Abstract
Purpose
The purpose of this review was to assess the quality of evidence on inspiratory muscle training (IMT) in patients with heart failure and to provide an overview on subject selection, training protocols, and outcome achieved with IMT.
Methods
Literature search was first performed via the PubMed database, and additional references were identified from the Scopus citation index. Articles of the review type and of clinical trials published in English were included. Quality of the articles was assessed using Sackett's levels of evidence and rigor of methodology was assessed using PEDro (Physiotherapy Evidence Database) criteria for randomized controlled trials and the Downs & Black tool for cohort studies.
Results
Twelve articles of clinical trials were included. Typical training protocols involved daily training with intensity greater than 30% of maximal inspiratory pressure (PImax), duration of 20 to 30 minutes (continuous or incremental) and using a pressure threshold muscle trainer. The effect sizes of PImax, walk test distance, and dyspnea were moderate to large across these studies. Effects on quality of life scores were inconsistent.
Conclusion
Inspiratory muscle training is beneficial for improving respiratory muscle strength, functional capacity, and dyspnea in patients with stable heart failure and respiratory muscle weakness.
Key Words: inspiratory muscle training, heart failure, maximal inspiratory pressure
INTRODUCTION
Chronic heart failure (CHF) is a clinical condition where the heart is unable to provide adequate tissue perfusion, especially to the vital organs (eg, the brain, the liver, and the kidneys).1 In the adult population, about 3% to 6% are diagnosed with CHF, and in adults more than 60 years old, approximately 13% have been diagnosed with CHF.2 With exercise, these patients exhibit increased airway resistance and ventilatory response. Fatigue and dyspnea are the common symptoms that lead to exercise intolerance and reduced quality of life in patients with heart failure.3
Inspiratory muscle weakness and peripheral skeletal muscle dysfunction have been thought to be part of the underlying mechanisms for fatigue, dyspnea, and exercise intolerance in patients with heart failure. Structural and biochemical alterations of the diaphragm muscle have been observed, including increased proportion of type I fibers, reduced type IIb fibers, and fiber atrophy due to chronic increase in diaphragm load and systemic myopathy.4 Altered intracellular calcium regulation and overexpression of cytokines, especially tumor necrosis factor-α (TNF-α), also contribute to respiratory muscle dysfunction. The TNF-α could stimulate mitochondrial production of reactive species, which in turn could cause degeneration of cristae and impaired electron transport.5, 6
Inspiratory muscle strength can be assessed by the maximal inspiratory pressure (PImax). It is measured at the mouth level by asking the subject taking a maximal inspiration while at residual volume and sustained for at least one second. According to the force-length relationship, the higher the position of the diaphragm (the longer the resting length of the diaphragm, or the lower the lung volume), the higher the PImax. This measure is independent of the patient's respiratory flow and is highly reproducible.7 In patients with heart failure, PImax lower than 70% of the predicted value indicates respiratory muscle weakness.8 Inspiratory muscle endurance refers to the ability to sustain a certain respiratory pressure over time which can be measured in several different ways. One common way is to ask the subject to sustain the PImax over time to obtain the sustained maximal inspiratory pressure.9, 10, 11 Another way is to measure the highest pressure that the subject can maintain for at least one minute (Pth max).8
Several different types of inspiratory muscle training (IMT) devices can be used for training, including the resistive type of muscle trainer, the pressure threshold muscle trainer, and the isocapnia hyperpnea maneuver. The resistive-type, hand-held inspiratory muscle trainer uses various small diameter orifices to provide resistance to inspiration, but its major disadvantage is that the pre-set resistance could vary with the subject's breathing flow rate. The pressure threshold type, hand-held, respiratory muscle trainer (Respironics Health Scan, Inc., Cedar Grove, NJ) is the most standard device, where the individual needs to generate the required flow-independent inspiratory pressure to open a valve in the device. The isocapnic hyperpnea method, which can only be performed in a well-equipped respiratory physiology laboratory, requires the individual to maintain a certain level of ventilation in the form of volitional hyperpnea for 12 minutes while various amounts of carbon dioxide are added to the inspired air to maintain isocapnia in the arterial blood.12 The target level of ventilation is then increased incrementally until the individual's volitional fatigue. For patients with preserved inspiratory muscle strength, a device that can reach a higher pressure is thought to be more beneficial (PowerBreathe, HaB International Ltd., Southam, Warwickshire, UK). For respiratory muscle endurance, a computer-controlled biofeedback device is commonly used (TRAINAIR, Project Electronics Ltd., Kent, UK),9, 11, 13 but it is expensive and is not commonly used in the home setting.
There is evidence of benefits from IMT in patients with chronic obstructive pulmonary disease,14, 15, 16 and such experience may be extended to patients with heart failure. Beneficial effects of IMT have been reported in patients with heart failure,3, 6, 17 including improved inspiratory muscle strength,8, 10, 13, 18, 19, 20 respiratory endurance,21 functional capacity,9, 18, 21 quality of life,18, 20, 22 and reduced dyspnea.13, 17, 18, 23, 24 Furthermore, a combination of IMT and aerobic exercise training can result in additional improvement in functional capacity.20 Yet to our knowledge, the systematic levels of evidence on IMT in patients with heart failure have not been reported before.
The purposes of this literature review are to: (1) examine the quality of evidence on IMT in patients with heart failure, (2) summarize the evidence and provide guidelines for clinicians, and (3) identify areas of future research on inspiratory muscle training.
METHODS
Literature Search
The literature search was completed using the PubMed database and Scopus citation index. Key words used in the initial search were: “inspiratory muscle training,” “respiratory muscle training,” and all literature published in English was included, including review articles and clinical trials. The second step for limiting and refining the initial search was to include only those articles on patients with “heart failure” or “congestive heart failure.” The third step involved identifying select articles of clinical trials cited by the review articles. Furthermore, articles from the Scopus index that cited other clinical trials we found were also identified. A total of 12 articles of clinical trials were included in this review.
Levels of Evidence and Methodological Rigor
We used Sackett's levels of evidence25 to rate the strength of evidence. The levels of evidence are listed in Table 1. For randomized controlled trials (RCTs), the methodological rigor, or the validity, was assessed using PEDro (Physiotherapy Evidence Database) scoring26 (Table 2). There are a total of 10 items in this scale, with each item given an individual possible score of 10 to give a total score of 100. Each article was evaluated by the 10 criteria. The score of each criterion was summed and divided by the total score of 100 points to derive the percentage defined as the rigor of that specific RCT study. For nonrandomized studies, the checklist of Downs & Black tool was used.27 All authors participated in the literature search, the critical appraisal for their assigned articles, and the writing of summary of articles. Discussions on the PEDro scoring and the appraisal process for each of the 12 articles occurred through several group meetings, and the levels of evidence were compiled.
Table 1.
Sackett's Levels of Evidence25
| 1A | Systemic Review of Randomized Controlled Trials |
| 1B | RCTs with Narrow Confidence Interval |
| 1C | All or None Case Series |
| 2A | Systemic Review Cohort Studies |
| 2B | Cohort Study/Low Quality RCTs |
| 2C | Outcomes Research |
| 3A | Systematic Review of Case-Controlled Studies |
| 3B | Case-controlled Study |
| 4 | Case Series, Poor Cohort Case Controlled |
| 5 | Expert Opinion |
Abbreviations: RCTs, randomized controlled trials
Table 2.
PEDro Scoring Criteria for Randomized Controlled Trials26
| 1 | Randomization |
| 2 | Inclusion and exclusion criteria were listed for subjects |
| 3 | Similarity of groups at baseline |
| 4 | The treatment protocol was sufficiently described to be replicable |
| 5 | Reliability of data obtained with the outcome measures was investigated |
| 6 | Validity data obtained with the outcome measures was addressed |
| 7 | Blinding of patient, and/or treatment provider, and/or assessor was performed (if possible and appropriate) |
| 8 | Dropouts were reported |
| 9 | Long-term (6 month or greater) results were addressed via follow-up |
| 10 | Adherence to home programs was investigated (if included in the intervention) |
To determine the magnitude of the intervention effects, we calculated the effect sizes on the following outcome variables: inspiratory muscle strength, peak oxygen consumption (VO2peak), functional capacity (walk test distance or exercise endurance), and dyspnea. The effect size (d) of treatment (pre- vs. post- or experimental vs. control) was calculated according to the following equation28:
where d is Cohen's effect size, meane refers to the mean of the experimental group, meanc refers to the mean of the control group, and Spool is the pooled variance estimate calculated as follows:
where n1, n2 are pre- and postintervention sample sizes, or experimental vs. control group sample sizes; S1, S2 are pre-and postintervention standard deviations, or experimental vs. control group standard deviations. According to Cohen, an effect size of 0.2 represents a small effect, 0.5 represents a moderate effect, and 0.8 represents a large effect.28
RESULTS
Three review articles on inspiratory muscle training in patients with heart failure were identified for the purpose of broad overview.3, 6, 17 These 3 articles did not involve details of critical appraisal, but provided a good summary of the pathophysiology of chronic heart failure, the mechanisms and the effects of respiratory muscle training. A number of articles were originally found using the search strategy mentioned above plus those identified from the reference list of the 3 review articles and citation articles. One article published in Spanish (2001 Martinez) was excluded. Two articles related to mechanisms of inspiratory muscle training were not included in our summary table.1, 29
Overall, a total of 12 articles were included for this review. Briefly, we divided these articles into 3 categories. The first category included those cohort studies involving pre/post experimental design. The second category involved those with a control group that was either randomly assigned, or age- and gender-matched. The control group included a sham IMT (no load), a low intensity fixed load, or receiving education only. The third category was inspiratory muscle training with/without an exercise training program. For the IMT training protocol, we mainly focused on training duration, frequency, intensity, and the training device used. The results of our summary are shown in Table 3.
Table 3.
Summary of Evidence
| Year, author, level of evidence and rigor | Subjects | Design | Protocol | outcomes |
|---|---|---|---|---|
|
Pre/post test |
|
||
|
Pre/post |
|
||
|
|
|
Treatment Group:
|
|
|
RCT |
|
Treatment Group:
|
|
|
|
|
Treatment Group:
|
|
|
|
|
Treatment group:
|
|
|
|
|
High Intensity Group:
|
|
|
|
|
||
|
RCT |
|
Treatment group increased:
Control group:(NS)
|
|
|
|
|
Treatment group:
Control group:
|
|
|
RCT |
|
Treatment group (AE+IMT):
|
|
|
|
|
Treatment group
|
<0.05;
<0.01;
<:0.001;
between groups; each value in the table was expressed as “mean (standard deviation)”.
Abbreviations: EF, ejection fraction; NYHA, New York Heart Association; F, frequency; T, treatment time; I, intensity; PImax, maximal inspiratory mouth pressure; PEmax, maximal expiratory mouth pressure; 6MWD, 6 min walk test distance; 12MWT, 12 minute walk test; (VO2 peak) peak O2 uptake; kPa, kilopascals; M/F, male/female; FEV1, forced expiratory volume in one second; IMT, inspiratory muscle training; SMIP, sustained maximal inspiratory pressure; LVEF, left ventricular ejection fraction; OUES, oxygen uptake efficiency slope; AE, aerobic exercise; n, number of patients.
The mean age of subjects in these 12 studies ranged from 47.1 to 71.4 years old, so these studies focused primarily on middle-aged to older adults. One study did not give the age of their participants.19 For a total of 265 subjects in the 12 studies, the sample sizes were all relatively small with an average of 24. Among all the subjects, 171 of them were male and 53 were female. For the degree of cardiovascular impairments, the ejection fraction ranged from 23% to 39%, and the average New York Heart Association (NYHA) functional classification was 2.3, indicating that participants were either Class II or Class III, except in one study which included patients who were waiting for transplant.23 For the maximal inspiratory pressure, some studies used the abbreviation of PImax and some used MIP. We standardized it to PImax in this review. PImax was usually expressed in cmH2O, but it can also be expressed in kilopascals (1 kPa = 10.19 cmH2O).3 The PImax of these studies ranged from 58.0 to 80.8 cmH2O indicating that most of participants had a certain degree of respiratory muscle weakness. These studies excluded patients with pulmonary pathology based on spirometry data.
The workout regimen was largely based on a certain percentage of the initial maximal inspiratory pressure (PImax) or sustained maximal inspiratory pressure (SMIP).9, 10, 11, 13 The training intensity ranged from 20% to 60% in the treatment group, with 30% of PImax being the most common. The intensity was readjusted based on a new PImax assessed weekly. The sustained maximal inspiratory pressure (SMIP) ranged from 15% to 60% of SMIP. For the control group, the training intensity varied from no load (Sham) to a 15% PImax (or SMIP). The total training period ranged from 8 to 12 weeks and the training duration at each session varied from 15 minutes 2 times per day (for the majority) to 30 minutes once daily. Some treatments were incremental accomplishment based and not time based. Only one study examined the long term effect at one-year follow up,8 and another study examined whether the respiratory muscle training provided additional benefits to the aerobic exercise training.20 Most of these 12 studies used pressure threshold respiratory muscle trainer except for one study that used isocapnic hyperpnea method.12 Three studies assessed respiratory muscle endurance using an incremental protocol via a computer-controlled biofeedback device (TRAINAIR, Project Electronics Ltd., Kent, UK).9, 11, 13
All studies included in this review outlined the training procedure and testing methods in details. Each study used validated and standardized outcome measures to assess the benefits of IMT such as PImax, maximal expiratory pressure (PEmax), dyspnea, sustained maximal inspiratory pressure, and peak oxygen consumption (VO2peak). Dyspnea was measured using the Borg Rating of Perceived Exertion scale12, 30, 31 or the Modified Medical Research Council dyspnea scale.22, 31, 32 Fatigue was evaluated using the Fatigue Severity Scale.33 Depression severity was evaluated using Montgomery Depression Rating Scale.34, 35, 36 Quality of life was assessed using the Minnesota Living with Heart Failure Questionnaire33 or the SF-36.37, 38 Functional exercise capacity was assessed by cardiopulmonary symptom-limited exercise test with gas analysis using bike or treadmill protocol, the 12-minute walk test, treadmill test time, corridor walk time, or 6-minute walk test.39, 40
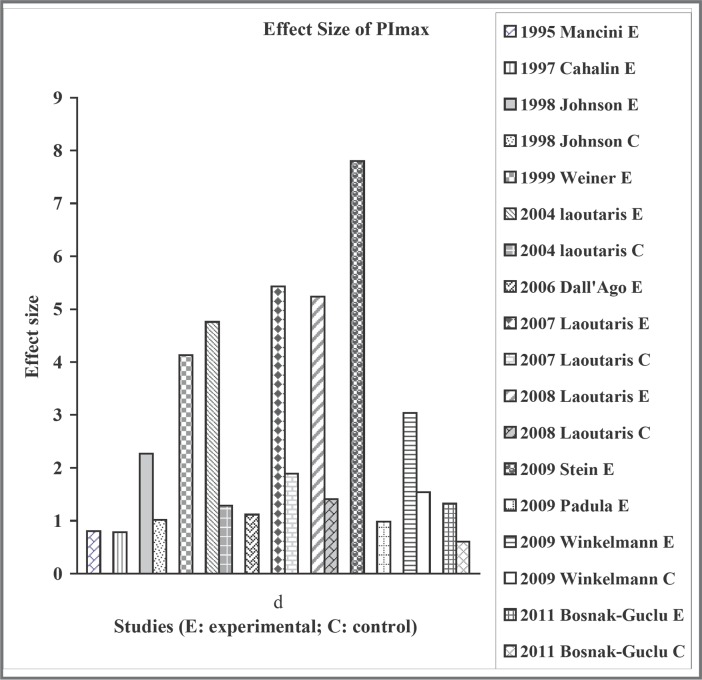
All studies reported on PImax. The effect sizes were large, ranging from 0.78 to 5.43 for the experimental groups (Figure 1). Studies with the largest effect sizes involved incremental training protocol up to 60% PImax of training intensity.9, 11, 13 The effect sizes for the control groups using either the fixed or incremental protocol up to 15% PImax ranged from 0.6 to 1.89 equivalent to the magnitude of “moderate” to “large.”9, 11, 13
Figure 1.
Effect size of maximal inspiratory pressure.
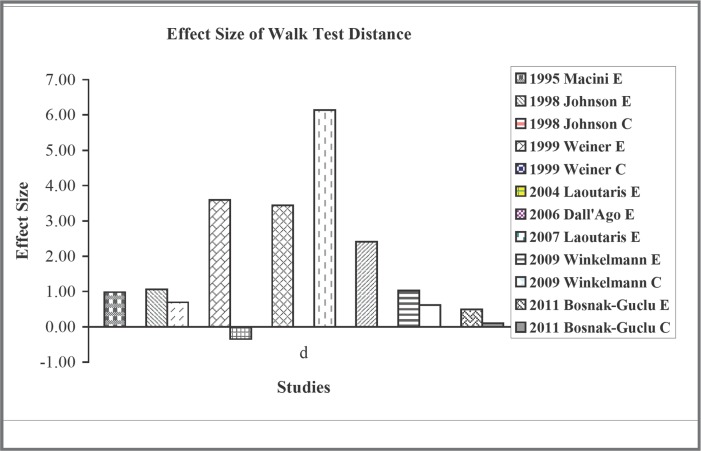
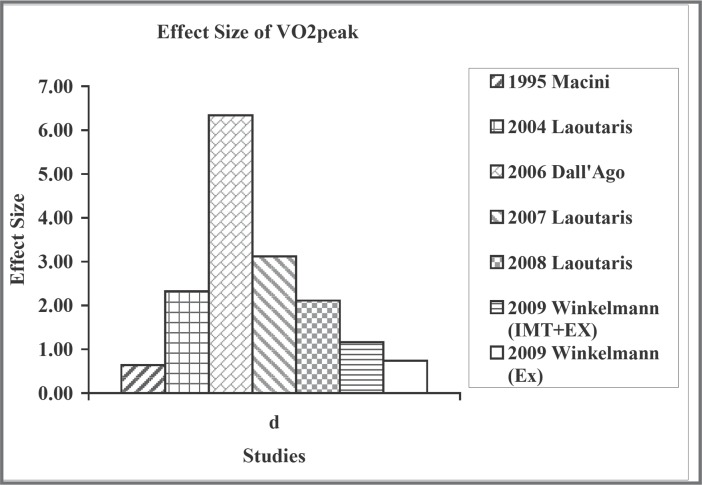
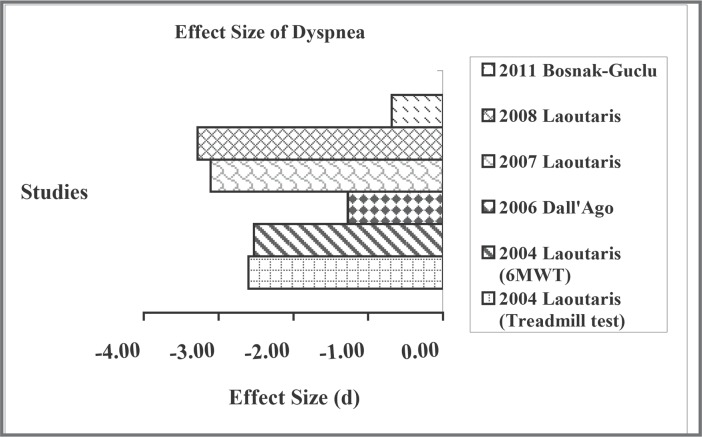
For the effect on functional capacity indicated by the walk test distance, the effect size was also large for the experimental groups, with the values ranging from 0.49 to 6.14 (Figure 2). But the effect size was minimal for the control group, ranging from −0.35 to 0.10, except for the study with aerobic exercise intervention.20 Similarly, there were moderate to large effect sizes (0.49 to 5.94) on VO2peak for the experimental group18, 21 (Figure 3). For the reduction of dyspnea, the effect sizes were also large, ranging from − 0.68 to − 3.28 (Figure 4). However, not all studies reported this outcome. For quality of life, because different questionnaires were used, we did not compare their effect sizes.
Figure 2.
Effect size of walk test distance.
Figure 3.
Effect size of Vo2peak.
Figure 4.
Effect size of dyspnea.
DISCUSSION
Selective inspiratory muscle training is effective in patients with chronic heart failure, based on the quality of evidence we found, where the methodological rigor ranged from 70% to 90% for the randomized controlled trials8, 9, 10, 11, 13, 18, 19, 20, 21, 22, 24 and 68% for nonrandomized trials.12, 23 Mechanisms underlying these beneficial effects of IMT included attenuated metaboreflex,29, 41 improved ventilatory efficiency, and lower ventilatory oscillations during incremental exercise.8 It is proposed that the metabolic products accumulated from fatiguing respiratory muscle contraction could increase sympathetic vasoconstriction activity (the inspiratory muscle metaboreflex),41 and the attenuation of the metaboreflex could then improve blood flow redistribution to skeletal muscles in the body, thereby delaying the time to fatigue and decreasing workload on the heart.29
The effect of IMT on respiratory muscle strength in patients with stable heart failure is evident. In all 12 articles, subjects in the experimental groups showed significant improvements in PImax. Based on their baseline PImax values, almost all subjects had a certain degree of respiratory muscle weakness. This probably explained why they benefited from progressive IMT at 30% PImax, the minimal training threshold recommended for patients with COPD and respiratory muscle weakness.14 Subjects who did not seem to have respiratory muscle weakness9, 11, 13 received a high intensity IMT incremental protocol at 60% PImax, also within the training intensity of 50% to 80% PImax reported in patients with COPD.14 Interestingly, we also found significant improvements in PImax for subjects who were trained at 15% PImax in the control groups.9, 11, 13, 18, 22 This probably was due to the fact that they also had respiratory muscle weakness at baseline.
The 3 previous review articles on IMT in patients with heart failure did not analyze the effect size.3, 6, 17 Our review provides detailed analysis of the effect size on the following outcomes: PImax, walk test distance, VO2peak, and dyspnea. We found consistently moderate to large effect sizes for the experimental groups. It seemed that the improvement of respiratory muscle strength could be translated to an improvement in functional capacity.8, 9, 11, 12, 13, 18, 20, 21, 23 However, we did not perform meta-analysis, so future studies would be needed in order to provide a stronger claim and to further identify modifiers and confounders of the results. The effect of IMT on quality of life in patients with heart failure was inconsistent. Two studies showed improvements13, 18 while one study did not.24 This probably was due to the lack of statistical power or due to the different quality of life questionnaires being used.
There were several limitations in the studies we reviewed. First, the sample sizes of all studies were relatively small. Therefore, there is a need for large randomized controlled trials in the future. Secondly, these studies excluded patients who had impaired lung function. Yet many patients with heart failure often also have a certain degree of chronic obstructive pulmonary disease. Whether similar IMT protocols would be effective is unknown. Furthermore, we only found one study that examined the effect of IMT plus exercise training. For patients with heart failure who have already engaged in structured aerobic exercise training, more studies are needed to determine if IMT could have additional benefits on exercise tolerance.
CONCLUSIONS
Selective inspiratory muscle training is beneficial for patients with CHF. The benefits include increased inspiratory muscle strength, increased inspiratory endurance, improved exercise capacity, reduced dyspnea, and probably improved quality of life. The effect sizes were considered “large” for improvements of respiratory muscle strength, functional exercise capacity, and reduction of dyspnea. It is recommended that patients with CHF should be screened for inspiratory muscle weakness routinely. If it is present, inspiratory muscle training via pressure threshold muscle trainer should be implemented. But the studies included in this review were limited by relatively small sample sizes. Future randomized controlled trials with large samples are needed. In addition, whether patients with CHF and preserved respiratory muscle strength could benefit from IMT is not known
ACKNOWLEDGEMENTS
The authors would like to thank Ms. Shelly Burns, our librarian, for her assistance with the literature search.
REFERENCES
- 1.MacIver DH, Dayer MJ. An alternative approach to understanding the pathophysiological mechanisms of chronic heart failure. Int J Cardiol. 2012;154((2)):102–110. doi: 10.1016/j.ijcard.2011.05.075. [DOI] [PubMed] [Google Scholar]
- 2.Krum H, Tonkin AM, Currie R, Djundjek R, Johnston CI. Chronic heart failure in australian general practice. the cardiac awareness survey and evaluation (CASE) study. Med J Aust. 2001;174((9)):439–444. doi: 10.5694/j.1326-5377.2001.tb143369.x. [DOI] [PubMed] [Google Scholar]
- 3.Ribeiro JP, Chiappa GR, Neder JA, Frankenstein L. Respiratory m26uscle function and exercise intolerance in heart failure. Curr Heart Fail Rep. 2009;6((2)):95–101. doi: 10.1007/s11897-009-0015-7. [DOI] [PubMed] [Google Scholar]
- 4.Tikunov B, Levine S, Mancini D. Chronic congestive heart failure elicits adaptations of endurance exercise in diaphragmatic muscle. Circulation. 1997;95((4)):910–916. doi: 10.1161/01.cir.95.4.910. [DOI] [PubMed] [Google Scholar]
- 5.Sutbeyaz ST, Koseoglu F, Inan L, Coskun O. Respiratory muscle training improves cardiopulmonary function and exercise tolerance in subjects with subacute stroke: A randomized controlled trial. Clin Rehabil. 2010;24((3)):240–250. doi: 10.1177/0269215509358932. [DOI] [PubMed] [Google Scholar]
- 6.Wong E, Selig S, Hare DL. Respiratory muscle dysfunction and training in chronic heart failure. Heart Lung Circ. 2011;20((5)):289–294. doi: 10.1016/j.hlc.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 7.ATS/ERS statement on respiratory muscle testing Am J Respir Crit Care Med. 2002;166((4)):518–624. doi: 10.1164/rccm.166.4.518. doi: 10.1164/ rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 8.Dall'Ago P, Chiappa GR, Guths H, Stein R, Ribeiro JP. Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: A randomized trial. J Am Coll Cardiol. 2006;47((4)):757–763. doi: 10.1016/j.jacc.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 9.Laoutaris ID, Dritsas A, Brown MD, et al. Immune response to inspiratory muscle training in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2007;14((5)):679–685. doi: 10.1097/HJR.0b013e3281338394. [DOI] [PubMed] [Google Scholar]
- 10.Laoutaris ID, Dritsas A, Adamopoulos S, Brown MD, Cokkinos DV. Effects of inspiratory muscle training in patients with chronic heart failure. J Am Coll Cardiol. 2008;52((23)):1888–1889. doi: 10.1016/j.jacc.2008.08.044. author reply 1889. [DOI] [PubMed] [Google Scholar]
- 11.Laoutaris ID, Dritsas A, Brown MD, et al. Effects of inspiratory muscle training on autonomic activity, endothelial vasodilator function, and N-terminal pro-brain natriuretic peptide levels in chronic heart failure. J Cardiopulm Rehabil Prev. 2008;28((2)):99–106. doi: 10.1097/01.HCR.0000314203.09676.b9. [DOI] [PubMed] [Google Scholar]
- 12.Mancini DM, Henson D, La Manca J, Donchez L, Levine S. Benefit of selective respiratory muscle training on exercise capacity in patients with chronic congestive heart failure. Circulation. 1995;91((2)):320–329. doi: 10.1161/01.cir.91.2.320. [DOI] [PubMed] [Google Scholar]
- 13.Laoutaris I, Dritsas A, Brown MD, Manginas A, Alivizatos PA, Cokkinos DV. Inspiratory muscle training using an incremental endurance test alleviates dyspnea and improves functional status in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2004;11((6)):489–496. doi: 10.1097/01.hjr.0000152242.51327.63. [DOI] [PubMed] [Google Scholar]
- 14.Gosselink R, De Vos J, van den Heuvel SP, Segers J, Decramer M, Kwakkel G. Impact of inspiratory muscle training in patients with COPD: What is the evidence? Eur Respir J. 2011;37((2)):416–425. doi: 10.1183/09031936.00031810. [DOI] [PubMed] [Google Scholar]
- 15.Hill K, Cecins NM, Eastwood PR, Jenkins SC. Inspiratory muscle training for patients with chronic obstructive pulmonary disease: A practical guide for clinicians. Arch Phys Med Rehabil. 2010;91((9)):1466–1470. doi: 10.1016/j.apmr.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Shoemaker MJ, Donker S, Lapoe A. Inspiratory muscle training in patients with chronic obstructive pulmonary disease: The state of the evidence. Cardiopulm Phys Ther J. 2009;20((3)):5–15. [PMC free article] [PubMed] [Google Scholar]
- 17.Padula CA, Yeaw E. Inspiratory muscle training: Integrative review of use in conditions other than COPD. Res Theory Nurs Pract. 2007;21((2)):98–118. doi: 10.1891/088971807780852039. [DOI] [PubMed] [Google Scholar]
- 18.Bosnak-Guclu M, Arikan H, Savci S, et al. Effects of inspiratory muscle training in patients with heart failure. Respir Med. 2011;105((11)):1671–1681. doi: 10.1016/j.rmed.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Stein R, Chiappa GR, Guths H, Dall'Ago P, Ribeiro JP. Inspiratory muscle training improves oxygen uptake efficiency slope in patients with chronic heart failure. J Cardiopulm Rehabil Prev. 2009;29((6)):392–395. doi: 10.1097/HCR.0b013e3181b4cc41. [DOI] [PubMed] [Google Scholar]
- 20.Winkelmann ER, Chiappa GR, Lima CO, Viecili PR, Stein R, Ribeiro JP. Addition of inspiratory muscle training to aerobic training improves cardiorespiratory responses to exercise in patients with heart failure and inspiratory muscle weakness. Am Heart J. 2009;158((5)):768. doi: 10.1016/j.ahj.2009.09.005. e1-768.e7. [DOI] [PubMed] [Google Scholar]
- 21.Weiner P, Magadle R, Berar-Yanay N, Pelled B. The effect of specific inspiratory muscle training on the sensation of dyspnea and exercise tolerance in patients with congestive heart failure. Clin Cardiol. 1999;22((11)):727–732. doi: 10.1002/clc.4960221110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson PH, Cowley AJ, Kinnear WJ. A randomized controlled trial of inspiratory muscle training in stable chronic heart failure. Eur Heart J. 1998;19((8)):1249–1253. doi: 10.1053/euhj.1998.1024. [DOI] [PubMed] [Google Scholar]
- 23.Cahalin LP, Semigran MJ, Dec GW. Inspiratory muscle training in patients with chronic heart failure awaiting cardiac transplantation: Results of a pilot clinical trial. Phys Ther. 1997;77((8)):830–838. doi: 10.1093/ptj/77.8.830. [DOI] [PubMed] [Google Scholar]
- 24.Padula CA, Yeaw E, Mistry S. A home-based nurse-coached inspiratory muscle training intervention in heart failure. Appl Nurs Res. 2009;22((1)):18–25. doi: 10.1016/j.apnr.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Straus SE, Richardson WS, Glasziou P, Haynes RB. 3rd. Toronto: Elsevier Churchill Livingstone; 2005. Evidence-based Medicine: How to Practice and Teach EBM. [Google Scholar]
- 26.Moseley AM, Herbert RD, Sherrington C, Maher CG. Evidence for physiotherapy practice: A survey of the physiotherapy evidence database (PEDro) Aust J Physiother. 2002;48((1)):43–49. doi: 10.1016/s0004-9514(14)60281-6. [DOI] [PubMed] [Google Scholar]
- 27.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52((6)):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurley WL, Denegar CR, Hertel J. 1st. Baltimore, MD: Wolters Kluwer|Lippincott Williams & Wilkins; 2011. Research Methods: A Framework for Evidence-based Clinical Practice; p. 426. [Google Scholar]
- 29.Chiappa GR, Roseguini BT, Vieira PJ, et al. Inspiratory muscle training improves blood flow to resting and exercising limbs in patients with chronic heart failure. J Am Coll Cardiol. 2008;51((17)):1663–1671. doi: 10.1016/j.jacc.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 30.Borg GA, Psychophysical bases of perceived exertion Med Sci Sports Exerc. 1982;14((5)):377–381. [PubMed] [Google Scholar]
- 31.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93((3)):580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 32.Mahler DA, Mejia-Alfaro R, Ward J, Baird JC. Continuous measurement of breathlessness during exercise: Validity, reliability, and responsiveness. J Appl Physiol. 2001;90((6)):2188–2196. doi: 10.1152/jappl.2001.90.6.2188. [DOI] [PubMed] [Google Scholar]
- 33.Austin J, Williams WR, Hutchison S. Patterns of fatigue in elderly heart failure patients measured by a quality of life scale (Minnesota living with heart failure) Eur J Cardiovasc Nurs. 2011 May 4; doi: 10.1016/j.ejcnurse.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Montgomery S, Asberg M, Jornestedt L, et al. Reliability of the CPRS between the disciplines of psychiatry, general practice, nursing and psychology in depressed patients. Acta Psychiatr Scand Suppl. 1978;(271)((271)):29–32. doi: 10.1111/j.1600-0447.1978.tb02358.x. [DOI] [PubMed] [Google Scholar]
- 35.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 36.Gromoske AN, Oldridge N, Brondino MJ. Readability of 3 heart disease health-related quality of life questionnaires. J Cardiopulm Rehabil Prev. 2011;31((4)):245–248. doi: 10.1097/HCR.0b013e31821c13c6. [DOI] [PubMed] [Google Scholar]
- 37.Ware JE, Kosinski M. Interpreting SF-36 summary health measures: A response. Qual Life Res. 2001;10((5)):405–413. doi: 10.1023/a:1012588218728. discussion 415-420. [DOI] [PubMed] [Google Scholar]
- 38.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-item short-form health survey (SF-36): II. psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31((3)):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 39.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166((1)):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 40.Dolmage TE, Hill K, Evans RA, Goldstein RS. Has my patient responded? interpreting clinical measurements such as the six minute walk test. Am J Respir Crit Care Med. 2011;184((6)):642–646. doi: 10.1164/rccm.201103-0497CC. [DOI] [PubMed] [Google Scholar]
- 41.Callegaro CC, Martinez D, Ribeiro PAB. Brod M. Ribeiro JP. Augmented peripheral chemoreflex in patients with heart failure and inspiratory muscle weakness. Respir Physiol Neurobiol. 2010;171((1)):31–35. doi: 10.1016/j.resp.2010.01.009. [DOI] [PubMed] [Google Scholar]