SUMMARY
Initiation of pancreatic ductal adenocarcinoma (PDA) is definitively linked to activating mutations in the KRAS oncogene. However, PDA mouse models show that mutant Kras expression early in development gives rise to a normal pancreas, with tumors forming only after a long latency or pancreatitis induction. Here we show that oncogenic KRAS upregulates endogenous EGFR expression and activation, the latter being dependent upon the EGFR ligand sheddase, ADAM17. Genetic ablation or pharmacological inhibition of EGFR or ADAM17 effectively eliminates KRAS-driven tumorigenesis in vivo. Without EGFR activity, active RAS levels are not sufficient to induce robust MEK/ERK activity, a requirement for epithelial transformation.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDA) is almost universally fatal, but its remarkable genetic homogeneity has aided greatly in our understanding of its genesis and progression. Most PDA samples harbor oncogenic mutations in the KRAS gene, from early pancreatic intraepithelial neoplasia (PanIN) to PDA, marking mutant KRAS as the most prominent PDA initiating gene. Pancreas-specific mutant Kras expression in mice results in mouse PanIN (mPanIN) formation, eventually leading to PDA. Strikingly, even with universal expression of mutant Kras in early organogenesis, the pancreas develops normally, giving rise to a functional, tumor-free pancreas with preneoplastic lesions and mPanINs forming stochastically only after several weeks (Hingorani et al., 2003). Consistent with this, oncogenic KRAS mutations are found in human pancreata with no signs of PDA (Luttges et al., 1999). Together, these observations suggest that expression of mutant Kras requires ill-defined secondary events to initiate pancreatic tumorigenesis.
The ductal nature of PanIN and PDA suggests their derivation via transformation of normal duct epithelium or of progenitor cells capable of assuming a ductal morphology. Confounding this hypothesis, KrasG12D expression directed to specific cellular compartments has shown that duct, islet and acinar cells can all give rise to mPanIN lesions (Gidekel Friedlander et al., 2009), but expression in the adult acinar or islet cell compartments requires pancreatitis induction (Carriere et al., 2009; Gidekel Friedlander et al., 2009; Guerra et al., 2007). This acquired sensitivity to transformation is attributed to acinar cell transdifferentiation to metaplastic ducts, which have progenitor-like characteristics (Miyamoto et al., 2003; Sharma et al., 1999) that may make them more susceptible to KRAS-induced oncogenesis. Consistent with this hypothesis, Hebrok and colleagues have shown that KRASG12D expression hijacks the regeneration process after tissue damage, promoting the metaplasia-to-PanIN transition (Morris et al., 2010).
Aberrant signal transduction pathways that control acinar-to-ductal metaplasia (ADM) are under intense study. Examination of chronic pancreatitis (CP) and PDA patient samples has shown an upregulation of epidermal growth factor receptor (EGFR, ERBB1) (Fjallskog et al., 2003; Korc et al., 1994; Tobita et al., 2003) and several of its ligands (Kobrin et al., 1994; Zhu et al., 2000). The relevance of this correlation is bolstered by the induction of metaplasia and desmoplasia in vivo by transgenic EGFR ligand overexpression (Means et al., 2003; Sandgren et al., 1990). In vitro, treatment of acinar cell explants with EGFR ligands, such as transforming growth factor alpha (TGFA), results in ADM (De Lisle and Logsdon, 1990; Means et al., 2005). Lineage tracing experiments confirm that metaplastic ducts arise in part from acinar cell transdifferentiation in response to tissue injury (Strobel et al., 2007), supporting a physiological relevance to acinar cell transdifferentiation. However, it is not known whether endogenous ligand activation of EGFR plays a role in ADM in pancreatic disease.
Taking advantage of the reproducible kinetics of tumor formation in PDA mouse models, we set out to address the contribution of EGFR activity to disease progression using genetic and pharmacological approaches.
RESULTS
EGFR Pathway Upregulation Precedes Tumorigenesis in KrasG12D mice
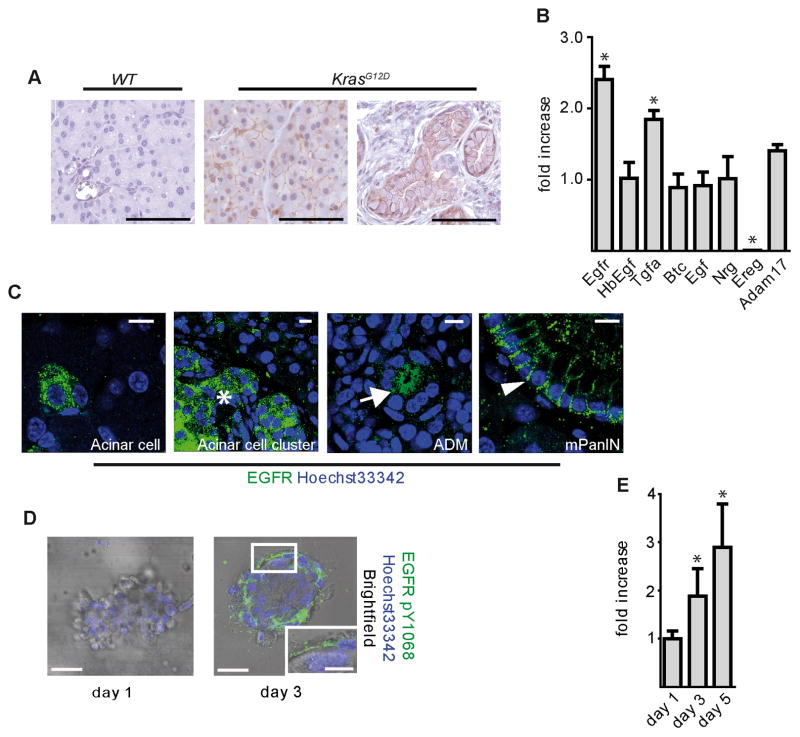
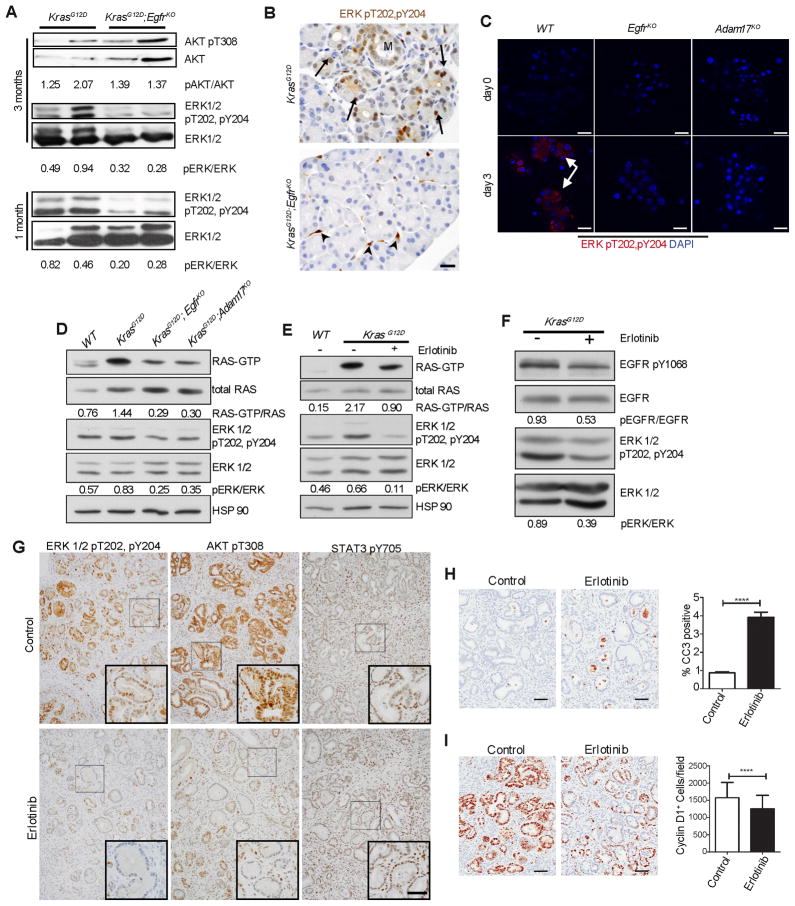
EGFR is upregulated in PDA and PDA mouse models (Fjallskog et al., 2003; Korc et al., 1994; Tobita et al., 2003), Figure S1) though its function primarily has been associated with enhanced proliferation and invasiveness (Jaganathan et al., 2010; Larbouret et al., 2007; Pino et al., 2006; Zhao et al., 2010). To better dissect the role of EGFR in PDA progression, we tested if EGFR was activated in the KrasLSL-G12D/+;Ptf1aCre/+ mouse model (referred to henceforth as KrasG12D), which reproducibly shows metaplasia and mPanIN formation beginning at ~8 weeks of age, with progression to PDA at ~1 year (Hingorani et al., 2003). Immunohistochemistry (IHC) for active EGFR (pY1068) was undetectable in wild-type pancreata, but easily detectable in acinar areas prior to mPanIN formation in 30 day old KrasG12D mice and in mPanINs in 3 month old KrasG12D mice (Figure 1A). To test if EGFR itself was upregulated, we analyzed mRNA isolated from 6 week old KrasG12D pancreata by qRT-PCR, a time prior to significant metaplasia or neoplasia. Transcripts for both EGFR and TGFA, an EGFR ligand, were consistently upregulated ~2-fold (Figure 1B). Amphiregulin (AREG), another EGFR ligand, was also upregulated relative to wild-type controls, which had undetectable AREG levels (data not shown). Immunofluorescence staining (IF) for total EGFR showed upregulation in discrete acinar cell clusters in KrasG12D pancreata (Figure 1C), becoming very prominent in larger acinar clusters, especially near areas of metaplasia and mPanIN, and was particularly high in metaplasia and mPanINs. Thus, EGFR pathway upregulation is a very early event in pancreatic tumorigenesis. Moreover, the stochasticity of EGFR overexpression in acini prior to mPanIN formation was reflected the pattern of tumor formation, suggesting a role for EGFR signaling in transformation of the acinar cell compartment.
Figure 1. EGFR signaling during mPanIN development.
(A) IHC for EGFR pY1068 in wild-type and KrasG12D pancreata. Scale bars = 50 μm. (B) qRT-PCR analysis for Egfr, its ligands and Adam17. Error bars are +/− SEM. (n = 3, * p < 0.05). (C) Confocal IF staining for total EGFR in distinct single acinar cells, acinar cell clusters (*), ADM (arrow), and mPanINs (arrowhead) of KrasG12D pancreata. Scale bars = 10 μm. (D) IF for EGFR pY1068 and (E) qRT-PCR analysis of Egfr in KrasG12D acinar cell explants. Scale bars = 10 μm for micrographs, 50 μm for inset. Error bars are +/− SEM (n = 3, * p < 0.05). See also Figure S1.
To test if acinar cell EGFR activation coincided with ductal transdifferentiation, we examined primary acinar cell explants isolated from KrasG12D mice, which spontaneously transdifferentiate into duct cells when embedded in fibrillar collagen. On day 1 of culture, active pY1068 EGFR was undetectable (Figure 1D), but was strongly positive by day 3, as transdifferentiation took place. Activation correlated with increased EGFR expression, as determined by qRT-PCR (Figure 1E). Thus, EGFR upregulation and activation is initiated by KRAS in vitro and in vivo in a manner consistent with its involvement in preneoplastic duct formation.
Inhibition of EGFR limits pancreatic tumorigenesis but not progression
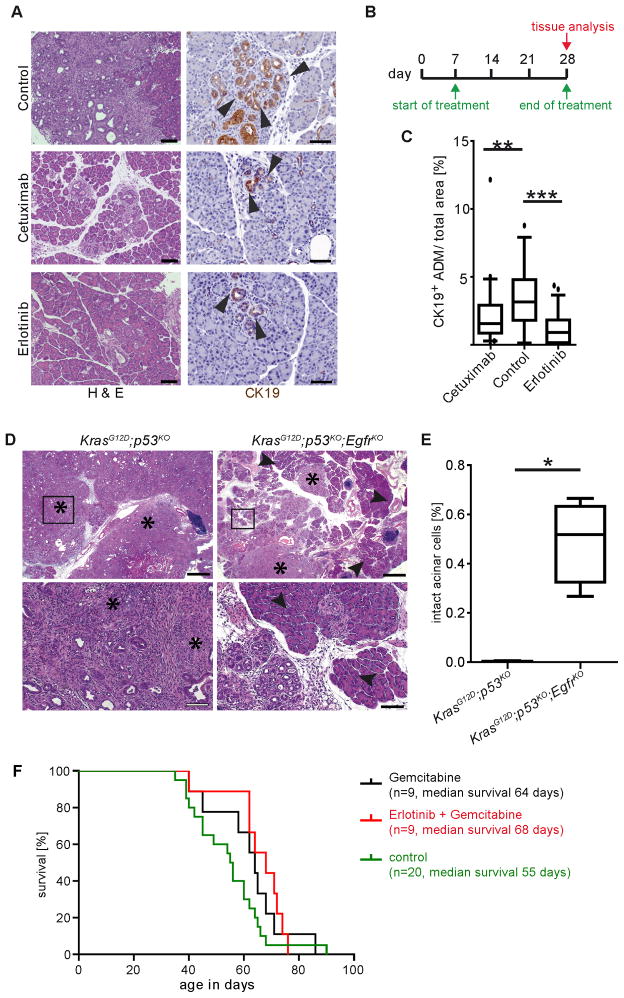
To test if EGFR activity is required for pancreatic preneoplastic lesion formation, we examined the effects of pharmacological EGFR inhibition in a highly aggressive PDA model. KrasLSL-G12D/+;Ptf1aCre/+;Trp53fl/fl mice, referred to as KrasG12D;p53KO, develop invasive PDA at 4–6 weeks of age (Bardeesy et al., 2006). Starting at 1 week of age, KrasG12D;p53KO mice were treated daily with either cetuximab, a monoclonal antibody that blocks ligand interaction with the receptor; erlotinib, a small molecule EGFR tyrosine kinase inhibitor; or vehicle for 3 weeks. Histological examination showed substantial areas of normal, non-transformed tissue with either treatment, and a significantly reduced number of CK19+ ductal lesions compared to control (Figure 2A–C).
Figure 2. Inhibition of EGFR signaling blocks KRAS-driven tumorigenesis but not PDA progression.
(A) H&E and CK19 IHC of 4 week old KrasG12D;p53KO control, cetuximab and erlotinib treated pancreata. Arrowheads indicate CK19+ structures. Scale bars = 100 μm for H&E and 50 μm for CK19 panels. (B) Schematic of cetuximab and erlotinib treatment protocols. (C) Quantitation of CK19+ structures (n = 3, ** p < 0.01, *** p < 0.001). (D) Histology of 9 week old KrasG12D;p53KO and KrasG12D;p53KO;EgfrKO pancreata. Asterisks indicate areas of invasive PDA. Arrowheads highlight areas of normal acinar tissue. Scale bars, upper panels = 200 μm, lower panels = 50 μm. (E) Quantitation of normal acinar area in KrasG12D;p53KOand KrasG12D;p53KO;EgfrKO pancreata. (F) Kaplan-Meier curve depicting survival of KrasG12D;p53KO mice treated with gemcitabine or erlotinib plus gemcitabine after progression to PDA. See also Figure S2.
Retention of substantial areas of normal tissue with EGFR inhibition supported a role for EGFR signaling in tumor initiation. To test this possibility definitively, we mated KrasG12D;p53KO mice with conditional Egfr knockout mice (Lee and Threadgill, 2009; Natarajan et al., 2007). Mice with EGFR ablated from the pancreas (Egfrfl/fl; Ptf1aCre/+, referred to as EgfrKO) were viable and showed no gross pancreatic abnormalities. Consistent with the inhibitor experiments, KrasG12D;p53KO; EgfrKO pancreata retained substantial areas of normal acinar tissue, whereas age-matched KrasG12D;p53KO pancreata were completely replaced by tumorous tissue (Figure 2D&E). IHC for EGFR confirmed that ~50% of mPanINs that formed in these mice were EGFR negative (Figure S2A), with the other half likely a product of incomplete recombination of the Egfr locus. To gain insight into the escape from EGFR dependency, we established cell lines from KrasG12D;p53KO;EgfrKO pancreata. Confirming EGFR loss by western blot, we assessed other signaling and differentiation changes compared to EGFR wild-type controls. Two pathways in particular showed consistent upregulation and activation in the absence of EGFR: the Notch pathway and MET, both of which have been implicated in pancreatic tumor progression (Figure S2B), most notably in the ADM process (Means et al., 2005; Miyamoto et al., 2003).
EGFR inhibition has been shown to be minimally effective in PDA patients. To test if the KrasG12D;p53KO model reflected the clinical data, we treated mice with MRI-detectable advanced PDA with gemcitabine or erlotinib plus gemcitabine. Combination-treated mice showed no significant difference in lifespan compared to gemcitabine treatment alone (Figure 2F) and showed no reduction in tumor burden by MRI, consistent with independence from EGFR after progression to PDA.
EGFR inhibition most clearly affected pancreatic tumorigenesis, leading us to explore its effects more thoroughly in the slower-progressing KrasLSL-G12D/+ model. Tumor burden of KrasG12D and Egfr knockout KrasG12D mice (KrasG12D;EgfrKO) was assessed at various ages by relative pancreatic mass, histology, loss of normal acinar (amylase+) tissue and appearance of MUC5AC+ ductal lesions (Figures 3A–C and S3A&B). By all criteria, Egfr ablation almost completely blocked tumorigenesis. Unlike KrasG12D;p53KO;EgfrRKO mice, all metaplasia and mPanIN that formed in KrasG12D;EgfrKO mice were EGFR positive (Figure S3D), suggesting incomplete recombination of the Egfr locus and reinforcing the need for EGFR in tumorigenesis. These data also suggested that p53 inactivation itself allows for escape from EGFR dependency. The tumorigenesis block was not due to a failure to recombine the silenced KrasG12D allele (Figure S3E).
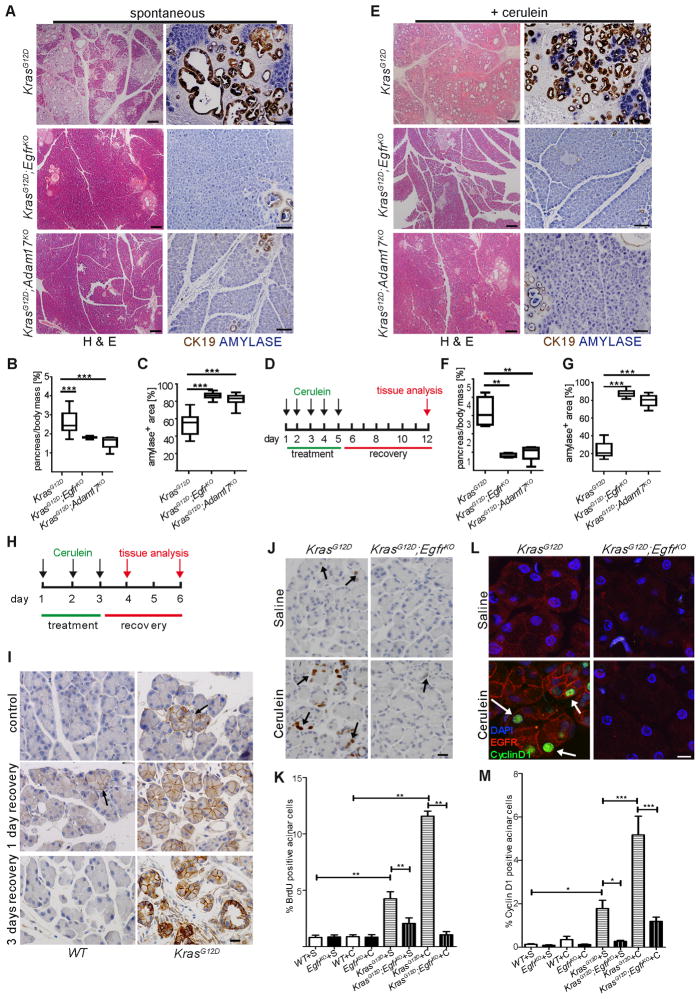
Figure 3. Genetic ablation of EGFR activity prevents KRAS-driven PDA development.
(A–C) Spontaneous and (D–G) cerulein-induced mPanIN formation in KrasG12D;EgfrKO and KrasG12D;Adam17KO mice compared to KrasG12D controls, shown by H&E (left panels, scale bars = 200 μm) and dual IHC for CK19 (brown) and amylase (blue) (right panels, scale bars = 100 μm). Quantitation of pancreas-to-body weight ratios (B&F) and amylase positive area (C&G) (n > 6, ** p < 0.01, *** p < 0.001) compared to control. (H) Truncated cerulein treatment protocol for analysis of premetaplastic signaling. (I) EGFR IHC in saline-treated (control) and cerulein treated WT (left panels) and KrasG12D (right panels) pancreata with 1 day and 3 days recovery. Arrows indicate focal areas of high EGFR expression. n = 3, scale bars = 20 μm. (J) BrdU incorporation measured by IHC in acinar cells of saline or cerulein-treated KrasG12D and KrasG12D;EgfrKO pancreata with 3 days recovery. Arrows indicate positive acinar nuclei. Scale bar = 20 μm (K) Counts of BrdU+ acinar cells, n=3, **p<0.01. (L) Co-IF for CyclinD1 (green) and EGFR (red) in saline or cerulein-treated KrasG12D and KrasG12D;EgfrKO. Arrows indicate some positive acinar nuclei. Scale bar = 10 μm (M) Quantitation of CyclinD1 expression (*p<0.05,***p<0.001, all other differences were not significant. n=3). Error bars in all panels are +/− SEM. See also Figure S3.
Coordinated upregulation of Tgfa, Areg and Egfr in KrasG12D pancreata, together with the effectiveness of cetuximab, suggested that receptor ligation is a necessary step in pancreatic tumorigenesis. EGFR ligands are generally shed from the cell surface by metalloproteinase “sheddases”, but can also activate receptor in their membrane-bound forms in a juxtacrine manner (Singh and Harris, 2005). TGFA and AREG share a common primary sheddase in ADAM17 (Sahin et al., 2004). To test if EGFR ligand shedding was necessary for tumorigenesis, we crossed a conditional Adam17 knockout mouse line (Adam17ΔEx5/ΔEx5) into the KrasG12D background (KrasG12D;Adam17KO). Pancreas-specific Adam17 knockout mice (Adam17ΔEx5/ΔEx5;Ptf1aCre/+, referred to as Adam17KO) were viable and showed no gross pancreatic abnormalities. To assess tumor formation in the KrasG12D;Adam17KO background, we aged mice and examined their pancreata for tumor development. Indeed, KrasG12D;Adam17KO mice phenocopied KrasG12D;EgfrKO mice, showing a similar degree of protection from tumorigenesis (Figures 3A–C and S3A&C), with no effect on KrasG12D recombination (Figure S3E). These results are consistent with EGFR activation by ADAM17-dependent ligand shedding being a necessary step in KRAS-driven pancreatic tumorigenesis.
Pancreatitis-associated Tumorigenesis Requires EGFR and ADAM17
Oncogenic Kras expression confined to acinar or islet cell compartments, requires pancreatitis for PDA formation (Gidekel Friedlander et al., 2009; Guerra et al., 2007). EGFR overexpression in KrasG12D acinar cells suggested that EGFR ablation blocks transformation of this cellular compartment. To test if EGFR activity is required for pancreatitis-dependent, acinar cell-derived tumorigenesis, we treated 30 day old KrasG12D, KrasG12D;EgfrKO and KrasG12D;Adam17KO mice with 250 μg/kg cerulein, a known inducer of pancreatic damage, daily for 5 days, followed by 7 days recovery. With cerulein treatment, KrasG12D mice showed an almost complete replacement of normal pancreatic tissue with fibrotic, inflamed tissue and the majority of epithelia replaced by metaplasia and mPanIN. Both KrasG12D;EgfrKO and KrasG12D;Adam17KO mice were almost completely protected from this dramatic transition (Figure 3D–G).
Noting the earlier correlation between the patterns of EGFR overexpression and spontaneous tumor formation, we tested if cerulein treatment affected EGFR expression. Using a truncated cerulein treatment protocol (Figure 3H), we examined EGFR expression prior to rampant epithelial morphogenesis (Figure 3I). In 34 day old saline-treated controls, EGFR was minimally expressed in wild-type pancreata. As noted previously, in KrasG12D controls, EGFR was elevated in discrete acinar clusters (1.3% of acinar cells). In contrast, 3 days of cerulein treatment in wild-type mice induced EGFR in discrete acini (<1% of total) after 1 day recovery, which returned to baseline levels with 3 days recovery. Strikingly, cerulein treatment of KrasG12D mice induced very high EGFR expression in 73% of acinar cells with one day recovery, which was sustained after 3 days recovery and elevated further in metaplasia that had begun to form. Once again, EGFR overexpression in acinar cells reflected the pattern of the eventual tumor formation in this model.
The striking upregulation of EGFR in acinar cells suggested that this cellular compartment is prominently affected by its activity. Besides inducing acinar cell transdifferentiation, EGFR is known to both protect cells from apoptotic cell death and to induce proliferation. Cleaved caspase-3 IHC showed no significant upregulation of apoptosis in 6 week old KrasG12D pancreata (data not shown). In contrast, proliferation, as measured by BrdU incorporation, increased ~4 fold in KrasG12D acinar cells compared to wild-type, with this increase reduced by half in KrasG12D;EgfrKO acinar cells (Figure 3J&K). This difference was exaggerated in cerulein-treated mice, where >10% of acinar cells in KrasG12D mice incorporated BrdU, with no increase under the same conditions in KrasG12D;EgfrKO pancreata. Consistent with this, CyclinD1 was upregulated in acinar cells of KrasG12D and cerulein-treated KrasG12D mice, but remained unchanged in KrasG12D;EgfrKO pancreata with or without cerulein (Figure 3L&M).
EGFR activity is required for metaplastic duct formation
The dependency of acinar cell derived pancreatic tumorigenesis on pancreatitis has been attributed to the need for ADM prior to transformation (Gidekel Friedlander et al., 2009). Chronic activation of EGFR is sufficient to induce ADM in vitro (De Lisle and Logsdon, 1990) and in vivo (Sandgren et al., 1990). To test if EGFR signaling is necessary for this process, we employed several in vivo and in vitro models of ADM. Chronic overexpression of the EGFR ligand TGFA in the Ela-Tgfa transgenic model induces substantial ductal metaplasia and fibrosis after several weeks of transgene expression (Sandgren et al., 1990). Ablation of either Egfr or Adam17 effectively eliminated TGFA-induced metaplasia in vivo even at one year of age (Figure S3F), reinforcing the necessity for ligand shedding in the ADM process.
Turning to metaplasia associated with experimental pancreatitis, we employed a cerulein treatment protocol that produced a chronic-pancreatitis-like phenotype in wild-type mice, marked by replacement of acinar tissue with metaplastic ducts and a reactive and inflamed stroma (Figure S3G). Both EgfrKO and Adam17KO mice were strongly protected from most aspects of pancreatitis, including ADM and the parallel stromal response. Quantitation of inflammatory cell infiltration into the damaged tissue confirmed a significant loss of this response (Figure S3H&I). Protein arrays revealed that several cytokines were significantly lower in cerulein treated EgfrKO pancreata compared to wild-type, including RANTES, IL16, CXCL13 and IL23 (data not shown). However, the common expression of each of these factors by reactive inflammatory and other stromal cells suggested that these lower levels were possibly a secondary effect of the reduction in inflammatory infiltrates. Two pro-inflammatory proteins that were definitively produced by the metaplastic epithelia, as well as the stroma, were COX-1 and COX-2 (Figure S3J), suggesting that the inability of EgfrKO pancreata to form metaplastic ducts contributes to the reduced inflammatory response.
The block in the pancreatitis phenotype was not due to gross loss of cerulein-induced signal transduction. When treated with cerulein to induce acute pancreatitis, mice of all genotypes showed a gain of pancreatic wet weight associated with edema, increased serum amylase levels, areas of tissue necrosis and induction of acinar cell SOX9 expression (Figure S3K–N).
We then tested if in vitro ADM required EGFR activation. In vitro ADM in collagen-embedded acinar cell explants is usually induced by addition of ectopic EGFR ligand. To more closely simulate the in vivo animal models, and to bypass direct EGFR stimulation, we chose to induce in vitro ADM either by expression of KrasG12D or by addition of low concentrations of cerulein into the culture medium.
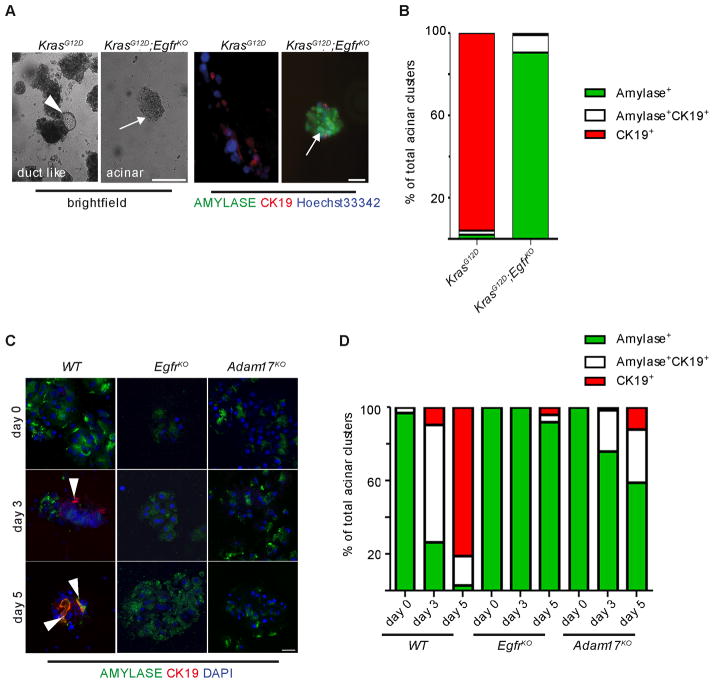
Acinar cell explants derived from KrasG12D mice transdifferentiated spontaneously in culture within 3 days, as determined by coimmunofluorescence for the acinar and duct cell markers, amylase and CK19, respectively. This transition was almost entirely absent in explants derived from KrasG12D;EgfrKO mice (Figure 4A&B), arresting at a nestin-positive intermediate stage (Miyamoto et al., 2003); Figure S4A&B).
Figure 4. EGFR signaling is required for acinar transdifferentiation in three dimensional primary culture.
(A) Light microscopy (left panels) and co-IF for amylase (green) and CK19 (red, right panels) in KrasG12D and KrasG12D;EgfrKO acinar cell explants after three days in three-dimensional collagen culture. Arrowhead indicates area of ductal morphology in KrasG12D explants, compared to maintained acinar cell morphology and amylase expression in KrasG12D;EgfrKO explants (arrows). Scale bars = 20 μm. (B) Quantitation of amylase and CK19 positive acinar clusters on day 3 of culture. (C) Co-IF for amylase (green) and CK19 (red) in cerulein-treated WT (left panels) EgfrKO (middle panels) and Adam17KO acinar cell explants (right panels). Arrowheads indicate CK19+ cells, scale bars = 20 μm. (D) Quantitation of amylase and CK19 positive acinar clusters in cerulein-treated explant cultures. See also Figure S4.
We found also that treatment of wild-type acinar cell explants with cerulein induced ADM within 5 days (Figure 4C). Both EgfrKO and Adam17KO explants were strongly resistant to this effect, although EgfrKO explants were more so (Figure 4C&D), indicating possible compensation from other sheddases. Like KRASG12D (Figure 1C), cerulein also induced EGFR activation, in an ADAM17 dependent manner (Figure S4C). We then examined expression of the EGFR ligands and ADAM17 substrates TGFA and AREG in acinar explants. While AREG was not detected (data not shown), TGFA was induced by the third day of cerulein treatment (Figure S4D), independent of genotype, confirming that the mutant acinar cells remain cerulein responsive. Finally, because ADAM17 is known to shed several bioactive cell surface molecules, we confirmed that the Adam17KO ADM blockade did not require shedding of other substrates by rescuing the wild-type phenotype with addition of TGFA to the culture medium (Figure S4E).
The dependency of pancreatitis-associated and in vitro ADM upon acinar cell EGFR and ADAM17 led us to test the relevance of these findings to human disease. Expression of components of the EGFR pathway in human CP, including active EGFR (pY1068), ADAM17 and TGFA and AREG was determined by IHC (Figure S4F). Regardless of CP type (alcoholic, familial or spontaneous), active EGFR, ADAM17 and TGFA were consistently upregulated, particularly in acinar cells adjacent to inflamed and fibrotic stroma in 10/10 CP samples. AREG was consistently expressed in ductal metaplasia adjacent to non-expressing acinar cells. Thus, activation of the EGFR circuitry in vivo and in vitro is reflected in human CP.
EGFR-dependent ERK activation is Required for Pancreatic Tumorigenesis
Initially, we hypothesized that EGFR was responsible for activation of some of the pathways that have been shown to be important for pancreatic tumorigenesis, including STAT3 and RAC1. Contrary to these possibilities, we observed substantial pSTAT3 in KrasG12D;EgfrKO pancreata comparable to KrasG12D tissue (Figure S5A). We also saw no change in active RAC pulldown experiments (data not shown). Recent data suggesting that RAS activation must reach a minimum threshold in order to transform the pancreas (Ji et al., 2009) led us to examine if EGFR and ADAM17 inhibition or ablation is consistent with this model by examining downstream effectors of RAS, including PI3K/AKT (pAKT) and MAPK (pERK1/2). KrasG12D;EgfrKO mice showed no diminution of active AKT compared to control KrasG12D mice (Figure 5A), suggesting no difference in PI3K activity. In contrast, we observed ~2-fold lower levels of pERK in 3 month old KrasG12D;EgfrKO pancreatic lysates (Figure 5A). Since this difference may be an indirect effect of enhanced pERK levels in tumors that form only in KrasG12D controls, we confirmed a similar loss of pERK in 4 week old pancreatic lysates, prior to substantial transformation (Figure 5A, lower panel). IHC for pERK in 4 week old KrasG12D mice revealed several positive isolated acinar regions (0.8% of total), whereas in KrasG12D;EgfrKO mice, pERK was limited to stromal cells (Figure 5B). ERK activation was also clearly detectable in 88% of acinar cell clusters in wild-type cerulein-treated acinar explants, compared to 4.2% and 10.8% in EgfrKO and Adam17KO explants, respectively (Figure 5C).
Figure 5. Endogenous EGFR is required for robust activation of RAS and ERK.
(A) Western blot analysis of whole pancreatic lysates for active pAKT and pERK in KrasG12D and KrasG12D;EgfrKO mice, at 3 months (upper and middle panels) and pERK at 1 month of age (lower panels). Numbers indicate densitometric quantitation of pERK/total ERK and pAKT/AKT ratios, of representative blots shown. n=4. (B) IHC for active pERK in 6 week old KrasG12D and KrasG12D;EgfrKO pancreata. Arrows indicate areas of focal acinar cell staining. Metaplasia is indicated (M). Arrowheads indicate stromal cell staining. Micrographs are representative of 6 mice. Scale bar = 20 μm (C) IF staining for active pERK in acinar cell explants of cerulein treated wild-type (WT), EgfrKO and Adam17KO explants at 0 and 3 days of culture. Arrows indicate areas of positive staining. Scale bar = 20 μm. (D) Active RAS pulldown assays from lysates of isolated primary acinar cells from wild-type (WT), KrasG12D, KrasG12D;EgfrKO and KrasG12D;Adam17KO mice. Numbers indicate densitometric quantitation of the ratio of GTP-bound and total RAS of representative blots shown. Western blots show concomitant relative ERK activity (pERK/ERK) in these lysates. n=3. (E) GTP-bound RAS/total RAS and pERK/total ERK ratios in isolated acinar cells treated with erlotinib for 6 hours. Numbers indicate quantitation of representative blots shown. n=3. (F) KrasG12D mice were cerulein-treated as in Figure 3D to induce uniform tumorigenesis, then treated with vehicle or 100 mg/kg erlotinib 12, 6 and 2 hours before sacrifice. Shown are western blots of pY1068 and total EGFR and pERK and total ERK. Numbers indicate ratios of representative blots shown. n=3. (G) IHC for active pERK, pAKT and pSTAT3 in vehicle or erlotinib treated mice described in (F). Scale bar = 100μm for primary micrographs, 50μm for insets. (H&I) IHC and quantitation for cleaved caspase-3 (CC3, H) and CyclinD1 (I) in vehicle or erlotinib treated mice described in (F). Scale bars = 50μm. Error bars are +/− SEM. See also Figure S5.
KRAS oncogenic mutations are dominant, usually only affecting a single allele. Thus, approximately half the KRAS protein expressed is wild-type and turns over GTP at a rapid rate thus inactivating signaling. Active EGFR is known to relocalize wild-type KRAS to the plasma membrane via Grb2/Sos where it is activated (Basu et al., 1994; Gale et al., 1993). Consistent with this, co-immunoprecipitation and co-immunofluorescence experiments showed clear interaction and colocalization of KRAS and EGFR in KrasG12D expressing tumor cell lines and acinar explants (Figure S5B&C). Using isolated acinar cells from WT, KrasG12D, KrasG12D;EgfrKO and KrasG12D;ADAM17KO mice, we found an upregulation of both total and active RAS in KrasG12D acinar cells (Figure 5D), consistent with previous reports (Ji et al., 2009). However, in KrasG12D;EgfrKO and KrasG12D;ADAM17KO cells, active RAS was reduced by more than 50%, while the upregulation of total RAS remained, leaving the active/total RAS ratios an average of 55% and 45% lower than KrasG12D cells, respectively.
We next tested if EGFR activity was required for maintenance of ERK activity when Kras is mutated. Treatment of isolated KrasG12D acinar cells with erlotinib reduced the active RAS/total RAS ratio by an average of 39.8% compared to control, with no effect on total RAS expression (Figure 5E). We then treated KrasG12D mice with cerulein to induce rampant transformation (Figure 3D) then treated acutely with erlotinib by oral gavage 12, 6 and 2 hours prior to tissue harvest. Compared to vehicle, erlotinib treatment reduced pERK levels by an average of 46% (Figure 5F). Treated pancreata showed an obvious reduction of pERK IHC, especially in isolated mPanINs (Figure 5G). Unlike our observations in EgfrKO mice, acute erlotinib treatment dramatically reduced pAKT staining in mPanINs (Figure 5G), suggesting an acute effect on PI3K signaling that is apparently compensated for under conditions of chronic inhibition. Consistent with the knockout mice, no alteration in pSTAT3 was observed (Figure 5G). Erlotinib-treated pancreata also showed a dramatic ~4-fold upregulation of cleaved caspase-3 IHC positive cells (Figure 5H) and a significant decrease in CyclinD1 expression (Figure 5I), indicating that EGFR activity is critical for survival and proliferation of metaplastic and mPanIN epithelia. EGFR dependency is also demonstrable in human PDA cell lines, with both RAS and ERK activity responsive to EGFR inhibition and activation, even in KRAS mutant cells (Figure S5D). We also found upregulation of pERK IHC in acinar cells of human CP samples (Figure S5E), consistent with a role in ADM in human disease.
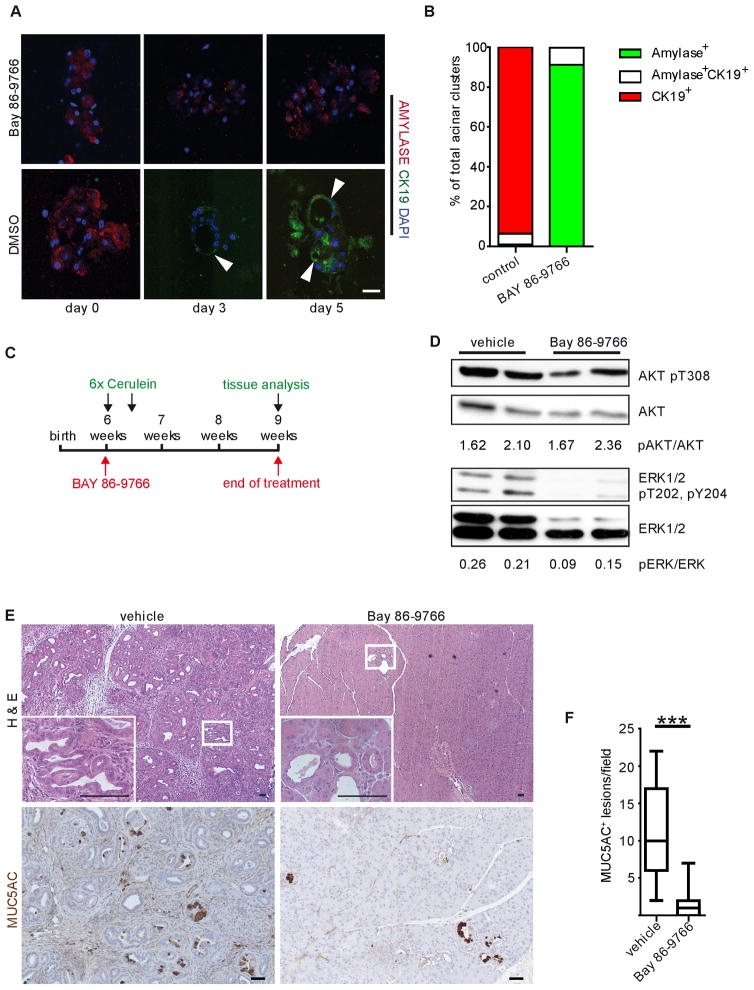
We have shown that EGFR is required for ADM and ERK activation and that EGFR and pERK are upregulated in acinar cells in human CP. To test if ERK activation is involved in ADM in vitro and pancreatic tumorigenesis in vivo, we employed the allosteric MEK1/2 inhibitor BAY 86-9766 (Iverson et al., 2009). BAY 86-9766 treatment of KrasG12D acinar explants strongly blocked transdifferentiation (Figure 6A&B). We then treated 6-week old KrasG12D mice with cerulein to induce pancreatitis. Concomitant with pancreatitis induction and continuing for 3 weeks after, mice were treated daily with 25 mg/kg BAY86-9766 or vehicle by oral gavage (Figure 6C). As expected, pERK levels in BAY86-9766-treated pancreata were reduced (Figure 6D) and vehicle-treated KrasG12D mice developed fibrotic, inflamed tissue with the majority of epithelia replaced by metaplasia and mPanIN. In contrast, BAY86-9766-treated mice retained mostly phenotypically normal tissue with only rare MUC5AC+ mPanINs (Figure 6E&F).
Figure 6. ERK activity is critical for metaplasia and neoplasia.
(A) Co-IF for amylase and CK19 in acinar cell explants of KrasG12D mice treated with either the MEK inhibitor BAY 86-9766 or vehicle. Arrowheads point to CK19+ duct-like structures. Scale bar = 20 μm. (B) Quantitation of amylase+ and CK19+ cell clusters is representative of at least 3 independent experiments per treatment. (C) Schematic illustration of pancreatitis induction in KrasG12D mice with a BAY 86-9766 treatment regimen. In 6 week old KrasG12D mice pancreatitis was induced by 6 hourly injections with 50 μg/kg cerulein on two consecutive days. Mice were treated either with a daily oral dose of BAY 86-9766 or vehicle, 6 days per week for 3 consecutive weeks, beginning on the first day of cerulein treatment. (D) Western blot analysis for active pERK and pAKT in control and BAY 86-9766 treated mice. Numbers indicate quantitation of ratios of blots shown, as indicated. (E) Histological analysis and IHC for the PanIN marker MUC5AC in vehicle and BAY 86-9766 treated mice (inset, n = 3). Scale bars = 50 μm. (F) Quantitation of MUC5AC+ lesions in BAY 86-9766 treated mice compared to controls (*** p < 0.001).
DISCUSSION
The reproducible tumor onset and progression in the KrasG12D PDA model has allowed us to explore how EGFR affects the transition of normal epithelia to neoplastic lesions. We find that KRAS upregulates EGFR in distinct, phenotypically normal acinar clusters prior to formation of metaplasia and mPanINs. Acinar cell upregulation of EGFR is rapid in cerulein-induced pancreatitis, with KRASG12D activity able to sustain the elevated expression. Most importantly, blocking EGFR activity effectively eliminates KRAS-initiated pancreatic tumorigenesis, with or without pancreatitis induction, due to its critical role in amplifying ERK activation within the pancreas. Further, EGFR is critical for acinar cell proliferation and its stimulation of MEK is necessary for transdifferentiation of transformation-resistant acinar cells to a transformation-sensitive, progenitor cell-like, metaplastic duct cell. Thus, we propose that EGFR’s major role in pancreatic tumorigenesis lies in its control of the differentiation of neoplastic precursors and, after tumor initiation, maintenance of ERK activity.
EGFR has been implicated in the pathogenesis of several epithelial cancers (Normanno et al., 2006). Inappropriate EGFR activation results from mutation of the receptor or overexpression of its ligands and their ADAM sheddases. Its contributions in RAS-mutated tumors are presumed to be through activation of complementary oncogenic pathways, as its abilities to activate the RAS pathway would be redundant. Nonetheless, upregulation of EGFR, its ligands and ADAM17 have been reported in PDA (Friess et al., 1996; Ringel et al., 2006), consistent with their importance in these usually Kras-mutant cancers. Several studies show that constitutive RAS signaling is not sufficient to compensate for EGFR activity. EGFR is necessary for growth (Dlugosz et al., 1997) and survival (Sibilia et al., 2000) of RAS-initiated cutaneous squamous cell carcinoma and maintains the stem-cell like nature of transformed keratinocytes (Hansen et al., 2000). In H-RAS initiated melanoma, an EGFR autocrine loop is required for tumor cell maintenance and survival (Bardeesy et al., 2005). In PDA cell lines, the unique activities of EGFR promote cell proliferation and invasion even when KRAS is mutated (Jaganathan et al., 2010; Larbouret et al., 2007; Pino et al., 2006; Zhao et al., 2010). Overall, the majority of studies support models where EGFR in RAS-mutated tumors is generally involved in post-transformation functions. Two studies provide notable exceptions where, in vitro, RAS transformation of otherwise normal immortalized cells requires EGFR activity (Gangarosa et al., 1997; Sibilia et al., 2000). In addition, concomitant pancreatic activation of oncogenic KRAS and EGFR signaling leads to accelerated formation of high-grade preneoplastic lesions and PDA, (Siveke et al., 2007). Our data support a model where endogenous EGFR signaling is required to maintain the critical threshold of RAS activity required for tumorigenesis (Ji et al., 2009). Based on numerous experimental approaches, loss of EGFR signaling resulted in a ~ 50% drop in active RAS, consistent with continual stimulation of GTP loading of the highly catalytically active wild-type RAS proteins, though we cannot eliminate the possibility that it is required for the occasional reactivation of the catalytically-impaired mutant KRAS.
Much has been made recently of experimental pancreatitis being required for transformation of oncogenic KRAS expressing acinar cells (Carriere et al., 2009; Gidekel Friedlander et al., 2009; Guerra et al., 2011; Guerra et al., 2007). In this model, cerulein induces intracellular activation of digestive enzymes, leading to cellular stress and necrosis. Necrosis attracts inflammatory cells, which cooperate with oncogenic KRAS in the epithelia to induce tumor formation by overcoming cellular senescence (Guerra et al., 2011; Lee and Bar-Sagi, 2010). While this provides a satisfying connection between PDA and one of its primary risk factors, our data show that KRAS and cerulein induce transdifferentiation of acinar cells in vitro by inducing EGFR expression and ADAM17-dependent activation, demonstrating that the earliest steps in this program are initiated cell-autonomously. Even so, since parenchymal EGFR ablation also dampens the stromal response, definitive separation of epithelial and stromal contributions to tumorigenesis is complicated. EGFR is known to influence the expression of inflammatory cytokines (Mascia et al., 2010; Monick et al., 2005) and expression of the proinflammatory COX proteins in metaplastic ducts suggests that reactive epithelia promote the host response. Unlike EGFR or pERK activity, however, we do not find COX upregulation in acinar cells, suggesting this is not direct control of gene expression by EGFR signaling, but is inherent to the metaplastic duct phenotype.
The role of EGFR as a therapeutic target in PDA is complex. In unselected metastatic PDA patients, anti-EGFR therapy has only a modest survival effect, however subgroups of patients and defined molecular subtypes respond to EGFR targeting (Collisson et al., 2011; Jimeno et al., 2008; Moore et al., 2007). In our study, EGFR-negative PDA developed in KrasG12D;p53KO;EgfrKO mice, albeit with reduced efficiency. In addition, KrasG12D;p53KOmice showed no additional survival advantage with erlotinib treatment, indicating that development and progression to PDA can be EGFR independent in a p53-null setting. While we have some indication of what may be compensating for EGFR loss, such as MET activation, this activity was detectable in only a minority of EGFR negative tumors, possibly suggesting multiple routes for bypassing EGFR deficiency. Defining the underlying molecular cues and the role of p53 inactivation as a potential contraindication of responsiveness to EGFR inhibition are important issues to be resolved.
In summary, we have identified a critical role for EGFR in KRAS’ reprogramming of the pancreatic epithelia en route to tumorigenesis. EGFR activation in this context requires ADAM17 and results in a substantial amplification of MEK signaling. The ADAM17/EGFR/MEK signaling axis is critical for some of the fundamental pathologies associated with PDA risk, such as formation of metaplastic ducts in pancreatitis, suggesting that there may be benefit in targeting the pathway in these at-risk patients, to restore homeostasis and thereby reduce the chance of tumorigenesis.
EXPERIMENTAL PROCEDURES
Mice
KrasLSL-G12D/+, Ptf1aCre/+, Ela-Tgfa, Trp53fl/fl, Adam17ΔEx5/ΔEx5 and Egfrfl/fl strains have been described previously (Hingorani et al., 2003; Kawaguchi et al., 2002; Lee and Threadgill, 2009; Marino et al., 2000; Natarajan et al., 2007; Sandgren et al., 1990; Siveke et al., 2007; Tang et al., 2011). Experiments were conducted in accordance with the Office of Laboratory Animal Welfare, the German Federal Animal Protection Laws and approved by the Institutional Animal Care and Use Committees at the Technical University of Munich, Stony Brook University and Mayo Clinic.
Histology, IHC, IF and Western Blot
Distribution and use of all human samples was approved by the Institutional Review Boards of Vanderbilt University Medical Center and the Mayo Clinic. IHC was performed on a Ventana XT (Tuscon, AZ) autostainer or as previously described (Siveke et al., 2007). For total EGFR and phospho-ERK IHC, mice were anesthetized and perfused with 4% paraformaldehyde. All IHC was counterstained with hematoxylin except amylase/CK19 dual IHC. Quantitation of amylase positive area was performed on singly DAB stained sections using Olympus cellSens Dimension software. Quantitation represents the average of 15–20 20X fields of view from 3 mice of each genotype, treatment regimen or time point, as indicated. IF and western blot were performed according to standard protocols (Siveke et al., 2007). Confocal IF images were collected on a Leica SP2 or a Ziess-LSM-510 Meta confocal microscope at consistent gain and offset. Antibodies are in Supplemental Information.
RAS activity assay
GST-Raf-1-RBD fusion protein was prepared by modifying procedures published elsewhere (Castro et al., 2005). Details of cell preparation and protocol modifications are found in Supplemental Information.
Quantitative RT-PCR
Real-Time PCR was performed as previously described (Siveke et al., 2007). For protocol details, see Supplemental Information.
Preparation of Pancreatic Epithelial Explants Culture
Pancreatic epithelial explants were as described previously (Heid et al., 2011). Either recombinant human TGF-α (rh TGFα, R&D Systems, final concentration 50 ng/ml) or cerulein (American Peptide Company, Sunnyvale, CA, 2.6 pM final concentration) were added as indicated. For MEK inhibition, BAY 86-9766 (provided by Bayer Schering) was added at indicated concentrations.
For each genotype, experiments with acinar epithelial explants were performed with the following numbers of mice: WT n=15, EgfrKO n=5, Adam17KO n=10, KrasG12D n=7, KrasG12D;EgfrKO n=10. WT plus BAY 86-9766 n=3, KrasG12D plus BAY 86-9766 n=3. For quantification, acinar explants were seeded in triplicate and cell clusters were counted from at least 3 optical fields/well.
Cerulein treatments
To induce a CP-like phenotype, 250 μg/kg twice daily injections of cerulein were administered i.p. for three weeks, with 24 hr recovery. KrasG12D and knockout derivatives were treated with 250 μg/kg cerulein once daily for 5 consecutive days and allowed to recover for 1 wk before tissue harvesting. For examining EGFR protein induction, 1 daily 250 μg/kg i.p. dose was administered for 3 days and allowed to recover for 1 or 3 days, as indicated.
In vivo inhibition of MEK in pancreatitis-induced tumors
6 week old KrasG12D mice were treated to induce pancreatitis, as described previously (Morris et al., 2010). On day 1 post cerulein injection, mice received a single daily dose of 25 mg/kg BAY 86-9766 or vehicle by oral gavage, 6 days per week for three weeks.
In vivo treatment of KrasG12D;p53KO mice with erlotinib or cetuximab
7 day old KrasG12D;p53KO mice were injected i.p. with either cetuximab (twice per week, 2 mg/kg), erlotinib (daily, 25 mg/kg) or vehicle (daily) for three weeks and sacrificed at 28 days of age. To assess efficacy of gemcitabine alone and in combination with erlotinib, mice with detectable tumor burden by MRI, determined by a clinical 1,5T MRI device, were treated daily with gemcitabine i.p. (120 mg/kg, 4 doses every third day) or gemcitabine i.p. (100 mg/kg, 4 doses every third day) in combination with erlotinib (daily, 50 mg/kg) by oral gavage. Tumor burden was monitored by MRI. For acute erlotinib treatment, KrasG12D were treated with 5 daily doses of 250 μg/kg cerulein, allowed to recover for 1 week and then treated with 100 mg/kg erlotinib 12, 6 and 2 hrs before sacrifice and tissue harvest.
Statistical analysis
Statistical analyses were performed using the Mann-Whitney-test for non-normally distributed, unpaired data. Tumor burden was compared between strains using one-way ANOVA. With all box plots, the bottom and top of the box are the 25th and 75th percentile and the central line is the median of the data. The whiskers represent maximum and minimum values, with the remaining dots being outliers.
Supplementary Material
HIGHLIGHTS.
KRAS-mediated tumorigenesis is inhibited in pancreatic Egfr and Adam17 knockouts
Pancreatitis-induced tumors are blocked by pancreatic Egfr or Adam17 ablation
Pancreatic Egfr and Adam17 knockouts resist metaplasia in vitro and in vivo
KRAS requires EGFR to robustly activate ERK, which is necessary for tumorigenesis
SIGNIFICANCE.
The almost universal lethality of PDA has led to the intense study of genetic mutations responsible for its formation and progression. The most common oncogenic mutations associated with all PDA stages are found in the KRAS gene, suggesting it as the primary initiator of pancreatic neoplasia. However, mutant Kras expression throughout the mouse pancreatic parenchyma shows that the oncogene remains largely indolent until secondary events, such as pancreatitis, unlock its transforming potential. We find KRAS requires an inside-outside-in signaling axis that involves ligand-dependent EGFR activation to initiate the signal transduction and cell biological changes that link PDA and pancreatitis.
Acknowledgments
This work was supported by a VA Merit Award, the Knapp Chair for Pancreatic Cancer Research and NIH grants R01CA129579 and R03CA129579 to HCC; NIH grants P01HL018645 and R01HL067267 to EWR; a pilot and feasibility award from University of Washington Nutrition and Obesity Center grant DK035816 to CLW; FWF (W1212), the EC (LSHC-CT-2006-037731) and the GEN-AU program “Austromouse” (GZ200.147/1-VI/1a/2006 and 820966) to MS; R01CA092479 and P50CA106991 to DWT; R01CA140290 to NRM; R01CA140182 to PS; by P50CA095103 for the Vanderbilt University Medical Center GI Spore Tissue Core; by Deutsche Krebshilfe (Grant 109992 to JTS:), by the German Federal Ministry of Education and Research (National Genomic Research Network (NGFN-Plus), 01GS08115 to JTS and RMS); by the German Research Foundation (SFB824/C4 to JTS). TROMAIII antibody, developed by Rolf Kemmler, was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242.
Footnotes
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bardeesy N, Aguirre AJ, Chu GC, Cheng KH, Lopez LV, Hezel AF, Feng B, Brennan C, Weissleder R, Mahmood U, et al. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc Natl Acad Sci U S A. 2006;103:5947–5952. doi: 10.1073/pnas.0601273103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeesy N, Kim M, Xu J, Kim RS, Shen Q, Bosenberg MW, Wong WH, Chin L. Role of epidermal growth factor receptor signaling in RAS-driven melanoma. Mol Cell Biol. 2005;25:4176–4188. doi: 10.1128/MCB.25.10.4176-4188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu T, Warne PH, Downward J. Role of Shc in the activation of Ras in response to epidermal growth factor and nerve growth factor. Oncogene. 1994;9:3483–3491. [PubMed] [Google Scholar]
- Carriere C, Young AL, Gunn JR, Longnecker DS, Korc M. Acute pancreatitis markedly accelerates pancreatic cancer progression in mice expressing oncogenic Kras. Biochem Biophys Res Commun. 2009 doi: 10.1016/j.bbrc.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro AF, Rebhun JF, Quilliam LA. Measuring Ras-family GTP levels in vivo--running hot and cold. Methods. 2005;37:190–196. doi: 10.1016/j.ymeth.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, Cooc J, Weinkle J, Kim GE, Jakkula L, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nature Med. 2011;17:500–503. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lisle RC, Logsdon CD. Pancreatic acinar cells in culture: expression of acinar and ductal antigens in a growth-related manner. Eur J Cell Biol. 1990;51:64–75. [PubMed] [Google Scholar]
- Dlugosz AA, Hansen L, Cheng C, Alexander N, Denning MF, Threadgill DW, Magnuson T, Coffey RJ, Jr, Yuspa SH. Targeted disruption of the epidermal growth factor receptor impairs growth of squamous papillomas expressing the v-ras(Ha) oncogene but does not block in vitro keratinocyte responses to oncogenic ras. Cancer Res. 1997;57:3180–3188. [PubMed] [Google Scholar]
- Fjallskog ML, Lejonklou MH, Oberg KE, Eriksson BK, Janson ET. Expression of molecular targets for tyrosine kinase receptor antagonists in malignant endocrine pancreatic tumors. Clin Cancer Res. 2003;9:1469–1473. [PubMed] [Google Scholar]
- Friess H, Berberat P, Schilling M, Kunz J, Korc M, Buchler MW. Pancreatic cancer: the potential clinical relevance of alterations in growth factors and their receptors. J Mol Med. 1996;74:35–42. doi: 10.1007/BF00202070. [DOI] [PubMed] [Google Scholar]
- Gale NW, Kaplan S, Lowenstein EJ, Schlessinger J, Bar-Sagi D. Grb2 mediates the EGF-dependent activation of guanine nucleotide exchange on Ras. Nature. 1993;363:88–92. doi: 10.1038/363088a0. [DOI] [PubMed] [Google Scholar]
- Gangarosa LM, Sizemore N, Graves-Deal R, Oldham SM, Der CJ, Coffey RJ. A raf-independent epidermal growth factor receptor autocrine loop is necessary for Ras transformation of rat intestinal epithelial cells. J Biol Chem. 1997;272:18926–18931. doi: 10.1074/jbc.272.30.18926. [DOI] [PubMed] [Google Scholar]
- Gidekel Friedlander SY, Chu GC, Snyder EL, Girnius N, Dibelius G, Crowley D, Vasile E, DePinho RA, Jacks T. Context-dependent transformation of adult pancreatic cells by oncogenic K-Ras. Cancer Cell. 2009;16:379–389. doi: 10.1016/j.ccr.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra C, Collado M, Navas C, Schuhmacher AJ, Hernandez-Porras I, Canamero M, Rodriguez-Justo M, Serrano M, Barbacid M. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell. 2011;19:728–739. doi: 10.1016/j.ccr.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Hansen LA, Woodson RL, 2nd, Holbus S, Strain K, Lo YC, Yuspa SH. The epidermal growth factor receptor is required to maintain the proliferative population in the basal compartment of epidermal tumors. Cancer research. 2000;60:3328–3332. [PubMed] [Google Scholar]
- Heid I, Lubeseder-Martellato C, Sipos B, Mazur PK, Lesina M, Schmid RM, Siveke JT. Early requirement of Rac1 in a mouse model of pancreatic cancer. Gastroenterology. 2011;141:719–730. 730 e711–717. doi: 10.1053/j.gastro.2011.04.043. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- Iverson C, Larson G, Lai C, Yeh LT, Dadson C, Weingarten P, Appleby T, Vo T, Maderna A, Vernier JM, et al. RDEA119/BAY 869766: a potent, selective, allosteric inhibitor of MEK1/2 for the treatment of cancer. Cancer research. 2009;69:6839–6847. doi: 10.1158/0008-5472.CAN-09-0679. [DOI] [PubMed] [Google Scholar]
- Jaganathan S, Yue P, Turkson J. Enhanced sensitivity of pancreatic cancer cells to concurrent inhibition of aberrant signal transducer and activator of transcription 3 and epidermal growth factor receptor or Src. J Pharmacol Exp Ther. 2010;333:373–381. doi: 10.1124/jpet.109.162669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji B, Tsou L, Wang H, Gaiser S, Chang DZ, Daniluk J, Bi Y, Grote T, Longnecker DS, Logsdon CD. Ras activity levels control the development of pancreatic diseases. Gastroenterology. 2009;137:1072–1082. 1082 e1071–1076. doi: 10.1053/j.gastro.2009.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimeno A, Tan AC, Coffa J, Rajeshkumar NV, Kulesza P, Rubio-Viqueira B, Wheelhouse J, Diosdado B, Messersmith WA, Iacobuzio-Donahue C, et al. Coordinated epidermal growth factor receptor pathway gene overexpression predicts epidermal growth factor receptor inhibitor sensitivity in pancreatic cancer. Cancer research. 2008;68:2841–2849. doi: 10.1158/0008-5472.CAN-07-5200. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- Kobrin MS, Funatomi H, Friess H, Buchler MW, Stathis P, Korc M. Induction and expression of heparin-binding EGF-like growth factor in human pancreatic cancer. Biochem Biophys Res Commun. 1994;202:1705–1709. doi: 10.1006/bbrc.1994.2131. [DOI] [PubMed] [Google Scholar]
- Korc M, Friess H, Yamanaka Y, Kobrin MS, Buchler M, Beger HG. Chronic pancreatitis is associated with increased concentrations of epidermal growth factor receptor, transforming growth factor alpha, and phospholipase C gamma. Gut. 1994;35:1468–1473. doi: 10.1136/gut.35.10.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larbouret C, Robert B, Navarro-Teulon I, Thezenas S, Ladjemi MZ, Morisseau S, Campigna E, Bibeau F, Mach JP, Pelegrin A, Azria D. In vivo therapeutic synergism of anti-epidermal growth factor receptor and anti-HER2 monoclonal antibodies against pancreatic carcinomas. Clin Cancer Res. 2007;13:3356–3362. doi: 10.1158/1078-0432.CCR-06-2302. [DOI] [PubMed] [Google Scholar]
- Lee KE, Bar-Sagi D. Oncogenic KRas suppresses inflammation-associated senescence of pancreatic ductal cells. Cancer Cell. 2010;18:448–458. doi: 10.1016/j.ccr.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TC, Threadgill DW. Generation and validation of mice carrying a conditional allele of the epidermal growth factor receptor. Genesis. 2009;47:85–92. doi: 10.1002/dvg.20464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttges J, Reinecke-Luthge A, Mollmann B, Menke MA, Clemens A, Klimpfinger M, Sipos B, Kloppel G. Duct changes and K-ras mutations in the disease-free pancreas: analysis of type, age relation and spatial distribution. Virchows Arch. 1999;435:461–468. doi: 10.1007/s004280050428. [DOI] [PubMed] [Google Scholar]
- Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 2000;14:994–1004. [PMC free article] [PubMed] [Google Scholar]
- Mascia F, Cataisson C, Lee TC, Threadgill D, Mariani V, Amerio P, Chandrasekhara C, Souto Adeva G, Girolomoni G, Yuspa SH, Pastore S. EGFR regulates the expression of keratinocyte-derived granulocyte/macrophage colony-stimulating factor in vitro and in vivo. The Journal of investigative dermatology. 2010;130:682–693. doi: 10.1038/jid.2009.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means AL, Meszoely IM, Suzuki K, Miyamoto Y, Rustgi AK, Coffey RJ, Jr, Wright CV, Stoffers DA, Leach SD. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development. 2005;132:3767–3776. doi: 10.1242/dev.01925. [DOI] [PubMed] [Google Scholar]
- Means AL, Ray KC, Singh AB, Washington MK, Whitehead RH, Harris RC, Jr, Wright CV, Coffey RJ, Jr, Leach SD. Overexpression of heparin-binding EGF-like growth factor in mouse pancreas results in fibrosis and epithelial metaplasia. Gastroenterology. 2003;124:1020–1036. doi: 10.1053/gast.2003.50150. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Maitra A, Ghosh B, Zechner U, Argani P, Iacobuzio-Donahue CA, Sriuranpong V, Iso T, Meszoely IM, Wolfe MS, et al. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3:565–576. doi: 10.1016/s1535-6108(03)00140-5. [DOI] [PubMed] [Google Scholar]
- Monick MM, Cameron K, Staber J, Powers LS, Yarovinsky TO, Koland JG, Hunninghake GW. Activation of the epidermal growth factor receptor by respiratory syncytial virus results in increased inflammation and delayed apoptosis. J Biol Chem. 2005;280:2147–2158. doi: 10.1074/jbc.M408745200. [DOI] [PubMed] [Google Scholar]
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- Morris JPt, Cano DA, Sekine S, Wang SC, Hebrok M. Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J Clin Invest. 2010;120:508–520. doi: 10.1172/JCI40045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan A, Wagner B, Sibilia M. The EGF receptor is required for efficient liver regeneration. Proc Natl Acad Sci U S A. 2007;104:17081–17086. doi: 10.1073/pnas.0704126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Pino MS, Shrader M, Baker CH, Cognetti F, Xiong HQ, Abbruzzese JL, McConkey DJ. Transforming growth factor alpha expression drives constitutive epidermal growth factor receptor pathway activation and sensitivity to gefitinib (Iressa) in human pancreatic cancer cell lines. Cancer research. 2006;66:3802–3812. doi: 10.1158/0008-5472.CAN-05-3753. [DOI] [PubMed] [Google Scholar]
- Ringel J, Jesnowski R, Moniaux N, Luttges J, Ringel J, Choudhury A, Batra SK, Kloppel G, Lohr M. Aberrant expression of a disintegrin and metalloproteinase 17/tumor necrosis factor-alpha converting enzyme increases the malignant potential in human pancreatic ductal adenocarcinoma. Cancer Res. 2006;66:9045–9053. doi: 10.1158/0008-5472.CAN-05-3287. [DOI] [PubMed] [Google Scholar]
- Sahin U, Weskamp G, Kelly K, Zhou HM, Higashiyama S, Peschon J, Hartmann D, Saftig P, Blobel CP. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164:769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandgren EP, Luetteke NC, Palmiter RD, Brinster RL, Lee DC. Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell. 1990;61:1121–1135. doi: 10.1016/0092-8674(90)90075-p. [DOI] [PubMed] [Google Scholar]
- Sharma A, Zangen DH, Reitz P, Taneja M, Lissauer ME, Miller CP, Weir GC, Habener JF, Bonner-Weir S. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes. 1999;48:507–513. doi: 10.2337/diabetes.48.3.507. [DOI] [PubMed] [Google Scholar]
- Sibilia M, Fleischmann A, Behrens A, Stingl L, Carroll J, Watt FM, Schlessinger J, Wagner EF. The EGF receptor provides an essential survival signal for SOS-dependent skin tumor development. Cell. 2000;102:211–220. doi: 10.1016/s0092-8674(00)00026-x. [DOI] [PubMed] [Google Scholar]
- Singh AB, Harris RC. Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cellular signalling. 2005;17:1183–1193. doi: 10.1016/j.cellsig.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Siveke JT, Einwachter H, Sipos B, Lubeseder-Martellato C, Kloppel G, Schmid RM. Concomitant pancreatic activation of Kras(G12D) and Tgfa results in cystic papillary neoplasms reminiscent of human IPMN. Cancer Cell. 2007;12:266–279. doi: 10.1016/j.ccr.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Strobel O, Dor Y, Alsina J, Stirman A, Lauwers G, Trainor A, Castillo CF, Warshaw AL, Thayer SP. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology. 2007;133:1999–2009. doi: 10.1053/j.gastro.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Zarbock A, Gomez I, Wilson CL, Lefort CT, Stadtmann A, Bell B, Huang LC, Ley K, Raines EW. Adam17-dependent shedding limits early neutrophil influx but does not alter early monocyte recruitment to inflammatory sites. Blood. 2011;118:786–794. doi: 10.1182/blood-2010-11-321406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobita K, Kijima H, Dowaki S, Kashiwagi H, Ohtani Y, Oida Y, Yamazaki H, Nakamura M, Ueyama Y, Tanaka M, et al. Epidermal growth factor receptor expression in human pancreatic cancer: Significance for liver metastasis. Int J Mol Med. 2003;11:305–309. [PubMed] [Google Scholar]
- Zhao S, Wang Y, Cao L, Ouellette MM, Freeman JW. Expression of oncogenic K-ras and loss of Smad4 cooperate to induce the expression of EGFR and to promote invasion of immortalized human pancreas ductal cells. Int J Cancer. 2010;127:2076–2087. doi: 10.1002/ijc.25412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Kleeff J, Friess H, Wang L, Zimmermann A, Yarden Y, Buchler MW, Korc M. Epiregulin is Up-regulated in pancreatic cancer and stimulates pancreatic cancer cell growth. Biochem Biophys Res Commun. 2000;273:1019–1024. doi: 10.1006/bbrc.2000.3033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.