Summary
A prediction-based error signal, neurally computed as the difference between predicted and observed movement outcomes, has been proposed as the driving force for motor learning. This suggests that the generation of predictive saccades to periodically paced targets – whose performance accuracy is actively maintained using this same error signal – invokes the motor learning network. We examined whether a simple predictive-saccade task (implicit double-step adaptation, in which targets are gradually displaced outward to exaggerate normal hypometric movement errors) can stand in place of a traditional double-step saccade-adaptation task to induce an increase in saccade gain. We find that the implicit double-step adaptation task can induce significant gain-increase adaptation (of comparable magnitude to that of the standard double-step task) in normal control subjects. Unlike control subjects, patients with impaired cerebella are unable to adapt their saccades in response to this paradigm; this implies that the cerebellum is crucial for processing prediction-based error signals for motor learning.
Keywords: predictive saccade, motor learning, adaptation, spinocerebellar ataxia type 6
INTRODUCTION
Error-based motor learning is crucial for maintaining movement accuracy. This process enables the brain to adjust future movements in response to prior errors, to keep motor plans accurate. Such learning is thought to be driven by a prediction-based error-signal: that is, motor plans are updated according to the difference between observed and expected movement outcomes at the conclusion of the movement, rather than the errors directly observed as the sensory consequences of actions (Bahcall and Kowler 2000; Tseng et al. 2007; Collins and Wallman 2012). This error signal may be computed using a forward model, which uses a copy of the motor command (efference copy) to estimate movement outcome (Miall and Wolpert 1996; Mehta and Schaal 2002). Trial-to-trial adaptation involves updating the motor plan in response to these computed prediction-based error signals.
Forward-model predictions can also be used to drive real-time movement corrections in a closed-loop fashion, by continually updating the motor command as it is being executed until the computed movement outcome is predicted to reach the intended goal. These corrections are different from trial-to-trial learning because they strictly occur in response to inaccuracies of the issued motor plan. It is difficult to ascertain whether consistent late-movement changes arise from trial-to-trial adaptation of the late phase of the movement, or repeated single-trial online corrections. For simplicity, we will assume the latter to be true since these kinematic adjustments generally compensate for changes of the early portion of the movement trajectory, maintaining overall movement accuracy. For example, with repeated execution of saccadic eye movements there is a gradual change of the motor plan, resulting in a decline of peak velocity (“fatigue”) across long blocks of targets (Golla et al. 2008; Xu-Wilson et al. 2009). If left uncompensated, such fatigue would produce hypometric saccades that fall short of the target. Forward-model predictions of this hypometria, however, can be used to modify the motor command online. This results in a delayed deceleration phase, which extends movement duration to compensate for the reduction in velocity such that the saccade lands on target. Such online “steering” of the movement to a desired endpoint is unlikely to depend on sensory feedback, which is delayed and – in the case of saccades – is thought to arrive too late to be used for modifying such rapid movements. Thus, two important modes of motor learning exist in synergy – trial-by-trial adaptation and online error corrections – both of which depend upon accurate predictions of movement outcome to improve overall movement accuracy.
Adaptation of saccadic eye movements incorporates a realistic expectation of movement inaccuracy (Henson 1978); Bahcall and Kowler 2000; Wong and Shelhamer 2011c; Collins and Wallman 2012). Saccades are intentionally hypometric (i.e., they systematically fall short of the target, at least in a laboratory setting). When stimulus manipulations change observed post-saccadic errors, subjects adapt in a direction that restores their expected hypometria, even if this increases observed visual errors. Furthermore, during a predictive-saccade task (in which subjects execute a saccade in anticipation of the next requested movement by accumulating experience across many previous trials), subjects maintain the accuracy of their predictive movements by using an error signal derived as the observed accuracy of their predictions (Wong and Shelhamer 2011a). In other words, motor learning in the predictive-saccade task is based on the difference between movement expectation (for a predictive saccade, this is the stimulus that directs the anticipatory movement) and the observation of prediction accuracy (or, post-saccadic visual errors). This difference between prediction and observation is the same error signal attributed to adaptation: a prediction-based error. In short, motor learning depends on the ability to produce accurate predictions, regardless of the specific task details.
Motor learning in the saccadic system is typically studied using the traditional double-step paradigm, in which the systematic displacement of the target during the saccade produces an unexpected movement error. Over hundreds of trials, this adapts saccade gain such that in response to the initial target, the motor system plans and executes a movement to the displaced target (Mclaughlin 1967). We observed that the predictive-saccade paradigm might be used to create a similar situation. Predictive saccades are invoked by stepping a target periodically between two locations at a rapid rate (0.6–1 Hz); each saccade is generated before visual information about the current stimulus can be processed to guide behavior (typical predictive-saccade latencies are <80 ms). The motor system learns from prior behavior to anticipate the next intended movement (Stark et al. 1962; Shelhamer and Joiner 2003; Wong and Shelhamer 2011a). Prediction accuracy is computed according to the error observed at the end of the movement, when visual information about the current target is available. Thus, a standard predictive-saccade task actually involves two targets. First, there is an initial (implicit) target that invokes the planning of a motor command – and in this case, the execution of the predicted movement. Second, there is the visual (presented) target, which provides feedback about prediction accuracy. During normal reactive saccades the initial target is explicit, and is identical to the feedback target; in a conventional adaptation paradigm both targets are explicitly presented, with the latter displaced from the former during the saccade (i.e., the double step). In contrast, the novel adaptation paradigm that we present here – the implicit double-step adaptation task – is based on a series of predictive saccades in which subjects make movements in anticipation of target appearance. The initial target in our task is implicit, and composed of the predicted target location; it is derived according to prior experience. Only the feedback target is explicitly presented to the subject. Steadily moving the location of feedback targets outward suggests to the motor system that the eye is hypometric to the initial, implied target. This generates a continuous and consistent error signal about the inaccuracy of the prediction. In this manner, the predictive-saccade based, implicit adaptation task is roughly equivalent to a traditional double-step adaptation paradigm, except that the initial saccade target is implied. Learning in this task is akin to the work of Mellis and van Gisbergen (1996), who showed that saccade adaptation can be induced by creating a mismatch between the expected endpoint of a saccade that is evoked by microstimulation of the superior colliculus in the absence of a visual target, and an explicit but displaced visual target following the saccade. Note, a copy of the motor command from the superior colliculus passes through the cerebellum (Leigh and Zee 2006). Thus, the error signal that drives learning seems equivalent in all these cases: it is derived as the difference between predicted and observed movement outcomes.
The cerebellum is of particular interest in motor learning because it is thought to be the site at which movement predictions are computed. Error-based adaptation depends upon the cerebellum (Miall 1998); Wolpert et al. 1998; Bastian 2006): patients with impaired cerebella exhibit motor-learning deficits such as the inability to adapt in response to sudden perturbations (Straube et al. 2001; Golla et al. 2008; Xu-Wilson et al. 2009). Some of these adaptation deficits can be directly attributed to the loss of predictive abilities. For example, during a split-belt treadmill paradigm in which one leg learns to walk faster than the other, cerebellar patients show impaired production of predictive but not reactive components of the typical adaptation response exhibited by normal control subjects (Morton and Bastian 2006). Furthermore, the cerebellum is important for generating online movement adjustments to correct improperly issued motor commands, such as to compensate for fatigue (Golla et al. 2008; Xu-Wilson et al. 2009). Therefore, the cerebellum seems critical for prediction-based learning.
On the other hand, cerebellar patients can successfully complete some motor-learning tasks that involve prediction. For example, they can generate predictive saccades with reasonably appropriate latencies; thus, they can plan and execute anticipatory behaviors, albeit with greater inter-trial variability (Joiner et al. 2005; Lasker et al. 2005; Nagel et al. 2008). Additionally, if adaptive stimuli are introduced gradually, cerebellar patients can learn almost as well as do normal control subjects (Criscimagna-Hemminger et al. 2010; Izawa et al. 2012). Since the slow increase in stimulus size means experienced errors are never very large, this may keep observed errors in close alignment with expected movement outcomes (i.e., prediction errors remain small) to effectively promote adaptation. If cerebellar patients can adapt under the circumstances described above, the cerebellum may not necessarily be required for prediction-based motor learning. Thus, it is useful to study whether normal control subjects and patients with cerebellar deficits can adapt in response to a prediction-based error signal.
Using the implicit double-step task, we can explore two questions. First, can this predictive-saccade task with an implied target step drive adaptation in normal subjects? This would further support the idea that adaptation depends on predicting movement outcomes, as the implicit double-step task drives behavior in response to prediction errors. Second, does the cerebellum contribute to the processing of a prediction-based error signal? By comparing the behavior of patients with cerebellar degeneration to that of control subjects during a standard predictive-saccade task, we can search for evidence of online cerebellar-based fatigue compensation during the saccade. This would demonstrate that the cerebellum is necessary to steer saccades toward intended movement goals by updating the predictive motor response. Furthermore, if cerebellar patients cannot adapt their saccades in a similar manner as do control subjects in the implicit double-step task, this confirms the general role of the cerebellum in facilitating trial-to-trial motor learning according to a prediction-based error signal.
MATERIALS AND METHODS
Informed consent was obtained from each participant, according to the local institutional review board. All subjects were naive to the purposes of this experiment.
Eleven patients with spinocerebellar ataxia type 6 (SCA6) participated in this study. SCA6 is an autosomal dominant, neurodegenerative disease that specifically targets the cerebellum, including the vermis and cerebellar hemispheres (Sasaki et al. 1998; Honjo et al. 2004). SCA6 results in a severe loss of Purkinje cells, a mild loss of granule, stellate, and basket cells, and little to no loss of cells in the inferior olive (Ishikawa et al. 1999). In this study, the average patient age was 60 (range, 41–72); there were four males and seven females. Ten of the SCA6 patients participated in a separate study that included evaluating the severity of their impairment using the International Cooperative Ataxia Rating Scale (ICARS; Trouillas et al. 1997). Since this assessment was administered during the same visit as our experiment, these ICARS scores reflect the motor abilities of these patients at the time this study was conducted. For these ten patients, the average ICARS score was 30.6 (range, 4–58.5).
Eleven age- and gender-matched control subjects also participated in this experiment; the mean age was 59 (range, 39–67; average age difference between age-matched pairs, 0.82 ± 2.82 years). Data from one control subject were discarded because, unlike the other control subjects and SCA6 patients, that subject generated a majority of anticipatory saccades (latency < −200 ms; see Results). The remaining control population did not differ in age from that of the patients (t-test, p = 0.67), and no correlation was found between the main findings (extent of adaptation) and age for either the control or SCA6 population (controls: r2 = 0.00, p = 0.94; SCA6: r2 = 0.07, p = 0.45), suggesting that our control population was fairly uniform and well-matched with the patient population.
Horizontal and vertical eye movements were recorded using a scleral search coil in a magnetic field (Chronos Vision, Berlin, Germany) from either the right or left eye (Robinson 1963). Data were acquired at 1000 Hz on a PC-compatible computer running real-time experiment control software developed in-house. Horizontal eye position was computed in real time using a piecewise-linear calibration tailored to each subject, based on eye position while fixating seven points over a range of ±25° along the horizontal axis. Subjects sat in a dark room in a stationary chair, and a bite bar was used to minimize head movements. Targets were presented using a mirror-controlled laser dot 2 mm in diameter that was rear-projected onto a screen 1 m in front of the subject.
Experimental Paradigm
Throughout the experiment, subjects were given no instructions regarding target location or time of appearance; they were simply asked to look at each target. The experiment proceeded in two parts. The first part involved one block (Pred) in which the subject made saccades to a continuous sequence of 300 targets. Targets were located at ±5° to either side of the vertical midline and paced at 0.7 Hz with the intent to evoke predictive saccades; target timing and position were predictable throughout the block. This enabled us to assess steady-state behavior and observe the effects of fatigue due to task repetition. This portion of the experiment was conducted first, to prevent any residual adaptation from the implicit double-step task from interfering with the analysis of these trials.
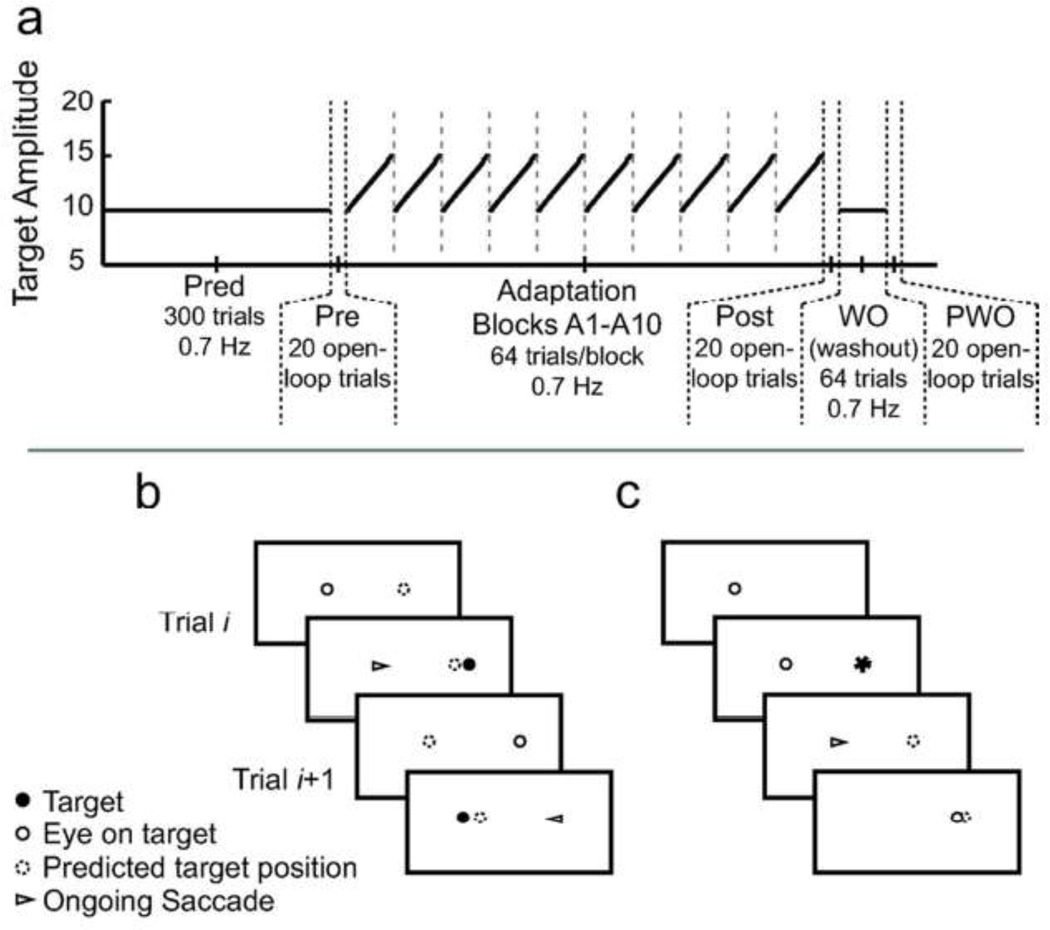
The second part of the experiment was the implicit double-step adaptation task (Figure 1). This portion involved 11 predictive-saccade blocks of 64 targets each, paced at 0.7 Hz. The first 10 blocks (A1 to A10) were implicit double-step adaptation blocks. During each block, the target locations slowly increased from ±5° to ±7.5° symmetrically on either side of the vertical midline: each target stepped outward on every third trial to produce a total amplitude change of 0.25°, ultimately yielding a cumulative change of 5° across the block. The eleventh block (WO) was a washout block, in which the targets alternated between fixed locations ±5° to either side of the vertical midline for the entire block. All blocks were separated by a rest period of about 30 s, during which subjects remained in the dark with their eyes closed. The vertical target location was fixed at 0� on all trials.
Figure 1.
Experimental paradigm. a: Time course of the experiment. In the first part of the experiment, subjects executed a long block of 300 predictive saccades (Pred) at 0.7 Hz. In the adaptation portion of the experiment, saccade gain was assessed using blocks of open-loop trials (Pre, Post, and PWO). Adaptation proceeded in 10 implicit double-step blocks (A1–A10), in which target endpoints gradually stepped outward throughout the block. Washout (WO) was conducted with a single, short block of predictive saccades with fixed endpoints. Target amplitude is plotted as solid black lines; vertical dashed lines denote breaks between blocks. b: Sequence of implicit double-step adaptation trials. On each trial, subjects fixating the current target (open circle) plan a saccade to an estimated target location (dashed circle). The saccade is initiated before the target moves (triangle); however, the target actually appears at a position beyond the estimated target location (filled circle). This post-saccadic target provides error feedback about prediction accuracy. c: Target sequence for open-loop trials. Subjects fixate a target. The next target appears, allowing subjects to plan a saccade to that location; visual and predicted target positions overlap (filled dashed circle). The target is blanked upon saccade initiation; after 600 ms the target reappears at the current eye position to begin the next trial.
Subjects repeated many short implicit double-step adaptation blocks during this task, with targets in each block starting at the same initial amplitude and moving outward at the same rate. Shifting the location of the targets outward in small steps from trial to trial mimicked a gradual saccade-adaptation paradigm in keeping post-saccadic errors small but always larger than expected (Alahyane and Pelisson 2005; Wong and Shelhamer 2011b). Block repetition was performed to expose subjects to the adaptation stimulus for a sufficient number of trials. In particular, since blocks are very short and subjects only generate saccades of a particular amplitude a few times per block, repetition ensured that subjects experienced several prediction errors for each implicit-target amplitude. Furthermore, repeating blocks promoted the transfer of learning from the rhythmic predictive saccades to the discrete reactive saccades that were used to assess learning before and after the adaptation task.Ikegami et al. (2010) have shown that adaptation during rhythmic movements transfers poorly to discrete movements, but such transfer might be improved with repeated exposure to the rhythmic adapting stimulus.
The gains of saccades made in response to 10° target steps were assessed throughout the paradigm using three blocks of open-loop trials (Ethier et al. 2008; Wong and Shelhamer 2011c): prior to adaptation (Pre), immediately following the ten adapting blocks (Post), and at the end of the paradigm following the washout block (post-washout, PWO; see Figure 1). Open-loop trials assess primary-saccade gain while reducing the washout of adaptation by removing the visual target during the saccade, such that no feedback is available to evaluate errors and drive further learning. At the end of each trial, there was a delay of 600 ms before the target reappeared at the current eye position to begin the next trial. This delay is sufficient to minimize undesired adaptation in response to stimulus feedback when the target reappears (Fujita et al. 2002).
Data Analysis
Eye-tracking data were analyzed offline using an interactive MATLAB computer program (The MathWorks, Natick MA). Saccades were detected using a velocity threshold (>15°/s). Primary-saccade amplitudes and latencies were computed for further analysis. When subjects blinked during a saccade, that saccade was discarded. Data were collapsed across saccade direction for analysis.
Gain for each trial was computed as the size of the primary saccade divided by the size of the initial target step, except during the implicit double-step task when all trials were normalized to the initial 10° target step. Changes in saccade gain were assessed using a two-way ANOVA with factors of Subject and Block (Pre, Post, and PWO). Post-hoc pairwise multiple-comparisons between blocks were performed using the Holm-Sidak test.
Kinematics (amplitude, peak velocity, peak acceleration, absolute value of peak deceleration) and timing parameters (latency, duration, time of peak velocity, time of peak acceleration, time of peak deceleration) were measured during the long Pred block for both controls and SCA6 patients. For this analysis, the first 30 trials of the Pred block after predictive behavior was established (about 10% of the way into the block, when saccade latencies and amplitudes became reasonably consistent across trials) comprised the “early” data set for each subject, while the last 30 trials of the Pred block comprised the “late” data set. Statistical comparisons of kinematics and timing parameters were assessed using paired t-tests.
RESULTS
All subjects completed the predictive-saccade tasks. One subject produced anticipatory saccades (latencies < −200 ms) during all predictive portions of the experiment. Saccades with latencies in this range are thought to reflect reliance on a cognitive “guessing” strategy. This comes from the observation that when the stimulus pacing rate becomes quite slow, the percentage of movements with predictive latencies falls but the percentage of movements with reactive and anticipatory latencies both rise (Isotalo et al. 2005; Shelhamer 2005). Due to this classification of movement latencies, the subject was excluded from further analysis. The remaining subjects, during both the long prediction block (Pred) and the ten implicit double-step adaptation blocks (A1–A10), produced saccades with latencies in the appropriate range (controls: −7.6 ± 75.2 ms; SCA6: 53.9 ± 76.4 ms), and latencies during the adaptation blocks were not significantly different from those during the long predictive block (t-test, controls: p = 0.30; SCA6: p = 0.24). Note that although SCA6 patients on average produced predictive saccades, individually these patients occasionally alternated between predictive and non-predictive saccades. Only one patient was able to consistently produce predictive saccades throughout all blocks of the recording session. Nevertheless, all subjects generated a majority of predictive saccades throughout the experiment. Subjects generally completed their saccades after the target appeared (time on target; controls: 51.8 ± 74.4 ms; SCA6: 119.1 ± 67.3 ms). Thus, the movement of the target effectively occurred while the eyes were mid-flight as in conventional adaptation paradigms, such that there was no delay between the conclusion of the saccade and the onset of the current target.
Learning in response to a prediction-based adaptation stimulus
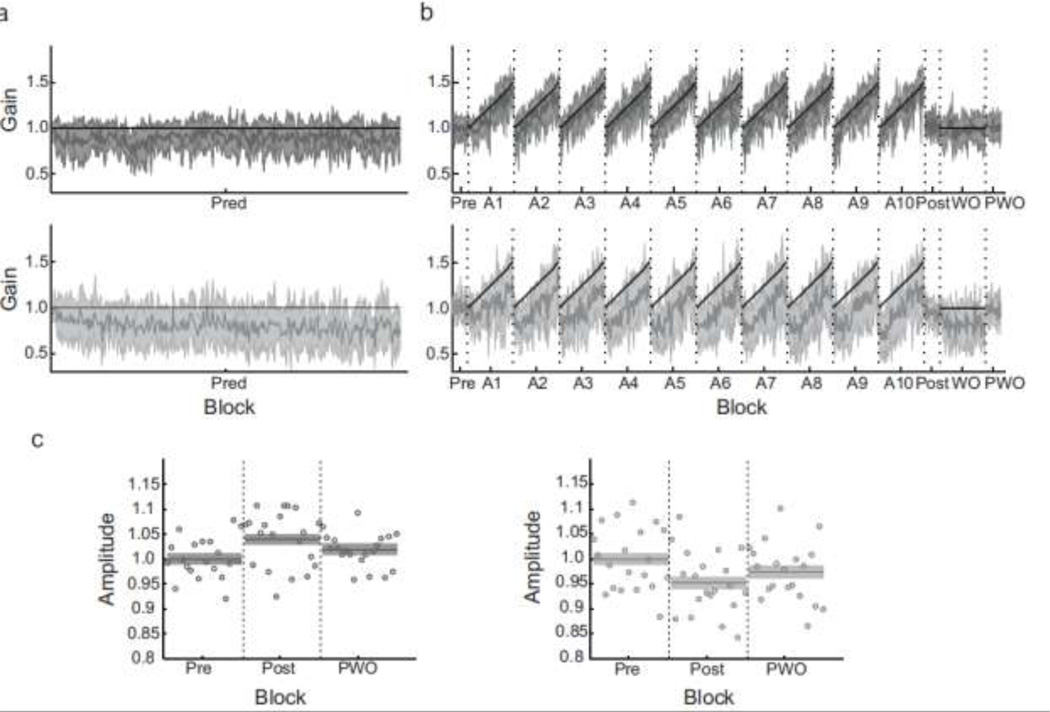
Control subjects successfully completed the implicit double-step adaptation task. They responded to the changing target stimulus by increasing the gain of their saccades as expected (Figure 2B). This task produced an average gain increase of 3.93% (Post compared to Pre; range, 1.0% to 8.4%). Using a two-way ANOVA, there was a significant main effect of block (p = 0.02), but no main effect of subject (p = 0.96) and a non-significant interaction. Pairwise testing between blocks revealed that this small gain change from Pre to Post is significant (p < 0.01), although it was mostly reversed after a short washout block (gain change between Pre and PWO, 1.95%, p = 0.20). These data indicate that the implicit double-step task can drive adaptation in control subjects, despite lacking an explicit visual target to initiate the saccade response. Although no clear evidence of learning (gain increase) throughout the session is evident when comparing saccade gains at the start of each adaptation block, this may be due to the mix of predictive and reactive saccades that occur within the first several trials of each block. Since predictive saccades are generally more hypometric and variable than reactive saccades (Bronstein and Kennard 1987), their presence may mask any ongoing learning across blocks. Nevertheless, error feedback about the accuracy of a predictive response (the expected movement goal) is sufficient to drive adaptation of movement gain.
Figure 2.
Time courses of saccade gain throughout the two experimental phases. a: Saccade gain for control subjects (dark gray, top) and SCA6 patients (light gray, bottom) in the Pred block. Whereas control subjects maintain a stable saccade gain, patients exhibit a gradual gain decrease across the block. b: Saccade gain during the implicit double-step adaptation. Control subjects exhibited a small gain increase, although saccade gain returned to baseline after a washout block. In contrast, SCA6 patients exhibited a gain decrease following adaptation; these changes may be in part related to a lack of adaptation and the presence of uncompensated fatigue. In the two time course plots, gray lines are means and shaded regions are SD; vertical dashed lines denote breaks between blocks. c. Direct comparison of average saccade gain across the three blocks of open-loop trials, for controls and SCA6 patients. The mean and SE (shaded region) is plotted, along with the average saccade gain for each trial. In all plots, saccades were normalized to subjects’ corresponding Pre-adaptation saccade gain (gain of Pre block was set to 1 and all other blocks were scaled accordingly), then averaged across subjects.
Cerebellar contributions to prediction-based learning
In response to the implicit double-step adaptation task, SCA6 patients as a group did not exhibit a gain increase. In fact, saccade gain decreased slightly, by 5.03% (Figure 2B). A two-way ANOVA revealed a significant main effect of both Subject (p = 0.04) and Block (p = 0.02), but no significant interaction. Pairwise testing revealed that this gain decrease is significant (p < 0.01), although there was no significant difference in saccade gain between the Post and PWO blocks (gain change, 2.46%, p = 0.14). There was also no significant difference between the Pre and PWO blocks (gain change, -2.58%, p = 0.11). Thus, the observed gain decrease may be related to the inability to compensate online for fatigue, as examined below, or to large intra-subject variability. Nevertheless, these data confirm that the cerebellum is important for processing a prediction-based adaptation error signal that regulates trial-to-trial learning, since SCA6 patients show no gain increases following the implicit double-step adaptation paradigm.
The cerebellum is also thought to generate online movement adjustments that “steer” movements to their goals. These corrections are produced if the expected movement outcome (predicted online using a copy of the motor command) is not aligned with the intended movement goal. This becomes particularly important when making large numbers of movements, as fatigue reduces movement velocity and – if uncompensated – results in increasingly inaccurate saccades (Golla et al. 2008; Xu-Wilson et al. 2009). If there is a difference in fatigue compensation between normal subjects and SCA6 patients in response to a standard predictive-saccade task (in which the targets remain fixed in space across the block), this confirms that the cerebellum drives corrections to an implicit target. That is, this compensatory effort may minimize anticipated errors to improve the accuracy of the predictive movement. Fatigue during the Pred block can be observed in the time course of saccade amplitudes presented in Figure 2A, and by comparing saccades made early and late during that block (group data are presented in Table 1). Representative kinematic data from one control subject and one SCA6 patient are presented in Figure 3.
Table 1.
Comparison of kinematic and timing parameters, Pred block
| Parameter | Early | Late | p | Parameter | Early | Late | p |
|---|---|---|---|---|---|---|---|
| Kinematic parameters | Timing parameters | ||||||
| Amp (°) | Latency (ms) | ||||||
| Control | 7.93 ± 1.55 | 7.85 ± 1.65 | 0.57 | Control | 23.43 ± 156.34 | −14.09 ± 164.91 | 0.01 |
| SCA6 | 7.97 ± 2.79 | 7.27 ± 2.71 | <0.01 | SCA6 | 110.23 ± 132.12 | 13.33 ± 196.40 | <0.01 |
| Peak velocity (°/s) | Time of peak velocity (ms) | ||||||
| Control | 261.39 ± 53.41 | 231.04 ± 55.77 | <0.01 | Control | 27.63 ± 6.79 | 31.19 ± 17.35 | 0.01 |
| SCA6 | 265.49 ± 99.00 | 228.16 ± 97.78 | <0.01 | SCA6 | 29.26 ± 15.94 | 33.58 ± 25.58 | 0.01 |
| Peak acceleration (×104 °/s2) | Time of peak acceleration (ms) | ||||||
| Control | 2.36 ± 0.57 | 2.12 ± 0.58 | <0.01 | Control | 12.10 ± 4.52 | 13.35 ± 13.17 | 0.12 |
| SCA6 | 2.41 ± 0.80 | 2.13 ± 0.78 | <0.01 | SCA6 | 15.40 ± 16.25 | 18.64 ± 27.88 | 0.08 |
| Peak deceleration (×104 °/s2) | Time of peak deceleration (ms) | ||||||
| Control | −1.76 ± 0.54 | −1.59 ± 0.45 | <0.01 | Control | 41.61 ± 8.35 | 45.43 ± 17.35 | <0.01 |
| SCA6 | −1.83 ± 0.63 | −1.62 ± 0.59 | <0.01 | SCA6 | 43.96 ± 20.87 | 45.67 ± 27.44 | 0.34 |
| Duration (ms) | |||||||
| Control | 45.22 ± 9.22 | 54.10 ± 28.31 | <0.01 | ||||
| SCA6 | 55.11 ± 26.60 | 61.88 ± 42.04 | 0.02 | ||||
Values are means ± SD. Velocity, acceleration, and deceleration are measured at their peak (maximum) values (first column), and are reported along with their corresponding time of occurrence (second column).
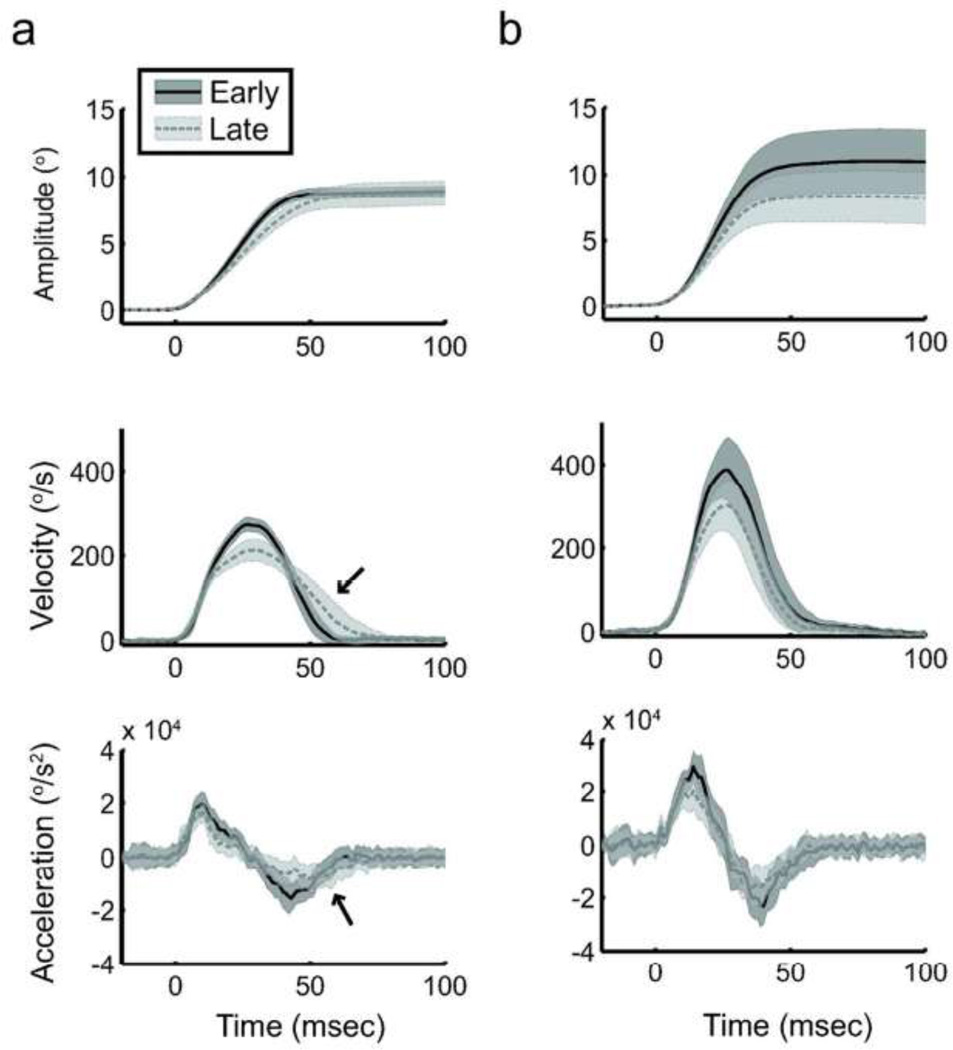
Figure 3.
Saccade kinematics during the long prediction block. Amplitude, velocity and acceleration for 30 consecutive saccades early (solid dark gray) or late (dashed light gray) in the prediction bock. Data are from a representative control subject (a) and SCA6 patient (b) respectively; plotted are means ± SD. The control subject exhibits fatigue in the form of decreased peak velocity, acceleration, and deceleration, but compensates by stretching the deceleration phase (black arrows) such that the saccade remains accurate. In contrast, the SCA6 patient exhibits similar signs of fatigue but cannot compensate for this, and resulting saccades are severely hypometric.
Fatigue is noted by examining the changes in both the kinematics and the timing of major kinematic events (e.g., time to peak velocity) during movements made across a large number of trials, and is primarily characterized as a decrease in saccadic peak velocity with trial repetition. Control subjects exhibited fatigue during the long Pred block: decreased peak velocity by 30.4°/s (p = 0.03), along with decreased peak acceleration by 2400°/s2 (p = 0.02) and absolute peak deceleration by 1700°/s2 (p = 0.05). Control subjects compensated for this fatigue by “stretching” the saccade. That is, they primarily increased the duration of the second half of the movement (Figure 3A, note arrows), as there was no change in the time of occurrence of peak acceleration (p = 0.56), but the time of peak deceleration was delayed by about 3.8 ms (p = 0.03). Thus, control subjects maintained consistent saccade amplitudes across a long continuous block of predictive saccades (decrease by 0.08°, p = 0.91). They accomplish this by invoking the same compensatory mechanisms that are active during long sequences of reactive saccades, even though predictive saccades are produced in expectation of the next requested movement and are not explicitly guided by visual stimulus information. This implies that online corrections during saccadic movements are produced to bring the eyes to an intended location, in the sense that a forward model estimates movement outcome and any predicted errors in that estimate drive performance corrections.
In contrast, across the Pred block SCA6 patients exhibit decreased saccade amplitudes (reduction of 0.7°, p < 0.03) and peak velocities (reduction by 37.3°/s, p = 0.01), coupled with decreases in peak acceleration (by 2800°/s2, p < 0.01) and absolute peak deceleration (by 2000°/s2, p < 0.01). However, saccades exhibited no compensatory temporal changes, as the timing of the peak acceleration and peak deceleration did not differ significantly (p > 0.48; see Figure 3B). If these changes are fatigue-related, this implies that unlike control subjects, SCA6 patients are unable to successfully compensate for top-down fatigue.
On the other hand, these kinematic changes might simply be attributed to the production of more predictive saccades later in the Pred block, as noted by the decrease in saccade latency across this block (see Table 1). Predictive saccades have lower peak velocities compared to reactive saccades of the same amplitude, although such differences are generally observed only for saccades larger than 15° (Bronstein and Kennard 1987). To explore this alternative hypothesis, we classified saccades as anticipatory (latency < −200 ms), predictive (latency between −200 ms and 80 ms), or reactive (latency > 80 ms) and examined kinematic changes within each group. For reactive saccades, there were significant decreases in amplitude (decrease of 0.64°; p = 0.04) and peak velocity (decrease of 27.71°/s; p = 0.02) with no compensatory timing-parameter changes (in particular, time of peak acceleration and time of peak deceleration did not change; p > 0.2). Predictive saccades exhibited a similar trend, although the changes in amplitude (decrease of 0.25°; p = 0.25) and peak velocity (decrease of 11.53°/s; p = 0.3) did not reach significance. This might be due to the increased trial-to-trial variability observed for predictive saccades (see also Bronstein and Kennard 1987). There were not enough anticipatory saccades to perform reliable statistical analyses. These data suggest that the observed kinematics changes may be a combination of fatigue and spontaneous transitions from reactive to predictive-saccade tracking.
Predictive saccades, therefore, may exhibit top-down fatigue effects that are similar to those of reactive saccades (Golla et al. 2008; Xu-Wilson et al. 2009). The cerebellum seems important for fatigue compensation, implying that it contributes to online corrections that guide saccades toward the intended movement goal. This improves single-trial accuracy. Together with the results from the implicit double-step adaptation task, which relate to trial-to-trial adaptation, such findings suggest that the cerebellum facilitates motor learning according to a prediction-based movement error signal. This error signal can be used online to generate realtime motor corrections, as well as after the movement concludes to support trial-to-trial adaptation.
DISCUSSION
We present a novel method for adapting movement gain using a predictive-saccade paradigm: the implicit double-step adaptation task. In this task, normal subjects significantly adapt their saccades in the requested gain-increase direction. Since this adaptation is driven by predictive movements, the result establishes a connection between prediction and adaptation and lends support to the notion that movements are adaptively modified according to a comparison of actual and predicted motor outcomes. This task is advantageous because it does not depend on triggering target steps using eye movements, so it constitutes an adaptation paradigm that is robust to eye-movement artifacts (e.g., blinks, drift, nystagmus). This makes it a useful tool for studying the adaptive behavior of patients with movement disorders – particularly those with cerebellar impairments, who may make multiple saccades to reach a target.
To compare the adaptation in our paradigm with that of conventional double-step tasks, we must compute the size of the implicit double-step. During the task, control subjects generate post-saccadic errors of 1–2° with respect to the target. Let us assume this saccade endpoint is identical to the predicted target location. Since the cerebellum is intact for control subjects, it should steer the saccade to reach the intended movement endpoint, which corresponds roughly to the expected target location (i.e., the anticipated requested saccade size). In fact, we know this is not true; our previous work suggests that predictions of saccade outcome include an estimate of hypometria, so subjects anticipate that the target will appear approximately 10% beyond the saccade endpoint (Wong and Shelhamer 2011c). Including this predicted hypometria, however, simply reduces the estimate of unexpected error (i.e., the implicit double-step size) computed in this approximation. Therefore, this approximation will, at worst, underestimate the effectiveness of our paradigm.
Using this assumption, the post-saccadic error is equivalent to stepping the target by that same magnitude during the saccade. In other words, the conventional double-step task encourages adaptation by presenting to the motor system an unexpected error equal to the size of the unpredicted double-step; similarly, during the implicit double-step task, the unexpected error that drives adaptation is approximately the observed post-saccadic (prediction) error. Thus, relative to the corresponding predictive-saccade amplitudes, the “requested” gain change due to the implicit double step is a 15% gain increase on average. Control subjects responded to this implicit target step by adaptively increasing their saccade amplitudes by ~5%, comparable to the proportion of the requested gain change that was acquired in a standard reactive-saccade adaptation paradigm (for example, see Miller et al. 1981).
In this study, we assume that the adaptation error signal is neurally computed as the difference between the implicit target that initiates the predictive saccade and the observed error at the end of the saccade (that is, the prediction error). However, adaptation might instead be driven by only the observed post-saccadic visual error: the distance between the target and the fovea as measured on the retina. Although lacking explicit data to distinguish between these hypotheses, we note that adaptation only occurs during the implicit double-step portion of the paradigm when target stimuli are displaced. During the Pred block when control subjects make predictive-saccades to fixed targets, they can observe large retinal errors but do not exhibit changes in saccade gain to improve their accuracy (recall Figure 2B). Thus, retinal error alone seems insufficient to induce adaptive changes. Instead, it requires the unpredicted retinal error resulting from the target shift during the implicit double-step paradigm. In order words, adaptation may indeed be driven by a prediction error, not a visual error. This agrees with previous research suggesting that subjects adapt to an error signal that is informed by their expectations: a prediction error (Henson 1978); Bahcall and Kowler 2000; Wong and Shelhamer 2011c; Collins and Wallman 2012).
This task also enabled us to demonstrate that while SCA6 patients can generate predictive saccades, they cannot adapt or maintain consistent long-term movement accuracy in the same manner as do control subjects. Both the production of online corrections and trial-to-trial adaptation are thought to depend upon the generation of accurate movement predictions, and were examined in this study. First, SCA6 patients do not exhibit gain increases in response to the implicit double-step task. The predictive saccades they generate exhibit highly variable movement amplitudes, suggesting that observations of saccadic errors alone cannot be used to maintain consistent and accurate performance. This adaptation deficit further implies that the cerebellum handles prediction-based error signals. Therefore, the implicit double-step adaptation paradigm helps to identify the prediction error signal as the cause of this learning deficit, since this task explicitly relies upon the observation of prediction accuracy (or, the difference between predicted and observed movement outcomes) to drive trial-to-trial adaptation.
Furthermore, the cerebellum is critical for modifying saccade gain online to drive movements toward an intended movement goal. During the long block of predictive saccades with fixed target amplitudes (Pred block), saccade amplitudes decrease in part because SCA6 patients become fatigued. Control subjects, in contrast, maintain predictive-saccade amplitude stability in compensation for the hallmark reduction in peak velocity that indicates fatigue by “stretching” the latter half of saccades to delay the deceleration phase. Such corrections seem to rely on the presence of an intact cerebellum; cerebellum-dependent compensation also occurs during typical reactive saccades (Golla et al. 2008; Xu-Wilson et al. 2009). Taken together, the data from SCA6 patients in both the implicit double-step adaptation task as well as the standard predictive-saccade task – when compared to controls – support the hypothesis that the cerebellum facilitates motor learning in response to movement predictions.
ACKNOWLEDGEMENTS
We gratefully acknowledge DC Roberts for technical assistance. We also thank SH Ying, AX Du, BC Jung, E Bryant-Cavazos, and E Murray for their assistance in recruiting SCA6 patients and conducting ICARS tests. This work was supported by NSF grant BCS-1126957, NIH grant R21-EY019713, and NIH grant T32 DC000023.
References
- Alahyane N, Pelisson D. Long-lasting modifications of saccadic eye movements following adaptation induced in the double-step target paradigm. Learn Mem. 2005;12:433–443. doi: 10.1101/lm.96405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahcall DO, Kowler E. The control of saccadic adaptation: implications for the scanning of natural visual scenes. Vision Research. 2000;40:2779–2796. doi: 10.1016/s0042-6989(00)00117-6. [DOI] [PubMed] [Google Scholar]
- Bastian AJ. Learning to predict the future: the cerebellum adapts feedforward movement control. Curr Opin Neurobiol. 2006;16:645–649. doi: 10.1016/j.conb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Bronstein AM, Kennard C. Predictive eye saccades are different from visually triggered saccades. Vision Research. 1987;27:517–520. doi: 10.1016/0042-6989(87)90037-x. [DOI] [PubMed] [Google Scholar]
- Collins T, Wallman J. The relative importance of retinal error and prediction in saccadic adaptation. J Neurophysiol. 2012 doi: 10.1152/jn.00746.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Bastian AJ, Shadmehr R. Size of error affects cerebellar contributions to motor learning. J Neurophysiol. 2010 doi: 10.1152/jn.00822.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier V, Zee DS, Shadmehr R. Spontaneous recovery of motor memory during saccade adaptation. J Neurophysiol. 2008;99:2577–2583. doi: 10.1152/jn.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Amagai A, Minakawa F, Aoki M. Selective and delay adaptation of human saccades. Cogn Brain Res. 2002;13:41–52. doi: 10.1016/s0926-6410(01)00088-x. [DOI] [PubMed] [Google Scholar]
- Golla H, Tziridis K, Haarmeier T, Catz N, Barash S, Thier P. Reduced saccadic resilience and impaired saccadic adaptation due to cerebellar disease. Eur J Neurosci. 2008;27:132–144. doi: 10.1111/j.1460-9568.2007.05996.x. [DOI] [PubMed] [Google Scholar]
- Henson DB. Corrective saccades: effects of altering visual feedback. Vision Research. 1978;18:63–67. doi: 10.1016/0042-6989(78)90078-0. [DOI] [PubMed] [Google Scholar]
- Honjo K, Ohshita T, Kawakami H, et al. Quantitative assessment of cerebral blood flow in genetically confirmed spinocerebellar ataxia type 6. Arch Neurol. 2004;61:933–937. doi: 10.1001/archneur.61.6.933. [DOI] [PubMed] [Google Scholar]
- Ikegami T, Hirashima M, Taga G, Nozaki D. Asymmetric transfer of visuomotor learning between discrete and rhythmic movements. J Neurosci. 2010;30:4515–4521. doi: 10.1523/JNEUROSCI.3066-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Watanabe M, Yoshizawa K, et al. Clinical, neuropathological, and molecular study in two families with spinocerebellar ataxia type 6 (SCA6) J Neurol Neurosurg Psychiatry. 1999;67:86–89. doi: 10.1136/jnnp.67.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isotalo E, Lasker AG, Zee DS. Cognitive influences on predictive saccadic tracking. Exp Brain Res. 2005;165:461–469. doi: 10.1007/s00221-005-2317-7. [DOI] [PubMed] [Google Scholar]
- Izawa J, Criscimagna-Hemminger SE, Shadmehr R. Cerebellar contributions to reach adaptation and learning sensory consequences of action. J Neurosci. 2012;32:4230–4239. doi: 10.1523/JNEUROSCI.6353-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WM, Shelhamer M, Ying SH. Cerebellar influence in oculomotor phase-transition behavior. Ann N Y Acad Sci. 2005;1039:536–539. doi: 10.1196/annals.1325.062. [DOI] [PubMed] [Google Scholar]
- Lasker AG, Isotalo EH, Zee DS. Predictive saccades to a regularly alternating target in cerebellar patients. Ann N Y Acad Sci. 2005;1039:544–547. doi: 10.1196/annals.1325.064. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. The neurology of eye movements. New York: Oxford University Press; 2006. [Google Scholar]
- Mclaughlin SC. Parametric Adjustment in Saccadic Eye Movements. Perception & Psychophysics. 1967;2:359–362. [Google Scholar]
- Mehta B, Schaal S. Forward models in visuomotor control. J Neurophysiol. 2002;88:942–953. doi: 10.1152/jn.2002.88.2.942. [DOI] [PubMed] [Google Scholar]
- Melis BJ, van Gisbergen JA. Short-term adaptation of electrically induced saccades in monkey superior colliculus. J Neurophysiol. 1996;76:1744–1758. doi: 10.1152/jn.1996.76.3.1744. [DOI] [PubMed] [Google Scholar]
- Miall RC. The cerebellum, predictive control and motor coordination. Novartis Found Symp. 1998;218:272–284. doi: 10.1002/9780470515563.ch15. discussion 284-290. [DOI] [PubMed] [Google Scholar]
- Miall RC, Wolpert DM. Forward models for physiological motor control. Neural Netw. 1996;9:1265–1279. doi: 10.1016/s0893-6080(96)00035-4. [DOI] [PubMed] [Google Scholar]
- Miller JM, Anstis T, Templeton WB. Saccadic plasticity: parametric adaptive control by retinal feedback. J Exp Psychol Hum Percept Perform. 1981;7:356–366. doi: 10.1037//0096-1523.7.2.356. [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci. 2006;26:9107–9116. doi: 10.1523/JNEUROSCI.2622-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel M, Behrmann H, Zangemeister WH. Disturbance of predictive response initiation of eye and head movements in cerebellar patients. Eur Neurol. 2008;60:179–185. doi: 10.1159/000148245. [DOI] [PubMed] [Google Scholar]
- Robinson DA. A Method of Measuring Eye Movement Using a Scleral Search Coil in a Magnetic Field. IEEE Trans Biomed Eng. 1963;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Kojima H, Yabe I, et al. Neuropathological and molecular studies of spinocerebellar ataxia type 6 (SCA6) Acta Neuropathol. 1998;95:199–204. doi: 10.1007/s004010050787. [DOI] [PubMed] [Google Scholar]
- Shelhamer M. Sequences of predictive saccades are correlated over a span of approximately 2 s and produce a fractal time series. J Neurophysiol. 2005;93:2002–2011. doi: 10.1152/jn.00800.2004. [DOI] [PubMed] [Google Scholar]
- Shelhamer M, Joiner WM. Saccades exhibit abrupt transition between reactive and predictive; predictive saccade sequences have long-term correlations. J Neurophysiol. 2003;90:2763–2769. doi: 10.1152/jn.00478.2003. [DOI] [PubMed] [Google Scholar]
- Stark L, Young LR, Vossius G. Predictive control of eye tracking movements. Ire Trans Hum Factors Electron Hfe3. 1962:52–57. [Google Scholar]
- Straube A, Deubel H, Ditterich J, Eggert T. Cerebellar lesions impair rapid saccade amplitude adaptation. Neurology. 2001;57:2105–2108. doi: 10.1212/wnl.57.11.2105. [DOI] [PubMed] [Google Scholar]
- Trouillas P, Takayanagi T, Hallett M, et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci. 1997;145:205–211. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol. 2007;98:54–62. doi: 10.1152/jn.00266.2007. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998;2:338–347. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- Wong AL, Shelhamer M. Exploring the fundamental dynamics of error-based motor learning using a stationary predictive-saccade task. PLoS ONE. 2011a;6:e25225. doi: 10.1371/journal.pone.0025225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AL, Shelhamer M. Saccade adaptation improves in response to a gradually introduced stimulus perturbation. Neurosci Lett. 2011b;500:207–211. doi: 10.1016/j.neulet.2011.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AL, Shelhamer M. Sensorimotor adaptation error signals are derived from realistic predictions of movement outcomes. J Neurophysiol. 2011c;105:1130–1140. doi: 10.1152/jn.00394.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Wilson M, Chen-Harris H, Zee DS, Shadmehr R. Cerebellar contributions to adaptive control of saccades in humans. J Neurosci. 2009;29:12930–12939. doi: 10.1523/JNEUROSCI.3115-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]