Abstract
Parkinson’s disease (PD) is characterized by widespread alpha-synuclein pathology and neuronal loss, primarily of the nigrostriatal dopaminergic neurons. Inflammation has been implicated in PD, and alpha-synuclein can initiate microglial activation; however, the kinetics and distribution of inflammatory responses to alpha-synuclein overexpression in vivo are not well understood. We have examined the regional and temporal pattern of microglial activation and pro-inflammatory cytokine production in mice over-expressing wild-type human alpha-synuclein driven by the Thy1-promoter (Thy1-aSyn mice). Increased number of activated microglia, and increased levels of TNF-α mRNA and protein were first detected in the striatum (1 month of age) and later in the substantia nigra (5–6 months), but not cerebral cortex or cerebellum; in contrast, IL-1β and TGFβ remained unchanged in striatum and substantia nigra at all ages examined. Microglial activation persisted up to 14 months of age in these regions and only minimal increases were observed in other regions at this later age. Increased concentrations of serum TNF-α were observed at 5–6 months, but not 1 month of age. The expression of toll-like receptors (TLR) 1, TLR 4 and TLR 8, which are possible mediators of microglial activation, was increased at 5–6 months in the substantia nigra but not in the cerebral cortex, and TLR 2 was increased in the substantia nigra at 14 months of age. With the exception of a slight increase in the striatum of 14 months old Thy1-aSyn mice, MHCII staining was not detected in the regions and ages examined. Similarly, peripheral CD4 and CD8-postive T cells were increased in the blood but only at 22 months of age, suggesting later involvement of the adaptive immune response. These data indicate that, despite the presence of high levels of alpha-synuclein in other brain regions, alpha-synuclein overexpression caused a selective early inflammatory response in regions containing the axon terminals and cell bodies of the nigrostriatal pathway. Our results suggest that specific factors, possibly involving a regionally and temporally selective increase in TLRs, mediate alpha-synuclein-induced inflammatory responses in the SN, and may play a role in the selective vulnerability of nigrostriatal dopaminergic neurons in PD.
Keywords: Parkinson’s disease, alpha-synuclein, overexpression, transgenic, neurodegeneration, microglia, cytokines, inflammation
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by degeneration of dopaminergic neurons in the substantia nigra (SN), pars compacta and the resulting loss of their axon terminals in the striatum (Fearnley and Lees, 1991). In addition, widespread pathological changes, in particular, the accumulation and aggregation of alpha-synculein, are present in many central and peripheral neurons in PD (Braak et al., 2003). Evidence from epidemiological and animal studies suggest that neuroinflammatory changes participate in disease pathology (Czlonkowska et al., 2002; Dauer and Przedborski, 2003; Bartels and Leenders, 2007; Wahner et al., 2007a; Przedborski, 2010; Tansey and Goldberg, 2010). Postmortem analysis and brain imaging studies have revealed increased numbers of activated microglia in the SN of PD patients (McGeer et al., 1988; Mogi and Nagatsu, 1999; Gerhard et al., 2006; Ouchi et al., 2009). Increased expression of pro-inflammatory cytokines that ultimately may lead to neuronal injury (Theodore et al., 2008) has been observed in SN and striatum, cerebrospinal fluid, and serum of PD patients (Mogi et al., 1994; Nagatsu et al., 2000; Scalzo et al., 2009). Polymorphisms in the TNF-a and IL-1 genes produce a 2–3 fold increased risk for PD (Wahner et al., 2007b), and early use of non-steroidal anti-inflammatory agents is protective (Gao et al., 2011). Additionally, there is also evidence for activation of adaptive immune responses in PD, as shown by increased numbers of CD4-positive (CD4+) and CD8-positive (CD8+) T cells in postmortem samples of PD brains (Brochard et al., 2009). Together, these data suggest that chronic inflammation may contribute to the pathogenesis of PD.
Alpha-synuclein has been associated with several neurodegenerative diseases, collectively known as synucleinopathies, which include PD (Trojanowski and Lee, 2003). Missense mutations and multiplication in the gene encoding alpha-synuclein cause autosomal dominant familial PD, and polymorphisms are strongly associated with an increased risk for the disease (Polymeropoulos et al., 1997; Kruger et al., 1998; Chartier-Harlin et al., 2004; Fuchs et al., 2007; Rhodes et al., 2011). The presence of alpha-synuclein in Lewy bodies, the prototypical insoluble inclusions in the brain of patients with PD, further strengthen the link between this protein and sporadic PD (Braak et al., 2003). Treatment of microglial cultures and monocytic cell lines with exogenous alpha-synuclein resulted in microglial activation and the release of the pro-inflammatory molecules TNF, IL-1, IL-6, COX2, NOX2 and iNOS (Zhang et al., 2005; Klegeris et al., 2008; Su et al., 2008), pointing to a role of alpha-synuclein in microglial activation in vitro. Furthermore, localized alpha-synuclein overexpression in vivo in mice, using viral vectors (Theodore et al., 2008; Sanchez-Guajardo et al., 2010) or driven by the tyrosine-hydroxylase promoter (Su et al., 2008; Su et al., 2009) can cause early and progressive microglial activation, T cell activation, and pro-inflammatory cytokine production in the SN and striatum even before the onset of neuronal pathology.
Collectively, these data suggest a link between alpha-synuclein and inflammation in PD pathogenesis. However, little is known about the time course and distribution of microglial activation and cytokine production in response to widespread alpha-synuclein pathology, as observed in the brain of patients with PD. To address this issue, we have examined the location, onset, progression and mechanisms of microglial activation and inflammatory mediators in mice overepressing wild-type (WT) human alpha-synuclein driven by the Thy-1 promoter (Thy1-aSyn mice; Rockenstein et al., 2002). The Thy1-aSyn mice display widespread over-expression of alpha-synuclein in neurons across the brain, proteinase K resistant aggregates in various brain regions including the SN (Fernagut et al., 2007), progressive synaptic and behavioral anomalies, and delayed loss of striatal dopamine that coincides with l-dopa responsive behavioral deficits (Fleming and Chesselet, 2006; Wu et al. 2010; Lam et al., 2011; Chesselet and Richter, 2011). Microglial activation and expression of activation related molecules were analyzed in striatum, SN, cerebral cortex, and cerebellum in very young mice (1 month of age), when motor and non-motor behavioral deficits are present but dopamine levels remain normal (5–6 months), and in the presence of striatal neuropathology and dopamine loss (14 months) (Lam et al. 2011). We show that despite broad over-expression of WT human alpha-synuclein, microglial activation and TNF-α production are regionally specific to the nigrostriatal pathway of young Thy1-aSyn mice, with alterations in the striatum preceding changes in the SN, and are paralleled by regionally specific increases in toll-like receptors (TLRs) that could play a role in the inflammatory changes induced by alpha-synuclein overexpression. In contrast to microglial activation and innate immune system changes that occurred many months before the onset of striatal dopamine loss, increases in adaptive immune involvement were not apparent until much older ages.
Materials and Methods
Animals
Thy1-aSyn mice were developed previously by the laboratory of Dr E. Masliah at UCSD and maintained on a hybrid C57BL/6-DBA/2 background as described previously (Rockenstein et al., 2002; Fleming et al., 2004; Fleming and Chesselet, 2006; Fernagut et al., 2007). The genotypes of all Thy1-aSyn and wild-type (WT) mice were determined by polymerase chain reaction (PCR) amplification analysis of tail DNA at one month of age and verified at the end of the experiment. Animals were maintained on a reverse light/dark cycle with lights off at 10 am. Food and water were available ad libitum. Animal care was conducted in accordance with the United States Public Health Service Guide for the Care and Use of Laboratory Animals. Male transgenic and wild-type (WT) littermates were used in this study.
Immunohistochemical analyses were performed in 1 month, 5–6 month and 14 month old male WT and Thy1-aSyn mice. The 5–6 month old mice were subjected to behavioral testing before sacrifice as part of another study but did not receive any treatment or injection. Mice were deeply anesthetized with sodium pentobarbital [100mg/kg, intraperitoneal (ip)] and transcardially perfused with 0.1M phosphate buffered saline (PBS; 137mM NaCl, 2.7mM KCl, 4.3mM Na2HPO4, 1.47mM KH2P04, pH 7.4), followed by 4% paraformaldehyde (PFA). Brains were quickly removed, post-fixed with PFA for 2 hours, cryoprotected in 30% sucrose in 0.1M PBS, frozen on powdered dry ice and stored at −80°C. Free-floating coronal sections (40μm) were cut on a cryostat and collected for analysis.
mRNA analysis was performed in separate cohorts of 1 month, 5–6 month (including a subset of animals that were part of the previous behavioral trial) and 14 month old male WT and Thy1-aSyn mice . Protein analysis was performed in a separate cohort of 5–6 month old male WT and Thy1-aSyn mice. Mice were deeply anesthetized with sodium pentobarbital [100mg/kg, ip] and blood was collected via retro-orbital bloodletting and cardiac puncture into tubes containing gel clot activator for serum analysis. Mice were trans-cardially perfused with 0.1M phosphate buffered saline (PBS), brains were quickly removed and placed on an ice-cold plate. Coronal blocks of the striatum, SN, cerebral cortex and cerebellum were micro-dissected using an acrylic mouse brain slicer matrix with 1.0mm coronal intervals (Mouse Brain Matrix, AL-1175; Roboz Surgical Instrument Co., USA). Tissue was frozen on powdered dry ice and stored at −80°C.
Alpha-synuclein expression in human T lymphocytes
Human peripheral blood samples were acquired after informed consent, in accordance with the University of California Los Angeles Institutional Review Board. After centrifugation on a ficoll hypaque gradient, the mononuclear cell layer was carefully removed and washed twice with PBS. T cells were isolated by negative selection (EasySep by StemCell), RNA was extracted using the Qiagen Mini RNA Isolation kit, quantified using the Invitrogen Ribogreen assay, and the cDNA was prepared using BioRad iScript. Alpha-synuclein mRNA expression was determined using the real-time reaction with BioRad’s EvaGreen supermix. The primer sequences were designed with the aid of Primer3 Design software and synthesized at Integrated DNA Technologies. The specificity was confirmed by examining the efficiency of the PCR run, the housekeeping gene had an efficiency of 97.3% and the SNCA had an efficiency of 95.6%. The specific primer sequences for the 36B4 housekeeping gene are: AATCTGCAGACAGACACTGG (forward) and TCTACAACCCTGAAGTGCTTGAT (reverse), and the primers for alpha-synuclein (SNCA) are AAATGTTGGAGGAGCAGTGG (forward) and TCCAGAATTCCTTCCTGTGG (reverse).
Immunohistochemistry for alpha-synuclein
Alpha-synuclein was evaluated by immunohistochemistry in the striatum, SN, cerebral cortex and cerebellum of 1 month (WT n=6, Thy1-aSyn n=6), 5–6 month (WT n=6, Thy1-aSyn n=6) and 14 month (WT n=4, Thy1-aSyn n=4) old mice. Sections were washed in 0.1 M PBS, incubated in 0.1 M PBS containing “mouse on mouse” blocking reagent (Vector Laboratories, Burlingame, CA) for 1 hour, followed by blocking with 5% NGS in 0.1M PBS for 1 hour. Sections were then incubated with a primary antibody against mouse and human alpha-synuclein (mouse anti-alpha-synuclein; 1:500; BD Biosciences, San Jose, CA) at 4°C in the presence of 2% normal goat serum overnight. Sections were washed in 0.1 M PBS and incubated in the dark in a Cy5-conjugated goat anti-mouse secondary antibody (1:600, Jackson Immunoresearch) for 2 hours at room temperature. Sections were rinsed in 0.1 M PBS followed by tap water to avoid salt crystals. Sections were mounted onto plain glass slides (instead of frosted) to avoid non-specific background signal from the lasers. Following staining sections were scanned using an Agilent microarray scanner. This requires sections to be completely dry and consequently they were mounted with no mounting medium and coverslip. Slides were stored at 4°C in the dark until ready for scanning.
Quantification of alpha-synuclein fluorescence intensity
Images of alpha-synuclein immunofluorescence-labeled sections from striatum, SN, cerebral cortex and cerebellum of 1, 5–6 and 14 months old WT and Thy1-aSyn mice were taken using an Agilent microarray scanner equipped with a krypton/argon laser (647 nm) at 10 μm resolution with the photomultiplier tube set at 5%. Immunofluorescence intensity of alpha-synuclein staining was quantified by an investigator blind to genotype using ImageJ (NIH). Values are expressed as the mean pixel intensity for each region.
Immunohistochemistry for microglial activation
For morphological assessment of microglial activation sections from PFA-perfused WT and Thy1-aSyn mice from all ages were stained with an antibody against ionized calcium binding adaptor molecule 1 (IBA-1). At one month of age, staining was performed in two sections of the striatum (WT n=5–6, Thy1-aSyn n=5–6), SN (WT n=4, Thy1-aSyn n=4) and cerebral cortex (WT n=4, Thy1-aSyn n=4) ; at 5–6 months of age, in two sections of the striatum (WT n=4–6, Thy1-aSyn n=4–6), cerebral cortex (WT n=6, Thy1-aSyn n=6) and cerebellum (WT n=6, Thy1-aSyn n=6) and 1 section of SN (WT n=6, Thy1-aSyn n=6); at 14 month of age mice, two sections of striatum, SN and cerebral cortex (WT n=3, Thy1-aSyn n=3). Additional sections of striatum and SN from 1 month (WT n=4, Thy1-aSyn n=4), 5–6 month (WT n=5, Thy1-aSyn n=5) and 14 month (WT n=5, Thy1-aSyn n=6) old PFA perfused mice were stained for major histocompatability marker II (MHCII). Sections were washed in 0.1M PBS, incubated in 0.5% H2O2 in methanol for 30 min to inhibit endogenous peroxidase activity, washed in PBS. For IBA-1 immunostaining, sections were incubated for 1 hour in a blocking solution containing 0.1M PBS, 10% normal goat serum and 0.5% Triton-X and for MHCII immunostaining, sections were incubated for 1 hour in 0.1 M PBS containing “mouse on mouse” blocking reagent (Vector Laboratories, Burlingame, CA). Sections were then incubated overnight with a primary antibody against IBA-1 (polyclonal rabbit anti-IBA-1; 1:500 dilution; Wako Pure Chemical Industries Ltd., Japan) or a primary antibody against MHCII (polyclonal mouse anti-Ox-6; 1:500 dilution; Serotec, USA) at 4° C in the presence of 5% normal goat serum. Sections were washed in 0.1M PBS followed by a 2-hour incubation with a biotinylated secondary antibody for IBA-1, goat anti-rabbit IgG (1:200 dilution; Vector Laboratories, Inc., Burlingame, CA) or for MHCII, goat anti-mouse IgG (1:250 dilution; Vector Laboratores, Inc., Burlingame, CA) at room temperature in the presence of 5% normal goat serum. Sections were washed in 0.1M PBS and subsequently incubated in avidin-biotin complex (ABC; Vector Laboratories, Burlingame, CA) for 45 minutes and washed again in 0.1M PBS followed by an incubation in 0.05M Tris buffered saline (TBS) containing 3-3′diamino benzidine (DAB; Sigma) and 0.3% H2O2 (Sigma) to reveal staining. Sections were washed with 0.1M PBS. Sections were mounted onto charged glass slides (to increase adhesion), dehydrated in ethanol and cleared with xylene. Mounted sections were coverslipped with Eukit mounting medium (Calibrated Instruments, Hawthorne, NY).
Quantification of microglial activation
Microglial activation was assessed by an investigator blind to genotype in sections of striatum, SN, cerebral cortex and cerebellum from 1 month, 5–6 month and 14 month old WT and Thy1-aSyn mice stained with IBA-1 as described above. IBA-1 is expressed by both resting and activated microglia (Rappold et al., 2006) and morphologically distinct classes of IBA-1-positive cells can be distinguished based on cell body diameter. Microglia with cell body diameters less than 5 μm have a resting morphology characterized by multiple ramified processes, hyper-ramified microglia/partially activated microglia had mean cell body diameters of 5–6 μm, and fully activated amoeboid microglia had mean cell body diameters of 7–14 μm have an activated morphology characterized by ameboid cell bodies with few, short processes (Batchelor et al., 1999). Accordingly, microglial activation was quantified by measuring the diameter of IBA-1-positive cells. Analysis was done on a Leica DM-LB microscope with a Ludl XYZ motorized stage and z-axis microcator (MT12, Heidenheim, Traunreut, Germany). The StereoInvestigator software (MicroBrightField, Colchester, VT) was used for ease of counting and to enable measurements of microglial diameters with a systematic, non-random, sampling protocol . A contour was drawn to delineate the striatum, SN, cerebral cortex and cerebellum under the 5x objective lens to ensure anatomical accuracy. Following delineation, the diameters of microglial cell bodies were measured in the first counting frame (100μm) and then in every fifth counting frame at 40x magnification. This sampling frequency was chosen in order to count approximately 30–50 IBA-1+ cells in each side of the SN. The first counting frame was always positioned in the upper left corner of the contour and systematically moved from left to right and from top to bottom. Microglia were counted and quantified in every 5th counting frame of the delineated contour regions. Diameters were determined by outlining IBA-1+ cell bodies using the “quick measure circle” tool from the StereoInvestigator software, making sure the screen image was very sharp for optimal accuracy. Measurements were made at 40X, being careful to accurately overlay the circle over each cell diameter. We determined the frequency of IBA-1+ microglia cells with cell diameters ranging from 1μm to 14μm. The number of cells with each diameter was normalized to the total number counted in each section and expressed as a percentage.
Assessment of inflammatory markers by quantitative real-time PCR
RNA was isolated from microdissected fresh frozen striatum, SN, cerebral cortex and cerebellum from 1 month (WT n=6–7, Thy1-aSyn n=7), 5–6 month (WT n=6–7, Thy1-aSyn n=5–6), 14-month (WT n=11–13, Thy1-aSyn n= 7) old mice using Nucleospin RNAII isolation kit (Machery-Nagel, Inc., Germany) according to the manufacturer’s instructions. RNA concentration was equalized across genotypes before cDNA synthesis using a High Capacity cDNA RT Kit (Applied Biosystems, USA).
For cytokines, real-time PCR primers were obtained as “Taqman® Gene Expression Assays” containing forward and reverse primers, and a FAM-labeled MGB Taqman probe for each gene (Applied Biosystems, US). Primers were purchased from Applied Biosystems and were the following accession numbers: CD11b (Mm00434455_m1), (TNF-α (Mm0043258_m1), IL-1β (Mm00434228_m1), transforming growth factor beta (TGF-β) (Mm00441724_m1), β-actin (Mm00607939_s1), HPRT mM01545399_m1 and Atp5b (Mm01160396_g1). A 1:4 dilution of cDNA was prepared and real-time PCR performed using Applied Biosystems 7900 Real-time PCR System. cDNA was mixed with qPCR™ Mastermix Plus (Applied Biosystems, US) and the respective gene assay. Mouse β-actin, HPRT and Atp5b were used as an endogenous controls and expression was measured using a gene expression assay containing forward and reverse primers. For quantification, real-time quantitative PCR was performed using the Applied Biosystems 7900 real-time PCR system. Forty to sixty cycles were run as follows: 10 min at 95 °C and for each cycle, 15 s at 95 °C and 1 min at 60 °C. Expression of the different genes was calculated using the 2−ΔΔCT method. The geometric mean of a combination of the housekeeping genes β-actin, HPRT or Atp5b was used for normalization at each time point.
For analysis of toll-like receptors (TLRs), RNA was isolated and cDNA prepared from microdissected fresh frozen SN of 1 month (WT n=6, Thy1-aSyn n=6), 5–6 month (WT n=4, Thy1-aSyn n=4) and 14 month (WT n=13, Thy1-aSyn n=7) old mice, and from the cerebral cortex of 5–6 month old (WT n=4, Thy1-aSyn n=4) mice. cDNA reactions were diluted 1/10 and 3μl of diluted cDNA reaction was added to a 7μl mastermix containing 5ul SYBR®GreenER™ qPCR SuperMix (Invitrogen, USA) and 200nM of each primer. The primer sequences for TLR 1, TLR 2, TLR 4 and TLR 8 were previously published (Letiembre et al., 2009). For quantification, real-time quantitative PCR was performed using the Applied Biosystems 7900 real-time PCR system, following forty cycles of amplification (95 °C for 15 s and 60 °C for 60 s). Expression of the different genes was calculated using the 2−ΔΔCT method and normalized to the geometric means of the relative concentration of the housekeeping genes EIF, HPRT and Atp5b.
Measurement of TNF-α concentration by ELISA
TNF-α concentration was measured by ELISA in homogenates from microdissected fresh frozen striatum from 1 month (WT n=5, Thy1-aSyn n=4) and 5–6 month (WT n=5, Thy1-aSyn n=6) old mice and from substantia nigra from 5–6 month (WT n=4, Thy1-aSyn n=4) old mice. Serum levels of TNF-α concentration were also measured in 1 month (WT n=4, Thy1-aSyn n=7) and 5–6 month (WT n=11, Thy1 n=7) old mice. Homogenates were equalized for protein concentration and samples (50 μl) of this homogenate, as well as standards, were added to 96-well plates coated with antibody (rat anti-mouse TNF-α antibody; 50 μl; 0.8 μg/ml; BD Biosciences, CA, USA). Samples were incubated overnight, washed and incubated for 1 hour with assay diluent (300 μl; PBS containing 10% FBS for TNF-α). Triplicate samples and standards (50 μl; 0–2000pg/ml recombinant mouse TNF-α; BD Biosciences, CA, USA) were added, incubation proceeded for 2 hours and samples were washed and incubated for 2 hours in the presence of detection antibody (50 μl; 150 ng/ml biotinylated rat anti-mouse TNF-α; BD Biosciences, CA, USA). Detection reagent (50 μl; HRP conjugated streptavidin; 1:250 dilution assay diluent for TNF-α) was added, incubation continued for 20 minutes, samples were washed and substrate solution (50 μl; 1:1 mixture of H2O2 and tetramethylbenzidine) was added. Samples were incubated in the dark for 20 to 30 min and the reaction was stopped using 25 μl 1M H2SO4. Plates were read at 450 nm and cytokine concentrations were estimated from the appropriate standard curve and expressed as pg/mg protein or pg/ml.
Measurement of circulating T cells by flow cytometry
Blood CD4+ T helper cells and CD8+ T cytotoxic cells were measured by flow cytometry in 1 month (WT n=4, Thy1-aSyn n=5), 5–6 month (WT n=5; Thy1-aSyn n=6), 14 month (WT n=13, Thy1-aSyn n=6) and 22-month old (WT n=3, Thy1-aSyn n=5) mice. Whole blood was collected via retro-orbital bleeding and cardiac puncture into tubes containing heparin from mice deeply anesthetized with sodium pentobarbital (100mg/kg, ip) just before perfusion. Erythrocyte lysis was performed by incubating blood for 10 minutes at 4°C with red blood cell lysis buffer (Sigma Aldrich, USA). Single cell suspensions from blood were prepared equalized to 1 × 106 cells/ml. Cells were incubated with the following fluorochrome-labelled monoclonal antibodies for 1 hour at room temperature: anti-CD4 coupled to phycoerythin cyanine dye 7 (PeCy7) and anti-CD8 coupled to Allophycocyanin (APC) and the respective isotype controls. Data were acquired on a BD Calibur flow cytometer equipped with three lasers operating at 488nm, 633nm and 407nm. Infrared fluorescence emission from PeCy7 was collected through a 780nm filter and red fluorescence emission from APC was collected through a 660nm filter. Data were collected using linear amplifiers for forward and side scatter and logarithmic amplifiers for each fluorescence emission spectrum. Cells were selectively analyzed using forward and side scatter properties. For each sample, multiparametric data were acquired for 10,000 events. Single fluorochrome-stained preparations were used for color compensation.
Statistics
To determine the degree of microglial activation in WT and Thy1-aSyn mice, we used the bootstrapping method (Efron and Tibshirani, 1991) using the MATLAB program, which requires no special probability assumptions regarding the shape of the populations being sampled from, and thus is fitting to use for populations that are not normally distributed. We began by calculating the group mean of microglial diameters in the WT mice. The frequency of distribution of the WT means was our test statistic, M. We re-sampled this test statistic by using a box model for simulation, and generated pseudo M values. This resampling process was repeated 1000 times, and the pseudo M values of the WT microglial diameters were plotted onto a histogram. The 2.5% and 97.5% cut-off values of the pseudo M values were calculated to find the 95% confidence interval (CI). These 95% CI bands were utilized to assess whether the actual mean microglial diameters of the Thy1-aSyn mice were statistically significant at the p<0.05 level, independent of assumptions about probability distributions.
Two-way repeated measures (RM) ANOVAs were performed on all data for cytokine mRNA (genotype x cytokine), TLR mRNA (genotype x TLR), T cell markers (genotype x T cell marker) and alpha synuclein immunoreactivity (genotype x region), with cytokine, TLR, T cells markers, and region being the repeated measures. Two-way randomized ANOVA was used for TNF-α protein in the striatum (genotype x age). Post hoc tests performed were Fisher’s least significant difference (LSD) or planned comparison Student’s t test. For analysis of TNF-α protein in the SN, a single Student’s t test was used as this was the only time-point assessed. The expression of TLRs in Thy1-aSyn mice was normalized to WT values for graphical purposes but statistics were performed on absolute values. Statistical analyses were conducted using MATLAB (MathWorks, Natick, MA, USA) and GB-STAT software (Dynamic Microsystems, Inc. Silver Spring, MD). The level of significance was set at p<0.05.
Results
Thy1-aSyn mice exhibit regionally-specific microglial activation
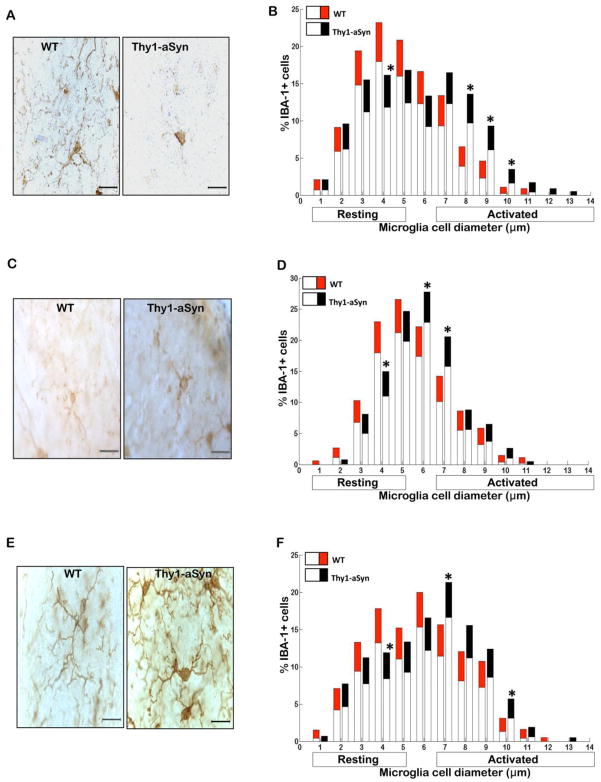
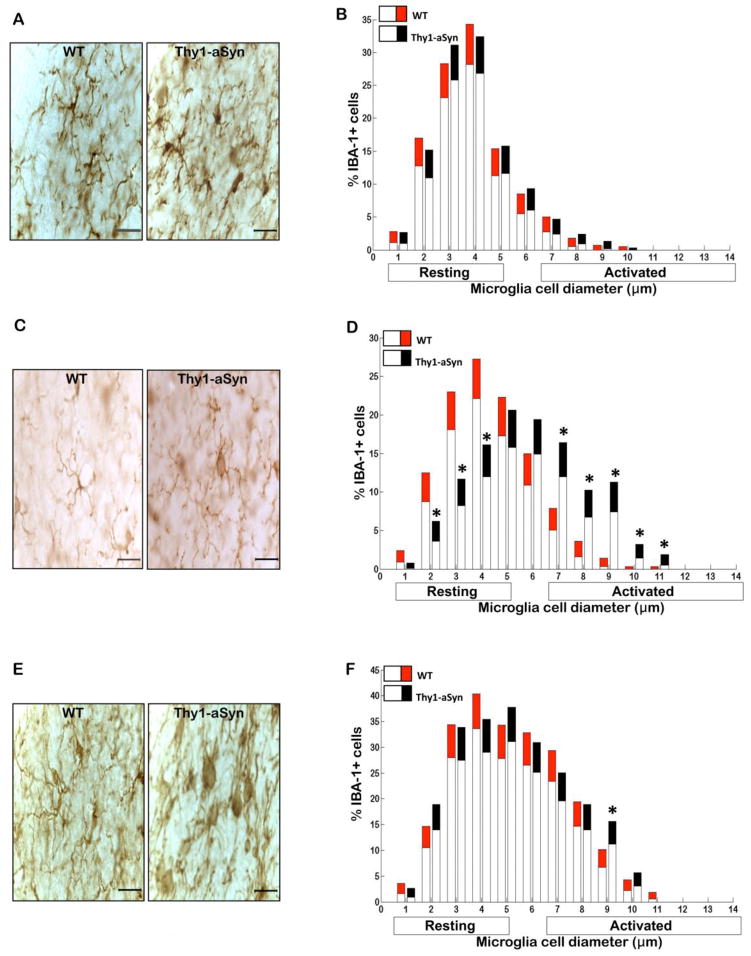
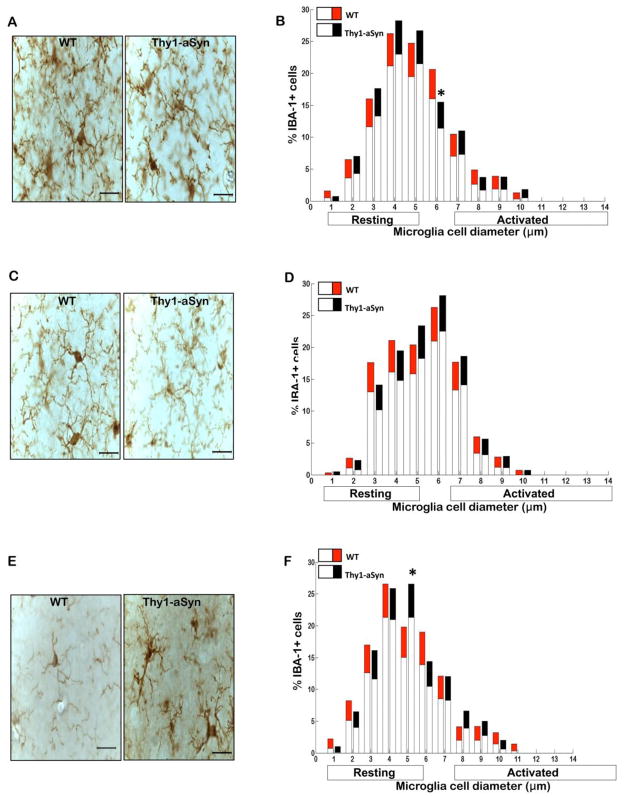
To assess microglial activation, we first labeled microglia by immunostaining with an antibody against IBA-1, an antigen expressed by both resting and activated microglia (Rappold et al., 2006). Microscopic analysis revealed that microglia in the striatum of the Thy1-aSyn mice exhibited a highly activated phenotype as early as 1 month of age (Fig. 1A). By contrast, there was no difference in microglial activation between WT and Thy1-aSyn mice in SN (Fig. 2A) and cerebral cortex (Fig. 3A) at that age. The level of activation was assessed quantitatively based upon the correspondence between cell body diameters with cell morphology, with the mean cell body diameters of resting, hyper-ramified and activated microglia measuring 1–4 μm, 5–6 μm, 7–14 μm, respectively. We measured the diameter of IBA-1 positive microglial cells in the striatum, SN, cerebral cortex and cerebellum of WT and Thy1-aSyn mice at 1, 5–6 and 14 months of age. Quantitative analysis documented a significant decrease in resting IBA-1-positive microglia and a striking increase in activated IBA-1-positive microglia in the striatum of 1 month old Thy1-aSyn mice compared to age-matched WT mice (Fig. 1B), whereas no such activation was observed in the SN (Fig. 2B), nor was there any increase in microglial activation in the cerebral cortex of Thy1-aSyn mice at this age (Fig. 3B).
Fig. 1. Microglial activation in the striatum of Thy1a-Syn mice.
(A) Representative images of IBA-1+ microglia in striatum of 1 month, (C) 5–6 month and (E) 14 month old WT and Thy1-aSyn mice (100x, scale bar 20μm). Diameter of IBA-1+ microglial cells measured in two sections of the striatum at 1 month (WT n=5–6, Thy1-aSyn n=5–6), 5–6 months (WT n=4–6, Thy1-aSyn n=4–6) and 14 months (WT n=3, Thy1-aSyn n=3) of age. Significance is represented if black confidence intervals (Thy1-aSyn) fall outside of 95% confidence interval (red segment of bar means for WT mice), calculated by bootstrapping (*p<0.05). (B) Significant decrease in resting and significant increase in activated IBA-1+ microglial cells in striatum of 1 month old Thy1-aSyn compared with WT mice. (D) In comparison to WT mice, Thy1-aSyn mice displayed a significant decrease in resting and significant increase in activated IBA-1+ microglial cells in the striatum at 5–6 months of age. (F) There was a significant decrease in resting and significant increase in activated IBA-1+ microglial cells in striatum of 14 month old Thy1-aSyn mice. No such changes were observed in the striatum of 14 month old WT mice. Values are mean ± 95%CI.
Fig. 2. Microglial activation in the substantia nigra of Thy1a-Syn mice.
(A) Representative images of IBA-1+ microglia in SN of 1 month, (C) 5–6 month and (E) 14 month old WT and Thy1-aSyn mice (100x, scale bar 20μm). Diameter of IBA-1+ microglial cells measured in two sections of the SN at 1 month (WT n=4, Thy1-aSyn n=4) and 14 months (WT n=3, Thy1-aSyn n=3) and in one section at 5–6 months (WT n=6, Thy1-aSyn n=6) of age. Significance is represented if black confidence intervals (Thy1-aSyn) fall outside of 95% confidence interval (red segment of bar means for WT mice), calculated by bootstrapping (*p<0.05). (B) Similar levels of resting and activated IBA-1+ microglial cells in SN of 1 month old WT and Thy1a-Syn mice. (D). Resting IBA-1+ microglia were decreased and activated IBA-1+ microglia were increased in SN of 5–6 month old Thy1-aSyn mice, this effect was not observed in WT mice. (F) In contrast to WT mice, activated IBA-1+ microglial cells were persistently increased in SN of 14 month old Thy1-aSyn mice. Values are mean ± 95%CI
Fig. 3. Microglial phenotype is similar in the cerebral cortex of Thy1a-Syn mice.
(A) Representative images of IBA-1+ microglia in cerebral cortex of 1 month, (C) 5–6 month and (E) 14 month old WT and Thy1-aSyn mice (100x, scale bar 20μm). Diameter of IBA-1+ microglial cells measured in two sections of the cerebral cortex at 1 month (WT n=4, Thy1-aSyn n=4), 5–6 months (WT n=6, Thy1-aSyn n=6), and 14 months (WT n=3, Thy1-aSyn n=3) of age. Significance is represented if black confidence intervals (Thy1-aSyn) fall outside of 95% confidence interval (red segment of bar means for WT mice), calculated by bootstrapping (*p<0.05). (B) Compared with WT mice, Thy1-aSyn mice had a significant reduction in the percentages of hyperramified microglial cells in the cerebral cortex at 1 month of age. (D) WT and Thy1-aSyn mice had similar levels of resting and activated IBA-1+ microglial cells in cerebral cortex at 5–6 months of age. (F) At 14 months of age, there was a significant increase in the percentages of hyperramified microglial cells in the cerebral cortex of Thy1-aSyn mice compared with WT mice. Values are mean ± 95%CI.
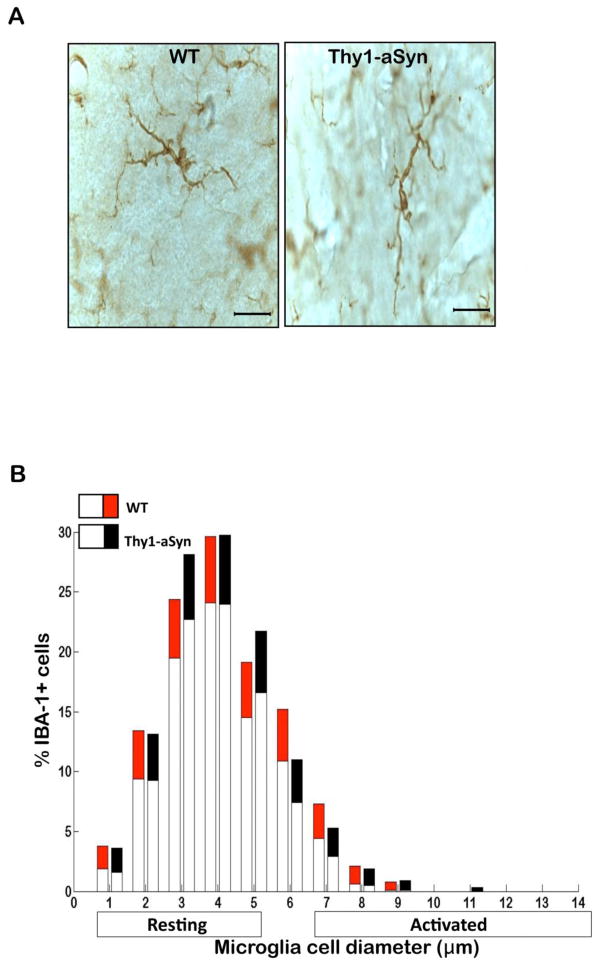
At age 5–6 months, microglia had become highly activated in the SN of Thy1-aSyn mice, as shown by their enlarged cell body size (Fig. 2C). Indeed, the number of resting microglia decreased (p<0.05; Bootstrapping; Fig. 2D) whereas the number of activated microglia with enlarged cell bodies increased in the SN of 5–6 month old Thy1-aSyn mice (Fig. 2D). Microglia remained significantly activated in the striatum of Thy1-aSyn mice at that age (Fig. 1C,D) but no differences were noted in the cerebral cortex (Fig. 3C) or cerebellum (Fig. 4A), an observation confirmed by quantitative analysis of cell body diameters (Fig. 3D; Fig. 4B).
Fig. 4. Microglial phenotype is similar in the cerebellum of Thy1a-Syn mice.
(A) Representative images of IBA-1+ microglia in the cerebellum of 5–6 month old WT and Thy1-aSyn mice. Diameter of IBA-1+ microglial cells measured in two sections of the cerebellum at 5–6 months of age (WT n=6, Thy1-aSyn n=6, 100x, scale bar 20μm). Significance is represented if black confidence intervals (Thy1-aSyn) fall outside of 95% confidence interval (red segment of bar means for WT mice), calculated by bootstrapping (*p<0.05). (B) Similar levels of resting and activated IBA-1+ microglial cells in the cerebellum of 5–6 month old WT and Thy1a-Syn mice. Values are mean ± 95%CI
By 14 months of age, microglia of the Thy1-aSyn mice were persistently activated in the striatum (Fig. 1E) and SN (Fig. 2E) whereas no activation was noted in the age-matched WT mice. At this time point, the transgenic mice showed a reduced number of resting microglia and increased number of activated microglia in the striatum (Fig. 1F), together with an increase in SN activated microglia (Fig. 2F). Minimal changes in microglial activation in the cerebral cortex began to emerge by age 14 months in the Thy1-aSyn mice, with an increase in the percentage of hyper-ramified microglia with cell body diameters of 5μm (Fig. 3E, F).
MHCII-positive microglia are increased in the striatum of 14 month old Thy1-aSyn mice
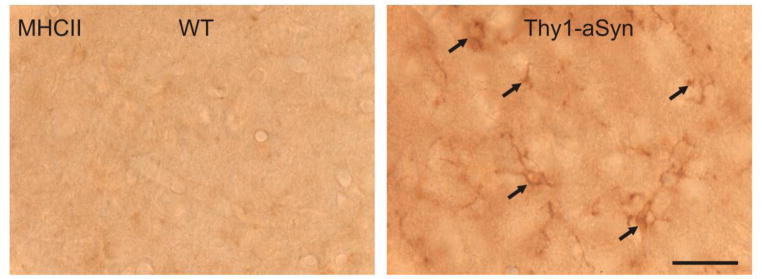
To further characterize the activated microglia in Thy1-aSyn mice, sections of the SN and striatum from WT and Thy1-aSyn mice were stained for MHCII, a receptor expressed on activated microglial cells that is involved in antigen presentation to T cells, As shown in the Fig. 5, compared with WT mice, MHCII immunoreactivity in the striatum was slightly increased in 14 month old Thy1-aSyn mice. However, no MHCII immunoreactivity was detected in the SN and striatum of WT and Thy1-aSyn mice at 1 and 5–6 months of age, and in the SN at 14 months of age, with either one of the two antibodies used (data not shown).
Fig. 5. MHCII-positive microglia are increased in the striatum of 14 month old Thy1-aSyn mice.
MHCII-positive (MHCII+) microglia were examined using antibodies against Ox-6. Representative images from WT and Thy1-aSyn mice at 14 months of age show increased MHCII+ microglia in striatum of Thy1-aSyn mice. Solid arrows indicated MHCII+ microglia, 100x, scale bar = 20μm.
TNF-α expression is increased in the striatum and SN of Thy1-aSyn mice
Previous studies have coupled increased microglial activation with increased production of pro- an anti-inflammatory cytokines (Clarke et al., 2007; Tambuyzer et al., 2009; Smith et al., 2011). To determine whether changes in microglial morphology had functional consequences, we examined the expression of pro- and anti-inflammatory mediators by quantitative real-time TaqMan PCR in the striatum, SN and cerebral cortex of 1 month, in the striatum, SN, cerebral cortex and cerebellum of 5–6 month and in the striatum and SN of 14 month old WT and Thy1-aSyn mice. Assessment of cytokines in the striatum at 1 month of age by two-way RM ANOVA revealed an overall cytokine effect F(3,36)=4.88, p<0.0403 but no genotype effect F(1,36)=0.4786, p=0.5022 or interaction effect of cytokine and genotype F(1,44)=0.61, p=0.5486. Fisher’s LSD revealed no significant differences in the cytokines between WT and Thy1-aSyn mice. However, considering the robust microglial activation in the striatum at this age, we conducted a planned comparison Student’s t test to determine if the pro-inflammatory cytokines were also increased. We found a selective increase in TNF-α mRNA in the striatum of Thy1-aSyn compared with WT mice (p<0.05; planned comparison Student’s t test; Table 1). In contrast to the changes observed in TNF-α, the expression of the pro-inflammatory cytokine (IL-1β), the anti-inflammatory cytokine (TGF-β), and of the microglia activation receptor (CD11b) remained unchanged in striatum of Thy1-aSyn mice compared to WT at all ages (Table 1). Similar to the increased TNF-α mRNA expression in the striatum, two-way ANOVA revealed a main effect of age F(1,16), p=<0.0161 and genotype F(1,16)=12.61982, p<0.0027 on TNF-α protein levels, but no interaction effect F(1,16)=2.67537, p=0.1214. Based on the mRNA analysis, planned comparison Student’s t test was also conducted and indicated that the increase in mRNA was paralleled by an increase in the level of TNF-α protein in the striatum of 1 month old Thy1-aSyn mice (p<0.05; planned comparison Student’s t test, Fig. 6A). No such changes were observed in the SN or cerebral cortex at this age (Table 1), consistent with the observed absence of microglia activation. Although two-way RM ANOVA revealed an overall significant cytokine effect F(3,36)=9.0988, p<0.0001 at 1 month of age in the SN, no genotype F(1,36)=1.37033, p=0.2645 or interaction effect of cytokine and genotype F(3,36)=0.32142, p=0.8098 were observed, and Fisher’s LSD showed no significant differences in any of the cytokines (TNF-α, IL-1β, TGF-β and CD11b) between WT and Thy1-aSyn mice. Similarly RM ANOVA analysis of cytokines in the cerebral cortex revealed an overall cytokine effect F(3,33)=10.86398, p<0.0001, but no genotype F(1,33)=1.22354, p=0.2923, or interaction effect of cytokine and genotype F(3,33)=0.85559 p=0.4737 and Fisher’s LSD revealed no differences in TNF-α, IL-1β, TGF-β and CD11b between WT and Thy1-aSyn mice at 1 month of age in this region.
Table 1. TNF-α expression and concentration are increased in Thy1-aSyn mice.
Total RNA was purified from microdissected striatum, substantia nigra, cerebral cortex and cerebellum from 1 month (WT n=6–7, Thy1-aSyn n=7), 5–6 month (WT n=6–7, Thy1-aSyn n=5–6) and 14-month (WT n=11–13, Thy1-aSyn n= 7) old mice. cDNA was generated and Q-PCR for CD11b, TNF-α, IL-1β and TGF-β was carried out. TNF-α mRNA expression was significantly increased in striatum of 1 month old Thy1-aSyn mice (*p<0.05; RM ANOVA followed by planned comparison Student’s t test) and 5–6 month old Thy1-aSyn mice (*p<0.05; RM ANOVA followed by Fisher’s LSD) compared to WT mice. TNF-α mRNA expression was significantly increased in the substantia nigra of 5–6 and 14 month old Thy1-aSyn mice compared to WT mice of the corresponding age (**p<0.01; RM ANOVA followed by Fisher’s LSD). TNF-α mRNA expression was unchanged in the cerebral cortex and cerebellum of 5–6 month old Thy1-aSyn mice. No changes were observed in the expression of CD11b, IL-1β and TGF-β. Serum TNF-α protein was significantly increased in 5–6 month old Thy1-aSyn mice compared with WT mice of the same age (*p<0.05; Student’s t test, WT n=11, Thy1-aSyn n=7), with no change at 1 month. (Values are mean ± SEM).
| WT | Thy1-aSyn | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 1 month | 5–6 months | 14 months | 1 month | 5–6 months | 14 months | ||||
|
| |||||||||
| Region | Marker | mRNA | mRNA | mRNA | mRNA | mRNA | mRNA | ||
| Striatum | CD11b | 0.949±0.160 | 0.512±0.170 | 0.763±0.048 | 0.935±0.056 | 0.725±0.076 | 0.758±0.059 | ||
| TNF-α | 0.597±0.064 | 0.260±0.082 | 0.405±0.062 | 0.809±0.060* | 0.528±0.110* | 0.495±0.088 | |||
| IL-1β | 0.669±0.090 | 0.336±0.221 | 0.580±0.077 | 0.631±0.067 | 0.195±0.064 | 0.572±0.062 | |||
| TGF-β | 0.784±0.029 | 0.347±0.125 | 0.194±0.029 | 0.780±0.042 | 0.417±0.165 | 0.337±0.121 | |||
| Substantia nigra | CD11b | 0.439±0.038 | 0.100±0.023 | 0.580±0.029 | 0.538±0.058 | 0.100±0.019 | 0.654±0.064 | ||
| TNF-α | 0.470±0.074 | 0.508±0.078 | 0.314±0.033 | 0.634±0.082 | 0.804±0.053** | 0.517±0.094** | |||
| IL-1β | 0.652±0.062 | 0.100±0.012 | 0.620±0.071 | 0.675±0.011 | 0.134±0.040 | 0.565±0.082 | |||
| TGF-β | 0.804±0.087 | 0.359±0.231 | 0.123±0.007 | 0.879±0.131 | 0.100±0.064 | 0.171±0.042 | |||
| Cortex | CD11b | 0.728±0.092 | 0.685±0.099 | nd | 0.821±0.072 | 0.551±0.048 | nd | ||
| TNF-α | 0.437±0.064 | 0.460±0.054 | nd | 0.589±0.073 | 0.632±0.109 | nd | |||
| IL-1β | 0.470±0.087 | 0.783±0.085 | nd | 0.433±0.062 | 0.577±0.072 | nd | |||
| TGF-β | 0.587±0.081 | 1.181±0.045 | nd | 0.684±0.035 | 0.342±0.135 | nd | |||
| Cerebellum | CD11b | nd | 0.487±0.213 | nd | nd | 0.454±0.034 | nd | ||
| TNF-α | nd | 0.472±0.275 | nd | nd | 0.177±0.051 | nd | |||
| IL-1β | nd | 0.370±0.315 | nd | nd | 0.100±0.054 | nd | |||
| TGF-β | nd | 0.655±0.194 | nd | nd | 0.459±0.091 | nd | |||
| Serum | TNF-α | 12.13±3.093pg/ml | 10.06±1.871pg/ml | nd | 13.96±2.890pg/ml | 40.51±17.47pg/ml* | nd | ||
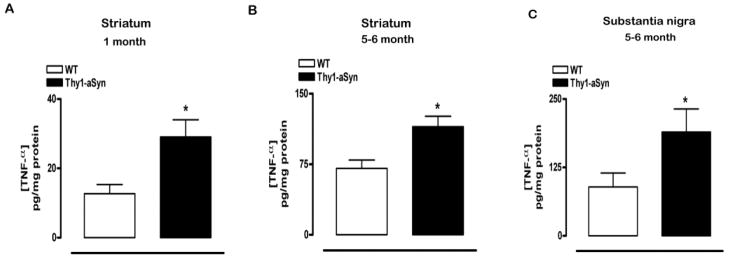
Fig. 6. TNF-α protein in striatum and substantia nigra of Thy1-aSyn mice.
(A) Compared to WT mice, TNF-α protein measured by ELISA was significantly increased in the striatum of 1 month old Thy1-aSyn mice (*p<0.05, RM ANOVA followed by planned comparison Student’s t test, WT n=5, Thy1-aSyn n=4). (B) Similar increases in TNF-α protein were observed in striatum of 5–6 month old Thy1-aSyn mice (*p<0.05, RM ANOVA followed by Fisher’s LSD, WT n=5, Thy1-aSyn n=6). (C) Increases in TNF-α protein were also observed in the SN of 5–6 month old Thy1-aSyn mice compared with WT mice (*p<0.05, Student’s t test, WT n=4, Thy1-aSyn n=4). Values are mean ± SEM.
At 5–6 months, when microglial activation was evident in both the striatum (Fig. 1C) and SN (Fig. 2C), two-way RM ANOVA revealed a cytokine effect F(3,48)=11.68536, p<0.0001 and an interaction effect of cytokines and genotype F(3,48)=4.46846, p<0.0076 in the striatum (but no effect of genotype alone F(1,48)=1.68747, p=0.2123). Fisher’s LSD showed that TNF-α mRNA expression was significantly increased in the striatum of 5–6 month old Thy1-aSyn mice compared with WT mice (p<0.05; Table 1). There were no changes in the other cytokines assessed. Two-way ANOVA also showed an age-related effect F(1,16), p=<0.0161 and genotype effect F(1,16)=12.61982, p<0.0027 on TNF-α protein concentration in the striatum, when measured at 1 and 5–6 months of age without interaction of age and genotype F(1,16)=2.67537, p=0.1214. Fisher’s LSD showed that a significant increase in TNF-α protein (p<0.05; Fig 6B) accompanied the increase in TNF-α mRNA expression in the striatum at 5–6 months of age. Similarly, analysis of cytokines in the SN at 5–6 months revealed a strong overall cytokine effect F(3,33)=42.59673, p<0.0001 and a strong interaction of cytokine and genotype F(3,33)=7.77112, p<0.0005 but no genotype effect F(1,33)=0.1396, p=0.7158. TNF-α mRNA expression was significantly increased in the SN of Thy1-aSyn mice compared with WT mice (p<0.01; Fisher’s LSD, Table 1) and this was accompanied by an increase in TNF-α protein (p<0.05; Student’s t test; Fig 6C). No changes in IL-1β, TGF-β and CD11b were observed in the SN at 5–6 months of age (Table 1).
In accord with the lack of microglial activation in the cerebral cortex and cerebellum of Thy1-aSyn mice at 5–6 months of age (Figs 3 and 4), the expression of the pro- and anti-inflammatory cytokines remained unchanged in these regions (Table 1). For the cerebral cortex, two-way RM ANOVA revealed an overall cytokine effect F(3,30)=12.64211, p<0.0001 and an interaction effect of cytokine and genotype F(3,30)=3.58707, p<0.0251 but no genotype effect F(1,30)=0.00072, p=0.9792 and Fisher’s LSD revealed no significant differences in any of the cytokines between WT and Thy1-aSyn mice. For the cerebellum, there was an effect of genotype F(1,21)=28.03644, p<0.0001 but no cytokine effect F(3,21)=2.0137, p=0.1428 and no interaction effect of cytokine and genotype F(3,21)=0.33518, p=0.4537. Fisher’s LSD revealed no significant changes in any of the cytokines in WT and Thy1-aSyn mice in this region.
Although microglial activation was sustained in both the striatum and SN up to 14 months of age in the transgenic mice, the increased expression of TNF-α mRNA was restricted to the SN (p<0.01, Fisher’s LSD, Table 1). Analysis of cytokines in the striatum at 14 months of age revealed an overall cytokine effect F(3,48)=28.03644, p<0.0001 but no effect of genotype F(1,48)=0.58083, p=0.4571 or interaction of cytokine and genotype F(3,48)=0.88882, p=0.4537 (two-way RM ANOVA) and Fisher’s LSD showed no alterations in any of the cytokines measured. For cytokines in the SN at 14 months of age, two-way repeated measures ANOVA showed an overall cytokine effect F(3,54)=33.63056, p<0.0001, no effect of genotype F(1,54)=2.29915, p=0.1468 or interaction effect of cytokine and genotype F(3,54)=2.00817, p=0.1237. Fisher’s LSD revealed a robust increase in TNF-α mRNA expression in the SN at 14 months of age, correlating with microglial activation in this region (p<0.01, Fisher’s LSD, Table 1).
Localized changes in microglial gene expression in Thy1-aSyn mice do not parallel regional differences in alpha-synuclein expression
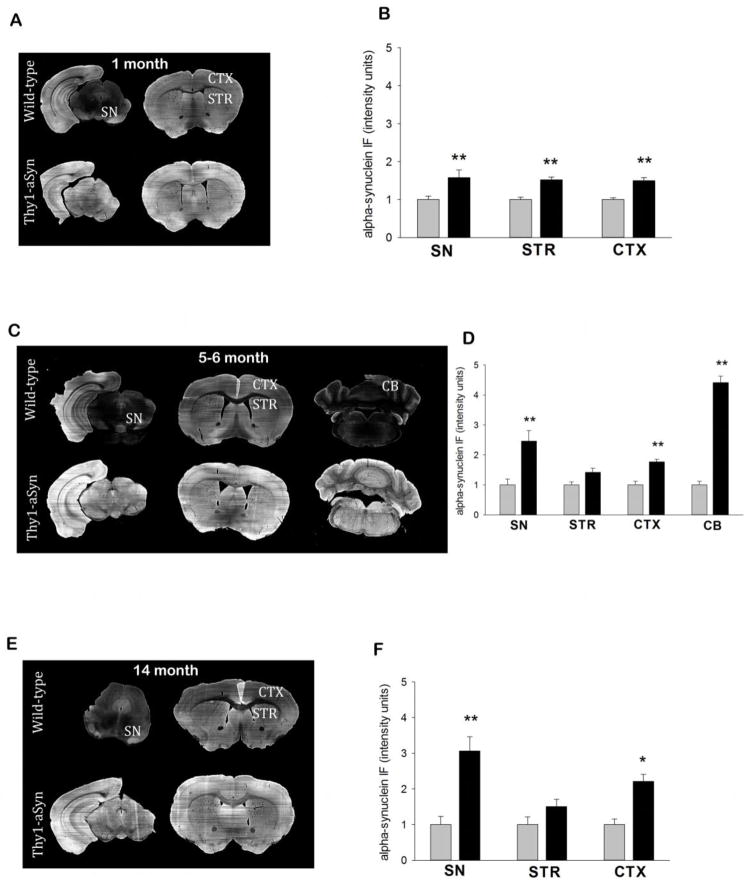
To exclude the possibility that the observed regional differences in microglial activation in Thy1-aSyn mice were caused by differential alpha-synuclein expression levels, we measured alpha-synuclein immunofluorescence in the striatum, SN, cerebral cortex and cerebellum of 1, 5–6 and 14 month old WT and Thy1-aSyn mice. A Two-way RM ANOVA, with genotype and region as factors revealed no effect of region F(2,20)=0.0659, p=0.936 and no significant interaction effect of region and genotype on alpha synuclein levels in the SN, striatum and cerebral cortex at 1 month of age F(2,20)=0.0659, p=0.936, however, a significant genotype effect was present, as expected (two-way RM ANOVA, F(1.20)=40.469, p<0.001) with a significant increase of alpha synuclein levels all three regions of Thy1-aSyn mice compared with WT mice (p<0.01; Fisher’s LSD, Fig. 7A,B). At 5–6 months of age, two-way RM ANOVA revealed a significant genotype F(1.32)=48.16, p<0.001, region F(3.32)=50.328, p<0.001 and interaction effect F(3.32)=50.497, p<0.001 on alpha synuclein levels across all regions. Compared with WT mice, alpha synuclein levels were significantly increased in SN, cerebral cortex and cerebellum of Thy1-aSyn mice (p<0.01; Fisher’s LSD, Fig. 7C,D). However, Fisher’s LSD also revealed that alpha synuclein levels are significantly increased in the cerebellum compared with the SN, striatum and cerebral cortex (p<0.001) and in the SN compared with the striatum (p<0.001) and the cerebral cortex (p<0.05). Thus localized changes in microglial activation in Thy1-aSyn mice do not parallel the regional differences in alpha synuclein levels served at this age since the cerebellum has the highest expression of alpha synuclein and microglial activation was not observed in this region. At older ages (14 months), the magnitude of alpha-synuclein expression remained similar in SN, cerebral cortex and cerebellum and RM ANOVA demonstrated no effect of region F(2,18)=3.044, p=0.073 and no significant interaction effect of region and genotype on alpha synuclein levels F(2,18)=3.044, p=0.073 whereas the significant genotype effect F(1.18=22.585, p<0.001 on alpha synuclein levels was maintained (p<0.01; Fisher’s LSD, Fig. 7E,F). We noted that in the striatum, at older ages, the immunofluorescence level was no longer increased compared to the WT mice, which is likely due to the presence of numerous myelinated fiber tracts within this region. Overall, these experiments confirm that, differences in microglial expression and cytokine levels we observed are not due to regional variations in alpha-synuclein expression levels in the Thy1-aSyn mice.
Fig. 7. Alpha-synuclein levels across different brain regions in Thy1-aSyn mice.
Alpha-synuclein is broadly over-expressed in the brain of Thy1-aSyn mice. (A) Representative images of alpha-synuclein immunofluorescence in the striatum, SN and cerebral cortex of 1 month old WT and Thy1-aSyn mice. (B) Alpha synuclein immunofluorescent intensity is increased in the striatum, SN and cerebral cortex of 1 month old Thy1-aSyn mice compared with WT mice. (C) Representative images of alpha-synuclein immunofluorescence in the striatum, SN, cerebral cortex and cerebellum of 5–6 month old WT and Thy1-aSyn mice (D) Compared with WT mice alpha synuclein immunofluorescent intensity is increased in the SN, cerebral cortex and cerebellum of 5–6 month old Thy1-aSyn mice (E) Representative images of alpha-synuclein immunofluorescence in the striatum, SN and cerebral cortex 14 month old WT and Thy1-aSyn mice (F) Compared with WT mice alpha synuclein immunofluorescent intensity is increased in the SN and cerebral cortex of 14 month old Thy1-aSyn mice. (n=4–6, values are expressed as the mean +SEM pixel intensity for each region, RM ANOVA followed by Fisher’s LSD).
Toll-like receptors are selectively increased in the substantia nigra
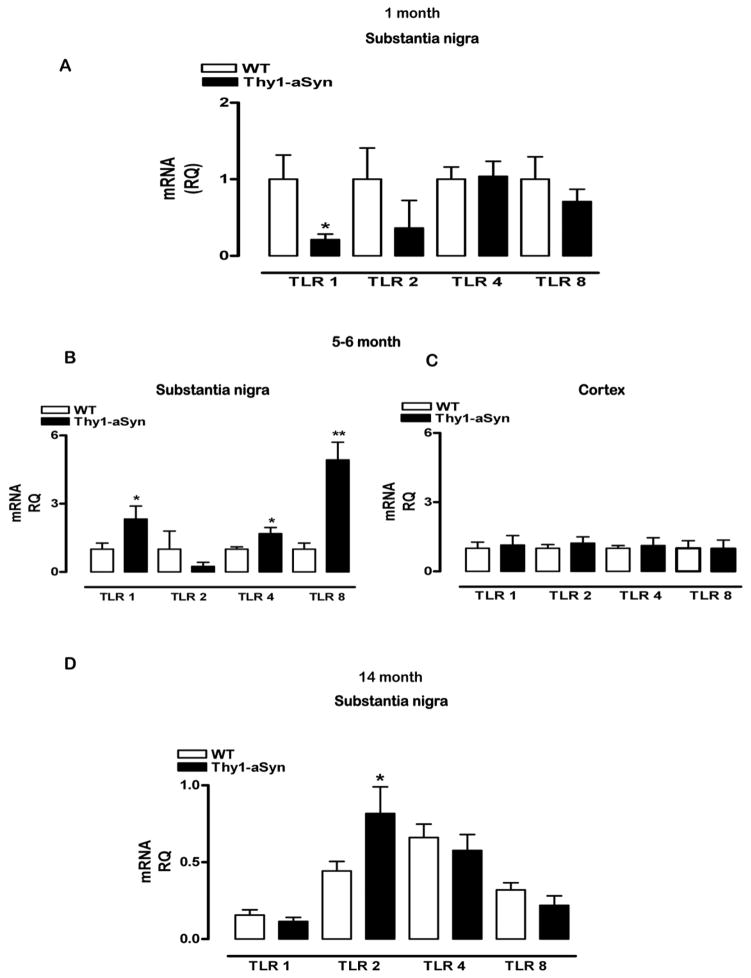
Toll-like receptors (TLRs) have been recently implicated in the activation of microglia by alpha-synuclein in vitro (Beraud et al., 2011). Therefore, we examined the expression of TLR mRNA in the striatum and SN of 1 month, 5–6 month and 14 month old Thy1-aSyn mice and WT littermates. Despite microglial activation in the striatum of the transgenic mice at 1 month of age, the expression of TLR 1, TLR 4 and TLR 8 at this time-point did not differ from that of the control mice (data not shown). In the 1 month old SN, two-way RM ANOVA revealed an overall genotype effect F(1,30)=5.23656, p=<0.0451, no TLR effect F(3,30)=0.96233, p=0.4233 and no interaction effect of TLR and genotype F(3,30)=1.10464, p=0.3625. At that age, TLR 1 mRNA expression in the SN was significantly lower in the Thy1-aSyn mice compared with WT mice (p<0.05; Fisher’s LSD, Fig. 8A), without differences in TLR 2, 4 and 8. At 5–6 months of age, two-way RM ANOVA showed an interaction of genotype and TLR F(3,18)=6.30141, p<0.0041 but no genotype F(1,18)=4.26964, p=0.0843 or TLR effect F(3,18)=3.02987, p=0.0563. A significant increase in TLR 1 and TLR 8 mRNA expression was present in Thy1-aSyn mice at this age (p<0.05, p<0.01; Fisher’s LSD, Fig. 8B). Furthermore, planned comparison Student’s t test showed that there was also a significant increase in TLR 4 mRNA expression (p<0.05, Fig. 8B). Thus, selective increases in TLRs coincided with microglial activation in this region (Fig. 2D). In line with the lack of microglial activation in the cerebral cortex (Fig. 3D), there were no differences in TLR 1, TLR 2, TLR 4 and TLR 8 mRNA expression at 5–6 months of age in this region: two-way RM ANOVA showed no genotype effects F(1,18)=0.00013, p=0.9912, no TLR effects F(3,18)=1.93588, p=0.16 and no interaction effects of genotype and TLRs F(3,18)=0.54398, p=0.6584 (Fig 8C), indicating a regional and temporal specificity of TLR expression in Thy1-aSyn mice. At 14 months of age, two-way ANOVA showed a significant interaction of TLR and genotype F(3,42)=3.44027, p<0.0252 and a TLR effect F(3,42)=12.84069, p<0.0001 but no effect of genotype alone F(1,42)=-.51996, p=0.4827. Although the expression levels of TLR 1, TLR 4 and TLR 8 were no longer different in the SN of Thy1-aSyn mice compared with WT mice, a significant increase in TLR 2 mRNA expression emerged at this older age in the SN of Thy1–aSyn mice compared to WT (p<0.05; Fisher’s LSD, Fig. 8D).
Fig 8. TLR 1, TLR 2, TLR 4 and TLR 8 mRNA expression in the substantia nigra and cerebral cortex of Thy1-aSyn mice.
The mRNA expression of TLR 1, TLR 2, TLR 4 and TLR 8 were measured by Q-PCR using SyberGreenER chemistry in the SN of 1 month (WT n=6, Thy1-aSyn n=6), 5–6 month (WT n=4, Thy1-aSyn n=4) and 14 month (WT n=13, Thy1-aSyn n=7) old mice and in the cerebral cortex of 5–6 month (WT n=4, Thy1-aSyn n=4) old mice. (A) We observed a significant decrease in the expression of TLR 1 mRNA in the SN of 1 month old Thy1-aSyn mice (*p<0.05, RM ANOVA followed by Fisher’s LSD). (B) The expression of TLR 1 and TLR 8 were significantly increased (*p<0.05, **p<0.01, RM ANOVA followed by Fisher’s LSD) in the SN and the expression of TLR 4 was also increased (*p<0.05; Student’s t test) in the SN of 5–6 month old Thy1-aSyn mice. (C) However, the expression of TLR 1, 4 and 8 was similar in the cerebral cortex of 5–6 month old WT and Thy1-aSyn mice. (D) Compared with WT mice, the expression of TLR 2 was significantly increased in SN of 14 month old Thy1-aSyn mice (*p<0.05; RM ANOVA followed by Fisher’s LSD). Values are mean ± SEM
TNF-α protein is increased in the serum of Thy1-aSyn mice
High levels of inflammatory cytokines have been identified in the serum of PD patients (Reale et al., 2009; Scalzo et al. 2009). Therefore, we compared the serum levels of TNF-α in the transgenic and WT mice. Although serum levels of TNF-α were similar in 1 month old WT and Thy1-aSyn mice, a significant increase was observed at 5–6 months of age (p<0.05, Student’s t test, Table 1).
Recent evidence suggests that circulating T cells are altered in the blood of PD patients (Baba et al., 2005) and recent evidence suggests that T cells have a role in PD pathogenesis (Brochard et al., 2009). Therefore we sought to determine the effect of widespread over-expression of alpha-synuclein on peripheral T cell responses. To verify that the use of the Thy1 promoter did not drive ectopic expression in T cells (which, like neurons, express Thy1), we tested T cells isolated from two healthy individuals for alpha-synuclein mRNA. Using triplicate quantitative PCR reactions, we show that the expression of alpha-synuclein in both samples was significantly greater than that of the housekeeping gene. For donor #1, the fold-increase was 1.6, and for donor #2, it was 5.9. Thus, alpha-synuclein is normally expressed in T cells.
The percentages of CD4+ and CD8+ T cells were measured in the blood of 1 month, 5–6 month, 14 month and 22-month old WT and Thy1-aSyn mice. At 1 month of age, ANOVA revealed an overall T cell effect F(1,4)=16.01053, p=<0.0161 but no genotype effect F(1,4)=0.02128, p=0.8911 or interaction F(1,4)=1.27368, p=0.3222. Post hoc Fisher’s LSD showed no differences in the percentages of blood CD4+ and CD8+ T cells between WT and Thy1-aSyn mice (Table 2). At 5–6 months of age, we observed a strong T cell effect F(1,15)=21.92857 p=<0.0003 but no gentoype F(1,15)=2.13785, p=0.1643 or interaction effect F(1,15)=0.8444, p=0.3727 and Fisher’s LSD showed similar percentages of CD4+ and CD8+ T cells in WT and Thy1-aSyn mice (Table 2). Similarly at 14 months of age, there was a strong T cell effect F(1,19)=34.66638p=<0.0001 but no genotype F(1,19)=1.44056 p=0.2448 or interaction effect F(1,19)=033661, p=0.5686 and despite the observed microglial activation and increased TNF-α in brain, we did not observe any increases in the percentages of CD4+ and CD8+ T cells in Thy1-aSyn mice compared with WT mice (Fisher’s LSD, Table 2). In contrast, at 22 months of age, RM ANOVA, revealed a strong T cell effect F(1,6)=152.18542, p=<0.0001 and interaction effect of genotype and T cells F(1,6)=6.3271, p<0.0456, but no genotype effect F(1,6)=4.69193 p=0.0735. Fisher’s LSD showed a highly significant increase in the percentages of both CD4+ and CD8+ T cells (p<0.01, Fisher’s LSD, Table 2). However, no differences in the densities of CD8+cells were observed in striatal tissue sections from WT and Thy1-aSyn mice after immunostaining for CD8 (data not shown).
Table 2. The percentages of CD4+ and CD8+ T cells are increased in the blood of 22 month old Thy1-aSyn mice.
The percentages of CD4+ and CD8+ T cells were measured by flow cytometry in the blood of 1 month (WT n=4, Thy1 n=5), 5–6 month (WT n=5; Thy1 n=6), 14 month (WT n=13, Thy1 n=6) and 22-month old (WT n=3, Thy1 n=5) mice. The percentages of CD4+ and CD8+ T cells were significantly increased in the blood of 22 month old Thy1-aSyn mice. (Values are mean ± SEM, **p<0.01, ANOVA followed by Fisher’s LSD).
| Age (month) | WT | Thy1-aSyn | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 5–6 | 14 | 22 | 1 | 5–6 | 14 | 22 | |
| %CD4 | 16.33±4.10 | 10.2±1.96 | 15.72±1.09 | 6.50±0.88 | 13.67±4.70 | 14.4±2.5 | 17.1±1.66 | 9.04±1.18** |
| %CD8 | 8.0±2.30 | 4.8±1.24 | 8.59±0.59 | 9.76±1.24 | 9.0±5.13 | 6.3±1.38 | 10.8±1.65 | 13.98±0.87** |
Discussion
Our studies provide the first demonstration that microglial activation and the pro-inflammatory cytokine, TNF-α, are selectively increased in the striatum and SN of young Thy1-aSyn mice and persist in both regions up to 14 months of age. By contrast, neither microglial activation nor changes in TNF-α were detected in the cerebral cortex or cerebellum, despite levels of alpha- synuclein overexpression that were similar to, or higher than, those in the striatum and SN. Importantly, the inflammatory response occurred earlier in the striatum (1 month of age) than in the substantia nigra (5–6 months of age), suggesting that terminals of nigrostriatal neurons are exposed to an inflammatory reaction and cytotoxic TNF-α earlier than their cell bodies. Finally, the parallel and selective increase in microglial activation and the expression of three TLRs (1,4,8) in the SN suggests that these receptors may contribute to the regionally specific microglial response observed in the SN of Thy1-aSyn mice, whereas other mechanisms may be involved in the earlier activation observed in the striatum.
Temporal profile of microglial response in Thy1-aSyn mice
One of the critical, but unresolved, issues in PD research is the role of inflammation during the early stages of the disease. Activated microglia and pro-inflammatory cytokines have been observed in the brains of PD patients (McGeer et al., 1988; Mogi and Nagatsu, 1999; Imamura et al., 2003). However, whether inflammation is involved early during the course of the disease is not known, because few pathological studies of inflammation in brains from early stage patients have been reported. Traditional models of PD, based on toxin administration, show early microglial activation, but the relevance of inflammation following these acute insults to the slow pathophysiological process of PD development is unclear.
Alpha-synuclein pathology is widespread in the brain of PD patients and in vitro studies suggest a link between alpha-synuclein and microglial activation (Klegeris et al., 2008; Alvarez-Erviti et al., 2011). In vivo evidence also indicates that regional increases in alpha-synuclein expression can trigger microglial activation and cytokine production prior to the loss of dopaminergic neurons, suggesting that inflammation can contribute to the pathological process that eventually leads to cell death (Su et al., 2008; Theodore et al., 2008; Sanchez-Guajardo et al., 2010). However, this hypothesis has not been tested in a model of widespread expression of alpha-synuclein that more closely mimics the pattern of pathology observed in the brains of patients with PD.
The Thy1-aSyn mouse model expresses human alpha-synuclein in neurons of all brain regions, providing a unique opportunity to model the early events of PD in humans, which also shows widespread alpha-synuclein expression (Rockenstein et al. 2002; Braak et al. 2003). Confirming observations in mice with localized alpha-synuclein overexpression, we noted a marked increase in activated microglia in the striatum and SN many months prior to the loss of dopamine and decreased tyrosine hydroxylase, which both occur at 14 months of age in the striatum of these mice (Lam et al., 2011). The increased number of activated microglia in striatum and SN at 5–6 months of age was accompanied by a decrease in the number of resting microglia, suggesting a switch to the activated phenotype rather than cell proliferation. The greatest microglia activation was observed in the SN of Thy1-aSyn mice at 5–6 months, with a microglial morphology resembling that of PD patients, including enlarged cell body and short stubby processes (McGeer et al., 1988).
Microglial activation persisted in both the striatum and SN of 14 month old Thy1-aSyn mice, which coincides with the time at which striatal dopamine and tyrosine hydroxylase loss becomes evident in these mice (Lam et al., 2011). MHCII-positive microglia were also observed in the striatum of 14 month old Thy1-aSyn mice, suggesting a switch in the microglial phenotype in this region from ‘activated’ to ‘antigen-presenting cell’. In contrast to the early presence of MHC II positive cells in models of alpha-synuclein over-expression by viral transfection which result in rapid neuronal cell loss (Sanchez-Guajardo et al. 2010), we did not observe MHC II immunoreactivity in the striatum or SN of WT and Thy1-aSyn mice at 1 and 5–6 months of age. This is compatible with evidence for minimal and late involvement of the adaptive immune response in this model, as indicated by the delayed (22 months of age) increase in C4+T cells in blood. Similar to the early morphological changes, MHCII staining of microglia was not observed outside the striatum, including the SN at 14 months, again suggesting earlier inflammatory changes in the region that contains the terminals of the nigrostriatal dopaminergic neurons.
Although microglia were not activated in the cerebral cortex of 14 month old Thy1-aSyn mice, alterations in the microglial phenotype were first observed in this region at this age, with a significant increase in microglia with cell body diameters of 5μm. These microglia have an intermediate morphology between ‘resting’ and ‘activated’ microglia, and are often termed ‘hyper-ramified’ due to their medium-size cell body and long branching processes. Overall, our observations suggest that, similar to what is observed in PD patients (Ouchi et al., 2005), microglial activation may occur first in the nigrostriatal pathway of the Thy1-aSyn mice, and then spread to cortical areas at later ages.
Regional selectivity of microglial activation in young Thy1-aSyn mice
An unexpected observation in this study was the regional specificity of microglial activation in the Thy1-aSyn mice, despite the sustained broad distribution of alpha-synuclein over-expression. This suggests that alpha-synuclein expression per se is not sufficient to cause microglia activation, because otherwise microglia should have been activated in the cerebral cortex and cerebellum of the Thy1 mice. Similarly, the pattern of microglial activation does not parallel the density of alpha-synuclein aggregates, which are dense in the cerebellum (C. Zhu and M-F Chesselet, unpublished observations). The underlying mechanism for this specificity is not known, but one possibility is a clear difference in microglia densities between different brain regions. Studies have shown that under normal conditions the SN, putamen and hippocampus have the highest densities of microglia in the brain (Lawson et al., 1990). Pathological processes in neurons of the nigrostriatal pathway, as for example accumulation of alpha-synuclein, might result in relatively fast and pronounced response of the dense microglia population. Indeed, several lines of evidence support the idea that alpha-synuclein is released from neurons and activates microglia directly. Indeed, alpha-synuclein can be released from cells in response to neural activity or via endoplasmic reticulum/Golgi-independent exocytosis (Fortin et al., 2005; Lee et al., 2005; Desplats et al., 2009; Alvarez-Erviti et al., 2011, Emmanouilidou et al., 2011). Once released, alpha-synuclein can bind to and activate microglial cells, and this has been demonstrated in rat primary cultures, human microglial cultures and a monocytic cell line, THP-1, following treatment with exogenous alpha-synuclein (Zhang et al., 2005; Klegeris et al., 2008).
Recent evidence suggests that alpha-synuclein can increase the expression of TLRs on microglia, resulting in microglial activation and pro-inflammatory cytokine production (Beraud et al., 2011). In particular, activation of TLR2 signaling by alpha synuclein was demonstrated using computation modeling of signaling network and by experimental validation in TLR2-deficient microglia in vitro (Seung-Jae Lee, 2011). Stimulation of microglia with TLR agonists leads to activation and up regulation of innate and effector immune cytokines (Olson and Miller, 2004). Here, we show that TLR 1, TLR 4 and TLR 8 are significantly increased in the substantia nigra of 5–6 month old Thy1-aSyn mice and this coincides with the initiation of microglial activation and TNF-α expression in the SN. In view of the absence of TLR expression and microglia activation in the cerebral cortex at this age, our data suggest that TLR expression may account for the regional specificity of microglial activation observed in this model. However, these TLR were also not activated at 1 month in the striatum, despite the early microglial activation observed in this region. This suggests that mechanisms that trigger the very early inflammatory response observed in the striatum of Thy1 aSyn mice may differ from those triggering microglial activation in the region that contains the cell bodies of the nigrostriatal dopaminergic neurons. Alternatively, TLR activation in the striatum may precede microglial activation and not be detected in our studies.
At variance with data suggesting that TLR2 is critical for alpha-synuclein-induced microglial activation (Seung-Jae Lee, 2011), TLR2 mRNA was not increased in the SN at 1 or 5–6 months, i.e., before or during microglia activation in this region. However, TLR2 mRNA was significantly increased in SN at 14 months, suggesting that, rather than initiating microglial activation, TLR2 may be involved in the maintenance of microglia activation.
Pattern of cytokine expression in Thy1-aSyn mice
Our study also addressed the functional significance of microglial activation in young Thy1-aSyn mice. Microglia have multiple phenotypes, such as a classically activated phenotype that produces pro-inflammatory cytokines and an alternatively activated phenotype that produces mainly anti-inflammatory/regulatory cytokines (Appel et al., 2010). We found that, whereas TNF alpha, a pro-inflammatory cytokine, was increased in parallel to microglial activation in striatum and SN, other pro- (IL-1β) and anti-inflammatory (TGF-β) cytokines remained unchanged. This suggests that the activated microglia specifically identified in the striatum are of the ‘pro-inflammatory’ phenotype and may play a role early in disease pathogenesis. There is a large body of in vitro evidence demonstrating the release of TNF-α upon stimulation of microglial cells with wild-type alpha-synuclein (Zhang et al., 2005; Klegeris et al., 2008). Importantly, dopaminergic neurons have been shown to be sensitive to the toxic effects of TNF-α, and specific blockade of TNF-α signaling via administration of a soluble dominant negative TNF-α factor inhibitor is sufficient to reduce dopaminergic cell loss induced by the oxidative neurotoxin 6-hydroxydopamine (6-OHDA) in rats (McCoy et al., 2008). Furthermore, both genetic ablation of TNF-α and pharmacological inhibition of TNF-synthesis attenuated MPTP toxicity in mouse striatum (Ferger et al., 2004).
In contrast to our own data using the Thy-1 mediated over-expression of wildtype alpha-synuclein, overexpression induced with a viral vector caused the increased production of a broader range of pro-inflammatory cytokines, including TNF-α, IL-6. IL-1β and ICAM (Theodore et al., 2008). Viral vector-induced overexpression of alpha-synuclein leads to rapid and overt neuronal loss whereby neuropathology in the Thy1-aSyn mice develops over 14 months, before dopamine loss becomes evident (Lam et al. 2011). Therefore, it is not surprising that the inflammatory response differs in the two models and the response seen in the slowly progressing transgenics may be more akin to what occurs at early stages of the human disease, during the early “pre-manifest” phase that precedes the death of dopaminergic neurons. In contrast to our observations of a lack of microglial activation in cerebral cortex of Thy1-aSyn mice, Lee et al. (2011) reported a qualitative increase in immunostaining for CD11b, a microglial marker, and iNOS, a marker of microglial activation, in the cerebral cortex of Thy1-aSyn mice compared to WT. However, Lee and colleagues did not analyze the activation state of microglia using morphological parameters. Therefore the increased staining they have observed may represent increased expression of these markers in resting microglia. It is worthy to note, however, that we did not see any differences in the expression levels of the mRNA for the microglial marker CD11b between wildtype and Thy1-aSyn mice, at any age, in any of the regions examined.
It has been suggested that in the early stages of PD, activated microglia and cytokines may exert neuroprotective effects but may later become neurotoxic and promote the progression of PD (Sawada et al., 2006). However, our studies did not uncover any increases in anti-inflammatory cytokines, even in very young mice, and the data suggest that activated microglia with a ‘pro-inflammatory’ phenotype emerge long before the decline in dopamine and tyrosine hydroxylase levels, which occurs at 14 months of age in these mice (Lam et al., 2011). The temporal disconnect between microglial activation and loss of dopamine in this model challenges the notion that inflammation plays a causal role in the demise of dopaminergic neurons that occurs in PD. Overwhelming epidemiological evidence, however, demonstrates that the long term use of anti-inflammatory drugs is protective against the risk of developing PD (Chen et al., 2005; Wahner et al., 2007a; Gao et al., 2011). Indeed, inflammation could be playing a major role at very early stages of the disease, which may occur decades before the onset of typical neurological symptoms (Lang and Obeso, 2004). This is strongly suggested by the early increase in microglial activation and proinflammatory cytokines we have observed, especially in the striatum. An early role of inflammation in PD is further supported by the observation that, in contrast to anti-inflammatory treatments started in young adulthood which show protection (Chen et al., 2005; Wahner et al., 2007a; Gao et al., 2011), the use of non-steroidal anti-inflammatory agents later in life does not decrease the risk of PD Rugbjerg et al. 2010). Together with these epidemiological data, our findings suggest that the use of anti-inflammatory agents in PD could only be preventive and is unlikely to ameliorate the outcome in symptomatic patients.
Peripheral markers of inflammation in Thy1-aSyn mice
Associated with microglial activation and the increased TNF-α observed in the striatum and SN, a significant increase in TNF-α protein concentration in the serum of Thy1-aSyn mice was documented in our study. This increase was not present at 1 month of age and is compatible with recent evidence for increased serum cytokines in PD patients (Brodacki et al., 2008; Reale et al., 2009). These studies have shown that, in addition to the central nervous system, the microenvironment of the peripheral immune system is also modified in PD. Since microglial activation and TNF-α production in the striatum precede the peripheral increase in TNF-α, it is possible that changes in the brain could affect immune cells in vivo and these effects could be transferred to the peripheral immune system. The increase in blood TNF-α in the Thy1-aSyn mice makes this model an ideal platform to assess the effects of anti-inflammatory compounds on PD pathology using a non-invasive peripheral index that could be translated to the clinic.
To further assess peripheral inflammation in the Thy1-aSyn mice, we measured the percentages of peripheral T cells in the blood. We confirmed that T cells endogenously express alpha synuclein, indicating that the use of the Thy1 promoter did not artificially drive the expression of alpha synuclein in these cells (which express Thy1) to cause a stimulated T cell response. We observed no differences in the percentages of circulating CD4+ and CD8+ T cells in blood between WT and Thy1-aSyn mice up to 14 months of age. However, we found a significant increase in the percentages of CD4+ and CD8+ T cells in the blood of 22 month old Thy1-aSyn mice, without simultaneous increase in CD8+ cells in tissue sections of the striatum. These observations, coupled with the late expression of the MHCII in the striatum of Thy1-aSyn mice, suggest that alpha-synuclein overexpression results in early activation of the innate immune system, and later adaptive immune activation, a scenario that may mimic the pathogenic process in PD patients. Indeed MHC class I and II molecules as well as T cells have been observed in the SN and STR of PD patients (Mogi et al., 1995; McGeer et al., 1988; Brochard et al., 2009). Whether microglial activation dictates this T cell response or vice versa in PD patients is unknown. However, toxin (MPTP) and other transgenic alpha synuclein mouse models have lent support to the idea that microglial proliferation and activation precedes T cell recruitment to the brain (Brochard et al., 2009, Sanchez-Guajardo et al., 2010).
In summary, our results indicate an early regionally specific microglial response in the striatum and SN of a mouse model of pre-manifest PD (summarized in Fig. 9). This work provides insight into the involvement of activated microglia, TLRs and TNF-α-mediated inflammation in the progression of PD-related pathology and may potentially herald new therapeutic targets. Importantly, these data help define the time at which anti-inflammatory interventions would most likely be effective in this disease by supporting evidence that anti-inflammatory agents are protective against the risk of developing PD only when administered long before the disease onset in humans (Chen et al., 2005; Wahner et al., 2007a; Rugbjerg et al. 2010; Gao et al., 2011).
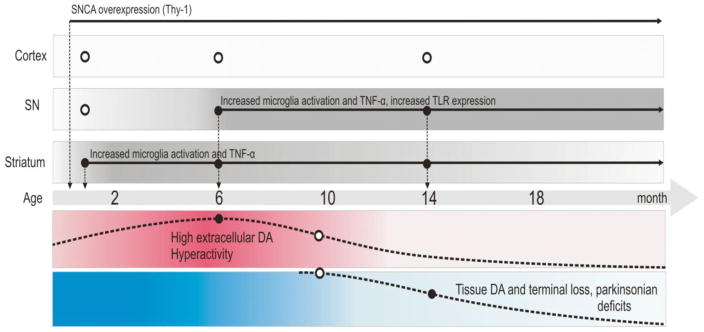
Fig. 9. Timeline of microglial activation and TNF-α in Thy1-aSyn mice.
Figure depicts the timeline of microglial activation; TNF-α production and TLR expression described in this manuscript in relation to other previously published phenotypic markers in the Thy1-aSyn mice (Lam et al., 2011, Chesselet and Richter, 2011). At 1 month of age, there is increased microglial activation and TNF-α expression in the striatum of Thy1-aSyn mice. By 5–6 months of age, increased microglial activation, TNF-α production occurs in both the striatum and substantia nigra and increased expression of TLR 1, 4 and 8 in the substantia nigra of Thy1-aSyn mice. These changes coincide with increased extracellular dopamine in the striatum of Thy1-aSyn mice and hyperactivity in the open field test (Lam et al., 2011). At 14 months of age, microglial activation is sustained in the striatum and substantia nigra with increased TNF-α expression and TLR 2 expression in the substantia nigra. This is accompanied by a reduction in tissue dopamine content, terminal loss and parkinsonian motor deficits (Lam et al., 2011). No increases in microglial activation or TNF-α expression were observed in the cerebral cortex at any age (open circles indicate no change).
Highlights.
Mouse model of PD that overexpresses alpha synuclein show microglial activation
Microglial activation occurs early and is progressive with age
Microglial activation is confined to the striatum and substantia nigra
The pro-inflammatory cytokine TNF-alpha is increased with microglia activation
Toll-like receptors are increased in substantia nigra and may activate microglia
Acknowledgments
The authors are grateful to Dr. Alan Garfinkel, UCLA for his help with statistical analyses. This work was supported by Morris K Udall Centre of Excellence in Parkinson’s disease research at UCLA (P50 NS38367), the centre for Gene Environment in Parkinson’s disease (P01 ES016732), the American Parkinson Disease Association Advances Centre at UCLA and gifts to the Center for the Study of Parkinson’s Disease at UCLA. None of the sponsors had any role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Abbreviations
- CRB
Cerebellum
- CTX
Cerebral cortex
- DA
dopaminergic
- IBA-1
ionized calcium binding adaptor molecule 1
- IL-1β
Interleukin-1 beta
- IP
intraperitoneal
- ND
not determined
- PBS
Phosphate buffered saline
- PFA
paraformaldehdye
- PD
Parkinson’s disease
- SPECT
Single photoemission tomography
- SN
Substantia nigra
- STR
Striatum
- TBS
Tris buffered saline
- TH
tyrosine hydroxylase
- Thy1-aSyn
mice overexpressing human wild-type alpha-synuclein under the Thy-1 promoter
- TGFβ
Transforming growth factor beta
- TNF-α
Tumor necrosis factor alpha
- TLR
Toll-like receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez-Erviti L, Couch Y, Richardson J, Cooper JM, Wood MJ. Alpha-synuclein release by neurons activates the inflammatory response in a microglial cell line. J Neurosci Res. 2011;69:337–342. doi: 10.1016/j.neures.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Appel SH, Beers DR, Henkel JS. T cell-microglial dialogue in Parkinson’s disease and amyotrophic lateral sclerosis, are we listening? Trends Immunol. 2010;31:7–17. doi: 10.1016/j.it.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba Y, Kuroiwa A, Uitti RJ, Wszolek ZK, Yamada T. Alterations of T-lymphocyte populations in Parkinson disease. Parkinsonism Relat Disord. 2005;11:493–498. doi: 10.1016/j.parkreldis.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Bartels AL, Leenders KL. Neuroinflammation in the pathophysiology of Parkinson’s disease: evidence from animal models to human in vivo studies with [11C]-PK11195 PET. Mov Disord. 2007;22:1852–1856. doi: 10.1002/mds.21552. [DOI] [PubMed] [Google Scholar]
- Batchelor PE, Liberatore GT, Wong JY, Porritt MJ, Frerichs F, Donnan GA, Howells DW. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci. 1999;19:1708–1716. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraud D, Twomey M, Bloom B, Mittereder A, Ton V, Neitzke K, Chasovskikh S, Mhyre TR, Maguire-Zeiss KA. Alpha-Synuclein Alters Toll-Like Receptor Expression. Front Neurosci. 2011;5:80. doi: 10.3389/fnins.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodacki B, Staszewski J, Toczylowska B, Kozlowska E, Drela N, Chalimoniuk M, Stepien A. Serum interleukin (IL-2, IL-10, IL-6, IL-4), TNFalpha, and INFgamma concentrations are elevated in patients with atypical and idiopathic parkinsonism. Neurosci Lett. 2008;441:158–162. doi: 10.1016/j.neulet.2008.06.040. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- Chen H, Jacobs E, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ, Ascherio A. Nonsteroidal antiinflammatory drug use and the risk for Parkinson’s disease. Ann Neurol. 2005;58:963–967. doi: 10.1002/ana.20682. [DOI] [PubMed] [Google Scholar]
- Chesselet MF, Richter F. Modeling of Parkinson’s disease in mice. Lancet Neurology. 2011;10:1108–1118. doi: 10.1016/S1474-4422(11)70227-7. [DOI] [PubMed] [Google Scholar]
- Clarke RM, O’Connell F, Lyons A, Lynch MA. The HMG-CoA reductase inhibitor, atorvastatin, attenuates the effects of acute administration of amyloid-beta1–42 in the rat hippocampus in vivo. Neuropharmacology. 2007;52:136–145. doi: 10.1016/j.neuropharm.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Czlonkowska A, Kurkowska-Jastrzebska I, Czlonkowski A, Peter D, Stefano GB. Immune processes in the pathogenesis of Parkinson’s disease - a potential role for microglia and nitric oxide. Med Sci Monit. 2002;8:RA165–177. [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. Statistical data analysis in the computer age. Science. 1991;253:390–395. doi: 10.1126/science.253.5018.390. [DOI] [PubMed] [Google Scholar]
- Emmanouilidou E, Elenis D, Papasilekas T, Stranjalis G, Gerozissis K, Ioannou PC, Vekrellis K. Assessment of alpha-synuclein secretion in mouse and human brain parenchyma. PLoS One. 2011;6:e22225. doi: 10.1371/journal.pone.0022225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Ferger B, Leng A, Mura A, Hengerer B, Feldon J. Genetic ablation of tumor necrosis factor-alpha (TNF-alpha) and pharmacological inhibition of TNF-synthesis attenuates MPTP toxicity in mouse striatum. J Neurochem. 2004;89:822–833. doi: 10.1111/j.1471-4159.2004.02399.x. [DOI] [PubMed] [Google Scholar]
- Fernagut PO, Hutson CB, Fleming SM, Tetreaut NA, Salcedo J, Masliah E, Chesselet MF. Behavioral and histopathological consequences of paraquat intoxication in mice: effects of alpha-synuclein over-expression. Synapse. 2007;61:991–1001. doi: 10.1002/syn.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Chesselet MF. Behavioral phenotypes and pharmacology in genetic mouse models of Parkinsonism. Behav Pharmacol. 2006;17:383–391. doi: 10.1097/00008877-200609000-00004. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, Chesselet MF. Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J Neurosci. 2004;24:9434–9440. doi: 10.1523/JNEUROSCI.3080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin DL, Nemani VM, Voglmaier SM, Anthony MD, Ryan TA, Edwards RH. Neural activity controls the synaptic accumulation of alpha-synuclein. J Neurosci. 2005;25:10913–10921. doi: 10.1523/JNEUROSCI.2922-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs J, Nilsson C, Kachergus J, Munz M, Larsson EM, Schule B, Langston JW, Middleton FA, Ross OA, Hulihan M, Gasser T, Farrer MJ. Phenotypic variation in a large Swedish pedigree due to SNCA duplication and triplication. Neurology. 2007;68:916–922. doi: 10.1212/01.wnl.0000254458.17630.c5. [DOI] [PubMed] [Google Scholar]
- Gao X, Honglei C, Schwarzchild M, Ascherio A. Use of ibuprofen and risk of Parkinson’s disease. Neurology. 2011;76:863–869. doi: 10.1212/WNL.0b013e31820f2d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis. 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Distribution of Major Histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 2003;106:518–26. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- Klegeris A, Pelech S, Giasson BI, Maguire J, Zhang H, McGeer EG, McGeer PL. Alpha-synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiol Aging. 2008;29:739–752. doi: 10.1016/j.neurobiolaging.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Lam HA, Wu N, Cely I, Kelly RL, Hean S, Richter F, Magen I, Cepeda C, Ackerson LC, Walwyn W, Masliah E, Chesselet MF, Levine MS, Maidment NT. Elevated tonic extracellular dopamine concentration and altered dopamine modulation of synaptic activity precede dopamine loss in the striatum of mice overexpressing human α-synuclein. J Neurosci Res. 2011;89:1091–102. doi: 10.1002/jnr.22611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci. 2005;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Chen W, Junn E, Im JY, Grosso H, Sonsalla PK, Feng X, Ray N, Fernandez JR, Chao Y, Masliah E, Voronkov M, Braithwaite SP, Stock JB, Mouradian MM. Enhanced phosphatase activity attenuates alpha-Synucleinopathy in a mouse model. J Neurosci. 2011;31:6963–6971. doi: 10.1523/JNEUROSCI.6513-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letiembre M, Liu Y, Walter S, Hao W, Pfander T, Wrede A, Schulz-Schaeffer W, Fassbender K. Screening of innate immune receptors in neurodegenerative diseases: a similar pattern. Neurobiol Aging. 2009;30:759–768. doi: 10.1016/j.neurobiolaging.2007.08.018. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Ruhn KA, Martinez TN, McAlpine FE, Blesch A, Tansey MG. Intranigral lentiviral delivery of dominant-negative TNF attenuates neurodegeneration and behavioral deficits in hemiparkinsonian rats. Mol Ther. 2008;16:1572–1579. doi: 10.1038/mt.2008.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–91. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, Nagatsu T. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett. 1994;180:147–150. doi: 10.1016/0304-3940(94)90508-8. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Kondo T, Riederer P, Nagatsu T. Brain beta 2-microglobulin levels are elevated in the striatum in Parkinson’s disease. J Neural Transm Park Dis Dement Sect. 1995;9:87–92. doi: 10.1007/BF02252965. [DOI] [PubMed] [Google Scholar]
- Mogi M, Nagatsu T. Neurotrophins and cytokines in Parkinson’s disease. Adv Neurol. 1999;80:135–139. [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson’s disease. J Neural Transm Suppl. 2000:277–290. doi: 10.1007/978-3-7091-6301-6_19. [DOI] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Yagi S, Yokokura M, Sakamoto M. Neuroinflammation in the living brain of Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S200–204. doi: 10.1016/S1353-8020(09)70814-4. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, Torizuka T. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann Neurol. 2005;57:168–175. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Przedborski S. Inflammation and Parkinson’s disease pathogenesis. Mov Disord. 2010;25(Suppl 1):S55–57. doi: 10.1002/mds.22638. [DOI] [PubMed] [Google Scholar]
- Rappold PM, Lynd-Balta E, Joseph SA. P2X7 receptor immunoreactive profile confined to resting and activated microglia in the epileptic brain. Brain Res. 2006;1089:171–178. doi: 10.1016/j.brainres.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Reale M, Iarlori C, Thomas A, Gambi D, Perfetti B, Di Nicola M, Onofrj M. Peripheral cytokines profile in Parkinson’s disease. Brain Behav Immun. 2009;23:55–63. doi: 10.1016/j.bbi.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Rhodes SL, Sinsheimer JS, Bordelon Y, Bronstein JM, Ritz B. Replication of GWAS associations for GAK and MAPT in Parkinson’s disease. Ann Hum Genet. 2011;75:195–200. doi: 10.1111/j.1469-1809.2010.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, Masliah E. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res. 2002;68:568–578. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- Rugbjerg K, Friis S, Jørgensen TL, Ritz B, Korbo L, Olsen JH. Risk for Parkinson’s disease among patients with osteoarthritis: a Danish cohort study. Mov Disord. 2010;25:2355–60. doi: 10.1002/mds.23274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Guajardo V, Febbraro F, Kirik D, Romero-Ramos M. Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson’s disease. PLoS One. 2010;5:e8784. doi: 10.1371/journal.pone.0008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M, Imamura K, Nagatsu T. Role of cytokines in inflammatory process in Parkinson’s disease. J Neural Transm. 2006;70:373–381. doi: 10.1007/978-3-211-45295-0_57. [DOI] [PubMed] [Google Scholar]
- Scalzo P, Kummer A, Cardoso F, Teixeira AL. Serum levels of interleukin-6 are elevated in patients with Parkinson’s disease and correlate with physical performance. Neurosci Lett. 2009;468:56–58. doi: 10.1016/j.neulet.2009.10.062. [DOI] [PubMed] [Google Scholar]
- Seung-Jae LeE. Pathological Neuron-glia interaction through extracellular alpha-synuclein. Satelite meeting of the 23rd Biennial meeting: From genes to pathogenesis: The evolving spectrum of syncleinopathies; 2011. Abstract. [Google Scholar]
- Smith JA, Das A, Ray SK, Banik NL. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull. 2011;87:10–20. doi: 10.1016/j.brainresbull.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Federoff HJ, Maguire-Zeiss KA. Mutant alpha-synuclein overexpression mediates early proinflammatory activity. Neurotox Res. 2009;16:238–254. doi: 10.1007/s12640-009-9053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ. Synuclein activates microglia in a model of Parkinson’s disease. Neurobiol Aging. 2008;29:1690–1701. doi: 10.1016/j.neurobiolaging.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambuyzer BR, Ponsaerts P, Nouwen EJ. Microglia: gatekeepers of central nervous system immunology. J Leukoc Biol. 2009;85:352–370. doi: 10.1189/jlb.0608385. [DOI] [PubMed] [Google Scholar]
- Tansey MG, Goldberg MS. Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis. 2010;37:510–518. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore S, Cao S, McLean PJ, Standaert DG. Targeted overexpression of human alpha-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. J Neuropathol Exp Neurol. 2008;67:1149–1158. doi: 10.1097/NEN.0b013e31818e5e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski JQ, Lee VM. Parkinson’s disease and related alpha-synucleinopathies are brain amyloidoses. Ann NY Acad Sci. 2003;991:107–110. doi: 10.1111/j.1749-6632.2003.tb07468.x. [DOI] [PubMed] [Google Scholar]
- Wahner AD, Bronstein JM, Bordelon YM, Ritz B. Nonsteroidal anti-inflammatory drugs may protect against Parkinson disease. Neurology. 2007a;69:1836–1842. doi: 10.1212/01.wnl.0000279519.99344.ad. [DOI] [PubMed] [Google Scholar]
- Wahner AD, Sinsheimer JS, Bronstein JM, Ritz B. Inflammatory cytokine gene polymorphisms and increased risk of Parkinson disease. Arch Neurol. 2007b;64:836–840. doi: 10.1001/archneur.64.6.836. [DOI] [PubMed] [Google Scholar]
- Wu N, Joshi PR, Cepeda C, Masliah E, Levine MS. Alpha synuclein overexpression in mice alters synaptic communication in the corticostriatal pathway. J Neurosci Res. 2010;88:1764–76. doi: 10.1002/jnr.22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]