Abstract
Introduction
Tryptophan oxidation via the kynurenine pathway is an important mechanism of tumoral immunoresistance. Increased tryptophan metabolism via the serotonin pathway has been linked to malignant progression in breast cancer. In this study, we combined quantitative positron emission tomography (PET) with tumor immunohistochemistry to analyze tryptophan transport and metabolism in breast cancer.
Methods
Dynamic alpha-[11C]methyl-L-tryptophan (AMT) PET was performed in 9 women with stage II-IV breast cancer. PET tracer kinetic modeling was performed in all tumors. Expression of L-type amino acid transporter 1 (LAT1), indoleamine 2,3-dioxygenase (IDO; the initial and rate-limiting enzyme of the kynurenine pathway), and tryptophan hydroxylase 1 (TPH1; the initial enzyme of the serotonin pathway) was assessed by immunostaining of resected tumor specimens.
Results
Tumor AMT uptake peaked at 5–20 min post-injection in 7 tumors; the other 2 cases showed protracted tracer accumulation. Tumor SUVs varied widely (2.6–9.8) and showed a strong positive correlation with volume of distribution values derived from kinetic analysis (p<0.01). Invasive ductal carcinomas (n=6) showed particularly high AMT SUVs (range: 4.7–9.8). Moderate to strong immunostaining for LAT1, IDO and TPH1 was detected in most tumor cells.
Conclusions
Breast cancers show differential tryptophan kinetics on dynamic PET. SUVs measured 5–20 minutes post-injection reflect reasonably the tracer’s volume of distribution. Further studies are warranted to determine if in vivo AMT accumulation in these tumors is related to tryptophan metabolism via the kynurenine and serotonin pathways.
Keywords: breast cancer, positron emission tomography, immunohistochemistry, tryptophan, kynurenine, serotonin
BACKGROUND
Tryptophan oxidation via the kynurenine pathway has been implicated in tumoral immune resistance [1,2]. Recent studies have consistently shown expression of indoleamine 2,3-dioxygenase (IDO), the initial and rate-limiting enzyme of the kynurenine pathway, in a variety of human tumors, including breast cancer [1, 3–5]. A high level of IDO expression also correlated with advanced stage breast cancer [4]. Serotonin is also derived from tryptophan, via a two-step enzymatic conversion. The first and rate-limiting enzyme in the serotonin pathway is tryptophan hydroxylase (TPH). Increased expression of TPH isoform 1 (TPH1) is associated with malignant phenotype in breast tumors [6]. L-tryptophan, the main substrate for both IDO and TPH1, is transported into cells via the L-type amino acid transporter 1 (LAT1) [7]. LAT1 is an amino acid exchange transporter that can be upregulated in breast cancers at both primary and metastatic sites [3,8,9]. Elevated expression of LAT1 in breast cancers correlates with poor prognosis [10,11]. It has been suggested that IDO, LAT1, and serotonergic markers could serve as potential molecular targets to inhibit tumor cell growth [2,6,12,13]. Therefore, selective inhibitors of IDO, LAT1, and TPH1 are being explored for potential use in new cancer treatments [14–17]. Future clinical trials using such inhibitors could benefit from molecular imaging methods capable of non-invasively monitoring in vivo expression and activity of these important proteins as well as assessing therapeutic response.
α-[11C]methyl-L-tryptophan (AMT) is a positron emission tomography (PET) radiotracer used to study in vivo transport and metabolism of tryptophan. AMT is not incorporated during protein synthesis [18,19]. However, it is a substrate for TPH (leading to synthesis of alpha-methyl-serotonin) [19,20] and IDO (due to its low substrate specificity) [21,22]. In PET studies of brain tumors, elevated tumoral AMT metabolic rates were associated with increased expression of IDO [23]. Recently, dynamic AMT PET/CT scanning of non-small cell lung cancers demonstrated prolonged uptake and retention of AMT, suggesting increased tryptophan metabolism in these tumors [24].
In the present study, we explored the use of AMT PET/CT scanning in imaging human breast cancers. We hypothesized that these tumors would show high AMT transport and metabolism as determined by kinetic analysis of dynamic PET images. In addition, we examined the expression of LAT1, IDO, and TPH1 in resected breast cancer samples and compared the immunohistochemical results with tissue resected from control subjects with benign breast disease.
MATERIALS AND METHODS
Subjects
Nine women (age: 29–63 years) with a diagnosis of breast cancer underwent AMT PET/CT scanning. Clinical data of the subjects are summarized in Table 1. Eight women had a newly diagnosed tumor, while one subject (patient No. 9) had a prior history of breast cancer (resected 5 years earlier) and a recent local tumor recurrence along with extensive metastatic disease (including brain metastasis). Diagnosis was established by histopathological examination of biopsy specimens in all 9 cases. Systemic treatment was started after completing the AMT PET scan. The study was approved by the Wayne State University Institutional Review Board and by the Protocol Review Monitoring Committee at the Karmanos Cancer Institute. Written informed consent was obtained from all participants.
Table 1.
Clinical data and alpha-[11C]methyl-L-tryptophan (AMT) PET kinetic values of the 9 patients with breast cancer. Patients are listed from the lowest to the highest AMT standardized uptake values (SUV).
| Patient | age (years) |
Tumor type | Tumor grade |
Tumor stage |
ER | PR | HER2/ neu |
VD’ | K1 ml/g/min |
SUV |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 63 | carcinosarcoma | I | IIIB | − | − | − | 0.74 | 0.14 | 2.6 |
| 2. | 57 | invasive microp. cc. | III | IV | + | + | − | 1.07 | 0.13 | 3.1 |
| 3. | 50 | carcinosarcoma | III | IIIA | − | − | − | 1.13 | 0.17 | 4.2 |
| 4. | 29 | invasive ductal cc. | III | IIIA | + | + | +++ | 1.23 | 0.22 | 4.7 |
| 5. | 59 | invasive ductal cc. | II | IIA | − | + | +++ | 1.52 | 0.23 | 5.0 |
| 6. | 56 | invasive ductal cc. | II | IIIA | − | − | − | 1.32 | 0.21 | 5.2 |
| 7. | 33 | invasive ductal cc. | III | IIIA | − | + | − | 1.45 | 0.21 | 6.5 |
| 8. | 60 | invasive ductal cc. | III | IIIB | − | + | − | 2.01 | 0.12 | 6.6* |
| 9. | 44 | invasive ductal cc.† | III | IV | + | + | − | 1.61 | 0.23 | 9.8* |
Abbreviations: microp.: micropapillary; cc: carcinoma; ER: estrogen receptors; PR: progesterone receptors; VD’: volume of distribution on PET; SUV: standardized uptake values;
patient with extensive metastases (brain, lung, liver, bone);
patients with prolonged (>20 min) AMT accumulation in tumor
PET data acquisition
PET imaging was performed with a Discovery STE PET/CT scanner (GE Healthcare), using a previously described protocol [24]. The AMT tracer was synthesized as outlined previously [25]. Patients fasted for 6 h prior to the AMT PET studies to ensure stable plasma levels of tryptophan and large neutral amino acids. Initially, a venous line was established for administration of AMT (14.8 MBq [0.4 mCi/kg]). A low-intensity scout CT scan (120 keV, 10 mA) was first acquired. Based on this scout image, one bed position was selected at the level of the breasts/myocardium, and a low-dose CT scan (120 keV, 100 mA) was subsequently acquired for attenuation correction. Thereafter, a 60-min dynamic PET scan (12 × 10, 3 × 60, 3 × 300, and 4 × 600 sec) coinciding with AMT injection was initiated. The reconstructed isotropic spatial resolution of the dynamic AMT PET study was approximately 7 mm full width at half maximum (FWHM).
Image data processing and analysis
Noninvasive determination of the arterial blood input function using dynamic PET of the left ventricle was obtained using a previously described approach [26,27]. In this technique, a small region of interest (ROI) at the center of the left ventricle is used to derive a time–activity curve representing the arterial blood input function. Breast tumors were visually identified by an experienced observer based on CT and FDG PET (where available) images. Using this information, tumors were visually identified on the averaged AMT PET images (10 to 30 min post-injection). In order to objectively determine tumor ROIs, we initially determined the voxel with the highest AMT tracer concentration as well as a background region in close proximity to the location of the tumor. A software developed in-house was then used to define an isocontour at the level of activity half way between the most active pixel in the tumor and the background region in three-dimension. The volume of the so defined volumes of interest (VOI) were on average ~7 cm3 (2.1–10 cm3). The so defined VOIs were subsequently overlaid to all time frames of the dynamic AMT PET sequence in order to obtain tumoral time-activity curves. Considering the relatively large tumor sizes, with a diameter more than twice the FWHM of our PET system, no correction for partial volume effects was performed.
Semi-quantitative analysis of summed PET images (in the time frame where AMT uptake peaked; see below) was performed using the mean standardized uptake value (SUV). The SUV calculation relates tracer concentration in tissue to the dose injected and the patient’s mass (SUV = tissue concentration in ROI [kBq/cm3]/injected dose per weight [MBq/kg]).
Compartmental modeling and identifiability analysis
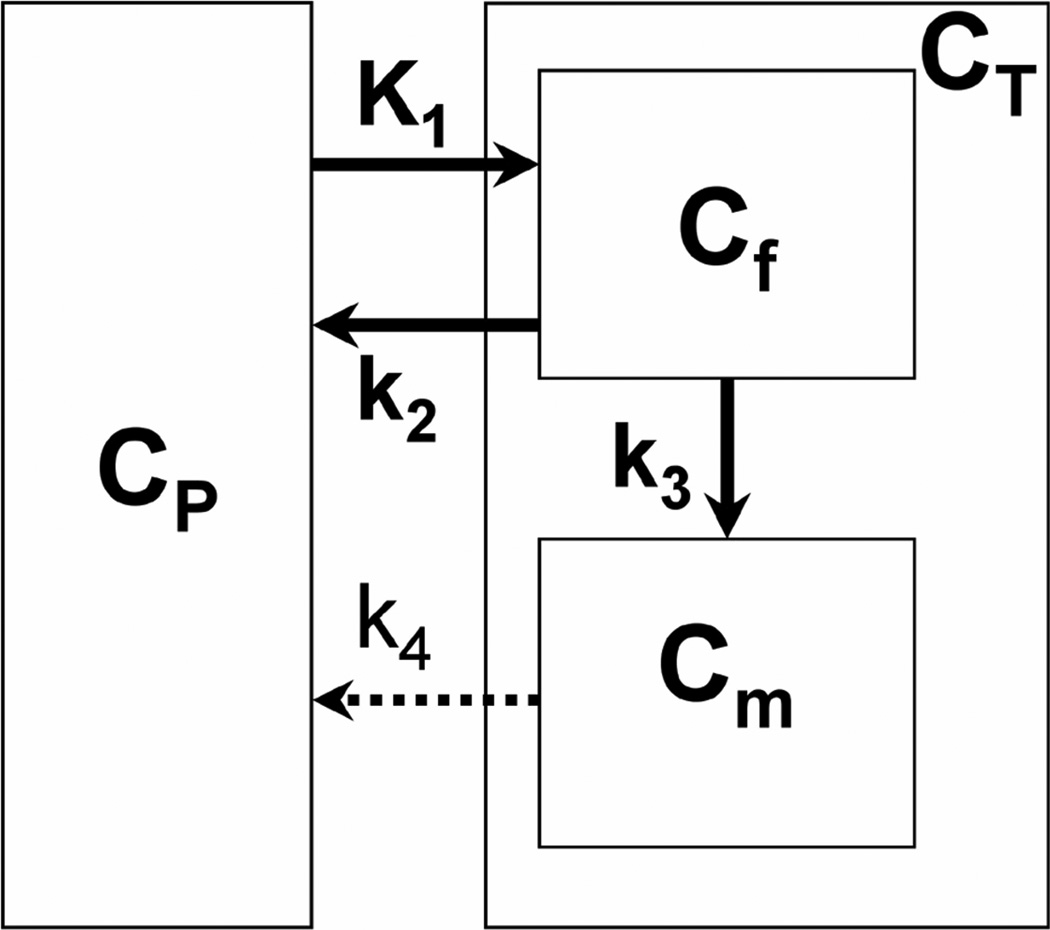
The tracer kinetics of AMT in breast tumors were assessed using a three-compartment model (i.e., blood plus two tissue compartments, see Figure 1) characterized by the parameter vector K1–k4 as well as the blood volume (BV). The rate constant K1 (mL/g/min) represents the forward and k2 (min−1) represents the reverse combined transport of AMT across the blood vessel, interstitial space, and cell membrane into the cytoplasm, in which it comprises the free compartment (Cf). Forward tryptophan transport may be facilitated by high tumoral blood flow and up-regulation of the LAT1 transporter and/or the recently described high affinity tryptophan-specific transporter [28,29]; reverse transport may be due to efflux of unmetabolized tryptophan back to the blood. Irreversible enzymatic conversion of AMT to its metabolites and accumulation in the metabolic compartment (Cm) is characterized by the metabolic rate constant k3 (min−1); this parameter may increase in case of tryptophan metabolism via the kynurenine and serotonin pathways, facilitated by IDO and/or TPH1, respectively, if the products are trapped in tissue as studied in the brain [19,20,22]. Finally, the rate constant k4 (min−1) characterizes the reverse transport of AMT metabolites (e.g., kynurenine and other metabolites formed as a result of IDO activity) across the interstitial space and blood vessel back into the blood pool. The apparent efflux rate constant k2’ can be then calculated as (k2 + k3k4/(k3+k4)), yielding the apparent volume of distribution VD’ (= K1/k2’) which represents the ratio of tracer concentrations in tissue and blood at dynamic equilibrium.
Figure 1.
Three-compartment model for alpha-[11C]methyl-L-tryptophan (AMT) kinetics in breast tumor tissue using first-order rate constants. The rate constant K1 (mL/g/min) represents the forward and k2 (min−1) represents the reverse combined transport of AMT across the blood vessel, interstitial space, and cell membrane into the cytoplasm, in which it comprises the free compartment (Cf). Irreversible enzymatic conversion of AMT to its metabolites and accumulation in the metabolic compartment (Cm) is characterized by the metabolic rate constant k3 (min−1). Finally, the rate constant k4 (min−1) characterizes the reverse transport of AMT metabolites across the interstitial space and blood vessel back into the blood pool. CP: plasma compartment; CT: tissue compartment.
The structure of the linearized model in the neighborhood of the computed solution was used for analysis of parameter identifiability. A singular value decomposition of the parameter sensitivity matrix (Xij = dfi/dpj, where fi(pj) is the tissue model function at time i dependent on the parameter vector pj) was computed, yielding the condition number (CN) of the model [26]. The CN of a model with orthonormal parameters is unity; hence, CN equals 1 in the ideal case. It was determined empirically [30] that weak dependencies are associated with CNs below 10, whereas strong dependencies are associated with CNs greater than 20. Finally, the quality of the fits was assessed based on the “Goodness-of-Fit” (GoF) measure which is defined as 1 – SSQ/SSQmean, where SSQ is the sum-of-squares of residuals with respect to the compartmental model and SSQmean is the sum-of-squares of residuals with respect to the curve mean. The closer the value of GoF approximates the value of 1.0, the better the fit.
Immunostaining
Formalin-fixed, paraffin-embedded sections (5 µm) of both breast cancer and benign breast disease were used for immunostaining for three proteins that may play a role in tumoral tryptophan uptake and metabolism: LAT1, IDO, and TPH1. Sections were available from 5 of the 9 breast cancer patients (patient #1, 2, 6, 8, and 9 in Table 1) and 14 benign breast disease controls. For patient #9, we stained sections both from the original breast surgery specimen and a brain metastasis resected 5 years later. Control sections were obtained from patients with benign breast disease who underwent biopsy for a suspicious finding on mammography. Twelve out of the 14 control cases were diagnosed with fibrocystic disease and the remaining 2 with fibroadenoma.
All sections were deparaffinized in xylene and rehydrated, followed by epitope retrieval performed by heating in 0.1M citrate buffer (pH 6.0) for 30 min. Sections were permeabilized with 0.5% (v/v) Triton X-100 in PBS and blocked in 4% normal donkey serum, 0.05% Triton X-100 in PBS. Primary antibodies used were as follows: rabbit polyclonal anti-human IDO (AB9898, Millipore Corporation, Billerica, MA), rabbit polyclonal anti-SLC7A5/LAT1 (ab85226, Abcam, Cambridge, MA), and rabbit polyclonal anti-TPH1 (ab78969, Abcam, Cambridge, MA). Sections were incubated overnight at 4°C with primary antibody at 1:100 dilution in blocking solution. Secondary detection employed Vectastain Elite ABC Kit (PK-6101, Vector Laboratories, Burlingame, CA) and DAB Enhanced Liquid Substrate System (D3939, Sigma-Aldrich, St. Louis, MO). After immunostaining, sections were counterstained with hematoxylin. Adjacent sections from each specimen were stained with hematoxylin and eosin. Sections were imaged at 20× magnification using an Olympus BX51 microscope (Olympus Corporation, Center Valley, PA) equipped with a DP25 color digital camera.
Immunohistochemical analysis
For semi-quantitative analysis, staining intensity of tumor and duct cells was rated separately from 0 to 3 (0 = no staining; 1 = mild staining; 2 = moderate staining; 3 = strong staining) in at least 3 representative 20× magnification fields of view of all samples by experienced pathologists who were blinded to the identity of the specimens. In addition, % frequency of stained tumor cells was estimated.
Statistical analysis
Tumoral SUVs and VD’ as well as K1 values were correlated using Pearson’s correlation and linear regression analyses. A p value less than 0.05 was considered significant.
RESULTS
Time-activity curves and identifiability of compartmental model parameters
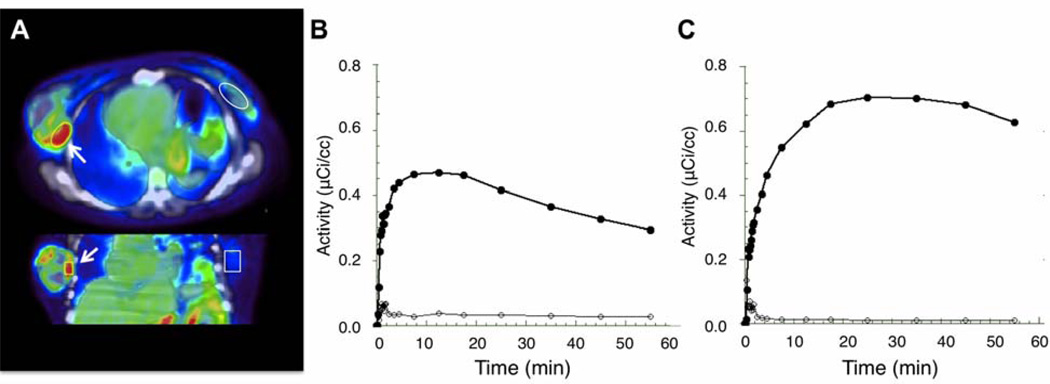
All tumors showed high AMT uptake compared to surrounding breast tissue (Figure 2A). Analysis of time-activity curves showed two distinct patterns of AMT accumulation in tumor tissue. Most of the subjects (G1: N1 = 7; patients #1–7 in Table 1) showed initial tracer accumulation peaking at 5–20 min after injection, followed by a steady decline (Figure 2B). The washout of the AMT tracer in tumor tissue beyond the initial time period indicates that most of the tracer was only transiently bound in tissue with negligible irreversible binding. In the remaining 2 patients (G2, patients #8 and 9 in Table 1), a more prolonged accumulation of AMT, reaching a higher maximal value, was seen with a peak at 20–30 min, followed by a moderate decline (Figure 2C). The observed time-activity curves were consistent with two reversible tissue compartments with different efflux constants (Figure 1), as initial fits using a simple two-compartment (i.e., blood and one tissue compartment) model (2Comp: k3 = k4 = 0) with only one reversible compartment resulted in poor fits with biased residuals (GoF = 0.91 ± 0.4).
Figure 2.
(A) AMT tracer accumulation in breast tumor between 5 and 20 minutes post injection. There is a good contrast between AMT tracer accumulation in tumor tissue and surrounding breast tissue. (B). Corresponding time-activity curve (black circles) shows high initial uptake, which peaks within the first 20 minutes with subsequent steady decline, in the same tumor. This time course was seen in most patients with breast cancer. (C). In two subjects a more prolonged accumulation was seen with a peak at 20–30 minutes post injection. The curves with open circles indicate the very low uptake in normal breast tissue (contralateral to the tumor).
Application of a three-compartment model (K1 – k4, CBV) yielded excellent fits (3Comp: GoF = 0.97 ± 0.15; p = 0.01 versus 2Comp) that were also superior to a three-compartment model with one irreversible compartment (k4 =0; GoF = 0.94 ±0.3, p = 0.035 versus 3Comp). Although application of the three-compartment model improved the data fit, it also resulted in poor identifiability of the overall parameter vector for patients with both kinetic patterns. The average CN for the three-compartmental model was calculated as 38.4 ± 11.5, indicating instability of the parameter values and rendering them highly dependent on noise and methodological parameters such as starting values and termination criterion. In contrast, the identifiability of the simple two-compartmental model (K1, k2, BV) was excellent with an average CN of 4.2 ± 0.9. Thus, in order to stabilize the three-compartment model fit, the K1 and BV parameters were initially derived from the simple two-compartment model and subsequently preset in the full three-compartment model fit, thus reducing the number of fitted parameters to three (k2 – k4). This resulted in a significant improvement in model identifiability, decreasing the CN to 16.6 ± 7.9. On the basis of this identifiability analysis, this stabilized three-compartment model was chosen for analysis of AMT kinetics in breast tumor tissue.
AMT kinetics in breast tumors
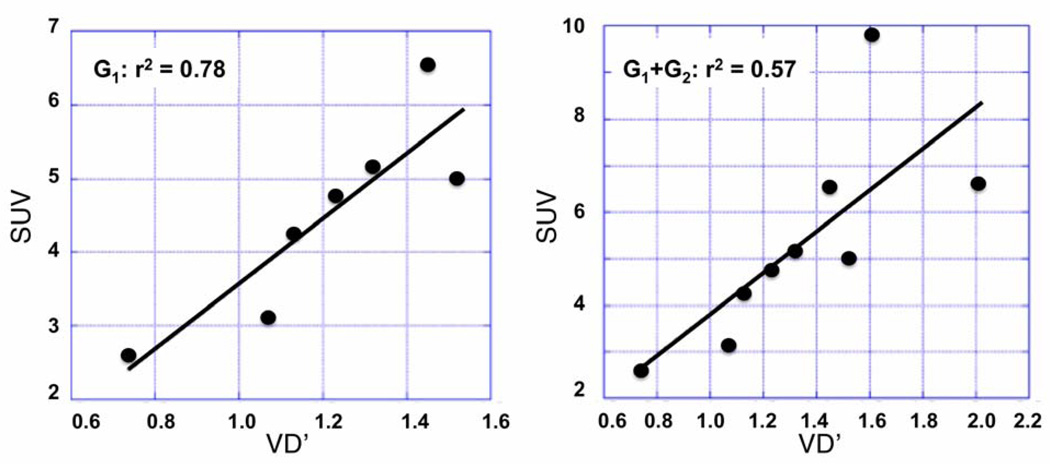
In both tumor groups (G1 with an early decline and G2 with a late decline of AMT accumulation), individual rate constants were fitted to dynamic time–activity curves obtained from tumor tissue, and the apparent volume of distribution (VD’= K1/[k2 + k3k4/(k3+k4)]) was calculated. Moreover, the SUV was calculated in both groups using the peak times of the time-activity curves (5–20 min for G1 and 20–30 min for G2). As expected from the time-activity curves (see Figure 2B–C), the 2 patients with a late decline had the highest SUV values. The SUVs (ranging widely between 2.6–9.8 across tumors; mean: 5.3) showed a strong positive correlation with VD’ values in both the whole group (G1+G2; r2=0.57, p = 0.02) and the subgroup (G1) with early decline (r2 = 0.78, p = 0.009; Figure 3); no significant correlation was found between SUV and K1 values in the whole group (r2= 0.22, p=0.21), but a significant positive correlation was found between SUV and K1 values in the subgroup with early decline (G1; r2=0.69, p=0.02). When both VD’ and K1 were entered as predictors of SUV in a regression analysis of the whole group, only VD’ (but not K1) showed independent partial correlation with SUV (for VD’: r2=0.61, p=0.02; for K1: r2=0.31, p=0.15). These results indicated that in clinical application a whole body scan starting at 5 min post injection can be used to predict the distribution volume (VD’) of AMT in tumor tissue, while SUV is a less accurate measure of K1.
Figure 3.
Correlations between VD’ values and SUV. Left panel: 7 patients (Group 1; G1) with an early plateau and then decline of tumoral radioactivity 20 min after injection; right panel: whole patient group (n=9).
Although the limited number of tumors precluded a systematic correlation between tumor types and AMT kinetic variables, we observed that all 6 invasive ductal carcinomas (patients #1–6 in Table 1) had higher VD’ and SUV values (range: VD’: 1.23–2.01; SUV: 4.75–9.81) than the three other rarer tumor types (two carcinosarcomas, one invasive micropapillary carcinoma; VD’: 0.74–1.13; SUV: 2.59–4.24; Table 1).
Immunostaining with LAT-1, IDO, and TPH1
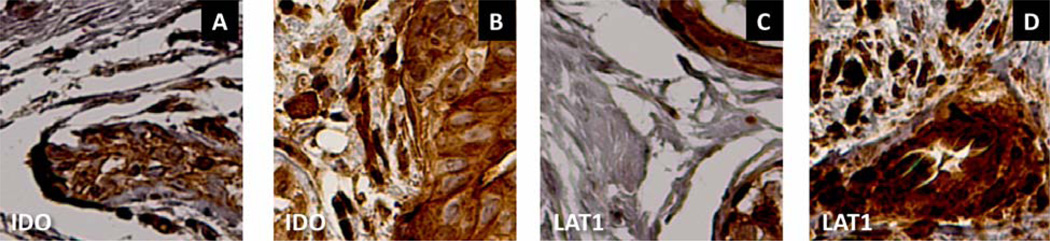
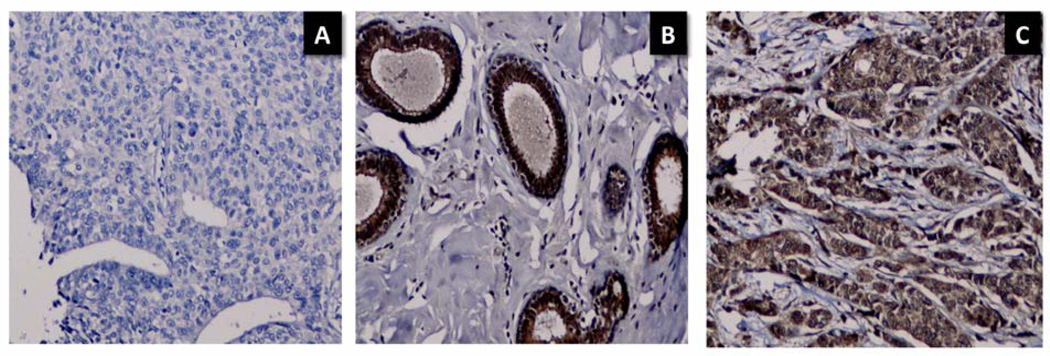
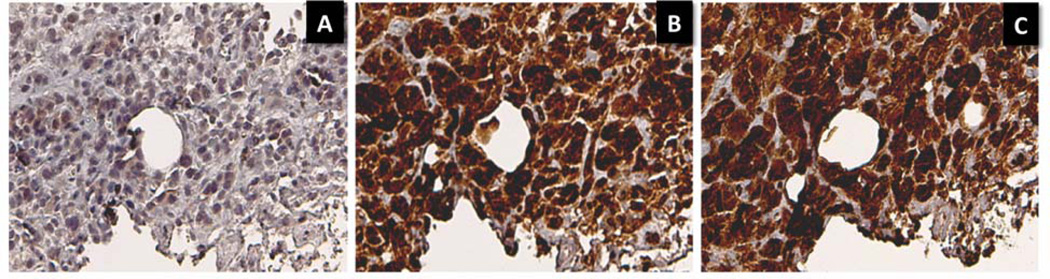
In both benign breast disease and primary breast cancer, immunoreactivity for LAT-1, IDO, and TPH1 was present in ductal epithelial cells (Figures 4, 6). However, in breast cancer specimens, tumor cells infiltrating the parenchyma surrounding the ducts were also positive for LAT-1, IDO, and TPH1 (Figures 4–6). Tumor cell staining intensity was moderate to strong for all three antibodies in most breast cancer specimens (Table 2). The brain metastasis in patient #9 also showed moderate to strong LAT1 and IDO staining and mild TPH1 staining in tumor cells.
Figure 4.
IDO and LAT1 staining in benign breast disease (A, C) vs. breast cancer (carcinosarcoma, patient #1 [B, D]). A. Ductal cells in benign breast disease demonstrate IDO immunoreactivity. B. Section from the breast cancer shows extensive IDO immunoreactivity in both ductal cells and infiltrating tumor cells. C. LAT1 staining in duct cells from benign breast disease. D. Strong LAT1 staining in both duct and infiltrating tumor cells in breast cancer. Original magnification 20×.
Figure 6.
TPH1 immunostaining in benign breast disease vs. invasive ductal breast carcinoma (patient #9; original resected breast cancer specimen). A. No-primary control staining. B. Ductal epithelial cells from benign breast disease demonstrate TPH1 immunoreactivity. C. Both ductal epithelial and infiltrating tumor cells show extensive, strong TPH1 immunoreactivity. Original magnification 20×.
Table 2.
Immunohistochemistry results from the tumor specimens (patient numbers refer to table 1)
| Patient | Specimen | Histology | LAT1 | IDO | TPH1 | |||
|---|---|---|---|---|---|---|---|---|
| intensity | % positive | intensity | % positive | intensity | % positive | |||
| 1. | breast | carcinosarcoma | 3+ | 100 | 2–3+ | 100 | n.a. | n.a. |
| 2. | breast | invasive micropapillary carcinoma | 3+ | 100 | 3+ | 100 | 2–3+ | 100 |
| 6. | breast | invasive ductal carcinoma | 3+ | 100 | 3+ | 100 | 3+ | 100 |
| 8. | breast | invasive ductal carcinoma | 2–3+ | 100 | 3+ | 100 | 3+ | 80 |
| 9. | breast | invasive ductal carcinoma | 2–3+ | 100 | 1–2+ | 100 | 3+ | 100 |
| brain metastasis | 2–3+ | 100 | 2–3+ | 100 | 1+ | 100 | ||
Abbreviations LAT1: L-type amino acid transporter 1; IDO: indoleamine 2,3-dioxygenase; TPH1: tryptophan hydroxylase 1; staining intensity scores: 1+ = mild staining; 2+ = moderate staining; 3+ = strong staining; n.a.: no further specimen was available for staining.
DISCUSSION
This was a study to explore the potential use of AMT PET in detection of breast cancers of various types and grades, and also to study potential mechanisms of tryptophan accumulation in these tumors. The main results of the study are as follows: (1) Breast cancers show high AMT accumulation with a wide range of SUVs regardless of grade, histology type, or receptor status. (2) Most breast cancers show a rapid influx of the radiotracer and an early peak (less than 20 min after AMT tracer injection) followed by a subsequent decline of radioactivity, as measured by dynamic AMT-PET scanning. This pattern of AMT tracer kinetics is different from that previously seen in gliomas and lung cancers, which typically show a more prolonged AMT accumulation [24,31]. (3) SUVs, obtained at peak concentrations (5–20 min after tracer injection in most tumors), are excellent predictors of the distribution volume, a measure of the ratio of tracer concentrations in tissue and blood at dynamic equilibrium. (4) The findings also provide preliminary data suggesting that invasive ductal carcinomas may have particularly high AMT accumulation. However, it is not clear if this is related to tumor type or is a reflection of different cellularity. Correlations between tumor type and AMT uptake need to be performed in a much larger patient cohort that also includes some other common tumor types. (5) All available breast cancer tissues showed extensive, moderate to strong expression of LAT1, IDO, and TPH1, which may play a role in tryptophan transport and tumoral metabolism and are potential targets of specific inhibitors of these proteins.
Entry of L-tryptophan into IDO-expressing breast cancers is mostly driven by system L [3,10], a transport protein complex consisting of the light chain LAT1 (and also LAT2, which shows little expression in cancers) and the heavy chain CD98. Increased activity of system L supports cell growth [12] and also provides the substrate for kynurenine and serotonin synthesis in breast cancer cells. Consistent with our results, IDO expression was found in all surgically-resected tumor specimens in a study of 30 patients with breast cancer [4]. The authors noted that strong expression of IDO correlates with advanced tumor stage. Another study showed differential IDO mRNA expression in breast cancer samples, one-half of which contained at least twice the IDO level found in normal reference breast samples [32]. The present study is biased toward high-grade tumor and advanced disease stage. This may explain why all available tissue samples showed moderate to strong, extensive protein expression of LAT1, IDO, and also TPH1, whose upregulation may indicate a poor prognosis in breast cancers [4,6,10,11]. On the other hand, we cannot make conclusions for IDO or TPH1 expression in low-grade tumors and early disease stage from the present data.
All breast cancers in our study showed a rapid AMT influx on dynamic PET images, and all but two samples showed an early peak followed by decreased activity consistent with efflux of radiolabeled molecules. This rapid washout may involve both unmetabolized AMT as well as metabolites derived from AMT (most likely in the form of kynurenine metabolites and derivatives of the serotonin pathway). Increased kynurenine and decreased tryptophan levels reported in the blood of breast cancer patients is consistent with an increased kynurenine efflux from the site of tumoral tryptophan degradation [33]. This process would require an effective tryptophan/kynurenine exchange mechanism that keeps the amount of kynurenine metabolites relatively low in the tumor microenvironment. Such tryptophan influx/kynurenine efflux cycle may be executed by LAT1 itself [34,35]. Therefore, high LAT1 expression may result in increased forward tryptophan transport, but also, depending on the dominant pathway of metabolism, a variable amount of radioactive products may be transported out of the tissue in the form of kynurenine metabolites by the same transport system. Therefore, it is difficult to predict how high LAT1 expression would affect individual kinetic parameters such as K1, k2, or k4. Still, coupling of high LAT1 expression with strong IDO activity would be consistent with a system that takes up available tryptophan rapidly, converts it to kynurenine and/or serotonin, and then removes the metabolites in exchange to further tryptophan absorption. In addition, there is evidence that IDO induces expression of a novel high affinity tryptophan transporter, that is biochemically distinct from LAT1, in mouse and human tumor cell lines [29]. A similar high affinity, tryptophan-specific transport system has also been described in human macrophages [28]. In contrast, more prolonged tumoral AMT accumulation, seen in two ductal carcinomas (including one with multiple metastases), could indicate a less effective kynurenine efflux process and/or retention of the radiotracer in the form of alpha-methyl-serotonin, which is distributed in the same tissue compartment as serotonin itself [20]. This latter could contribute to prolonged AMT accumulation in patient #9 with the advanced metastatic disease, where IDO expression was relatively mild while TPH1 showed a strong expression pattern in all tumor cells. In those with strong, extensive IDO expression, tryptophan depletion coupled with tumoral accumulation of kynurenine metabolites may contribute to suppression of anti-tumor immune response via blocking T-cell activation and promoting regulatory T-cells [36,37]. Since AMT PET cannot differentiate between tryptophan metabolism via the kynurenine vs. serotonin pathways, further tissue studies measuring enzyme activity and metabolite concentrations could be helpful to better understand the metabolic fate of tryptophan in various stages of breast cancer.
Altogether, our results indicate that LAT1-, IDO- and TPH1-positive stage II-IV breast cancers show rapid AMT uptake on PET, followed by an early peak and then efflux within 20 min after tracer injection in most cases. Since SUV values obtained from images summed 5–20 min after injection show a strong correlation with VD’ values, future studies could utilize a simplified, shorter scanning protocol to obtain AMT SUV values without full kinetic analysis requiring blood input function. Using such a simple image acquisition protocol, one could further address the histopathological correlates and prognostic significance of lower vs. higher tumoral AMT SUV values. Further studies of AMT PET scanning in breast and other solid cancers could determine the potential role of this imaging modality for future clinical trials of emerging LAT1, IDO, and TPH inhibitors.
Figure 5.
Strong IDO and LAT1 staining in an invasive ductal carcinoma (patient #8). A. No-primary control staining. B. IDO staining at 3+ intensity in 100% of tumor cells. C. LAT1 staining at 3+ intensity in 100% of cells. Original magnification 20×.
Acknowledgment
This project has been funded in part with Federal Funds from the National Cancer Institute, National Institutes of Health, under Contract No. NO1-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The study was also supported by a grant from the National Cancer Institute (#CA123451 to C. Juhasz).
The authors thank Cathie Germain MA, Melissa Burkett, CNMT, Jane Cornett, RN, Anna DeBoard, RN, Kelly Forcucci, RN, Carole Klapko, CNMT, Mei-li Lee, MS, Xin Lu, MS, Andrew Mosqueda CNMT, Galina Rabkin, CNMT, and Angela Wigeluk, CNMT, for their assistance in patient recruitment, scheduling, and preparation, as well as for their technical assistance in performing the PET studies. We also thank Sam Kiousis for his skilled technical assistance with the immunohistochemistry studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 2.Munn DH, Mellor AL. IDO and tolerance to tumors. Trends Mol Med. 2004;10:15–18. doi: 10.1016/j.molmed.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Travers MT, Gow IF, Barber MC, Thomson J, Shennan DB. Indoleamine 2,3-dioxygenase activity and L-tryptophan transport in human breast cancer cells. Biochim Biophys Acta. 2004;1661:106–112. doi: 10.1016/j.bbamem.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Sakurai K, Amano S, Enomoto K, Kashio M, Saito Y, Sakamoto A, et al. Study of indoleamine 2,3-dioxygenase expression in patients with breast cancer. Gan To Kagaku Ryoho. 2005;32:1546–1549. [PubMed] [Google Scholar]
- 5.Jacquemier J, Bertucci F, Finetti P, Esterni B, Charafe-Jauffret E, Thibult ML, et al. High expression of indoleamine 2,3-dioxygenase in the tumour is associated with medullary features and favourable outcome in basal-like breast carcinoma. Int J Cancer. 2012;130:96–104. doi: 10.1002/ijc.25979. [DOI] [PubMed] [Google Scholar]
- 6.Pai VP, Marshall AM, Hernandez LL, Buckley AR, Horseman ND. Altered serotonin physiology in human breast cancers favors paradoxical growth and cell survival. Breast Cancer Res. 2009;11:R81. doi: 10.1186/bcr2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuchs BC, Bode BP. Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol. 2005;15:254–266. doi: 10.1016/j.semcancer.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Shennan DB, Thomson J, Barber MC, Travers MT. Functional and molecular characteristics of system L in human breast cancer cells. Biochim Biophys Acta. 2003;1611:81–90. doi: 10.1016/s0005-2736(03)00028-2. [DOI] [PubMed] [Google Scholar]
- 9.Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, Hisada T, et al. l-type amino acid transporter 1 and CD98 expression in primary and metastatic sites of human neoplasms. Cancer Sci. 2008;99:2380–2386. doi: 10.1111/j.1349-7006.2008.00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esseghir S, Reis-Filho JS, Kennedy A, James M, O'Hare MJ, Jeffrey R, et al. Identification of transmembrane proteins as potential prognostic markers and therapeutic targets in breast cancer by a screen for signal sequence encoding transcripts. J Pathol. 2006;210:420–430. doi: 10.1002/path.2071. [DOI] [PubMed] [Google Scholar]
- 11.Ring BZ, Seitz RS, Beck R, Shasteen WJ, Tarr SM, Cheang MCU, et al. Novel prognostic immunohistochemical biomarker panel for estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3039–3047. doi: 10.1200/JCO.2006.05.6564. [DOI] [PubMed] [Google Scholar]
- 12.Shennan DB, Thomson J. Inhibition of system L (LAT1/CD98hc) reduces the growth of cultured human breast cancer cells. Oncol Rep. 2008;20:885–889. [PubMed] [Google Scholar]
- 13.Kaji M, Kabir-Salmani M, Anzai N, Jin CJ, Akimoto Y, Horita A, et al. Properties of L-type amino acid transporter 1 in epidermal ovarian cancer. Int J Gynecol Cancer. 2010;20:329–336. doi: 10.1111/IGC.0b013e3181d28e13. [DOI] [PubMed] [Google Scholar]
- 14.Jia L, Schweikart K, Tomaszewski J, Page JG, Noker PE, Buhrow SA, et al. Toxicology and pharmacokinetics of 1-methyl-d-tryptophan: absence of toxicity due to saturating absorption. Food Chem Toxicol. 2008;46:203–211. doi: 10.1016/j.fct.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Q, Yang Q, Sun W, Vogel P, Heydorn W, Yu XQ, et al. Discovery and characterization of novel tryptophan hydroxylase inhibitors that selectively inhibit serotonin synthesis in the gastrointestinal tract. J Pharmacol Exp Ther. 2008;325:47–55. doi: 10.1124/jpet.107.132670. [DOI] [PubMed] [Google Scholar]
- 16.Imai H, Kaira K, Oriuchi N, Shimizu K, Tominaga H, Yanagitani N, et al. Inhibition of Ltype amino acid transporter 1 has antitumor activity in non-small cell lung cancer. Anticancer Res. 2010;30:4819–4828. [PubMed] [Google Scholar]
- 17.Yi YM, Zhang G, Zeng J, Huang SC, Li LL, Fang R, et al. A new tumor vaccine: FAPτ-MT elicits effective antitumor response by targeting indolamine2,3-dioxygenase in antigen presenting cells. Cancer Biol Ther. 2011;11:866–873. doi: 10.4161/cbt.11.10.15179. [DOI] [PubMed] [Google Scholar]
- 18.Diksic M, Tohyama Y, Takada A. Brain net unidirectional uptake of alpha-[14C]methyl-L-tryptophan (alpha-MTrp) and its correlation with regional serotonin synthesis, tryptophan incorporation into proteins, and permeability surface area products of tryptophan and alpha-MTrp. Neurochem Res. 2000;25:1537–1546. doi: 10.1023/a:1026654116999. [DOI] [PubMed] [Google Scholar]
- 19.Tohyama Y, Takahashi S, Merid MF, Watanabe A, Diksic M. The inhibition of tryptophan hydroxylase, not protein synthesis, reduces the brain trapping of alpha-methyl-L-tryptophan: an autoradiographic study. Neurochem Int. 2002;40:603–610. doi: 10.1016/s0197-0186(01)00132-2. [DOI] [PubMed] [Google Scholar]
- 20.Diksic M. alpha-Methyl tryptophan as a tracer for in vivo studies of brain serotonin system, from autoradiography to positron emission tomography. J Chem Neuroanat. 1992;5:349–354. doi: 10.1016/0891-0618(92)90026-m. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu T, Nomiyama S, Hirata F, Hayaishi O. Indoleamine 2,3-dioxygenase. Purification and some properties. J Biol Chem. 1978;253:4700–4706. [PubMed] [Google Scholar]
- 22.Chugani DC, Muzik O. α̃[C-11]Methyl-L-tryptophan PET maps brain serotonin synthesis and kynurenine pathway metabolism. J Cereb Blood Flow Metab. 2000;20:2–9. doi: 10.1097/00004647-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Batista CE, Juhász C, Muzik O, Kupsky WJ, Barger G, Chugani HT, et al. Imaging correlates of differential expression of indoleamine 2,3-dioxygenase in human brain tumors. Mol Imaging Biol. 2009;11:460–466. doi: 10.1007/s11307-009-0225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juhász C, Muzik O, Lu X, Jahania MS, Soubani AO, Khalaf M, et al. Quantification of tryptophan transport and metabolism in lung tumors using PET. J Nucl Med. 2009;50:356–363. doi: 10.2967/jnumed.108.058776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakraborty PK, Mangner TJ, Chugani DC, Muzik O, Chugani HT. A high-yield and simplified procedure for the synthesis of alpha-[11C]methyl-L-tryptophan. Nucl Med Biol. 1996;23:1005–1008. doi: 10.1016/s0969-8051(96)00127-8. [DOI] [PubMed] [Google Scholar]
- 26.Muzik O, Behrendt DB, Mangner TJ, Chugani HT. Design of a protocol for quantitative brain FDG studies with PET not requiring invasive blood sampling [abstract] J Nucl Med. 1994;35:104P. [Google Scholar]
- 27.Suhonen-Polvi H, Ruotsalainen U, Kinnala A, Bergman J, Haaparanta M, Teras M, et al. FDG-PET in early infancy: simplified quantification methods to measure cerebral glucose utilization. J Nucl Med. 1995;36:1249–1254. [PubMed] [Google Scholar]
- 28.Seymour RL, Ganapathy V, Mellor AL, Munn DH. A high-affinity, tryptophan-selective amino acid transport system in human macrophages. J Leukoc Biol. 2006;80:1320–1327. doi: 10.1189/jlb.1205727. [DOI] [PubMed] [Google Scholar]
- 29.Silk JD, Lakhal S, Laynes R, Vallius L, Karydis I, Marcea C, et al. IDO induces expression of a novel tryptophan transporter in mouse and human tumor cells. J Immunol. 2011;187:1617–1625. doi: 10.4049/jimmunol.1000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belsley D. Conditioning diagnostics: collinearity and weak data in regression. New York: Wiley & Sons; 1991. [Google Scholar]
- 31.Juhász C, Chugani DC, Muzik O, Wu D, Sloan AE, Barger G, et al. In vivo uptake and metabolism of alpha-[11C]methyl-L-tryptophan in human brain tumors. J Cereb Blood Flow Metab. 2006;26:345–357. doi: 10.1038/sj.jcbfm.9600199. [DOI] [PubMed] [Google Scholar]
- 32.Godin-Ethier J, Pelletier S, Hanafi LA, Gannon PO, Forget MA, Routy JP, et al. Human activated T lymphocytes modulate IDO expression in tumors through Th1/Th2 balance. J Immunol. 2009;183:7752–7760. doi: 10.4049/jimmunol.0901004. [DOI] [PubMed] [Google Scholar]
- 33.Lyon DE, Walter JM, Starkweather AR, Schubert CM, McCain NL. Tryptophan degradation in women with breast cancer: a pilot study. BMC Res Notes. 2011;4:156. doi: 10.1186/1756-0500-4-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaper T, Looger LL, Takanaga H, Platten M, Steinman L, Frommer WB. Nanosensor detection of an immunoregulatory tryptophan influx/kynurenine efflux cycle. PLoS Biol. 2007;5:e257. doi: 10.1371/journal.pbio.0050257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serbecic N, Lahdou I, Scheuerle A, Höftberger R, Aboul-Enein F. Function of the tryptophan metabolite, L-kynurenine, in human corneal endothelial cells. Mol Vis. 2009;15:1312–1324. [PMC free article] [PubMed] [Google Scholar]
- 36.Zamanakou M, Germenis AE, Karanikas V. Tumor immune escape mediated by indoleamine 2,3-dioxygenase. Immunol Lett. 2007;111:69–75. doi: 10.1016/j.imlet.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Newton RC, Friedman SM, Scherle PA. Indoleamine 2,3-dioxygenase, an emerging target for anti-cancer therapy. Curr Cancer Drug Targets. 2009;9:938–952. doi: 10.2174/156800909790192374. [DOI] [PubMed] [Google Scholar]