Abstract
Globoid Cell Leukodystrohpy (GLD, Krabbe Disease) is a lysosomal storage disease, resulting from the genetic deficiency of Galactosylceramidase (GALC). This disease is marked by accumulation of the cytotoxic lipid psychosine (Psy). Psychosine is known to induce oxidative stress in cultured cells, and this stress can be ameliorated through co-treatment with the antioxidant N-acetyl cysteine (NAC). Oxidative stress has also been observed in vivo in the mouse model of GLD, the Twitcher mouse (Twi). We hypothesized that treating oxidative stress with NAC; either alone or in combination with bone marrow transplant (BMT) would improve the course of disease. All breeding cages were maintained on water containing NAC. Once born, the pups received IP boluses of NAC three times per week, and were maintained on NAC-containing water. A separate cohort of animals received the same regimen of NAC in addition to a BMT on post-natal day 2–3. Although NAC lowers the level of oxidized proteins in the brains of Twi mice, and dramatically improves immunohistochemical markers of disease, neither treatment results in any clinical improvements in the Twi mouse. Our data suggest that oxidative stress may be sufficiently down-stream in the pathogenic cascade initiated by Psy accumulation as to be difficult or impossible to treat with standard pharmacologic agents. It is possible that NAC may synergize with other therapies or combinations of therapies. A better understanding of the initiating effects of Psy toxicity and oxidative damage may uncover treatable therapeutic targets.
Keywords: Krabbe Disease, N-acetyl cysteine, bone marrow transplant, Twitcher, Lysosomal storage disease, oxidative stress
INTRODUCTION
Globoid Cell Leukodystrophy (GLD, Krabbe Disease) is a lysosomal storage disorder caused by the deficiency of the glactosylsphingolipid-degrading enzyme galactosylceramidase (GALC). In the most common infantile form of GLD, children appear normal at birth and present with neurological symptoms by six months of age (Wenger, et al., 2001). Myelin loss is notable in GLD, and clinical signs include irritability, neuropathy, sensory deficits and seizure (Wenger, et al., 2001). A biochemically authentic mouse model of GLD is available and is commonly referred to as the Twitcher (Twi) mouse (Kobayashi, et al., 1980).
In the absence of GALC, glactosylsphingosine (Psychosine or psy) accumulates to high levels in the central (CNS) and peripheral nervous systems (PNS) (Igisu and Suzuki, 1984). Psychosine is a highly cytotoxic lipid (Taketomi and Nishimura, 1964), and its accumulation is presumed to be the cause of oligodendrocyte apoptosis in GLD (Haq, et al., 2003, Suzuki, 1998, Zaka and Wenger, 2004). While the primary mechanism of psy toxicity is still largely unknown, many effects of the lipid have been documented. Notably, cultured cells treated with psy have elevated levels of oxidative stress, which may be decreased in vitro by co-treating with a strong anti-oxidant, N-acetyl cysteine (NAC) (Giri, et al., 2008, Haq, et al., 2003, Khan, et al., 2005).
N-acetyl cysteine is a potent anti-oxidant, and may function either directly as a reactive oxygen species (ROS) scavenger or indirectly as a cysteine source and glutathione precursor (Atkuri, et al., 2007). This molecule is readily available and is well tolerated in humans, including pediatric patients (Bebarta, et al., 2010). N-acetyl cysteine is active in the CNS, although it is unclear whether it crosses the blood-brain-barrier or functions more indirectly (McLellan, et al., 1995, Pocernich, et al., 2000). Moreover, NAC has shown promise in treating other neurologic diseases (Farr, et al., 2003, Viscomi, et al., 2010). While anti-oxidant treatment alone would not be expected to cure an inborn error of metabolism, numerous recent reports have demonstrated enhanced or synergistic efficacy when multiple therapies are combined (Hawkins-Salsbury, et al., 2011). Therefore, we hypothesized that NAC treatment in concert with bone marrow transplant (BMT), the only currently available therapy for GLD, could be a promising therapy.
In the current study, we utilized the Twitcher mouse to investigate whether NAC therapy might, either alone or in combination with BMT, be an efficacious therapy for GLD. Antioxidant was administered to mice simultaneously via multiple routes and at high doses. Treatment with NAC decreases oxidative stress in the brain of treated Twi mice and improves many cellular markers of disease. However, this reduction in oxidative stress is not associated with an improvement in any clinical features of GLD in the Twi mouse.
MATERIALS AND METHODS
Animals
Mice heterozygous for the GALC mutation (GALC+/−) on a C57Bl/6 background were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). All animals were housed at Washington University School of Medicine, under the direction of MSS. Animals were housed under standard conditions with unlimited access to food and water. Twitcher mice (GALC −/−) were obtained through double GALC+/− matings. The genotype of animals was determined before post-natal day (PND) 2 by PCR specific for the Twitcher mutation (Sakai, et al., 1996). Experimental animals in this study were restricted to those surviving past weaning (post-natal day 21). The Institutional Animal Care and Use Committee at Washington University School of Medicine approved all animal studies. All analysis was performed on terminal Twi animals (day 37) unless otherwise stated.
Therapeutic Protocol
NAC Treatment
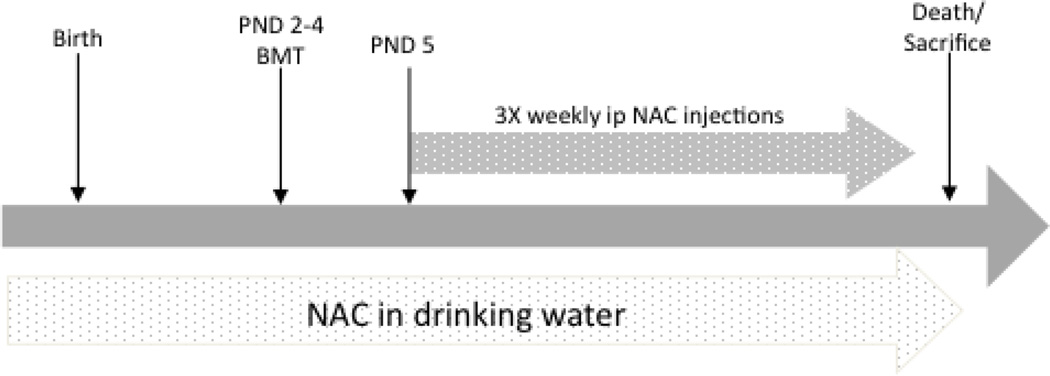
Prior to this study, a pilot investigation into mouse water consumption was performed. Bottles were filled with a standard volume of fresh water, then weighed and changed after 48hrs. We observed that the average mouse consumed approximately 7mLs of water per day. The NAC-dosing regimen is shown in Figure 3. All treated groups in this study were maintained on water containing NAC (Sigma-Aldrich, St. Louis, MO USA) at 5mg/mL (Cotter, et al., 2007). Therefore, each mouse received approximately 35mg NAC per day orally. This included breeding pairs before, during and after pregnancy, as well as through weaning. Once weaned, treated mice continued to receive NAC water. Water containing NAC was prepared fresh three times per week. As a result, NAC-treated animals received a continuous dose of NAC trans-placentally, in mother’s milk and finally in their drinking water when they began consuming it. The same cohort of NAC-treated animals also received an intraperitoneal (i.p.) bolus of NAC three times per week (150mg/kg), beginning on PND5 (Abdelsaid, et al., 2010) in addition to NAC in the drinking water. NAC for i.p. injections was prepared immediately prior to injection in sterile PBS, then filter sterilized. Untreated normal and Twi controls were maintained on water without NAC.
Figure 3.
Therapeutic regimen. Animals were treated with NAC or NAC+BMT as described in materials and methods. NAC was provided to experimental animals in the drinking water throughout life. This included the breeding pairs pre- and post-natally and through weaning. In addition to NAC drinking water, all experimental animals received bolus ip injections of NAC starting on PND 5 and continuing three times per week through the life of the animal. BMTs were performed on PND 2–4.
Bone Marrow Transplant
Animals receiving BMT were exposed to 400 rads of total body irradiation from a 137Cs source on PND2-4. Immediately following radiation, mice were injected intravenously (Sands and Barker, 1999) with 106 nucleated bone marrow cells from a congenic, GALC+/+, sex-matched donor constitutively expressing GFP. Animals receiving BMT have 15–25% donor chimerism (data not shown), consistent with previous reports using this conditioning regimen (Lin, et al., 2007, Reddy, 2011).
Lifespan and Behavioral Testing
Lifespan was recorded as the age in days of the animal on the date of death or sacrifice. Animals were sacrificed when they became moribund, determined as losing greater than 25% of maximal body weight, hind limb paralysis, or unresponsive to tactile stimulus. Body weight was recorded every five days. Rotarod testing was preformed as previously reported (Lin, et al., 2007). Animals were tested on the rotarod every 5 days starting on PND 25. The rotarod was set at a constant speed of 3rpm. Animals were placed on the rod while it was stationary, then after the rod began rotating, latency for the animal to fall was recorded. The maximum latency was 60 seconds. Testing was discontinued when the animals could no longer stand on the stationary rod.
Measurement of Protein Carbonyl
All animals were perfused transcardially with phosphate buffered saline (PBS) prior to tissue harvest. Protein carbonyls were measured using the Oxyblot kit (Chemicon International, Temecula, CA), as well as the quantitative Protein Carbonyl Assay Kit (Cayman Chemical, Ann Arbor, MI). For either assay, the sample to be assayed (brain tissue or cells) was homogenized in 50mM phosphate, 1mM EDTA, pH 6.70. Protein concentration was determined using a coomasie dye-binding assay (Bio-Rad, Hercules, CA).
The Oxyblot assay was performed as previously described (Reddy, 2011, Wagner, et al., 2005). Briefly, equal amounts of protein were reacted with dinitrophenyl hydrazine (DNPH), to derivatize the carbonyl residues. The derivatized sample was loaded onto a 12% SDS-PAGE gel, resolved, and then blotted onto a PVDF (Amersham, Buckinghamshire) membrane. The membrane was then probed with an anti-DNP primary antibody and corresponding secondary antibody. The signal was visualized using an ECL kit (Peirce, Rockford, IL). Equal protein loading was confirmed using Ponseau S (Sigma, St. Louis, MO) staining. Unless otherwise noted, all investigations of protein carbonyl content were preformed on whole brain homogenates.
The colorimetric Protein Carbonyl assay was performed according to manufacturer’s instructions (Cayman Chemical). Briefly, equal amounts of protein were reacted with DNPH to derivatize carbonyl residues. The derivatized protein was precipitated with trichloroacetic acid (TCA) then washed three times with 1:1 ethanol: ethyl acetate to remove unbound DNPH. The washed sample was resuspended in guanidine hydrochloride and absorbance at 370nm measured using a plate reader. The protein carbonyl content was normalized to protein concentration for each sample.
Measurement of Glutathione Concentration
Brain tissue was harvested and processed as described above for protein carbonyl measurements. Glutathione was quantified using the Oxis Glutathione kit (OxisResearch Portland, OR) according to manufacturer’s directions (Tietze, 1969). This kit relies on the generation of a colored product through the reaction of glutathione with 5,5'-dithiobis-2-nitrobenzoic acid (DNTB). The rate at which the colored product is generated is proportional to the concentration of glutathione in the reaction. Briefly, 5% meta-phosphoric acid was used to precipitate proteins and the sample centrifuged. The supernatant was diluted 1:10, then mixed with enzyme and DNTB. Following the addition of NADPH the absorbance at 412nm was recorded over 3min. The slope of this curve was used to calculate glutathione concentration from a standard curve generated during the same test.
Cell Culture
Cells from the human Glial (oligodendrocytic) cell line MO3.13 (Cedarlane Labratories, Burlington NC) were maintained in Dubelcco’s Modified Eagle Medium (DMEM) with 10% fetal bovine serum. Two hours before Psy treatment, media was removed and replaced with either fresh media or fresh media containing 10mM NAC. Psychosine (Matreya, Pleasant Gap, PA) was added to the cell medium (10uM). Cells were incubated for 16hrs, then harvested with Trypsin and homogenized in 50mM phosphate, 1mM EDTA, pH 6.70. This procedure for psy exposure was empirically determined. It was utilized because it was gentle enough to allow cell survival, but produced marked changes in protein carbonyl content as well as cell morphology, an indicator of psy toxicity. Longer exposures or exposures at higher psy concentrations resulted in significant cell death, making further analysis impossible. Protein measurements and protein carbonyl content measurements were performed as described above.
Immunohistochemistry
Animals were deeply anesthetized, then perfused with PBS until the liver was clear. Brains were harvested immediately following perfusion and post-fixed in 4% paraformaldehyde for 24 hours. Tissues were then cryoprotected in 30% sucrose in Tris-buffered saline for 24–48 hours. Following cryoprotection, tissues were embedded in tissue-tek optimal cutting medium (OCT, Sakura Finetek, Torrance, CA), frozen and cryosectioned. Sections were immunostained with rabbit anti-iNOS, rabbit anti-GFAP or rat anti-CD68 antibody (abCAM, Cambridge, MA) according to standard protocols. Briefly, sections were incubated with 1% hydrogen peroxide to quench endogenous peroxidase activity. Sections were blocked in normal goat serum then incubated with the primary antibody, followed by a vectastain IgG secondary antibody conjugated to horseradish peroxidase (PK-6101, Vector laboratories, Burlingame, CA) and developed using Peroxidase kit (Vector laboratories, Burlingame, CA). Sections were mounted, dehydrated and cover slipped. Immunoreactivity was quantified with ImageJ software. All images were captured under constant variables with an Olympus BX41 microscope and Olympus DP20 camera. For iNOS immunostaining, four serial photos of the corpus callosum were collected. For CD68 and GFAP staining, images of the hippocampus (10X), deep cerebellar nucleus (20X) and brainstem (20X) were collected. Sections from three different animals (three sections per animal) per treatment group were analyzed. In the case of iNOS, the corpus callosum was manually traced and only the staining in that region was measured. For all staining, the percentage of the area above a constant threshold value was measured and called area fraction. The average area fraction for each experimental and control group was calculated. Statistical significance was calculated with a one-way ANOVA followed by a Bonferonni multiple comparisons test.
Histology
Brains were harvested and fixed as described above and embedded in paraffin. Ten micron-thick saggital sections were collected and stained with Luxol-Fast Blue and Periodic Acid Schiff according to standard procedures (Lin, et al., 2007). A pathologist blinded to genotype and treatment performed analysis of these sections and determined if any differences were apparent.
Statistical Analysis
All data are presented as the mean of 3 measurements unless otherwise noted. Graphpad Prism software (GraphPad Software, Inc, La Jolla, CA) was used to generate all graphs and perform all statistical analysis. Lifespan curves were generated through the Kaplan-Meier method and analyzed with the log-rank test. Comparisons between multiple groups were performed using ANOVA, followed by either Bonferroni’s or Tukey’s multiple comparisons procedures. Student’s T-tests were used to analyze protein carbonyl content measurements.
RESULTS
Oxidative Stress in the Twi Mouse
In Vitro
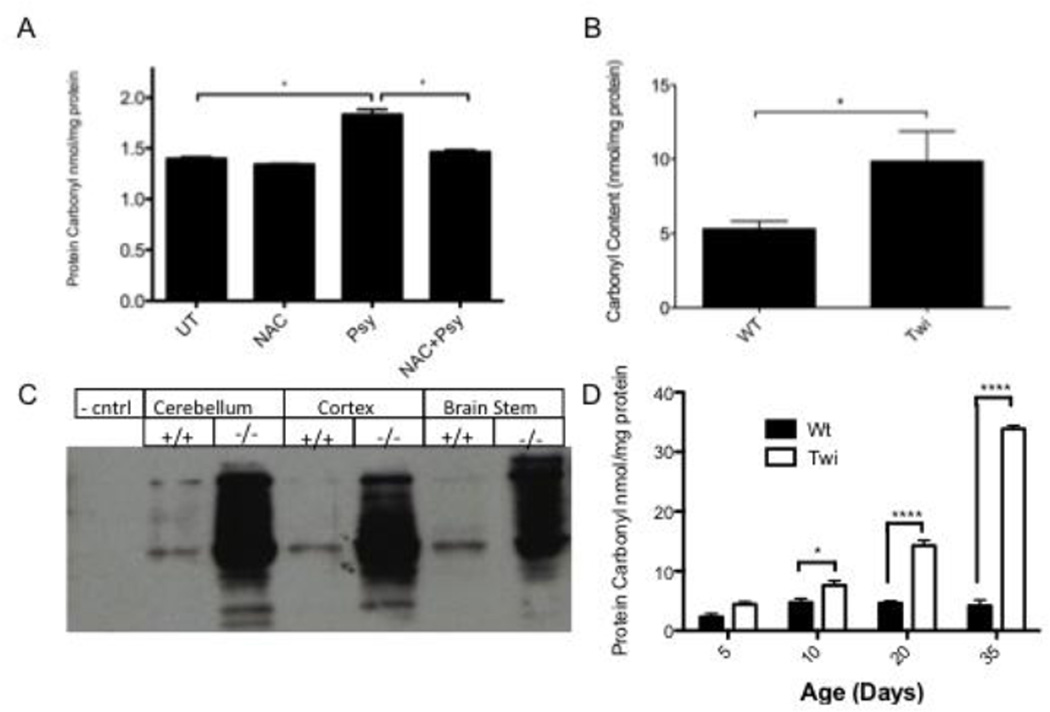
Elevated ROS following psy exposure has been documented in cellulo (Giri, et al., 2008, Haq, et al., 2003, Khan, et al., 2005). Oxidative stress can be identified in Psy-treated cells by measuring protein carbonyl content. Proteins may be carbonylated in oxidizing cellular conditions, and this marker is relatively stable. The oligodendrocytic cell line MO3.13 has been utilized in the vast majority of past psy-toxicity experiments and is used here for consistency. These cells showed increased protein carbonyls when treated with Psy. When Psy-treated cells were pre- and co-treated with 10mM NAC (Fig 1A), protein carbonyl content was indistinguishable from normal (Fig 1A). This concentration of NAC was selected to be consistent with previously published reports using this molecule to reduce oxidative stress in psy-treated cells.
Figure 1.
A) Protein carbonyl content in Psy- and NAC-treated MO3.13 cells. Psy treatment results in an elevation of protein carbonyls compared to either untreated or NAC-treated cells (p < .005). Cells treated with 10mM NAC along with Psy results in protein carbonyl levels that are indistinguishable from untreated cells (p > .09, n=4 per treatment group). B, C) Oxidative stress in the brain of Twi mice. Protein carbonyl content was measured quantitatively (B and D) through a colorimetric assay and qualitatively (C) through the OxyBlot kit, as described in Materials and Methods B) Protein carbonyl content is significantly elevated in whole brain homogenates from Twi mice (n=5) compared to wild-type controls (n=6). C) Elevated protein carbonyls are apparent in all regions of the Twi brain, including cerebellum, cortex and brain stem. D) Protein carbonyl levels increase as mice age and are significantly increased by PND10. * = p<0.05, **** = p<0.001.
In Vivo
A previous study showed reduced glutathione content in the Twi mouse, indicating in vivo oxidative stress (Khan, et al., 2005). We further investigated the accumulation of oxidative damage in vivo. Protein carbonyl levels were significantly elevated in terminal (36–40 day) Twi brains compared with age-matched controls (Fig 1B). The spatial distribution of protein carbonyl elevation was investigated in the cerebellum, cortex and brainstem. All brain regions investigated showed elevated protein carbonyl content in Twi mice (Fig 1C). There was no apparent difference in protein carbonylation between brain regions. In order to determine when oxidative stress begins in the Twi mouse, we measured protein carbonyl content at 5, 10, 20 and 35 days. Protein carbonyls were elevated as early as PND10, and increased as the animals aged (Fig 1D).
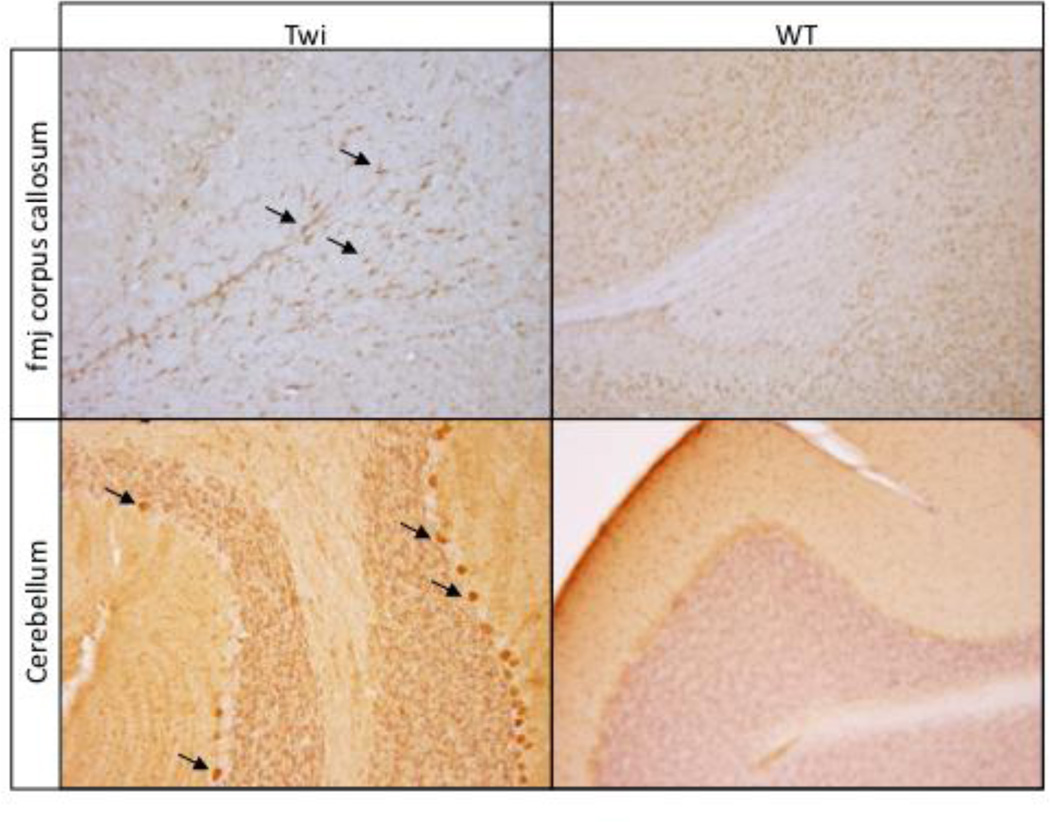
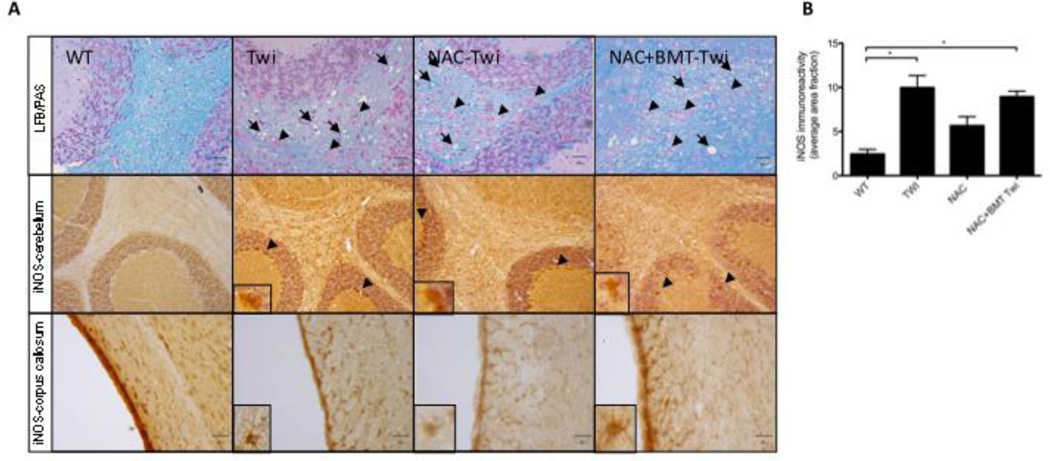
Increased expression of the oxidant-sensitive enzyme, inducible nitric oxide synthase (iNOS), has been reported in the Twi mouse as well as other animal models of GALC deficiency (Borda, et al., 2008, Ripoll, et al.). High levels of iNOS activity produces excess nitric oxide (NO) (Beckman and Koppenol, 1996) which in turn can lead to peroxynitrite generation, an unstable molecule and strong oxidant. We observed iNOS-positive cells throughout the corpus callosum, in the white matter tracts of the cerebellum, as well as Purkinjie cells in Twi mice (Fig 2). In contrast, iNOS-positive cells were rare in the brains of wild-type mice (Fig 2).
Figure 2.
iNOS positive cells are evident in the forcepts major corpus callosum (fmj) and cerebellum of Twi mice (arrows). Similar profiles are absent from WT mice.
Impact of NAC treatment on the Twi mouse
Reduction of Oxidative Stress in the Brain
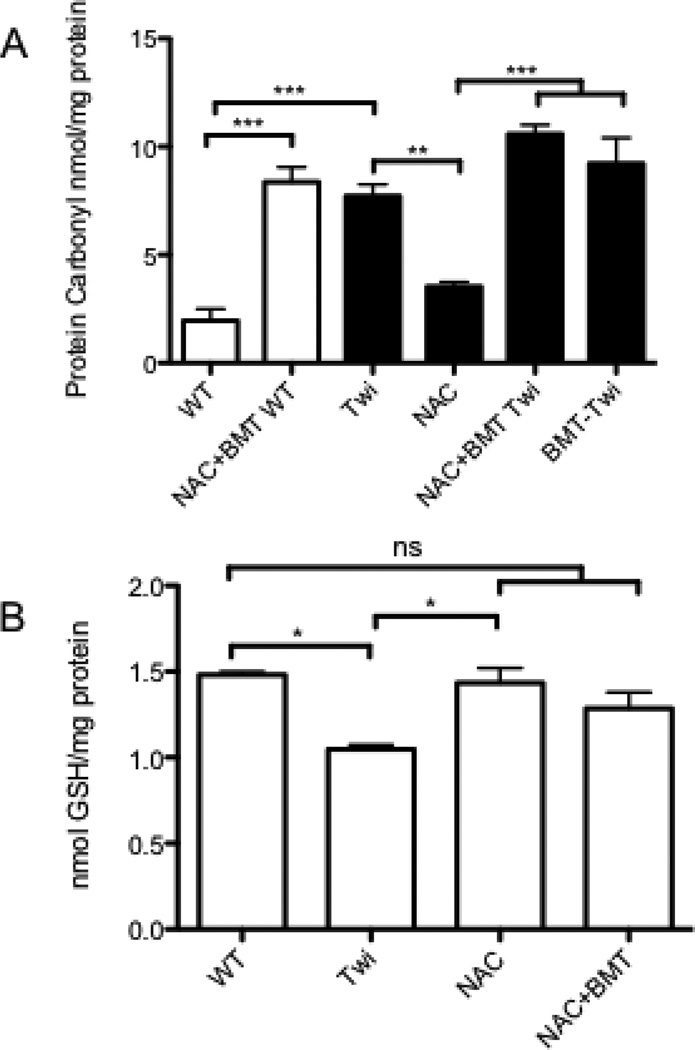
Animals were treated with NAC throughout life (Fig 3). To determine whether sufficient NAC reached the nervous system to decrease oxidative stress, the carbonyl content of treated brains was determined (Fig 4A). Treatment with NAC alone significantly reduced protein carbonyls in the brain of Twi mice compared with untreated Twi. Interestingly, the addition of BMT to the treatment regimen abolished this decrease. Increased protein carbonyls were also observed in the brains of BMT-treated WT and Twi mice compared with untreated controls. Glutathione levels are normalized in NAC treated mice, with BMT having no further effect (Fig 4B).
Figure 4.
Treatment with NAC decreases oxidative stress in the brains of day mice. A) NAC-treated Twi mice (NAC Twi) have significantly (p< .05) reduced brain carbonyl content compared to either untreated Twi (Twi) mice. This decreased is abolished when BMT is added to the treatment regimen (NAC+BMT-Twi) (black bars). Wildtype (NAC+BMT WT) animals that received BMT have elevated protein carbonyl content compared to untreated controls.(white bars) B) Twi mice have reduced glutathione (GSH) compared with normal controls. NAC treatment, either alone or in combination with BMT increases the GSH content of the brain to normal. * = p<0.05, ** = p<0.01, *** = p<0.005.
Histology
The hallmark of GLD, present in both Twi mice and human patients, is infiltration of multi-nucleated globoid cells in white matter, along with loss and disorganization of myelin. Brain sections from all treated and untreated groups were stained with Periodic Acid-Schiff (PAS) for globoid cells and Luxol Fast Blue (LFB) for myelin. No apparent improvement in myelin quantity or organization, or decrease in the number of infiltrating globoid cells was observed in any treated Twi group when compared to untreated Twi (Fig 5A).
Figure 5.
A) No apparent improvements were observed with respect to myelin organization (LFB/PAS arrows) or infiltrating PAS-positive globoid cells (LFB/PAS, arrowheads) in NAC (NAC Twi) or NAC+BMT treated (NAC+BMT Twi) mice. Similarly, no apparent reduction in iNOS immunoreactivity was seen in the cerebellum of treated animals. B) iNOS immunostaining was quantified in the corpus callosum. While iNOS-reactive cellular profiles were still observed in the corpus callosum of treated animals, there is a trend (p=.06) towards reducted iNOS immunoreactivity in NAC-treated Twi (Twi) animals. Both untreated Twi and NAC+BMT Twi have significantly higher levels of iNOS immunoreactivity compared with wildtypes (WT). *= p<0.05
iNOS expression
Cells reactive to an anti-iNOS antibody were found throughout the corpus callosum and cerebellar white matter of Twi mice. Brain sections from all treated groups were immunostained for iNOS to determine whether treatment reduced expression of this redox-sensitive enzyme. Profiles similar to those observed in untreated Twi mice were seen in NAC-Twi and combination-treated Twi brains (Fig 5A). A small decrease (p=.06) in iNOS immunoreactivity was observed in the corpus callosum of mice treated with NAC, however this reduction is not evident in mice receiving NAC and BMT (Fig 5B).
GFAP and CD68 expression
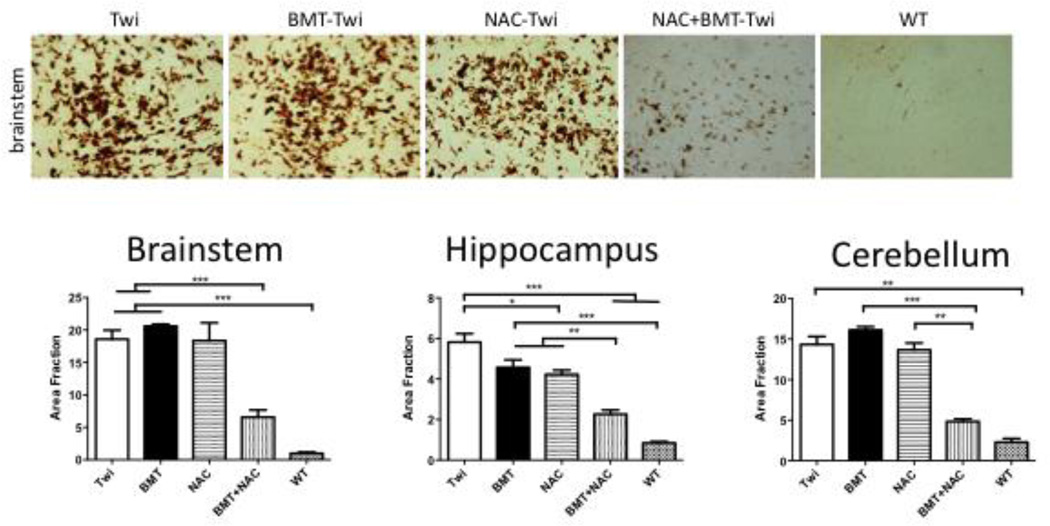
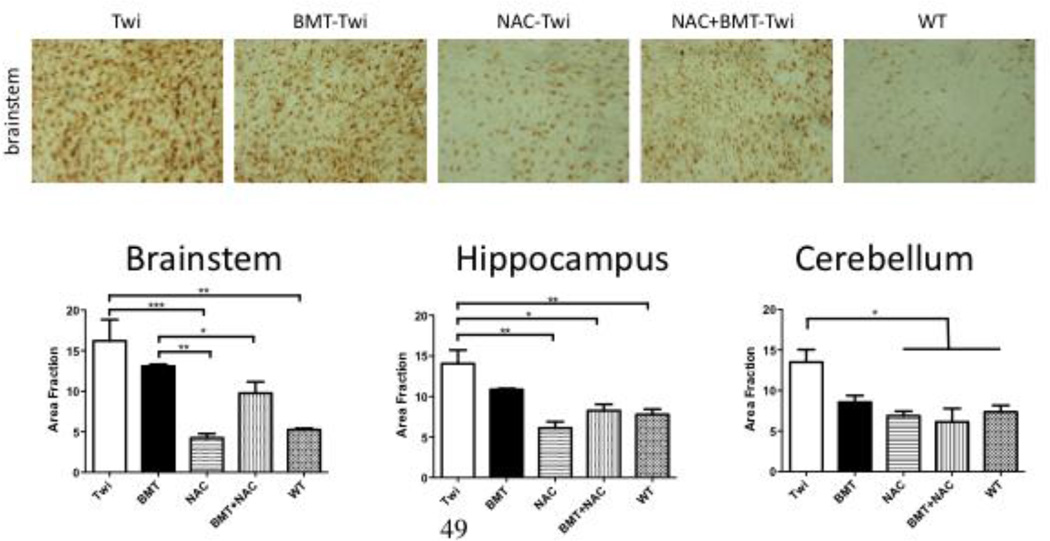
GFAP and CD68-postitive cells were observed throughout the brains of Twi mice, but not WT animals. Treatment with either NAC or BMT reduced the load of GFAP positive cells, but had little to no effect on CD68 staining. Co-treatment with NAC+BMT reduced CD68 immunostaining (Fig 6), but did not further reduce GFAP (Fig 7) immunoreactivity.
Figure 6.
CD68 (macrophages and activated microglia) staining in treated and untreated mice. Treatment with NAC and BMT reduces markers of inflammation in the brains of Twi mice. is significantly reduced in the cerebellum and hippocampus following either BMT or NAC treatment. The combination of these two therapies further decreased the number of CD68-positive cells. In the brainstem, CD68 staining was only decreased when the two treatments were combined. * = p<0.05, ** = p<0.01, *** = p<0.005.
Figure 7.
Treatment with NAC and BMT reduces markers of inflammation in the brains of Twi mice. Staining for GFAP (activated astrocytes) was significantly reduced in all brain regions studied following either NAC or BMT treatment. The combination of these two therapies did not further reduce GFAP expression. * = p<0.05, ** = p<0.01, *** = p<0.005
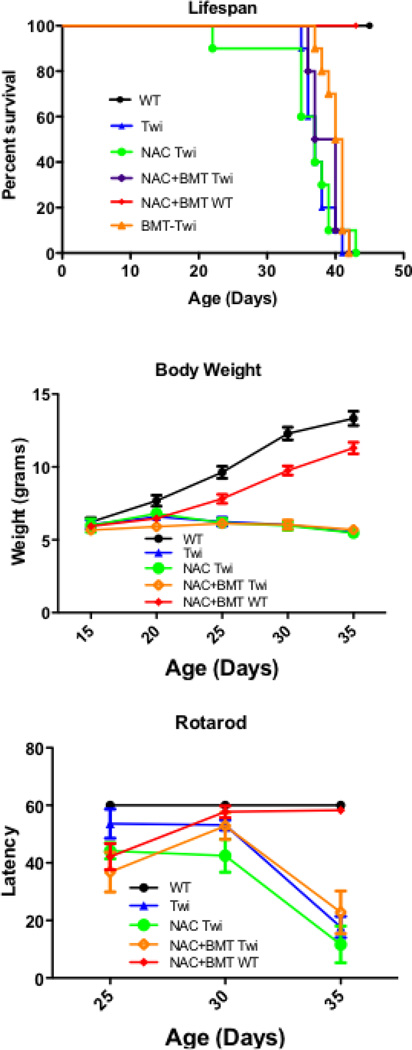
Lifespan
A cohort of Twi mice (n=12) was treated with NAC alone, and another cohort (n= 12) was treated with NAC in combination with BMT. In addition, a cohort of normal (n=10) animals was treated with NAC and BMT as a toxicity control. A final cohort (n=10) of Twi mice receiving only BMT was generated. The treatment paradigm and NAC dosing (Fig 3) were based on protocols previously shown to be effective in treating CNS and other diseases in mice (Abdelsaid, et al., 2010, Cotter, et al., 2007, Farr, et al., 2003). Untreated, NAC-treated, or combination-treated animals had median lifespans of 37, 37 and 38.5 days, respectively (Fig 8A). No change in lifespan was observed in Twi mice following NAC or NAC+BMT treatment. Similarly to previous studies, a small but statistically significant increase in lifespan was observed with BMT alone (median lifespan 40d, p < 0.001), and engraftment rates were comparable to previous reports. Animals treated with NAC+BMT had no significant increase or decrease in lifespan compared with BMT-treated Twi mice. In comparison, no normal animals, treated or untreated, died during the course of this study. Weight gain was indistinguishable between the Twi groups, and greatly reduced compared to untreated or combination-treated normal littermate controls (Fig 8B).
Figure 8.
NAC treatment results in no clinical improvement in the Twi mouse. No significant improvements were observed in NAC (green open circles) or NAC+BMT (purple open diamonds) or BMT only (orange open triangles) treated Twi mice with respect to lifespan (A), rotarod performance (B) or body weight (C) when compared with untreated Twi mice (blue closed triangles). NAC+BMT treated WT (red closed diamonds) show a small delay in weight gain and defect in motor performance, but they quickly recover and perform similarly to untreated wild type mice (black closed circles). Animals were not tested after 35d as they could no longer remain on the stationary rotarod. Body weight and rotarod measurements were not performed for BMT-Twi animals.
Behavior
Twitcher mice show progressive peripheral neuropathy and hind limb wasting (Wenger, et al., 2001). As a result, their performance on the rotarod, a measure of motor coordination and control, is significantly impaired. Neither NAC treatment nor combination treatment improved performance on the rotarod (Fig 8C). Combination-treated normal mice show a small delay in motor function when first tested on the rotarod, however they quickly improve. By the second test combination-treated normal animals are indistinguishable from untreated normal mice.
DISCUSSION AND CONCLUSIONS
In this study we further characterize an elevation of oxidative damage and markers of oxidative stress in the nervous system of Twi mice. This is consistent with an increase in oxidative damage in cultured cells exposed to psy and with previous reports. Through crude dissection of brain regions into cerebellum, brain stem and cortex, we observed no differences in the spatial distribution of oxidative stress. However, future more detailed dissections and measurements could uncover differences on a smaller scale, such as between white and grey matter. Additionally, we see that the elevation in oxidative stress is progressive, becoming more pronounced as the mice age. The elevation of protein carbonyl content is already significant by PND10, a full 10–12 days before symptoms are evident, highlighting the importance of early treatment for GLD.
It is generally accepted that oxidative stress and damage have deleterious effects on cells. Therefore it was reasonable to hypothesize that directly treating oxidative stress in vivo with a potent anti-oxidant, NAC, would have a beneficial effect on the lifespan or other markers of disease in the Twi mouse. This is supported by the fact that psy-induced oxidative stress can be significantly reduced in vitro by treatment with NAC (Giri, et al., 2008, Haq, et al., 2006). Additionally, NAC has proven to be effective in treating other diseases of the CNS (Abdelsaid, et al., 2010, Farr, et al., 2003). For example, the lifespan of mice suffering from ethylmalonic encephalopathy was extended by nearly 100% when treated with NAC in the drinking water alone (Viscomi, et al., 2010). While antioxidant therapy could not be expected to cure an inborn error of metabolism alone, we hypothesized that it might have some effect, and could augment the effect of established therapies. Recent studies have reported additive or even synergistic effects when multiple therapies are applied to the Twi mouse (Biswas and LeVine, 2002, Galbiati, et al., 2009, Lin, et al., 2007). Bone Marrow transplant was particularly appealing, as it is the only clinically available treatment for GLD. It was hypothesized that through this combination therapy, BMT would provide some enzyme as well as immunomodulatory effects (Lin, et al., 2007, Reddy, 2011), and NAC would reduce oxidative stress.
In this study, animals were treated with relatively large doses of NAC, simultaneously through multiple routes (orally and ip). The goal of this dosing regimen was to provide as much NAC as early in life as possible. The NAC in the drinking water provided a continuous source of antioxidant while the ip injections provided a measureable bolus, particularly important in neonatal mice that are not yet directly drinking water. The level of oxidative stress was significantly reduced in the brains of NAC-treated Twi mice, to a level indistinguishable from normal mice. Clearly, sufficient NAC was given to reduce the oxidative load in the brains of NAC-treated Twi mice. Unexpectedly, this decrease in oxidative stress was no longer observed in NAC+BMT treated Twi mice. The damaging effects of ionizing radiation needed for BMT conditioning could cause increased oxidative stress. Indeed, ionizing radiation has been shown to induce oxidative stress in mice (Navarro, et al., 1997). The slight elevation in protein carbonyls observed in BMT only and NAC+BMT-WT mice supports this hypothesis. It is possible that a higher dose of NAC, sufficient to bring down the level of stress in BMT-treated animals, would have therapeutic benefit. Alternatively, different conditioning regimens that induce less oxidative damage could be employed. In addition, glutathione levels are normalized in NAC treated mice, with BMT having no further effect, further evidence that sufficient NAC reached the nervous system to reduce the oxidative load.
Significant improvements in immunohistochemical markers of disease were observed. Expression of iNOS is increased in Twi mice compared with normal controls. iNOS immunostaining appeared to be decreased in NAC-treated Twi mice, however, this decrease was not significant. More impressively, however, was the reduction in the inflammatory markers CD68 (macrophages and activated microglia) and GFAP (activated astrocytes). Untreated Twi mice show dramatic elevations of both CD68 and GFAP immunoreactivity throughout the brain. The combination of NAC and BMT significantly reduced the number of CD68-positive microglia and macrophages in the brain, however either treatment alone had little effect. While both NAC and BMT reduced the number of activated astrocytes, the combination of these two therapies did not lead to any additional reduction as was observed in the CD68 staining. One explanation for this could be that some percentage of CD68-positive cells are peripherally-derived (macrophages). Perhaps these two therapies, which are both administered systemically, have a more significant synergy on the peripheral components of disease, as observed through a decreased number of infiltrating macrophages in the brain. Why this is not observed with PAS staining is unclear. Astrocytes may be somewhat less affected, although clearly they do derive some benefit from these therapies. Through these findings, it is clear that NAC treatment does have some effect on the Twi mouse, both with respect to oxidative state and immunohistochemical markers of disease.
Unfortunately, all of these biochemical and immunohistological benefits did not lead to any improvements in the clinical course of disease. In this study, we observed no improvements in lifespan with NAC or NAC+BMT treatment. As previously reported (Lin, et al., 2007, Reddy, et al., 2011), there is a very small but significant increase in lifespan following BMT alone (p <0.01). However, the additional manipulation and stress associated with NAC co-treatment (animal handling, intraperitoneal injections, etc.) might have negated any benefit of BMT since the effect size is so small. Another possibility is that the observed decreases in oxidative stress and glial activation are actually detrimental. Recent studies have indicated that immune activation in the form of infiltrating macrophages (Kondo, et al., 2011) and activated astrocytes (Macauley, et al., 2011) may actually be beneficial to disease in GLD and other LSDs. The reason for this is not fully known, however the activated cells may be clearing debris or otherwise maintaining the brain. Although a decrease in gliosis and macrophage infiltration has generally been considered positive, in light of these recent findings, decreasing immune activation through NAC treatment could have an inadvertent negative effect on disease.
Although there is still room for future investigation, these data suggest that oxidative stress is not an effective primary target for GLD therapy. Oxidative stress has been identified in a large number of LSDs, indicating that it is either a common result of lysosomal dysfunction or a marker of a sick and dying cell. In the specific case of GLD, it is likely that generation of ROS is a down-stream effect of Psy toxicity, rather than a disease causing mechanism. Therefore, treating this aspect of pathology has very limited effects. A better understanding of psy toxicity would be helpful in identifying more efficacious therapeutic targets. Indeed, recent publications have indicated that lipid rafts (White, et al., 2009), peroxisomal systems (Contreras, et al., 2008, Haq, et al., 2006, Khan, et al., 2005), and numerous cellular pathways are involved in psy toxicity and GLD pathogenesis. It is possible that NAC could be a useful adjunct therapy alongside more efficacious regimens, such as BMT combined with gene therapy, that target the disease-initiating events. The possibility for synergy between even non-efficacious single therapies can not be discounted, as recent reports have shown that this is possible (Macauley, et al., 2012). These findings also call into question whether antioxidant therapy will hold much promise in treating other LSDs. It is likely that some LSDs will respond well to antioxidants, while some will not, as has been the case with most other therapies tested for the disorders.
Highlights for Hawkins-Salsbury et al.
Oxidative Stress is evident in the nervous system of Twitcher mice.
We treated Twi mice with N-acetyl cysteine, both alone and in combination with bone marrow transplantation.
NAC treatment lowered oxidative stress, and improved many histological markers of disease.
However, neither NAC nor NAC+BMT improved any clinical markers of disease in the Twi mouse.
Oxidative stress may bee too far downstream of the initiating events in GLD for it to be a suitable therapeutic target.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Abdelsaid MA, Pillai BA, Matragoon S, Prakash R, Al-Shabrawey M, El-Remessy AB. Early intervention of tyrosine nitration prevents vaso-obliteration and neovascularization in ischemic retinopathy. J Pharmacol Exp Ther. 2010;332:125–134. doi: 10.1124/jpet.109.157941. [DOI] [PubMed] [Google Scholar]
- 2.Atkuri KR, Mantovani JJ, Herzenberg LA. N-Acetylcysteine--a safe antidote for cysteine/glutathione deficiency. Curr Opin Pharmacol. 2007;7:355–359. doi: 10.1016/j.coph.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bebarta VS, Kao L, Froberg B, Clark RF, Lavonas E, Qi M, Delgado J, McDonagh J, Arnold T, Odujebe O, O'Malley G, Lares C, Aguilera E, Dart R, Heard K, Stanford C, Kokko J, Bogdan G, Mendoza C, Mlynarchek S, Rhyee S, Hoppe J, Haur W, Tan HH, Tran NN, Varney S, Zosel A, Buchanan J, Al-Helial M. A multicenter comparison of the safety of oral versus intravenous acetylcysteine for treatment of acetaminophen overdose. Clin Toxicol (Phila) 2010;48:424–430. doi: 10.3109/15563650.2010.486381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. American Journal of Physiology. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 5.Biswas S, LeVine SM. Substrate-reduction therapy enhances the benefits of bone marrow transplantation in young mice with globoid cell leukodystrophy. Pediatr Res. 2002;51:40–47. doi: 10.1203/00006450-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Borda JT, Alvarez X, Mohan M, Ratterree MS, Phillippi-Falkenstein K, Lackner AA, Bunnell BA. Clinical and immunopathologic alterations in rhesus macaques affected with globoid cell leukodystrophy. Am J Pathol. 2008;172:98–111. doi: 10.2353/ajpath.2008.070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Contreras MA, Haq E, Uto T, Singh I, Singh AK. Psychosine-induced alterations in peroxisomes of twitcher mouse liver. Arch Biochem Biophys. 2008;477:211–218. doi: 10.1016/j.abb.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotter MA, Thomas J, Cassidy P, Robinette K, Jenkins N, Florell SR, Leachman S, Samlowski WE, Grossman D. N-acetylcysteine protects melanocytes against oxidative stress/damage and delays onset of ultraviolet-induced melanoma in mice. Clin Cancer Res. 2007;13:5952–5958. doi: 10.1158/1078-0432.CCR-07-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E, Butterfield DA, Morley JE. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003;84:1173–1183. doi: 10.1046/j.1471-4159.2003.01580.x. [DOI] [PubMed] [Google Scholar]
- 10.Galbiati F, Givogri MI, Cantuti L, Lopez Rosas A, Cao H, van Breemen R, Bongarzone ER. Combined hematopoietic and lentiviral gene-transfer therapies in newborn Twitcher mice reveal contemporaneous neurodegeneration and demyelination in Krabbe disease. J Neurosci Res. 2009 doi: 10.1002/jnr.22006. [DOI] [PubMed] [Google Scholar]
- 11.Giri S, Khan M, Nath N, Singh I, Singh AK. The role of AMPK in psychosine mediated effects on oligodendrocytes and astrocytes: implication for Krabbe disease. J Neurochem. 2008;105:1820–1833. doi: 10.1111/j.1471-4159.2008.05279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haq E, Contreras MA, Giri S, Singh I, Singh AK. Dysfunction of peroxisomes in twitcher mice brain: a possible mechanism of psychosine-induced disease. Biochem Biophys Res Commun. 2006;343:229–238. doi: 10.1016/j.bbrc.2006.02.131. [DOI] [PubMed] [Google Scholar]
- 13.Haq E, Giri S, Singh I, Singh AK. Molecular mechanism of psychosine-induced cell death in human oligodendrocyte cell line. J Neurochem. 2003;86:1428–1440. doi: 10.1046/j.1471-4159.2003.01941.x. [DOI] [PubMed] [Google Scholar]
- 14.Hawkins-Salsbury JA, Reddy AS, Sands MS. Combination therapies for lysosomal storage disease: is the whole greater than the sum of its parts? Human Molecular Genetics. 2011 doi: 10.1093/hmg/ddr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Igisu H, Suzuki K. Progressive accumulation of toxic metabolite in a genetic leukodystrophy. Science. 1984;224:753–755. doi: 10.1126/science.6719111. [DOI] [PubMed] [Google Scholar]
- 16.Khan M, Haq E, Giri S, Singh I, Singh AK. Peroxisomal participation in psychosine-mediated toxicity: implications for Krabbe's disease. J Neurosci Res. 2005;80:845–854. doi: 10.1002/jnr.20529. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi T, Yamanaka T, Jacobs JM, Teixeira F, Suzuki K. The Twitcher mouse: an enzymatically authentic model of human globoid cell leukodystrophy (Krabbe disease) Brain Res. 1980;202:479–483. doi: 10.1016/0006-8993(80)90159-6. [DOI] [PubMed] [Google Scholar]
- 18.Kondo Y, Adams JM, Vanier MT, Duncan ID. Macrophages counteract demyelination in a mouse model of globoid cell leukodystrophy. Journal of Neuroscience. 2011;31:3610–3624. doi: 10.1523/JNEUROSCI.6344-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin D, Donsante A, Macauley S, Levy B, Vogler C, Sands MS. Central nervous system-directed AAV2/5-mediated gene therapy synergizes with bone marrow transplantation in the murine model of globoid-cell leukodystrophy. Mol Ther. 2007;15:44–52. doi: 10.1038/sj.mt.6300026. [DOI] [PubMed] [Google Scholar]
- 20.Macauley SL, Pekny M, Sands MS. The role of attenuated astrocyte activation in infantile neuronal ceroid lipofuscinosis. Journal of Neuroscience. 2011;31:15575–15585. doi: 10.1523/JNEUROSCI.3579-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macauley SL, Roberts MS, Wong AM, McSloy F, Reddy AS, Cooper JD, Sands MS. Synergistic effects of central nervous system-directed gene therapy and bone marrow transplantation in the murine model of infantile neuronal ceroid lipofuscinosis. Annals of Neurology. 2012 doi: 10.1002/ana.23545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLellan LI, Lewis AD, Hall DJ, Ansell JD, Wolf CR. Uptake and distribution of N-acetylcysteine in mice: tissue-specific effects on glutathione concentrations. Carcinogenesis. 1995;16:2099–2106. doi: 10.1093/carcin/16.9.2099. [DOI] [PubMed] [Google Scholar]
- 23.Navarro J, Obrador E, Pellicer JA, Aseni M, Vina J, Estrela JM. Blood glutathione as an index of radiation-induced oxidative stress in mice and humans. Free Radical Biology and Medicine. 1997;22:1203–1209. doi: 10.1016/s0891-5849(96)00554-0. [DOI] [PubMed] [Google Scholar]
- 24.Pocernich CB, La Fontaine M, Butterfield DA. In-vivo glutathione elevation protects against hydroxyl free radical-induced protein oxidation in rat brain. Neurochemistry International. 2000;36:185–191. doi: 10.1016/s0197-0186(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 25.Reddy A, Kim J, Hawkins-Salsbury JA, Macauley SL, Tracy E, Vogler C, Han X, Song SK, Wozniak D, Fowler SC, Klein R, Sands MS. Bone Marrow Transplantation augments the effect of brain and spinal cord-directed AAV2/5 gene therapy by altering inflammation in the murine model of Globoid-cell Leukodystrophy. Journal of Neuroscience. 2011 doi: 10.1523/JNEUROSCI.1802-11.2011. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy AS, Kim JH, Hawkins-Salsbury JA, Macauley SL, Tracy ET, Vogler CA, Han X, Song SK, Wozniak DF, Fowler SC, Klein RS, Sands MS. Bone marrow transplantation augments the effect of brain- and spinal cord-directed adeno-associated virus 2/5 gene therapy by altering inflammation in the murine model of globoid-cell leukodystrophy. Journal of Neuroscience. 2011;31:9945–9957. doi: 10.1523/JNEUROSCI.1802-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ripoll CB, Flaat M, Klopf-Eiermann J, Fisher-Perkins JM, Trygg CB, Scruggs BA, McCants ML, Leonard HP, Lin AF, Zhang S, Eagle ME, Alvarez X, Li YT, Li SC, Gimble JM, Bunnell BA. Mesenchymal lineage stem cells have pronounced anti-inflammatory effects in the twitcher mouse model of Krabbe's disease. Stem Cells. 29:67–77. doi: 10.1002/stem.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakai N, Inui K, Tatsumi N, Fukushima H, Nishigaki T, Taniike M, Nishimoto J, Tsukamoto H, Yanagihara I, Ozono K, Okada S. Molecular cloning and expression of cDNA for murine galactocerebrosidase and mutation analysis of the twitcher mouse, a model of Krabbe's disease. Journal of Neurochemistry. 1996;66:1118–1124. doi: 10.1046/j.1471-4159.1996.66031118.x. [DOI] [PubMed] [Google Scholar]
- 29.Sands MS, Barker JE. Percutaneous intravenous injection in neonatal mice. Lab Anim Sci. 1999;49:328–330. [PubMed] [Google Scholar]
- 30.Suzuki K. Twenty five years of the "psychosine hypothesis": a personal perspective of its history and present status. Neurochem Res. 1998;23:251–259. doi: 10.1023/a:1022436928925. [DOI] [PubMed] [Google Scholar]
- 31.Taketomi T, Nishimura K. Physiological Activity of Psychosine. Jpn J Exp Med. 1964;34:255–265. [PubMed] [Google Scholar]
- 32.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Analytical Biochemistry. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 33.Viscomi C, Burlina AB, Dweikat I, Savoiardo M, Lamperti C, Hildebrandt T, Tiranti V, Zeviani M. Combined treatment with oral metronidazole and N-acetylcysteine is effective in ethylmalonic encephalopathy. Nature Medicine. 2010;16:869–871. doi: 10.1038/nm.2188. [DOI] [PubMed] [Google Scholar]
- 34.Wagner KR, Dean C, Beiler S, Bryan DW, Packard BA, Smulian AG, Linke MJ, de Courten-Myers GM. Plasma infusions into porcine cerebral white matter induce early edema, oxidative stress, pro-inflammatory cytokine gene expression and DNA fragmentation: implications for white matter injury with increased blood-brainbarrier permeability. Curr Neurovasc Res. 2005;2:149–155. doi: 10.2174/1567202053586785. [DOI] [PubMed] [Google Scholar]
- 35.Wenger D, Suzuki K, Suzuki Y. Galactosylceramide Lipidosis: Globoid Cell Leukodystrophy (Krabbe Disease) In: Scriver C, Beaudet A, Sly W, Valle D, Childs B, Kinzler K, Vogelstein B, editors. The Metabolic & Molecular Basis of Disease. McGraw-Hill Medical Publishing Division; New York: 2001. pp. 2669–3694. [Google Scholar]
- 36.White AB, Givogri MI, Lopez-Rosas A, Cao H, van Breemen R, Thinakaran G, Bongarzone ER. Psychosine accumulates in membrane microdomains in the brain of krabbe patients, disrupting the raft architecture. J Neurosci. 2009;29:6068–6077. doi: 10.1523/JNEUROSCI.5597-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaka M, Wenger DA. Psychosine-induced apoptosis in a mouse oligodendrocyte progenitor cell line is mediated by caspase activation. Neurosci Lett. 2004;358:205–209. doi: 10.1016/j.neulet.2003.12.126. [DOI] [PubMed] [Google Scholar]