Abstract
Objective
Nigrostriatal reserve refers to the threshold of neuronal injury to dopaminergic cell bodies and their terminal fields required to produce parkinsonian motor deficits. Inferential studies have estimated striatal dopamine reserve to be at least 70%. Knowledge of this threshold is critical for planning interventions to prevent symptom onset or reverse nigrostriatal injury sufficient to restore function in people with Parkinson disease. In this study, we determine the nigrostriatal reserve in a non-human primate model that mimics the motor manifestations of Parkinson disease.
Methods
Fifteen macaque monkeys received unilateral randomized doses of the selective dopaminergic neuronal toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. We compared blinded validated ratings of parkinsonism to in vitro measures of striatal dopamine and unbiased stereologic counts of nigral neurons after tyrosine hydroxylase immunostaining.
Results
The percent of residual cell counts in lesioned nigra correlated linearly with the parkinsonism score at 2 months (r = −0.87, p <0.0001). The parkinsonism score at 2 months correlated linearly with the percent residual striatal dopamine (r = −0.77, p = 0.016) followed by a flooring effect once nigral cell loss exceeded 50%. A reduction of about 14 to 23% of nigral neuron counts or 14 to 37% of striatal dopamine was sufficient to induce mild parkinsonism.
Conclusions
The nigral cell body and terminal field injury needed to produce parkinsonian motor manifestations may be much less than previously thought.
Keywords: Parkinsonism, MPTP, substantia nigra, dopamine
Introduction
Degeneration of nigrostriatal neurons with striatal dopamine deficiency produces many of the motor manifestations of idiopathic Parkinson disease (PD) (Ehringer and Hornykiewicz, 1960). The functional reserve of the nigrostriatal pathway, which is the required degree of loss of either dopaminergic nigral cell bodies or terminal fields in the striatum to produce manifestations of parkinsonism, is critical for planning interventions to prevent progression of pathology before producing symptoms or reverse nigrostriatal injury to restore function.
Early studies in humans suggested that motor symptoms do not manifest until striatal dopamine deficiency reaches at least 70% loss (Bernheimer et al., 1973; Riederer and Wuketich, 1976). An often quoted postmortem study (Bernheimer et al., 1973) reported people with a variety of diseases developed mild parkinsonism after striatal dopamine decreased 70–80% in caudate with a much greater loss in putamen. Another study (Riederer and Wuketich, 1976) in 1976 measured dopamine in the caudate rather than putamen although it is now known that PD preferentially affects putamen (Scherman et al., 1989) and that motor function depends more upon putamen than caudate integrity (Mink, 1996). By extrapolating dopamine content from age at death to age at symptom onset in a group of chronically treated patients, they calculated that symptoms began when dopamine loss in the caudate was 68% to 82%. Postmortem studies in people with incidental Lewy bodies and no history of parkinsonism revealed concordant reduction of about 50% to 70% striatal dopamine and about 50% of marker of vesicular monoamine transporter type 2 (VMAT2) (Scherman et al., 1989) and tyrosine hydroxylase protein (Beach et al., 2008). Such postmortem studies likely result in inaccurate dopamine measurements (Carlsson and Winblad, 1976).
Other research focused on dopaminergic cell body loss in substantia nigra (SN). By extrapolating neuronal counts at death in people with different durations of illness, estimates of the extent of the loss of nigral neurons at symptom onset were calculated to be as high as 50% (Ross et al., 2004). However, nigral neurons were counted only on a single, transverse caudal midbrain section for each of 10 PD patients. A similar study of 20 PD patients (Fearnley and Lees, 1991) estimated that only a 31% of neuronal loss was sufficient to trigger clinical manifestations whereas asymptomatic people with incidental Lewy bodies had an average of 27% neuron loss in SN. Two postmortem studies using more rigorous quantitative methods, such as using a tessellation method (Greffard et al., 2006) and the dissector-based neuromorphometry (Ma et al., 1997), found that about 30% of nigral neuronal loss appeared sufficient to cause motor symptoms in PD (Beach et al., 2008). Thus, substantial variability in estimates of nigrostriatal reserve in humans may reflect imprecision in retrospective identification of symptom onset and in quantification of either nigral cell loss or striatal dopamine content.
Molecular imaging with radioligands to measure striatal dopamine or nigral neurons in vivo could help determine the degree of nigrostriatal injury needed to produce parkinsonism or permit measurement of pathologic progression of disease. However, these methods still need further validation to determine how accurately they measure striatal dopamine or nigral cell loss (Brooks et al., 2003; Ravina et al., 2005; Yee et al., 2001). Despite these limitations, molecular imaging studies suggest PD symptom onset begins with a loss of uptake in putamen of 20–50% measured with 6-[18 F] fluorodopa (FD), 50–70% measured with radioligands for the dopamine transporter (DAT) or VMAT2 (Bohnen et al., 2006; Nandhagopal et al., 2008; Lee et al., 2000). Interestingly, PET measures of the striatal uptake of the VMAT2 radioligand [11C]dihydrotetrabenazine contralateral to the clinically unaffected body side was reduced by 73% in 16 patients with early PD at Hoehn and Yahr stage 1 or 1.5 (Bohnen et al., 2006). The authors concluded that the development of motor manifestations required a substantial degree of nigrostriatal pathology due to a high nigrostriatal reserve. The different findings regarding nigrostriatal reserve among radiotracers may be due to differences in sensitivity of the different methods or compensatory upregulation of aromatic amino acid decarboxylase increasing FD uptake, should such differential compensation occur.
One-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) produces a reasonably faithful nonhuman primate model of PD with regard to reduction of nigrostriatal neurons (Tabbal et al., 2006; Langston et al., 1983; Ballard et al., 1985), motor behavior (Tabbal et al., 2006) and response to pharmacotherapy including development of drug-induced dyskinesias. This animal model provides a good system to investigate the interplay between motor behavior, striatal dopamine and nigral dopaminergic neuron loss. Although the mechanism of injury of nigrostriatal neurons differs between PD in humans and MPTP-induced injury in non-human primates, the animal model permits prospective measures of motor behavior that correspond with measurements of striatal dopamine and nigral neuronal counts at various degrees of nigrostriatal injury in a measurable and controlled fashion compared to observational human studies.
The goal of this study is to determine the thresholds of nigral cell body and terminal field injury needed to produce parkinsonian motor manifestations in MPTP-treated monkeys that have never been treated with any dopaminergic drugs.
Subjects and Methods
Ethics Statement
We used the minimum number of animals necessary for this research and followed all relevant national and international guidelines. In accordance with the recommendations of the Weatherall report “The use of non-human primates in research,” we took all steps to minimize any discomfort or suffering to the animals. The welfare of the animals conformed to the requirements of National Institutes of Health (NIH), and we followed the guidelines prescribed by the NIH Guide for the Care and Use of Laboratory Animal. This study was conducted at the Nonhuman Primate Facility of Washington University in St. Louis with approval from its Institutional Animal Care and Use Committee (IACUC). The protocol number is 20110161 and was last approved on July 8, 2011. All animals were housed in the same room in cages meeting or exceeding the stipulated size requirements. Animals were maintained in facilities with 12-hour dark and light cycles, given access to food and water ad libitum; all animals were equally engaged with a variety of psychologically enriching tasks such as watching movies or playing with appropriate toys to avoid effects of sensory deprivation. No animal was knowingly exposed to potential infection. Humane endpoints were pre-defined in this protocol and applied as a measure if necessary to reduce any discomfort.
Subjects
We studied 15 male macaque monkeys, 12 M. fascicularis and 3 M. nemestrina (monkey #4, #5 and #13) before and after unilateral internal carotid artery infusion of various doses of MPTP. The mean age of the animals was 5.3 ±1 years (3.5 to 6.4 years).
MPTP Infusion
Unilateral internal carotid artery infusion was done under angiographic control in anesthetized animals, as described elsewhere (Tabbal et al., 2006). A randomized dose between 0 to 0.31 mg/kg of MPTP (Sigma, St. Louis, MO.) in normal saline was infused in a concentration of 0.1 mg/ml no faster than 1 ml per minute. Angiograms done before and after the infusion documented maintenance of proper catheter position and good distal blood flow. Proper safety procedures were followed for handling MPTP and all contaminated tissues and waste products (Tian et al., 2012).
Behavioral evaluations
Each animal was videotaped at the same time of day (early morning) several times before MPTP treatment for baseline behavioral ratings, then several times per week after MPTP until euthanasia. Animals were trained using positive conditioning only to follow a behavioral protocol that consisted of a) walking back and forth 3 times in a straight corridor for 15 feet, b) walking in a circle 6 times in a room (3 clockwise and 3 counterclockwise turns) and c) reaching 10 times with each hand at full arm length for a food item held by the trainer. Each behavioral session was video recorded and the same observer blinded to the MPTP dose rated each video using a scale previously developed and validated for non-human primate studies (Tabbal et al., 2006; Perlmutter et al., 1997). Briefly, ratings included a 0 to 3 scale (0 = unaffected, 1 = mildly affected, 2 = moderately affected and 3 = severely affected) for parkinsonian features (bradykinesia, tremor and flexed posturing). This yielded a composite parkinsonism score ranging from 0 to 18 per side. We used for the analysis the parkinsonism score at 2 months after MPTP just prior to euthanasia. Prior to data collection, we conservatively defined the threshold of behavioral parkinsonism as a score of at least 2 points to avoid possible overlap with normal behavior since we had not previously blindly rated any control animal with a score of greater than zero (Tabbal et al., 2006; Perlmutter et al., 1997). Animals were always able to care for themselves despite not receiving any anti-parkinsonian drugs at any time.
In Vitro Measures
Animals were euthanized 2 months after MPTP with intravenous pentobarbital (100 mg/kg; Somnasol Euthanasia, Butler Schein Animal Health, Dublin, OH). The brain was removed within 10 minutes and the hemispheres and midbrain were separated. The hemispheres were sliced in the coronal plane. Standard punch biopsies were collected from caudate and putamen bilaterally, frozen quickly on dry ice snow and stored in a −80 degrees Celsius freezer until dopamine assay. The midbrain was fixed in 4% paraformaldehyde in 0.1 M phosphate buffered solution (PBS). After 7 days, the midbrain was transferred to 30% sucrose for 7 days, and then cut on a freezing microtome in serial, free-floating 50 µm-thick transverse sections. A random series of every 6th section was processed for tyrosine hydroxylase (TH) immunocytochemistry.
Tyrosine hydroxylase immunohistochemistry and stereology
Sections were incubated with rabbit anti-TH (1:1000; Chemicon International, Inc., Temecula, CA), reacted with biotin-conjugated universal secondary antibody provided in Universal Vectastain (1:200; Vector Laboratories, Burlingame, CA), incubated in streptavidin-biotin complex (Vectastain ABC Kit Elite, Vector Laboratories, CA) and then incubated in 3,3'-diaminobenzidine (DAB) solution with nickel enhancement (Vector Laboratories, Burlingame, CA) for visualization as previously described (Tian et al., 2012). Sections were counterstained with cresyl violet. Unbiased stereological counts of nigral neurons followed procedures defined by Gundersen et al (Gundersen et al., 1988a; Gundersen et al., 1988b) using an Olympus BX41 Microscope with a proscan stage kit and DP70 digital camera with newCAST™ software (Visiopharm, Denmark). The SN pars compacta was defined as the region ventral to the medial leminiscus and lateral to the third cranial nerve fibers. We used a Visiopharm Integrator System (version 3.2.10.0) to count TH-stained nigral cells as described elsewhere (Tian et al., 2012). The percent residual cell count in nigra on the MPTP injected side was calculated for each animal as the ratio of cell count on the MPTP-treated side divided by that on the control side, respectively. These measures were done blinded to MPTP dose, clinical ratings or knowledge of striatal dopamine content. We repeated the nigral cell counts in control and severely-lesioned monkeys to determine the coefficient of error (CE) of the stereological method. CE values were ≤ 0.045 for control and lesioned monkeys, which meet the criteria of acceptable CE values as previously established elsewhere (Gundersen and Jensen, 1987).
Striatal dopamine measurements
A blinded investigator measured dopamine levels in striatal tissue samples using high-performance liquid chromatography (HPLC) with electrochemical detection (Karimi et al., 2006; Namavari et al., 1992; Taylor et al., 1991; Tian et al., 2012). All measures were done in duplicate. Striatal dopamine levels were expressed in microgram per gram of wet tissue. The striatal dopamine and nigral cell counts of 14 of these animals have been reported in a previous publication that did not include the behavioral measures (Tian et al., 2012).
Statistics
Averages are listed with standard errors. Regression analyses were performed using OriginLab software (version 7.5 SR4) to calculate the Pearson’s coefficients (r). Two-sample paired t-tests were used to compare data sets between the MPTP-treated and control sides.
Results
All 15 monkeys completed the study protocol successfully and were euthanized at 2 months after MPTP infusion. Table 1 summarizes the demographic characteristics, behavioral, biochemical and morphological profiles of all monkeys.
Table 1.
Demographic, behavioral, biochemical and morphological profile of all monkeys.
| Striatal dopamine (in microgram/gram of wet tissue) |
TH-staining nigral cell counts (in 1000 cells per side) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Monkey number |
Age at MPTP |
MPTP dose in mg/kg |
Parkinsonism Score at 2 months |
Control side |
MPTP- lesioned side |
% residual dopamine in injected side |
Control side |
MPTP- lesioned side |
% residual cell count in injected nigra |
| 1 | 5.6 | 0 | 0 | 15.1 | 15.6 | 103 | 32.3 | 28.6 | 89 |
| 2 | 5.8 | 0 | 0 | 26.7 | 28.7 | 107 | 51.5 | 40.1 | 78 |
| 3 | 3.5 | 0.07 | 3 | 13.9 | 5.2 | 37 | 50.5 | 34.4 | 68 |
| 4 | 5.6 | 0.08 | 3 | 25.5 | 21.9 | 86 | 48.4 | 39.0 | 81 |
| 5 | 6.4 | 0.12 | 3 | 14.0 | 7.0 | 50 | 54.6 | 39.6 | 73 |
| 6 | 3.5 | 0.13 | 0 | 21.8 | 13.8 | 64 | 50.9 | 44.0 | 86 |
| 7 | 3.7 | 0.13 | 2 | 16.4 | 13.9 | 84 | 50.1 | 43.1 | 86 |
| 8 | 5.3 | 0.13 | 6 | 27.3 | 0.4 | 2 | 31.7 | 15.5 | 49 |
| 9 | 6.3 | 0.14 | 8 | 21.0 | 0.2 | 1 | 40.8 | 8.0 | 20 |
| 10 | 6.3 | 0.14 | 4 | 25.2 | 14.8 | 59 | 39.5 | 31.9 | 81 |
| 11 | 6.4 | 0.14 | 5 | 14.3 | 0.2 | 1 | 37.2 | 18.6 | 50 |
| 12 | 4.1 | 0.19 | 6 | 27.6 | 1.1 | 4 | n/a | n/a | n/a |
| 13 | 5.1 | 0.20 | 7 | 16.0 | 0.3 | 2 | 32.4 | 12.9 | 40 |
| 14 | 5.5 | 0.24 | 6 | 20.2 | 0.7 | 3 | 49.5 | 7.9 | 16 |
| 15 | 5.6 | 0.31 | 7 | 30.9 | 0.5 | 2 | 39.9 | 18.7 | 47 |
Behavioral responses to MPTP
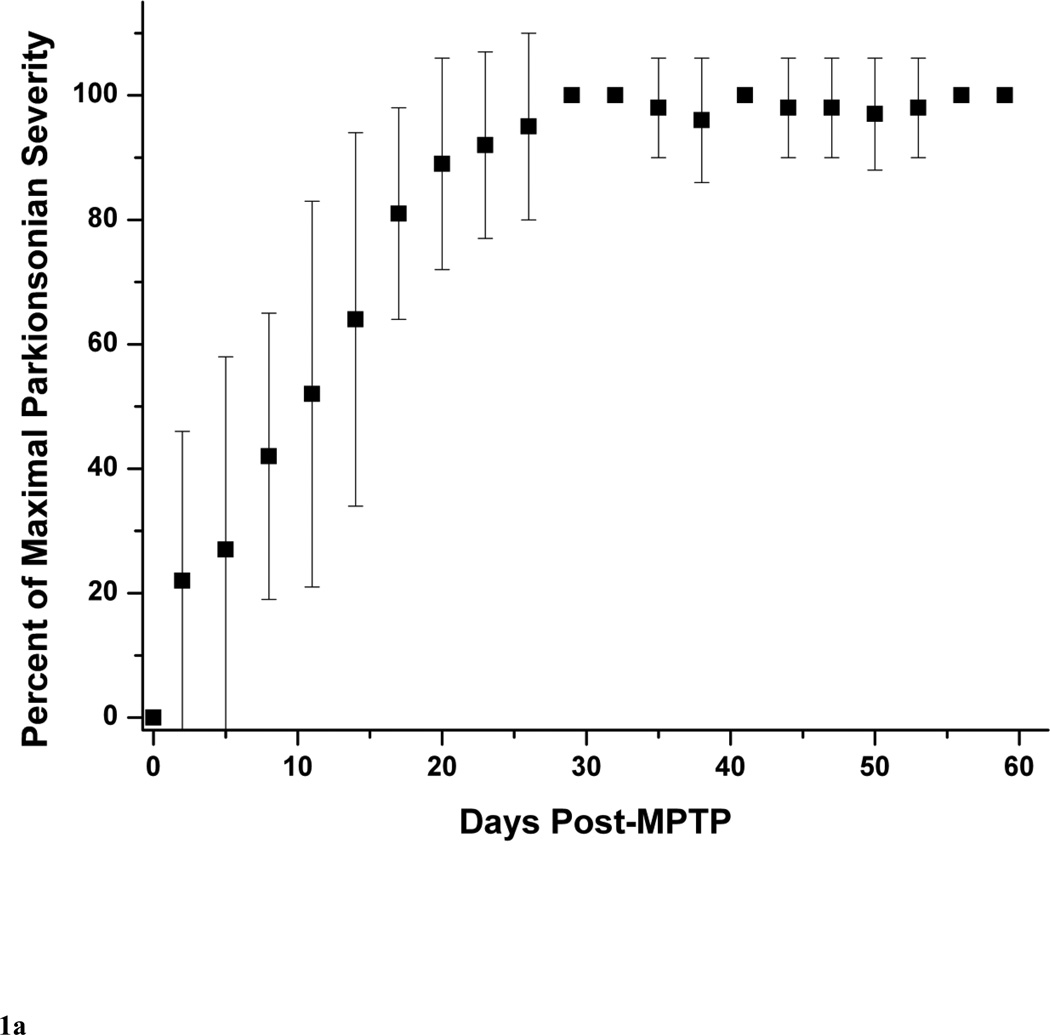
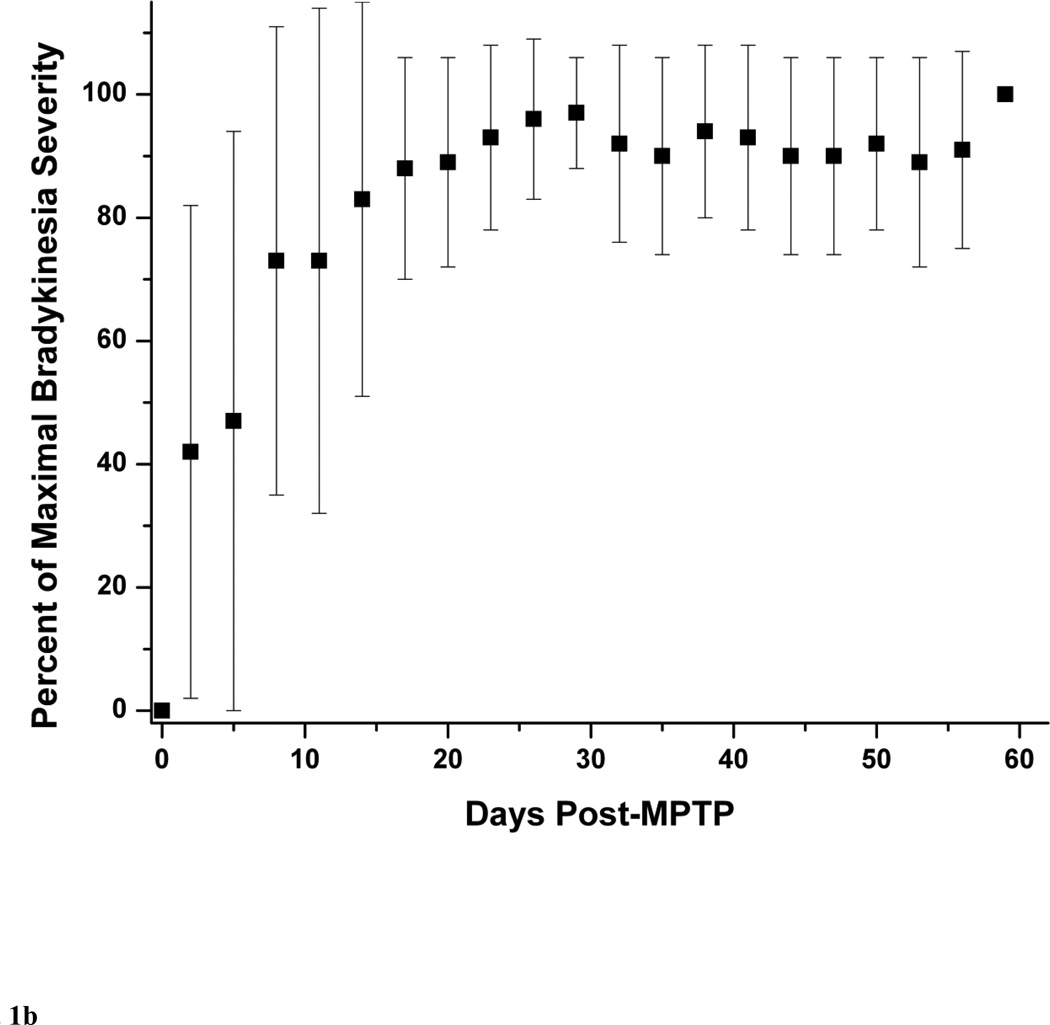
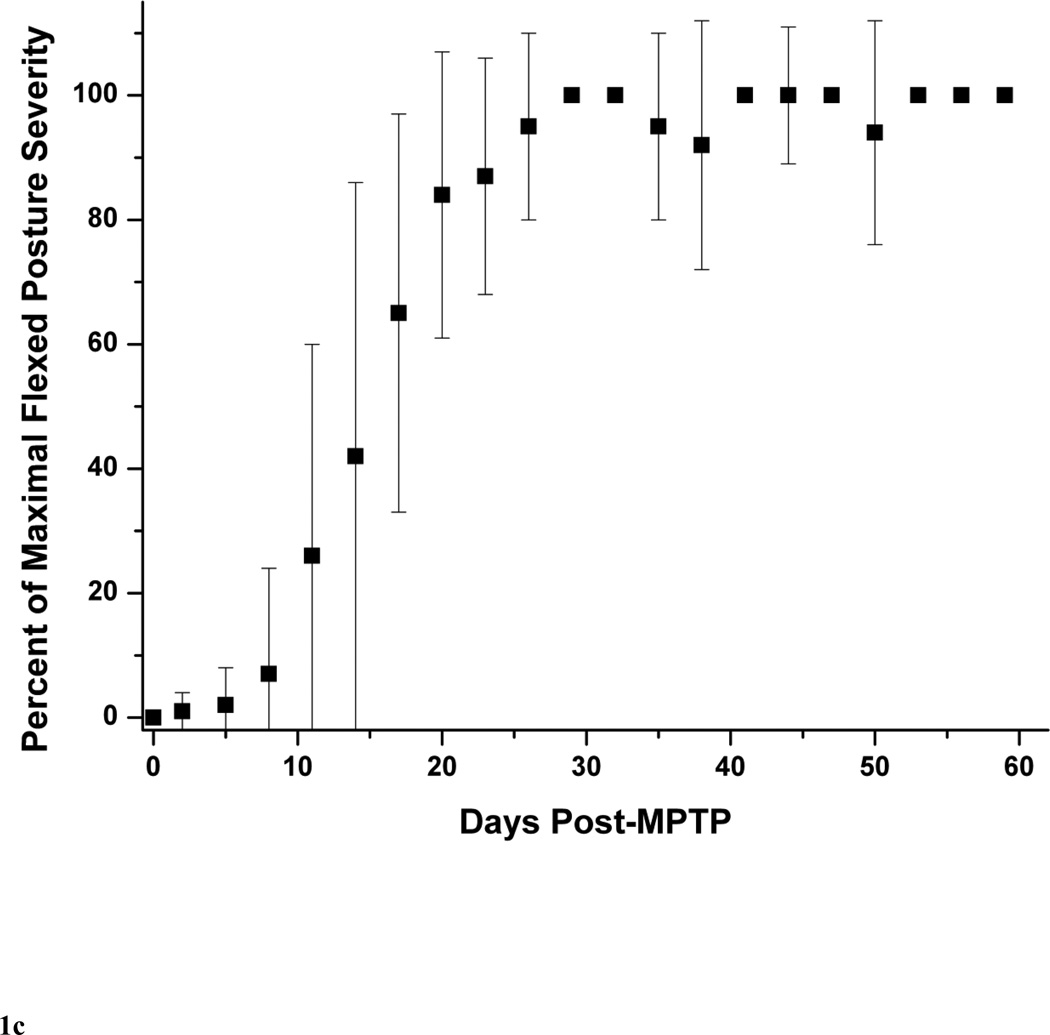
The typical behavioral response to unilateral MPTP injection included contralateral transient dystonia in the limbs followed by parkinsonism (Tabbal et al., 2006; Perlmutter et al., 1997). As expected the severity of these manifestations varied with the dose of MPTP (Table 1). The parkinsonism score just prior to euthanasia ranged from 0 to 8 points. None of the animals had a motor rating of 1. Figure 1A illustrates the progression of parkinsonism over 2 months represented by the percent of maximal parkinsonian severity rating calculated for each monkey with a least two measures per monkey per week. We defined percent of maximal parkinsonian severity for each monkey as the average of parkinsonian score over 3 days divided by the maximal parkinsonian score of that monkey over the course of the study. All the MPTP-treated monkeys reached over 90% of their maximal parkinsonism score about 3 weeks after MPTP and their parkinsonism scores remained stable until euthanasia at 2 months after MPTP. Although relatively low in magnitude, individual components of the rating scale (bradykinesia, tremor and flexed posture) followed the same pattern than that of the total parkinsonian scores (Fig. 1B and C). The onset of bradykinesia tended to precede the onset of flexed posture. The wider standard deviations early in the study reflects the variability in the time of onset of parkinsonian motor deficits as expected due to the variability in the administered MPTP dose among animals. Only two monkeys (monkey# 8 and #9) had a mild left hand action tremor rated as 1 starting on day 29 and day 16 after MPTP, respectively, that did not change for the duration of the study. At euthanasia, no animals had any dystonic manifestations.
Figure 1.
Behavioral response over 2 months after unilateral intra-carotid MPTP administration, including (A) total parkinsonian score, (B) bradykinesia subscore and (C) flexed posture subscore. Each data point is an average of percent of maximal parkinsonian score severity calculated from several monkeys within a 3-day interval. Percent of maximal parkinsonian score severity for each monkey was defined as the average of parkinsonian score over 3 days divided by the maximal parkinsonian score of that monkey over the course of the study. The error bars represent standard deviations.
MPTP-induced nigral neuron loss
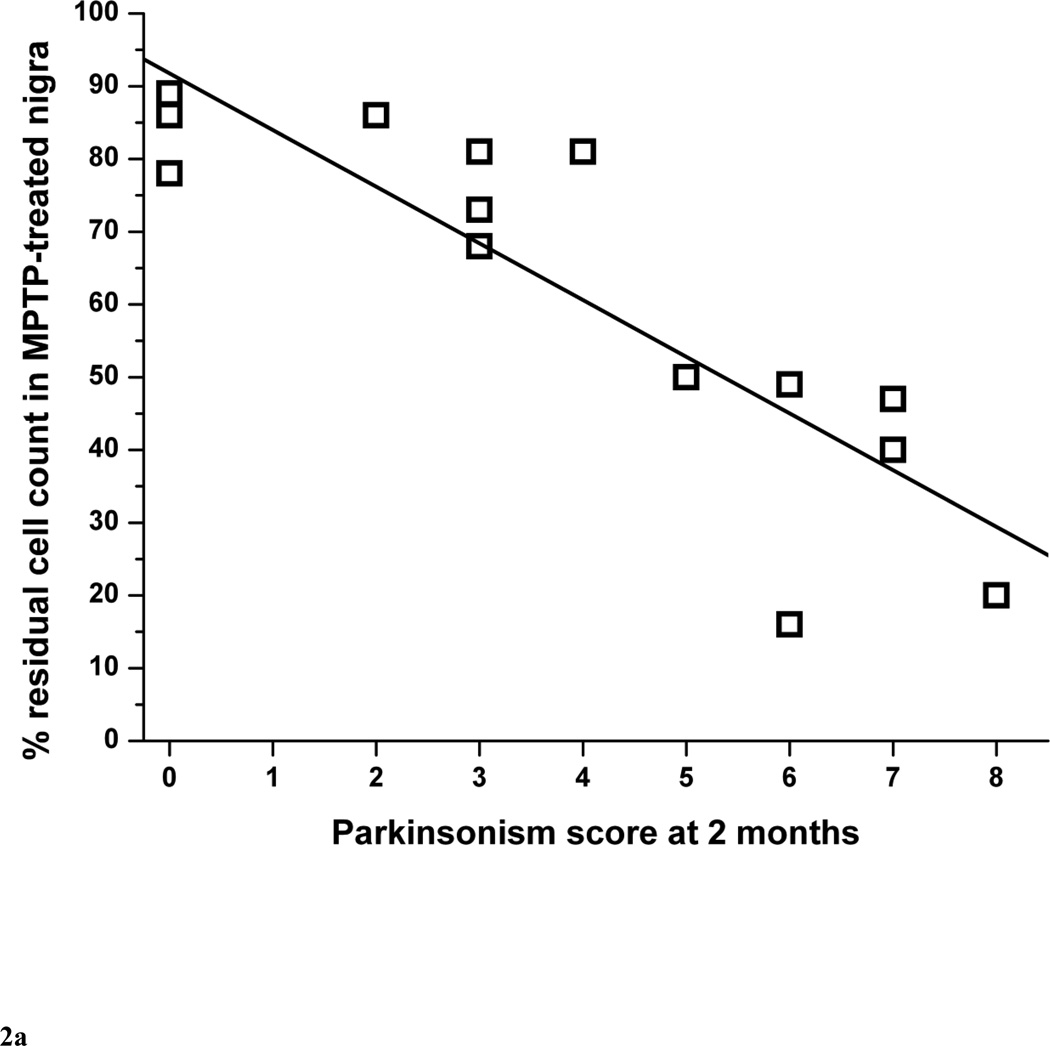
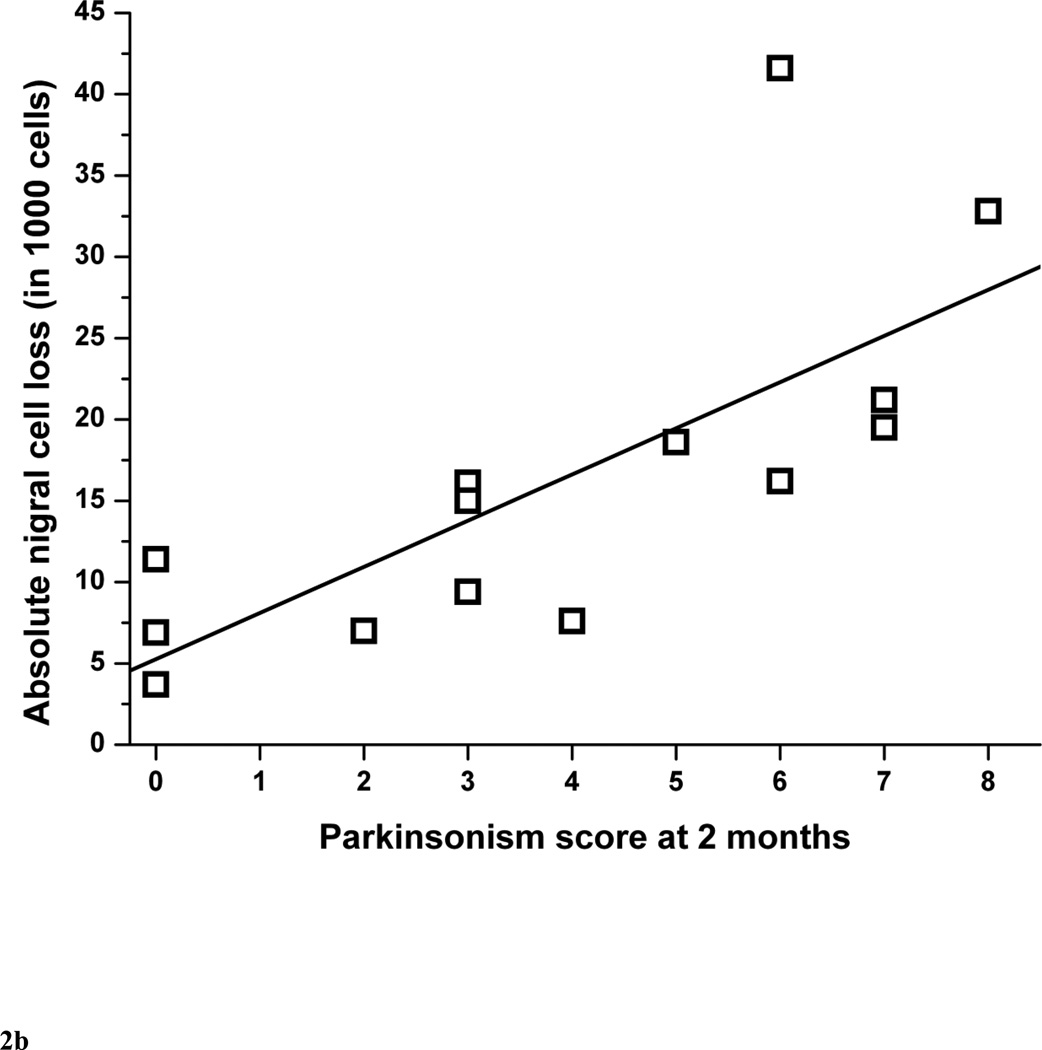
The quantification of TH-stained nigral neurons in 14 monkeys is summarized in Table 1. Monkey #12 was excluded from the nigral neuron count analysis because the nigral tissue was damaged during processing. The percent of residual cell counts in lesioned nigra correlated linearly with the parkinsonism score at 2 months (r = −0.87, p < 0.0001, n = 14) (Fig. 2A). The threshold of nigral cell loss to produce parkinsonism was about 14 to 23%. The lower end of this range was based on the animal with the least cell loss associated with development of parkinsonism. The upper end of the range was estimated as slightly more loss than that found in the animal with the greatest cell loss that did not have parkinsonism. Since there is normal side-to-side variability in the two control animals and each animal may start with a different number of nigral neurons, we also determined the relationship between parkinsonism score and absolute number of residual nigral neurons (Fig. 2B). They also correlated linearly (r = 0.74, p = 0.0024, n = 14).
Figure 2.
Parkinsonism score at 2 months versus (A) percent of residual dopaminergic cell counts and (B) absolute number of nigral cell loss in MPTP-lesioned nigra (n=14). In figure 2A, the linear equation y = A + B*x has A = 91.8±6.1, B = −7.8±1.3, r = −0.87, p <0.0001. In figure 2B, the linear equation y = A + B*x has A = 5.3±3.5, B = 2.8±0.7, r = 0.74, p = 0.0024.
MPTP-induced striatal dopamine deficit
The striatal dopamine concentrations are summarized in Table 1. There were no significant differences between the mean (±standard error) caudate and putamen dopamine concentrations (Table 2) on the control side (11.2 ± 1.1 µg/g vs 9.9 ±0.7 µg/g of wet tissue, respectively; two-sample paired t-test: t = 1.12, p = 0.28) or on the MPTP-lesioned side (4.0 ±1.2 µg/g vs 4.3 ± 1.3 µg/g of wet tissue; two-sample paired t-test: t = −0.57, p = 0.58). There was also no significant difference between caudate and putamen percent residual dopamine concentration (38 ± 10 % vs 44 ± 12%, respectively; two-sample paired t-test: t = −1.16, p = 0.27). Since the individual caudate and putamen dopamine measures were relatively noisy, we used the average of the caudate and putamen dopamine concentrations for each monkey to calculate the percent residual striatal dopamine concentration (100 × right/left ratio) in the MPTP-lesioned striatum.
Table 2.
Striatal, caudate and putamen dopamine levels in all monkeys.
| Caudate | Putamen | Striatum | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Monkey number |
MPTP dose in mg/kg |
Uninjeted side |
Injected side |
% residual dopamine in injected side |
Uninjeted side |
Injected side |
% residual dopamine in injected side |
Uninjeted side |
Injected side |
% residual dopamine in injected side |
| 1 | 0 | 8.4 | 6.9 | 83 | 6.7 | 8.7 | 129 | 15.1 | 15.6 | 103 |
| 2 | 0 | 13.7 | 11.6 | 84 | 13.0 | 17.1 | 132 | 26.7 | 28.7 | 107 |
| 3 | 0.07 | 8.4 | 1.9 | 22 | 5.4 | 3.3 | 61 | 13.9 | 5.2 | 37 |
| 4 | 0.08 | 13.1 | 11.4 | 87 | 12.4 | 10.5 | 85 | 25.5 | 21.9 | 86 |
| 5 | 0.12 | 3.5 | 1.6 | 46 | 10.5 | 5.4 | 51 | 14.0 | 7.0 | 50 |
| 6 | 0.13 | 10.8 | 7.5 | 70 | 10.9 | 6.3 | 58 | 21.8 | 13.8 | 64 |
| 7 | 0.13 | 6.9 | 6.7 | 97 | 9.5 | 7.2 | 76 | 16.4 | 13.9 | 84 |
| 8 | 0.13 | 20.0 | 0.1 | 0 | 7.3 | 0.4 | 5 | 27.3 | 0.4 | 2 |
| 9 | 0.14 | 13.3 | 0.1 | 0 | 7.6 | 0.1 | 2 | 21.0 | 0.2 | 1 |
| 10 | 0.14 | 16.3 | 10.3 | 63 | 8.9 | 4.5 | 50 | 25.2 | 14.8 | 59 |
| 11 | 0.14 | 7.6 | 0.1 | 1 | 6.6 | 0.0 | 1 | 14.3 | 0.2 | 1 |
| 12 | 0.19 | 12.7 | 0.5 | 4 | 14.8 | 0.6 | 4 | 27.6 | 1.1 | 4 |
| 13 | 0.20 | 7.2 | 0.2 | 3 | 8.9 | 0.1 | 1 | 16.0 | 0.3 | 2 |
| 14 | 0.24 | 9.4 | 0.2 | 2 | 10.8 | 0.4 | 4 | 20.2 | 0.7 | 3 |
| 15 | 0.31 | 16.6 | 0.3 | 2 | 14.3 | 0.2 | 1 | 30.9 | 0.5 | 2 |
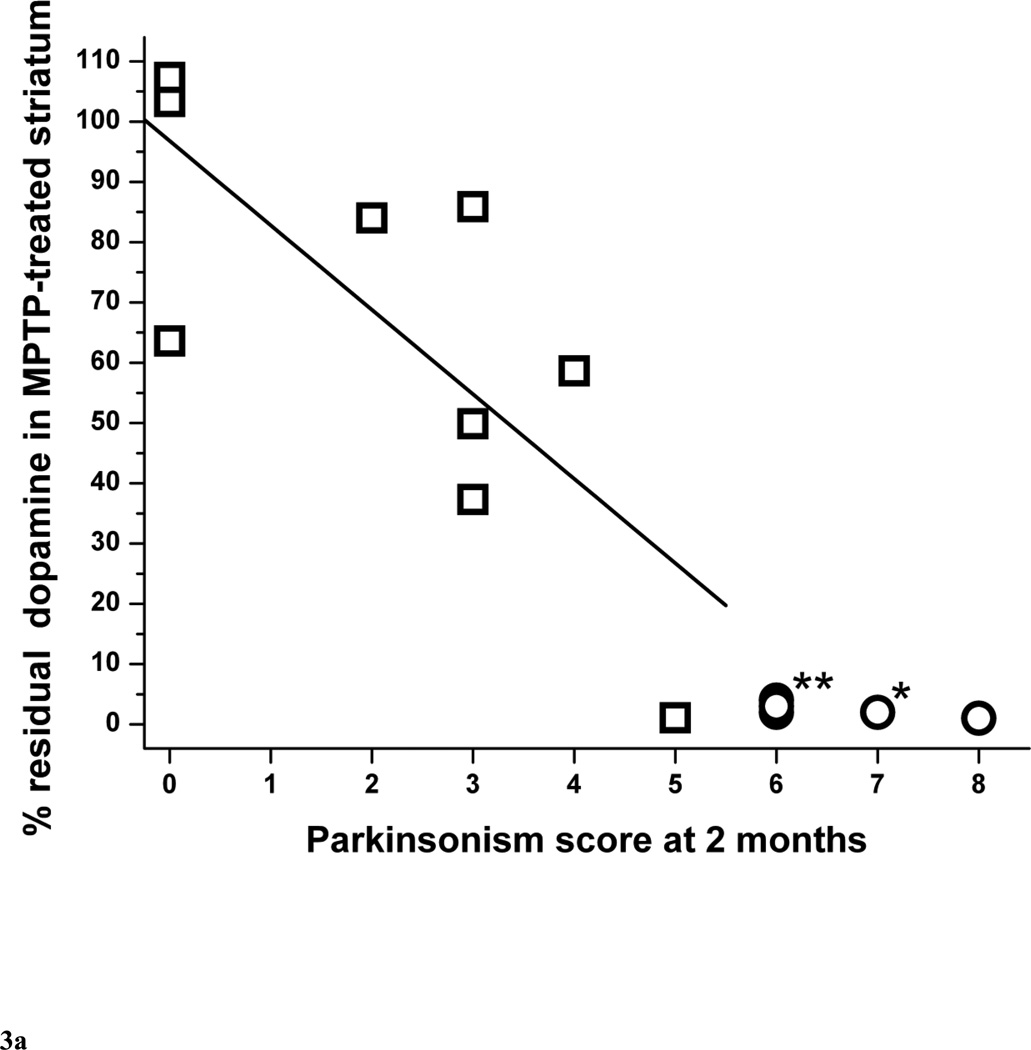
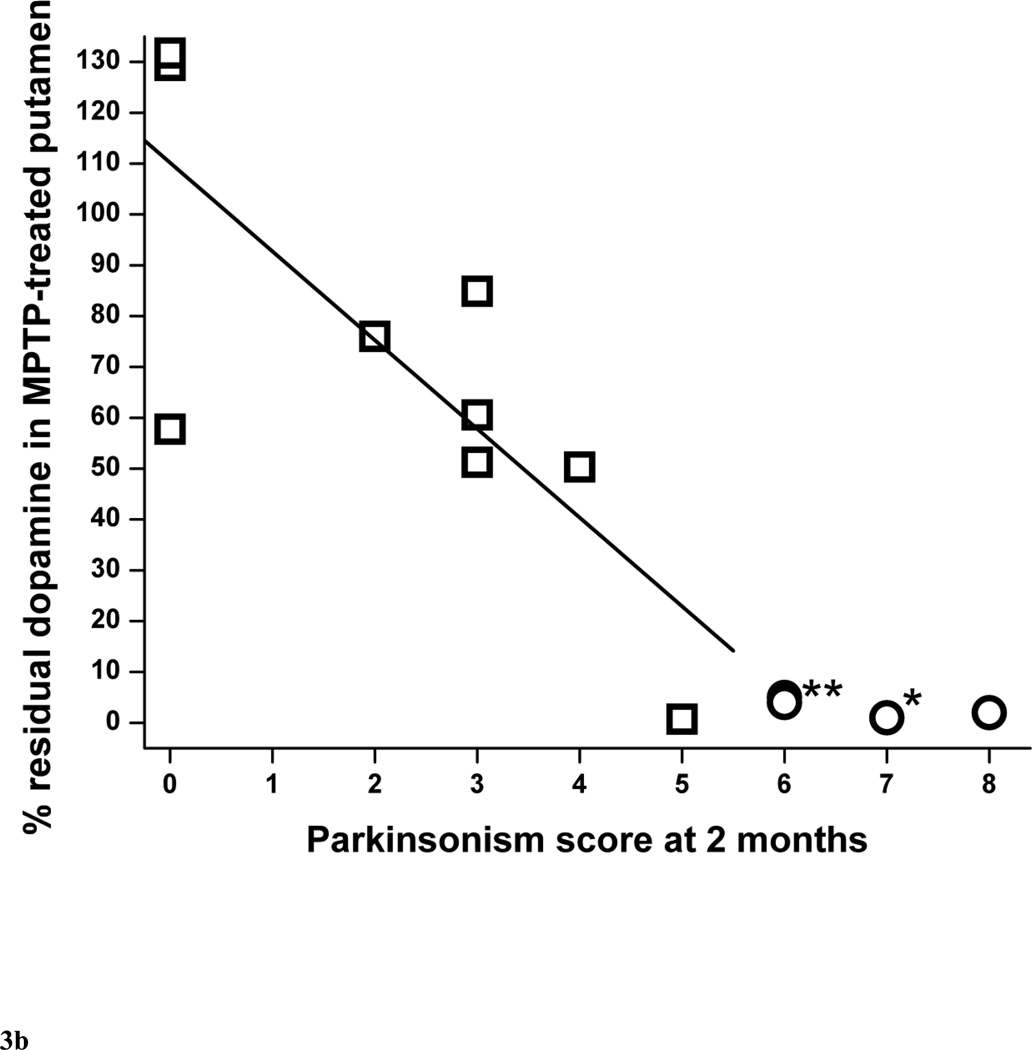
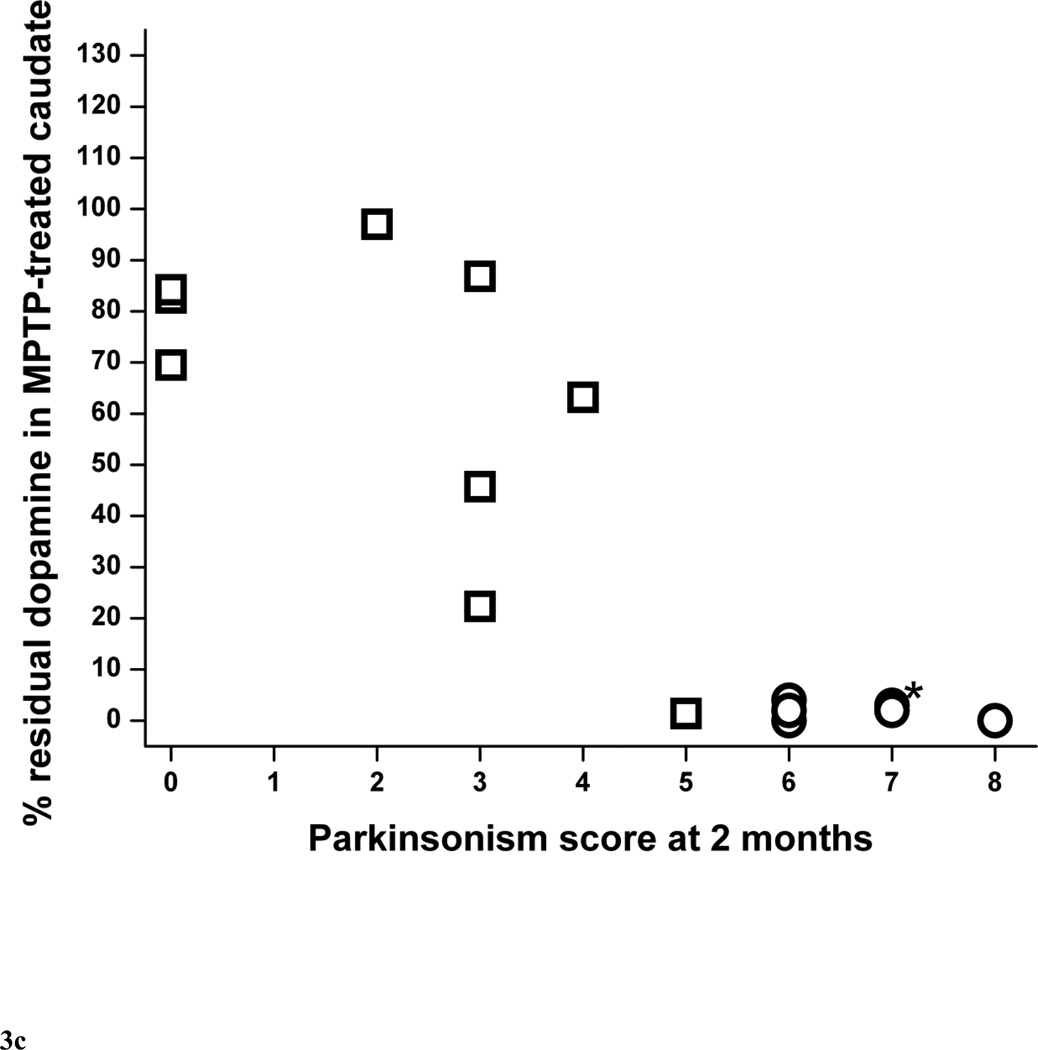
The percent residual striatal dopamine dropped linearly (r = −0.77, p = 0.016, n = 9) as parkinsonism score worsened followed by a plateau or flooring effect once residual striatal dopamine was less than 5% (Fig. 3A). This flooring effect occurred in monkeys that had more than 50% nigral cell count loss, as indicated by the circle symbols in figure 3A. The threshold of striatal dopamine loss to produce parkinsonism was about 14 to 37%. The lower end of this range was based on the animal with the least dopamine loss associated with development of parkinsonism. The upper end of the range was estimated as slightly more loss than that found in the animal with the greatest dopamine loss that did not have parkinsonism. The relationship of percent residual dopamine in putamen and parkinsonism score was similar to the relationship between striatal dopamine and parkinsonism score, dropping linearly initially (r = −0.79, p = 0.011, n = 9), then reaching a plateau once residual striatal dopamine was less than 5% (Fig. 3B) in animals with more than 50% nigral cell count loss. However, we found was no significant correlation between percent residual caudate dopamine and parkinsonism score (r = −0.65, p = 0.060, n = 9) (Fig. 3C).
Figure 3.
Parkinsonism score at 2 months versus percent residual dopamine content in (A) the striatum, (B) putamen and (C) caudate (n=15). Data from monkeys with residual nigral cell counts of less than 50% are depicted by circles (n=6). Squares represent data from monkeys with residual nigral cell counts of 50% or more (n=9). In figure 3A, the linear equation y = A + B*x has A = 96.8±12.5, B = −14.0±4.4, r = −0.77, p = 0.016. In figure 3B, the linear equation has A = 110±14, B = −17.5±5.1, r = −0.79, p = 0.011). In figure 3C, the linear equation has A = 86.4±14.2, B = −11.2±5.0, r = −0.65, p = 0.060. * denotes 2 overlapping data points; ** denotes 3 overlapping data points.
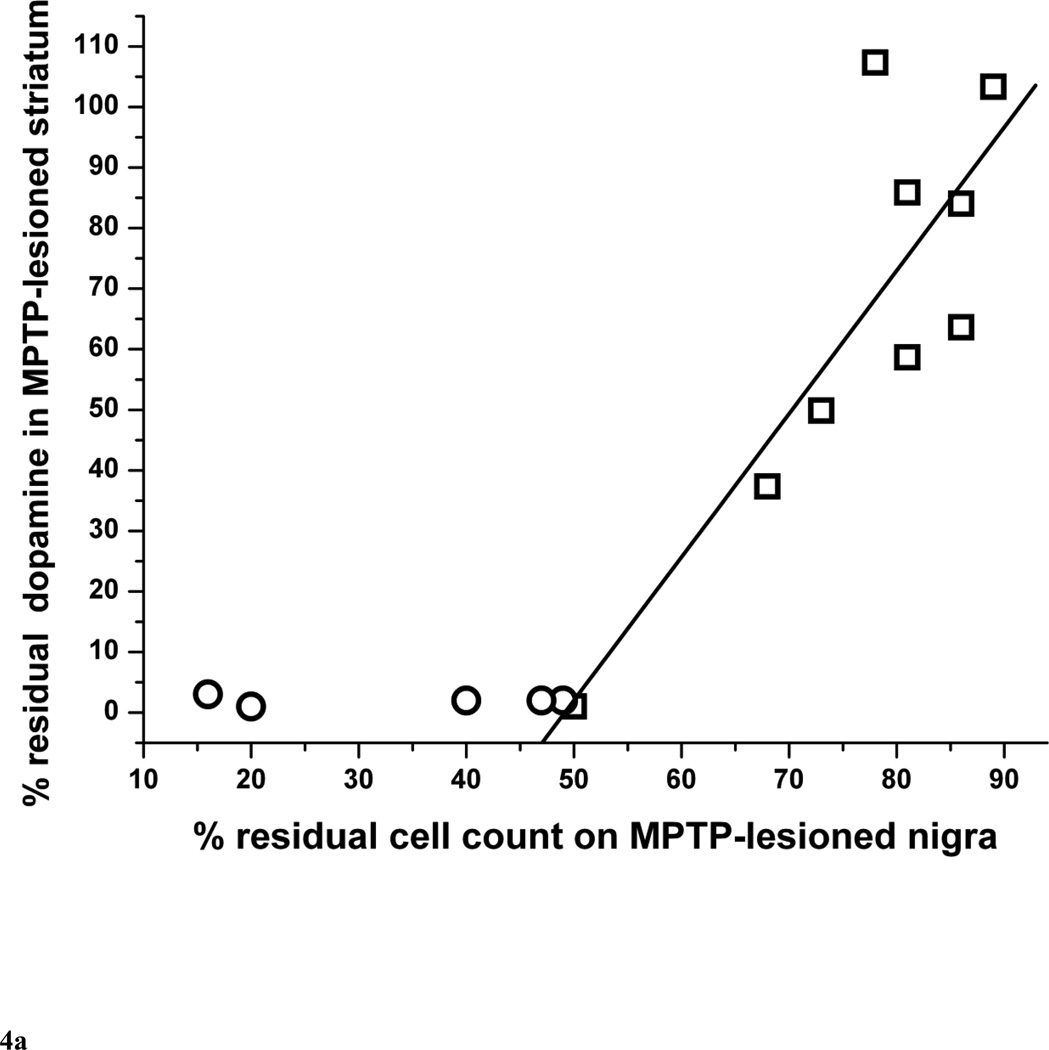
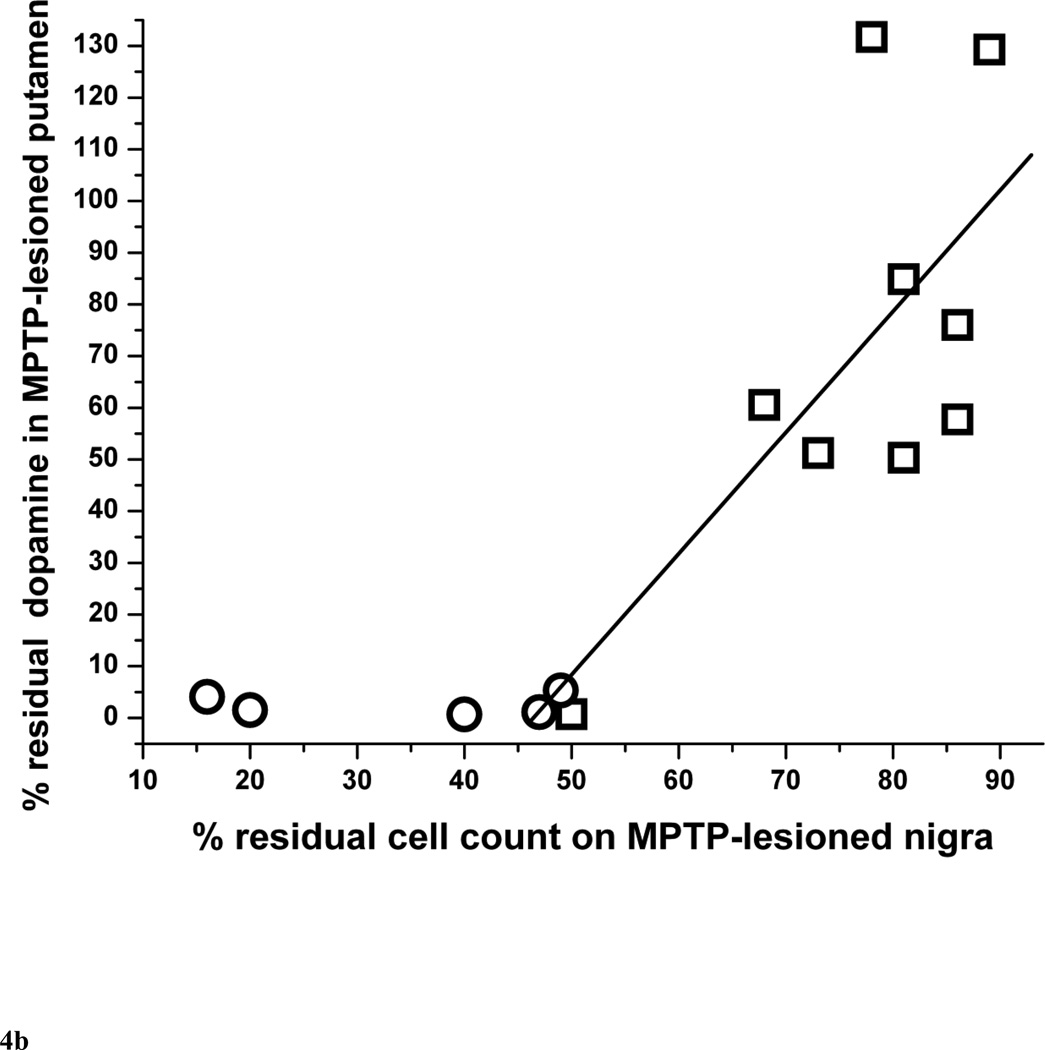
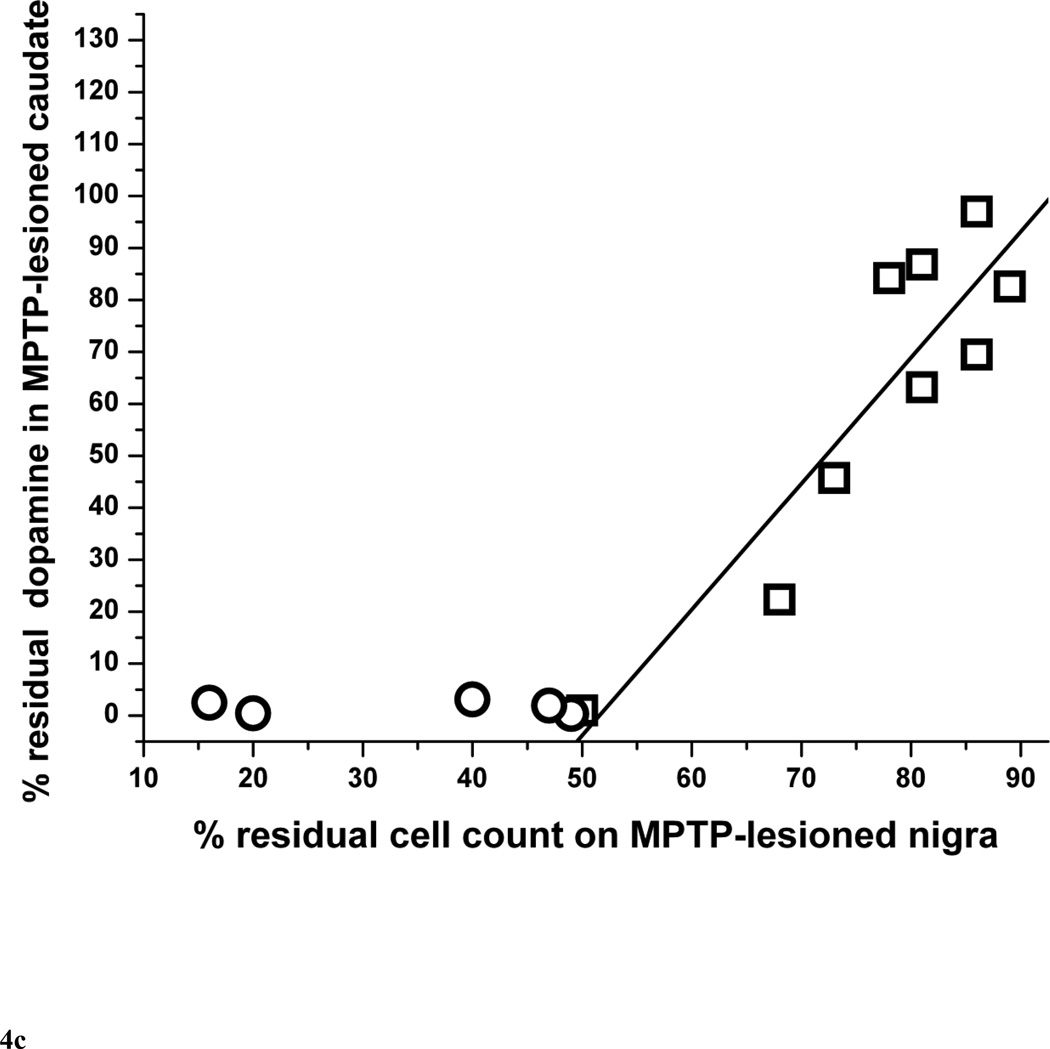
The residual nigral neuron counts correlated linearly with striatal dopamine only in monkeys that did not have more than 50% nigral cell loss (r = 0.84, p = 0.0043, n = 9) (Fig. 4A). Those monkeys with more than 50% nigral loss are indicated by circles in figure 4A. The residual nigral neuron counts also correlated linearly with putamen and caudate dopamine levels in a similar manner (r = 0.69, p = 0.039 and r = 0.90, p = 0.0008, respectively, n = 9) (Fig. 4B and 4C, respectively).
Figure 4.
Percent of residual dopaminergic cell counts in injected nigra versus percent residual dopamine in (A) the striatum, (B) putamen and (C) caudate (n=14). Data from monkeys with residual nigral cell counts of less than 50% are depicted in circles (n=6). Squares represent data from monkeys with residual nigral cell counts of 50% or more (n=9), In figure 4A, the linear equation y = A + B*x has A = −116±44, B = 2.37±0.57, r = 0.84, p = 0.0043. In figure 4B, the linear equation y = A + B*x has A = −109±72, B = 2.34±0.92, r = 0.69, p = 0.039. In figure 4C, the linear equation y = A + B*x has A = −125±34, B = 2.42±0.43, r = 0.90, p = 0.0008.
Discussion
We found a lower threshold in this unilateral MPTP non-human primate model of PD than previously reported for nigrostriatal injury that produces measureable parkinsonian features. This indicates that the nigrostriatal reserve may be much less than previously thought. If this applies to human PD, interventions to forestall onset of motor manifestations would need to target patients earlier in the pathophysiologic process than previously estimated. Our study demonstrated that as little as 14 to 23% drop in nigral neuron counts or 14 to 37% reduction of striatal dopamine was sufficient to induce mild parkinsonism (Table 1). This finding contradicts two earlier reports that suggested that a 70 to 80% striatal dopamine loss was necessary to induce PD symptoms (Bernheimer et al., 1973; Riederer and Wuketich, 1976). However, several issues limit the interpretation of these earlier reports. The PD patients in the first study (Bernheimer et al., 1973) had clinical ratings consistent with more severe parkinsonism. In addition, postmortem delay prior to removing the brain can reduce striatal dopamine measures (Carlsson and Winblad, 1976). The second report (Riederer and Wuketich, 1976) was based on extrapolation of cross sectional dopamine measures with the assumption that the time of onset of PD symptoms was established with an error of only one year. However, this assumption may not be accurate given the unreliability of historical medical data. Furthermore, there may be a substantial difference between when an individual retrospectively recalls onset of symptoms and when a movement disorder expert identifies development of subtle clinical signs such as flexed posturing of a limb. Finally, postmortem human studies of striatal dopamine also may be confounded by chronic dopaminergic drug therapy.
Our study revealed that only about a 14 to 23% loss of nigral dopaminergic neurons could produce mild parkinsonism in MPTP-treated monkeys (Fig. 2A). This was close to the 30% nigral cell loss estimated to occur at onset of motor symptoms in PD (Greffard et al., 2006; Ma et al., 1997) but these carefully done studies also highlight that the denominator for this calculation, the number of nigral neurons in people without PD, is highly variable. For example, one of these studies measured 163,238 cells with a standard deviation of 42,372 of TH-stained nigral neuron in 12 healthy controls (Ma et al., 1997). These studies as well as ours used careful quantitative morphologic techniques that involved systematic sampling of the nigra. Another human study (Ross et al., 2004) that counted nigral neurons on a single, transverse caudal midbrain section estimated the extent of the nigral cell loss as 50% at onset of PD. This overestimate highlights the importance of sampling the entire SN pars compacta with an unbiased counting method given the non-homogeneous distribution of affected cells in human PD (Damier et al., 1999). We found considerable variability in in vitro measures for a given behavioral deficit in MPTP treated and control animals. However, the low coefficient of error in nigral counts in our study suggests that the large variability in the number of nigral neurons for a given behavioral deficit is due to a biological variability in our primates rather than sampling bias or systematic errors in the stereological method. This is why we elected to discuss ranges of percentage neuronal cell or striatal dopamine loss rather than specific cutoff values at which parkinsonian features develop.
As expected, there was also variable sensitivity to MPTP across animals that likely reflects a variety of biological variables. Since our animals did not receive the same dose of MPTP, we cannot confirm or exclude that the initial number of nigral neurons accounts for the susceptibility to MPTP. Of interest, in the group of 3 monkeys that received 0.13 mg/kg of MPTP, monkey#8 has the worst parkinsonian score associated with the lowest initial number of nigral neurons as compared to monkey #6 and #7 who had a lower parkinsonian scores and a higher initial neuronal cell counts. This may suggest that nigrostriatal reserve potentially depends upon the initial number of nigral neurons. However, in another group of 3 monkeys who received a slightly higher dose of MPTP (0.14 mg/kg), monkey #9 had a slightly higher number of initial number of nigral neurons, yet it sustained a more severe nigral cell loss of 80% associated with a severe parkinsonism score of 8 as compared to monkey #10 who sustained a mild nigral cell loss of 19% with much lower parkinsonism score of 4. In this group, even monkey#11, that had the lowest initial number of nigral neurons, had a milder nigral cell loss of 50% with lower parkinsonism score of 5 compared to monkey #9 who had a higher initial number of nigral neurons. Therefore, we doubt that MPTP susceptibility depends upon the initial number of nigral neurons.
The effect of age on MPTP susceptibility cannot be determined in this study because we used different doses of MPTP in monkeys with in a relatively narrow age range. In animals that received the same dose, one may deduce from table 1 that the older monkey #8 (aged 5.3 years) was more susceptible to MPTP than monkeys #6 and #7 (aged 3.5 and 3.7 years) as reflected by his low residual striatal dopamine of 2% as compared to 64% and 84% in the other 2 younger monkeys, respectively. All 3 monkeys received 0.13 mg/kg of MPTP. However, monkey #3 (age 3.5 years) received a slightly lower dose than the older monkey #4 (aged 5.6 years), yet the former had a more severe striatal dopamine deficit (37% residual dopamine vs 86%, respectively). Therefore, at least in these small subgroups of animals, age alone would not account for the variability of nigral injury in response to MPTP. Thus, we doubt that MPTP susceptibility depends on the age of the animals or the initial number of nigral neurons and suspect that it is more likely due to other unknown sources of biological variability. Nevertheless, the purpose of this study is to compare behavioral responses to nigral counts and striatal dopamine, regardless of the dose of MPTP administered. This is why we compare motor behavior to the in vitro measures rather than to MPTP dose.
Of course, our measures of nigral neurons only include those that we identify with TH-staining. It is likely that nigral neurons that do not stain with TH remain but others have shown that their loss parallels the loss of TH-staining nigral neurons after MPTP (Bezard et al., 2001). Yet, these non-TH-staining neurons could be viable dopaminergic neurons with potential to recover, although we found no evidence of behavioral recovery during the course of our study.
Using an escalating dose of MPTP, we achieved the clinical spectrum of parkinsonism from mild, to moderate, to severe parkinsonian rating scores. This wide variation in severity of clinical manifestation correlated with the full range of residual nigral cell counts (Fig. 2A). However, there were no animals that exhibited a 5–35% residual striatal dopamine due to the variability in each animal’s response to MPTP (Table 1). Nevertheless, this does not negate the statistically significant linear correlation between striatal dopamine and mild parkinsonism and the clear flooring effects of striatal dopamine in moderate to severe parkinsonism (Fig. 3A).
MPTP-induced injury has a different rate of nigral neurons loss and a different pattern of injury to the striatum compared to human PD (Irwin et al., 1990; Kish et al., 1988; Brooks et al., 1990). We found, like some others (Bezard et al., 2001), that caudate and putamen were equally affected by MPTP in contrast to the greater involvement of the putamen in PD (Damier et al., 1999). However, unlike caudate dopamine levels, only putamen dopamine levels correlated significantly with parkinsonism scores (Fig. 3B and 3C). This supports the notion the caudate is less critical than the putamen for motor function (Mink, 1996) but could also just reflect the noise in the measurements of individual components of striatum.
An impressively large study that included 67 monkeys chronically treated with MPTP (Blesa et al., 2010) showed a significant correlation between 18F-DOPA or 11C-DTBZ PET measures and motor behavior using Statistical Parametric Mapping analysis. However, the authors do not show the scatter plots of their data that contributed to those correlations. Based upon our findings, we found a linear correlation between motor behavior and nigral counts but not a similar correlation with striatal dopamine. The relationship of motor behavior with striatal dopamine showed a clear flooring effect such that, after 50% of nigral cell loss, striatal dopamine reaches a nadir. Using all of our data points in Fig. 3A (i.e., irrespective of the extent of nigral cell loss) would still show a linear correlation between striatal dopamine and motor behavior, but this would be misleading. It would be interesting to know whether scatter plots of the data of Blesa et al (2010) are consistent with our findings. If true, this would then be further evidence that those striatal PET measures may only be a valid biomarker in early disease.
Animal studies, such as ours, control for potential confounding factors and have a major advantage of allowing close day-to-day observation of clinical manifestations. This permits the precise determination of development of subtle motor signs rather than relying on extrapolations of postmortem measures to time of symptom onset done in humans. One study using daily intravenous MPTP injections in monkeys found that the first manifestations of parkinsonism was associated with 43% nigral neurons loss and about 85% striatal dopamine loss (Bezard et al., 2001). This greater loss compared to our study may reflect the shorter time course of their study (from 6 to 25 days after up to 15 daily MPTP injections) whereas our measures were done after clinical manifestations had stabilized for 4 to 5 weeks before euthanasia at 2 months post MPTP. The stability of the parkinsonian manifestations prior to making in vivo measurements is critical for the interpretation of the findings, as we found no evidence of behavioral recovery in our animals that could otherwise alter interpretation of our findings.
Conclusions
Our study shows that striatal dopamine loss after MPTP usually exceeds the loss of nigral neurons, as illustrated on figure 4. With relatively mild nigral cell loss, striatal dopamine and nigral neurons losses appear similar with an 85% residual nigral neurons count corresponding to about 85% residual striatal dopamine. However, when severity of nigral neuron loss exceeds 50%, striatal dopamine loss is more than 95%. This supports the observation in MPTP-treated monkeys that striatal terminal injury precedes nigral cell loss although this study does not include measures of time-dependent changes (Herkenham et al., 1991). Finally, the severity of parkinsonism correlated linearly throughout the range of parkinsonism severity with the percent of and the absolute number of residual nigral cell counts (Fig. 2A and 2B) whereas the residual striatal dopamine reached a plateau under 5% in animals with parkinsonism score of more than 4 points (Fig. 3). These animals also had a nigral cell loss greater than 50%. This flooring effect may suggest that residual nigral cell counts may be a more suitable biomarker of clinical progression of advanced parkinsonism than residual striatal dopamine. This also raises the importance of using the proper metric for assessment of the efficacy of therapeutic interventions. A biomarker used as such a metric must be validated to clearly reflect the target of the intervention (nigral cells, axons or terminal fields).
Highlights.
-
-
Parkinsonism in monkeys occurs with about 14 to 23% of nigral dopamine neuron loss.
-
-
Parkinsonism in monkeys occurs with about a 14 to 37% loss of striatal dopamine
-
-
Nigrostriatal reserve for motor parkinsonism is low in MPTP-treated monkeys.
-
-
Loss of nigral neurons correlates with parkinsonism through a wide range of severity.
-
-
Striatal dopamine only correlates with parkinsonism with <50% nigral neuron loss.
Acknowledgments
We thank Hugh Flores, Huchuan Xia, Christina Zukas, Darryl Craig, and Terry Anderson for expert technical assistance.
Funding:
This study was funded by NIH (NS050425, NS058714, NS41509, and NS075321); Michael J Fox Foundation; Murphy Fund; American Parkinson Disease Association (APDA) Center for Advanced PD Research at Washington University; Greater St. Louis Chapter of the APDA; McDonnell Center for Higher Brain Function; Hartke Fund; Howard Hughes Medical Institute; Barnes-Jewish Hospital Foundation Elliot H. Stein Family Fund and PD Research Fund). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ballard PA, Tetrud JW, Langston JW. Permanent Human Parkinsonism Due to 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP) - 7 Cases. Neurology. 1985;35:949–956. doi: 10.1212/wnl.35.7.949. [DOI] [PubMed] [Google Scholar]
- 2.Beach TG, Adler CH, Sue LI, Peirce JB, Bachalakuri J, Dalsing-Hernandez JE, Lue LF, Caviness JN, Connor DJ, Sabbagh MN, Walker DG. Reduced striatal tyrosine hydroxylase in incidental Lewy body disease. Acta Neuropathologica. 2008;115:445–451. doi: 10.1007/s00401-007-0313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain Dopamine and Syndromes of Parkinson and Huntington - Clinical, Morphological and Neurochemical Correlations. Journal of the Neurological Sciences. 1973;20:415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- 4.Bezard E, Dovero S, Prunier C, Ravenscroft P, Chalon S, Guilloteau D, Crossman AR, Bioulac B, Brotchie JM, Gross CE. Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson's disease. Journal of Neuroscience. 2001;21:6853–6861. doi: 10.1523/JNEUROSCI.21-17-06853.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blesa J, Juri C, Collantes M, Penuelas I, Prieto E, Iglesias E, Marti-Climent J, Arbizu J, Zubieta JL, Rodriguez-Oroz MC, Garcia-Garcia D, Richter JA, Cavada C, Obeso JA. Progression of dopaminergic depletion in a model of MPTP-induced Parkinsonism in non-human primates. An F-18-DOPA and C-11-DTBZ PET study. Neurobiology of Disease. 2010;38:456–463. doi: 10.1016/j.nbd.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Bohnen NI, Albin RL, Koeppe RA, Wernette KA, Kilbourn MR, Minoshima S, Frey KA. Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. Journal of Cerebral Blood Flow and Metabolism. 2006;26:1198–1212. doi: 10.1038/sj.jcbfm.9600276. [DOI] [PubMed] [Google Scholar]
- 7.Brooks DJ, Frey KA, Marek KL, Oakes D, Paty D, Prentice R, Shults CW, Stoessl AJ. Assessment of neuroimaging techniques as biomarkers of the progression of Parkinson's disease. Experimental Neurology. 2003;184:S68–S79. doi: 10.1016/j.expneurol.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Brooks DJ, Ibanez V, Sawle GV, Quinn N, Lees AJ, Mathias CJ, Bannister R, Marsden CD, Frackowiak RSJ. Differing Patterns of Striatal F-18 Dopa Uptake in Parkinsons-Disease, Multiple System Atrophy, and Progressive Supranuclear Palsy. Annals of Neurology. 1990;28:547–555. doi: 10.1002/ana.410280412. [DOI] [PubMed] [Google Scholar]
- 9.Carlsson A, Winblad B. Influence of Age and Time Interval Between Death and Autopsy on Dopamine and 3-Methoxytyramine Levels in Human Basal Ganglia. Journal of Neural Transmission. 1976;38:271–276. doi: 10.1007/BF01249444. [DOI] [PubMed] [Google Scholar]
- 10.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain - II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain. 1999;122:1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 11.Ehringer H, Hornykiewicz O. Verteilung von Noradrenalin und Dopamin (3-Hydroxytyramin) im Gehirn des Menschen und ihr Verhalten bei Erkrankungen des Extrapyramidalen Systems. Klin Wochenschr. 1960;38:1236–1239. doi: 10.1007/BF01485901. [DOI] [PubMed] [Google Scholar]
- 12.Fearnley JM, Lees AJ. Aging and Parkinsons-Disease - Substantia-Nigra Regional Selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 13.Greffard S, Verny M, Bonnet AM, Beinis JY, Gallinari C, Meaume S, Piette F, Hauw JJ, Duychaerts C. Motor score of the unified Parkinson disease rating scale as a good predictor of Lewy body-associated neuronal loss in the substantia Nigra. Archives of Neurology. 2006;63:584–588. doi: 10.1001/archneur.63.4.584. [DOI] [PubMed] [Google Scholar]
- 14.Gundersen HJG, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, West MJ. The New Stereological Tools - Disector, Fractionator, Nucleator and Point Sampled Intercepts and Their Use in Pathological Research and Diagnosis. Apmis. 1988a;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 15.Gundersen HJG, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, West MJ. Some New, Simple and Efficient Stereological Methods and Their Use in Pathological Research and Diagnosis - Review Article. Apmis. 1988b;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 16.Gundersen HJG, Jensen EB. The Efficiency of Systematic-Sampling in Stereology and Its Prediction. Journal of Microscopy-Oxford. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 17.Herkenham M, Little MD, Bankiewicz K, Yang SC, Markey SP, Johannessen JN. Selective Retention of MPP+ Within the Monoaminergic Systems of the Primate Brain Following MPTP Administration - An Invivo Autoradiographic Study. Neuroscience. 1991;40:133–158. doi: 10.1016/0306-4522(91)90180-v. [DOI] [PubMed] [Google Scholar]
- 18.Irwin I, Delanney LE, Forno LS, Finnegan KT, DiMonte DA, Langston JW. The Evolution of Nigrostriatal Neurochemical Changes in the Mptp-Treated Squirrel-Monkey. Brain Research. 1990;531:242–252. doi: 10.1016/0006-8993(90)90780-f. [DOI] [PubMed] [Google Scholar]
- 19.Karimi M, Carl JL, Loftin S, Perlmutter JS. Modified high-performance liquid chromatography with electrochemical detection method for plasma measurement of levodopa, 3-O-methyldopa, dopamine, carbidopa and 3,4-dihydroxyphenyl acetic acid. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences. 2006;836:120–123. doi: 10.1016/j.jchromb.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 20.Kish SJ, Shannak K, Hornykiewicz O. Uneven Pattern of Dopamine Loss in the Striatum of Patients with Idiopathic Parkinsons-Disease - Pathophysiologic and Clinical Implications. New England Journal of Medicine. 1988;318:876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- 21.Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in Humans Due to A Product of Meperidine-Analog Synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 22.Lee CS, Samii A, Sossi V, Ruth TJ, Schulzer M, Holden JE, Wudel J, Pal PK, Fuente-Fernandez R, Calne DB, Stoessl AJ. In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson's disease. Annals of Neurology. 2000;47:493–503. [PubMed] [Google Scholar]
- 23.Ma SY, Roytta M, Rinne JO, Collan Y, Rinne UK. Correlation between neuromorphometry in the substantia nigra and clinical features in Parkinson's disease using disector counts. Journal of the Neurological Sciences. 1997;151:83–87. doi: 10.1016/s0022-510x(97)00100-7. [DOI] [PubMed] [Google Scholar]
- 24.Mink JW. The basal ganglia: Focused selection and inhibition of competing motor programs. Progress in Neurobiology. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 25.Namavari M, Bishop A, Satyamurthy N, Bida G, Barrio JR. Regioselective Radiofluorodestannylation with [F-18] F2 and [F-18] Ch3Coof - A High-Yield Synthesis of 6-[F-18]Fluoro-L-Dopa. Applied Radiation and Isotopes. 1992;43:989–996. doi: 10.1016/0883-2889(92)90217-3. [DOI] [PubMed] [Google Scholar]
- 26.Nandhagopal R, McKeown MJ, Stoessl AJ. Functional imaging in Parkinson disease. Neurology. 2008;70:1478–1488. doi: 10.1212/01.wnl.0000310432.92489.90. [DOI] [PubMed] [Google Scholar]
- 27.Perlmutter JS, Tempel LW, Black KJ, Parkinson D, Todd RD. MPTP induces dystonia and parkinsonism - Clues to the pathophysiology of dystonia. Neurology. 1997;49:1432–1438. doi: 10.1212/wnl.49.5.1432. [DOI] [PubMed] [Google Scholar]
- 28.Ravina B, Eidelberg D, Ahlskog JE, Albin RL, Brooks DJ, Carbon M, Dhawan V, Feigin A, Fahn S, Guttman M, Gwinn-Hardy K, McFarland H, Innis R, Katz RG, Kieburtz K, Kish SJ, Lange N, Langston JW, Marek K, Morin L, Moy C, Murphy D, Oertel WH, Oliver G, Palesch Y, Powers W, Seibyl J, Sethi KD, Shults CW, Sheehy P, Stoessl AJ, Holloway R. The role of radiotracer imaging in Parkinson disease. Neurology. 2005;64:208–215. doi: 10.1212/01.WNL.0000149403.14458.7F. [DOI] [PubMed] [Google Scholar]
- 29.Riederer P, Wuketich S. Time Course of Nigrostriatal Degeneration in Parkinsons-Disease - Detailed Study of Influential Factors in Human-Brain Amine Analysis. Journal of Neural Transmission. 1976;38:277–301. doi: 10.1007/BF01249445. [DOI] [PubMed] [Google Scholar]
- 30.Ross GW, Petrovitch H, Abbott RD, Nelson J, Markesbery W, Davis D, Hardman J, Launer L, Masaki K, Tanner CM, White LR. Parkinsonian signs and substantia nigra neuron density in decendents elders without PD. Annals of Neurology. 2004;56:532–539. doi: 10.1002/ana.20226. [DOI] [PubMed] [Google Scholar]
- 31.Scherman D, Desnos C, Darchen F, Pollak P, Javoyagid F, Agid Y. Striatal Dopamine Deficiency in Parkinsons-Disease - Role of Aging. Annals of Neurology. 1989;26:551–557. doi: 10.1002/ana.410260409. [DOI] [PubMed] [Google Scholar]
- 32.Tabbal SD, Mink JW, Antenor JA, Carl JL, Moerlein SM, Perlmutter JS. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced acute transient dystonia in monkeys associated with low striatal dopamine. Neuroscience. 2006;141:1281–1287. doi: 10.1016/j.neuroscience.2006.04.072. [DOI] [PubMed] [Google Scholar]
- 33.Taylor MD, Deceballos ML, Rose S, Chong PN, Jenner P, Marsden CD. Neuropeptide Levels in the Basal Ganglia of Aged Common Marmosets Following Prolonged Treatment with Mptp. Journal of Neural Transmission-Parkinsons Disease and Dementia Section. 1991;3:99–108. doi: 10.1007/BF02260885. [DOI] [PubMed] [Google Scholar]
- 34.Tian LL, Karimi M, Loftin SK, Brown CA, Xia HC, Xu JB, Mach RH, Perlmutter JS. No Differential Regulation of Dopamine Transporter (DAT) and Vesicular Monoamine Transporter 2 (VMAT2) Binding in a Primate Model of Parkinson Disease. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yee RE, Irwin I, Milonas C, Stout DB, Huang SC, Shoghi-Jadid K, Satyamurthy N, Delanney LE, Togaski DM, Farahani KF, Delfani K, Janson AM, Phelps ME, Langston JW, Barrio JR. Novel observations with FDOPA-PET imaging after early nigrostriatal damage. Movement Disorders. 2001;16:838–848. doi: 10.1002/mds.1168. [DOI] [PubMed] [Google Scholar]