Abstract
Purpose
The purpose of the present review was to use existing, published data to provide an estimate of the amount of change in six-minute walk test distance (Δ6MWT) that represents a clinically meaningful change in individuals with chronic heart failure (CHF).
Methods
The present review included two separate literature searches of the CINAHL and Medline databases for articles that: (1) reported the intraclass correlation coefficient (ICC) of the 6MWT in individuals with CHF, and (2) used the 6MWT along with either aerobic capacity or health-related quality of life (HRQL) as study endpoints in randomized controlled trials (RCTs) of exercise-based intervention for individuals with CHF. The ICCs were used to calculate the minimum detectable difference (MDD) at the 95% confidence interval for each included study. The Δ6MWT associated with aerobic capacity and HRQL within-group effect sizes for the intervention and control groups in each included RCT was analyzed using receiver operating characteristic (ROC) curves.
Results
Thirteen articles reported the ICC for the 6MWT. The mean (standard deviation) MDD calculated based on these data was 43.1(16.8) m. Eighteen RCTs measured the 6MWT and either aerobic capacity and/or HRQL. A Δ6MWT of 40–45 m was associated with at least moderate aerobic capacity and HRQL effect sizes in the intervention groups. The Δ6MWT thresholds that discriminated between intervention and control groups using ROC curves revealed the following sensitivity/specificity for the respective thresholds: 19 m, 94.4/83.3%, 32 m, 83.3/94.4%, and 48 m 44.4/100% (AUC = .935, p = .009, CI95% .855, 1.015).
Conclusions
A Δ6MWT of approximately 45 m appears to exceed measurement error and be associated with significant changes in either aerobic capacity and/or HRQL.
Key Words: heart failure, six-minute walk test, clinically meaningful change, minimum detectable difference
INTRODUCTION
Chronic heart failure (CHF) affects nearly 4 million U.S. residents and more than 15 million people worldwide.1 Symptoms and limitations experienced by individuals with CHF include fatigue, dyspnea on exertion, muscle atrophy, weakness, reduced health-related quality of life (HRQL), and poor exercise tolerance,2, 3, 4 with exercise intolerance reported as the most frequent and debilitating symptom.5 Rehabilitation interventions are primarily directed at improving exercise tolerance with an anticipated reduction in the other symptoms and limitations associated with CHF.6 One of the most common instruments for measuring changes in exercise tolerance following exercise-based interventions is the six-minute walk test (6MWT). Interpretation of clinically meaningful change in the 6MWT is important for clinicians for making decisions about the effectiveness of interventions in individual patients, and is important for researchers in estimating required sample sizes and making conclusions regarding magnitude of treatment effect. Unfortunately, the amount of change in 6MWT distance (Δ6MWT) that should be considered to be clinically meaningful has not been well studied.
The first estimate of clinically meaningful Δ6MWT was intially reported by Guyatt et al7 in 1988 who reported the within-person standard deviation of 22.5 m. Opasich et al8 and Pinna et al,9 in two papers reporting on the same data set, calculated the minimum detectable difference (MDD) for the 6MWT in patients with CHF to be between 32 and 55 m depending on the confidence level (95% versus 99%) and number of trials (average vs. single) used, with 45 m representing the 95% confidence interval calculated based on two trials. In attempting to estimate the minimum clinically important difference (MCID) of the 6MWT, O'Keefe et al10 used a 5-point, patient-rated Global Rating Scale (GRS) and found mean changes of 24 m and 43 m, respectively, for those who reported being “a little worse” or “a little better” over the course of a 4-week follow-up period. Spertus et al11 used a 15-point, clinician-rated GRS collapsed into either large, moderate, small, and negligible improvement or decline. They found that mean Δ6MWT distances for moderate improvement or decline were 55 m and 90 m, respectively.
The aforementioned studies using the within-person standard deviation, MDD, or MCID suggest a somewhat large range of 22.5 m to 90 m that may represent meaningful Δ6MWT in individuals with CHF.12 Other methods for determining clinically meaningful change can include systematic review and meta-analysis of Δ6MWT in response to interventions in randomized controlled trials (RCTs). Rees et al13 and an update by Davies et al,14 found a weighted mean difference of 41 m for Δ6MWT between control and intervention groups, as well as statistically significant changes in other measures of exercise capacity (eg, maximum oxygen consumption and peak work) and HRQL. However, the weighted mean difference of 41 m only accounts for differences between intervention and control groups, and there was no analysis of concurrent changes between the 6MWT and other measures. Olsson et al12 examined Δ6MWT concurrent with changes in other study end points in a systematic review of clinical trials investigating pharmacologic and cardiac resynchronization interventions in individuals with CHF. They found that the 6MWT appeared to be a responsive study endpoint for cardiac resynchronization therapy trials, and that changes in symptoms were concordant with Δ6MWT. Unfortunately, Olsson et al12 did not report sufficient data to suggest a specific threshold or threshold range associated with these concordant changes that might constitute clinically meaningful change.
Because an optimal method for determining clinically meaningful change has not been established, some have recommended triangulation using several approaches to determine a threshold or threshold range.15, 16, 17 In addition to the MDD and MCID, other methods can include the analysis of concordant changes between the measure of interest and other measures.12 Therefore, the purpose of this review is to use a novel approach for triangulating the Δ6MWT that should be considered to be clinically meaningful.
METHODS
Two literature reviews were conducted to assist in meeting the planned overall objective of triangulating a threshold for clinically meaningful Δ6MWT. First, a literature search for articles that reported intra-class coefficients (ICCs) for the 6MWT in patients with CHF was conducted to allow for the calculation of MDD estimates. A second literature search investigating the effect of exercise interventions in patients with CHF was conducted to allow for an ecological analysis using group-level data from multiple trials. The objectives of this analysis were (1) to examine whether Δ6MWT is associated with concurrent changes in exercise capacity and HRQL, and (2) to determine if there is a Δ6MWT threshold that discriminates between intervention and placebo groups.
Literature Search for Intraclass Correlation Coefficients
The literature was searched on April 14, 2011, for English-language articles using Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus with Full-Text (EBSCO) and MEDLINE (via PubMed) databases using “heart failure” and the following key words: “walk test,” “walking test,” “reproducibility,” and “reliability.” Articles were included if they reported the ICC for the 6MWT in patients with CHF. Reference lists from articles found during the search were also used for discovering potentially relevant articles.
Calculation of the MDD
The MDD95% was calculated as follows:18 MDD95% = z * SEM * √2 where z = 1.96 and SEM (standard error of measurement) = σbaseline √(1-ICC).
Analysis of Findings
In anticipation of a range of values, the MDD95% was plotted as a function of several variables including mean age, baseline 6MWT distance, mean left ventricular ejection fraction, mean New York Heart Association Functional Class, sample size, and test-retest interval to determine the potential influence of these variables on MDD.
Literature Search for Concurrent Changes in Exercise-based Controlled Trial Outcomes
The literature was also searched on April 15, 2011, for English-language articles using CINAHL Plus with Full-Text (EBSCO) and MEDLINE (via PubMed) databases using “heart failure” and the following key words: “exercise,” “training,” and “rehabilitation.” All years of publication were included, and randomized controlled trial (RCT) filters were applied when available. Reference lists from articles found during the search were also used for discovering potentially relevant articles
Study Selection Criteria
For inclusion into the present review, studies met the following criteria: (1) participants included adults diagnosed with HF, (2) randomized study design, (3) exercise-related intervention, and (4) use of the 6MWT as a study endpoint in addition to cardiopulmonary exercise testing or HRQL measures. Studies were excluded if the language was anything other than English, or if they were not RCTs.
Methodological Quality of Reviewed Studies
The methodological quality and rigor of each article was evaluated using a 10-point scale adopted from Medlicott and Harris.19 Item 7 of the original scale, “blinding of the patient, treatment provider, and assessor,” was modified to “blinding of the assessor” to account for exercise studies where it is difficult to blind patients to exercise-based interventions.20
Articles were scored based on the number of “yes” answers for each of the 10 items21 and were rated as “strong” for scores of 80% or higher, “moderate” for scores of 60% to 79%, or “weak” for scores of 59% or less.
Analysis of Findings
An ecological approach using group-level data included both visual and quantitative analysis. Visual analysis was conducted using scatterplots of the mean Δ6MWT for intervention and placebo groups plotted against the exercise capacity and HRQL within-group effect sizes (conservatively assuming a pre/post measure correlation of 0.5) to account for variability in samples and the instruments used for measuring these variables. Quantitative analysis included comparison of Δ6MWT, aerobic capacity effect size, and HRQL effect size between intervention and control groups using the appropriate independent groups comparison strategy (independent t-tests or Mann Whitney U), as well as receiver operating characteristic (ROC) curves to determine the optimal threshold for Δ6MWT that discriminates between intervention and control groups.
RESULTS
Literature Search for Intra-class Correlation Coefficients
Eight-hundred twenty-five unique articles were found using the aforementioned search strategy, yielding 13 articles8, 9, 10, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 that reported the ICC for the 6MWT in patients with CHF. The results of the search strategy are outlined in Figure 1. A summary of each included study is presented in Table 1. Of the 13 articles,8, 9, 10, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 one reported on two different samples,26 and two different articles8, 9 reported on the same sample. Five studies20, 21, 22, 23, 27 did not report the standard deviation of the 6MWT for the subgroup of subjects in whom the ICC was calculated. Therefore, the standard deviation from the entire sample was used.
Figure 1.
Results of search strategy for Intraclass Correlation Coefficients.
Table 1.
Summary of Literature Search for Intraclass Correlation Coefficients
| AUTHOR YEAR | PURPOSE OF STUDY | STUDY POPULATION | TEST-RETEST INTERVAL | BASELINE 6MWT ICC CALCULATED MDD | NOTES |
|---|---|---|---|---|---|
| Cahalin et al 199620 | 62 week longitudinal study to determine if 6MWT predicts peak VO2 and survival | N = 45 Age: 49(8) years LVEF: 20(6)% NYHA-FC: 3.3(.6) | “Later” on same day | 6MWT: 310(100)m ICC: 0.96 MDD: 55.4 | Used subgroup of 20 subjects used for ICC Selection method NR Baseline data from entire sample Standard procedure except: no encouragement provided |
| O'Keefe et al 199810 | Cross-sectional study with 3-8 week f/u to determine reproducibility and responsiveness of the 6MWT | N = 60 Age: median 81 Years LVEF: NR NYHA-FC: 2.5(.85) | Median 4 weeks (range 3-8) | 6MWT: 237(49)m ICC: 0.91 MDD: 40.7 | Used subgroup of 24 subjects who reported “No change” in status at follow-up Standard procedure except: no practice trial, 25 m course |
| Roul et al 199821 | Up to 3 year longitudinal study to determine if 6MWT predicts peak VO2 and survival | N = 121 Age: 59(11) years LVEF: 29.6(13)% NYHA-FC: 2.5(.5) | Same day | 6MWT: 433(108)m ICC: 0.82 MDD: 127.0 | Used subgroup of 40 subjects used for ICC Selection method NR Baseline data from entire sample Standard procedures except: course length unspecified, no practice trial |
| Pinna et al 19988 and Opasich et al 20009 | Cross sectional study to determine reproducibility of the 6MWT | N = 233 Age: 54(9) years LVEF: 26(7)% NYHA-FC: 2.2(.7) | 30 min for 202 Subjects Next day for 31 subjects | 6MWT: 400(69)m ICC: 0.96 MDD:38.3 | 2nd test was 20 m and 15 m farther for the 30 min and next day test-retest interval groups, respectively Standard procedure except: no encouragement provided, no practice trial |
| Morales et al 199922 | Cross-sectional study comparing the 6MWT to the Shuttle Walk test | N = 46 Age: 53(10) years LVEF: 28(8)% NYHA-FC: 2.8(.7) | Not well described; no more than 2 tests were given on the same day, and all tests were completed in ≤ 2 weeks | 6MWT: 496(91)m r: 0.98 MDD: 35.7 | Used the first 17 subjects who did 3 trials with the Pearson correlation coefficient calculated based on trials 2 and 3 3rd test was 9m farther than the 2nd test Baseline standard deviation from entire sample Standard procedure except: 20 m course |
| Zugck et al 200023 | Cross-sectional study to compare the 6MWT to peak VO2 with regard to prognosis | N = 113 Age: 54(12) years LVEF: 19(7)% NYHA-FC: 2.2(.8) | 3 consecutive days | 6MWT: 466(107)m ICC: 0.96 MDD: 59.3 | Used subgroup of 10 subjects who performed 3 trials Selection method NR Baseline data from entire sample Standard procedure except: no encouragement provided |
| Demers et al 200124 | 43 week longitudinal subanalysis of the RESOLVD trial to assess reliability, validity, and responsiveness of 6MWT | N = 768 Age: 63(11) years LVEF: 27(10)% NYHA-FC: 2.35(.5) | Within 5 days | 6MWT: 381(84)m ICC: 0.90 MDD: 73.6 | Distances at 18 and 43 weeks were within 6 m of each other with ICCs of .88 and .91, respectively Standard procedure except: 20 m course, no practice trial |
| Gary et al 200425 | Interventional study assessing the effect of medical intervention in patients with diastolic heart failure | N = 32 Age: 68(11) years LVEF: 55(8)% NYHA-FC: 2.6(.5) | 2 hours | 6MWT: 254(112)m ICC: 0.99 MDD: 31.0 | Follow-up ICC for control and intervention groups were .97 and .93, respectively Standard procedure except: 18 m course, no practice trial |
| Kervio et al 200426 | Cross-sectional study to determine reproducibility of the 6MWT in patients with and without cardiac pacing | N = 24 Age: 65(6) years LVEF: 27(8.6)% NYHA-FC: 2.3(.5) | Within 24 − 48 hours | 6MWT: 445(73)m and 407(55)m, non-paced/paced, respectively ICC: 0.99 and 0.98 for non-paced/paced, respectively MDD: 20.2, 21.6, respectively | 2nd test was 3 m farther in the non-paced group and 1 m farther in the paced group Standard procedure except: 18 m course no practice trial |
| Corvera-Tindel et al 200427 | 12 week home-based walking RCT | N = 79 Age: 63(10) LVEF: 28(10) NYHA-FC: 2.2(.4) | Not specified | 6MWT: 379(75)m ICC: 0.92 MDD: 58.8 m | Used 10% of the sample to assess test-retest reliability Selection method NR Baseline data from entire sample Standard procedure except: course length not specified, no encouragement provided, no practice trial |
| Ingle et al 200528 | Longitudinal 1 year f/u to determine reproducibility and sensitivity of the 6MWT over time | N = 1013 Age: 72(7) years LVEF: 33(8)% NYHA-FC: 2.25(.6) | 1 year | 6MWT: 285(122)m ICC: 0.80 MDD: 124.0 | Used subgroup of 74 subjects who reported no change in symptoms as defined by a pre-to-post change in the Euro Heart Failure Survey of ≤ 3 points at 1 year follow-up Standard procedure except: 15 m course, no practice trial |
| Ingle et al 200729 | Cross-sectional study of VO2 during the 6MWT and to compare to peak VO2 | N = 24 Age: 76(5) years LVEF: 36(5)% NYHA-FC: 78% III or IV | Next day | 6MWT: 340(100)m ICC: 0.98 MDD: 47.8 | 2nd test was 4 meters farther on average Standard procedure except: 15 m course, no practice trial |
Abbreviations: LVEF, left ventricular ejection fraction; NHYA-FC, New York Heart Association Functional Class; 6MWT, six-minute walk test; ICC, Intraclass Correlation Coefficient; MDD, minimum detectable difference; NR, not sufficiently reported; standard procedure = (1) subject encouraged to walk as far as possible, (2) course length is ≥ 33 m, (3) a practice trial is provided, (4) standardized encouragement is provided as regular intervals
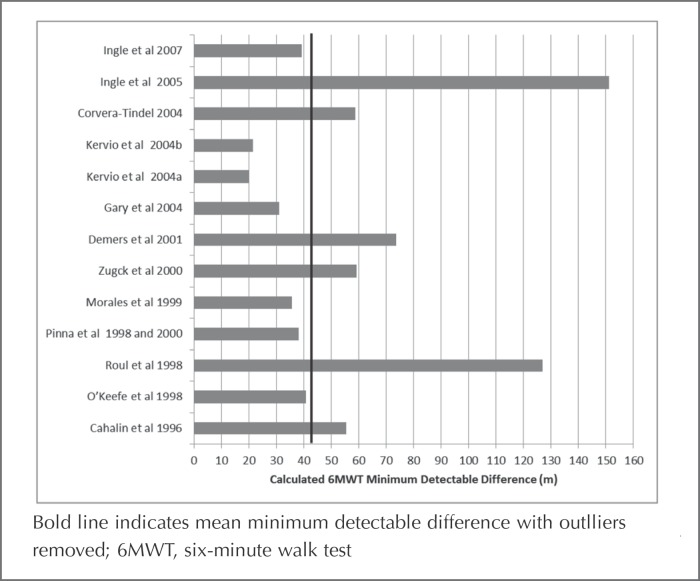
Overall, the search permitted calculation of 13 estimates of the MDD95% for the 6MWT, which ranged from 20 to 151 m with a mean (standard deviation) of 57.9 (37.5) m and a 95% confidence interval of 34.0 to 81.7 m (Figure 2). Given the wide range of calculated MDDs and two apparent outliers, potential explanatory factors were explored using scatterplots of the MDD95% plotted against test-retest interval, disease severity as measured by left ventricular ejection fraction, New York Heart Association Functional Class, baseline distance walked, sample size, and age. Additionally, several factors known to influence 6MWT distance,22 including instruction to walk as far as possible in 6 minutes, use of a practice trial, use of a course longer than 33 m, and providing encouragement were also explored using scatterplots. No relationships among the calculated MDD95% and any of the aforementioned variables emerged. It should be noted that Ingle et al 200528 used a one year test-retest interval that likely introduced other sources of variability, and although there were no discernable factors that contributed to the high MDD95% calculated based on the Roul et al21 data, it appeared to be an outlier. Therefore, these two outliers were removed for additional analysis. The mean (standard deviation) MDD95% was 43.1(16.8) m with a 95% confidence interval of 31.8 to 54.4 m in the revised sample.
Figure 2.
Calculated minimum detectable difference values.
Literature Search for Concurrent Changes in Exercise-based Controlled Trial Outcomes
Four-hundred seventy-two unique articles were found using the aforementioned search strategy, yielding 18 RCTs6, 27, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45 investigating exercise-based interventions for patients with CHF using the 6MWT as a study endpoint. The results of the search strategy are outlined in Figure 3. A summary of each included study is presented in Table 2.
Figure 3.
Search results for concurrent changes in exercise-based controlled trial.
Table 2.
Summary of Concurrent Changes in Exercise-based Controlled Trial Outcomes
| Author, Year, Design, and Rigor | Subjects at Baseline | Intervention |
Outcomes |
||
|---|---|---|---|---|---|
| 6MWT (m) | Peak VO2 (mL/kg/min) | MLHFQ/Other* | |||
| Tyni-Lenne et al 19966 M&HR = 60% | Treatment 1: N = 7 Age: 60(10)LVEF(%): 26(13) | 3x/week for 8 weeks Treatment 1: 15 min of continuous one-legged knee extensor training using one leg at a time at 35% of absolute peak twolegged work load using a modified ergometer cycle | Treatment 1: Pre: 469(39) Post: 525(38) Δ: 56 | Treatment 1: Pre: 13.8(1.1) Post: 14.2(1.1) Δ: 0.4 ESw-g 0.36 | Treatment 1: Pre: 10(6) Post: 8(6) Δ: −2 ESw-g 0.33 |
| Treatment 2: N = 7 Age: 57(10)LVEF(%): 29(9) | Treatment 2: Pre: 484(72) Post: 505(80) Δ: 21 | Treatment 2: Pre: 12.6(1.2) Post: 13.1(0.9) Δ: 0.5 ESw-g 0.46 | Treatment 2: Pre: 13(13) Post: 8(8) Δ: −5 ESw-g 0.44 | ||
| Control: N = 7 Age: 63(6) LVEF(%): 28(10) | Treatment 2: 15 min of continuous two-legged knee extensor training performed simultaneously at 65-75% of absolute peak two-legged work load using a modified ergometer cycle | Control: Pre: 467(58) Post: 460(65) Δ: −7 ES: 1.27 ES: 0.43 | Control: NR | Control: Pre: 10(6) Post: 12(8) Δ: 2 ESw-g 0.28 ES: 0.67 ES: 0.69 | |
| Control: habitual physical activity | *Sickness Impact Profile | ||||
| Tyni-Lenne et al 199730 M&HR = 80% | Treatment N = 8 Age: 62(10)LVEF(%): 30(5)NYHA-FC: 2.5(.5) | Treatment: 3x/week for ∼ 25 min (warm-up, exercise, cool down) for 8 weeks for endurance training of leg muscles at 65-75% of absolute baseline peak work rate. | Treatment: Pre: 466(85) Post: 503(72) Δ: 37 | Treatment: Pre: 10.0(2.4) Post: 12.0(2.3) Δ: 2.0 ESw-g 0.85 | Sickness Impact Profile NSR |
| Control N = 8 Age: 63(10) LVEF(%): 28(10)NYHA-FC: 2.4(.5) | Control: ordinary daily activities | Control: Pre: 465(67) Post: 463(61) Δ: −2 ES: 0.51 | Control: Pre: 8.9(1.8) Post: 8.8(1.8) Δ: −0.1 ESw-g 0.06 ES: 1.41 | ||
| Gottlieb et al 199931 M&HR = 80% | Treatment: N = 11 Age: 67(7) LVEF(%): 22(8) NYHA-FC: 2.7(.5) | Treatment: 3x/week for 6 months supervised graded aerobic exercise program using Schwinn Airdyne bike and treadmill, following a Borg scale of 12-13 | Treatment: Pre: 408.4(38.7) Post: NR Δ: 44.8(59.1) | Treatment: Pre: 14.1(1.9) Post: NR Δ: 2.4(2.8) ESw-g 0.86 | Treatment: Pre: 29(25) Post: 22(20) Δ: −7 ES w-g 0.31 |
| Control: N = 14 Ages = 64(10) LVEF(%) = 25(10) NYHA-FC: 2.5(.5) | Control: usual care | Control:Pre: 393.5(48.8)Post: NR Δ:-18.6(40.8). ES: 1.44 | Control: Pre: 14.0(2.9) Post: NR Δ: 0.1(2.6)ESw-g 0.04 ES: 0.94 | Control: Pre: 47(24) Post: NR Δ: NR | |
| Tyni-Lenne et al 200132 M&HR = 60% | Treatment: N = 16 Ages = 63(9) M:F = 1:1 LVEF(%) = 30(9) NYHA-FC: 2.3(.5) | Treatment: 3x/week for 60 min (warm-up, training, cool down) for 8 weeks for continuous repetitive muscle contraction using a thera-band for 2 × 25 repetitions in arm, leg and trunk muscles at a 70 bpm frequency using a Borg scale of 13-16 | Treatment: Pre: 500(64) Post: 555(59) Δ: 55 | Treatment: Pre: 14.8(4.2) Post: 15.9(4.3) Δ: 1.1 ESw-g 0.26 | NSR |
| Control:N = 8 Ages = 62(11)M:F = 5:3 LVEF(%) = 30(10)NYHA-FC: 2.3(.4) | Control: ordinary daily activities | Control: Pre: 504(30) Post: 504(27) Δ: 0 ES: 1.1 | Control: Pre: 16.4(4.0) Post: 14.4(2.8) Δ: −2.0 ESw-g −0.56 ES: 0.76 | ||
| Pu et al 200133 M&HR = 70% | Treatment:N = 9 Age: 76.6(2.0) LVEF(%): 36.3(2.7) NYHA-FC: 2.2(.1) | Treatment: 3x/week for 60 min (warm-up, exercise, stretching) for 10 weeks for progressive resistive training at 80% 1RM for 3 sets of 8 repetitions for upper and lower extremities | Treatment: Pre: 372(42) Post: 421(50) Δ: 49 | Treatment: Pre: 15.46(1.04) Post: 15.08(1.6) Δ: −0.42 ESw-g −0.27 | Not measured |
| Control:N = 7 Age: 76.6(2.4)LVEF(%): 36(2.9)NYHA-FC: 2.3(.2) | Control: 2x/week for 60 min (stretching) for 10 weeks for “sham exercise” | Control: Pre: 365(42) Post: 362(31) Δ: −3 ES: 1.22 | Control: Pre: 14.40(1.13) Post: 14.75(0.9) Δ: 0.35 ESw-g 0.34 ES: 0.71 (decline) | ||
| McKelvie et al 200234 M&HR = 90% | Treatment:N = 90 Age: 64.8(10) LVEF(%): 28.2(8) NYHA-FC: 2.3(.4) | Treatment: 2x/week for 3 months supervised 30 min aerobic exercise at 60-70% max HR using cycling, treadmill, and arm ergometry, and resistance training at 40-60% of 1RM for arm curls, knee extension, and leg press with a 1x/week walk at home. This was followed by a 3x/week for 9 months home-based training program of cycling, and free weights. | Pre: 434(66) Δ at 3 mos: 22(47) | NSR | Treatment: Pre: 32.5(23.7) Δ at 3 mos: −3.9(18.1) ESw-g 0.22 |
| Control:N = 91 Age: 66.1(9.4)LVEF(%): 27.7(9)NYHA-FC: 2.3(.4) | Control: usual level of physical activity | Control:Pre: 421(76)Δ at 3 mos: 15(48) ES: 0.10 | Control: Pre: 28.6(20) Δ at 3 mos: −1.2(14.3)ESw-g 0.08 ES: 0.12 | ||
| Parnell et al 200235 M&HR = 40% | Treatment: N = 11 Age: 57(15) LVEF(%): 25(7) NYHA-FC: 2.4(.5) | Treatment: 3x/week for 30 min to 5-7x/week for 60 min for 8 weeks at 50-60% max HR while walking, using light hand weights and stationary cycling | Treatment: Pre: 474(90) Post: 547(113) Δ: 73 | Not measured | Treatment: Pre: 46(23)Post: 24(17) Δ −22 ESw-g 1.06 |
| Control: N = 10 Age: 53(11) LVEF(%) = 24(10) NYHA-FC: 2.3(.5) | Control: continue with usual lifestyle | Control: Pre: 517(66) Post: 515(92) Δ: −2 ES: 0.95 | Control: Pre: 30(23) Post: 28(23) Δ: −2 ESw-g 0.09 ES: 0.87 | ||
| Van den Berg-Emons et al 200436 M&HR = 50% | Treatment: N = 18 Age: 59(12) LVEF(%): 24(9) NYHA-FC: 2.4(.5) | Treatment: 2x/week for 60 min at 60% HRR aerobic walking and cycling for 3 months | Treatment: Pre: 455(71) Post:501(96) Δ: 46 | Treatment: Pre: 16.6(4.2) Post:17.8(4.0) Δ: 1.2 ESw-g 0.29 | Treatment: Pre: 24.1(19.7) Post:18.1(18.5) Δ: −6 ESw-g 0.31 |
| Control: N = 16 Age: 59(11) LVEF(%) = 28(6) NYHA-FC: 2.4(.5) | Control: Usual care | Control: Pre: 435(77) Post:448(84) Δ: 13 ES: 0.53 | Control: Pre: 16.1(3.8) Post:15.8(2.9) Δ: −0.3 ESw-g −0.09 ES: 0.37 | Control: Pre: 27.5(13.9) Post: 26.5(12.7) Δ: −1 ESw-g 0.07 ES: 0.29 | |
| Corvera-Tindel et al 200427 M&HR = 70% | Treatment: N = 42 Age: 64(10) LVEF(%): 29(9) NYHA-FC: 2.2(.4) | Treatment: 5x/week for 60 min at 65% max home walking program for 12 weeks | Treatment: Pre: 372(74) Post:408(83) Δ: 36 | Treatment: Pre: 14.3(3.7) Post:15.3(3.8) Δ: 1.0 ESw-g 0.27 | Treatment: Pre: 6.4(1.4) Post:6.1(1.6) Δ: −0.3 ESw-g 0.20 |
| Control: N = 37 Age: 61(11) LVEF(%) = 25(9) NYHA-FC: 2.2(.4) | Control: Usual care | Control: Pre: 388(76) Post:385(78) Δ: −3 ES: 0.52 | Control: Pre: 14.2(3.4) Post:15.2(4.1) Δ: 1.0 ESw-g 0.26 ES: 0 | Control: Pre: 6.2(1.4) Post: 6.3(1.5) Δ: 0.1 ESw-g 0.07 ES: 0.29 *HFSSI | |
| Gary et al 200437 M&HR = 50% | Treatment: N = 32 Age: 67(11) LVEF(%): 54(7) NYHA-FC: 2.6(.5) | Treatment: 3x/week for 30 min at 60% max home walking program for 12 weeks with weekly supervision | Treatment: Pre: 256(112) Post: 318(97) Δ: 62 | Not Measured | Treatment: Pre: 41(26) Post: 24(18) Δ: −17 ES w-g 0.78 |
| Control: N = 32 Age: 69(11) LVEF(%) = 57(9) NYHA-FC: 2.6(.5) | Control: 12 home-based education visits | Control: Pre: 251(112) Post:223(124) Δ: 28 ES: 0.30 | Control: Pre: 27(18) Post: 28(22) Δ: 1 ESw-g 0.05 ES: 0.81 | ||
| Yeh et al 200438 M&HR = 70% | Treatment: N = 15 Age: 6(12) LVEF(%): 24(7) NYHA-FC: 2.2(1) | Treatment: 2x/week for 60 min group-based Tai Chi | Treatment: Pre: 327(106) Post:412(116) Δ: 85 | Treatment: Pre: 10.5(3) Post:11.4(3) Δ: 0.9 ESw-g 0.30 | Treatment: Pre: 43(21) Post: 26(23) Δ: −17 ES 0.77 |
| Control: N = 15 Age: 61(14) LVEF(%) = 22(8) NYHA-FC: 2.3(.6) | Control: Usual care | Control: Pre: 340(117) Post:289(165) Δ: −51 ES: 1.22 | Control: Pre: 11.1(6) Post:10.4(6) Δ: −0.7 ESw-g −0.12 ES: 0.34 | Control: Pre: 44(20) Post: 52(25) Δ: 8 ES −0.35 ES: 1.22 | |
| Witham et al 200539 M&HR = 70% | Treatment: N = 41 Age: 80(6) LVEF(%): NSR NYHA-FC: 2.4(.5) | Treatment: 2x/week for 3 months supervised moderate intensity aerobic exercise and strengthening followed 2-3x/week for 3 months home-based exercise | Treatment: Pre: 261(117) Post: 262(110) Δ: 1 | Not measured | Treatment: Pre: 67(13) Post: 65(10) Δ: −2 ESw-g 0.17 |
| Control: N = 41 Ages: 81(4) LVEF(%): NSR NYHA-FC: 2.4(.5) | Control: usual care | Control: Pre: 240(93) Post: 246(111) Δ: 6 ES: 0.05 | Control: Pre: 70(12) Post: 69(13) Δ: −1 ESw-g 0.08 ES: 0.08 *CHQ | ||
| Austin et al200540 M&HR = 70% | Treatment: N = 100 Age: 72(6) LVEF(%): NSR NYHA-FC: 2.5(.5) | Treatment: 2x/week for 8 weeks cardiac rehab program | Treatment: Pre: 276(119) Post: 320(112) Δ: 44 | Not measured | Treatment: Pre: 41(25.5) Post: 22.9(17.9) Δ: −18.1 ESw-g 0.80 |
| Control: N = 100 Ages: 72(7) LVEF(%): NSR NYHA-FC: 2.5(.5) | Control: standard care | Control: Pre: 259(118) Post: 253(132) Δ: −6 ES: 0.42 | Control: Pre: 44.3(24.5) Post: 36.9(24) Δ: −7.4 ESw-g 0.31 ES: 0.43 | ||
| Jonsdottir et al 200641 M&HR = 60% | Treatment: N = 21 Age: 68(7) LVEF(%): 42(14) NYHA-FC: NR | Treatment: 2x/week for 5 months of 15 min moderate intensity aerobic exercise and 20 min low intensity resistance exercise | Treatment: Pre: 489(75) Post:526(72) Δ: 37 | Treatment: Pre: 14.9(3) Post: 14.8(3) Δ: −0.1 ESw-g −0.03 | NSR |
| Control: N = 22 Ages: 69(5) LVEF(%): 41(14) NYHA-FC: NR | Control: Usual care | Control: Pre: 489(66) Post:495(66) Δ: 6 ES: 0.44 | Control: Pre: 16.3(3) Post:16.9(4) Δ: 0.6 ESw-g 0.17 ES: 0.23 (decline) | ||
| Brubaker et al 200942 M&HR = 70% | Treatment: N = 30 Age: 70(5) LVEF(%): 32(9) NYHA-FC: 2.5(.5) | Treatment: 3x/week for 16 weeks of 60 min moderate intensity aerobic exercise | Treatment: Pre: 406(166) Post:462(164) Δ: 56 | Treatment: Pre: 14.1(3.3) Post: 13.9(4.4) Δ: −0.2 ESw-g −0.05 | Treatment: Pre: 39.9(23) Post: 44.1(26) Δ: 4.2 ESw-g −0.17 |
| Control: N = 29 Ages: 70(6) LVEF(%): 30(9) NYHA-FC: 2.5(.5) | Control: Attention control | Control: Pre: 376(214) Post:423(128) Δ: 47 ES: 0.05 | Control: Pre: 13.5(3.2) Post:13.6(3.8) Δ: 0.1 ESw-g 0.03 ES: 0.09 (decline) | Control: Pre: 35.3(23) Post: 37.9(23) Δ: 2.6 ESw-g −0.11 ES: 0.07 | |
| Davidson et al 201043 M&HR = 70% | Treatment: N = 53 Age: 72(NR) LVEF(%): NR NYHA-FC: 2.6(.5) | Treatment: 1x/week for 12 weeks of 30 min endurance exercise | Treatment: Pre: 279(111) Post:361(132) Δ: 82 | Not Measured | Treatment: Pre: 44.1(23.7) Post: 27.9(12.5) Δ: −16.2 ESw-g 0.79 |
| Control: N = 52 Ages: 74(NR) LVEF(%): NR NYHA-FC: 2.7(.5) | Control: Usual care | Control: Pre: 251(113) Post: 275(107) Δ: 24 ES: 0.52 | Control: Pre: 53.4(26.4) Post: 36.9(16.2) Δ: −16.5 ESw-g 0.72 ES: 0.01 | ||
| Kitzman et al 201044 M&HR = 70% | Treatment: N = 26 Age: 70(5) LVEF(%): NR NYHA-FC: 2.2(.4) | Treatment: 3x/week for 16 weeks of 60 min moderate intensity aerobic exercise | Treatment: Pre: 455(68) Post:506(53) Δ: 51 | Treatment: Pre: 13.8(2.5) Post: 16.1(2.6) Δ: 2.3 ESw-g 0.90 | Treatment: Pre: 32(20) Post: 25(24) Δ: −7 ESw-g 0.31 |
| Control: N = 27 Ages: 70(6) LVEF(%): NR NYHA-FC: 2.4(.5) | Control: Attention control | Control: Pre: 430(116) Post:445(125) Δ: 15 ES: 0.38 | Control: Pre: 12.8(2.6) Post:12.5(3.4) Δ: −0.3 ESw-g −0.10 ES: 1.02 | Control: Pre: 25(22) Post: 27(19) Δ: 2 ES 0.10 ES: 0.43 | |
| Gary et al 201045 M&HR = 100% | Treatment: N = 17 Age: NSR LVEF(%):NSR NYHA-FC: NSR | Treatment: 3x/week for 12 weeks of 30-60 min home-based walking program and 3 sessions of Cognitive Behavioral Therapy | Treatment: Pre: 363(100) Post:401(103) Δ: 38 | Not Measured | Treatment: Pre: 34.3(23.7) Post: 27.3(16) Δ: −7 ESw-g 0.33 |
| Control: N = 18 Ages: NSR LVEF(%):NSR NYHAFC: NSR | Control: Usual care | Control: Pre: 347(166) Post: 287(125) Δ: −60 ES: 0.72 | Control: Pre: 28.1(17.3) Post: 26.4(23.7) Δ: −1.7 ESw-g 0.08 ES: 0.26 | ||
Abbreviations: M&HR, Medlicott and Harris rating scale of methodological rigor; LVEF, left ventricular ejection fraction; HF, heart failure; NR, not reported; 6MWT, six-minute walk test; VO2, oxygen consumption; HRQL, health-related quality of life; ESw-g, within-group effect size assuming correlation of 0.5; ES, between-group effect size; NYHA-FC, New York Heart Association functional class
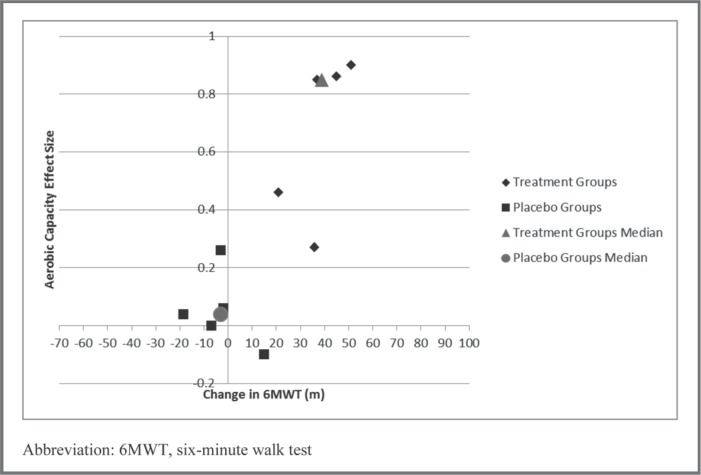
Eleven studies6, 27, 30, 31, 32, 33, 36, 38, 41, 42, 44 measured both the 6MWT and aerobic capacity. Visual analysis of the scatterplots depicting Δ6MWT versus the within-group effect size for aerobic capacity in both the treatment and intervention groups revealed that Δ6MWT for the intervention groups were all greater than 20 m compared to only one placebo group exceeding 20 m. However, most aerobic capacity effect sizes in the intervention groups were small (0.2–0.5), with some having no effect or a small effect in the opposite direction. In further exploring these results, only 56, 27, 30, 31, 44 of the 11 studies that measured aerobic capacity used an intervention that was of sufficient frequency (≥ 3 days per week), intensity (> 65% of predicted or measured maximal capacity), duration (≥ 25 minutes per session) and type (large muscle groups, rhythmic motion) to be reasonably expected to produce a change in aerobic capacity. Therefore, in plotting the data from only these 5 studies, moderate aerobic capacity effect sizes for the intervention group only appeared to be associated with a Δ6MWT greater than 40 m (Figure 4).
Figure 4.
Scatterplot of change in six-minute walk test distance and aerobic capacity within-group effect size.
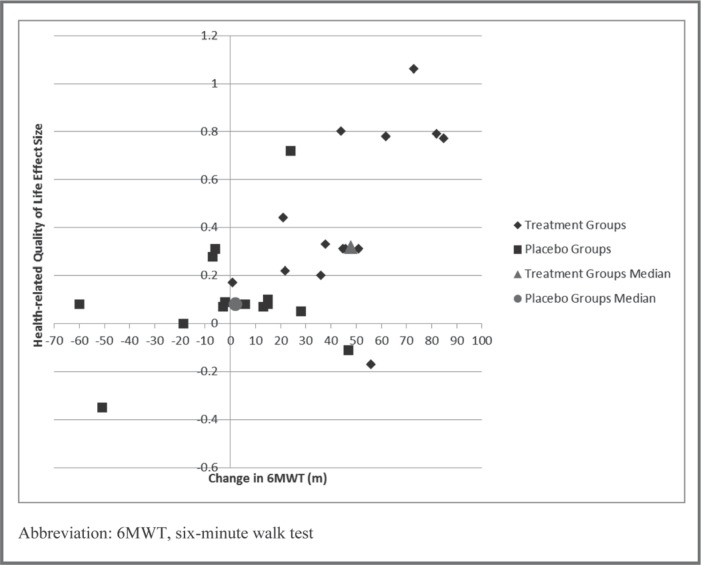
Fourteen studies6, 27, 31, 34, 35, 36, 37, 38, 39, 40, 42, 43, 44, 45 measured and sufficiently reported both 6MWT and HRQL. Visual analysis of the scatterplots depicting Δ6MWT versus the within-group effect size for HRQL in both the treatment and intervention groups revealed that Δ6MWT for the intervention groups were all greater than 20 m compared to only one placebo group exceeding 20 m (Figure 5). Reported Δ6MWT distance for the intervention groups were all more than 20 m compared to only one placebo group exceeding 20 m; however, moderate HRQL effect sizes for the intervention group only appeared to be associated with a Δ6MWT greater than 45 m. There appears to be a linear association between the magnitude of Δ6MWT and the magnitude of within-group effect size for HRQL, with an apparent threshold of 35 m discriminating between intervention and placebo groups. It should be noted that the intervention group in the Brubaker et al43 study had a HRQL effect size indicating decline. Brubaker et al42 also demonstrated statistically insignificant changes in 6MWT distances of 56 and 47 m for the intervention and placebo groups, respectively, and had near-zero between-subject effect sizes in all measures (6MWT, VO2, and HRQL).
Figure 5.
Scatterplot of change in six-minute walk test distance and health-related quality of life.
With regard to the quantitative analysis of the differences in Δ6MWT, aerobic capacity effect size, and HRQL, intervention and control groups were significantly different for Δ6MWT (Mdn = −1 vs 45.5, U= 21.0, p=.000, r=.74), aerobic capacity (Mdn = .04 vs .85, U= 0.0, p=.008, r=.83), and HRQL (Mdn = .08 vs .32, U= 28.5, p=.001, r=.60). The coordinates of the median for each variable are plotted in Figures 4 and 5 to provide quantitative support for the visual analysis.
Quantitative analysis of the Δ6MWT threshold that discriminated between intervention and control groups using ROC curves revealed the following sensitivity/specificity for the respective thresholds: 19 m, 94.4/83.3%, 32 m, 83.3/94.4%, and 48 m 44.4/100% (AUC = .935, p = .009, CI95% .855, 1.015).
DISCUSSION
Few studies have investigated the Δ6MWT that is necessary to be considered clinically meaningful. There are numerous methods for determining this threshold, and because no single method is considered to be optimal, triangulation of the threshold for clinically meaningful change is recommended.15, 16, 17 Therefore, the purpose of the present review was to use several different methods for analysis of existing data, including the calculation of the range of MDD values based on reported ICCs, as well as using an ecologic analysis of RCT group-level data to determine what amount of change discriminates between exercise intervention and control groups. Each method used in the present review resulted in similar estimates of a threshold for clinically meaningful Δ6MWT and were consistent with those already reported in the literature.
The initial range of calculated MDD95% values was quite large; however, the majority of MDD95% values were between 20 m and 40 m. Two outliers were apparent, with one (Ingle et al)28 likely due to the lengthy test-retest interval of one year that allows for innumerable confounding factors. With regard to the second outlier (Roul et al),21 numerous variables were examined to determine their association with the calculated MDD95% which is based on the ICC and sample standard deviation. Although there appeared to be no study or sample characteristic that may have been responsible for the relatively low ICC reported by Roul et al,21 this was considered to be an outlier given that the MDD95% was nearly 3 times greater than the others. With these outliers removed, the mean MDD95% was 43 m. This suggests that in order to have a reasonable degree of confidence that a Δ6MWT was not due to test-retest variability or measurement error, the amount of change must exceed 43 m. This is consistent with the MDD95% calculation by Pinna et al8 and with the MCID of 40 m for those subjects who reported clinical improvement over a 4-week period in the study by O'Keefe et al.10
With regard to the ecologic analysis of group-level data for subjects enrolled in exercise-based RCTs, the median change in 6MWT for intervention groups was 45.5 m compared to −1 m for the placebo groups. In plotting the individual group data points against the effect sizes for the concurrent changes in aerobic capacity and HRQL, visual analysis of the scatterplots suggest that only the intervention groups had a Δ6MWT of greater than 20 m. However, moderate effect sizes in aerobic capacity and HRQL did not emerge until 40 m. Additionally, the use of ROC curves to quantify the optimal Δ6MWT threshold to discriminate between exercise-based intervention and control groups revealed that, when biasing the threshold toward 100% specificity (and therefore ensuring that true clinical change has occurred), the optimal threshold was 48 m. This value aligns very well with the mean MDD95% (an index of measurement error) of 43 m calculated in the first analysis. Therefore, a threshold of approximately 45 m should be used for determining clinically meaningful change.
This proposed clinically meaningful threshold of 45 m based on the present review is consistent with Pinna et al8 and O'Keefe et al,10 and because of the good alignment of the thresholds determined by multiple methods used in the present review and those of two other studies, confidence in this threshold can be fairly high. However, no study to date has prospectively evaluated and reported different methods of determining clinically meaningful change in individuals with CHF, which would further reveal how these different methods relate to one another. Therefore, future studies should incorporate several different methods for determining clinically meaningful change in the same sample to help further elucidate advantages and disadvantages of different methods for determining clinically meaningful changes, and whether good alignment of meaningful change threshold estimates are consistently observed.
There are several limitations to the present analyses. Calculation of the MDD95% values for several studies required use of the baseline standard deviation for the entire sample because it was not reported for the sub-sample in whom the ICC was calculated. However, doing so did not reveal any consistent effect because the range of calculated MDD95% values from those 4 studies were similar to the others.
A potential limitation of the analysis based on the literature search for concurrent changes in exercise-based RCT outcomes is the underlying assumption that the 6MWT is in fact associated with these variables and that these variables are responsive to exercise interventions. However, previous research supports this assumption,13, 14, 20, 48, 49, 50 and changes in exercise capacity and/or HRQL likely represent meaningful changes resulting from an individual's participation in exercise interventions.
CONCLUSIONS
The present review used two different literature searches based on two different constructs for determining a clinically meaningful change threshold for the 6MWT. A change of 45 m in the 6MWT exceeds measurement error and is associated with moderate aerobic capacity and HRQL within-group effect sizes reported by exercise-based RCTs.
ACKNOWLEDGMENT
This paper was completed in partial fulfillment of the PhD in Interdisciplinary Health Sciences at Western Michigan University.
REFERENCES
- 1.Eriksson H. Heart failure: a growing public health problem. J Intern Med. 1995;237:135–141. doi: 10.1111/j.1365-2796.1995.tb01153.x. [DOI] [PubMed] [Google Scholar]
- 2.Perk J, Mathes P, Gohlke H, Monpere C, Hellemans I, McGee H, Sellier P, Saner H. Cardiovascular Prevention and Rehabilitation. London: Springer; 2007. [Google Scholar]
- 3.Califf RM, Prystowsky EN, Thomas JD, Thompson PD, Topol EJ. Textbook of Cardiovascular Medicine. 3rd. Philadelphia, PA: Lippincott Williams & Wilkins;; 2007. [Google Scholar]
- 4.Berra K, Hamm LF, Kavanagh T. Champaign, IL: Human Kinetics; 2006. AACVPR Cardiac Rehabilitation Resource Manual. [Google Scholar]
- 5.Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Philedelphia, PA: Lippincott Williams & Wilkins; 2005. Principles of Exercise Testing and Interpretation. [Google Scholar]
- 6.Tynni-Lenne R, Gordan A, Sylven C. Improved quality of life in chronic heart failure patients following local endurance training with leg muscles. J Cardiac Failure. 1996;2((2)):111–117. doi: 10.1016/s1071-9164(96)80029-7. [DOI] [PubMed] [Google Scholar]
- 7.Guyatt GH, Sullivan MJJ. Fallen EL, et al. A controlled trial of digoxin in congestive heart failure. Am J Cardiol. 1988;61:371–375. doi: 10.1016/0002-9149(88)90947-2. [DOI] [PubMed] [Google Scholar]
- 8.Opasich C, Pinna GD, Mazza A, et al. Reproducibility of the six-minute walking test in patients with chronic congestive heart failure: practical implications. Am J Cardiol. 1998;81:1497–1500. doi: 10.1016/s0002-9149(98)00218-5. [DOI] [PubMed] [Google Scholar]
- 9.Pinna GD, Opasich C, Mazza A, Tangenti A, Maestri R, Sanarico M. Reproducibility of the six-minute walking test in chronic heart failure patients. Statist Med. 2000;19:3087–3094. doi: 10.1002/1097-0258(20001130)19:22<3087::aid-sim628>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 10.O'Keeffe ST, Lye M, Donnellan C, Carmichael DN. Reproducibility and responsiveness of quality of life assessment and six minute walk test in elderly heart failure patients. Heart. 1998;80:377–382. doi: 10.1136/hrt.80.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spertus J, Peterson E, Conard MW, et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150:707–15. doi: 10.1016/j.ahj.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Olsson LG, Swedberg K, Clark AL, Witte KK, Cleland JGF. Six-minute corridor walk test as an outcome measure for the assessment of treatment in randomized, blinded intervention trials of chronic heart failure: a systematic review. Eur Heart J. 2005;25:778–793. doi: 10.1093/eurheartj/ehi162. [DOI] [PubMed] [Google Scholar]
- 13.Rees K, Taylor RS, Singh S, Coats AJS. Ebrahim S. Exercise based rehabilitation for heart failure. Cochrane Database Syst Rev. 2004;((3)) doi: 10.1002/14651858.CD003331.pub2. CD003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies EJ, Moxham T, Rees K, et al. Exercise based rehabilitation for heart failure. Cochrane Database Syst Rev. 2010;((4)) doi: 10.1002/14651858.CD003331.pub3. CD003331. [DOI] [PubMed] [Google Scholar]
- 15.Crosby RD, Kolotkin RL, Williams GR. An integrated method to determine meaningful changes in health-related quality of life. J Clin Edipemiol. 2004;57:1153–1160. doi: 10.1016/j.jclinepi.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61:102–109. doi: 10.1016/j.jclinepi.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Norman GR, Stratford P, Regehr Methodological problems in the retrospective computation of responsiveness to change: the lesson of Cronbach. J Clin Epidemiol. 1997;50:869–879. doi: 10.1016/s0895-4356(97)00097-8. [DOI] [PubMed] [Google Scholar]
- 18.Beaton DE, Bombardier C, Katz JN, et al. Looking for important change/differences in studies of responsiveness. OMERACT MCID Working Group. Outcome Measures in Rheumatology. Minimal Clinically Important Differences. J Rheumatol. 2001;28:400–405. [PubMed] [Google Scholar]
- 19.Medlicott MS, Harris SR. A systematic review of the effectiveness of exercise, manual therapy, electrotherapy, relaxation training, and biofeedback in the management of temporo-mandibular disorder. Phys Ther. 2006;86:955–973. [PubMed] [Google Scholar]
- 20.Cahalin LP, Mathier MA, Semigran MJ, Dec W, DiSalvo TG. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest. 1996;110:325–332. doi: 10.1378/chest.110.2.325. [DOI] [PubMed] [Google Scholar]
- 21.Roul G, Germain P, Bareiss P. Does the 6-minute walk test predict the prognosis in patients with NYHA class II or III chronic heart failure? Am Heart J. 1998;136:449–457. doi: 10.1016/s0002-8703(98)70219-4. [DOI] [PubMed] [Google Scholar]
- 22.Morales FJ, Martinez A, Mendez M, et al. A shuttle walk test for assessment of functional capacity in chronic heart failure. Am Heart J. 1999;138((2)):292–297. doi: 10.1016/s0002-8703(99)70114-6. [DOI] [PubMed] [Google Scholar]
- 23.Zugck C, Kruger C, Durr S, et al. Is the 6-minute walk test a reliable substitute for peak oxygen uptake in patients with dilated cardiomyopathy. Eur Heart J. 2000;21:540–549. doi: 10.1053/euhj.1999.1861. [DOI] [PubMed] [Google Scholar]
- 24.Demers C, McKelvie RS, Negassa A, Yusuf S. Reliability, validity, and responsiveness of the six-minute walk test in patients with heart failure. Am Heart J. 2001;142((4)):698–703. doi: 10.1067/mhj.2001.118468. [DOI] [PubMed] [Google Scholar]
- 25.Gary RA, Sueta CA, Rosenberg B, Cheek D. Use of the 6-minute walk test for women with diastolic heart failure. J Cardiopulm Rehabil. 2004;24:264–268. doi: 10.1097/00008483-200407000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Kervio G, Wille NS, Leclercq C, Daubert JC, Carre F. Intensity and daily reliability of the six-minute walk test in moderate chronic heart failure patients. Arch Phys Med Rehabil. 2005;85:1513–1518. doi: 10.1016/j.apmr.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 27.Corvera-Tindel T, Doering LV. Woo MA. Khan S. Dracup K. Effects of a home walking exercise program on functional status and symptoms in heart failure. Am Heart J. 2004;147((2)):339–346. doi: 10.1016/j.ahj.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Ingle L, Shelton RJ, Rigby AS, Nabb S, Clark AL, Cleland JGF. The reproducibility and sensitivity of the 6-minute walk test in elderly patients with chronic heart failure. Eur Heart J. 2005;26:1742–1751. doi: 10.1093/eurheartj/ehi259. [DOI] [PubMed] [Google Scholar]
- 29.Ingle L, Wilkinson M, Carroll S, Boyes C, et al. Cardiorespiratory requirements of the 6-min walk test in older patients with left ventricular systolic dysfunction and no major structural heart disease. Int J Sports Med. 2007;28:678–684. doi: 10.1055/s-2007-964886. [DOI] [PubMed] [Google Scholar]
- 30.Tyni-Lenne R, Gordon A, Jansson E, Bermann G, Sylven C. Skeletal muscle endurance training improves peripheral oxidative capacity, exercise tolerance, and health-related quality of life in women with chronic congestive heart failure secondary to either ischemic cardiomyopathy or idiopathic dilated cardiomyopathy. Am J Cardiol. 1997;80:1025–1029. doi: 10.1016/s0002-9149(97)00597-3. [DOI] [PubMed] [Google Scholar]
- 31.Gottlieb SS, Fisher ML, Freudenberger R, et al. Effects of exercise training on peak performance and quality of life in congestive heart failure patients. J Card Fail. 1999;5:188–194. doi: 10.1016/s1071-9164(99)90002-7. [DOI] [PubMed] [Google Scholar]
- 32.Tyni-Lenne R, Dencker K, Gordon A, Jansson E, Sylven C. Comprehensive local muscle training increases aerobic working capacity and quality of life and decreases neurohormonal activation in patients with chronic heart failure. Eur J Heart Fail. 2001;3:47–52. doi: 10.1016/s1388-9842(00)00087-8. [DOI] [PubMed] [Google Scholar]
- 33.Pu CT, Johnson MT, Forman DE, et al. Randomized trial of progressive resistance training to counteract the myopathy of chronic heart failure. J Appl Physiol. 2001;90:2341–2350. doi: 10.1152/jappl.2001.90.6.2341. [DOI] [PubMed] [Google Scholar]
- 34.McKelvie RS, Teo KK, Roberts R, et al. Effects of exercise training in patients with heart failure: the exercise rehabilitation trial (EXERT). Am Heart J. 2002;144((1)):23–30. doi: 10.1067/mhj.2002.123310. [DOI] [PubMed] [Google Scholar]
- 35.Parnell MM, Holst DP, Kaye DM. Exercise training increases arterial compliance in patients with congestive heart failure. Clin Sci. 2002;102((1)):1–7. [PubMed] [Google Scholar]
- 36.Van den Berg-Emons R. Balk A. Bussmann H. Stam H. Does aerobic training lead to a more active lifestyle and improved quality of life in patients with chronic heart failure? Eur J Heart Failure. 2004;6:95–100. doi: 10.1016/j.ejheart.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Gary RA, Sueta CA, Dougherty M, et al. Home-based exercise improves functional performance and quality of life in women with diastolic heart failure. Heart Lung. 2004;33((4)):210–218. doi: 10.1016/j.hrtlng.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Yeh GY, Wood MJ, Lorell BH, et al. Effects of tai chi mind-body movement therapy on functional status and exercise capacity in patients with chronic heart failure: a randomized controlled trial. Am J Med. 2004;117:541–548. doi: 10.1016/j.amjmed.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 39.Witham MD, Gray JM, Argo IS. Effect of a seated exercise program to improve physical function and health status in frail patients > 70 years of age with heart failure. Am J Cardiol. 2005;95:1120–1124. doi: 10.1016/j.amjcard.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 40.Austin J, Williams R, Ross L, Moseley L, Hutchison S. Randomised controlled trial of cardiac rehabilitation in elderly patients with heart failure. Eur J Heart Fail. 2005;7:411–417. doi: 10.1016/j.ejheart.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Jonsdottir S, Andersen KK, Sigurosson AF, Sigurosson SB. The effect of physical training in chronic heart failure. Eur J Heart Fail. 2006;8:97–101. doi: 10.1016/j.ejheart.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Brubaker PH, Moore JB, Stewart KP, Wesley DJ, Kitzman DW. Endurance exercise training in older patients with heart failure: results from a randomized, controlled single-blind trial. J Am Geriatr Soc. 2009;57((11)):1982–1989. doi: 10.1111/j.1532-5415.2009.02499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davidson PM, Cockburn J, Newton PJ, et al. Can a heart failure-specific cardiac rehabilitation program decrease hospitalizations and improve outcomes in high-risk patients? Eur J Cardiovasc Prev Rehabil. 2010;17((4)):393–402. doi: 10.1097/HJR.0b013e328334ea56. [DOI] [PubMed] [Google Scholar]
- 44.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2010;3((6)):659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gary RA, Dunbar SB, Higgins MK, Musselman DL, Smith AL. Combined exercise and cognitive behavioral therapy improves outcomes in patients with heart failure. J Psychosom Res. 2010;69:119–131. doi: 10.1016/j.jpsychores.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ott AE, Pate RR, Trost SG, Ward DS, Saunder R. The use of uniaxial and triaxial accelerometers to measure children's free play physical activity. Pediatr Exerc Sci. 2000;71:36–43. [Google Scholar]
- 47.American Thoracic Society, Board of Directors ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 48.Cahalin L, Pappagianopolous P, Prevost S, Wain J, Ginns L. The relationship of the six-minute walk test to maximal oxygen consumption in transplant candidates with end-stage lung disease. Chest. 1995;108:452–459. doi: 10.1378/chest.108.2.452. [DOI] [PubMed] [Google Scholar]
- 49.Riley M, McParland J, Stanford CF, et al. Oxygen consumption during corridor walk testing in chronic cardiac failure. Eur Heart J. 1992;13:789–793. doi: 10.1093/oxfordjournals.eurheartj.a060258. [DOI] [PubMed] [Google Scholar]
- 50.Lucas C, Stevenson LW, Johnson W, et al. The six-minute walk and peak oxygen consumption in advanced heart failure: aerobic capacity and survival. Am Heart J. 1999;138:618–624. doi: 10.1016/s0002-8703(99)70174-2. [DOI] [PubMed] [Google Scholar]