Abstract
Objectives
Toothbrushing, though aimed at biofilm removal, also affects the lubricative function of adsorbed salivary conditioning films (SCFs). Different modes of brushing (manual, powered, rotary–oscillatory or sonically driven) influence the SCF in different ways. Our objectives were to compare boundary lubrication of SCFs after different modes of brushing and to explain their lubrication on the basis of their roughness, dehydrated layer thickness, and degree of glycosylation. A pilot study was performed to relate in vitro lubrication with mouthfeel in human volunteers.
Materials and methods
Coefficient of friction (COF) on 16-h-old SCFs after manual, rotary–oscillatory, and sonically driven brushing was measured using colloidal probe atomic force microscopy (AFM). AFM was also used to assess the roughness of SCFs prior to and after brushing. Dehydrated layer thicknesses and glycosylation of the SCFs were determined using X-ray photoelectron spectroscopy. Mouthfeel after manual and both modes of powered brushing were evaluated employing a split-mouth design.
Results
Compared with unbrushed and manually or sonically driven brushed SCFs, powered rotary–oscillatory brushing leads to deglycosylation of the SCF, loss of thickness, and a rougher film. Concurrently, the COF of a powered rotary–oscillatory brushed SCF increased. Volunteers reported a slightly preferred mouthfeel after sonic brushing as compared to powered rotating–oscillating brushing.
Conclusion
Deglycosylation and roughness increase the COF on SCFs.
Clinical relevance
Powered rotary–oscillatory brushing can deglycosylate a SCF, leading to a rougher film surface as compared with manual and sonic brushing, decreasing the lubricative function of the SCF. This is consistent with clinical mouthfeel evaluation after different modes of brushing.
Keywords: Salivary conditioning film, Glycosylation, Toothbrushing, Friction, AFM, XPS
Introduction
The periodic removal of oral biofilm is essential in order to prevent dental caries and periodontal diseases. For centuries now, toothbrushing has been the most popular and effective method for oral biofilm removal. The development of powered toothbrushes has provided a means for more effective biofilm removal than what can be achieved by manual brushing [1–3], and has brought a variety of different rotary–oscillatory and sonically driven toothbrushes to the market. Although aimed to remove biofilm, brushing also affects the adsorbed salivary conditioning film (SCF) covering all surfaces exposed in the oral cavity. The SCF on oral surfaces is pivotal for oral health because it facilitates oral lubrication, as required for eating (mastication) and speaking [4, 5], and it protects against dental erosion [6, 7] and abrasion [8].
Mastication and speech are only possible if the articulation (relative motion) between various oral surfaces is not hampered by excessive friction, as in the case of xerostomic patients suffering from reduced salivary excretion. Friction between two surfaces is determined by the roughness of the surfaces and the adhesion force between them and can be minimized by applying boundary lubricants, as constituted on oral surfaces by the adsorbed SCF. Atomic force microscopy (AFM) of the nanoscopic friction between two hard silica surfaces indicated that the coefficient of friction (COF) decreased by a factor of 20 upon coating the surfaces with a SCF [4, 9]. Hahn Berg et al. [9] tentatively attributed the lubricating properties of a SCF to mucins, proline-rich proteins, histatins, and their structure in an adsorbed state, although in general biolubrication is attributed to the presence of adsorbed glycosylated proteins [10]. Glycosylated proteins can bind water molecules and, in an adsorbed state, can generate hydration pressure against applied normal forces, therewith acting as a lubricant between articulating surfaces [11, 12]. Although the role of glycosylation in the boundary lubrication in joints and ocular surfaces has been established [13], the role of glycosylation on boundary lubrication of SCFs has not yet been thoroughly established.
Not all energy generated by toothbrushing is utilized for biofilm removal. The properties of SCFs, including boundary lubrication, are subject to changes after brushing, although complete removal of the SCF by brushing is generally considered impossible. Cleanliness after brushing is often probed by moving the tongue over the tooth surface, and a slick and smooth feeling is generally preferred. However, any basis for an altered mouthfeel after brushing based on the boundary lubrication properties of the SCF is unknown.
Therefore, the aim of this study is to compare the boundary lubrication properties of SCFs in vitro after manual and powered (rotary–oscillatory and sonically driven) brushing. Boundary lubrication properties are studied using a colloidal probe AFM and are related to the adhesion energy upon contact between the lubricating surfaces, their surface roughness, dehydrated film thickness, and the degree of glycosylation of the SCF. Mouthfeel after manual and both modes of powered brushing was evaluated in a group of human volunteers.
Materials and methods
Preparation of adsorbed salivary conditioning films
After stimulation by chewing Parafilm®, human whole saliva from 20 healthy volunteers of both sexes was collected into ice-cooled beakers, pooled, centrifuged, dialyzed, and lyophilized for storage. Prior to lyophilization, phenylmethylsulfonylfluoride (final concentration of 1 mM) was added as a protease inhibitor in order to reduce protein breakdown and preserve high molecular weight mucins. For experiments, lyophilized saliva was dissolved at a concentration of 1.5 mg ml−1 in buffer (2 mM potassium phosphate, 50 mM potassium chloride, and 1 mM calcium chloride at pH 6.8). All volunteers gave their informed consent to saliva donation in accordance with the rules set out by the ethics committee at the University Medical Center Groningen.
Microscope glass slides (Thomas Scientific, NJ, USA) were cleaned by sonication in a 2% surfactant RBS 35 (Fluka Chemie, Buchs, Switzerland), followed by thorough rinsing with hot tap water and subsequently alternate rinsing with methanol and demineralized water. Glass surfaces were then placed in reconstituted human whole saliva for 16 h at room temperature in order to form a SCF. After adsorption, glass slides were rinsed three times with demineralized water to remove excess saliva.
Toothbrushes and brushing
After adsorption, SCF-coated slides were brushed with a sonically driven (Sonicare® Elite, Philips, Eindhoven, The Netherlands) and rotary–oscillatory (Oral-B®; EB-17, P&G, OH, USA) powered toothbrush. Brushing was done in both power ON and OFF condition to simulate manual brushing with an identical bristle configuration, under a clinically relevant mass of 90 g [14, 15]. All SCFs were brushed in a wetted state, i.e., with a thin film of water on the SCF surface for 30 s with the brushes attached to a home-made moving tray, involving 30 single strokes back and forth each over a length of 3 cm.
Atomic force microscopy
Coefficient of friction, roughness, and adhesion energy toward a colloidal AFM probe [16, 17] on brushed and unbrushed SCFs were measured with an AFM (Nanoscope IV DimensionTM 3100) equipped with a Dimension Hybrid XYZ SPM scanner head (Veeco, NY, USA). Rectangular tipless cantilevers (length (l), width (w), and thickness (t) of 300, 35, and 1 μm, respectively) with a stiffness of 0.05 N m−1 were calibrated for their exact torsional and normal stiffness using AFM Tune IT v2.5 software [18–20]. The normal stiffness (K n) was in the range of 0.01 to 0.04 N m−1, while the torsional stiffness (K t) was in the range of 2 to 4 × 10−9 N m rad−1.
Subsequently, a silica particle of 4.74 μm diameter (d) (Bangs Laboratories, IN, USA) was glued to a cantilever with an epoxy glue (Pattex, Brussels, Belgium) using a micromanipulator (manufactured by Narishige Groups, Tokyo, Japan) to prepare a colloidal probe. The deflection sensitivity (α) of the colloidal probe was recorded on bare glass in buffer to calculate the applied normal force (F n) using
| 1 |
where ∆V n is the voltage output from the AFM photodiode due to normal deflection of the colloidal probe.
The torsional stiffness and geometrical parameters of the colloidal probe were used to calculate the friction force (F f) [19, 21] according to
| 2 |
where t is the thickness of the cantilever, δ is the torsional detector sensitivity of the AFM and ΔV L corresponds to the voltage output from the AFM photodiode due to lateral deflection of the colloidal probe. Lateral deflection was observed at a scanning angle of 90° over a distance of 5 μm and a scanning frequency of 2 Hz. The scanning angle, distance, and frequency were kept constant throughout all friction force measurements.
The colloidal probe was incrementally loaded and unloaded in steps of 5 nN up to a maximal normal force of 30 nN. At each normal force, ten friction loops were recorded to yield the average friction force. Friction forces during loading and unloading were separately plotted against the normal forces applied, and linear least-squares fitting subsequently provided the coefficient of friction. Coefficients of friction were measured on three different locations on each SCF-coated glass slide. After each measurement of a coefficient of friction, force–distance curves were measured on a bare glass surface to verify that the colloidal probe had not become contaminated by proteins, i.e., if a soft contact was observed upon approach, the probe was discarded. Colloidal probes were scanned over SCF-coated glass slides to obtain topographic images from which the mean surface roughness at zero load was calculated. Surface roughness was measured on three different locations on one SCF-coated glass slide. Force–distance curves between a colloidal probe and the SCF were obtained at a trigger threshold force of 5 nN, and the adhesion energy between the two interacting surfaces was calculated from the area under about 50 retract force–distance curves.
X-ray photoelectron spectroscopy
The dehydrated thickness of a SCF was determined prior to and after brushing from the surface chemical composition of the SCF-coated glass slides as measured using X-ray photoelectron spectroscopy (XPS, S-probe, Surface Science Instruments, Mountain View, CA, USA). First, wet surfaces were dried in the pre-vacuum chamber of the XPS, and then subjected to a vacuum of 10−7 Pa. X-rays (10 kV, 22 mA), at a spot size of 250 × 1,000 μm, were produced using an aluminum anode. Scans of the overall spectrum in the binding energy range of 1–1,100 eV were made at low resolution (pass energy 150 eV). The area under each peak was used to yield elemental surface concentrations for Si, N, O, and C after correction with sensitivity factors provided by the manufacturer. The dehydrated layer thickness was estimated by an overlayer model [22] based on attenuation of the Si2s electrons arising from the glass surface with respect to N1s electrons from the overlaying adsorbed SCF.
The oxygen peak area for a SCF on glass (%Ototal) can be separated into three components arising from oxygen involved in amide functionalities (%Oamide), glycosylated oxygen (%Oglyco) and oxygen from the underlying glass surface (%Oglass) according to
| 3 |
The contribution to the total oxygen peak area from the glass surface is given by twice the observed Si peak area, while the oxygen contribution from amide functionalities follows from
| 4 |
in which 1.18 represents the average ratio between oxygen and nitrogen in amide functionalities [23]. Therewith, Eq. 3 provides a simple means to calculate the %Oglyco as an estimate of the degree of glycosylation of a SCF on glass.
In vivo evaluation of oral mouthfeel after different modes of brushing
Ten healthy volunteers (six females, age 30 ± 8 years and four males, age 34 ± 7 years) participated in this randomized, cross-over, split-mouth study according to the guidelines and independent review and approval by the Medical Ethics Committee of the University Medical Center Groningen, The Netherlands (METc 2005/197). All volunteers gave their written informed consent and had never used any rotary–oscillatory or sonically driven brush.
Sonic brushing and rotary–oscillatory brushing were independently compared with manual brushing in a split-mouth design to allow a direct comparison between two modes of powered brushing for each volunteer. The volunteers were requested to brush with a manual toothbrush using 1.5 g of a standard toothpaste (Crest® Regular, Proctor & Gamble, Mason, OH, USA) for 2 min twice per day during a one week period according to their routine habits. After this period, volunteers were provided with a manual and a powered sonically driven or rotary–oscillatory toothbrush. Volunteers were instructed to brush the left or right side of their dentition with a powered brush and the other side with the manual brush using the provided toothpaste. For each side of their dentition separately, volunteers completed questionnaires on days 1, 2, 3, 5, 8, 15, and 22 after commencing this split-mouth experiment. Subsequently, a wash-out period of 2 weeks was obeyed during which the volunteers brushed their full dentition with a manual brush again, after which the volunteers with a sonically driven brush received a rotary–oscillatory additional to a new manual brush and vice versa (volunteers with a rotary–oscillatory brush received a sonically driven brush) for use during another 22 days.
The study was carried out in the Department of Biomedical Engineering, UMCG, Groningen, The Netherlands. The volunteers were enrolled and assigned by the person who performed the study. The randomization schedule was generated using SAS 9.1.3. Mouthfeel was evaluated using a questionnaire immediately prior to and after brushing in the morning, involving the following questions:
How do you like the smoothness of your teeth?
How do you like the clean feeling of your teeth?
How do you like the moist feeling of your teeth?
Overall, how do you like the feeling of your mouth?
All questions were scored for the left and right side of the dentition on a seven point scale (0, dislike extremely; 1, dislike; 2, dislike somewhat; 3, neutral; 4, like somewhat; 5, like; 6, like extremely) and expressed in a single average score per evaluation relative to manual brushing.
Results
Figure 1 presents examples of AFM images of SCFs. The unbrushed SCF constitutes an uneven, knotted structure with a roughness of 0.41 nm, while after powered brushing, higher hills and deeper valleys developed that run along the direction of brushing. Manual brushing yields a more even SCF surface compared with powered brushing. Accordingly, the surface roughness of the films (see Table 1) increased only slightly after manual brushing (not statistically significant), while powered brushing significantly increased the surface roughness to 1.96 and 5.37 nm for sonic and rotary–oscillatory brushing, respectively. The dehydrated thickness of the unbrushed SCF was 4.9 nm and decreased somewhat after manual brushing and most after powered sonic and rotary–oscillatory brushing to 3.6 and 3.3 nm, respectively. Neither mode of brushing was able to remove a substantial amount of adsorbed protein and bring the dehydrated layer thickness anywhere close to 0.
Fig. 1.
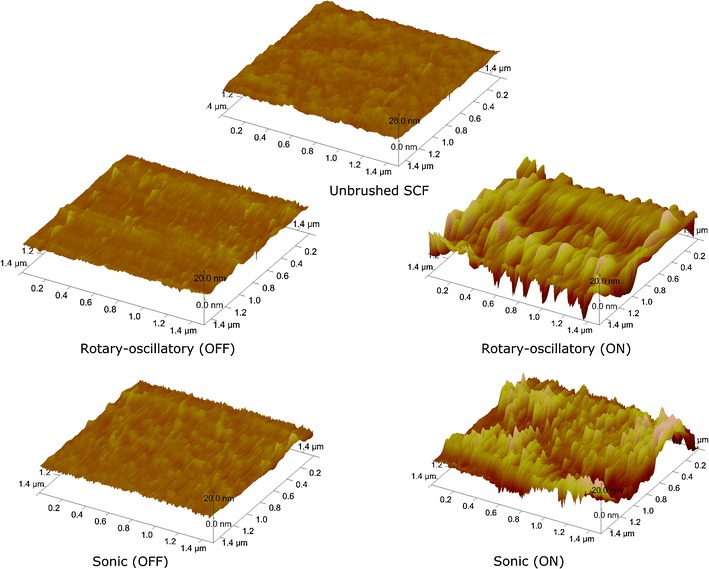
AFM topographic images of an unbrushed 16-h-old SCF and after manual (OFF) and powered (ON) brushing, with rotary–oscillatory and sonically driven toothbrush. Note that the valleys run in the direction of the brush movement
Table 1.
Surface roughness, dehydrated thickness, coefficient of friction, and adhesion energy for glass and SCFs after different modes of brushing
| Surface roughness (nm) | Dehydrated thickness (nm) | Coefficient of friction | Adhesion energy (10−18 J) | ||
|---|---|---|---|---|---|
| Glass | 0.13 ± 0.01 | 0.0 ± 0.0 | 0.450 ± 0.040 | −86 ± 14 | |
| Unbrushed 16-h SCF | 0.41 ± 0.02 | 4.9 ± 0.3 | 0.037 ± 0.000 | −14 ± 2 | |
| Manual | Rotary–oscillatory | 0.55 ± 0.08 | 4.1 ± 0.4 | 0.030 ± 0.000 | −9 ± 3 |
| OFF | |||||
| Sonic | 0.86 ± 0.12 | 4.2 ± 0.6 | 0.040 ± 0.000 | −6 ± 1 | |
| OFF | |||||
| Powered | Rotary–oscillatory | 5.37 ± 1.22 | 3.3 ± 0.4 | 0.630 ± 0.090 | −51 ± 30 |
| ON | |||||
| Sonic | 1.96 ± 0.55 | 3.6 ± 0.6 | 0.110 ± 0.010 | −24 ± 8 | |
| ON | |||||
Brushing was done manually (OFF) and powered (ON) with a rotary-oscillating and sonic toothbrush
± standard error over nine AFM and two XPS measurements
Figure 2 shows examples of the retract force–distance curves for an unbrushed SCF and a SCF after rotary–oscillatory brushing, as well as the friction forces measured for these SCFs at different loading forces. The unbrushed SCF exerts only a minor adhesion force on the colloidal probe (Fig. 2a), and accordingly the friction forces are small (Fig. 2b). The SCF after rotary–oscillatory brushing, on the other hand, attracts the colloidal probe more strongly (Fig. 2a), and the probe experiences a larger friction force (Fig. 2b).
Fig. 2.
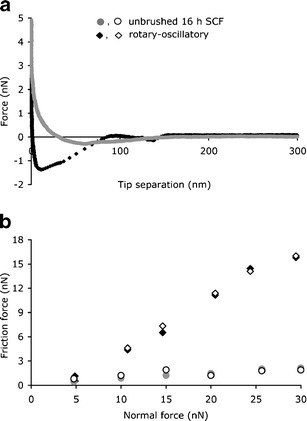
Examples of retract force–distance curves as measured using AFM (a) and of the friction force as a function of the normal force (b) for unbrushed 16-h-old SCF and rotary–oscillatory powered brushed SCF. Open and closed symbols represent friction force values during loading and unloading
The resulting coefficients of friction are summarized in Table 1 as well. The presence of a SCF clearly decreases the friction as compared with bare glass (p < 0.05, two-tailed Student’s t test), while manual brushing does not have a significant impact on the friction compared with the unbrushed SCF. Powered brushing increases the COF significantly (p < 0.05, two-tailed Student’s t test) to 0.110 and 0.630 for sonic and rotary–oscillatory brushing, which constitutes a statistically significant difference between the two modes of powered brushing (p < 0.05, two-tailed Student’s t test).
Integration of the retract force–distance curve yields the adhesion energy between the colloidal probe and the SCF, which amounts to −14 × 10−18 J for the unbrushed SCF (see also Table 1). The effects of the different modes of brushing on the adhesion energy follow the trend discussed above for the COF and accordingly the highest adhesion energy was measured on the rotary–oscillatory brushed SCF, i.e., −51 × 10−18 J.
The unbrushed SCF is composed of nitrogen, oxygen, and carbon, while the measurement of 4.1% Si attests to the fact that the underlying glass surface still contributes to the measured XPS composition (see Table 2). After brushing, the %Si increases, indicating that the dehydrated layer thickness of the SCF decreases after brushing, as summarized in Table 1. The elemental compositions in Table 2 can be employed in Eqs. 3 and 4 to yield the percentage of oxygen involved in glycosylated moieties (%Oglyco), as presented in Fig. 3. As can be seen, the coefficient of friction is highly sensitive to the degree of glycosylation of the SCF. Powered brushing, especially rotary–oscillatory brushing, strongly reduces the degree of glycosylation of the SCF, concurrent with a strong and abrupt increase in the coefficient of friction.
Table 2.
Elemental surface compositions of SCFs after different modes of brushing
| %C | %O | %N | %Si | ||
|---|---|---|---|---|---|
| Unbrushed 16-h SCF | 60.5 ± 1.2 | 25.5 ± 1.3 | 9.8 ± 0.2 | 4.1 ± 0.1 | |
| Manual | Rotary–oscillatory | 65.9 ± 2.9 | 21.7 ± 7.2 | 6.4 ± 2.2 | 6.0 ± 2.6 |
| OFF | |||||
| Sonic | 55.2 ± 1.2 | 29.0 ± 4.0 | 8.5 ± 1.5 | 7.3 ± 1.2 | |
| OFF | |||||
| Powered | Rotary–oscillatory | 57.0 ± 4.9 | 27.5 ± 3.4 | 4.4 ± 0.3 | 11.0 ± 1.8 |
| ON | |||||
| Sonic | 46.6 ± 0.3 | 33.4 ± 0.3 | 8.5 ± 0.7 | 11.5 ± 0.7 | |
| ON | |||||
Brushing was done manually (OFF) and powered (ON) with a rotary-oscillating and sonic toothbrush
± standard error over two measurements
Fig. 3.

The coefficient of friction (COF) between a colloidal AFM probe and differently brushed SCFs as a function of the degree of glycosylation of the film, expressed as the percentage of oxygen involved in glyconaceous moieties (%O glyco). Error bars represent the standard deviations over nine independent COFs and two independent measurements of the degree of glycosylation
Mouthfeel scores for the sonic and rotary–oscillatory toothbrushing were compared with the manual toothbrush scores at prior to brushing, post-brushing, pre-lunch, and post-lunch time points, over a 3-week period. For each time point, score differences for sonic minus manual and rotary–oscillatory minus manual are visually depicted using bar plots in Fig. 4a, b. Only in the post-brushing condition were the scores from sonic minus manual significantly (p < 0.1) higher than the scores from rotary–oscillatory minus manual, indicating the preference for sonic compared with rotary–oscillatory or manual toothbrush. Also, the mouthfeeling scores for sonic minus manual was higher (not significant) than the mouthfeeling scores for rotary–oscillatory minus manual brushing, over different time points in a day.
Fig. 4.
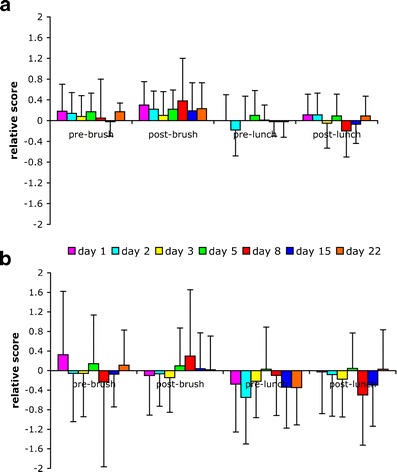
Average mouthfeel scores relative to manual brushing pre-brushing, post-brushing, pre-lunch, and post-lunch in a group of ten healthy volunteers for a sonically driven brushing and b rotary–oscillatory brushing. A positive difference indicates a preference for sonically driven or rotary–oscillatory brushing over manual brushing. Error bars indicate the SD over the ten volunteers
Discussion
This study addresses the boundary lubrication behavior of SCF and shows for the first time that boundary lubrication of SCF critically depends on the degree of glycosylation as well as on structural features of the adsorbed film, i.e., its surface roughness. Moreover, we show that powered rotary–oscillatory brushing yields deglycosylation and an increased roughness of the film, therewith increasing the COF of the film to above the level of unbrushed and otherwise brushed SCFs. Deglycosylation and increased roughness did not occur after sonic or manual brushing, in line with an oral mouthfeel evaluation after different modes of brushing in a group of human volunteers.
The compositional and structural changes in SCFs brought about by powered rotary-oscillating brushing suggest that this mode of brushing must deliver considerably more energy into the SCF than sonic or manual brushing. The amount of energy delivered by the various modes of brushing can be estimated by considering their mode of action more closely. Manual brushing causes abrasion in one dimension due to only sliding of the bristles over the SCF. Powered brushes cause abrasion both due to bristle contact in sliding, rotation (for rotary–oscillatory brushing at 340 Hz), and pulsation (for rotary–oscillatory at 73 Hz and for sonic brushing at 260 Hz). In addition to bristle motion, powered brush heads also generate acoustic pressure [24–26], which along with bristle motion may cause air bubbles that impinge on the SCF to abrade the film. The power transferred due to brushing can be roughly estimated as
| 5 |
where COF is the coefficient of friction for unbrushed SCF as determined in this paper (0.037 from Table 1), n B is the number of bristles, A B is the area of one bristle (taken as 0.018 mm2 and 0.019 mm2 for sonic and rotary–oscillatory), F is the applied normal force (1 N in the current experiments), V s is sliding velocity (1 mm s−1), and A BH is brush head area (220 mm2 and 78.5 mm2 for sonic and rotary–oscillatory, respectively). I ac is the acoustic intensity during non-contact brushing (1,995 W m−2 and 50,118 W m−2 for sonic and rotary–oscillatory brushing, respectively) [27] and V p is the pulsating velocity (m s−1), that can be calculated as
| 6 |
where f p is the pulsating frequency of powered sonic (250 Hz) and rotary–oscillatory (73 Hz) brushing, λ is the amplitude of bristle vibration amounting to 0.89 mm, and 1.55 mm for sonic and rotary–oscillatory brushing, respectively [28]. Power transfer during manual brushing can be calculated from Eq. 5 by setting the acoustic intensity and pulsating velocity at 0. According to Eq. 5, rotary–oscillatory brushing transfers 950 mW, which is about nine times more than sonic brushing (110 mW), and several orders of magnitude more than manual brushing (0.1 mW).
SCFs are generally strongly adsorbed and act as a load bearing film because the impinging surface causes confinement of proteins in smaller space, inducing an elastic repulsive force. The current data indicate that the lubrication provided by the SCF adsorbed on glass depends on its protein composition and structure. Similar behavior can be expected on tooth enamel because it possesses a similarly hydrophilic surface as glass with a correspondingly similar structure of the SCF [29]. Brushing of SCF causes an increase in roughness by disrupting the original protein conformation causing them to extend away from the surface. These extended proteins are now free to interact with the colloidal tip causing an increase in the interaction energy and hence its friction. Similarly, glycosylated proteins, like mucins, appeared to be the major lubricating component adsorbed in the SCF [4, 30]. A study by Jay et al. [13] has also shown that deglycosylation of synovial fluid decreased boundary lubrication between surfaces, similarly as demonstrated here for SCFs. Adsorbed mucins counteract the applied force by steric repulsion and local osmotic pressure due to the negatively charged sugar moieties and glycosylated groups extended on the peptide backbone, thereby assisting easy sliding at the interface [11, 12]. Mucins are high molecular weight proteins with multiple anchoring points on a surface that makes them more difficult to remove than smaller proteins. Removal of adsorbed proteins is a dynamic process of breaking anchorage points and renewed creation of anchoring points; only if all anchoring points are broken will larger proteins detach. Clearly, sonic and manual brushing do not deliver enough energy into a SCF to cause removal of large glyconaceous proteins.
Oral mouthfeel is the qualitative representation of different types of lubrication, including the boundary lubrication quantitatively measured in this paper. Daily mouthfeel depends on the interplay of chemical and mechanical effects of toothpaste ingredients and tooth brush action, respectively, on the SCF lubrication. Oral mouthfeel is unconsciously assessed several times a day by most people, and it is of interest that our split-mouth study, comparing sonically driven and rotary–oscillatory brushing, showed that sonic brushing yielded a preferred mouthfeel over rotary–oscillatory brushing. Therewith, this paper is the first to relate oral mouthfeel in humans with the nanoscopic structural and compositional features of SCFs found in vitro. The preferred mouthfeel after sonic brushing continued during the day despite the fact that the formation of a SCF on surfaces exposed to the oral cavity is assumed to occur within seconds and restoration of the pre-brushing SCF might thus be expected. However, adsorption of high molecular weight proteins is relatively a slow process as their adsorption involves removal of low molecular weight proteins that diffuse much faster to a surface than high molecular weight mucins [31].
Conclusion
Boundary lubrication in the oral cavity is influenced by the changes in the structure and protein composition of the SCFs upon brushing. A 16-h-old unbrushed SCF contains glycosylated mucins that assist in lubrication, thereby decreasing the COF. Powered rotary–oscillatory brushing leads to deglycosylation of the SCF along with the loss of thickness, indicating partial removal of mucins from the SCF which is in line with their higher amount of power transferred into the SCF. In addition, the higher surface roughness of these low mucinous films causes a high adhesion energy, contributing to a high COF. Relatively higher COFs observed on rotary–oscillatory brushed SCFs compared with sonic or manual brushed SCFs confirmed the outcome of a clinical evaluation on oral mouthfeel.
Acknowledgment
The authors would like to thank Dr. Norbert Schaefer from Oral-B, Germany, for his valuable discussions that have been implemented in this paper.
Conflicts of interest
The authors declare that there was no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Dorfer CE, von Bethlenfalvy ER, Pioch T, Galustians HJ, Qaqish JG, Sharma NC. Clinical evaluation of the efficacy of the battery-powered tooth brush: results from two independent studies. Am J Dent. 2001;14:273–277. [PubMed] [Google Scholar]
- 2.Farrell S, Terezhalmy GT, Bartizek RD, Biesbrock AR. Comparative plaque removal efficacy of a dual-action power toothbrush and a manual tooth brush: effects by toothbrush type. Am J Dent. 2006;19:195–200. [PubMed] [Google Scholar]
- 3.Sharma NC, Galustians HJ, Qaqish JG, Cugini MA. Safety and plaque removal efficacy of a battery-operated power toothbrush and a manual toothbrush. Am J Dent. 2001;14:9–15. [PubMed] [Google Scholar]
- 4.Hahn Berg IC, Rutland MW, Arnebrant T. Lubricating properties of the initial salivary pellicle—an AFM study. Biofouling. 2003;19:365–369. doi: 10.1080/08927010310001618571. [DOI] [PubMed] [Google Scholar]
- 5.Aguirre A, Mendoza B, Levine MJ, Hatton MN, Douglas WH. In vitro characterization of human salivary lubrication. Arch Oral Biol. 1989;34:675–677. doi: 10.1016/0003-9969(89)90024-1. [DOI] [PubMed] [Google Scholar]
- 6.Hannig C, Becker K, Hausler N, Hoth-Hannig W, Attin T, Hannig M. Protective effect of the in situ pellicle on dentin erosion—an ex vivo pilot study. Arch Oral Biol. 2007;52:444–449. doi: 10.1016/j.archoralbio.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Hannig M, Hess NJ, Hoth-Hannig W, de Vrese M. Influence of salivary pellicle formation time on enamel demineralization—an in situ pilot study. Clin Oral Investig. 2003;7:158–161. doi: 10.1007/s00784-003-0219-2. [DOI] [PubMed] [Google Scholar]
- 8.Joiner A, Schwarz A, Philpotts CJ, Cox TF, Huber K, Hannig M. The protective nature of pellicle towards toothpaste abrasion on enamel and dentine. J Dent. 2008;36:360–368. doi: 10.1016/j.jdent.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Hahn Berg CI, Lindh L, Arnebrant T. Intraoral lubrication of PRP-1, statherin and mucin as studied by AFM. Biofouling. 2004;20:65–70. doi: 10.1080/08927010310001639082. [DOI] [PubMed] [Google Scholar]
- 10.Tabak LA. Structure and function of human salivary mucins. Crit Rev Oral Biol Med. 1990;1:229–234. doi: 10.1177/10454411900010040201. [DOI] [PubMed] [Google Scholar]
- 11.Coles JM, Chang DP, Zauscher S. Molecular mechanisms of aqueous boundary lubrication by mucinous glycoproteins. Curr Opin Col Int Sci. 2010;15:406–416. doi: 10.1016/j.cocis.2010.07.002. [DOI] [Google Scholar]
- 12.Lee S, Spencer ND. Sweet, hairy, soft, and slippery. Science. 2008;319:575–576. doi: 10.1126/science.1153273. [DOI] [PubMed] [Google Scholar]
- 13.Jay GD. Characterization of a bovine synovial fluid lubricating factor I. Chemical, surface activity and lubricating properties. Connect Tissue Res. 1992;28:71–88. doi: 10.3109/03008209209014228. [DOI] [PubMed] [Google Scholar]
- 14.Heasman PA, McCracken GI. Clinical evidence for the efficacy and safety of powered toothbrushes. Adv Dent Res. 2002;16:9–15. doi: 10.1177/154407370201600104. [DOI] [PubMed] [Google Scholar]
- 15.Van der Weijden GA, Timmerman MF, Reijerse E, Snoek CM, Van der Velden U. Toothbrushing force in relation to plaque removal. J Clin Periodontol. 1996;23:724–729. doi: 10.1111/j.1600-051X.1996.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 16.Ducker WA, Senden TJ, Pashley MR. Direct measurement of colloidal forces using an atomic force microscope. Nature. 1991;353:239–241. doi: 10.1038/353239a0. [DOI] [Google Scholar]
- 17.Ralston J, Larson I, Rutland MW, Feiler AA, Kleijn M. Atomic force microscopy and direct surface force measurements. Pure Appl Chem. 2005;77:2149–2153. doi: 10.1351/pac200577122149. [DOI] [Google Scholar]
- 18.Green CP, Lioe H, Cleveland JP, Proksch R, Mulvaney P, Sader JE. Normal and torsional spring constants of atomic force microscope cantilevers. Rev Sci Instrum. 2004;75:1988–1997. doi: 10.1063/1.1753100. [DOI] [Google Scholar]
- 19.Pettersson T, Nordgren N, Rutland MW. Comparison of different methods to calibrate torsional spring constant and photodetector for atomic force microscopy friction measurements in air and liquid. Rev Sci Instrum. 2007;78:9–13. doi: 10.1063/1.2779215. [DOI] [PubMed] [Google Scholar]
- 20.Sader JE, Chon JWM, Mulvaney P. Calibration of rectangular atomic force microscope cantilevers. Rev Sci Instrum. 1999;70:3967–3969. doi: 10.1063/1.1150021. [DOI] [Google Scholar]
- 21.Pettersson T, Naderi A, Makuska R, Claesson PM. Lubrication properties of bottle-brush polyelectrolytes: an AFM study on the effect of side chain and charge density. Langmuir. 2008;24:3336–3347. doi: 10.1021/la703229n. [DOI] [PubMed] [Google Scholar]
- 22.Andrade JD. Surface and interfacial aspects of biomedical polymers. New York: Plenum; 1985. pp. 105–193. [Google Scholar]
- 23.Dufrene YF, Van der Wal A, Norde W, Rouxhet PG. X-ray photoelectron spectroscopy analysis of whole cells and isolated cell walls of gram-positive bacteria: comparison with biochemical analysis. J Bacteriol. 1997;179:1023–1028. doi: 10.1128/jb.179.4.1023-1028.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parini MR, Eggett DL, Pitt WG. Removal of Streptococcus mutans biofilm by bubbles. J Clin Periodontol. 2005;32:1151–1156. doi: 10.1111/j.1600-051X.2005.00836.x. [DOI] [PubMed] [Google Scholar]
- 25.Parini MR, Pitt WG. Dynamic removal of oral biofilms by bubbles. Colloids Surf B. 2006;52:39–46. doi: 10.1016/j.colsurfb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Pitt WG. Removal of oral biofilm by sonic phenomena. Am J Dent. 2005;18:345–352. [PubMed] [Google Scholar]
- 27.Busscher HJ, Jager D, Finger G, Schaefer N, Van der Mei HC. Energy transfer, volumetric expansion and removal of oral biofilms by non-contact brushing. Eur J Oral Sci. 2010;118:177–182. doi: 10.1111/j.1600-0722.2010.00723.x. [DOI] [PubMed] [Google Scholar]
- 28.Lea SC, Khan A, Patanwala HS, Landini G, Damien Walmsley A. The effects of load and toothpaste on powered toothbrush vibrations. J Dent. 2007;35:350–354. doi: 10.1016/j.jdent.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Hannig M, Herzog S, Willigeroth SF, Zimehl R. Atomic force microscopy study of salivary pellicles formed on enamel and glass in vivo. Colloid Polym Sci. 2001;279:479–483. doi: 10.1007/s003960000478. [DOI] [Google Scholar]
- 30.Yakubov GE, McColl J, Bongaerts JHH, Ramsden JJ. Viscous boundary lubrication of hydrophobic surfaces by mucin. Langmuir. 2009;25:2313–2321. doi: 10.1021/la8018666. [DOI] [PubMed] [Google Scholar]
- 31.Vroman L, Adams AL. Adsorption of proteins out of plasma and solutions in narrow spaces. J Colloid Interface Sci. 1986;111:391–402. doi: 10.1016/0021-9797(86)90042-1. [DOI] [Google Scholar]


