Abstract
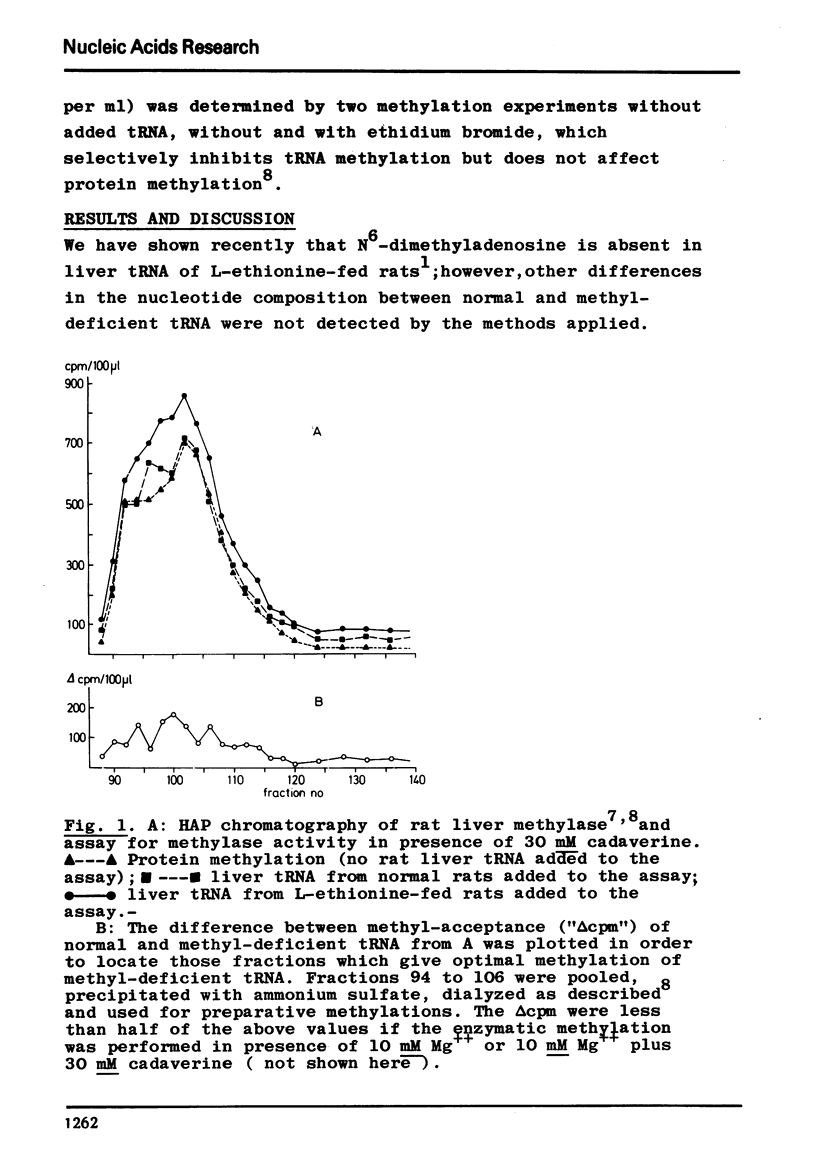
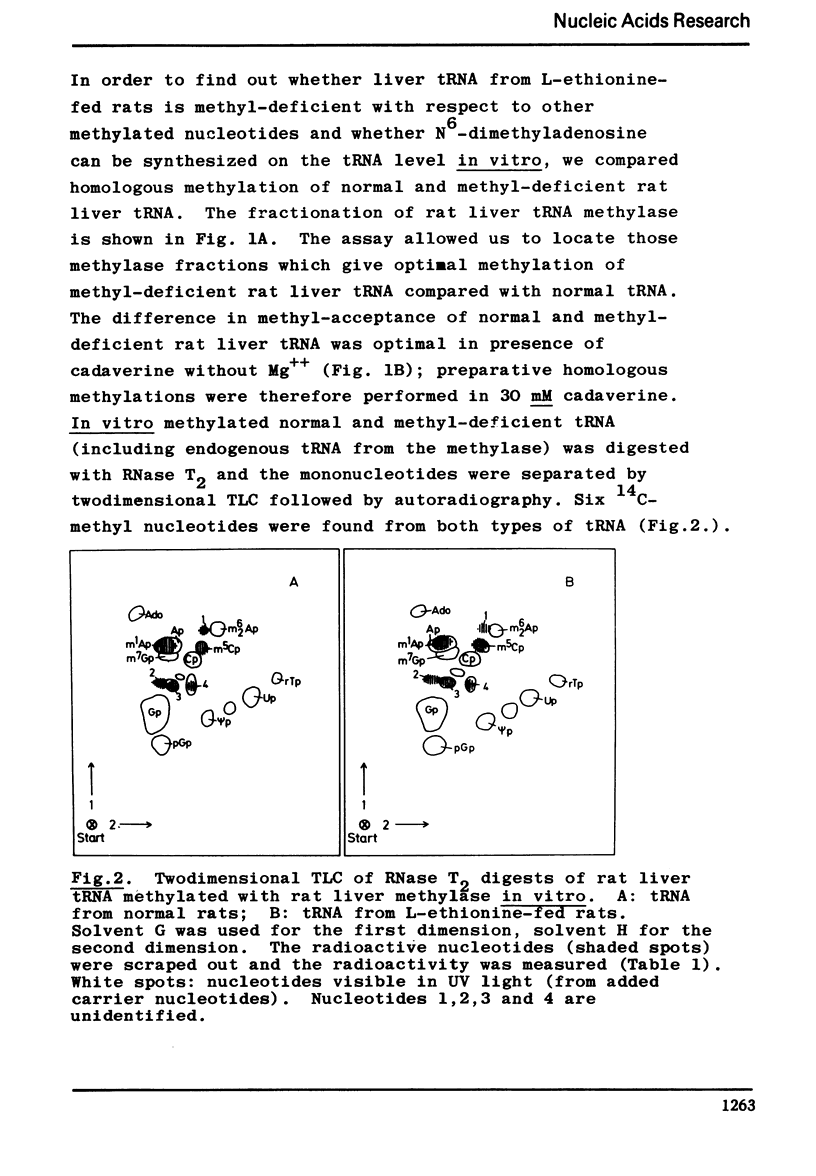
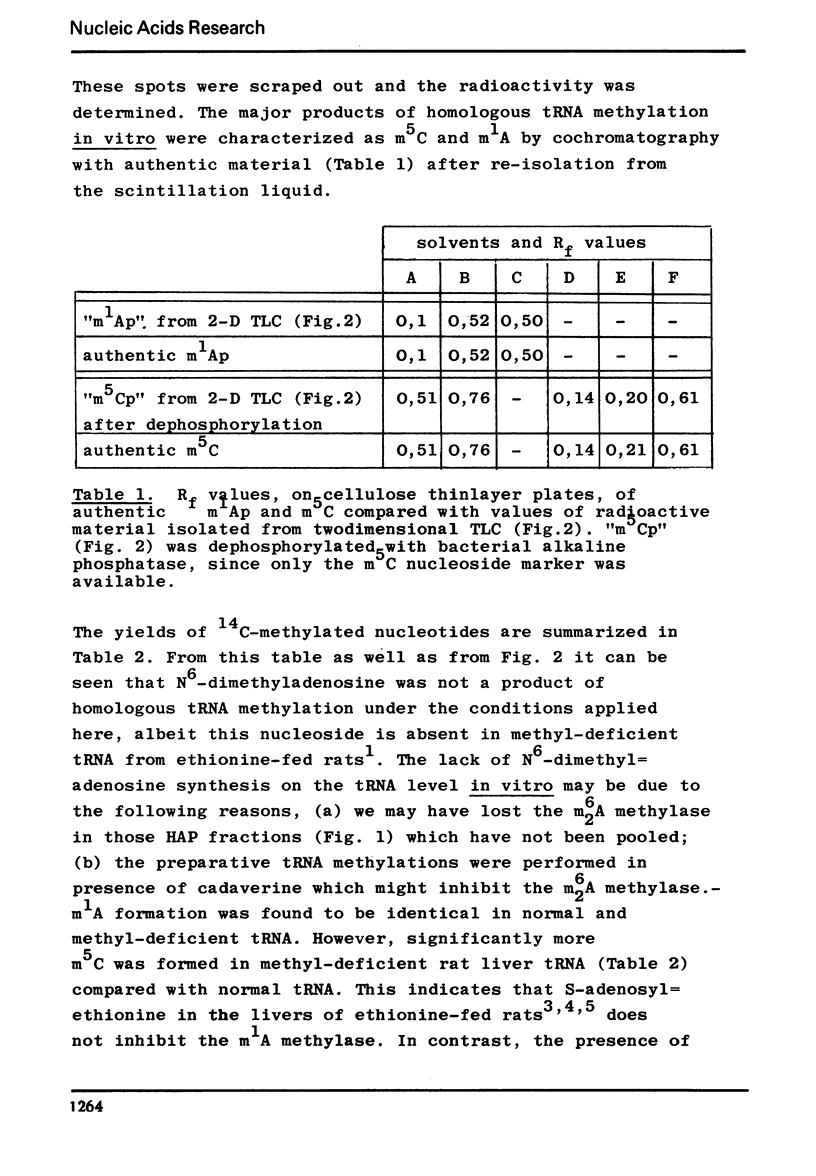
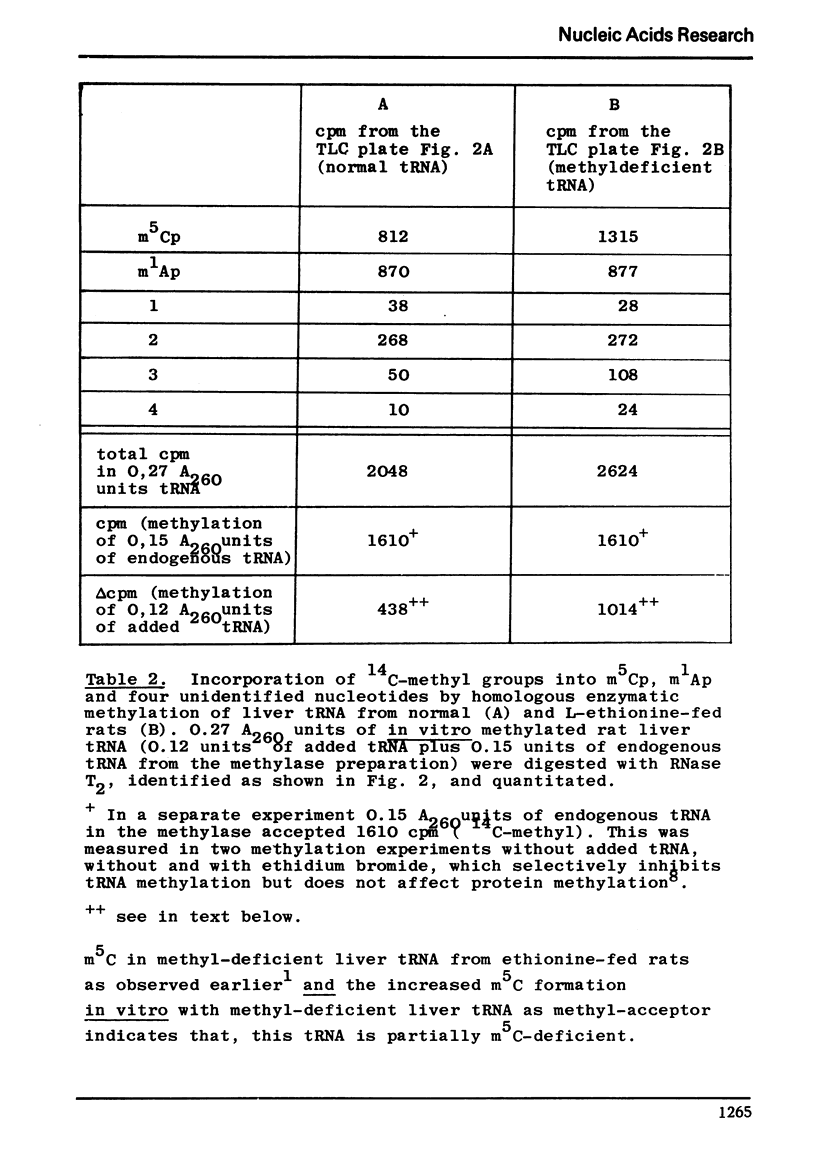
Following hydroxyapatite chromatography, rat liver tRNA methylase activity was assayed with liver tRNA from normal rats and with methyl-deficient liver tRNA from ethionine-fed rats. The difference in homologous methylation between normal and methyl-deficient tRNA was maximal in certain fractions in presence of cadaverine, and much less in presence of Mg++ or Mg++ plus cadaverine. These methylase fractions, which contained endogenous tRNA, were used for preparative homologous methylation of added normal and methyl-deficient tRNA in presence of 30 mM cadaverine. The 14C-methylated tRNA was digested with RNase T2 and the resulting methylated mononucleotides were characterized and quantitated after twodimensional thinlayer chromatography and autoradiography. The major products of homologous tRNA methylation were m5C and m1A. However, the methylase fraction used here did not catalyze the formation of m62A with m62A-deficient tRNA as substrate.- In addition to the previously described, analytically detectable m62A-deficiency, a partial m5C-deficiency was demonstrated in liver tRNA from ethionine-fed rats by measuring the methylacceptance in vitro. In presence of cadaverine, with the methylase fraction used here, methyl-deficient tRNA from ethionine-fed rats was a twofold more efficient methyl-acceptor in vitro than normal liver tRNA, while endogenous tRNA isolated from the methylase fraction was a threefold more efficient methyl-acceptor than normal liver tRNA. Homologous methylation of normal tRNA, as observed here, has not been described before.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold H., Kersten H. The occurrence of ribothymidine, 1-methyladenosine, methylated guanosines and the corresponding methyltransferases in E. coli and Bacillus subtilis. FEBS Lett. 1973 Oct 1;36(1):34–38. doi: 10.1016/0014-5793(73)80331-x. [DOI] [PubMed] [Google Scholar]
- FARBER E., SHULL K. H., VILLA-TREVINO S., LOMBARDI B., THOMAS M. BIOCHEMICAL PATHOLOGY OF ACUTE HEPATIC ADENOSINETRIPHOSPHATE DEFICIENCY. Nature. 1964 Jul 4;203:34–40. doi: 10.1038/203034a0. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Wildenauer D. Enzymatic methylations. I. Isonicotinic acid hydrazide: an inhibitor of tRNA and protein methylation. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1215–1220. doi: 10.1016/0006-291x(72)90964-3. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Wildenauer D. Enzymatic methylations. II. In vitro inhibition of tRNA and protein methylation by nicotinamide and isonicotinic acid hydrazide: activation of a s-adenosylmethionine-splitting enzyme in rat liver. Biochem Biophys Res Commun. 1972 Jul 11;48(1):58–64. doi: 10.1016/0006-291x(72)90343-9. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Nishimura S. Nucleotide sequence specificities of guanylate residue-specific tRNA methylases from rat liver. Biochem Biophys Res Commun. 1970 Jul 27;40(2):306–313. doi: 10.1016/0006-291x(70)91010-7. [DOI] [PubMed] [Google Scholar]
- Philipps G. R. Analysis of purified tRNA species by polyacrylamide gel electrophoresis. Anal Biochem. 1971 Dec;44(2):345–357. doi: 10.1016/0003-2697(71)90220-x. [DOI] [PubMed] [Google Scholar]
- Rogg H., Wehrli W., Staehelin M. Isolation of mammalian transfer RNA. Biochim Biophys Acta. 1969 Nov 19;195(1):13–15. doi: 10.1016/0005-2787(69)90597-8. [DOI] [PubMed] [Google Scholar]
- Shull K. H., McConomy J., Vogt M., Castillo A., Farber E. On the mechanism of induction of hepatic adenosine triphosphate deficiency by ethionine. J Biol Chem. 1966 Nov 10;241(21):5060–5070. [PubMed] [Google Scholar]
- Smith R. C., Salmon W. D. Formation of S-adenosylethionine by ethionine-treated rats. Arch Biochem Biophys. 1965 Jul;111(1):191–196. doi: 10.1016/0003-9861(65)90339-5. [DOI] [PubMed] [Google Scholar]
- Wildenauer D., Gross H. J. Methyldeficient mammalian 4s RNA: evidence for L-ethionine-induced inhibition of N6-dimethyladenosine synthesis in rat liver tRNA. Nucleic Acids Res. 1974 Feb;1(2):279–288. doi: 10.1093/nar/1.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]



