Abstract
Purpose
Intestinal adaptation following massive small bowel resection (SBR) permits improved absorption of enteral nutrition despite significant loss of bowel length. Epidermal growth factor (EGF) and its receptor (EGFR) have previously been established to play major roles in the pathogenesis of adaptation. This study tested the hypothesis that EGFR signaling within the epithelial cell compartment (enterocytes) is required for intestinal adaptation.
Methods
We developed a tamoxifen-inducible Villin-Cre/LoxP recombinant system for enterocyte-directed EGFR deletion using EGFR-floxed mice. EGFR-null mice and wild type (WT) littermates underwent either 50% proximal SBR or sham operation. Ileal tissue was harvested on post-operative day 7. To assess for adaptation, villus height and crypt depth as well as rates of crypt cell proliferation and apoptosis were measured.
Results
Adaptation after SBR occurred normally, as demonstrated by significant increases in villus height, crypt depth, crypt proliferative and apoptotic index in both the WT and EGFR-null mice.
Conclusion
Enterocyte EGFR expression is not required for the adaptation response to massive SBR. This novel finding suggests that enterocyte proliferation during adaptation is regulated by EGFR signaling in cells other than enterocytes, perhaps within the mesenchymal cell compartment of the bowel wall via factor(s) that are presently unknown.
Keywords: EGFR, adaptation, small bowel resection, enterocytes
INTRODUCTION
Intestinal adaptation is a compensatory response following massive small bowel resection (SBR) and allows for adequate absorption of enteral nutrition despite significant loss of bowel length. This phenomenon is characterized by significant increases in villus height and crypt depth, resulting in increased absorptive mucosal surface area [1].
Epidermal growth factor (EGF) and its receptor (EGFR) have previously been established to play major roles in the pathogenesis of adaptation. Systemic EGF stimulation enhances adaptation. If EGF is given exogenously after SBR or overexpressed in transgenic mice, a magnified adaptation response occurs [2, 3, 4]. Perturbed EGFR activity inhibits this important response. Removal of the major endogenous source of EGF via sialoadenectomy attenuates adaptation, an effect that is partially reversible with either systemic or oral administration of EGF [5]. Further, resection-induced adaptation after SBR is inhibited by systemic administration of EGFR inhibitors and in mutant mice (waved-2) that have generalized perturbed EGFR activity [6, 7]. While these experimental manipulations confirm an important role for EGFR signaling during adaptation, the exact cellular compartment within the intestinal wall (enterocyte versus underlying mesenchyme) that is governed by EGFR signaling to direct adaptation is presently unknown. The purpose of the present study, therefore, was to test the hypothesis that enterocyte-specific EGFR signaling is required for intestinal adaptation.
MATERIALS AND METHODS
Experimental design
Protocols for this study were approved by the Washington University Animal Studies Committee (Protocol 20100103) and were in accordance with the National Institute of Health laboratory animal care and use guidelines. Baseline intestinal morphological data was collected from enterocyte EGFR-null mice (n=5) and wild type (WT) littermates (n=5) at 7 and 30 days following mucosal EGFR deletion. Four experimental groups were studied: WT mice that underwent sham operation (n=9) or 50% proximal SBR (n=10), and EGFR-null mice that underwent sham operation (n=8) or 50% proximal SBR (n= 11). Ileal tissue was harvested on post-operative day 7. EGFR deletion was confirmed via Western blotting and RT-PCR of the purified epithelial cells. To assess for adaptation, villus height and crypt depth were measured via hematoxylin and eosin (H&E)-stained histology. Rates of crypt cell proliferation and apoptosis were also recorded.
Animals
Since disrupted EGFR expression in enterocytes has been shown to be embryonic lethal, we used a tamoxifen (TAM)-inducible Villin-Cre/LoxP recombinant system for enterocyte-directed EGFR deletion. EGFR-floxed mice [8] (Egfrtm1Dwt generously provided by David Threadgill, University of North Carolina, Chapel Hill) were crossed with mice harboring a TAM-inducible Cre-fusion protein under control of the villin promoter [9] (vil-Cre ERT2; obtained via generous donation from Sylvie Robine, Curie Institute, Paris, France). Both lines were on a C57BL/6 background. The construct is such that TAM administration causes the deletion of exon 3 in the EGFR gene in VC(+) mice. Mice with both the Cre fusion protein and floxed EGFR alleles (VC(+)/ EGFR(f/f)) were used for the knockout group (VC(+)). Littermates lacking Cre (VC(−)/EGFR(f/f)) were used as wild type (WT) controls. Male and female mice aged 10–12 weeks were used in this study with a weight range of 19.5 to 27.5 g. Mice were kept on a 12-hour light-dark schedule and were housed in a standard facility. Both WT and EGFR-floxed mice were injected with intra-peritoneal TAM (0.5 mg/day, Sigma, St. Louis, MO) for 3 days in a row starting 4 days prior to surgery. The mice were given a liquid rodent diet (Micro-Stabilized Rodent Liquid Diet LD101; Purina Mills, St Louis, MO) 1 day prior to surgery.
Operative technique
Mice underwent 50% proximal SBR or sham operation (transection and reanstomosis only) as previously described [1]. Briefly, mice that underwent SBR had transection of the bowel at a point 12 cm proximal to the ileal-cecal junction and also at a point 1 to 2 cm distal to the ligament of Treitz. The mesentery was ligated and the intervening bowel was removed. Intestinal continuity was restored with an end-to-end anastomosis using 9-0 monofilament suture. In mice undergoing sham operation the bowel was transected at a point 12 cm proximal to the ileal-cecal junction and intestinal continuity was restored with an end-to-end reanastomosis. Following the operation, mice were provided free access to water for the first 24 hours and then given a liquid rodent diet until sacrifice.
Tissue harvest and isolation of epithelial cells
On the seventh postoperative day, the mice were anesthetized with a subcutaneous injection of ketamine, xylazine, and acepromazine (4:1:1). A midline laparotomy was performed and the small bowel was flushed with ice-cold phosphate-buffered saline with protease inhibitors (0.2 mM phenylmethylsulfonyl fluoride, 5 μg/mL aprotinin, 1 mM benzamidine, 1 mM sodium orthovanadate, and 2 μM cantharidin; EMD, Gibbstown, NJ) and excised. The first 1 cm segment of bowel distal to the anastomosis was discarded. The next 2 cm segment of bowel was fixed in 10% neutral-buffered formalin for histology. The subsequent 5 cm of bowel was cut longitudinally and transferred into tubes containing 5 mL of ice-cold PBS with protease inhibitors for one hour at 4°C. Crypt and villus enterocytes were isolated using a calcium chelation, mechanical vibration, and cell straining protocol that we have beforehand described [10].
RT-PCR confirmation of EGFR mRNA knock-out in enterocytes
Total RNA was extracted from frozen isolated crypt and villus enteroocytes and underlying mesenchymal/muscularis fractions following the manufacturer’s protocol for the RNAqueous kit and total RNA concentration determined spectrophotometrically. The quality of the obtained RNA was evaluated using the Bio-Rad Experion System with an RNA StdSens Chip and reagents (Bio-Rad Laboratories, Richmond, CA). The RT2 First Strand Kit (SABioscience, Frederick, MD) was used per the manufacturer’s protocol to produce complementary DNA (cDNA) from the isolated mRNA. Quantification of cDNA was performed using Quanti-iT Oligreen ssDNA Assay Kit (Invitrogen, Carlsbad, CA). Equal amounts of cDNA were used for real-time PCR with B-actin as an endogenous control and a standard whole bowel sample was used as the calibrator. The EGFR gene expression was examined using primers specific for EGFR exon 3 (Forward: TCAACACCGTGGAGAGAATCCCTT, Reverse: TTGCTGAATCGCACAGCACCAATC) with reagents from SABioscience (Frederick, MD) using an Applied Biosystems 750 Fast Real-Time PCR System (Foster City, CA).
Western blot confirmation of EGFR protein deletion in enterocytes
Frozen isolated crypt and villus enterocyte fraction pellets prepared during the epithelial cell isolation above were thawed and reconstituted in Tris buffer. The samples were sonicated for 10 seconds, lysed with sodium dodecyl sulfate sample buffer (50 mmol/L Tris-HCl pH 6.8, 2% sodium dodecyl sulfate, 10% glycerol, and 5% mercaptoethanol), and then heated for 5 minutes at 100°C. The resultant protein concentration was determined using the RC DC kit (Bio-Rad, Hercules, CA) per the manufacturer’s protocol. The proteins were separated on 8% gels and blots probed for EGFR (Millipore, Billerica, MA) and β-Actin (Cell Signaling, Danvers, MA).
Adaptation measurements
Villus height and crypt depth were measured on H&E stained sections by a single investigator blinded to mouse strain or procedure using the image analysis software MetaMorph (Dowington, PA). At least twenty well-oriented villi and crypt per animal were measured.
Crypt cell proliferation
Ninety minutes prior to sacrifice, mice received an intraperitoneal injection of 5-bromodeoxyuridine (BrdU; 0.1 ml/10 gm body weight; Zymed Laboratories Inc., San Francisco CA). Incorporation of BrdU into proliferating crypt cells was detected in paraffin-embedded tissue sections by immunohistochemistry. The number of cells staining positive (incorporating BrdU) per crypt were counted and divided by the total number of cells per crypt to determine the proliferative rate. At least twenty well-oriented crypts were counted per animal by blinded scoring.
Crypt cell apoptosis
H&E stained sections were evaluated for the presence of apoptotic bodies as evidenced by pyknotic nuclei, condensed chromatin, and nuclear fragmentation. An apoptotic index was calculated as the number of apoptotic bodies per 100 crypts. One hundred well-oriented crypts were counted per animal by blinded scoring.
Statistical analysis
Values are presented as mean +/− SEM. The Sigma Stat statistical package (SPSS, Chicago, IL) was used for all statistical analyses. A p value of less than 0.05 was considered significant.
RESULTS
All mice appeared healthy at the time of harvest. Overall survival was 94% for mice undergoing operation. In the WT group, only mice that demonstrated adaptive villus growth after SBR of a magnitude greater than 20% were included for analysis [8]. In addition, one EGFR-null mouse from the sham group was excluded from analysis due to a large degree of deviation in villus height as compared to the remainder of the group.
Confirmation of enterocyte EGFR mRNA and protein deletion
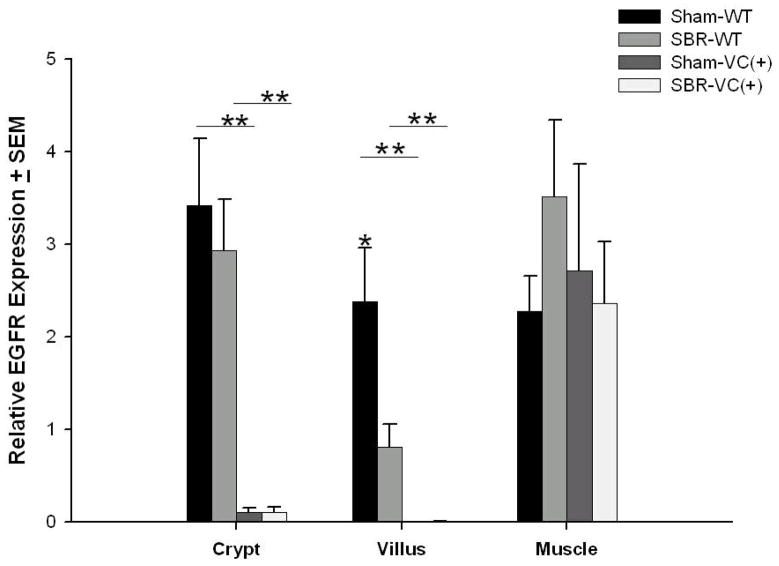
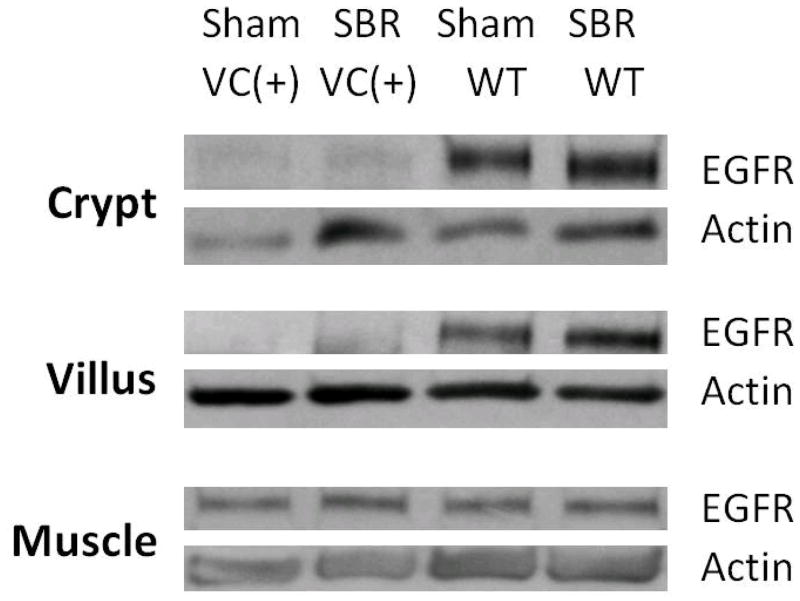
RT-PCR of crypt and villi cell fractions of the enterocyte EGFR-null mice that underwent SBR or sham procedures confirmed deletion of EGFR (Figure 1). The mRNA expression of EGFR was only seen in the WT mice. Preservation of EGFR mRNA expression in the mesenchymal/muscularis layer of all animals was also confirmed. Western blot of crypt and villi cell fractions confirmed deletion of EGFR protein in the enterocyte EGFR-null mice (Figure 2) while expression was confirmed in the crypt and villi cell fractions of WT mice. For all groups, the expected EGFR protein expression in the mesenchymal/muscular layer was identified.
Figure 1. Successful disruption of EGFR mRNA expression in enterocytes.
TaqMan real-time PCR for EGFR mRNA demonstrates nearly complete reduction in mRNA levels in crypt and villus enterocytes in EGFR null (VC(+)) compared to wild-type (WT) mice. The expression of EGFR within the underlying mesenchyme/smooth muscle layer was equivalent for all mice confirming that the EGFR gene deletion was successfully targeted to the intestinal mucosa. N=6–11 per group, *= p < .05, ** = p < .001.
Figure 2. Western blots confirming disrupted EGFR protein expression.
Western Blots for EGFR protein demonstrate minimal expression in crypt and villus enterocytes from EGFR null mice (VC(+)) compared to wild-type (WT) controls. Actin was used as a loading control. EGFR protein expression is equivalent for mice in both groups in the mesenchyme/smooth muscle layer confirming that the EGFR gene deletion was successfully targeted to the intestinal mucosa.
EGFR-null phenotype
Enterocyte-targeted EGFR deficiency induced in adult mice resulted in no demonstrable phenotype at 7 or 30 days after TAM injection. The EGFR-null mice demonstrated no difference in total body weight or weight change 30 days post TAM, as compared to WT. At 7 days post-TAM, villus heights were equivalent (182.2 +/− 12.4 EGFR-null vs 190.3 +/− 2.4 WT, p 0.54) as were crypt depths (97.9 +/− 9.7 EGFR-null vs 101.8 +/− 6.4 WT, p 0.74). A similar equivalency was seen at 30 days; villus heights (201.8 +/− 8.1 EGFR-null vs 197.1 +/− 15.1 WT, p 0.93) and crypt depths (98.4 +/− 9.4 EGFR-null vs 106.1 +/− 6.8 WT, p 0.25) were indistinguishable between groups. The total length of the bowel between the two groups was also equivalent. RT-PCR and Western blot confirmed deletion of EGFR in the crypt and villi cells isolated from these mice both at 7 and 30 days following TAM treatment.
Intestinal Adaptation in Enterocyte-directed EGFR-null Mice
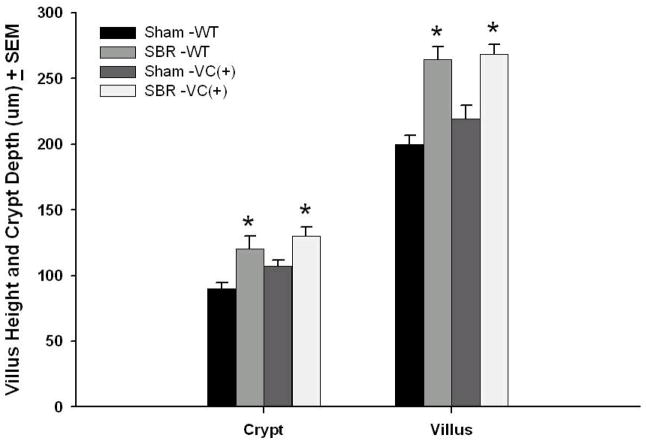
Adaptation occurred normally in both the WT and EGFR-null mice that underwent SBR (Figure 3). The difference in villus height and crypt depth between WT and EGFR-nulls after SBR was insignificant, consistent with equivalent degrees of morphological adaptation between these two groups.
Figure 3. Crypt depth and Villus height dimensions.
Wild type (WT) and EGFR-null mice (VC(+)) underwent either 50% proximal small bowel resection (SBR) or sham operation (transection and reanastomosis of the bowel alone). On post operative day 7, the ileum was harvested and crypt depth and villus height were measured from H&E-stained sections. N=6–11 per group, * = p < . 05.
Crypt Cell Proliferation in Enterocyte-directed EGFR-null Mice
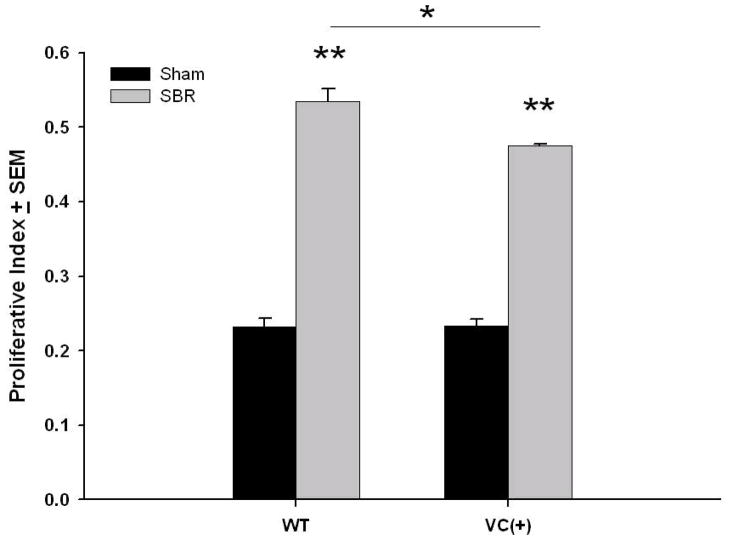
Crypt enterocytes demonstrated a significantly greater proliferation for both WT and EGFR null (VC (+)) mice after SBR (Figure 4). While the proliferation index for both sham groups was equivalent, a small but significantly lower proliferation rate was noted for EGFR null SBR mice when compared with WT SBR.
Figure 4. Crypt cell proliferation.
Wild type (WT) and EGFR-null mice (VC(+)) underwent either 50% proximal small bowel resection (SBR) or sham operation (transection and reanastomosis of the bowel alone). On post operative day 7, the ileum was harvested and number of BrdU-immunostained and total number of crypt cells were both recorded and a proliferative index calculated from the ratio of the two measurements. N=6 per group, *= p < .05, ** = p < .001.
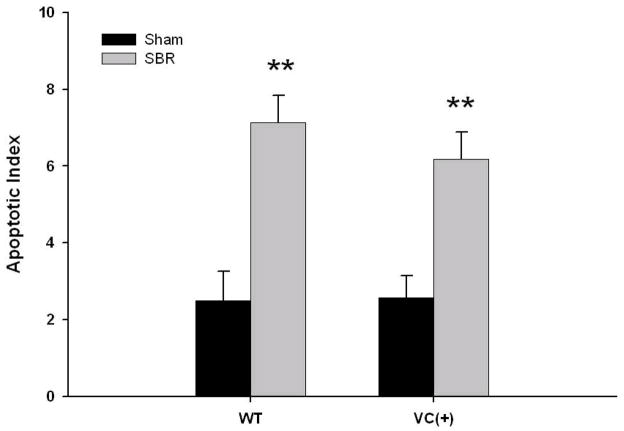
Rates of Apoptosis in Enterocyte-directed EGFR-null Mice
The apoptotic index presents a similar pattern as observed for proliferation (Figure 5). In both groups of mice, the apoptotic index was significantly greater after SBR versus their sham counterpart. There was a small, but statistically insignificant lower rate of apoptosis observed after SBR when comparing the EGFR null mice with WT animals.
Figure 5. Crypt cell apoptosis.
Wild type (WT) and EGFR-null mice (VC(+)) underwent either 50% proximal small bowel resection (SBR) or sham operation (transection and reanastomosis of the bowel alone). On post operative day 7, the ileum was harvested and apoptotic bodies were counted in H&E sections of ileum. An apoptotic index was derived from the number of apoptotic bodies per 100 crypts. N=6-11 per group, ** = p < .005.
DISCUSSION
The role of EGF and EGFR in the pathogenesis of adaptation has previously been established. Systemic EGF stimulation enhances adaptation while global models of perturbed EGFR activity results in attenuated adaptation responses [1–7]. In the present study, we selectively inactivated the expression of EGFR in the enterocytes of the adult mouse intestine in order to study the effects of mucosal EGFR deletion in both unoperated and post-resection conditions. Under normal (unoperated) conditions mice with enterocyte EGFR deletion demonstrated no change in phenotype as compared to WT littermates. Surprisingly, enterocyte-targeted EGFR ablation resulted in no difference obvious in adaptation potential when compared to WT littermates. Thus, despite significant data that impaired EGF signaling impairs adaptation, this study demonstrates that the enterocyte expression of EGFR is not required for normal resection-induced intestinal adaptation.
For this study, we developed an inducible, enterocyte-specific EGFR deletion mouse model, since germline EGFR deletion is associated with embryonic lethality [12]. Mice with global EGFR deletion that survive to delivery die within 8 days of birth with dark-colored, dilated, hemorrhagic intestinal loops and abdominal free fluid, with a condition resembling necrotizing enterocolitis [12]. These intestinal effects are likely to be strain-dependent [13, 14]. Waved-2 mice, with global diminished EGFR activity, demonstrate skin and eye abnormalities [15], and have been shown to have markedly increased susceptibility to dextran-sulfate induced colitis [16]. Notably, mucosal deletion of EGFR in our adult mouse model resulted in a normal phenotype. Mice followed for up to 30 days after disrupted EGFR expression appeared healthy with a standard body weight, uncharacteristic appearing intestine, and normal villus and crypt architecture. It is possible that functional overlap of the family of ErbB receptors may account for the normal phenotype observed in these mice. As such, ErbB 2, 3, and 4 may compensate for the deletion of EGFR (ErbB1) within the enterocytes. Along these lines, simultaneous deletion of several EGFR ligands including transforming growth factor alpha, amphiregulin, and EGF results in only a modest phenotype, with viable and fertile mice that display growth retardation, increased incidence of mucosal ulceration, shortened villi, and decreased crypt DNA synthesis [17]. Alternatively, EGFR signaling from compartments of the bowel other than the enterocytes may be responsible for maintenance of normal intestinal epithelium. Finally, enterocyte EGFR may be crucial for embryonic and neonatal gut development, but obsolete for normal homeostasis within the adult mouse intestinal mucosa.
We were surprised by our findings that EGFR-null mice adapted normally after SBR since all prior models of global EGFR inhibition have resulted in attenuated adaptation responses. Removal of the major endogenous source of EGF via sialoadenectomy resulted in a diminished increase in villus height, total protein, and DNA content following SBR [5]. Administration of EGFR inhibitors prevented increases in villus height and crypt depth as well as cell proliferation following SBR [6]. Further, waved-2 mutant mice with generalized perturbed EGFR activity, display reduced adaptation following SBR. Waved-2 mice have a slight increase in villus height and proliferation after SBR, however not to the level of WT controls [7]. Waved-2 mice also demonstrate significantly increased rates of apoptosis after SBR as compared to WT control [18]. In vitro treatment of intestinal epithelial cells (RIEC-18) with EGFR inhibitors and EGFR small interfering RNA knockdown both result in increased apoptosis [19].
Several factors may explain the normal adaptation observed in the enterocyte EGFR-null mice following SBR. As noted above, EGFR (ErbB1) is one of a family of 4 ErbB receptors and possible redundancy of function may exist. While EGF will only bind to the EGFR, it is also plausible that other growth factors preserve the effects of EGF following SBR. It must be considered that adaptation is regulated by EGFR signaling in cells other than the enterocytes, perhaps within the mesenchymal or muscularis compartments of the bowel wall via factor(s) that are presently unknown. Consistent with this notion is that former work from our lab has revealed a 3-fold increase in EGFR expression in the muscularis compartment of the bowel following SBR [20]. It is possible that EGFR signaling within the muscularis layer following SBR results in signaling to enterocytes that drives the increased villus height and crypt depth adaptation response.
In summary, despite significant evidence that perturbed EGFR activity attenuates intestinal adaptation following SBR, this study demonstrates for the first time that enterocyte EGFR expression is not required for resection-induced adaptation. This finding illustrates the complexity of intestinal adaptation in response to SBR. It is unlikely that a single gene or receptor accounts entirely for the process of adaptation, but rather the process is intricate and multifactorial. While the exact cellular compartment of the EGFR responsible for observed adaptation changes established by enterocytes remains elusive, the use of an inducible, cell-compartment-specific knock out model enables a more focused understanding for the role of EGFR signaling in this important response.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 DK 059288 (Wandu, Erwin, and Warner), T32 CA009621 (Wakeman, McMellen, and Rowland),and P30DK52574 - Morphology and Murine Models Cores of the Digestive Diseases Research Core Center of the Washington University School of Medicine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Helmrath MA, VanderKolk WE, Can G, et al. Intestinal adaptation following massive small bowel resection in the mouse. J Am Coll Surg. 1996;183:441–449. [PubMed] [Google Scholar]
- 2.Chaet MS, Arya G, Ziegler MM, et al. Epidermal growth factor enhances intestinal adaptation after massive small bowel resection. J Pediatr Surg. 1994;29:1035–1038. doi: 10.1016/0022-3468(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 3.Shin CE, Helmrath MA, Falcone RA, et al. Epidermal growth factor augments adaptation following small bowel resection: optimal dosage, route and timing of administration. J Surg Res. 1998;77:11–16. doi: 10.1006/jsre.1998.5336. [DOI] [PubMed] [Google Scholar]
- 4.Erwin CR, Helmrath MA, Shin CE, et al. Intestinal overexpression of EGF in transgenic mice enhances adaptation after small bowel resection. Am J Physiol. 1999;277:G535–540. doi: 10.1152/ajpgi.1999.277.3.G533. [DOI] [PubMed] [Google Scholar]
- 5.Helmrath MA, Shin CE, Fox JW, et al. Adaptation after small bowel resection is attenuated by sialoadenectomy: the role for endogenous epidermal growth factor. Surgery. 1998;124(5):848–854. [PubMed] [Google Scholar]
- 6.O’Brien DP, Nelson LA, Williams JL, et al. Selective inhibition of the epidermal growth factor receptor impairs intestinal adaptation after small bowel resection. J Surg Res. 2002;105(1):25–30. doi: 10.1006/jsre.2002.6440. [DOI] [PubMed] [Google Scholar]
- 7.Helmrath MA, Erwin CR, Warner BW. A defective EGF-receptor in waved-2 mice attenuates intestinal adaptation. J Surg Res. 1997;69:76–80. doi: 10.1006/jsre.1997.5033. [DOI] [PubMed] [Google Scholar]
- 8.Lee TC, Threadgill DW. Generation and validation of mice carrying a conditional allele of the epidermal growth factor receptor. Genesis. 2009;47:85–92. doi: 10.1002/dvg.20464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.el Marjou F, Janssen KP, Chang BH, et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39:186–193. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 10.Guo J, Longshore S, Nair R, et al. Retinoblastoma protein (pRb), but not p107 or p130, is required for maintenance of enterocyte quiescence and differentiation in small intestine. J Biol Chem. 2009;284(1):134–140. doi: 10.1074/jbc.M806133200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor JA, Martin CA, Nair R, et al. Lessons learned: optimization of a murine small bowel resection model. J Pediatr Surg. 2008;43:1018–1024. doi: 10.1016/j.jpedsurg.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miettinen PJ, Berger JE, Meneses J, et al. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995;376(6538):337–341. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- 13.Sibilia M, Wagner EF. Strain-dependent epithelial defects in mice lacking the EGF receptor. Science. 1995;269(5221):234–238. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- 14.Threadgill DW, Dlugosz AA, Hansen LA, et al. Targeted disruption of the mouse EGF receptor; effect of genetic background on mutant phenotype. Science. 1995;269(5221):230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 15.Luetteke NC, Phillips HK, Qiu TH, et al. The mouse waved-2 phenotype results from a point mutation in the EGF receptor kinase. Genes Dev. 1994;8:399–413. doi: 10.1101/gad.8.4.399. [DOI] [PubMed] [Google Scholar]
- 16.Egger B, Buchler MW, Lakshmanan J. Mice harboring a defective epidermal growth factor receptor (waved-2) have an increased susceptibility to acute dextran sulfate-induced colitis. Scand J Gastroenterol. 2000;35(11):1181–1187. doi: 10.1080/003655200750056664. [DOI] [PubMed] [Google Scholar]
- 17.Troyer KL, Luetteke NC, Saxon ML, et al. Growth retardation, duodenal lesions, and aberrant ileum architecture in triple null mice lacking EGF, amphiregulin, and TGF-alpha. Gastroenterology. 2001;121(1):68–78. doi: 10.1053/gast.2001.25478. [DOI] [PubMed] [Google Scholar]
- 18.Knott AW, Juno RJ, Jarboe MD, et al. EGF receptor signaling affects bcl-2 family gene expression and apoptosis after massive small bowel resection. J Pediatr Surg. 2003;38(6):875–880. doi: 10.1016/s0022-3468(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 19.Sheng G, Guo J, Warner BW. Epidermal growth factor receptor signaling modulates apoptosis via p38alpha MAPK-dependent activation of Bax in intestinal epithelial cells. Am J Physiol Gastrointest Physiol. 2007;293(3):G599–606. doi: 10.1152/ajpgi.00182.2007. [DOI] [PubMed] [Google Scholar]
- 20.Knott AW, Erwin CR, Profitt SA, et al. Localization of postresection EGF receptor expression using laser capture microdissection. J Pediatr Surg. 2003;38(3):440–445. doi: 10.1053/jpsu.2003.50076. [DOI] [PubMed] [Google Scholar]