Abstract
Objective
Amenorrhoea and low bone density are common in excessive exercisers, yet endocrine factors that differentiate adolescent amenorrhoeic exercisers (AE) from eumenorrhoeic exercisers (EE) are unclear. We have previously reported that high ghrelin and low leptin predict lower LH secretion in AE. Leptin and ghrelin impact cortisol secretion, and hypercortisolaemia can inhibit LH pulsatility. We hypothesized that higher cortisol secretion in young endurance weight-bearing AE compared with EE and non-exercisers predicts lower LH secretion, lower levels of a bone formation marker and higher levels of a bone resorption marker.
Design
Cross-sectional
Subjects
We studied 21 AE, 18 EE and 20 non-exercisers 14–21 years (BMI 10th–90th%iles).
Measurements
Subjects underwent frequent sampling (11 p.m. to 7 a.m.) to assess cortisol, ghrelin, leptin and LH secretory dynamics. Fasting levels of a bone formation (P1NP) and bone resorption (CTX) marker were measured.
Results
BMI did not differ among groups. Cortisol pulse amplitude, mass, half-life and area under the curve (AUC) were highest in AE (p=0.04, 0.007, 0.04 and 0.003) and were associated inversely with fat mass (r=−0.29, −0.28 and −0.35, p=0.03, 0.04 and 0.007). We observed inverse associations between cortisol and LH AUC (r= −0.36, p=0.008), which persisted after controlling for fat mass, leptin and ghrelin AUC. Cortisol correlated positively with CTX in EE and inversely with P1NP in non-exercisers.
Conclusions
Higher cortisol secretion in AE compared with EE and non-exercisers is associated with lower LH secretion. Effects of leptin and ghrelin on LH secretion may be mediated by increased cortisol.
Keywords: exercisers, amenorrhoea, adolescents, cortisol, energy
Introduction
Menstrual dysfunction occurs in up to 24% of adolescent athletes 1. Hypothalamic amenorrhoea is an extreme form of menstrual dysfunction that is particularly common in lean endurance athletes and exercisers, in whom it is often associated with low bone density 2. It is important to determine the factors that differentiate amenorrhoeic exercisers (AE) from eumenorrhoeic exercisers (EE) and non-exercisers, in order to develop potential therapeutic strategies for hypothalamic amenorrhoea. While factors associated with low energy availability such as low leptin and high ghrelin are believed to contribute to hypothalamic amenorrhoea in over-exercising adolescents and adults, based on our studies and those of others 3–6, there are conflicting data regarding the impact of alterations in cortisol secretory patterns on LH pulsatility and also bone metabolism in young exercisers. Additionally, exogenous ghrelin administration stimulates cortisol secretion in healthy adults 7, 8 and leptin administration to cell cultures reduces ACTH-induced cortisol secretion 9. It is thus possible that effects of ghrelin and leptin on LH puslatility are at least partially mediated through their effect on cortisol secretion.
In ovariectomized ewes, stress-like elevations in cortisol (regardless of exercise status) cause decreased GnRH pulse amplitude or frequency, and impaired gonadotrope responsiveness to GnRH 10, 11. This appears to be mediated by the type II glucocorticoid receptor within the pituitary independent of GnRH receptor expression 11. In another study in monkeys, a CRH receptor-1 antagonist was able to mitigate the deleterious effects of stress and cortisol on LH pulse frequency 12. In anorexia nervosa (a condition of markedly low weight and fat mass, as well as amenorrhoea), cortisol levels are higher than in normal-weight controls, and are inversely associated with fat mass 13. Relatively higher cortisol has also been reported in adult non-athletic women with functional hypothalamic amenorrhoea 14, and correlates inversely with the number of menses in the preceding year in adult AE 15. However, there are no studies examining cortisol pulsatility in relation to LH pulse parameters independent of ghrelin and leptin secretion in normal-weight exercisers 16, particularly in the adolescent/young adult age range.
Higher cortisol has deleterious effects on bone in anorexia nervosa 13 and other conditions of endogenous or exogenous glucocorticoid excess 17, 18. Adolescent AE have lower bone density than EE and non-athletic controls 19. It is not known whether relatively high cortisol in AE compared with EE and controls is a determinant of impaired bone metabolism in AE.
We assessed patterns of overnight cortisol pulsatility in adolescent female exercisers with amenorrhoea compared with EE and non-exercisers using deconvolution analysis 20. We hypothesized that adolescent AE have higher cortisol secretion than EE and non-exercisers, and that higher cortisol secretion in AE is associated with lower LH secretion, lower levels of a bone formation marker and higher levels of a bone resorption marker. We also hypothesized that associations of cortisol and LH would persist after controlling for ghrelin and leptin secretion.
Subjects and Methods
Subject Selection and Study Protocol
Subjects included 21 amenorrhoeic exercisers (AE), 18 eumenorrhoeic exercisers (EE), and 20 non-athletic controls 14–21 years old with BMI between the 10th–90th percentiles for age. Exercisers were mostly endurance athletes. Inclusion and exclusion criteria for exercisers and non-exercisers have been previously reported for this cohort 6. Subjects were screened to rule out conditions other than athletic activity that might affect cortisol secretion or cause hypogonadism. Subjects on medications containing oestrogen, progesterone, anabolic steroids and glucocorticoids within 3 months of the screen were excluded.
EE and non-exercisers were assessed in the early and mid follicular phase of their cycles (based on menstrual history). Frequent sampling was initiated at 11 p.m. and continued until 7 a.m. the following morning. Blood samples were drawn every 10 minutes for LH. Every other sample (at 20 minute intervals) was assessed previously for ghrelin and leptin 6, and subsequently for cortisol. We used deconvolution analysis as described by Veldhuis and Johnson 20, and specifically the AutoDecon program 21, to determine cortisol half-life, basal secretion (basal secretion rate x duration of sampling), number of secretory pulses over the sampling period, interval between secretory pulses, median pulse amplitude, median pulse mass, total pulsatile secretion (number of secretory pulses*pulse mass) and total area under the curve (AUC). LH, leptin and ghrelin (but not cortisol) data have been previously reported 6, and we report only area under the curve (AUC) for leptin and ghrelin in relation to cortisol secretion. Fasting samples were drawn for a marker of bone formation, N-terminal propeptide of type 1 procollagen (P1NP), and of bone resoprtion, C-telopeptide (CTX). Body composition was assessed using dual energy x-ray absorptiometry (DXA) (Hologic 4500A, Waltham, MA). Energy intake and expenditure were assessed using a 4-day food record and the 3-day Bouchard activity record22 respectively.
The study was approved by the Institutional Review Board of the Partners HealthCare system. Informed consent was obtained from subjects ≥18 years old and parents of subjects <18 years. Informed assent was obtained from subjects <18 years.
Biochemical Analysis
We used an Access chemiluminescent immunoassay (Beckman Coulter, Fullerton, CA) to assess cortisol (limit of detection 11.0 nmol/L, intra-assay CV is 4.4–6.7%, inter assay CV 6.4–7.9%). P1NP was assessed using a RIA (Orion Diagnositcs, Espoo, Finland; lower limit of detection 0.7 mcg/L, intra-assay CV 3.5–5.3%, inter-assay CV 3.6–5.4%), and CTX using an IRMA (Immunodiagnostics Systems, Fountain Hills, AZ; lower limit of detection 0.02 mcg/L, intra-assay CV 5.2–6.8%, inter-assay CV 5.6–7.4 %). Assay details for LH, ghrelin and leptin have been previously reported6.
Statistical Analysis
JMP Software (vesion 8; SAS Institute, Cary, NC) was used for statistical analysis. Results are reported as means ± SD. A P value < 0.05 was considered significant. In order to determine differences between AE, EE and NA, we used analysis of variance followed by the Tukey Kramer test (to control for multiple comparisons). LH AUC required logarithmic transformation to approximate a normal distribution. Transformations were not required for other variables. We used Pearson’s correlations to determine associations between covariates, and stepwise regression modeling to determine the contribution of cortisol secretory parameters to LH secretory parameters after controlling for potential confounders such as fat mass, leptin AUC and ghrelin AUC (p <0.10 to enter and leave the model).
Results
Clinical Characteristics
Clinical characteristics are described in Table 1 and have been previously reported (except for bone markers)6. Additionally, total caloric intake did not differ among groups. Daily energy expenditure was higher in AE and EE compared with non-exercisers (2805±678 and 2930±777 versus 2263±327 kcals/day, p=0.02).
Table 1.
Clinical Characteristics of Amenorrhoeic Exercisers, Eumenorrhoeic Exercisers and Non-Exercisers
| Amenorrhoeic Exercisers (AE) n = 21 |
Eumenorrhoeic Exercisers (EE) n = 18 |
Non-Exercisers n = 20 |
p | |
|---|---|---|---|---|
| Age (years) | 20.0±1.8 | 18.7±1.7 | 19.1±1.6 | NS |
| Bone age (years) | 17.7±0.7 | 17.5±0.9 | 17.6±0.9 | NS |
| Age of Menarche (years) | 13.9±2.3 | 13.1±1.4 | 12.2±1.6 | 0.02a |
| BMI (kg/m2) | 20.9±2.0 | 22.6±2.5 | 21.7±2.6 | NS |
| Fat Mass (kg) | 12.5±3.4 | 15.7±4.8 | 15.7±5.0 | 0.02a,b |
| Lean Mass (kg) | 44.7±60.6 | 48.0±8.2 | 41.3±3.8 | 0.008c |
| Percent Body Fat | 21.0±4.4 | 23.5±4.1 | 26.3±5.3 | 0.003a |
| Hours per week of sports activity (past 12 months) | 10.6±4.6 | 11.7±4.9 | 0.89±0.80 | <0.0001a,c |
| Leptin AUC (nmol. L−1.8h) | 279.6±188.4 | 473.3±232.5 | 502.9±229.4 | 0.005a,b |
| Ghrelin AUC (nmol. L−1.8h) | 112.5±56.2 | 87.1±36.5 | 72.8±20.8 | 0.01a |
| LH AUC (mIU.ml−1.8h)* | 1785.6±1782.1 | 2625.7±1658.6 | 4007.8±7495.2 | 0.02 a |
| P1NP (mcg/L) | 88.6±37.6 | 99.2±56.6 | 83.8±37.2 | 0.09 |
| CTX (ng/L) | 1078±329 | 1151±396 | 911±291 | NS |
p<0.05 AE versus non-exercisers,
p<0.05 AE versus EE,
p<0.05 EE versus non-exercisers
p value reported for log converted data
Cortisol Secretory Dynamics
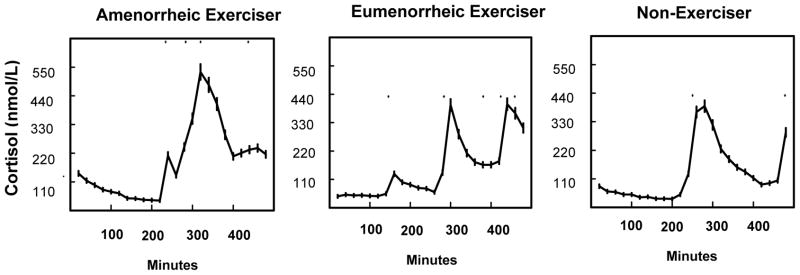
Cortisol secretory parameters are described in Table 2 and Figure 1. Cortisol AUC and pulse mass were higher in AE than EE and non-exercisers. Pulse amplitude was highest in AE, and significantly higher than in EE. Cortisol half-life was highest in AE and differed significantly from controls. Pulse frequency did not differ among groups. Of note, although AE had higher cortisol AUC than controls, only three subjects had values that exceeded the upper limit of the normal range (ULN) in controls, and none of these subjects exceeded 1.5*ULN.
Table 2.
Cortisol Secretory Parameters in Amenorrhoeic Exercisers, Eumenorrhoeic Exercisers and Non-Exercisers
| Amenorrhoeic Exercisers (AE) n = 21 |
Eumenorrhoeic Exercisers (EE) n = 18 |
Non-Exercisers n = 20 |
p | |
|---|---|---|---|---|
| Total Basal Secretion (nmol.L−1.8h) | 306.2±333.8 | 229.0±165.4 | 300.7±240.0 | NS |
| Half life (minutes) | 60.7±29.8 | 47.3±11.8 | 43.8±15.6 | 0.04 a |
| Secretory Pulse Height (nmol/L) | 18.2±15.7 | 9.1±6.3 | 11.6±7.7 | 0.04 b |
| Secretory Pulse Mass (nmol/L) | 325.6±245.6 | 162.8±85.5 | 190.4±104.8 | 0.007 a,b |
| Total Pulsatile Secretion (nmol.L−1.8h) | 1782.3±2709.3 | 822.2±458.0 | 954.6±557.3 | NS |
| Area Under Curve (AUC) (nmol.L−1.8h) | 99,048±36,584 | 61,388±30,404 | 72,369±31,701 | 0.003 a,b |
p<0.05 AE versus non-exercisers;
p<0.05 AE versus EE
Figure 1.
Cortisol concentrations over the sampling period in an amenorrhoeic exerciser, an eumenorrhoeic exerciser and a non- exerciser
Associations of Cortisol Secretory Parameters with Body Composition, Caloric Intake and Expenditure, and Other Hormones
For the group as a whole, cortisol pulse mass and AUC were associated inversely with BMI (r= −0.32 and −0.35, p=0.02 and 0.007) and cortisol pulse amplitude, mass and AUC with fat mass (r= −0.29, −0.28 and −0.35, p=0.03, 0.04 and 0.007). On subgroup analysis, cortisol AUC correlated inversely with BMI in AE (r=− 0.47, p=0.03). We found no associations of energy intake or expenditure with cortisol pulse parameters.
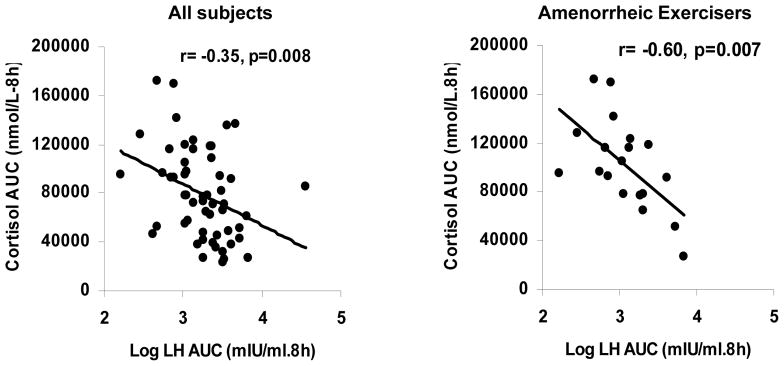
For the group as a whole, cortisol AUC correlated positively with ghrelin AUC (r=0.37, p=0.007), and inversely with leptin AUC (r= −0.30, p=0.03) and LH AUC (r= −0.36, p=0.008). Similarly, within AE there were inverse associations of cortisol AUC with LH AUC (r= −0.60, p=0.007) (Figure 2).
Figure 2.
Associations between cortisol area under the curve (AUC) with LH AUC for the group as a whole and within amenorrhoeic exercisers. The figure shows the inverse association of cortisol AUC with LH AUC for all subjects (left) and amenorrhoeic exercisers (right). Correlation coefficients and p values are indicated in the figure
We next performed stepwise regression to determine whether associations with LH AUC persisted after controlling for possible confounders. For the group as a whole and within AE, LH AUC was predicted by cortisol AUC even after controlling for fat mass (p=0.009 and 0.007 respectively). Additionally, cortisol AUC remained a significant predictor of LH AUC after controlling for ghrelin and leptin AUC for the group as a whole (r2=0.11, p=0.02) and within AE (r2=0.38, p=0.01). Leptin and ghrelin AUC were no longer significant predictors of LH AUC when cortisol AUC was included in the regression model.
Associations of Cortisol with Surrogate Markers of Bone Turnover
Within EE, there was a strong positive association between cortisol pulse mass and CTX (r= 0.66, p=0.003). In non-exercisers, there was a strong inverse association of cortisol pulse mass with P1NP (r= −0.59, p=0.01). We found no associations with bone turnover markers in AE.
Discussion
We demonstrate higher cortisol secretion in adolescent and young adult amenorrhoeic exercisers compared with eumenorrhoeic exercisers and non-exercisers, and also demonstrate inverse associations of cortisol AUC with LH AUC. The inverse associations between cortisol and LH persisted after controlling for fat mass. Similarly, inverse associations of cortisol with LH persisted after controlling for leptin and ghrelin AUC.
With the rising number of young girls participating in competitive sports, it is essential to gain a better understanding of factors that contribute to amenorrhoea in exercisers. Elevations in cortisol have the potential to impact both LH pulsatility and bone metabolism. Studies in animals indicate that stress like elevations in cortisol have a deleterious effect on LH secretion 10, 11, and a CRH receptor-1 antagonist is able to prevent these deleterious effects of stress and cortisol on LH pulsatility 12. Additionally, cortisol elevation has multiple deleterious effects on bone 17, 18.
We have previously reported higher cortisol in adolescent girls with anorexia nervosa, a condition of low fat mass associated with low weight and amenorrhoea, than in normal-weight controls 13. In that study, cortisol was associated inversely with fat mass. In this study of normal-weight, adolescent, amenorrhoeic exercisers, we again demonstrate elevations in cortisol and inverse associations with fat mass, despite the girls being normal-weight. Increased cortisol secretion in AE in the current study was consequent to greater pulse amplitude and mass, and a longer half-life. This is the first study assessing cortisol status using deconvolution analysis in adolescent and young adult exercisers.
Studies in adult exercisers have mostly assessed changes in cortisol in response to acute exercise 23–25 rather than chronic over activity, and report conflicting results. Only a few studies have examined chronic cortisol secretion in AE compared with EE and controls, or in relation to LH pulsatility 15, 16, 26, and these small studies report conflicting results. Two reported higher nighttime cortisol AUC (using Cluster analysis) in athletes (both AE and EE) compared with non-athletes 16, 26, and another reported higher baseline (but not pulsatile) secretion (using Pulsar) in adult AE compared with EE and controls 15. This lack of consistency may relate to differing methods used to assess cortisol pulsatility (Pulsar 15 or Cluster analysis 16, 26) and the small numbers of subjects in these studies. Deconvolution analysis differs from Pulsar 27 or Cluster 28 in that it takes into account hormone clearance unlike the other two methods. The refinement of devonvolution with AutoDecon also incorporates statistical methods to determine whether any estimated pulse is significant and ‘real’ 21. There are no studies that have examined cortisol pulsatility in exercisers using deconvolution analysis (and specifically the AutoDecon program).
Our study of frequent cortisol sampling over an 8-h period in a large group of adolescent and young adult exercisers clearly indicates higher nighttime cortisol pulsatile secretion and AUC using deconvolution analysis (AutoDecon) in AE compared with EE and non-exercisers (in contrast to two previous studies that reported higher cortisol AUC in both AE and EE compared with non-exercisers16, 26). Additionally, our data suggest that higher cortisol in AE is consequent to a longer half-life and greater pulse amplitude and mass (in contrast to a previous study that reported higher basal, but not pulsatile cortisol secretion in AE15). The increased half life in AE contributing to increased cortisol secretion is consistent with similar reports of a longer cortisol half-life in adult women with anorexia nervosa (a condition of severe undernutrition and low energy availability) using radio-isotope studies 29.
In addition, we observed inverse associations of cortisol AUC with LH AUC for the group as a whole, and this association was even stronger within AE. Our data are consistent with reports of stress-like elevations in cortisol suppressing LH pulse amplitude in animals 30. Two studies in adults reported inverse associations of LH AUC with baseline cortisol 15, or LH pulse frequency with cortisol AUC 26, using methods other than deconvolution. However, neither study assessed these associations after controlling for body fat. This is important because both LH and cortisol secretion are impacted by decreases in fat mass. In our study in adolescent and young adult exercisers, inverse associations of cortisol and LH AUC held after controlling for body fat. Although our study is cross-sectional and cannot prove causation, these strong associations between cortisol and LH suggest that stress-related elevations in cortisol may contribute to decreased LH pulsatile secretion. An animal study that used a CRH receptor-1 antagonist was able to prevent the deleterious effects of stress and cortisol on LH pulsatility 12. Similar studies are lacking in humans, but would be a definitive method of assessing whether higher cortisol in AE is a cause of lower LH pulsatility.
Disordered eating behavior is fairly common in AE and in association with excessive exercise is presumed to lead to a state of low energy availability. There were no associations of caloric intake or exercise energy expenditure with cortisol pulse parameters. While it is possible that food logs and exercise questionnaires are not completely accurate in determining energy intake or expenditure, there are no perfect ambulatory tools to assess these in a community setting. Accelerometers to assess energy expenditure are associated with significant variability when prediction equations are used 31, and measures of energy intake remain biased towards over-reporting in leaner populations and under-reporting in heavier populations 32.
In contrast, fat mass is a more objective measure of energy stores, and lower fat mass suggests increased utilization of fat stores subsequent to lower energy availability. Consistent with this, we observed that the AE group had the lowest fat mass amongst our subjects, and lower fat mass was associated with higher cortisol pulse parameters. It is possible that lower fat mass not only reflects lower energy availability, but also contributes to disruption of the H-P-G axis through altered secretion of hormones (including but not limited to cortisol) that impact the H-P-G axis. For example, the adipokine, leptin, facilitates GnRH secretion 33. Additionally, ghrelin, an orexigenic hormone that inhibits gonadotropin secretion, increases in conditions of low energy availability and correlates inversely with fat mass 6, 34. However, lower leptin and higher ghrelin in AE compared with EE and controls account only for a portion of the variability in gonadal steroid and LH secretion 5, 6. Importantly, ghrelin stimulates cortisol secretion in healthy adults 7, 8 and leptin reduces ACTH-induced cortisol secretion 9. In our study, associations of LH AUC with cortisol AUC persisted after controlling for ghrelin and leptin AUC, whereas associations of LH AUC with ghrelin and leptin AUC were lost. We thus speculate that effects of ghrelin and leptin on LH puslatility are at least partially mediated through their effect on cortisol secretion.
We observed positive associations of cortisol pulse mass with CTX (a bone resorption marker) in EE, and inverse associations of cortisol pulse mass with P1NP (a bone formation marker) in non-exercisers, consistent with known stimulatory effects of cortisol on osteoclasts and inhibitory effects on osteoblasts. The lack of associations of cortisol with bone turnover markers in AE may be secondary to greater variability in bone turnover markers in this subgroup.
There were certain limitations of our study. Group differences may have been more pronounced if we had sampled our subjects over a full 24 hours. However, even with nighttime sampling, we observed marked differences among groups for cortisol, leptin, ghrelin and LH, and significant inverse associations of cortisol with LH. Additionally, although we did collect information about nutritional status using the four-day food diary, such records are not always accurate. Percent fat mass, however, is a good surrogate for overall energy status. Finally, we defined our criteria for excessive activity based on hours of weight bearing endurance activity per week and miles per week of running. However, individuals may differ in their response to the same amount of exercise depending on energy intake, training status and individual variability. The size of our study cohort is a strength, as is the marked difference in cortisol parameters among groups.
In conclusion, our data demonstrate that adolescent and young adult amenorrhoeic exercisers have lower fat mass than eumenorrhoeic exercisers and non-exercisers associated with increased cortisol secretion. We speculate that this stress induced increase in cortisol contributes to lower LH secretion in AE, and this association is independent of ghrelin and leptin levels.
Acknowledgments
This study was supported by NIH grants 1 UL1 RR025758-01 and 1 R01 HD060827-01A1
We would like to thank the CRC dieticians for their help analyzing the food records and exercise questionnaires, and the CRC nurses for performing the frequent blood sampling required for this study. We would also like to thank our study subjects, without whom this study would not have been possible.
Footnotes
The authors have no conflicts of interest to declare
References
- 1.Nichols JF, Rauh MJ, Lawson MJ, Ji M, Barkai HS. Prevalence of the female athlete triad syndrome among high school athletes. Arch Pediatr Adolesc Med. 2006;160:137–142. doi: 10.1001/archpedi.160.2.137. [DOI] [PubMed] [Google Scholar]
- 2.Nichols JF, Rauh MJ, Barrack MT, Barkai HS, Pernick Y. Disordered eating and menstrual irregularity in high school athletes in lean-build and nonlean-build sports. Int J Sport Nutr Exerc Metab. 2007;17:364–377. doi: 10.1123/ijsnem.17.4.364. [DOI] [PubMed] [Google Scholar]
- 3.De Souza MJ, Leidy HJ, O’Donnell E, Lasley B, Williams NI. Fasting ghrelin levels in physically active women: relationship with menstrual disturbances and metabolic hormones. J Clin Endocrinol Metab. 2004;89:3536–3542. doi: 10.1210/jc.2003-032007. [DOI] [PubMed] [Google Scholar]
- 4.Kaufman BA, Warren MP, Dominguez JE, Wang J, Heymsfield SB, Pierson RN. Bone density and amenorrhoea in ballet dancers are related to a decreased resting metabolic rate and lower leptin levels. J Clin Endocrinol Metab. 2002;87:2777–2783. doi: 10.1210/jcem.87.6.8565. [DOI] [PubMed] [Google Scholar]
- 5.Christo K, Cord J, Mendes N, Miller K, Goldstein M, Klibanksi A, Misra M. Acylated Ghrelin and Leptin in Adolescent Athletes with Amenorrhoea, Eumenorrheic Athletes and Controls: A Cross-sectional Study. Clin Endocrinol. 2008;69:628–633. doi: 10.1111/j.1365-2265.2008.03237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackerman KE, Slusarz K, Guereca G, Pierce L, Slattery M, Mendes Estella N, Herzog DB, Misra M. Higher Ghrelin and Lower Leptin Secretion is Associated with Lower LH Secretion in Young Amenorrheic Athletes Compared with Eumenorrheic Athletes and Controls. Am J Physiol Endocrinol Metab. 2012 doi: 10.1152/ajpendo.00598.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takaya K, Ariyasu H, Kanamoto N, Iwakura H, Yoshimoto A, Harada M, Mori K, Komatsu Y, Usui T, Shimatsu A, Ogawa Y, Hosoda K, Akamizu T, Kojima M, Kangawa K, Nakao K. Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab. 2000;85:4908–4911. doi: 10.1210/jcem.85.12.7167. [DOI] [PubMed] [Google Scholar]
- 8.Tassone F, Broglio F, Destefanis S, Rovere S, Benso A, Gottero C, Prodam F, Rossetto R, Gauna C, van der Lely AJ, Ghigo E, Maccario M. Neuroendocrine and metabolic effects of acute ghrelin administration in human obesity. J Clin Endocrinol Metab. 2003;88:5478–5483. doi: 10.1210/jc.2003-030564. [DOI] [PubMed] [Google Scholar]
- 9.Glasow A, Haidan A, Hilbers U, Breidert M, Gillespie J, Scherbaum WA, Chrousos GP, Bornstein SR. Expression of Ob receptor in normal human adrenals: differential regulation of adrenocortical and adrenomedullary function by leptin. J Clin Endocrinol Metab. 1998;83:4459–4466. doi: 10.1210/jcem.83.12.5337. [DOI] [PubMed] [Google Scholar]
- 10.Oakley AE, Breen KM, Clarke IJ, Karsch FJ, Wagenmaker ER, Tilbrook AJ. Cortisol reduces gonadotropin-releasing hormone pulse frequency in follicular phase ewes: influence of ovarian steroids. Endocrinology. 2009;150:341–349. doi: 10.1210/en.2008-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breen KM, Davis TL, Doro LC, Nett TM, Oakley AE, Padmanabhan V, Rispoli LA, Wagenmaker ER, Karsch FJ. Insight into the neuroendocrine site and cellular mechanism by which cortisol suppresses pituitary responsiveness to gonadotropin-releasing hormone. Endocrinology. 2008;149:767–773. doi: 10.1210/en.2007-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herod SM, Pohl CR, Cameron JL. Treatment with a CRH-R1 antagonist prevents stress-induced suppression of the central neural drive to the reproductive axis in female macaques. Am J Physiol Endocrinol Metab. 2011;300:E19–27. doi: 10.1152/ajpendo.00224.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Misra M, Miller KK, Almazan C, Ramaswamy K, Lapcharoensap W, Worley M, Neubauer G, Herzog DB, Klibanski A. Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2004;89:4972–4980. doi: 10.1210/jc.2004-0723. [DOI] [PubMed] [Google Scholar]
- 14.Laughlin GA, Dominguez CE, Yen SS. Nutritional and endocrine-metabolic aberrations in women with functional hypothalamic amenorrhoea. J Clin Endocrinol Metab. 1998;83:25–32. doi: 10.1210/jcem.83.1.4502. [DOI] [PubMed] [Google Scholar]
- 15.Rickenlund A, Thoren M, Carlstrom K, von Schoultz B, Hirschberg AL. Diurnal profiles of testosterone and pituitary hormones suggest different mechanisms for menstrual disturbances in endurance athletes. J Clin Endocrinol Metab. 2004;89:702–707. doi: 10.1210/jc.2003-030306. [DOI] [PubMed] [Google Scholar]
- 16.Loucks A, Mortola J, Girton L, Yen S. Alterations in the hypothalamic-pituitary-ovarian and the hypothalamic-pituitary-adrenal axes in athletic women. J Clin Endocrinol Metab. 1989;68:402–411. doi: 10.1210/jcem-68-2-402. [DOI] [PubMed] [Google Scholar]
- 17.Abad V, Chrousos GP, Reynolds JC, Nieman LK, Hill SC, Weinstein RS, Leong GM. Glucocorticoid excess during adolescence leads to a major persistent deficit in bone mass and an increase in central body fat. J Bone Miner Res. 2001;16:1879–1885. doi: 10.1359/jbmr.2001.16.10.1879. [DOI] [PubMed] [Google Scholar]
- 18.Hermus AR, Smals AG, Swinkels LM, Huysmans DA, Pieters GF, Sweep CF, Corstens FH, Kloppenborg PW. Bone mineral density and bone turnover before and after surgical cure of Cushing’s syndrome. J Clin Endocrinol Metab. 1995;80:2859–2865. doi: 10.1210/jcem.80.10.7559865. [DOI] [PubMed] [Google Scholar]
- 19.Ackerman KE, Nazem T, Chapko D, Russell M, Mendes N, Taylor AP, Bouxsein ML, Misra M. Bone microarchitecture is impaired in adolescent amenorrheic athletes compared with eumenorrheic athletes and nonathletic controls. J Clin Endocrinol Metab. 2011;96:3123–3133. doi: 10.1210/jc.2011-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veldhuis J, Johnson M. Deconvolution analysis of hormone data. Methods Enzymol. 1992;210:539–575. doi: 10.1016/0076-6879(92)10028-c. [DOI] [PubMed] [Google Scholar]
- 21.Johnson ML, Pipes L, Veldhuis PP, Farhy LS, Boyd DG, Evans WS. AutoDecon, a deconvolution algorithm for identification and characterization of luteinizing hormone secretory bursts: description and validation using synthetic data. Anal Biochem. 2008;381:8–17. doi: 10.1016/j.ab.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart TL, Ainsworth BE, Tudor-Locke C. Objective and subjective measures of sedentary behavior and physical activity. Med Sci Sports Exerc. 2011;43:449–456. doi: 10.1249/MSS.0b013e3181ef5a93. [DOI] [PubMed] [Google Scholar]
- 23.De Souza MJ, Maguire MS, Maresh CM, Kraemer WJ, Rubin KR, Loucks AB. Adrenal activation and the prolactin response to exercise in eumenorrheic and amenorrheic runners. J Appl Physiol. 1991;70:2378–2387. doi: 10.1152/jappl.1991.70.6.2378. [DOI] [PubMed] [Google Scholar]
- 24.Kanaley JA, Boileau RA, Bahr JM, Misner JE, Nelson RA. Cortisol levels during prolonged exercise: the influence of menstrual phase and menstrual status. Int J Sports Med. 1992;13:332–336. doi: 10.1055/s-2007-1021276. [DOI] [PubMed] [Google Scholar]
- 25.Loucks AB, Horvath SM. Exercise-induced stress responses of amenorrheic and eumenorrheic runners. J Clin Endocrinol Metab. 1984;59:1109–1120. doi: 10.1210/jcem-59-6-1109. [DOI] [PubMed] [Google Scholar]
- 26.Laughlin G, Yen S. Nutritional and endocrine-metabolic aberrations in amenorrheic athletes. J Clin Endocrinol Metab. 1996;81:4301–4309. doi: 10.1210/jcem.81.12.8954031. [DOI] [PubMed] [Google Scholar]
- 27.Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion. Am J Physiol. 1982;243:E310–318. doi: 10.1152/ajpendo.1982.243.4.E310. [DOI] [PubMed] [Google Scholar]
- 28.Veldhuis JD, Johnson ML. Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol. 1986;250:E486–493. doi: 10.1152/ajpendo.1986.250.4.E486. [DOI] [PubMed] [Google Scholar]
- 29.Boyar RM, Hellman LD, Roffwarg H, Katz J, Zumoff B, O’Connor J, Bradlow HL, Fukushima DK. Cortisol secretion and metabolism in anorexia nervosa. N Engl J Med. 1977;296:190–193. doi: 10.1056/NEJM197701272960403. [DOI] [PubMed] [Google Scholar]
- 30.Breen KM, Karsch FJ. Does cortisol inhibit pulsatile luteinizing hormone secretion at the hypothalamic or pituitary level? Endocrinology. 2004;145:692–698. doi: 10.1210/en.2003-1114. [DOI] [PubMed] [Google Scholar]
- 31.Trost SG, Way R, Okely AD. Predictive validity of three ActiGraph energy expenditure equations for children. Med Sci Sports Exerc. 2006;38:380–387. doi: 10.1249/01.mss.0000183848.25845.e0. [DOI] [PubMed] [Google Scholar]
- 32.Pietilainen KH, Korkeila M, Bogl LH, Westerterp KR, Yki-Jarvinen H, Kaprio J, Rissanen A. Inaccuracies in food and physical activity diaries of obese subjects: complementary evidence from doubly labeled water and co-twin assessments. Int J Obes (Lond) 2010;34:437–445. doi: 10.1038/ijo.2009.251. [DOI] [PubMed] [Google Scholar]
- 33.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant Human Leptin in Women with Hypothalamic Amenorrhoea. N Engl J Med. 2004;351:987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 34.Kluge M, Schussler P, Uhr M, Yassouridis A, Steiger A. Ghrelin suppresses secretion of luteinizing hormone in humans. J Clin Endocrinol Metab. 2007;92:3202–3205. doi: 10.1210/jc.2007-0593. [DOI] [PubMed] [Google Scholar]