Abstract
Background and Purpose
Due to the increasing prevalence of heart failure (HF) and medical advances extending the life expectancy of patients with HF, physical therapists are often consulted to assist in the management of these patients in light of the benefits of exercise for them. The purpose of this paper is to review the equipment and measures often encountered in patients with Stage D HF in the intensive care unit (ICU) and to discuss the care provided to two patients in this setting.
Case Descriptions
One patient was a 40-year-old man with Stage D HF on multiple inotropes receiving physical therapy intervention while awaiting a cardiac transplant. The second patient was a 57-year-old man with Stage D HF due to ischemic cardiomyopathy, treated by physical therapy while receiving inotropic and intra-aortic balloon pump support, awaiting a renal and cardiac transplant.
Discussion
Different physical therapy interventions were safely provided to both patients during their cardiac medical ICU courses. Despite multiple medical issues requiring more complex decision making skills, this patient population may greatly benefit from physical therapy intervention.
Key Words: heart failure, exercise, intensive care unit
INTRODUCTION
As people are living longer, heart failure (HF) is an increasing health concern in the United States.1 Further, medical advances in telemedicine, pharmacotherapy, and ventricular assistive devices have resulted in increased patient survival rates in individuals with severe HF.2, 3, 4, 5, 6, 7, 8 While the outpatient medical management of patients with HF has improved, HF related hospital admissions have increased.9 Recent evidence reports 83% of patients with HF were admitted once, while 43% of patients were admitted at least 4 times per year.9 Given this combination of factors, there is an increased likelihood that physical therapists practicing in acute care settings will be responsible for treating patients with HF.
The New York Heart Association (NYHA) created a functional capacity based system for categorizing patients with HF in 1928.10 This original classification system can change within minutes to hours depending upon the patient's symptoms, medical management, and a host of other factors. In 1994, the Criteria Committee of the NYHA released its ninth revision, which accounted for objective data to assist in the classification of these patients.10, 11 These two systems are often used in concert to best describe a patient's status. The patients in this case report have been classified exclusively on the objective portion of the scale for multiple reasons. First and foremost, the patients’ multiple medical issues and fluctuating situations resulted in NYHA class ranged from II-IV throughout their length of stay. Secondly, due to intra-aortic balloon pump (IABP) placement, one patient was on bedrest and couldn't participate in any functional activities making it difficult to correctly categorize him. Finally, NYHA I-IV classes alone have poor reliability and validity.11, 12 The objective assessment is based on valid clinical tests.
There is an increasing body of literature supporting the concept that a combination of aerobic and resistive exercise improves function in HF patients. Recent evidence suggests that exercise is safe and beneficial for this patient population.2, 13, 14, 15, 16, 17, 18, 19, 20 The majority of studies often target patients that are classified as NYHA Class I-III and/or Stage B and C, and excludes hospitalized or recently hospitalized patients. It should be noted that these limitations exclude a number of patients with advanced or more acute, compensated HF, who could benefit from physical therapy (PT) intervention. Another understudied population is patients with Stage D HF; especially those in the intensive care unit (ICU) awaiting cardiac transplantation or those receiving long-term intravenous medications. Stage D HF patients may benefit from an exercise program designed and supervised by a PT; however, this has not been tested. Recent case studies support the concept that exercise programs designed by physical therapists may improve outcomes. For example, Kataoka et al21 described a single case study where a 53-year-old patient with HF on intravenous dobutamine benefited from a 32-week long exercise program. The patient demonstrated continued improvements on cardiopulmonary exercise testing throughout this period without adverse effects. Furthermore, Ricard et al22 provided another case report of a 67-year-old man in an acute care setting receiving intravenous dobutamine who demonstrated an increase in exercise capacity while participating in an exercise program. While not assessed over time, White et al23 demonstrated an increase maximal and submaximal response to exercise in patients with Class III or IV HF being treated with IV milrinone. In addition, there is increasing evidence that patients in ICUs can benefit from initiating exercise and mobilization early in their hospital stay despite multiple medical issues.24, 25, 26, 27
While these patients benefited from an exercise program, it should not go unmentioned that there is a large risk associated with exercise due to the patient's tenuous medical status. A physical therapist treating patients with Stage D HF in an ICU setting is responsible for making advanced clinical decisions. The physical therapist must be able to interpret real-time data and make decisions surrounding complex medical information in order to determine if the patient is able to participate in an exercise program at a particular point in time. Moreover, the therapist must also make real-time decisions quickly to determine if the patient demonstrates an appropriate hemodynamic response to program. Lastly, the physical therapist is responsible for managing all the equipment that is needed to provide the patient optimal medical care. Therefore, the purpose of these case presentations is two-fold:
To review the equipment that is unique to an ICU setting that a physical therapist will likely encounter when treating patients with Stage D HF. While this presentation is not aimed to make the reader competent in treating this patient population, it should highlight the implications of the equipment, lab values, medical tests, and medications to a physical therapists practice.
To present the exercise programs of two patients with Stage D HF in a cardiac medical ICU awaiting cardiac transplant at a large teaching hospital in Boston, MA.
EQUIPMENT AND MEASUREMENTS
As with any patient, it is important to locate and understand the purpose of all pieces of equipment connected to a patient during a PT session. This will assist the PT in determining the best course of action if equipment is mistakenly dislodged or unhooked, so that he or she can act appropriately and with the correct amount of urgency in order to maintain patient homeostasis and safety. It also provides the physical therapist the opportunity to use real-time data to assist in the management of the patient. Equipment frequently encountered in the ICU setting when treating patients with stage D HF will be discussed here.
Intra-aortic Balloon Pump
An IABP is comprised of a balloon that is inserted percutaneously in the femoral artery and advanced to the proximal aorta. There is a transducer at the insertion site and a catheter that extends to an external pneumatic device with hemodynamic monitor (Figure 1). It provides mechanical cardiovascular support in two ways. First, the balloon inflates during diastole that forces the residual blood volume directly into the coronary arteries, thereby maximizing coronary artery perfusion. The balloon then rapidly deflates at the initiation of systole. This creates a vacuum that facilitates systole and increases cardiac output by as much as 40%.28 As long as the transducer at the insertion site remains level, it provides real-time heart and blood pressure measurement. Depending upon the level of support that is indicated, the IABP can be set at a 1:2, 1:4 or 1:8 ratios. In other words, the balloon inflates with every other, every 4th, or every 8th ventricular contraction.
Figure 1.
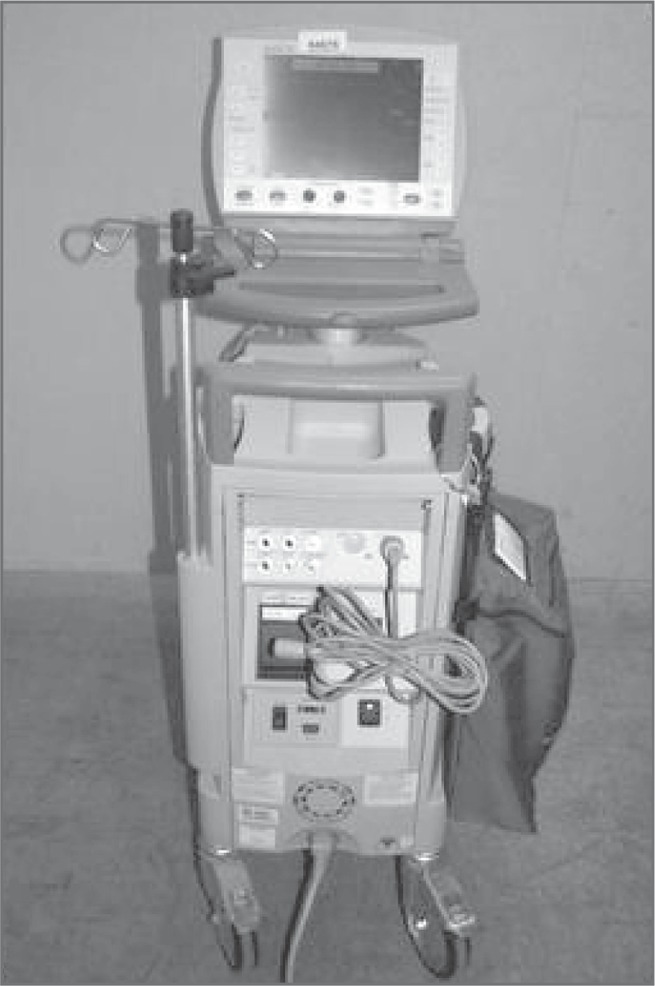
IABP.
An IABP is used in acute coronary ischemia that is not amenable to medical intervention, cardiogenic shock, ventricular arrhythmias, during catheterization procedures that are high risk or periacute myocardial infarctions or while awaiting surgical intervention.28 Patients with a femorally inserted IABP remain on bedrest with their head of bed height restricted, limiting their ability to participate in any activity. To date, there is not any literature with respect to mobility or exercise with patients with a femoral IABP in place.
The major risk associated with an IABP is vascular complications, including limb ischemia, laceration, or hemorrhage. An international review of 243 institutions with approximately 17,000 patients over a 4-year period showed a complication rate of 7% associated with an IABP, 2.6% being major complications, and an IABP related mortality of just 0.5%.29 Factors that increase the rate of complications associated with IABP are peripheral vascular disease, older age, female gender, diabetes mellitus, hypertension, prolonged support, larger catheter size (greater than 9.5 French), small body surface area (less than 1.8 m2) and low cardiac index (less than 2.2 L/min/m2).28 Another potential vascular issue is IABP induced thrombocytopenia. In a retrospective analysis published in 2008, there was an incidence of thrombocytopenia in 57.9% of the patients, with the nadir falling approximately 3 days after IABP insertion. Conversely, there was not an increased incidence of thrombocytopenia in patients on heparin.30 Therefore it is important to monitor the patient's lab values (eg, platelets, hematocrit, hemoglobin) and signs/symptoms of vascular compromise when treating them.
Due to the risks of prolonged bedrest, an ambulatory IABP was developed. Because it is inserted in the common iliac artery or left axillary artery, it was proposed to be used as a bridge to transplant as patients can mobilize with it in place.31, 32 Neither of these reports demonstrated any complications associated with this insertion technique, even with mobility and exercise. However, the sample size for each study were limited (4 and 13, respectively), thus making the results difficult to generalize.
Swan Ganz Catheter
This is a catheter inserted into the subclavian or internal jugular veins, or less commonly the femoral, basilic or brachial veins, which is passed through the right atrium, right ventricle, and terminates in the pulmonary artery. There are two lumens along the course of the catheter (19 cm and 30 cm from the tip of the catheter), which can give the pressures in the right ventricle, right atrium, or superior vena cava depending upon the patient's anatomy. The normal pressures can be found in Table 1. The catheter has a small balloon proximal to the end of the catheter and a small lumen located at the tip. This is advanced into a distal pulmonary artery where the balloon is then inflated. This prevents the blood from flowing through the artery and therefore into the capillary bed, pulmonary vein, and ultimately the left atrium. The pressure equalizes across this area and the pulmonary wedge capillary wedge pressure can be obtained via the lumen on the distal tip of the catheter. Because of the technique used, this is an estimation of the left atrium via the least invasive method. Measurements that are made possible by the Swan Ganz catheter include cardiac output and cardiac index as well as myocardial oxygen consumption. The transducer must be level to the 4th intercostal space or to the right atrium in order for the pressures to be valid.
Table 1.
Normative Values Obtained via Swan Ganz Catheter33
| Location | Normal Value |
|---|---|
| Right Atrium Pressure | 8 mmHg |
| Right Ventricle End Diastolic Pressure | 8 mmHg |
| Right Ventricle End Systolic Pressure | 15–30 mmHg |
| Pulmonary Artery Systolic Pressure | 15–30 mmHg |
| Pulmonary Artery Diastolic Pressure | 4–12 mmHg |
| Pulmonary Capillary Wedge Pressure | 4–12 mmHg |
| Left Atrium | 4–12 mmHg |
There are complications associated with the use of a Swan Ganz catheter. These can occur during insertion, use of the catheter, and/or misinterpretation of the data. The most common complications associated with catheter insertion are atrial or ventricular arrhythmias, with only 3% being sustained.34 This may be a consideration for PT treatments if the patient continues to demonstrate increased myocardial irritability.
Cardiac Output and Cardiac Index
Cardiac output is the volume of blood being pumped by the ventricle per minute. Normal cardiac output is 4–8 L/min, depending upon the patient's body size. To standardize this data between patients, the cardiac index is often used. It is the cardiac output divided by the body surface area. A normal cardiac index is 3.5 L/min/m2 ± 0.7 L/min/m2.35
Cardiac output can be measured directly or indirectly. Thermodilution is the direct measurement technique. It is based on the principle that the flow rate will be inversely proportional to an indicator (cooler substance) injected at a downstream site. In practice, patients are injected through a Swan Ganz catheter proximally with dextrose or saline at a temperature cooler than blood. The tip of the Swan Ganz catheter has a thermistor on the end that measures resistance based on temperature changes. A plot is generated based on temperature and time. The area under the curve is inversely proportional to the flow rate in the pulmonary artery, which is where the thermistor is located.36 The use of the Fick equation [CO=VO2/(CaO2-CvO2)] is the most often employed indirect measure. It is gathered using data obtained via Swan-Ganz catheter and estimation of oxygen consumption based on age, sex, height, and weight.36 Patients whose cardiac output and cardiac index are very low will have a limited ability to tolerate increased activity levels.
Central Venous Pressure
Central venous pressure (CVP) is a measurement obtained through a central catheter threaded to the distal third of the superior vena cava. This is a different catheter than a Swan Ganz catheter. It measures the pressure in the superior vena cava and right atrium, and as a result indicates the pressure throughout the entire venous system. Like a Swan Ganz catheter, the line is attached to a transducer that must be level to the 4th intercostal space for an accurate measurement. It is usually high when overall blood volume and venous return are high, as in HF or over-hydration. After an acute decompensation or change in medical status, the CVP can assist in determining the amount of additional volume in the venous system, which indicates the severity of the HF. Central venous pressure should decrease with medical management and aggressive diuresis.
Myocardial Oxygen Consumption
Myocytes have high oxygen consumption and therefore are at increased risk of ischemia if coronary artery blood flow is inadequate. Any activity that increases tension in myocytes will increase myocardial oxygen consumption (MVO2). For example, increased heart rate or increased afterload both increase myocyte activity due to increased demand, and therefore increase MVO2. This value can be invasively measured using data collected from a Swan-Ganz catheter and the Fick Principle. Normal MVO2 is approximately 8–9mlO2/100g/min,37 while with exercise, MVO2 increases significantly. In concert with the medical team, it can be helpful to assess MVO2 at rest and immediately after exercise to determine if the exercise intensity is too great, especially in the absence of other clinical signs or symptoms.
LAB VALUES
Brain Natriuretic Peptide and NT-pro BNP
Brain natriuretic peptide (BNP) is a hormone that is released from the ventricles during times of increased ventricular pressures. This hormone assists with the regulation of salt and water, and is increased in an attempt to reduce the ventricular pressures.38 The NT-pro BNP is the degradation product of BNP. While both are released into the blood stream and are increased in the setting of ventricular dysfunction, the level of NT-pro BNP increases approximately 4 times when compared to BNP.38 Normative values are lab dependent and age based; therefore, reference values should be assessed in individual institutions. For example, NT-pro BNP is higher in women and older adults, and both values are lower in obese patients.38 Brain natriuretic peptide and NT-pro BNP levels are often elevated in multiple disorders; therefore, they are not used singularly to diagnose HF. In patients with chronic HF and/or with concomitant kidney disease, BNP may be chronically elevated. In this case, it is helpful to compare a baseline BNP to the value obtained in decompensated situations to assist in determining the severity of a particular episode.39
MEDICATIONS
Patients with HF are initially managed on a combination of oral ACE inhibitors, beta blockers, angiotension receptor blockers, aldosterone blockers, and diuretics depending upon their individual needs. As the patient's disease progresses and it becomes refractory to these medications, additional medications are used.
Inotropes
Oral inotropic supports such as digoxin and others in the drug class are often initiated earlier than Stage D HF. Beta adrenergic agonists, dobutamine and dopamine for example, are often added in acute HF in this patient population as well as in chronic management. These are administered by continuous IV infusion as they are not active in their oral form. Dobutamine is often preferred for patients in shock whereas dopamine is used more with patients who have ischemic heart disease. Phosphodiesterase-III inhibitors, which act by increasing cell calcium concentration and therefore increasing contractility, also act as inotropes. Milrinone is the most commonly used medication in this drug class. Common side effects include hypertension, tachycardia, headache, and ventricular arrhythmias.
PATIENT CASES
Patient #1: AP
AP was a 40-year-old man admitted 2/21/10 to a tertiary medical center with nausea, anorexia, malaise, and right upper quadrant pain. AP had a toxicology screen positive for amphetamine use. He was found to have acute hepatitis and liver failure, and a work-up was initiated for a liver transplant. This revealed end-stage HF and the patient transferred to the cardiac medical intensive care unit for tailored therapy (intravenous Lasix and dobutamine) with a Swan Ganz line. AP initially demonstrated acute confusion, which was attributed to hepatic encephalopathy, and this as well as his liver failure resolved by the time of the initial PT exam. He was also treated with antibiotics for fevers, but that was discharged once his cultures were negative. He was medically maximized in the ICU for 5 days, and the patient was transferred to the step-down unit for further medical management. He returned to the cardiac catheterization lab 5 days later after he had increasing symptoms and was found to have severe three vessel coronary artery disease. He had an IABP placed and returned to the cardiac medical ICU. AP was restarted on dobutamine and milrinone as well as IV Diuril and IV Lasix to manage his refractory HF. The patient was then listed status 1A on heart transplant list. After two weeks of this medical management, the IABP was able to be removed. Physical therapy was consulted to assist with exercise training while AP awaited cardiac transplant. The exam completed on 4/1/10 is shown in Table 2.
Table 2.
Exam Results for Patient AP
| Past Medical History | Diabetes Mellitus I Psoriasis |
| Relevant Tests | 4/1/10 White blood cell count: 11 × 103 gg/dl Hematocrit: 26.0% Hemoglobin A1C: 8.4% |
| 4/1/10 Chest film: interval development of mild interstitial edema | |
| 3/12/10 Viability Study: septum, inferior wall and proximal to mid anterior wall remain viable. Mid to distal anterior, lateral walls and apex are non-viable | |
| 3/3/10 Head CT scan: no intracranial hemorrhage, mass lesion or evidence of acute territorial infarction. | |
| 2/26/10 Transesophageal echocardiogram: ejection fraction 19%, mild to moderate mitral regurgitation, left ventricle is diffusely hypokinetic, severe triscupid insufficiency, right ventricle is diffusely hypokinetic, and abnormal interventricular septal motion consistent with interventricular conduction delay | |
| Medications | Milrinone, Dobutamine, Argatroban, Insulin aspart, Insulin, Lidocaine patch, Prilosec, Lipitor As needed: Ativan, Compazine, Trazodone, Oxycodone, Lactulose |
| Social History | AP has a history of excessive alcohol use, which he stopped 6 years prior to admit. He reported active recreational marijuana use, 2 to 3 times per month, but denies other drug use. He is divorced without any children. His parents were his primary social support. |
| Functional Status | AP lives alone and was independent with all activity prior to his admit to the hospital. He worked full-time in lumber sales where heavy lifting was frequently required. He didn't engage in a regular exercise program outside of work. |
| Patient's Goal | To get stronger. |
| Tests and Measures | |
| Arousal, Attention, Communication and Cognition | Within normal limits (WNL), wears glasses at all times which effectively compensates for baseline nearsightedness |
| Pain | Denies throughout examination |
| Integument | Lines: R subclavian Swan Ganz line, R UE Peripherally Inserted Central Catheter (PICC) line; R hallux and along 1st ray with ecchymosis with minimal ecchymosis on other R toes; L 2nd toe with ecchymosis on tip and ½ dollar size area on lateral plantar aspect of L foot with ecchymosis with all toe tips with ecchymosis (all due to ischemia) |
| Sensation | Denies paresthesia, intact to light touch |
| Circulation | No peripheral edema, 2+ dorsal pedis pulses, PA: 44/28 mmHg |
| Range of Motion | WNL |
| Muscle Performance | WNL except B 4/5 hip flexion, hip extension, hip abduction, knee flexion and knee extension |
| Motor function | WNL with functional activities, no further assessment |
| Ventilation |
|
| Functional Mobility |
|
| Balance | Sitting and static standing are WNL, dynamic standing unable to be assessed as patient requires bilateral UE assist |
| Gait | Slow cadence, decreased step length, increased base of support, decreased heel strike and push off |
| Aerobic capacity | 125 feet in 4:50 with multiple standing rests due to overall, full body fatigue (vital sign response below) |
| Activity | HR (b/min) | BP (mmHg) | RR | Spo2(%) | Fio2 flow |
|---|---|---|---|---|---|
| Supine | 132 | 86/54 | 12 | 98% | RA |
| Sit at edge of bed after 2 minutes | 134 | 80/56 | 12 | - | RA |
| Sit edge of bed after 4 minutes | 134 | 82/54 | 12 | - | RA |
| Stand, rest after 2 minutes | 134 | 86/50 | 12 | - | RA |
| Stand, peak during activity | 134-136 | 86/52 | 16 | 97% | RA |
| Sit, 1 minute rest | 134 | 78/52 | 12 | - | RA |
| Supine, 5 minute rests | 132 | 94/66 | 12 | 97% | RA |
| HR, heart rate; b/min, beats per minute; BP, blood pressure; mmHg, millimeters of mercury; RR, respiratory rate; SpO2, oxygenation saturation; FiO2, fraction of inspired oxygen; RA, room air | |||||
International Classification of Functioning, Disability and Health (ICF):
Impairments: aerobic capacity/endurance, balance, gait, integumentary integrity, muscle performance
Activity limitations: inability to walk long distances in lumbar yard, inability to carry objects in lumbar yard, inability to climb stairs or ladder
Participation Restrictions: inability to work
Physical Therapy Intervention
In order to address the patient's impairments, activity limitations, and participation restrictions, a comprehensive exercise program was initiated. The patient's preferred method of exercise was walking, so a supervised walking program was started. As noted in the exam, the patient started at 125 feet, which was a maximal effort limited by fatigue. Unfortunately, due to persistently low myocardial oxygen consumption in the setting of worsening HF, Swan Ganz line frailty, and the patient's subjective complaints, his ambulation program was unable to be continued after the initial examination. In lieu of this, a seated lower extremity ergometer program was initiated. He started with 40 seconds of pedaling with no resistance at 60 revolutions per minute (RPM). At this intensity, he demonstrated an abnormal hemodynamic response: 10 beat per minute drop in heart rate, 10 mm Hg drop in systolic blood pressure, and subjective complaints of dizziness. After 4 minutes of recovery, the patient's vital signs returned to baseline. A second trial of 20 seconds followed with an appropriate response with full recovery after one minute. This interval was repeated for a total of 4 trials. This program was subsequently progressed on the following treatments to one minute pedaling and 3 to 4 minute rest intervals. The rest periods were gradually decreased to two minutes while the number of intervals was progressed to 10, two to 3 times per day. The patient was taught how to take his blood pressure after each interval given his tenuous status and instructed on parameters that were appropriate for exercise. He was able to complete this program independently, with assistance from caregivers only for equipment set-up given his multiple lines.
In combination with his aerobic program, the patient was started on a resistive exercise program. Due to his long length of stay and complex medical issues, his exercise program was initiated conservatively. He started with elbow flexion, elbow extension, mini-squats, sit-stand, standing hip abduction and standing hip flexion without resistance for 15 repetitions each, one set, and all against gravity. After waiting 24 hours for any delayed adverse response, including increased muscle soreness or decreased myocardial oxygen consumption, the patient was guided to complete a 10-repetition maximum for the same exercises and weight was added as able. The patient started with 2.2 kg for elbow flexion and 1.4 kg for elbow extension. He continued to complete the remaining exercises against gravity, undergoing re-evaluation on a weekly basis where his resistance was modified to maintain a 10-repetition maximum. Due to ongoing medical issues, his progress was variable but he was to maintain his status until his transplant.
During the patient's preoperative time, there were no adverse effects of exercise. On one occasion, after transferring from supine to sit, his Swan Ganz catheter inadvertently advanced into the wedge position. The change in waveform was immediately noted by the PT, who directed the patient to maintain his position on the edge of the bed while calling his primary nurse. The nurse assisted with repositioning and reassessment occurred by the medical team via chest film. There was no harm to the patient or equipment as a result and activity was continued as planned on the next treatment. The only other limiting factor was the patient's subjective complaints of nausea with frequent vomiting and recurrent Swan Ganz malfunction unrelated to activity. Both of these issues were managed by the medical team. AP successfully underwent an orthotopic heart transplant 5 weeks after beginning the exercise program. He was able to resume his interval gait training on postoperative day two and was discharged home with an independent walking program on postoperative day 9, which was hospital day 79.
Patient #2: NC
NC was a 57-year-old male admitted to an out-of-state acute care hospital 12/15/09 with a non-ST elevated myocardial infarction and decompensated HF. He underwent a cardiac catheterization, but was found to have no revascularization options. NC sustained an automated internal cardiac defibrillator (AICD) firing and was transferred to another acute care hospital for electrophysiology (EP) evaluation. A biventricular upgrade was attempted but failed, and the patient was discharged to rehab where he continued to be optimized medically and physically. He was readmitted 1/2/10 to an acute care hospital for a repeat attempt at a biventricular pacer upgrade, which was successful. However, the procedure was complicated by volume overload due to decompensated HF, ventricular tachycardia, and atrial fibrillation, which culminated in ICD firing × 12 on 1/17/10 despite optimal medical management. NC was transferred to a large area medical center 1/18/10 for further management of his stage D HF and for consideration of a left ventricular assist device (LVAD). NC decided to forgo LVAD placement to avoid additional surgery (prior to heart transplant). The patient was found to have concomitant kidney failure and was listed 1A for heart and kidney transplant. NC was managed in the cardiac ICU for tailored therapy. Due to further cardiac decompensation, NC returned to the cath lab 3/14/10 for IABP placement. His course was further complicated by line sepsis from an unknown source requiring IV antibiotics for vancomycin resistant enterococcus (VRE). NC also sustained IABP induced thrombocytopenia, which was treated conservatively. Due to these events, the patient was listed status 7 (inactive) on the transplant list for a 2-week period, but was reactivated as 1A after these acute medical issues stabilized. Patient required maximal doses of dobutamine, Lasix, and milrinone to maximize his cardiac function. The PT exam initially completed on 2/18/10, but due to persistent medical issues the patient had minimal ability to participate until a 4/14/10 re-evaluation, which is presented in Table 3.
Table 3.
Exam Results for Patient NC
| Past Medical History |
|
| Relevant Tests |
|
| Medications |
|
| Social History | Lives with his niece and nephew. Niece and nephew split working morning and evenings so that someone is usually home with NC, although he had not needed physical assistance prior to admit. NC alludes to fear associated with complexity of his cardiac issues, specifically frequent ICD firing for a period of time. Has a dog whom he misses. |
| Functional Status | IADLs. His niece does cooking and cleaning. Worked as animal cage cleaner in a large university research building but on medical leave since due to medical issues. Pt was getting out of house almost daily to visit friend's body shop. No regular exercise. Reports he hasn't been home since 11/09 and hasn't walked in approximately one month prior to admission. |
| Patient Goals | To return home again and see his dog |
| Tests and Measures | |
| Arousal, Attention, Communication and Cognition | WNL, appropriate throughout, follows all of commands |
| Pain | Denies throughout examination |
| Integument | PICC line right brachium; L femoral IABP and arterial line |
| Sensation | Denies paresthesia, intact to light touch |
| Circulation | No peripheral edema, 2+ dorsal pedis pulses, HR: 80, BP: 82/48, PA: 48/20 mmHg |
| Range of Motion | WNL except B DF = neutral with firm endfeels |
| Muscle Performance | WNL except 4/5 B elbow extension, L LE not examined due to IABP |
| Motor function | WNL with functional activities, no further assessment warranted |
| Ventilation |
|
| Functional Mobility, Balance, Gait | Deferred due to IABP (on bedrest) |
International Classification of Functioning, Disability and Health (ICF):
Impairments: aerobic capacity/endurance (anticipated), integumentary integrity, muscle performance, range of motion, balance (anticipated), gait (anticipated)
Activity limitations: inability to carry objects, inability to reach into cages, inability to walk long distance in animal facility
Participation Restrictions: inability to work in research facility, unable to play with his dog
Physical Therapy Intervention
NC was significantly deconditioned due to prolonged bedrest as well as the sequela of end-stage HF. NC initiated an exercise program in a similar fashion to AP. After initial days of antigravity exercises, NC completed a 10-repetition maximum for elbow flexion (1.4 kg), elbow extension (0.9 kg), shoulder flexion (no resistance), hip flexion (R only, 2.3 kg), hip abduction (2.3 kg), short arc quad (R only, 2.3 kg), and one leg bridging (R only). He completed two sets of 10 repetitions, with reassessment and progression on a weekly basis as able. NC also performed a towel stretch for bilateral plantarflexor muscle length, which he completed independently. Patient consistently had an appropriate hemodynamic response to this activity.
In order to reacclimate the patient to the upright position, increase his quality of life, increase weight bearing, and to assist with his postoperative recovery, NC was initiated on a tilt table standing program, after being able to tolerate maximal Trendelenburg position in the bed for two hours. After discussion with the medical team, this program was initiated under the close supervision of the physical therapist, nurse, and IABP balloon technician. His standing angle was gradually increased by 10° increments, maintaining each position for 3 to 5 minutes with continual hemodynamic assessment. His first trial was well tolerated, increasing to 40° before increased symptoms of dizziness in setting of orthostatic response. On all subsequent trials, he was able to tolerate 60° prior to symptoms with stable hemodynamic response. While in standing, he was able to complete heel raises and mini-squats to fatigue (5 repetitions, 2 sets). This was progressed to trials of upper body ergometer training the upright position. NC tolerated 15 second intervals with two minute rest periods, 3 trials. NC reported increased satisfaction with being able to mobilize out of bed after more than 5 months of bedrest. There were no adverse effects to his exercise program during his 7-week course of PT, where he was seen 3 to 4 times per week.
Discussions were initiated with the medical team early in his PT course to investigate transitioning his femoral IABP to a brachial IABP. While all agreed this would be beneficial in order to promote mobility to maximize the patient's function prior to transplant surgery, it was determined that he was not a good candidate for this device due to his medical instability. Therefore, his femoral IABP remained.
During his PT course, he continued to experience multiple medical complications associated with his Stage D HF: ventricular tachycardia requiring defibrillation, kidney failure requiring dual transplant, and maximal mexiletine, dobutamine, milrinone, and Lasix dosing. Ultimately, NC suffered irreversible liver failure, which would have required a liver transplant in addition to the cardiac and renal transplants. The medical team determined that he would not be able to survive the surgery and in accordance with NC's wishes, he was made comfortable and passed away.
DISCUSSION
Both of these patients participated in a program that combined functional mobility, aerobic exercise, and resisted exercise. Patient NC had a femoral IABP for a prolonged period of time, which drastically altered exercise options that were available. These modifications forced some creativity in creating his plan of care and increased the interprofessional collaboration surrounding his care.
There were similarities between these patients. Both were young, with good social supports. This helped to motivate them throughout their extensive hospital stays. Each had a tenuous medical status for a prolonged period of time as well as multisystem involvement. This required daily re-evaluation by the physical therapist and the medical team, often resulting in medication titration or additional procedures, making scheduling more challenging. This also drove the PT plan of care to be malleable based on the each patient's daily status. Lastly, it was critical for the physical therapist and ICU teams to work closely together due to the increased frequency of symptoms that could potentially be attributed to the activity.
The invasive monitoring was pivotal in the management of these patients. Both had PA lines throughout, which gave real-time measurements that assisted the PT with clinical decision making. Their MVO2 was closely monitored to assist with medical management. Since it was drawn at frequent intervals post-activity, it was helpful in determining if the workload was too great, which may not have been captured by the patient's symptoms or vital sign response (which was often flat). Subtle changes in their CVP were noted, as increased volume in such brittle patients could easily overload the heart, drastically decreasing their activity tolerance and explaining new symptoms at rest.
The goal of PT intervention with both patients was to maximize function and strength in order to prepare for a heart transplantation surgery and recovery. There are other intervention techniques that may have been beneficial for these patients that were not trialed: functional electrical stimulation (FES) and inspiratory muscle training (IMT). Inspiratory muscle training has been shown to decrease dyspnea at rest and with activity,40 increase peripheral blood flow,41 increase maximum inspiratory pressures,40, 41 increase maximum oxygen consumption,42 and increase oxygen uptake.43 Functional electrical stimulation yields improvements in VO2 max, increases muscle strength and 6 minute walk test distances.44 This combination of results would have been beneficial for NC and AP, and were feasible options for these patients as they can be completed even with a low functional capacity. It should be noted that NC had both an AICD and IABP, so FES should be used with increased caution.
The presentation of these cases suggests that further research regarding exercise programs for patients with Stage D HF on inotropes is worthy of investigation. It may be helpful to determine appropriate dosages of conventional PT interventions or other interventions altogether that may be more effective and beneficial for this patient population. These cases add to the literature that patients with stage D HF can benefit from a supervised exercise program despite multiple ongoing medical issues. When engaging this patient population in exercise, coordination of all ICU team members as well as a high level of PT clinical decision making improves patient outcomes prior to heart surgery.
Figure 2.
Swan Ganz catheter.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics–2011 update: A report from the American Heart Association. Circulation. 2011;123((4)):e18–e209. doi: 10.1161/CIR.0b013e3182009701. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.hilbin EF. Comprehensive multidisciplinary programs for the management of patients with congestive heart failure. J Gen Intern Med. 1999;14((2)):130–135. doi: 10.1046/j.1525-1497.1999.00291.x. [DOI] [PubMed] [Google Scholar]
- 3.Sohn S, Helms TM, Pelleter JT, Muller A, Krottinger AI, Schoffski O. Costs and benefits of personalized healthcare for patients with chronic heart failure in the care and education program “telemedicine for the heart.”. Telemed J E Health. 2012;18((3)):198–204. doi: 10.1089/tmj.2011.0134. [DOI] [PubMed] [Google Scholar]
- 4.Dendale P, De Keulenaer G, Troisfontaines P, et al. Effect of a telemonitoring-facilitated collaboration between general practitioner and heart failure clinic on mortality and rehospitalization rates in severe heart failure: The TEMA-HF 1 (TElemonitoring in the MAnagement of heart failure) study. Eur J Heart Fail. 2012;14((3)):333–340. doi: 10.1093/eurjhf/hfr144. [DOI] [PubMed] [Google Scholar]
- 5.Park SJ, Milano CA, Tatooles AJ, et al. Outcomes in advanced heart failure patients with left ventricular assist devices for destination therapy. Circ Heart Fail. 2012;5((2)):241–248. doi: 10.1161/CIRCHEARTFAILURE.111.963991. [DOI] [PubMed] [Google Scholar]
- 6.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345((20)):1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 7.Allen LA, Stevenson LW, Grady KL, et al. Decision making in advanced heart failure: A scientific statement from the american heart association. Circulation. 2012;125((15)):1928–1952. doi: 10.1161/CIR.0b013e31824f2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: A report of the american college of cardiology foundation/american heart association task force on practice guidelines: Developed in collaboration with the international society for heart and lung transplantation. Circulation. 2009;119((14)):1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 9.Dunlay SM, Redfield MM, Weston SA, et al. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol. 2009;54((18)):1695–1702. doi: 10.1016/j.jacc.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Office of Scientific Affairs American Heart Association Classification of functional capacity and objective assessment http://my.americanheart.org/professional/StatementsGuidelines/ByPublicationDate/PreviousYears/Classification-of-Functional-Capacity-and-Objective-Assessment_UCM_423811_Article.jsp. Accessed June 19, 2012. [Google Scholar]
- 11.Bennett JA, Riegel B, Bittner V, Nichols J. Validity and reliability of the NYHA classes for measuring research outcomes in patients with cardiac disease. Heart Lung. 2002;31((4)):262–270. doi: 10.1067/mhl.2002.124554. [DOI] [PubMed] [Google Scholar]
- 12.Raphael C, Briscoe C, Davies J, et al. Limitations of the new york heart association functional classification system and self-reported walking distances in chronic heart failure. Heart. 2007;93((4)):476–482. doi: 10.1136/hrt.2006.089656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beckers PJ, Denollet J, Possemiers NM, Wuyts FL, Vrints CJ, Conraads VM. Combined endurance-resistance training vs. endurance training in patients with chronic heart failure: A prospective randomized study. Eur Heart J. 2008;29((15)):1858–1866. doi: 10.1093/eurheartj/ehn222. [DOI] [PubMed] [Google Scholar]
- 14.Mandic S, Tymchak W, Kim D, et al. Effects of aerobic or aerobic and resistance training on cardiorespiratory and skeletal muscle function in heart failure: A randomized controlled pilot trial. Clin Rehabil. 2009;23((3)):207–216. doi: 10.1177/0269215508095362. [DOI] [PubMed] [Google Scholar]
- 15.Feiereisen P, Delagardelle C, Vaillant M, Lasar Y, Beissel J. Is strength training the more efficient training modality in chronic heart failure? Med Sci Sports Exerc. 2007;39((11)):1910–1917. doi: 10.1249/mss.0b013e31814fb545. [DOI] [PubMed] [Google Scholar]
- 16.Smart NA, Steele M. Systematic review of the effect of aerobic and resistance exercise training on systemic brain natriuretic peptide (BNP) and N-terminal BNP expression in heart failure patients. Int J Cardiol. 2010;140((3)):260–265. doi: 10.1016/j.ijcard.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Jankowska EA, Wegrzynowska K, Superlak M, et al. The 12-week progressive quadriceps resistance training improves muscle strength, exercise capacity and quality of life in patients with stable chronic heart failure. Int J Cardiol. 2008;130((1)):36–43. doi: 10.1016/j.ijcard.2007.07.158. [DOI] [PubMed] [Google Scholar]
- 18.O'Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301((14)):1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piepoli MF, Davos C, Francis DP, Coats AJ. ExTraMATCH Collaborative. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH) BMJ. 2004;328((7433)):189. doi: 10.1136/bmj.37938.645220.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smart N, Marwick TH. Exercise training for patients with heart failure: A systematic review of factors that improve mortality and morbidity. Am J Med. 2004;116((10)):693–706. doi: 10.1016/j.amjmed.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 21.Kataoka T, Keteyian SJ, Marks CR, Fedel FJ, Levine AB, Levine TB. Exercise training in a patient with congestive heart failure on continuous dobutamine. Med Sci Sports Exerc. 1994;26((6)):678–681. doi: 10.1249/00005768-199406000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Paul EHR. Camarda R. Foley LL. Givertz MM. Cahalin LP. Case report: Exercise in a patient with acute decompensated heart failure receiving positive inotropic therapy. Cardiopulm Phys Ther J. 2011;22((2)):13–18. [PMC free article] [PubMed] [Google Scholar]
- 23.White HD, Ribeiro JP, Hartley LH, Colucci WS. Immediate effects of milrinone on metabolic and sympathetic responses to exercise in severe congestive heart failure. Am J Cardiol. 1985;56((1)):93–98. doi: 10.1016/0002-9149(85)90573-9. [DOI] [PubMed] [Google Scholar]
- 24.O'Connor ED, Walsham J. Should we mobilise critically ill patients? A review. Crit Care Resusc. 2009;11((4)):290–300. [PubMed] [Google Scholar]
- 25.Burtin C, Clerckx B, Robbeets C, et al. Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med. 2009;37((9)):2499–2505. doi: 10.1097/CCM.0b013e3181a38937. [DOI] [PubMed] [Google Scholar]
- 26.Kress JP. Clinical trials of early mobilization of critically ill patients. Crit Care Med. 2009;37((10 Suppl)):S442–447. doi: 10.1097/CCM.0b013e3181b6f9c0. [DOI] [PubMed] [Google Scholar]
- 27.Needham DM. Mobilizing patients in the intensive care unit: Improving neuromuscular weakness and physical function. JAMA. 2008;300((14)):1685–1690. doi: 10.1001/jama.300.14.1685. [DOI] [PubMed] [Google Scholar]
- 28.Laham RJ, Aroesty JM. Intraaortic balloon pump counterpulsation. Up to Date. 1/25/2008:3/30/2010. [Google Scholar]
- 29.Ferguson JJ, Cohen M. Freedman RJ, Jr, et al. The current practice of intra-aortic balloon counterpulsation: Results from the benchmark registry. J Am Coll Cardiol. 2001;38((5)):1456–1462. doi: 10.1016/s0735-1097(01)01553-4. [DOI] [PubMed] [Google Scholar]
- 30.Bream-Rouwenhorst HR, Hobbs RA, Horwitz PA. Thrombocytopenia in patients treated with heparin, combination antiplatelet therapy, and intra-aortic balloon pump counterpulsation. J Interv Cardiol. 2008;21((4)):350–356. doi: 10.1111/j.1540-8183.2008.00363.x. [DOI] [PubMed] [Google Scholar]
- 31.Cochran RP, Starkey TD, Panos AL, Kunzelman KS. Ambulatory intraaortic balloon pump use as bridge to heart transplant. Ann Thorac Surg. 2002;74((3)):746–751. doi: 10.1016/s0003-4975(02)03808-0. discussion 751-752. [DOI] [PubMed] [Google Scholar]
- 32.H'Doubler PB, Jr, H'Doubler WZ. Bien RC. Jansen DA. A novel technique for intraaortic balloon pump placement via the left axillary artery in patients awaiting cardiac transplantation. Cardiovasc Surg. 2000;8((6)):463–465. doi: 10.1016/s0967-2109(00)00052-1. [DOI] [PubMed] [Google Scholar]
- 33.Kern MJ. Cardiac catheterization techniques: Normal hemodynamics. Up To Date Online 17 3. 8/4/09. [Google Scholar]
- 34.Sprung CL, Pozen RG, Rozanski JJ, Pinero JR, Eisler BR, Castellanos A. Advanced ventricular arrhythmias during bedside pulmonary artery catheterization. Am J Med. 1982;72((2)):203–208. doi: 10.1016/0002-9343(82)90811-7. [DOI] [PubMed] [Google Scholar]
- 35.Shea M. Cardiac catheterization. http://www.merck.com/mmpe/sec07/ch070/ch070b.html. Accessed June 20, 2010. [Google Scholar]
- 36.Silvestry FE. Swan-ganz catheterization: Interpretation of tracings. Up To Date Online 17 3. 2010 June 20, 2010;18(1) [Google Scholar]
- 37.Klabunde R. Cardiovascular physiology concepts: Myocardial oxygen consumption. http://www.cvphysiology.com/CAD/CAD003.htm. Accessed June 22, 2010. [Google Scholar]
- 38.Colucci W, Chen H. Brain natriuretic peptide measurement in left ventricular dysfunction and other cardiac diseases. Up to Date 18 1. 2009 January 2010: June 22, 2010 [Google Scholar]
- 39.Colucci W. Evaluation of the patient with suspected heart failure. Up to date 18 1. 2009 January 2010: June 22, 2010. [Google Scholar]
- 40.Cahalin LP, Semigran MJ, Dec GW. Inspiratory muscle training in patients with chronic heart failure awaiting cardiac transplantation: Results of a pilot clinical trial. Phys Ther. 1997;77((8)):830–838. doi: 10.1093/ptj/77.8.830. [DOI] [PubMed] [Google Scholar]
- 41.Chiappa GR, Roseguini BT, Vieira PJ, et al. Inspiratory muscle training improves blood flow to resting and exercising limbs in patients with chronic heart failure. J Am Coll Cardiol. 2008;51((17)):1663–1671. doi: 10.1016/j.jacc.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 42.Winkelmann ER, Chiappa GR, Lima CO, Viecili PR, Stein R, Ribeiro JP. Addition of inspiratory muscle training to aerobic training improves cardiorespiratory responses to exercise in patients with heart failure and inspiratory muscle weakness. Am Heart J. 2009;158((5)):768. doi: 10.1016/j.ahj.2009.09.005. e1-768.e7. [DOI] [PubMed] [Google Scholar]
- 43.Stein R, Chiappa GR, Guths H, Dall'Ago P, Ribeiro JP. Inspiratory muscle training improves oxygen uptake efficiency slope in patients with chronic heart failure. J Cardiopulm Rehabil Prev. 2009;29((6)):392–395. doi: 10.1097/HCR.0b013e3181b4cc41. [DOI] [PubMed] [Google Scholar]
- 44.Sbruzzi G, Ribeiro RA, Schaan BD, et al. Functional electrical stimulation in the treatment of patients with chronic heart failure: A meta-analysis of randomized controlled trials. Eur J Cardiovasc Prev Rehabil. 2010;17((3)):254–260. doi: 10.1097/HJR.0b013e328339b5a2. [DOI] [PubMed] [Google Scholar]