Abstract
Cortical dopamine (DA) modulation of the gamma-amino butyric acid (GABA) system is closely associated with cognitive function and psychiatric disorders. We recently reported that the glycogen synthase kinase 3β (GSK-3β) pathway is required for hyperdopamine/D2 receptor-mediated inhibition of NMDA receptors in the prefrontal cortex. Here we explore whether GSK-3β is also involved in dopaminergic modulation of GABAA receptor-mediated inhibitory transmission. We confirmed that DA induces a dose-dependent, bidirectional regulatory effect on inhibitory postsynaptic currents (IPSCs) in prefrontal neurons. The modulatory effects of DA were differentially affected by co-application of GSK-3β inhibitors and different doses of DA. GSK-3β inhibitors completely blocked high-dose (20 μM) DA-induced depressive effects on IPSCs but exhibited limited effects on the facilitating regulation of IPSC in low-dose DA (200 nM). We also confirmed that surface expressions of GABAA receptor β2/3 subunits were significantly decreased by DA applied in cultured prefrontal neurons and in vivo administration of DA reuptake inhibitor. These effects were blocked by prior administration of GSK-3β inhibitors. We explored DA-mediated regulation of GABAA receptor trafficking and exhibited the participation of brefeldin A-inhibited GDP/GTP exchange factor 2 (BIG2) or dynamin-dependent trafficking of GABAA receptors. Together, these data suggest that DA may act through different signaling pathways to affect synaptic inhibition, depending on the concentration. The GSK-3β signaling pathway is involved in DA-induced decrease in BIG2-dependent insertion and an increase in the dynamin- dependent internalization of GABAA receptors, which results in suppression of inhibitory synaptic transmission.
Keywords: Receptor trafficking, inhibitory synaptic transmission, GSK-3, dopamine, neocortex
Introduction
Dopamine (DA), as well as glutamate and gamma-amino butyric acid (GABA) systems in the prefrontal cortex (PFC), plays an important role in normal prefrontal function and neuropsychiatric disorders (Lewis & Gonzalez-Burgos 2006, Harrison & Weinberger 2005, Goldman-Rakic et al. 2004, Goldman-Rakic 1995). A large body of evidence indicates that abnormality in cortical DA levels causes cognitive impairments similar to those associated with schizophrenia (Harrison & Weinberger 2005, Goldman-Rakic et al. 2004, Davis et al. 1991, Howes & Kapur 2009, Simpson et al. 2010, Egan & Weinberger 1997). It is known that dopamine regulation of prefrontal cortical inhibition plays an important role in the regulation of executive cognitive functions (Seamans & Yang 2004). Functional interaction between DA and GABAA receptor-mediation inhibition has been widely studied in PFC neurons (Wang et al. 2002, Law-Tho et al. 1994, Gonzalez-Islas & Hablitz 2001, Seamans et al. 2001, Trantham-Davidson et al. 2004, Kroner et al. 2007, Gao et al. 2003). It has been reported that DA has bidirectional effects on modulation of GABAA receptor-mediated inhibitory transmission and that the opposing effect of DA is dependent on activation of different DA receptor subtypes (Seamans et al. 2001, Seamans & Yang 2004, Trantham-Davidson et al. 2004, Kroener & Lavin 2010). Typically, GABAA receptor function is enhanced by activation of D1 receptors and depressed by activation of D2 receptors. The functions of DA receptors have been studied using the cyclic adenosine monophosphate (cAMP) protein kinase A (PKA)–phosphoprotein (DARPP-32)-dependent signaling pathway (Missale et al. 2006, Neve et al. 2004, Greengard et al. 1999, Li & Gao 2011). Activation of D1 and D2 receptors or the D1–D2 heterooligomer can also trigger other signaling molecules such as Ca2+, protein kinase C, and phospholipase C (PLC) (Greengard 2001, George & O’Dowd 2007). In addition, emerging evidence has shown that D2 receptors also exert their effects through the glycogen synthase kinase 3 (GSK-3) signaling cascade, a cAMP-independent mechanism (Beaulieu et al. 2007, Beaulieu et al. 2009, Li et al. 2009). Indeed, increasing attention is being paid to the role of GSK-3 in schizophrenia (Emamian et al. 2004, Freyberg et al. 2010, Bersudsky et al. 2008, Lovestone et al. 2007, Koros & Dorner-Ciossek 2007, Kozlovsky et al. 2002), especially in DA-associated behaviors (Beaulieu et al. 2007, Li et al. 2009, Li & Gao 2011, Beaulieu et al. 2005, Beaulieu et al. 2004).
We recently found that the GSK-3β pathway is required for hyperdopamine-induced inhibition of NMDA receptor-mediated excitatory synaptic transmission in the PFC (Li et al. 2009). In addition, GSK-3β was also reported to contribute to GABAergic synapse formation and plasticity (Tyagarajan et al. 2011). GABAA receptors coexist with NMDA receptors on the postsynaptic membrane and both are regulated by DA. Therefore, we hypothesized that GSK-3β pathway is also required for dopaminergic regulation of GABAA receptor-mediated inhibitory transmission. In this study, we investigated GSK-3β mediated mechanisms underlying DA regulation of inhibitory transmission by using a combination of techniques. We found that GSK-3β is involved in a high-dose DA-induced suppression of inhibitory synaptic transmission.
Experimental Procedures
Detailed experimental protocols can be found in the Supplemental Data.
Electrophysiological recording in prefrontal cortical slices
The postnatal day 15–30 SD rats were used and the brains were sectioned into 300 μm sections. Whole-cell patch-clamp recordings were conducted in the prefrontal neurons. The recordings were conducted at ~35°C and the resistance of the recording pipette was 5–7 MΩ The IPSCs were elicited by stimulating layer 2/3 with either a single pulse or a 10-pulse 20 Hz train (0.1 ms, 10–100 μA, 10 s inter-stimulus interval) through a bipolar electrode. The mIPSCs and sIPSCs at the layer 5 pyramidal neurons were recorded at −65 mV in the presence of AP5 (50 μM) and DNQX (20 μM) with or without TTX (0.5 μM), respectively. All neurons without stable baseline recording of IPSCs for 5 min and with input resistance increased more than 20% were discarded for further analysis. All drug effects were then normalized to the baseline levels. The data were analyzed by the Student t test and were presented as mean ± standard error.
Primary neuronal cultures
Primary neuron cultures were prepared from embryonic day 20 rat PFC. Cultured PFC neurons at 14 days in vitro were fixed with or without 0.25% Triton X-100 for total protein staining or surface protein staining, respectively. Cells were incubated with antibody against the N-terminal of GABAA receptor β2/3 subunits and cells were incubated with conjugated to fluorescein isothiocyanate (1:400). Dendritic processes were selected for analysis. Results were presented as mean ± standard error.
BS3 cross-linking assay
PFC tissues were sectioned as 400-μm slices, which were incubated with BS3 (1 mg/ml) and the surface expression was determined by Western blot analysis. PFC tissues were homogenized and centrifuged. The supernatants were resolved by electrophoresis on polyacrylamide gels and transferred onto nitrocellulose membranes. The membranes were incubated with primary antibodies against GABAA receptor β2/3, BIG2, and β-actin. The blots were incubated in HRP-conjugated goat anti-mouse IgG and the immunopositive protein bands were detected with the ECL Blotting System. The band densities were measured with ImageJ and normalized to β-actin. The results were presented as mean ± standard error.
Results
Effect of DA on GABAA receptor-mediated IPSCs is concentration-dependent
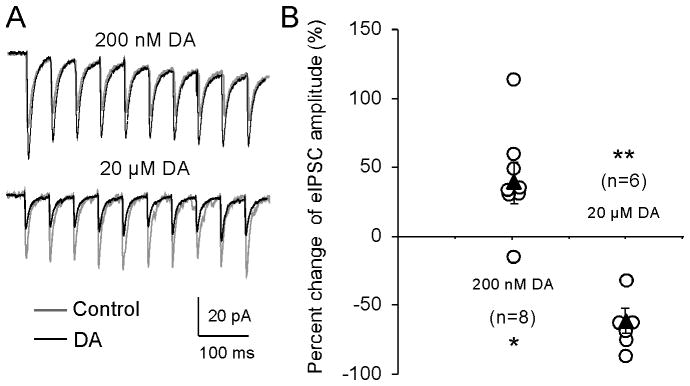
We recorded synaptically evoked GABA receptor-mediated IPSCs from layer 5 pyramidal neurons in the medial PFC by stimulating layer 2/3 in the presence of NMDA receptor antagonist AP5 (50 μM ) and AMPA receptor antagonist DNQX (20 μM). To test the effect of DA on inhibitory transmission, we used a 10-pulse, 20-Hz train at 10 s inter-stimulus interval (Fig. 1A) to elicit IPSCs with the input resistance of the cell monitored by an injected negative current (−100 pA, 200 ms). The evoked IPSCs were first recorded for 5 min as baseline. If the baselines were stable without clear changes in IPSC amplitude and input resistance (change < 20%), low- (200 nM) or high-dose DA (20 μM) was then applied to bath solution for 10 min, followed by an additional 10–20 min wash. As shown in Figure 1, a low concentration of DA (200 nM) gradually and significantly increased the amplitude of the first IPSCs by an average of 43.5% ± 12.78% in the second 5-min DA application (n = 8, *p < 0.05, also see Fig. 2). In contrast, when the concentration of DA was increased to 20 μM, the amplitude was significantly decreased by 64.1 ± 9.20% (n = 6, **p < 0.01). This bidirectional regulation of cortical inhibition by DA appeared to be long-lasting, persisting for more than 20 min without clear recovery during washout (see Fig. 2), consistent with the results from a previous report (Trantham-Davidson et al. 2004).
Figure 1.
DA induces dose-dependent and bidirectional effects in GABAA receptor-mediated IPSCs in PFC layer 5 pyramidal neurons. A, Representative traces showing IPSCs induced by a 20-Hz 10-pulse train before (gray) and 10 min after (black) bath-application of DA at two different concentrations. B, Summary graph shows the effects of DA on the amplitudes of the first IPSCs during application of DA for 10 min. The open circles denote the first IPSC amplitude of individual neurons, whereas the closed triangles represent the average changes of the first IPSC amplitudes at each concentration of DA. DA induced bidirectional effects on evoked IPSCs, with a significant enhancement at the lower concentration of 200 nM but with suppression at the higher concentration of 20 μM (*p < 0.05, **p < 0.01).
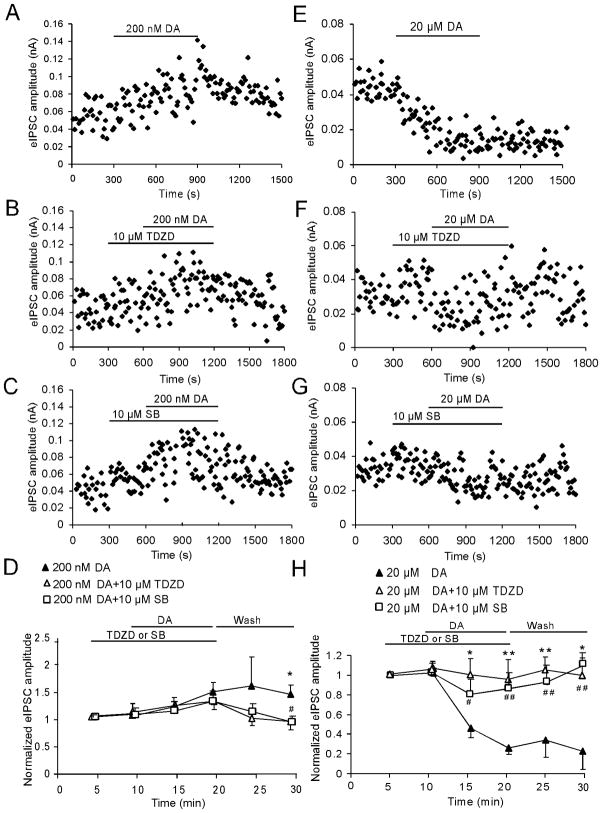
Figure 2.
GSK-3β signaling pathway is involved in the 20-μM DA-induced depression of GABA receptor-mediated IPSCs. A, B, and C, Representative samples show the changes of the 1st IPSC amplitudes before and after application of 200 nM DA alone (A) or co-applied with GSK inhibitors TDZD (10 μM, B) or SB216763 (10 μM, C). D, Summary graph shows that low-dose (200 nM) DA gradually increased the amplitude of the first IPSCs whereas the GSK-3β inhibitor TDZD (10 μM) or SB216763 (10 μM), when co-applied with DA, exhibited slow effects. Both of them could not completely block the facilitating effects induced by low-dose (200 nM) DA on the amplitude of the first IPSCs until 10-min wash (TDZD vs. DA, n = 6, *p < 0.05; SB vs. DA, n = 6, # p < 0.05).. E, F and G, Time course of the 1st IPSC amplitudes before and after application of 20 μM DA alone (E) or co-applied with GSK inhibitors TDZD (10 μM, F) or SB216763 (10 μM, G). H, Summary graph shows that both GSK-3β inhibitors TDZD and SB216763 completely blocked 20-μM DA’s inhibitory effect on IPSCs and this effect lasted for more than 10 min after washout (n = 6 for both drugs; * (TDZD vs. DA) # (SB vs. DA), * or # p < 0.05, ** or ## p < 0.01).
GSK-3β signaling pathway is involved in the DA modulation of inhibitory transmission
Recent studies have suggested that GSK-3β is critical for DA-dependent actions (Beaulieu et al. 2005). Our previous study reported that GSK-3β activity is enhanced by increasing DA concentrations and is required for hyperdopamine/D2 receptor-mediated inhibition of NMDA receptors in the prefrontal neurons (Li et al. 2009). We therefore attempted to test whether the GSK-3β signaling pathway is also involved in the modulation of inhibitory transmission by DA. Bath-application of GSK-3β inhibitor TDZD (10 μM) for 5 min did not show clear effects on the basal amplitudes of the first IPSC (10-pulse, 20-Hz, 10 s interval, increased by 4.33 ± 3.09%, p > 0.05). However, co-application of DA with TDZD for 10 min and then washing for another 10 min exerted various degrees of blockades on the effects of DA. As shown in Figure 2, The GSK-3β inhibitor TDZD (10 μM) slowly blocked 200 nM DA-induced increase in the first IPSC amplitude but only showed significance at the second 5 min of washing (n = 6, *p < 0.05; Fig. 2A, B, and D). In addition, the paired-pulse ratios (both 1st/2nd IPSC and 1st/10th IPSC) exhibited no significant differences either before or after application of drugs or between a single application of DA and co-application with the GSK-3β inhibitor (p > 0.05 for all time points; data not shown), indicating the postsynaptic effects of the drugs. In contrast, TDZD completely prevented 20 μM DA-induced decrease of IPSCs (n = 6, *p < 0.05, **p < 0.01; Fig. 2E, F, and H). Similarly, the paired-pulse ratios exhibited no significant differences during the drug application and washing period (p > 0.05 for both; data not shown).
We also examined another structurally different GSK-3β inhibitor SB216763 (10 μM). Pre-application of SB216763 for 5 min didn’t show significant effects on the baseline of IPSCs (increased by 3.76 ± 1.89%, p > 0.05). Similar to TDZD, SB216763 had no immediate effect on 200-nM DA-induced increase in the first IPSC amplitude even after 10-min DA+SB application (n = 6, p > 0.05, Fig. 2C). However, SB216763 exhibited significant blocking effect after washout for 10 min (n = 6, #p < 0.05; Fig. 2C and D). In contrast, 10 μM SB216763 completely abolished 20-μM DA’s inhibitory effect on IPSCs and this effect lasted more than 10 min after washout (n = 6, #p < 0.05, ##p < 0.01; Fig. 2G and F). These data suggest that GSK-3β signaling pathway is more involved in high-dose (20 μM) DA-mediated depression of IPSCs.
DA-induced depression in GABAA receptor-mediated IPSCs is mainly through activation of D2 receptors
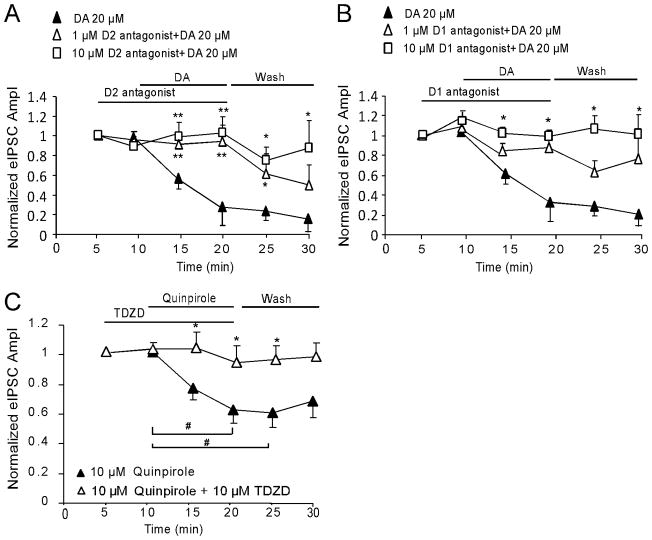
DA receptors are subdivided into the D1 family (D1 and D5 receptor) and the D2 family (D2, D3, and D4 receptor subtypes). To further determine which DA receptor subtype mediates DA-induced reduction in IPSC amplitudes, selective D1 or D2 antagonist was first applied for 5 min and then co-applied with 20 μM DA for 10 min. None of these antagonists, neither D2 antagonist L741,626 nor D1 antagonist SCH23390, exhibited significant effects on the baseline of single-pulse evoked IPSCs (decreased 5.26 ± 3.73 % and 9.79 ± 5.58% by 1 μM and 10 μM L741,626, respectively, p > 0.05; and increased 8.25 ± 4.01% and 16.8 ± 5.02% by 1 μM and 10 μM SCH23390, respectively, p > 0.05). The D2 antagonist L741,626 (1μM or 10μM) effectively blocked the suppressing effects of DA (20 μM) on GABAA receptor-mediated IPSCs (n = 6 for each concentration, **p < 0.01; Fig. 3A). Surprisingly, the D1 antagonist SCH23390 at 10 μM also partially abolished the inhibitory effect of DA on IPSCs (n = 6, *p < 0.05; Fig. 3B), but SCH23390 at 1 μM did not show a significant recovery effect (n = 6, *p > 0.05; Fig. 3B). These data suggested that 20-μM DA-induced depression in IPSCs was mainly mediated via D2 receptors because D1 antagonist SCH23390 at 1 μM (Ki = 0.2 nM for D1 and 0.3 nM for D5) exhibited no significant blocking effects on DA’s depression in IPSCs. Although SCH23390 at 10 μM had significant blocking effects, but SCH23390 at this concentration may also block D2-like receptors because the Ki values for D2, D3 and D4 receptors are 1100, 800, and 3000 nM, respectively.
Figure 3.
High-dose DA (20 μM) attenuates GABAa receptor-mediated IPSCs mainly through activation of D2 receptors. A, 20 μM DA-induced depression of evoked IPSCs was completely blocked by the D2 receptor antagonist L741,626 at both 1 and 10 μM. B, The inhibitory effects of DA were partially blocked by D1 receptor antagonist SCH 23390 with significance at 10 μM but without significance at 1 μM (n = 6 for each concentration for both A and B; *p < 0.05, **p < 0.01). Note: same group of neurons (n = 6) was used for 20 μM DA in A and B. C, Selective D2 agonist quinpirole at 10 μM (bath) mimicked high-dose DA’s action with a significant decrease of the amplitude of evoked IPSCs (n = 8, #p < 0.05), and this effect was completely abolished by co-application of 10 μM TDZD (n = 8, *p < 0.05 versus quinpirole).
To verify the action of D2 receptors in high-dose DA-induced suppressive effect on inhibitory transmission, we applied selective D2 agonist quinpirole (10μM, Ki values are 4.8, ~24, and ~30 nM at D2, D3, and D4 receptors, respectively) alone or with GSK-3β inhibitor TDZD (10 μM). As shown in Fig. 3C, quinpirole induced similar depressive effect as 20-μM DA on IPSCs and this effect was significantly abolished by co-application of TDZD (n = 8, *p < 0.05; Fig. 3C).
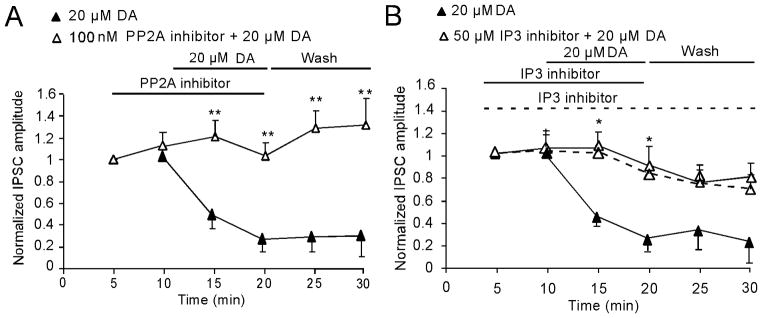
Activation of PP2A or IP3 is required for GSK-3β signaling-mediated inhibitory action of DA on IPSCs
Next we investigated the upstream GSK-3β signaling-mediated inhibitory action of high-dose DA on single-pulse evoked IPSCs. Because PP2A is identified as an upstream regulator of GSK-3β (Beaulieu et al. 2009), we tested the effects of the PP2A inhibitor okadaic acid on DA-induced inhibition of GABAA receptor-mediated IPSCs. The baseline of IPSCs was not affected by pre-applied okadaic acid (100 nM; increased 9.48 ± 5.29%, p > 0.05). In contrast, as shown in Fig. 4, okadaic acid completely prevented the 20 μM DA-induced decrease of IPSC when both were co-applied together (n = 6, **p < 0.01; Fig. 4A). Earlier studies indicated that D2 or high-dose DA-mediated reduction of IPSCs in the PFC could be blocked by either IP3 or PP1/2A receptors, indicating involvement in the PLC-IP3-PP1/2A signaling pathway (Trantham-Davidson et al. 2004). To determine whether PLC-IP3-PP1/2A is upstream of GSK-3β or whether they are two independent signaling pathways, we applied the IP3 inhibitor 2-APB (50 μM) for 5 min and then co-administered it with DA (20 μM) for 10 min. Interestingly, 2-APB did not affect the baseline of IPSCs when it was pre-applied alone (increased by 8.25±11.27%, p > 0.05), but it also completely prevented the effect of DA on IPSCs (n = 11, *p < 0.05; Fig. 4B), although 2-APB’s blocking action was soon dissipated by either washing out DA alone (n = 5, p > 0.05; Fig. 4B dashed line) or both DA and 2-APB (n = 6, p > 0.05; Fig. 4B). These data suggest that both PP2A and IP3 play a key role in 20μM DA-mediated depression of inhibitory transmission.
Figure 4.
GSK-3β signaling pathway mediates DA-induced reduction of IPSCs through activation of PP2A and/or IP3. A, Co-application of the PP2A inhibitor okadaic acid (100 nM) significantly and completely blocked the depressing effects of DA (20 μM) on IPSCs (n = 6, **p < 0.01). B, Similarly, co-application of the IP3 inhibitor 2-APB (50μM) with DA (20 μM) also blocked DA-induced inhibition of GABAa receptor-mediated IPSCs (n = 11; *p < 0.05). However, the blocking effect of IP3 inhibitor was transient and easily washed out, different from that of PP2A inhibitor. Dashed line showed that 2-APB significantly blocked DA’s effect but only lasted for 10 min even under condition of continuous application without washout (dashed line). Note: the 20-μM DA data presented in A and B were from same group of cells (n = 6).
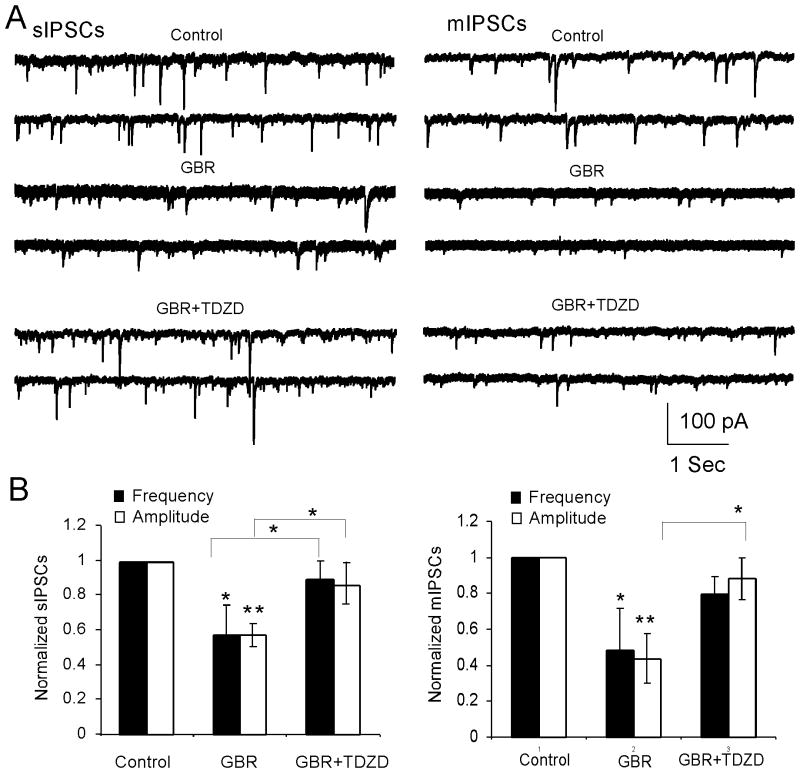
Increase of endogenous DA attenuates both sIPSCs and mIPSCs through activation of GSK-3β in vivo
To further examine the role of GSK-3β in the regulation of inhibitory transmission by DA, GBR12909, a selective DA reuptake inhibitor, was systemically administered to animals to enhance endogenous DA, as we reported previously (Li et al. 2009). A single dose of GBR12909 (10 mg/kg, intraperitoneally [i.p.]) was injected with saline as vehicle control; the rats were killed for physiological recordings after 1 h. We found that GBR12909 significantly decreased the amplitude and frequency of spontaneous IPSCs (sIPSCs) in the layer 5 pyramidal neurons compared with those in the control (n = 6, sIPSC amplitude: 36.3 ± 4.03 pA in control vs. 20.8 ± 6.24 pA in GBR12909, p < 0.01; sIPSC frequency: 1.24 ± 0.27 Hz in control vs. 0.71±0.21 Hz in GBR12909, *p < 0.05,; Fig. 5). Pre-injection of TDZD (1 mg/kg, i.p.) for 30 min before administration of GBR12909 (10 mg/kg, i.p.) completely blocked GBR12909-induced depression in sIPSCs (n = 6, sIPSC amplitude: 20.8 ± 6.24 pA in GBR12909 vs. 31.3 ± 8.18 pA in GBR12909+TDZD, p < 0.05; sIPSC frequency: 0.71±0.21 Hz in GBR12909 vs. 0.99 ± 0.29 Hz in GBR12909+TDZD, * p < 0.05 for both; Fig. 5). Similarly, administration of GBR12909 also decreased the amplitude and frequency of miniature IPSCs (mIPSCs) compared with those in the controls (n = 6, mIPSC amplitude: 32.27±4.86 pA in control vs. 14.29 ± 4.54 pA in GBR12909, **p < 0.01; mIPSC frequency: 0.45 ± 0.17 Hz in control vs. 0.22 ± 0.11 Hz in GBR12909,* p < 0.05; Fig. 5). TDZD pre-administration effectively recovered the decreased amplitude of mIPSCs induced by GBR12909 but did show significant recovery effect on the frequency of mIPSCs (n = 10, mIPSC amplitude: 14.29 ± 4.54 pA in GBR12909 vs. 20.33 ± 7.71 pA in GBR12909+TDZD, *p<0.05; mIPSC frequency: 0.22 ± 0.11 Hz in GBR12909 vs. 0.29±0.08 Hz in GBR12909+TDZD, p> 0.05; Fig. 5). These data further suggested that GSK-3β is involved in the regulation of inhibitory transmission by DA.
Figure 5.
Systemic administration of GBR12909 to increase endogenous DA significantly decreased both sIPSCs and mIPSCs; the effects were reversed by systemic prior administration of the GSK-3β inhibitor TDZD. A, Representative traces of the sIPSCs (left panel) and mIPSCs (right panel). B, Left panel, GBR12909 (10 mg/kg, i.p.) significantly decreased both frequency (p < 0.05) and amplitude (p < 0.01) of sIPSCs; these effects were completely blocked by TDZD (1 mg/kg, i.p.). Right panel, both frequency (p < 0.05) and amplitude (p < 0.01) of mIPSCs were significantly decreased by GBR12909 but only the amplitude was significantly prevented by TDZD.
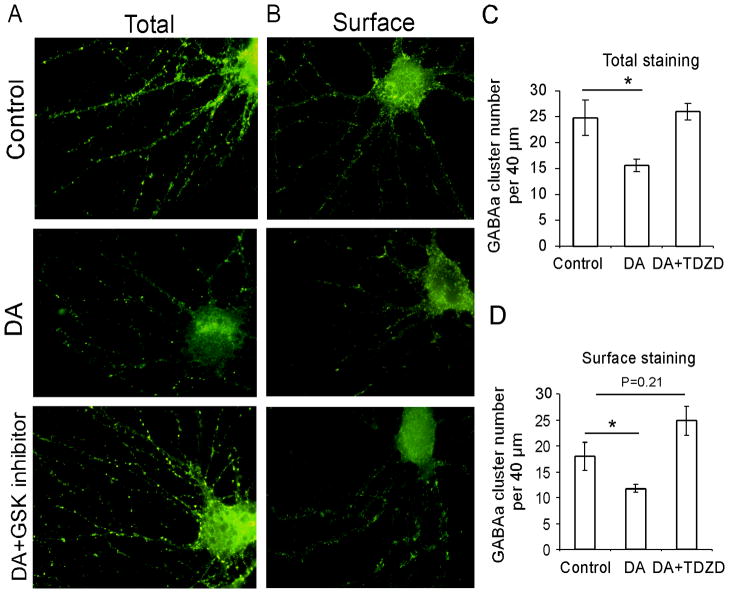
GSK-3β mediates DA-induced decrease of both total and surface expression of GABAA receptors in cultured PFC neurons
GABA receptor trafficking is a key determinant in the efficiency of GABAergic synaptic transmission. To examine whether GSK-3β-mediated DA depression of GABAA receptor function was attributable to the alteration of receptor expression, total and surface GABAA receptors in cultured PFC cells were labeled using N-terminal GABAA β2/3 antibody under permeabilizing and nonpermeabilizing conditions, respectively. As illustrated in Figure 6, either total or surface staining for GABAA β2/3 exhibited a punctate pattern that was expressed on the soma and dendrites. Treatment with DA (20μM) for 10 min significantly decreased both total and surface cluster numbers on the dendrites (Fig. 6A and B). The total cluster numbers on the dendrites decreased from 24.9 ± 3.40 to 15.7 ± 1.21 per 40 μm (n = 12, *p < 0.05; Fig. 6C) and the surface cluster number decreased from 18.0 ± 2.68 to 11.8 ± 0.79 (n = 12, *p < 0.05; Fig. 6D). Treatment with the GSK-3β inhibitor TDZD (10 μM) for 5 min did not show any effects on either total or surface GABAA clusters (data not shown) but further co-administration of TDZD (10 μM) with DA (20 μM) for 10 min effectively blocked the effects of DA on total and surface GABAA receptor expression. The total cluster number per 40 μm dendrites was 26.0 ± 1.69 (n = 12), a level comparable with that in the controls (vs. 24.9 ± 3.40, p > 0.05; Fig. 6C). The surface cluster numbers on the 40-μm dendrites were slightly increased to 24.9 ± 2.82 (n= 12) but were not significant compared with the controls (vs. 18.0 ± 2.68 p > 0.05; Fig. 6D). These results suggest that GSK-3β mediates the DA-induced decrease of GABAA receptor expression at the neuronal dendrites, which is associated with reduction of GABA function.
Figure 6.
GSK-3β inhibitor prevented DA-induced decrease of total and surface GABAA receptor expressions in the cultured prefrontal neurons. A and B, Cultured PFC neurons were treated with vehicle, DA (20 μM), or DA (20 μM) with TDZD (10 μM) for 10 min and then stained for GABAA receptors using anti-N-terminal GABAa β2/3 antibody in permeabilizing (A) and impermeablizing (B) conditions, respectively. C and D, Quantification of GABAA receptor clusters in the immunofluorescence staining. DA significantly decreased both total (A) and surface (B) GABAA β2/3 cluster numbers compared with those in the control (n = 12, *p < 0.05). The GSK-3β inhibitor TDZD (10 μM) completely blocked the effect of DA on both total (C) and surface (D) GABAA cluster numbers (n = 12,*p < 0.05).
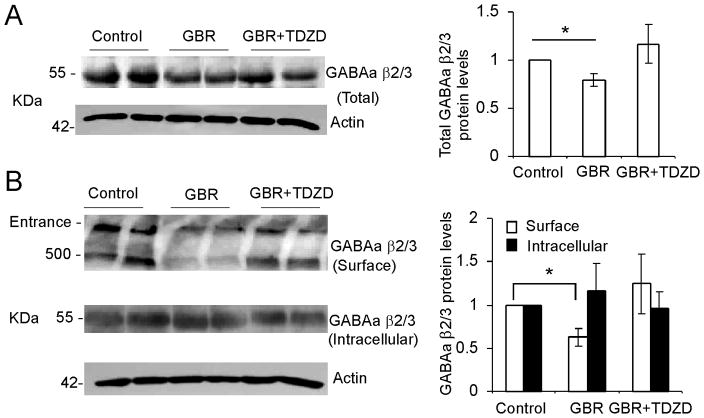
DA-induced depression of inhibitory transmission is through GSK-3β-mediated trafficking of GABAA receptors
We further examined the change in GABAA protein levels in vivo using Western blot analysis. As shown in Figure 7A, systemically administered GBR12909 (10 mg/kg, i.p, 1 h) significantly decreased the total protein levels of GABAA receptors by 20.3% ± 6.01% compared with the saline vehicle control (n = 4 rats in each group, *p < 0.05). Consistent with the electrophysiological changes in prefrontal cortical slices, pretreatment with the GSK-3β inhibitor TDZD for 30 min (1 mg/kg, i.p.) completely prevented the decrease of total protein expressions of the GABAA receptor (n = 4 rats in each group, p > 0.05).
Figure 7.
Hyperdopamine induced by GBR12909 decreases both surface and intracellular GABAA β2/3 subunit proteins in the PFC and the GSK-3β inhibitor blocks the effect of DA. A, Left panel, representative blots showing the protein levels of GABAA β2/3 and actin after systemic administration of vehicle or GBR12909 or coinjection of GBR12909 and the GSK-3 β inhibitor TDZD. Right panel, quantification of the total GABAA β2/3 protein levels relative to the controls (n = 6, **p < 0.05). B, Left panel, BS3 cross-linking analysis of surface and intracellular GABAA β2/3 proteins after systemic administration of vehicle or GBR12909 or coinjection of GBR12909 and the GSK-3β inhibitor TDZD. Right panel, quantification of surface and intracellular GABAA β2/3 indicated a significant reduction of surface protein level and a slightly higher but not significant level of intracellular protein.
The BS3-cross-linking technique enables us to separate surface and intracellular proteins. Therefore, the relative changes in the surface and intracellular GABAA receptors can be detected by Western blot assay. We found that GBR12909, which increases the endogenous DA level in the brain, induced a significant decrease in the surface GABAA protein level. As shown in Figure 7B, GBR12909 reduced the surface expression of GABAA protein by 44.4% ± 11.22% (n = 3 rats in each group; *p < 0.05) and there was a trend of increase in intracellular GABAA protein levels but not significant. The decrease in GABAA receptors on the dendritic surface may result from alterations in receptor trafficking. Thus, this data strongly indicate that GBR12909 significantly affected the trafficking of GABAA receptors. However, the intracellular GABAA protein levels were only slightly increased without significance, which led to the decrease of total protein. Therefore, protein synthesis, degradation, or other mechanisms that affect the trafficking of GABA A receptors cannot be excluded. However, the decreased expression of surface GABAA protein was rescued by the GSK-3β inhibitor TDZD (n = 3 rats, p > 0.05), suggesting that a GSK-3β-mediated trafficking mechanism is involved in the DA-induced decrease of inhibitory transmission.
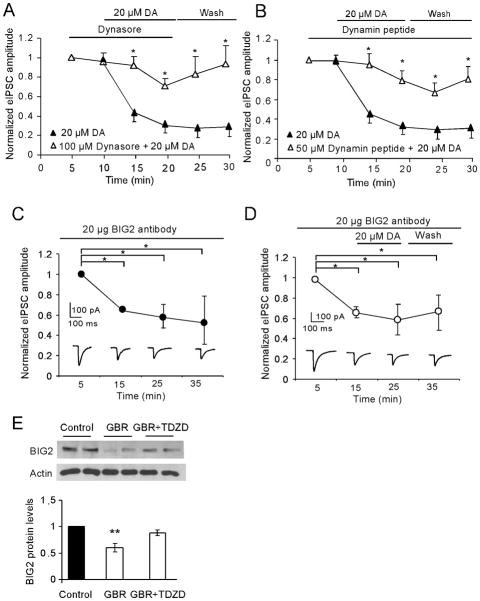
Both dynamin and BIG 2 protein are involved in the GSK-3β-mediated trafficking of GABAA receptors
Receptor trafficking involves insertion or internalization. The level of receptors on the surface is maintained by balancing these two opposing trafficking processes. Modulation of either insertion or internalization will change the receptor expression at the cell surface. Exocytosis and endocytosis of GABAA receptors occur mainly via clathrin-coated vesicles. Dynamin, a GTPase, is responsible for newly formed clathrin-coated vesicles moving from the membrane into the cell. On the contrary, BIG2 plays a role in moving clathrin-coated vesicles to the synaptic plasma membrane. Because dynamin and BIG2 have been implicated in the mediation of the trafficking of GABAA receptors (Charych et al. 2004), we examined whether GSK-3β-mediated trafficking of GABAA receptors is regulated by either or both of them. As shown in the in vitro electrophysiology study illustrated in Figure 8, application by bath of dynasore (100 μM), a cell membrane-permeable inhibitor of dynamin, significantly recovered the depressive effect of DA (20 μM) on IPSCs (n = 6, *p < 0.05; Fig. 8A) while pre-application of dynasore for 5 min exhibited no significant effect on single-pulse evoked IPSCs (−2.91 %±1.93 %, p > 0.05). The dynamin inhibitory peptide (QVPSRPNRAP; Biomatik Corporation; 50 μM), when applied intracellularly through a recording pipette, exhibited a similar effect (n = 6, *p < 0.05; Fig. 8B). Because there is no specific BIG2 protein inhibitor, we loaded anti-BIG2 antibody (Bioworld Technology) into a recording pipette to determine its effects on the evoked IPSCs. We initially applied 10 μg/ml and observed no significant effect (n = 4; p > 0.05). When we then increased the concentration to 20μg/ml, the amplitude of IPSCs decreased by 35.0% ± 3.21% after 15 min and the inhibitory effect was stable for at least another 20 min (# p < 0.05, n = 6; Fig. 8C). However, application of DA (20 μM) in a bath 15 min after loaded anti-BIG2 antibody did not cause further decrease in IPSC amplitude (decreased by 37.2% ± 14.12%; p >0.05, n = 6; Fig. 8C and D). This result confirmed that BIG-2 is involved in GABAA receptor trafficking. Consistently, as shown in an in vivo study (Fig. 8E), the expression of BIG2 protein levels was significantly decreased by systemically administered GBR12909 (10 mg/kg, i.p); this effect was blocked by pre-application of TDZD for 30 min (1 mg/kg, i.p). Together, these results indicated that DA-induced reduction of inhibitory transmission is effected through GSK-3β-mediated GABAA receptor trafficking, which involves the potentiation of dynamin-dependent internalization and attenuation of BIG2-dependent insertion mechanisms.
Figure 8.
Both dynamin and BIG 2 protein are involved in the GSK-3β-mediated trafficking of GABAA receptors induced by high-dose DA. A, Time course data showed that dynasore (100 μM), a cell membrane-permeable inhibitor of dynamin-mediated endocytosis, significantly blocked 20 μM DA-induced inhibition of GABAA receptor-mediated IPSCs (n = 6; *p < 0.05). B, Similarly, the inhibitory effects of DA on IPSCs were blocked by applying dynamin inhibitory peptide (50 μM) in a recording pipette (n = 6, *p < 0.05, **p < 0.01). Note: the 20-μM DA data presented in A and B were from same groups of cells for this experiment (n = 6). C and D, Bottom panel, representative traces showing the effects of BIG2 antibody (20 μg in pipette, C) and BIG2 antibody with DA on IPSCs (D). Top panel, the amplitude of the IPSCs was significantly decreased after loading BIG2 antibody for 15 min and this effect lasted for more than 20 min (n = 4,# p < 0.05, C). In contrast, application of DA for 10 min by bath after loaded the BIG2 antibody for 15 min did not cause a further decrease in IPSC amplitude (n = 6, p > 0.05, D). E, Top panel, representative images of BIG2 and β-actin from Western blot assay. Bottom panel, systemic administration of GBR12909 caused significant decrease of BIG2 protein level, but coinjection of GBR12909 and GSK-3β inhibitor TDZD prevented the decrease of BIG2 protein (n = 8, *p < 0.05, **p < 0.01).
Discussion
We found that GSK-3β is involved in high-dose DA-induced depression of IPSCs but had limited effects on the facilitating action of low-dose DA in the prefrontal neurons. Further study indicated that in an often applied dose (20 μM) of DA, the depressive effects on IPSCs were mediated predominantly by D2 receptors via the PP2A/IP3-GSK-3β signaling pathway. The results of both in vivo and in vitro studies suggested that DA triggers attenuation of BIG2-dependent insertion and dynamin-dependent endocytosis of GABAA receptor β2/3 subunits and that these processes required the activation of GSK-3β.
Numerous studies have reported the regulatory effects of DA on the inhibitory synaptic transmission in various brain regions, including the PFC (Penit-Soria et al. 1987, Law-Tho et al. 1994, Zhou & Hablitz 1999, Gonzalez-Islas & Hablitz 2001, Seamans et al. 2001, Gao et al. 2003, Kroner et al. 2007, Trantham-Davidson et al. 2004, Wang et al. 2002), hippocampus (Swant et al. 2008, Romo-Parra et al. 2005), striatum (Janssen et al. 2009, Delgado et al. 2000, Hjelmstad 2004), ventral tegmentum area (Michaeli & Yaka 2010, Pan et al. 2008), amygdala (Diaz et al. 2011, Naylor et al. 2010, Floresco & Tse 2007), basal forebrain (Momiyama & Sim 1996), septal nucleus (Asaumi et al. 2006), and subthalamic nucleus (Baufreton & Bevan 2008). In the PFC, DA induced a bidirectional regulation in GABAA receptor-mediated IPSCs, i.e., low DA (< 500 nM) increased the amplitude of IPSCs whereas high doses of DA (>10 μM) decreased the amplitude of IPSCs by activation of either D1 or D2 receptors, respectively (Seamans et al. 2001, Trantham-Davidson et al. 2004). Our result supports these findings: 200 nM DA increased the amplitude of IPSCs whereas 20 μM DA decreased the IPSCs.
We further investigated the specificity of DA receptors involved in the inhibitory effect of high-dose DA at 20 μM and found that the effect of DA was completely prevented by either 1 μM or 10 μM D2 antagonists and partially blocked by 10 μM D1 antagonist but not by 1 μM D1 antagonist. It was reported that D1-mediated reduction of IPSCs occurred postsynaptically in neostriatal neurons (Flores-Hernandez et al. 2000) and presynaptically in PFC neurons (Gonzalez-Islas & Hablitz 2001, Gao et al. 2003). In addition, DA also exhibited a biphasic modulation of IPSPs via the combined effects of D1 and D2 (Seamans et al. 2001, Trantham-Davidson et al. 2004) or a D4 receptor-mediated postsynaptic modulation (Wang et al. 2002). Similarly, synapse-specific DA regulation was also found in inhibitory circuitry (Kroner et al. 2007, Gao et al. 2003). In the present study, however, the paired-pulse ratios were not significantly altered, indicating that the effect of DA on the evoked IPSCs is mainly through postsynaptic mechanisms, although presynaptic effects cannot be completely excluded. Another possibility is that a high concentration (10 μM) of the D1 antagonist may exert nonselective effects beyond those of D1 receptors on other receptor subtypes, such as D2 and 5-HT receptors. Indeed, administration of selective D2 agonist quinpirole mimics high-dose DA’s depressive effect on inhibitory transmission.
We next focused on identifying the mechanisms involved in high-dose (20 μM) DA because hyperdopaminergia is usually associated with activation of D2 receptors (Li & Gao 2011, Li et al. 2009). The classic D2-linked signaling pathway was believed to be Gαi/o-mediated inhibition of adenylate cyclase, which causes a decrease in PKA-dependent phosphorylation of DARPP-32. In addition, many other signaling pathways such as the K+/Ca2+ channel, phospholipases, and MAP kinase are also reportedly regulated by Gβγ (Neve et al. 2004). Indeed, several previous studies reported that activation of D2 receptors inhibited IPSCs in the prefrontal neurons by regulating cAMP-PKA signaling pathway (Swant et al. 2008, Wang et al. 2002). Another possible mechanism was reported that D2 activation via platelet-derived growth factor receptor-associated PLC and IP3 decreases GABAA currents (Trantham-Davidson et al. 2004). Indeed, we found that co-application of IP3 inhibitor 2-APB with 20 μM DA also completely blocked the decrease in IPSC amplitude, consistent with previous report (Trantham-Davidson et al. 2004).
A novel finding in this study is the fact that DA triggers a GSK-3β-dependent modulation of GABAA receptor trafficking. Increasing attention is being focused on the role of GSK-3β in hyperdopaminergia and psychiatric disorders (Li & Gao 2011). Recent investigations have linked downstream signaling of D2 receptor to GSK-3 (Li & Gao 2011, Li et al. 2009). We found that DA regulation of IPSCs was blocked by inhibiting GSK-3β, suggesting that GSK-3β plays a critical role in high-dose DA-induced reduction of IPSCs. In addition, it was reported that DA transporter inhibitors such as cocaine and amphetamine, which increase DA level in the brain, alter D2-GSK-3β signaling to cause psychotic symptoms and cognitive dysfunction (Beaulieu et al. 2005, Beaulieu et al. 2007, Beaulieu et al. 2009, Lute et al. 2008). Similar to the effect of high-dose DA-induced depression in IPSCs in brain slices, we provided evidence that administration of DA transporter inhibitor GBR12909 in vivo significantly decreased the expression of GABAA receptor subunits and inhibitory synaptic transmission and that these effects were effectively blocked by prior administration of GSK-3β inhibitor, further validating the role of GSK-3β in the modulation of GABAA receptors.
A wealth of evidence indicates that GSK-3β plays an important role in AMPA receptor trafficking and synaptic plasticity (Peineau et al. 2007, Peineau et al. 2008, Zhu et al. 2007, Chen et al. 2007). GSK-3β appears to regulate the function and trafficking of NMDA receptors and AMPA receptors in cortical neurons (Peineau et al. 2009, Chen et al. 2007, Zhu et al. 2007, Wei et al. 2010). We recently reported that GSK-3β is involved in hyperdopamine-triggered internalization of NR2B subunits in the prefrontal neurons (Li et al. 2009). Likewise, the number of GABAA receptors in the postsynaptic membrane represents a key determinant in establishing synaptic transmission and plasticity. Insulin, brain-derived neurotrophic factor (BDNF), and Wnt signaling have been implicated as downstream effectors in the regulation of GABAA receptors. Interestingly, all of them regulate the trafficking of GABAA receptor via interaction with GSK-3β-related protein kinases. Insulin-induced insertion of GABAA receptors to the cell surface requires activation of AKT or PI3K, which in turn inhibits GSK-3β (Wang et al. 2003, Fujii et al.2010, Cross et al. 1995, Vetiska et al. 2007); BDNF facilitates endocytosis of GABAA receptors to reduce surface expression by PP2A-mediated dephosphorylation of GABAA receptors (Kittler et al. 2005, Kanematsu et al. 2006); in contrast, Wnt-5a, which is involved in the inhibition of GSK3β, increases clustering of GABAA receptors on membrane surfaces (Cuitino et al. 2010). These studies strongly suggest that GSK-3β is an important mediator in the regulation of GABAA receptor trafficking. In agreement, our data indicate that administration of GSK-3β inhibitors blocked a decrease of GABAA receptor expression on the membrane caused either by in vitro application of DA or by in vivo injection of DA transport inhibitor in the prefrontal neurons. However, it is unknown if similar mechanism is implicated in the striatum which receives much more DA innervations than the PFC. In addition, DA receptors are highly enriched in the striatum and increased activity of D2 receptors in this region has been associated with schizophrenia (Li et al. 2011). Thus, it is possible that GSK-3β will mediate dopaminergic regulation of GABAergic transmission in the medium striatal neurons but further study on this issue is needed.
GABAA receptor trafficking includes processes involving receptors inserted into and internalized from the cell surface. The rates of insertion and internalization decide the number of GABAA receptors on the membrane. AP2/clathrin/dynamin-mediated endocytosis is the major internalization mechanism for neuronal GABAA receptors (Kittler et al. 2000, Kittler & Moss 2003, Tehrani & Barnes 1993, Herring et al. 2003). Our data showed that blocking dynamin significantly but not completely inhibited DA-induced depression in IPSCs, indicating that another mechanism may coexist with dynamin-dependent endocytosis. The trafficking of GABAA receptors is a highly regulated process that is controlled by receptor-associated proteins. Among these proteins, BIG2 has recently been shown to play a role in the transport of newly assembled GABAA receptors by clathrin/AP-1-coated vesicles from the Golgi apparatus to endosomes and/or the plasma membrane by interacting with the β subunits of the GABAA receptor (Charych et al. 2004). We therefore investigated whether DA-induced downregulation of inhibitory transmission occurs via GSK-3β-mediated reduction of BIG2 and found that it was. This finding suggests that both reduction of BIG2-mediated insertion and enhancement of dynamin-dependent endocytosis contributed to the DA-induced decrease of GABAA expression on the cell surface.
In summary, DA-induced downregulation of GABAA receptor-mediated inhibitory transmission in the PFC is mediated by activation of PP2A/IP3-GSK-3β signaling pathway and this process is through the promotion of GABAA receptor internalization and reduction of insertion. Thus, the concentration of DA, by acting through separate signaling cascades, may determine the relative amount of cortical inhibition (Trantham-Davidson et al. 2004) and excitation (Li et al. 2009), thereby differentially regulating the tuning of cortical networks and cognitive functions.
Supplementary Material
Acknowledgments
We thank Ms. Pamela Fried at DUCOM Academic Publishing Services for editorial work. This study was supported by a NARSAD young investigator award to YCL from the Brain and Behavior Research Foundation and by NIH R01MH232395 to W-J Gao.
Footnotes
Conflict of interest
The authors claim no financial conflicts of interest.
References
- Asaumi Y, Hasuo H, Akasu T. Dopamine presynaptically depresses fast inhibitory synaptic transmission via D4 receptor-protein kinase A pathway in the rat dorsolateral septal nucleus. J Neurophysiol. 2006;96:591–601. doi: 10.1152/jn.00966.2005. [DOI] [PubMed] [Google Scholar]
- Baufreton J, Bevan MD. D2-like dopamine receptor-mediated modulation of activity-dependent plasticity at GABAergic synapses in the subthalamic nucleus. The Journal of Physiology. 2008;586:2121–2142. doi: 10.1113/jphysiol.2008.151118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, Caron MG. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci. 2007;28:166–172. doi: 10.1016/j.tips.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol. 2009;49:327–347. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, Caron MG. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci U S A. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersudsky Y, Shaldubina A, Kozlovsky N, Woodgett JR, Agam G, Belmaker RH. Glycogen synthase kinase-3beta heterozygote knockout mice as a model of findings in postmortem schizophrenia brain or as a model of behaviors mimicking lithium action: negative results. Behav Pharmacol. 2008;19:217–224. doi: 10.1097/FBP.0b013e3282feb099. [DOI] [PubMed] [Google Scholar]
- Charych EI, Yu W, Miralles CP, Serwanski DR, Li X, Rubio M, De Blas AL. The brefeldin A-inhibited GDP/GTP exchange factor 2, a protein involved in vesicular trafficking, interacts with the beta subunits of the GABA receptors. J Neurochem. 2004;90:173–189. doi: 10.1111/j.1471-4159.2004.02481.x. [DOI] [PubMed] [Google Scholar]
- Chen P, Gu Z, Liu W, Yan Z. Glycogen synthase kinase 3 regulates N-methyl-D-aspartate receptor channel trafficking and function in cortical neurons. Mol Pharmacol. 2007;72:40–51. doi: 10.1124/mol.107.034942. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Cuitino L, Godoy JA, Farias GG, Couve A, Bonansco C, Fuenzalida M, Inestrosa NC. Wnt-5a modulates recycling of functional GABAA receptors on hippocampal neurons. J Neurosci. 2010;30:8411–8420. doi: 10.1523/JNEUROSCI.5736-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- Delgado A, Sierra A, Querejeta E, Valdiosera RF, Aceves J. Inhibitory control of the GABAergic transmission in the rat neostriatum by D2 dopamine receptors. Neuroscience. 2000;95:1043–1048. doi: 10.1016/s0306-4522(99)00495-9. [DOI] [PubMed] [Google Scholar]
- Diaz MR, Chappell AM, Christian DT, Anderson NJ, McCool BA. Dopamine D3-like receptors modulate anxiety-like behavior and regulate GABAergic transmission in the rat lateral/basolateral amygdala. Neuropsychopharmacology. 2011;36:1090–1103. doi: 10.1038/npp.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Weinberger DR. Neurobiology of schizophrenia. Curr Opin Neurobiol. 1997;7:701–707. doi: 10.1016/s0959-4388(97)80092-x. [DOI] [PubMed] [Google Scholar]
- Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- Flores-Hernandez J, Hernandez S, Snyder GL, Yan Z, Fienberg AA, Moss SJ, Greengard P, Surmeier DJ. D(1) dopamine receptor activation reduces GABA(A) receptor currents in neostriatal neurons through a PKA/DARPP-32/PP1 signaling cascade. J Neurophysiol. 2000;83:2996–3004. doi: 10.1152/jn.2000.83.5.2996. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MT. Dopaminergic regulation of inhibitory and excitatory transmission in the basolateral amygdala-prefrontal cortical pathway. J Neurosci. 2007;27:2045–2057. doi: 10.1523/JNEUROSCI.5474-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyberg Z, Ferrando SJ, Javitch JA. Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am J Psychiatry. 2010;167:388–396. doi: 10.1176/appi.ajp.2009.08121873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii M, Kanematsu T, Ishibashi H, Fukami K, Takenawa T, Nakayama KI, Moss SJ, Nabekura J, Hirata M. Phospholipase C-related but catalytically inactive protein is required for insulin-induced cell surface expression of gamma-aminobutyric acid type A receptors. J Biol Chem. 2010;285:4837–4846. doi: 10.1074/jbc.M109.070045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao WJ, Wang Y, Goldman-Rakic PS. Dopamine modulation of perisomatic and peridendritic inhibition in prefrontal cortex. J Neurosci. 2003;23:1622–1630. doi: 10.1523/JNEUROSCI.23-05-01622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SR, O’Dowd BF. A novel dopamine receptor signaling unit in brain: heterooligomers of D1 and D2 dopamine receptors. ScientificWorldJournal. 2007;7:58–63. doi: 10.1100/tsw.2007.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl) 2004;174:3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Hablitz JJ. Dopamine inhibition of evoked IPSCs in rat prefrontal cortex. J Neurophysiol. 2001;86:2911–2918. doi: 10.1152/jn.2001.86.6.2911. [DOI] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- Herring D, Huang R, Singh M, Robinson LC, Dillon GH, Leidenheimer NJ. Constitutive GABAA receptor endocytosis is dynamin-mediated and dependent on a dileucine AP2 adaptin-binding motif within the beta 2 subunit of the receptor. J Biol Chem. 2003;278:24046–24052. doi: 10.1074/jbc.M301420200. [DOI] [PubMed] [Google Scholar]
- Hjelmstad GO. Dopamine excites nucleus accumbens neurons through the differential modulation of glutamate and GABA release. J Neurosci. 2004;24:8621–8628. doi: 10.1523/JNEUROSCI.3280-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen MJ, Ade KK, Fu Z, Vicini S. Dopamine modulation of GABA tonic conductance in striatal output neurons. J Neurosci. 2009;29:5116–5126. doi: 10.1523/JNEUROSCI.4737-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanematsu T, Yasunaga A, Mizoguchi Y, et al. Modulation of GABA(A) receptor phosphorylation and membrane trafficking by phospholipase C-related inactive protein/protein phosphatase 1 and 2A signaling complex underlying brain-derived neurotrophic factor-dependent regulation of GABAergic inhibition. J Biol Chem. 2006;281:22180–22189. doi: 10.1074/jbc.M603118200. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Chen G, Honing S, et al. Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proc Natl Acad Sci U S A. 2005;102:14871–14876. doi: 10.1073/pnas.0506653102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Delmas P, Jovanovic JN, Brown DA, Smart TG, Moss SJ. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci. 2000;20:7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Moss SJ. Modulation of GABAA receptor activity by phosphorylation and receptor trafficking: implications for the efficacy of synaptic inhibition. Curr Opin Neurobiol. 2003;13:341–347. doi: 10.1016/s0959-4388(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Koros E, Dorner-Ciossek C. The role of glycogen synthase kinase-3beta in schizophrenia. Drug News Perspect. 2007;20:437–445. doi: 10.1358/dnp.2007.20.7.1149632. [DOI] [PubMed] [Google Scholar]
- Kozlovsky N, Belmaker RH, Agam G. GSK-3 and the neurodevelopmental hypothesis of schizophrenia. Eur Neuropsychopharmacol. 2002;12:13–25. doi: 10.1016/s0924-977x(01)00131-6. [DOI] [PubMed] [Google Scholar]
- Kroener S, Lavin A. Altered dopamine modulation of inhibition in the prefrontal cortex of cocaine-sensitized rats. Neuropsychopharmacology. 2010;35:2292–2304. doi: 10.1038/npp.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroner S, Krimer LS, Lewis DA, Barrionuevo G. Dopamine increases inhibition in the monkey dorsolateral prefrontal cortex through cell type-specific modulation of interneurons. Cereb Cortex. 2007;17:1020–1032. doi: 10.1093/cercor/bhl012. [DOI] [PubMed] [Google Scholar]
- Law-Tho D, Hirsch JC, Crepel F. Dopamine modulation of synaptic transmission in rat prefrontal cortex: an in vitro electrophysiological study. Neurosci Res. 1994;21:151–160. doi: 10.1016/0168-0102(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Pathophysiologically based treatment interventions in schizophrenia. Nat Med. 2006;12:1016–1022. doi: 10.1038/nm1478. [DOI] [PubMed] [Google Scholar]
- Li YC, Gao WJ. GSK-3beta activity and hyperdopamine-dependent behaviors. Neurosci Biobehav Rev. 2011;35:645–654. doi: 10.1016/j.neubiorev.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Kellendonk C, Simpson EH, Kandel ER, Gao WJ. D2 receptor overexpression in the striatum leads to a deficit in inhibitory transmission and dopamine sensitivity in mouse prefrontal cortex. Proc Natl Acad Sci U S A. 2011;108:12107–12112. doi: 10.1073/pnas.1109718108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Xi D, Roman J, Huang YQ, Gao WJ. Activation of glycogen synthase kinase-3 beta is required for hyperdopamine and D2 receptor-mediated inhibition of synaptic NMDA receptor function in the rat prefrontal cortex. J Neurosci. 2009;29:15551–15563. doi: 10.1523/JNEUROSCI.3336-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovestone S, Killick R, Di Forti M, Murray R. Schizophrenia as a GSK-3 dysregulation disorder. Trends in Neurosciences. 2007;30:142–149. doi: 10.1016/j.tins.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Lute BJ, Khoshbouei H, Saunders C, Sen N, Lin RZ, Javitch JA, Galli A. PI3K signaling supports amphetamine-induced dopamine efflux. Biochem Biophys Res Commun. 2008;372:656–661. doi: 10.1016/j.bbrc.2008.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaeli A, Yaka R. Dopamine inhibits GABA(A) currents in ventral tegmental area dopamine neurons via activation of presynaptic G-protein coupled inwardly-rectifying potassium channels. Neuroscience. 2010;165:1159–1169. doi: 10.1016/j.neuroscience.2009.11.045. [DOI] [PubMed] [Google Scholar]
- Missale C, Fiorentini C, Busi C, Collo G, Spano PF. The NMDA/D1 receptor complex as a new target in drug development. Curr Top Med Chem. 2006;6:801–808. doi: 10.2174/156802606777057562. [DOI] [PubMed] [Google Scholar]
- Momiyama T, Sim JA. Modulation of inhibitory transmission by dopamine in rat basal forebrain nuclei: activation of presynaptic D1-like dopaminergic receptors. J Neurosci. 1996;16:7505–7512. doi: 10.1523/JNEUROSCI.16-23-07505.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor JC, Li Q, Kang-Park MH, Wilson WA, Kuhn C, Moore SD. Dopamine attenuates evoked inhibitory synaptic currents in central amygdala neurons. Eur J Neurosci. 2010;32:1836–1842. doi: 10.1111/j.1460-9568.2010.07457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Pan B, Hillard CJ, Liu QS. D2 dopamine receptor activation facilitates endocannabinoid-mediated long-term synaptic depression of GABAergic synaptic transmission in midbrain dopamine neurons via cAMP-protein kinase A signaling. J Neurosci. 2008;28:14018–14030. doi: 10.1523/JNEUROSCI.4035-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peineau S, Bradley C, Taghibiglou C, Doherty A, Bortolotto ZA, Wang YT, Collingridge GL. The role of GSK-3 in synaptic plasticity. Br J Pharmacol. 2008;153(Suppl 1):S428–437. doi: 10.1038/bjp.2008.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peineau S, Nicolas CS, Bortolotto ZA, Bhat RV, Ryves WJ, Harwood AJ, Dournaud P, Fitzjohn SM, Collingridge GL. A systematic investigation of the protein kinases involved in NMDA receptor-dependent LTD: evidence for a role of GSK-3 but not other serine/threonine kinases. Mol Brain. 2009;2:22. doi: 10.1186/1756-6606-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peineau S, Taghibiglou C, Bradley C, et al. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Penit-Soria J, Audinat E, Crepel F. Excitation of rat prefrontal cortical neurons by dopamine: an in vitro electrophysiological study. Brain Res. 1987;425:263–274. doi: 10.1016/0006-8993(87)90509-9. [DOI] [PubMed] [Google Scholar]
- Romo-Parra H, Aceves J, Gutierrez R. Tonic modulation of inhibition by dopamine D4 receptors in the rat hippocampus. Hippocampus. 2005;15:254–259. doi: 10.1002/hipo.20049. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001;21:3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65:585–596. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swant J, Stramiello M, Wagner JJ. Postsynaptic dopamine D3 receptor modulation of evoked IPSCs via GABA(A) receptor endocytosis in rat hippocampus. Hippocampus. 2008;18:492–502. doi: 10.1002/hipo.20408. [DOI] [PubMed] [Google Scholar]
- Tehrani MH, Barnes EM., Jr Identification of GABAA/benzodiazepine receptors on clathrin-coated vesicles from rat brain. J Neurochem. 1993;60:1755–1761. doi: 10.1111/j.1471-4159.1993.tb13400.x. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Neely LC, Lavin A, Seamans JK. Mechanisms underlying differential D1 versus D2 dopamine receptor regulation of inhibition in prefrontal cortex. J Neurosci. 2004;24:10652–10659. doi: 10.1523/JNEUROSCI.3179-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagarajan SK, Ghosh H, Yevenes GE, et al. Regulation of GABAergic synapse formation and plasticity by GSK3β-dependent phosphorylation of gephyrin. Proceedings of the National Academy of Sciences. 2011;108:379–384. doi: 10.1073/pnas.1011824108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetiska SM, Ahmadian G, Ju W, Liu L, Wymann MP, Wang YT. GABAA receptor-associated phosphoinositide 3-kinase is required for insulin-induced recruitment of postsynaptic GABAA receptors. Neuropharmacology. 2007;52:146–155. doi: 10.1016/j.neuropharm.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liu L, Pei L, Ju W, Ahmadian G, Lu J, Wang Y, Liu F, Wang YT. Control of synaptic strength, a novel function of Akt. Neuron. 2003;38:915–928. doi: 10.1016/s0896-6273(03)00356-8. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhong P, Yan Z. Dopamine D4 receptors modulate GABAergic signaling in pyramidal neurons of prefrontal cortex. J Neurosci. 2002;22:9185–9193. doi: 10.1523/JNEUROSCI.22-21-09185.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Liu W, Yan Z. Regulation of AMPA receptor trafficking and function by glycogen synthase kinase 3. J Biol Chem. 2010;285:26369–26376. doi: 10.1074/jbc.M110.121376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Hablitz JJ. Dopamine modulation of membrane and synaptic properties of interneurons in rat cerebral cortex. J Neurophysiol. 1999;81:967–976. doi: 10.1152/jn.1999.81.3.967. [DOI] [PubMed] [Google Scholar]
- Zhu LQ, Wang SH, Liu D, Yin YY, Tian Q, Wang XC, Wang Q, Chen JG, Wang JZ. Activation of glycogen synthase kinase-3 inhibits long-term potentiation with synapse-associated impairments. J Neurosci. 2007;27:12211–12220. doi: 10.1523/JNEUROSCI.3321-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.