Abstract
Alterations in neural synchrony and oscillations may contribute to the pathophysiology of schizophrenia and reflect aberrations in cortical glutamatergic and GABAergic neurotransmission. We tested the effects of a GABA agonist and a NMDA antagonist on auditory steady state responses (ASSRs) in awake rats with neonatal ventral hippocampal lesions (NVHLs) as a neurodevelopmental model of schizophrenia. NVHL vs. SHAM lesioned rats were injected with saline then either ketamine (NMDA antagonist) or muscimol (GABAA agonist). Time-frequency analyses examined alterations in phase locking (consistency) across trials and changes in total power (magnitude). ASSRs were compared at 5 stimulation frequencies (10, 20, 30, 40, and 50 Hz). In SHAM rats, phase locking and power generally increased with stimulation frequency. Both ketamine and muscimol also increased phase locking and power in SHAM rats, but mostly in the 20 to 40 Hz range. NVHL and ketamine altered the frequency dependence of phase locking, while only ketamine changed power frequency dependence. Muscimol affected power, but not phase locking, in the NVHL rats. NVHL and ketamine models of schizophrenia produce similar independent effects on ASSR, potentially representing similar forms of cortical network/glutamatergic dysfunction, albeit the effects of ketamine were more robust. Muscimol produced NVHL-dependent reductions in ASSR measures, suggesting that cortical networks in this model are intolerant to post-synaptic GABAergic stimulation. These findings suggest the utility of combining lesion, pharmacological, and ASSR approaches in understanding neural mechanisms underlying disturbed synchrony in schizophrenia.
Keywords: ASSRs, GABA, NMDA, NVHL, schizophrenia, synchronization
1. Introduction
Schizophrenia is a severe and chronic psychiatric disorder for which full understanding of the biobehavioral mechanisms remains elusive. Altered neural synchronization commonly measured via electroencephalographic (EEG) and magnetoencephalographic (MEG) recordings is thought to play a significant role in information and sensory processing abnormalities in schizophrenia (Ford & Mathalon, 2008; Uhlhaas & Singer, 2010; Whittington, 2008). Periodic auditory stimuli entrain the EEG to a specific phase and frequency, often referred to as a steady-state response (Brenner et al., 2009). Auditory steady-state responses (ASSRs) can test the functional integrity of neural circuits that support synchronization (Lins & Picton, 1995; Regan, 1989) across frequencies in both human and animal models.
Several EEG (Brenner et al, 2003; Krishnan et al., 2009; Kwon et al., 1999; Light et al., 2006; Spencer et al., 2008; Vierling-Claasen et al., 2008) and MEG (Teale et al., 2008) studies demonstrated reduced ASSR power (magnitude) and phase locking (phase consistency across trials) in schizophrenia particularly in the gamma range (30 to 80 Hz). ASSR reduction in schizophrenia patients may reflect neurophysiological abnormalities in auditory pathways or cortex (Brenner et al., 2009; Teale et al., 2008) and developmental alterations in neurotransmission of excitatory (glutamate) and inhibitory (Gamma-amino butyric acid: GABA) transmitter systems (Uhlhaas & Singer, 2010). Much of the evidence implicating glutamate and GABAergic transmission in disturbances of oscillatory activity in schizophrenia has been extrapolated from in-vitro studies, which support the role of N-methyl d-aspartate (NMDA) and GABA subtype A (GABAA) receptors in synchronization (Bartos et al., 2007; Gonzales-Burgos & Lewis, 2008; Roopun et al., 2008; Whittington, 2008). It follows, therefore, that in-vivo manipulation of these receptors in animal models of schizophrenia provide a suitable translational assay for testing mechanisms underlying electrophysiological disturbances observed in patients and measurable across species (Brenner et al., 2009; Luck et al., 2011).
The Neonatal Ventral Hippocampal Lesion (NVHL) model is an established neurodevelopmental rat model of schizophrenia that has been shown to have endophenotypic similarities to patients, including behavioral and molecular changes, as well as a pharmacological response profiles (Lipska et al., 2004; Tseng et al., 2009). NVHL lesions affect both GABAergic (Francois et al, 2009; Tseng et al., 2008) neurotransmission and sensitivity to NMDA antagonists (Al-Amin et al, 2000; Tseng et al., 2007). Because anatomical studies have shown that the hippocampus CA1 field (Cenquizca & Swanson, 2007) and the entorhinal cortex (Insausti et al, 1997) project to the auditory cortex, it is plausible that neonatal ablation of the ventral hippocampus in rat pups would also developmentally alter projections to auditory cortex and excitatory and inhibitory neurotransmission relevant to both schizophrenia and ASSR generation.
The present investigation is among the first to utilize both a ‘multifaceted’ developmental and pharmacological rodent models of schizophrenia in vivo during ASSR elicitation and recording. ASSR’s across a range of frequencies (10 – 50 Hz) were systematically examined in NVHL and SHAM rats. Further, the role of two major neurotransmitter systems involved in both schizophrenia phenomenology and ASSR generation, maintenance, and perturbation (Whittington, 2008) were tested via ketamine (NMDA antagonist) and muscimol (GABAA agonist) administration. We hypothesized that pharmacological manipulation of either GABAergic or glutamatergic receptor systems would alter ASSR generation and maintenance. Data from this study are expected to replicate our previous finding that GABAA agonism differentiates cortical and hence oscillatory responses in NVHL vs. SHAM (decrease and increase response, respectively) rats (Vohs et al., 2010) and characterize the impact of both NMDA antagonism and GABAA agonism across a range of frequencies.
2. Materials and Methods
2.1 Animals
All procedures complied with the Guide for the Care and Use of Laboratory Animals and were approved by the Indiana University Animal Care and Use Committee (IACUC). Six pregnant Sprague-Dawley rats were obtained from Harlan Labs (Indianapolis, IN) at the gestational age of 14–17 days and individually housed for 7–14 days until birth. At post-natal day (PND) 7, a subset of male pups were taken into a surgical room for 2–3 hours, where they were hypothermically anesthetized and received either an ibotenic acid excitotoxic lesion (N = 12) to the ventral hippocampi, or a SHAM lesion (N = 10), following the same procedure less the excitotoxin (see Vohs et al, 2010). Pups were monitored and warmed before being returned to their home cages.
Rats were weaned (PND 25) and at PND 49, paired (2 per cage) and moved to standard plastic cages. Three days prior to implantation of EEG electrodes, rats were moved to single-house plexiglass cages. Rats were kept in a temperature-controlled room, maintained on a 12:12 hr light/dark cycle (7am-7pm), with food and water available ad libitum. To reduce stress, rats were handled daily until implantation of electrodes and twice weekly thereafter.
2.2 EEG Electrode Implantation
Upon reaching 350 g (+/− 50 g; approximately PND 70), rats underwent surgery for EEG electrode implantation. Rats were anesthetized via isoflurane/air mixture and positioned in a stereotaxic frame. A midline incision exposed the dorsal surface of the skull and another incision reflected the right temporalis muscle, exposing the temporal bone. Stainless steel screw electrodes with wire leads were implanted epidurally over the vertex (AP −4.0 mm, ML −1.0 mm, VD 0.0 mm), temporal cortex (AP −4.5 mm, ML −7.5 mm, VD −4.0 mm), cerebellum (ground) and frontal sinus (reference). Stainless steel lead wires were connected to an Amphenol plug and the entire assembly was secured to the skull with dental cement. All ASSRs reported were recorded from the temporal electrode, as the vertex electrode was implanted for another study (Vohs et al., 2009). After surgery rats were allowed 14 days recovery. All SHAM rats (N = 10) used in the present investigation survived surgery without incident, however one NVHL rat (final N = 11) was lost during surgery, due to anesthetic complications.
2.3 Drugs
The GABAA agonist muscimol hydrobromide (Sigma, St. Louis, MO) and NMDA receptor antagonist ketamine (Sigma, St. Louis, MO) were dissolved in physiological saline for subcutaneous injection. Physiological saline, with an altered pH of 3.5 to match the lower pH drug (muscimol), was used as a control condition at a volume of 0.3 ml per injection. Drug dosages were calculated based on animal weight and adjusted to dosages of 10 mg/kg for ketamine and 1mg/kg for muscimol. The subanesthetic ketamine dose was selected because it was previously demonstrated to increase cortical glutamate release up to 60 minutes post-injection (Moghaddam et al., 1999), while the muscimol dose was selected based on previous investigations showing behavioral alterations (Houston et al., 2002).
2.4 Stimuli and EEG Recording Procedure
Rats were placed in a 5-sided plastic mesh cage within an electrically shielded chamber. A flexible, insulated plug connector was attached to the Amphenol plug. Rats were allowed a 20 minute acclimation period, which was immediately followed by an injection of saline, NMDA antagonist (ketamine), or GABAA agonist (muscimol). ASSRs were elicited from all rats, with 3 sessions of data collection (at least 24 hours apart): 1) saline control; 2) ketamine (10 mg/kg); and 3) muscimol (1 mg/kg). Based on literature review (Houston et al., 2002; Moghaddam et al, 1997) and behavioral observations, a 20 minute drug absorption period was observed after muscimol administration, while a 3 minute drug absorption period was observed for saline and ketamine. While saline was always the first condition, drug type (ketamine/muscimol) was randomly assigned to balance against order effects.
ASSRs were elicited via the presentation of auditory stimuli (80 dB) through a small speaker mounted on the ceiling of the recording chamber. Stimuli consisted of ten 10 second trains of clicks, presented at a range of frequencies (10, 20, 30, 40, and 50 Hz), with an inter-trial-interval (ITI) of 1000 ms. EEG data were collected (band pass .1 – 300 Hz) and digitized (1,000 Hz). Test-retest reliability of ASSRs in lesion and SHAM rats has been demonstrated (Vohs et al., 2010).
2.5 Histology
Following completion of recordings, NVHL and SHAM rats were sacrificed (deep isoflurane anesthesia and decapitation) and brains were sectioned on a cryostat into coronal slices 40 micrometers thick spaced 400 micrometers apart. Once mounted and fixed with chloroform, each section was Nissl stained with .05% thionin. The extent of the lesions was determined by microscopic assessment from approximately 3.30 to 5.80 mm posterior to bregma, by a collaborator (R.A.C.) blind to electrophysiological results. Four NVHL rats did not meet criteria for successful hippocampal lesions and two SHAM rats with needle damage to the hippocampi or surrounding regions were excluded. A figure showing acceptable lesion size and placement for the NVHL rats used in this study has been previously published (Vohs et al., 2009) in an investigation during which all rats underwent EEG electrode implantation and recording, but no pharmacological manipulations were performed. Data from each of the remaining rats (SHAM, N = 8; NVHL, N = 7) were analyzed.
2.6 Data Analysis
ASSR data analysis was performed using specialized code for MatLab® (R2007a). ASSRs at each frequency of stimulation (+/− 1.9 Hz) were segmented to include the time period when the stimulus was presented (10 seconds). Data from each 10 second segment was then further segmented into 1 second epochs. Each epoch was baseline corrected to control for waveform polarity drift away from 0 mV and submitted to a 100% Hanning window, to reduce bias in estimation of both power and phase. The taper was applied to the windowed EEG segments before performing FFT. A time-frequency spectrogram, using the short time sliding ~ window (128 ms) FFT was then obtained. The FFT returns a complex number output (F̃(f,t)) for each frequency (f) and time step (t) in each trial. The sliding window FFT was then moved with 10 ms time steps to perform the spectrogram analysis.
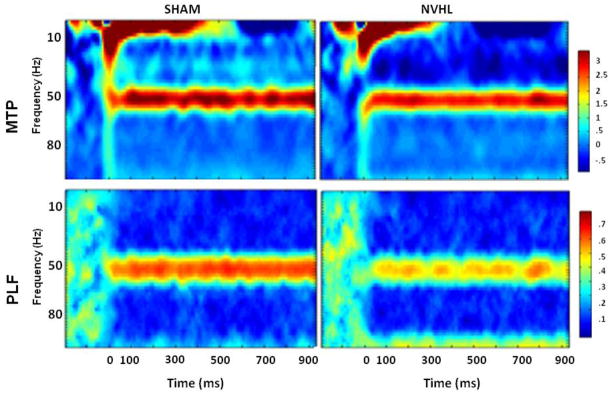
Two independent measures of ASSR activity, mean trial power (MTP) and phase locking factor (PLF), were obtained from the spectrogram by averaging across trials for the normalized complex output for every time period and frequency (Makeig et al., 2004). MTP was computed by determining the power, or squared amplitude, of individual trials, then subtracting the mean power of the baseline period (260 ms before stimulus onset) from the power of each trial. MTP scores were then log scored, as appropriate, to normalize the distribution. PFL measures phase synchronization of EEG activity across trials at particular time intervals and frequencies. To illustrate these measures, Figure 1 shows the average time-frequency spectrograms for NVHL and SHAM rats at 50 Hz stimulation, which was the frequency that best illustrated differential group responses.
Figure 1.
PLF = phase locking factor (consistency across trials); MTP = mean trial power (magnitude of response). This figure shows time-frequency plots of ASSRs at 50 Hz stimulation, which most robustly illustrated the responses of NVHL vs. SHAM rats.
Statistical analysis was performed using SPSS for Windows (18.0, 2010. Chicago: SPSS, Inc.). Effect size, as measured by partial eta squared (ηp2) was calculated to reflect strength of association. Partial Eta squared is the proportion of the effect variance to the effect plus error variance (ηp2 = SSfactor/(SSfactor + SSerror), with higher ηp2 values reflecting stronger effects. Statistical significance was determined at p < 0 .05. Repeated measures analyses of variance (ANOVAs) were performed on the dependent measures of phase locking and power in separate analyses. Focusing exclusively on lesion effects, repeated measure ANOVAs with the within subject factor frequency and between subjects factor of lesion status were performed on data collected after saline administration. Additional repeated measures ANOVAs examined lesion by drug by frequency effects in which ketamine and muscimol were examined separately.
3. Results
3.1 NVHL vs. SHAM rats after saline
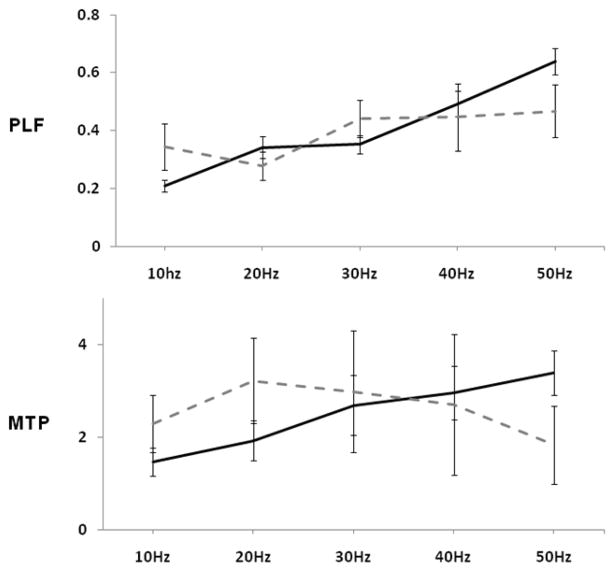
Phase locking increased with increases in stimulation frequency as shown in Figure 2 (upper panel) (F(4,52) = 10.28, ηp2 = .44, p < .001). This frequency dependence was altered by NVHL as indicated by a frequency × lesion interaction (F(5, 52) = 3.06, ηp2 = .19, p < .05) associated with a flattening of the phase locking frequency response curve in NVHL rats. Power did not show significant frequency dependent variation or a main lesion effect or frequency × lesion interaction (Figure 2, lower panels).
Figure 2.
PLF = phase locking factor (consistency across trials); MTP = mean trial power (magnitude of response). Data from each panel was used separately in repeated measure ANOVA. A frequency by lesion interaction (p<.05) was detected with PLF (upper panel). Error bars represent the standard error of the mean.
3.2 NVHL vs. SHAMs: ketamine effects
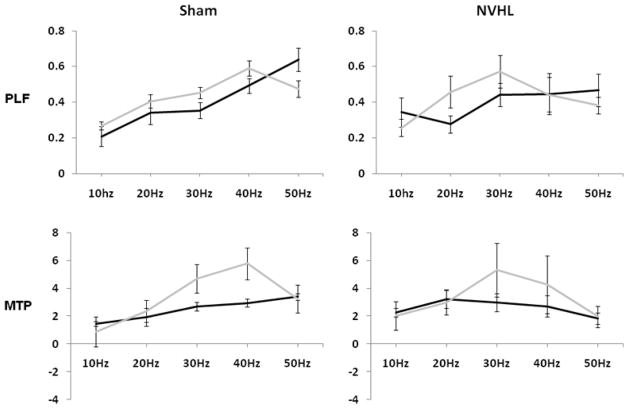
The dependence of phase locking on frequency (F(4,52) = 10.86, ηp2 = .46, p < .001) again interacted significantly with NVHLs (lesion × frequency: (F(4,52) = 2.91, ηp2 = .18, p < .05). Ketamine also significantly disrupted the frequency dependence of phase locking as indicated by drug × frequency interaction (F(4,52) = 4.86, ηp2 = .27, p < .01). As shown in Figure 3 (upper), phase locking values generally increased from low to high frequencies across all rats (albeit most robustly in SHAMs), while ketamine blunted phase locking magnitude at 50 Hz.
Figure 3.
PLF = phase locking factor (consistency across trials); MTP = mean trial power (magnitude of response). Data from the upper two and then lower two panels were each used in a repeated measure ANOVA to test for lesion and/or drug effects and interaction using PLF and MTP, respectively. Both lesion by frequency and drug (ketamine) by frequency interactions were detected using PLF, while main effects of frequency and drug, as well as a frequency by drug interaction were detected using the MTP measure. Effects and interactions were considered significant at p<.05. Error bars represent the standard error of the mean.
Power showed significant main effects of frequency (F(4, 52) = 4.51, ηp2 = .27, p < 0.01), ketamine (F(1, 12) = 5.11, ηp2 = .30, p <0.05), and a frequency × drug interaction (F(4, 52) = 3.86, ηp2 = .24, p < .01). As shown in Figure 3 (lower), ketamine-induced elevations were most pronounced at 30 and 40 Hz.
3.3 NVHL vs. SHAMs: muscimol effects
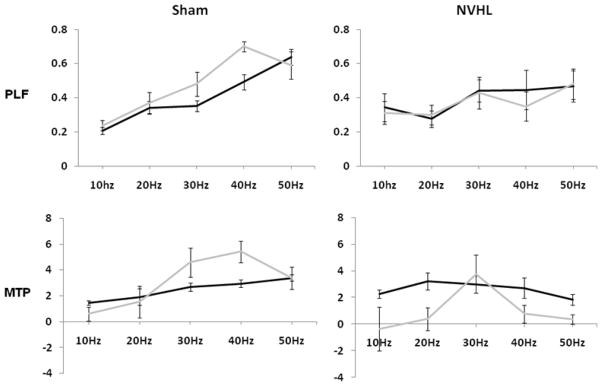
Phase locking was sensitive to muscimol administration and lesion status, and these two manipulations interacted to disrupt the frequency response function. These effects were revealed by a main effect of frequency (F(4,52) = 14.39, ηp2 = .53, p < .001); a frequency × lesion interaction (F(4, 52) = 4.13, ηp2 = .24, p < .01), a muscimol × lesion interaction (F(1, 13) = 9.25, ηp2 = .42, p < .01) and as 3-way interaction (muscimol × lesion × frequency interaction (F(4, 52) = 2.49, ηp2 = .16, p = .05). As indicated in Figure 4 (upper) these results indicate that muscimol increases 30 and 40 Hz phase locking in SHAM rats, but lacks a systematic effect on the frequency response function in NVHL rats.
Figure 4.
PLF = phase locking factor (consistency across trials); MTP = mean trial power (magnitude of response). Data from the upper two and then lower two panels was used in a repeated measure ANOVA to test for lesion and/or drug (muscimol) effects and interaction using PLF and MTP, respectively. PLF data revealed a frequency by lesion, drug by lesion, and three way drug, frequency, lesion interaction (p < .05). Using MTP frequency by drug and drug by lesion interactions were detected. Effects and interactions were considered significant at p<.05. Error bars represent the standard error of the mean.
Power was also affected by muscimol administration, as indicated by frequency dependent variation (F(4, 52) = 5.03, ηp2 = .28, p < .01) that was altered by drug effects (frequency × muscimol: (F(4, 52) = 4.22, ηp2 = .25, p < .01) and a muscimol × lesion interaction (F(1, 13) = 4.54, ηp2 = .26, p < .05). As shown in Figure 4 (lower) these interactions suggested that muscimol increased power, especially at 30 and 40 Hz, in SHAM rats, while decreasing overall power in NVHLs.
4. Discussion
ASSRs are usually abnormal in schizophrenia, with reductions consistently found in the gamma frequency range. This study showed that the frequency response function of ASSRs in rats was sensitive to NVHL developmental perturbation and pharmacological manipulation of neurotransmitter systems implicated in the generation of neural oscillations and the pathophysiology of schizophrenia. Ketamine resembled, and muscimol interacted with the NVHL model, suggesting actions on common neural systems in auditory cortical pathways. These findings build on previous work identifying EEG markers of auditory information processing abnormalities in the NVHL model (Vohs et al., 2009, 2010) that are analogous to those identified in schizophrenia.
In examining responses over a wide range of frequencies, NVHLs were shown for the first time to produce alterations in the frequency dependence of the ASSR. This effect manifested qualitatively as a failure of NVHL rats to demonstrate increasing phase locking with increasing stimulation frequency, analogous to schizophrenia-related ASSR abnormalities that occur predominantly in the gamma range (Brenner et al, 2003, 2009; Krishnan et al., 2009; Kwon et al., 1999; Spencer et al., 2008). These findings may reflect altered network dysfunction with greater stimulation frequencies, due to increased demand for temporal coordination of neural firing (Ford et al., 2007; Ford & Mathalon, 2008) hypothesized to be modulated by excitatory and inhibitory neurotransmission (Bartos et al., 2007; Lazarewicz et al., 2010; Vierling-Claasen et al., 2008; Whittington et al., 2010). Developmental disruption, inherent to the NVHL model (Tseng et al., 2007, 2008), of connections from the hippocampal regions to the temporal (Cenquizca et al, 2007; Insausti et al, 1997) and prefrontal cortex (Goto & Grace, 2008; Jay & Witter, 1991) and possibly other cortical areas, may underlie network dysfunction which affects both GABAergic and NMDA transmission. This interpretation is consistent with hypotheses and supporting data suggesting that cortical networks in schizophrenia (Benes & Beretta, 2001; Carlsson et al., 2001; Javitt, 2008; Javitt et al., 2007; Krystal et al., 2003) and NVHL rats (Endo et al., 2007; Lipska et al., 2004; Tseng et al., 2009) show subtle yet pervasive abnormalities of both glutamatergic and GABAergic organization and function. While there is a paucity of data regarding auditory cortex abnormalities and dysfunction in the NVHL model, it is notable that the lesion does result in acoustic hypersensitivity in adult rats (Macedo et al., 2010) and altered event-related potentials to paired click stimuli (Vohs et al., 2009).
In order to further explore the nature of the observed cortical dysfunction and its relationship to the model, we examined ASSRs after acute administration of ketamine. This and other non-competitive NMDA antagonists (PCP, MK-801) have long been appreciated as pharmacological models of the putative glutamatergic disturbance in schizophrenia (Carlsson & Svensson, 1990; Krystal et al., 1994). Like NVHLs, ketamine changed the frequency dependence of ASSR phase locking, tempering the trend for increased phase locking in the gamma range. This consistency between NVHL and ketamine ASSR response profiles may be indicative of similarities in their cortical network effects. It has been hypothesized that NMDA antagonists may acutely produce a functional state where NMDA blockade (on GABAergic interneurons) weakens inhibitory control of principal glutamatergic neurons (Coyle, 2004; Grunze et al., 1996; Homayoun & Moghaddam, 2007; Krystal et al., 2003; Moghaddam et al., 1997), which could manifest as altered phase locking at some frequencies. Similarly, NVHL rats show cellular and physiological markers of cortical GABAergic hypofunctionality (Endo et al., 2007; Francois et al., 2009; Tseng et al., 2008), and dysfunctional glutamatergic transmission associated with stimulus-dependent dis-coordination and/or hyper-active firing of cortical neurons (Chambers et al., 2010; O’Donnell et al., 2002; Tseng et al., 2007). These themes mirror histopathological and physiological evidence suggesting that GABAergic hypofunction and disorganization of excitatory transmission are themes characteristic of the schizophrenic cortex (Aghajanian, 2009; Coyle, 2004; Lewis & Moghaddam, 2006). Notably, we also observed a robust effect of ketamine on ASSR power, which was not observed as an independent NVHL effect. This difference may reflect the capacity of acute ketamine challenge to produce a more potent, uncompensated effect in altering cortical connectivity and network activity compared to NVHL, where developmental compensatory mechanisms may have minimized network dysfunction severity.
Analysis of the effects of the GABAA agonist muscimol provided an alternative perspective on the neurotransmitter basis of ASSR profiles and their derangements in NVHL rats. Consistent with our expectation to replicate previous findings (Vohs et al., 2010), GABAA agonism decreased gamma range activity in NVHL relative to the increased response observed in SHAM rats. The degradation of both phase locking and power that occurred only as a combined result of both muscimol and NVHL suggests that the low GABAergic functionality associated with the NVHL model could represent a compensatory adaptation to, or secondary consequences of other primary network aberrations. Then, GABAergic provocation in a NVHL cortical network that requires, or is inflexibly limited to, GABAergic hypoactivity may produce disruptions in cortical synchronization. Considering that muscimol and ketamine produce acute network effects that are to some extent in opposition (i.e. mucimol increases inhibitory/decreases excitatory activity; ketamine decreases inhibitory/increases excitatory activity), we posit that their differential effects on NVHL ASSRs could be further illustrative of the altered cortical dysfunction, particularly in auditory cortex, that is yet to be explored in this model.
Taking our results and interpretations in perspective, it is clear that follow-up investigations are needed to further explore network and neurotransmission alterations in the auditory cortex of NVHL rats. Further, additional data on ASSR measures in rats is needed to characterize dose-response relationships, cellular mechanisms, and predictive validity for anti-psychotic agents. This study is the first to characterize ASSRs in SHAM and NVHL rats across and range of frequencies and implement pharamacological manipulations to test the role of excitatory and inhibitory transmission, which could ultimately further understanding of this complex issue in patients with schizophrenia. Finally, this study highlights an experimental approach and rationale for using NVHLs and additional schizophrenia models, and alternative pharmacological challenges involving other dosing regimens and drug types. Providing a way to test mechanistic hypotheses that are not feasible in humans, or for generating new clinically relevant hypotheses, ASSR studies in rat models are potentially highly complementary to, and synergistic with parallel human studies.
Highlights.
NVHL, ketamine, and muscimol altered ASSRs frequency dependence
Results sometimes differ depending on measure used (power vs. phase locking)
Data may support receptor alterations in schizophrenia and ASSR generation
Data suggest utility of combining lesion, pharmacological, and ASSR approaches
Further study needed for mechanisms underlying disturbed ASSR in schizophrenia
Acknowledgments
Role of Funding Source
This work was supported in part by the Orvis Fund Intercampus IUMS Psychiatry Training Grant (SLM); NIDA K08 DA 019850 (RAC), NIMH R21 MH071876 (BFO). JLV was supported by the Research Training in Clinical Science Award, NIMH T32 MH17146 (Viken) and the Indiana CTSI Predoctoral Training Award; PHS (NCCR) Grant No: TL1RR02575 (Shekhar). These organizations had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
We would like to thank Sam Kaiser and Tammy Nelson for their technical assistance.
Footnotes
Contributors
Jenifer L. Vohs designed the study and wrote the protocol. Andrew Chambers and Sandra Morzorati contributed technical expertise on the NVHL model and rat EEG, respectively. Brian O’Donnell contributed significant expertise in schizophrenia research design and implementation. Giri Krishnan designed and programmed EEG collection and analysis software. All authors contributed to the literature searches and analyses. Jenifer L. Vohs undertook the statistical analysis, and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
The authors of this manuscript have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
R. Andrew Chambers, Email: achambers@iupui.edu.
Brian F. O’Donnell, Email: boddonel@indiana.edu.
Giri P. Krishnan, Email: pkrishn@gmail.com.
Sandra L. Morzorati, Email: smorzora@iupui.edu.
References
- Aghajanian GK. Modeling “psychosis” in vitro by inducing disordered neuronal network activity in cortical brain slices. Psychopharmacol (Berl) 2009;206:575–585. doi: 10.1007/s00213-009-1484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Amin HA, Weinberger DR, Lipska BK. Exaggerated MK-801-induced hyperactivity in rats with the neonatal lesion of the ventral hippocampus. Behav Pharmacol. 2000;11(3–4):269–278. doi: 10.1097/00008877-200006000-00010. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharm. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Krishnan GP, Vohs JL, Ahn WY, Hetrick WP, Morzorati SL, et al. Steady state responses: electrophysiological assessment of sensory function in schizophrenia. Schizophr Bull. 2009;35:1065–1077. doi: 10.1093/schbul/sbp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner CA, Sporns O, Lysaker PH, O’Donnell BF. EEG synchronization to modulated auditory tones in schizophrenia, schizoaffective disorder, and schizotypal personality disorder. Am J Psychiatry. 2003;160:2238–2240. doi: 10.1176/appi.ajp.160.12.2238. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Svensson A. Interfering with glutamatergic neurotransmission by means of NMDA antagonist administration discloses the locomotor stimulatory potential of other transmitter systems. Pharmacol Biochem Behav. 1990;36:45–50. doi: 10.1016/0091-3057(90)90123-y. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. 2001;41:237–260. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Spatial Organization of Direct Hippocampal Field CA1 Axonal Projections To the Rest of the Cerebral Cortex. Brain Res Rev. 2007;56(1):1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Sentir AM, Conroy SK, Truitt WA, Shekhar A. Cortical-striatal integration of cocaine history and prefrontal dysfunction in animal modeling of dual diagnosis. Biol Psychiatry. 2010;67:788–792. doi: 10.1016/j.biopsych.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT. The GABA-glutamate connection in schizophrenia: which is the proximate cause? Biochem Pharmacol. 2004;68:1507–1514. doi: 10.1016/j.bcp.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Endo K, Hori T, Abe S, Asada T. Alterations in GABA(A) receptor expression in neonatal ventral hippocampal lesioned rats: comparison of prepubertal and postpubertal periods. Synapse. 2007;61:357–366. doi: 10.1002/syn.20393. [DOI] [PubMed] [Google Scholar]
- Ford JM, Krystal JH, Mathalon DH. Neural synchrony in schizophrenia: from networks to new treatments. Schizophr Bull. 2007;33:848–852. doi: 10.1093/schbul/sbm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH. Neural synchrony in schizophrenia. Schizophr Bull. 2008;34:904–906. doi: 10.1093/schbul/sbn090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois J, Ferrandon A, Koning E, Angst MJ, Sandner G, Nehlig A. Selective reorganization of GABAergic transmission in neonatal ventral hippocampal-lesioned rats. Int J Neuropsychopharmacol. 2009;12:1097–1110. doi: 10.1017/S1461145709009985. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: Implications for understanding cotical dysfunction in schizophrenia. Schizophrenia Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopamine modulation of hippocampal prefrontal cortical interaction drives memory-guided behavior. Cerebral Cortex. 2008;18:1407–1414. doi: 10.1093/cercor/bhm172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunze HC, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, Greene RW. NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci. 1996;16(6):2034–43. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston AJ, Wong JCL, Ebenezer IS. Effects of subcutaneous administration of γ-aminobutyric acidA receptor agonist muscimol on water intake in water-deprived rats. Physiology & Behavior. 2002;77:445–450. doi: 10.1016/s0031-9384(02)00876-4. [DOI] [PubMed] [Google Scholar]
- Insausti R, Herrero MT, Witter MP. Entorhinal Cortex of the Rat: Cytoarchitectonic Subdivisions and the Origin and Distribution of Cortical Efferents. Hippocampus. 1997;7:146–183. doi: 10.1002/(SICI)1098-1063(1997)7:2<146::AID-HIPO4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajos M. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7:68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM, Witter MP. Distrbution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde tranport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1991;313:574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- Krishnan GP, Hetrick WP, Brenner CA, Shekhar A, Steffen AN, O’Donnell BF. Steady state and induced auditory gamma deficits in schizophrenia. Neuroimage. 2009;47:1711–1719. doi: 10.1016/j.neuroimage.2009.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, D’Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacol (Berl) 2003;169:215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarewicz MT, Ehrlichman RS, Maxwell CR, Gandal MJ, Finkel LH, Siegel SJ. Ketamine modulates theta and gamma oscillations. J Cogn Neurosci. 2010;22:1452–1464. doi: 10.1162/jocn.2009.21305. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63:1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, et al. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60:1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- Lins OG, Picton TW. Auditory steady-state responses to multiple simultaneous stimuli. Electroencephalogr Clin Neurophysiol. 1995;96:420–432. doi: 10.1016/0168-5597(95)00048-w. [DOI] [PubMed] [Google Scholar]
- Lipska BK. Using animal models to test a neurodevelopmental hypothesis of schizophrenia. Journal of Psychiatry and Neuroscience. 2004;29(4):282–286. [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Mathalon DH, O’Donnell BF, Hamalainen MS, Spencer KM, Javitt DC, et al. A Roadmap for the Development and Validation of Event-Related Potential Biomarkers in Schizophrenia Research. Biol Psychiatry. 2011;70(1):28–34. doi: 10.1016/j.biopsych.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo CE, Angst MJ, Guiberteau T, Brasse D, O’Brien TJ, Sandner G. Acoustic hypersensitivity in adult rats after neonatal ventral hippocampus lesions. Behav Brain Res. 2010;207:161–168. doi: 10.1016/j.bbr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Makeig S, Debener S, Onton J, Delorme A. Mining event-related brain dynamics. Trends Cognitive Sci. 2004;8:204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P, Lewis BL, Weinberger DR, Lipska BK. Neonatal hippocampal damage alters electrophysiological properties of prefrontal cortical neurons in adult rats. Cereb Cortex. 2002;12:975–982. doi: 10.1093/cercor/12.9.975. [DOI] [PubMed] [Google Scholar]
- Regan D. Human Brain Electrophysiology: Evoked Potentials and Evoked Magnetic Fields in Science and Medicine. Amsterdam: Elsevier; 1989. [Google Scholar]
- Roopun AK, Cunningham MO, Racca C, Alter K, Traub RD, Whittington MA. Region-specific changes in gamma and beta2 rhythms in NMDA receptor dysfunction models of schizophrenia. Schizophrenia Bulletin. 2008;34:962–973. doi: 10.1093/schbul/sbn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Salisbury DF, Shenton ME, McCarley RW. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. 2008;64:369–375. doi: 10.1016/j.biopsych.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teale P, Collins D, Maharajh K, Rojas DC, Kronberg E, Reite M. Cortical source estimates of gamma band amplitude and phase are different in schizophrenia. Neuroimage. 2008;42:1481–1489. doi: 10.1016/j.neuroimage.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2009;204:295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Lewis BL, Hashimoto T, Sesack SR, Kloc M, Lewis DA, et al. A neonatal ventral hippocampal lesion causes functional deficits in adult prefrontal cortical interneurons. J Neurosci. 2008;28:12691–12699. doi: 10.1523/JNEUROSCI.4166-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Lewis BL, Lipska BK, O’Donnell P. Post-pubertal disruption of medial prefrontal cortical dopamine-glutamate interactions in a developmental animal model of schizophrenia. Biol Psychiatry. 2007;62:730–738. doi: 10.1016/j.biopsych.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Vierling-Claassen D, Siekmeier P, Stufflebeam S, Kopell N. Modeling GABA alterations in schizophrenia: a link between impaired inhibition and altered gamma and beta range auditory entrainment. J Neurophysiol. 2008;99:2656–2671. doi: 10.1152/jn.00870.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohs JL, Chambers RA, Krishnan GP, O’Donnell BF, Berg S, Morzorati SL. GABAergic modulation of the 40 Hz auditory steady-state response in a rat model of schizophrenia. Int J Neuropsychopharmacol. 2010;13:487–497. doi: 10.1017/S1461145709990307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohs JL, Chambers RA, Krishnan GP, O’Donnell BF, Hetrick WP, Kaiser ST, et al. Auditory sensory gating in the neonatal ventral hippocampal lesion model of schizophrenia. Neuropsychobiology. 2009;60:12–22. doi: 10.1159/000234813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA. Can brain rhythms inform on underlying pathology in schizophrenia? Biol Psychiatry. 2008;63:728–729. doi: 10.1016/j.biopsych.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Cunningham MO, Lebeau FE, Racca C, Traub RD. Multiple origins of the cortical gamma rhythm. Dev Neurobiol. 2010;71(1):92–106. doi: 10.1002/dneu.20814. [DOI] [PubMed] [Google Scholar]