the perinatal period.
As a conclusion, the results of the study indicate that nNOS is present in rabbit OE during the first three postnatal weeks. Modulation of olfactory transduction by NO levels suggests a new role of OE nNOS in activity dependent functional maturation of olfactory system during the perinatal period. Perinatal H-I injury may affect early functional development of olfaction by decreasing NO-dependence of olfactory signal transduction.
Highlights.
nNOS transiently upregulated during 3 postnatal weeks in rabbits olfactory system
Manganese-enhanced functional MRI quantifies olfactory functional response
nNOS participates in olfactory functional response in perinatal rabbits
Perinatal neuronal injury decreases NO-dependent olfactory function
Novel role of nNOS proposed as activity dependent modulator of olfactory maturation
Acknowledgments
Acknowledgement statement (including conflict of interest and funding sources): Funding sources: NICHD grant R21HD054742 (ST), R01 GM049725 (RS)
Abbreviations
- MEMRI
manganese enhanced MRI
- OE
olfactory epithelium
- nNOS
neuronal nitric oxide synthase
- H-I
hypoxia - ischemia
- OB
olfactory bulb
- NO
nitric oxide
- ORN
olfactory receptor neuron
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Barrington KJ, Finer NN. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database Syst Rev. 2006;25:CD000509. doi: 10.1002/14651858.CD000509.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Bingham PM, Churchill D, Ashikaga T. Breast milk odor via olfactometer for tube-fed, premature infants. Behav Res Methods. 2007;39:630–634. doi: 10.3758/bf03193035. [DOI] [PubMed] [Google Scholar]
- 3.Bredt DS, Snyder SH. Transient nitric oxide synthase neurons in embryonic cerebral cortical plate, sensory ganglia, and olfactory epithelium. Neuron. 1994;13:301–313. doi: 10.1016/0896-6273(94)90348-4. [DOI] [PubMed] [Google Scholar]
- 4.Breer H, Shepherd GM. Implications of the NO/cGMP system for olfaction. Trends Neurosci. 1993;16:5–9. doi: 10.1016/0166-2236(93)90040-s. [DOI] [PubMed] [Google Scholar]
- 5.Brunert D, Kurtenbach S, Isik S, Benecke H, Gisselmann G, Schuhmann W, Hatt H, Wetzel CH. Odorant-dependent generation of nitric oxide in Mammalian olfactory sensory neurons. PLoS One. 2009;4:e5499. doi: 10.1371/journal.pone.0005499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Tu Y, Moon C, Matarazzo V, Palmer AM, Ronnett GV. The localization of neuronal nitric oxide synthase may influence its role in neuronal precursor proliferation and synaptic maintenance. Developmental Biology. 2004;269:165–182. doi: 10.1016/j.ydbio.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Cole FS, Alleyne C, Barks JD, Boyle RJ, Carroll JL, Dokken D, Edwards WH, Georgieff M, Gregory K, Johnston MV, Kramer M, Mitchell C, Neu J, Pursley DM, Robinson WM, Rowitch DH. NIH Consensus Development Conference statement: inhaled nitric-oxide therapy for premature infants. Pediatrics. 2011;127:363–369. doi: 10.1542/peds.2010-3507. [DOI] [PubMed] [Google Scholar]
- 8.Delker SL, Ji H, Li H, Jamal J, Fang J, Xue F, Silverman RB, Poulos TL. Unexpected binding modes of nitric oxide synthase inhibitors effective in the prevention of a cerebral palsy phenotype in an animal model. Journal of the American Chemical Society. 2010;132:5437–5442. doi: 10.1021/ja910228a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derrick M, Drobyshevsky A, Ji X, Tan S. A model of cerebral palsy from fetal hypoxia-ischemia. Stroke. 2007;38:731–735. doi: 10.1161/01.STR.0000251445.94697.64. [DOI] [PubMed] [Google Scholar]
- 10.Derrick M, Luo NL, Bregman JC, Jilling T, Ji X, Fisher K, Gladson CL, Beardsley DJ, Murdoch G, Back SA, Tan S. Preterm fetal hypoxia-ischemia causes hypertonia and motor deficits in the neonatal rabbit: a model for human cerebral palsy? J Neurosci. 2004;24:24–34. doi: 10.1523/JNEUROSCI.2816-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drobyshevsky A, Robinson AM, Derrick M, Wyrwicz AM, Ji X, Englof I, Tan S. Sensory deficits and olfactory system injury detected by novel application of MEMRI in newborn rabbit after antenatal hypoxia-ischemia. Neuroimage. 2006;32:1106–1112. doi: 10.1016/j.neuroimage.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Kabir MM, Beig MI, Baumert M, Trombini M, Mastorci F, Sgoifo A, Walker FR, Day TA, Nalivaiko E. Respiratory pattern in awake rats: effects of motor activity and of alerting stimuli. Physiology and Behavior. 2010;101:22–31. doi: 10.1016/j.physbeh.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Kuhn P, Astruc D, Messer J, Marlier L. Exploring the olfactory environment of premature newborns: a French survey of health care and cleaning products used in neonatal units. Acta Paediatrica. 2011;100:334–339. doi: 10.1111/j.1651-2227.2010.02076.x. [DOI] [PubMed] [Google Scholar]
- 14.Lowe G, Buerk DG, Ma J, Gelperin A. Tonic and stimulus-evoked nitric oxide production in the mouse olfactory bulb. Neuroscience. 2008;153:842–850. doi: 10.1016/j.neuroscience.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marks JD, Schreiber MD. Inhaled Nitric Oxide and Neuroprotection in Preterm Infants. Clinics in Perinatology. 2008;35:793–807. doi: 10.1016/j.clp.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mercier JC, Hummler H, Durrmeyer X, Sanchez-Luna M, Carnielli V, Field D, Greenough A, Van Overmeire B, Jonsson B, Hallman M, Baldassarre J. Inhaled nitric oxide for prevention of bronchopulmonary dysplasia in premature babies (EUNO): a randomised controlled trial. Lancet. 2010;376:346–354. doi: 10.1016/S0140-6736(10)60664-2. [DOI] [PubMed] [Google Scholar]
- 17.Michelakis ED, Archer SL. The measurement of NO in biological systems using chemiluminescence. In: Titheradge M, editor. Nitric Oxide Protocols Methods in Molecular Biology. Totowa, NJ: Humana Press Inc; 1998. pp. 111–127. [DOI] [PubMed] [Google Scholar]
- 18.Moulton DG. Spatial patterning of response to odors in the peripheral olfactory system. Physiological Reviews. 1976;56:578–593. doi: 10.1152/physrev.1976.56.3.578. [DOI] [PubMed] [Google Scholar]
- 19.Olivier P, Loron G, Fontaine RH, Pansiot J, Dalous J, Thi HP, Charriaut-Marlangue C, Thomas JL, Mercier JC, Gressens P, Baud O. Nitric oxide plays a key role in myelination in the developing brain. Journal of Neuropathology and Experimental Neurology. 2010;69:828–837. doi: 10.1097/NEN.0b013e3181ea5203. [DOI] [PubMed] [Google Scholar]
- 20.Pansiot J, Loron G, Olivier P, Fontaine R, Charriaut-Marlangue C, Mercier JC, Gressens P, Baud O. Neuroprotective effect of inhaled nitric oxide on excitotoxic-induced brain damage in neonatal rat. PLoS One. 2010;5:e10916. doi: 10.1371/journal.pone.0010916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pautler RG, Koretsky AP. Tracing odor-induced activation in the olfactory bulbs of mice using manganese-enhanced magnetic resonance imaging. Neuroimage. 2002;16:441–448. doi: 10.1006/nimg.2002.1075. [DOI] [PubMed] [Google Scholar]
- 22.Pautler RG, Silva AC, Koretsky AP. In vivo neuronal tract tracing using manganese-enhanced magnetic resonance imaging. Magn Reson Med. 1998;40:740–748. doi: 10.1002/mrm.1910400515. [DOI] [PubMed] [Google Scholar]
- 23.Romero-Grimaldi C, Moreno-Lopez B, Estrada C. Age-dependent effect of nitric oxide on subventricular zone and olfactory bulb neural precursor proliferation. Journal of Comparative Neurology. 2008;506:339–346. doi: 10.1002/cne.21556. [DOI] [PubMed] [Google Scholar]
- 24.Roskams AJ, Bredt DS, Dawson TM, Ronnett GV. Nitric oxide mediates the formation of synaptic connections in developing and regenerating olfactory receptor neurons. Neuron. 1994;13:289–299. doi: 10.1016/0896-6273(94)90347-6. [DOI] [PubMed] [Google Scholar]
- 25.Schaal B, Hummel T, Soussignan R. Olfaction in the fetal and premature infant: functional status and clinical implications. Clin Perinatol. 2004;31:261–285. vi–vii. doi: 10.1016/j.clp.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Scott JW. Sniffing and spatiotemporal coding in olfaction. Chemical Senses. 2006;31:119–130. doi: 10.1093/chemse/bjj013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith KD, Kallhoff V, Zheng H, Pautler RG. In vivo axonal transport rates decrease in a mouse model of Alzheimer's disease. Neuroimage. 2007;35:1401–1408. doi: 10.1016/j.neuroimage.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varendi H, Christensson K, Porter RH, Winberg J. Soothing effect of amniotic fluid smell in newborn infants. Early Human Development. 1998;51:47–55. doi: 10.1016/s0378-3782(97)00082-0. [DOI] [PubMed] [Google Scholar]
- 29.Varendi H, Porter RH, Winberg J. Does the newborn baby find the nipple by smell? The Lancet. 1994;344:989–990. doi: 10.1016/s0140-6736(94)91645-4. [DOI] [PubMed] [Google Scholar]
- 30.Varendi H, Porter RH, Winberg J. Natural odour preferences of newborn infants change over time. Acta Paediatrica. 1997;86:985–990. doi: 10.1111/j.1651-2227.1997.tb15184.x. [DOI] [PubMed] [Google Scholar]
- 31.Weruaga E, Brinon JG, Porteros A, Arevalo R, Aijon J, Alonso JR. Expression of neuronal nitric oxide synthase/NADPH-diaphorase during olfactory deafferentation and regeneration. European Journal of Neuroscience. 2000;12:1177–1193. doi: 10.1046/j.1460-9568.2000.00003.x. [DOI] [PubMed] [Google Scholar]
- 32.Wilson CH, Christensen TA, Nighorn AJ. Inhibition of nitric oxide and soluble guanylyl cyclase signaling affects olfactory neuron activity in the moth, Manduca sexta. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology. 2007;193:715–728. doi: 10.1007/s00359-007-0227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson DA, Sullivan RM. Neurobiology of associative learning in the neonate: early olfactory learning. Behavioral and Neural Biology. 1994;61:1–18. doi: 10.1016/s0163-1047(05)80039-1. [DOI] [PubMed] [Google Scholar]
- 34.Winberg J, Porter RH. Olfaction and human neonatal behaviour: clinical implications. Acta Paediatrica. 1998;87:6–10. doi: 10.1080/08035259850157787. [DOI] [PubMed] [Google Scholar]
- 35.Yu L, Derrick M, Ji H, Silverman RB, Whitsett J, Vasquez-Vivar J, Tan S. Neuronal nitric oxide synthase inhibition prevents cerebral palsy following hypoxia-ischemia in fetal rabbits: comparison between JI-8 and 7-nitroindazole. Developmental Neuroscience. 2011;33:312–319. doi: 10.1159/000327244. [DOI] [PMC free article] [PubMed] [Google Scholar]