Abstract
Objective
To analyze the mechanism for the therapeutic effects of TNFα inhibition in a murine model SLE.
Methods
We used the IFN induced NZB/W model of SLE nephritis and treated mice with TNFR2-Ig after TNF expression was detected in the kidneys. Autoantibodies were measured by ELISA and autoantibody forming cells by ELISpot. Activation of splenocytes was analyzed by flow cytometry. Kidneys were harvested and analyzed using flow cytometry, immunohistochemistry, ELISA, Western blot and real-time PCR.
Results
TNFR2-Ig treatment stabilized nephritis and markedly prolonged survival. Autoantibody production and systemic immune activation were not inhibited but the renal response to glomerular immune complex deposition was attenuated. This was associated with decreases in renal production of chemokines, renal endothelial cell activation, interstitial F4/80hi macrophage accumulation, tubular damage and oxidative stress. In contrast, perivascular lymphoid aggregates containing B cells, T cells and dendritic cells accumulated unabated.
Conclusions
Our data suggest that TNFα is a critical cytokine that amplifies the response of the nephron to immune complex deposition but it has less influence on the response of the systemic vasculature to inflammation.
Keywords: SLE, Macrophages, Cytokines, Rodent, TNF alpha
TNFα (TNF) is an important regulator of physiologic and inflammatory immune responses. Although TNF antagonists have remarkable therapeutic benefits in several autoimmune diseases including rheumatoid arthritis, psoriasis and Crohn’s disease, they also have been associated with the development of anti-nuclear antibodies and even clinical SLE, multiple sclerosis and other demyelinating diseases. TNF is protective in the early stages of SLE in some mouse models but is overexpressed in inflamed target organs in both mice and humans with SLE (1–2). Consistent with both protective and pro-inflammatory roles of TNF, blockade of TNF improved proteinuria in patients with refractory SLE nephritis but increased anti-DNA and anti-cardiolipin autoantibody titers (3).
In this study, we determined the clinical effects of TNFR2-Ig in a mouse model of lupus nephritis in which an increase in serum levels of TNF and renal production of TNF occurs concomitantly with the onset of renal disease. TNF inhibition introduced after the development of autoantibodies and at the onset of clinical nephritis stabilized the nephritis and resulted in a long period of relapsing and remitting proteinuria that was associated with a marked improvement in survival. Mechanistic studies revealed that there was no effect of TNF inhibition on autoantibody production or lymphocyte activation in the spleen or renal autoantibody deposition, however there was a marked decrease in renal periglomerular and interstitial accumulation of F4/80hi renal macrophages. Real-time PCR analysis revealed a significant decrease in renal expression of chemokines CCL2, CCL5 and CCL9, the endothelial activation marker VCAM-1, genes involved in tissue remodeling and the markers of proximal tubule damage lipocalin-2 and HAVCR1 (KIM-1). We conclude that TNF inhibition decreases the renal inflammatory response to immune complex deposition. This is associated with decreased accumulation of periglomerular and interstitial renal macrophages that play a role in tissue remodeling, and decreased damage of renal tubular cells. In contrast, perivascular aggregates containing CD11chi dendritic cells as well as B and T cells accumulate in the kidneys in a TNF independent manner and are not sufficient to induce terminal renal damage.
MATERIALS AND METHODS
IFNα adenovirus treatment of NZB/W mice
12 week old NZB/W females (Jackson Laboratory Bar Harbor, ME) were treated with a single i.v. injection of 2.2 x 108 particles of IFNα adenovirus (Ad-IFN, Qbiogene Morgan Irvine, CA) that induced proteinuria at a median time of 36 days (range 24–161 days; 75% developed proteinuria before Day 56) and death at a median time of 85 days. Serum was obtained and urine was tested weekly for proteinuria by dipstick (Multistick; Fisher Scientific, Pittsburg, PA) as previously described (4). Treatment with fully murine TNFR2-Ig 50ug (2mg/kg) three times weekly i.p. (a kind gift from Dr David Shealy, Centocor) was started at Day 25 after the first mice developed proteinuria. One group of 6 TNFR2-Ig treated mice was sacrificed at Day 86 after IFN induction by which time approximately half the control mice had died. Three groups of TNFR2-Ig treated mice (3–5 per group) were allowed to age and were sacrificed at Day 220–240. 12 control mice were sacrificed at Day 50 or Day 86 and the remaining 16 mice were followed for survival. Previous studies from our group have shown that administration of irrelevant monoclonal IgG2a has no effect on proteinuria or survival in NZB/W mice ((5) and unpublished studies) and that several other fusion proteins given just before the onset of proteinuria are ineffective in the IFN-induced model (6); therefore we used mainly untreated controls in this study. A group of 5 IgG2a treated controls was included in one of the experiments. These mice developed fixed proteinuria at the same time as untreated controls and were sacrificed at Day 50; data from this group is included in some of the figures. In a separate experiment, groups of 5 control mice were sacrificed weekly after Ad-IFN injection and perfused kidneys were harvested for real-time PCR analysis. All experiments using animals were carried out according to protocols approved by the Institutional Animal Care and Use Committee of the Feinstein Institute.
Serum levels of TNF
These were measured using a commercial ELISA (Assaygate, Inc. Ijamsville, MD) (4).
Anti-DNA Abs and ELISpot assay
These were performed as previously described (4)
Flow cytometric analysis of spleen and kidney cells
Spleen and PBMCs were analyzed for cell surface markers as previously described (7) using Abs to CD4, CD8 (both from Caltag Laboratories, Burlingame, CA), and CD19. Activated CD4 T cells were identified by double staining with FITC-anti-CD4 and PE-anti-CD69. Naive and activated/memory CD4 cells were identified by triple staining with FITC-anti-CD4, CyChrome-anti-CD44, and PE-anti-CD62L. Naïve B cell subsets were identified based on staining for CD19, IgM, IgD, CD21 and CD23 (7). Germinal center B cells were positive for Fas and PNA and plasma cells were positive for CD138. Single cell suspensions were prepared from perfused kidneys and stained with antibodies to CD11b, CD11c and F4/80 as previously described (8). Antibodies were obtained from BD Pharmingen (San Diego, CA) unless otherwise stated.
Immunohistochemistry and immunofluorescence
H&E sections of kidneys were scored for renal damage as previously described (7–9). Cryosections (6 μm) of kidney were stained as previously described (7) using: FITC-conjugated anti-mouse IgG2a (Southern Biotech) and PE-conjugated anti-mouse CD11b, CD11c, CD4, CD8, B220 (BD Pharmingen) or F4/80 (Invitrogen, Carlsbad, California). Images were captured using a Zeiss Axiocam digital camera connected to a Zeiss Axioplan2 microscope.
Real-time PCR of renal tissue
RNA was purified from lysates of perfused kidneys and real-time PCR was performed in triplicates as previously described (8). The average of the raw data for each sample (Ct value) was normalized to the internal control (housekeeping gene β-actin) as previously described (8). Normalized expression data was log2-transformed and scaled to the expression value for a single naïve mouse given an arbitrary value of 1 (0 by log scale, ΔCt for calculation 4.76). For display on the graph the mean value for the naïve controls was given an arbitrary value of 1.
Quantitation of renal lipocalin-2, CCL9 and VCAM-1
Kidney lysates were prepared by homogenization in a Dounce homogenizer using lysis buffer (Ray Biotech, Norcross, GA) with complete protease inhibitors (Roche, Mannheim, Germany). The homogenate was centrifuged at 14000 rpm and 4°C for 20 minutes and he pellet was discarded. The protein in the supernatant was quantified using Bradford dye binding method and proteins were run on a discontinuous Tris-Glycine-SDS gel system (5% stacking, 12 % resolving), followed by transfer to a nitrocellulose membrane. The membrane was then probed with anti-lipocalin-2 (ProSci, Poway, CA) followed by donkey anti-goat IR800 (LI-COR, Lincoln, Nebraska). The lysates were also used for mouse CCL9 and VCAM-1 using cytokine array (Raybiotech, Norcross, GA) and normalized lysates were used for highly sensitive quantitative VCAM-1 ELISA (R & D Systems, Minneapolis, MN), according to manufacturer’s instructions.
Renal oxidation
Renal oxidation was studied by immuno-labeling using an oxidation kit (Millipore Corporpation, Billerica, MA) and IR800 labeled secondary antibody (LI-COR, Lincoln, Nebraska). Briefly, 8μm frozen sections were fixed in ice cold acetone for 15 minutes followed by washing with PBS. The proteins were then derivatized using DNPH at room temperature for 25 minutes followed by neutralization. After washing with PBS the sections were blocked with 3% BSA followed by serial incubations with anti-DNPH antibody for 1 hr and goat anti-rabbit IR800 antibody for 1 hr. The slides were then washed and scanned in a LICOR Odyssey instrument. As a negative control, a second section was processed in parallel for each sample using the derivatization control solution.
Statistics
Survival data in Figure 1 was analyzed using Kaplan-Meier curves and log-rank test. Comparisons in the other figures were performed using Mann-Whitney test. p values ≤0.05 were considered significant.
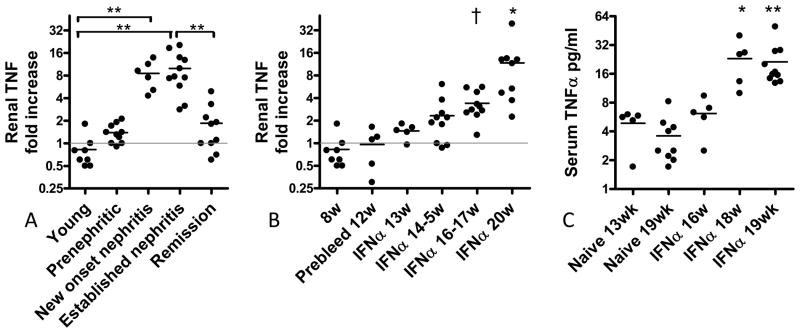
Figure 1.
A: TNF is expressed in the kidneys of nephritic NZB/W mice. A: Unmanipulated NZB/W mice: Young mice (<16w), 23w old prenephritic mice, 23w old mice with new onset nephritis and 36–40w old mice with nephritis for > 2 weeks were used as previously described (8) (** p<0.001). B: TNF is expressed in kidneys of IFN induced NZB/W mice concomitant with nephritis onset († p<0.01; ** p<0.001 vs. naïve controls). C: This is followed by an increase in serum levels of TNF (* p<0.05; ** p<0.001 vs. naïve controls).
RESULTS
Clinical results
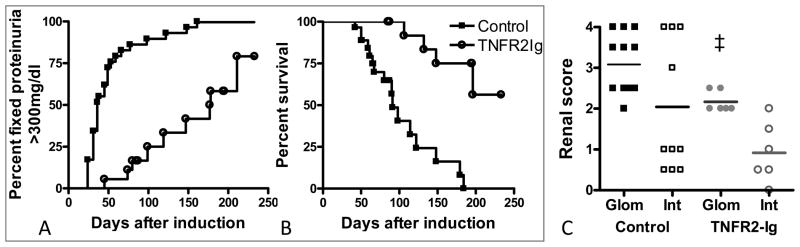
We have previously shown in unmanipulated NZB/W mice that renal expression of TNF increases at the onset of proteinuria and decreases with remission induction (Figure 1A) (8). We similarly showed that after induction with IFNα, renal expression of TNF increased after 6 weeks (Figure 1B); this was associated with an increase in serum levels of TNF (Figure 1C). When we administered TNFR2-Ig starting 3–4 weeks after IFN induction, proteinuria either remained stable or followed a remitting and relapsing course and survival was markedly prolonged (Figures 2A, B). This was associated with a decrease in the renal glomerular damage score (Figure 2C).
Figure 2.
TNFR2-Ig prevents renal damage and prolongs life. A: TNFR2-Ig delays the establishment of fixed proteinuria of > 300mg/dl (p < 0.0001) and B: death (p < 0.0001). C: TNFR2-Ig treatment results in a decrease in glomerular damage, calculated as previously described (9) (‡ p<0.02 vs. IFN controls).
Production of autoantibodies
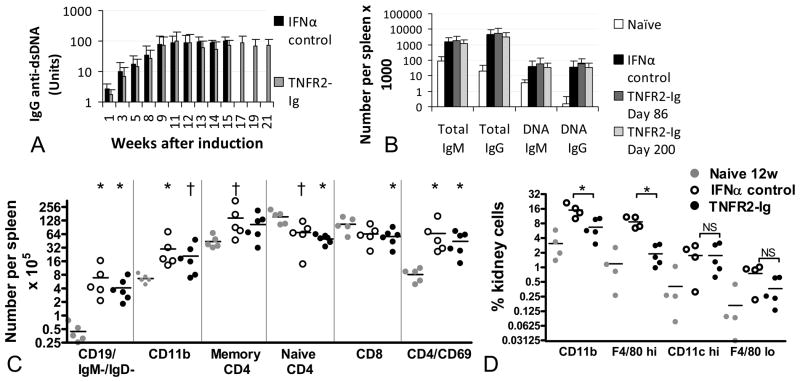
We have previously reported that IFN induces serum autoantibodies to dsDNA within several weeks and this is associated with a marked increase in antibody producing B cells in the spleen compared with naïve controls (4). Serum levels of IgG autoantibodies to dsDNA, measured weekly throughout treatment, were the same in IFN induced controls and TNFR2-Ig treated mice (Figure 3A). Similarly, at sacrifice on Day 86 or Day 200 after IFN induction there was no difference between TNFR2-Ig treated mice and IFN controls in the number of IgG anti-dsDNA antibody producing B cells per spleen as measured by ELISpot. (Figure 3B).
Figure 3.
TNFR2-Ig does not change B or T cell activation but prevents renal accumulation of mononuclear phagocytes. A: TNFR2-Ig does not affect serum titers of anti-dsDNA antibodies (Mean + 1SD shown n = 10 per group). Most control mice had either been sacrificed or died by Day 86 (>15w). B: There is no difference in total or anti-dsDNA antibody producing B cells/spleen spleen between TNFR2-Ig treated mice and controls at Day 86. Both groups have more spots than 20w old naïve controls (Mean + 1SD shown, n = 5–10 per group - * p<0.05; † p<0.01 vs. naïve controls). C: TNFR2-Ig does not alter the number of class switched (IgM-/IgD-) B cells, activated CD4 T cells or CD11b/CD11c dendritic cells induced by IFN compared with IFN treated controls. Only significant p values are shown (n = 5–7 per group;. * p<0.05; † p<0.01 vs. naïve controls). There was no difference between 12w naïve controls and 20w naïve or Ad-LacZ treated controls (not shown). D: TNFR2-Ig treated mice have fewer renal F4/80hi cells at Day >220 day than Day 86 IFN treated controls (* p<0.01) but have the same percentage of renal CD11chi dendritic cells (NS = not significant).
Flow cytometric evaluation of spleens and kidneys
Flow cytometric analysis revealed no differences between IFN induced controls and TNFR2-Ig treated mice (Figure 3C) or between IgG2a treated and TNFR2-Ig treated mice (data not shown) with respect to accumulation of class switched (IgM-/IgD-) B cells, early activation of CD4 T cells (as assessed by CD69 positivity), generation of memory (CD44+/CD62L-) CD4 T cells or increase in splenic CD11b+ dendritic cells, all features of IFN induced disease (4).
The onset of proteinuria in the NZB/W model is associated with the expansion and activation of a dominant population of resident renal mononuclear phagocytes that have a resting phenotype of CD11b+/CD11cint/F4/80hi. In addition, nephritis onset is associated with the influx of a population of CD11b+/CD11chi myeloid dendritic cells that accumulate in perivascular lymphoid aggregates (10) that also contain T and B cells. Using flow cytometry we found a marked increase in the F4/80hi population in IFN-induced controls compared with naïve NZB/W mice; this increase was prevented by TNFR2-Ig. In contrast there was no difference in the percentage of renal CD11chi cells between control and TNFR2-Ig treated mice (Figure 3D).
Localization of renal infiltrating mononuclear phagocytes
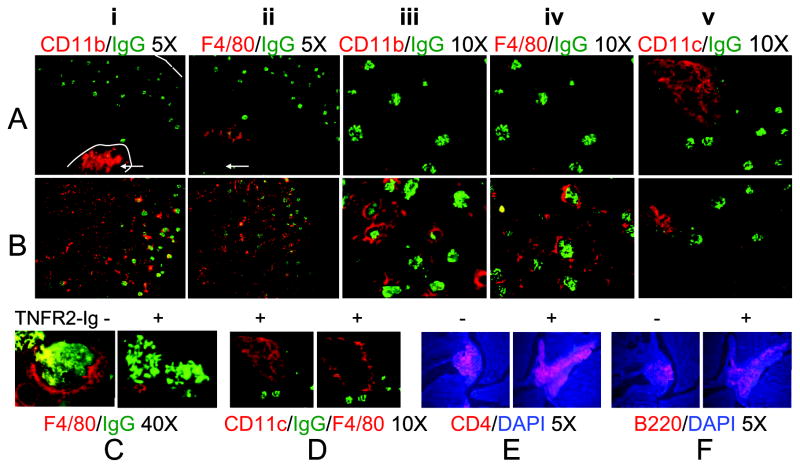
To confirm and extend these findings, we performed immune fluorescence analysis of kidneys from control and TNFR2-Ig treated mice sacrificed at Day 86 or at Day >220. Stains from TNFR2-Ig treated mice (Figure 4A) are compared with those from control IFN treated mice (Figure 4B). In control mice CD11bhi cells (panels i and iii) were located in the periglomerular regions and were abundant in the interstitium as well as within perivascular and large perihilar lymphoid aggregates (arrows); in contrast, these cells were found only in perihilar aggregates in TNFR2-Ig treated mice. In accord with these results F4/80hi cells were virtually absent from the interstitium (panels ii and iv) and periglomerular regions (Figure 4C) of TNFR2-Ig treated mice compared with controls and were restricted to a ring around the perihilar aggregates (Figure 4D), a location in which they are also found in unmanipulated nephritic NZB/W mice (11). In contrast, CD11chi cells (panel v and Figure 4D) were located in the perivascular aggregates of both controls and TNFR2-Ig treated mice. These aggregates also contained abundant CD4 T cells (Figure 4E) and B cells (Figure 4F). Very few neutrophils were present in the inflamed kidneys of control mice and there was no difference between control and TNFR2-Ig treated mice (data not shown).
Figure 4.
Immunofluorescence staining of Day 86 kidneys from TNFR2-Ig treated mice (A) and IFN induced controls (B) stained with antibodies as indicated (red) and either anti-IgG (green) or DAPI (blue). Note equivalent glomerular IgG deposition in control and TNFR2-Ig treated mice. Periglomerular and interstitial CD11b (panels i and iii) and F4/80hi cells (panels ii and iv) are decreased in TNFR2-Ig treated mice compared with controls (also see 40X magnification in 4C) but CD11b+/CD11c+ cells still accumulate in the hilar region and F4/80hi cells still form cuffs around lymphoid aggregates (4D - adjacent sections stained with anti-CD11c and anti-F4/80). There is no difference in the perihilar accumulation of CD4 T cells (E) or B cells (F). Representative of at least 5 mice per group. Similar features were present in TNFR2-Ig treated mice harvested at Day >220. Mice treated with control IgG2a appeared similar to controls (not shown).
Decreased inflammation in renal tissue of TNFR2-Ig treated mice
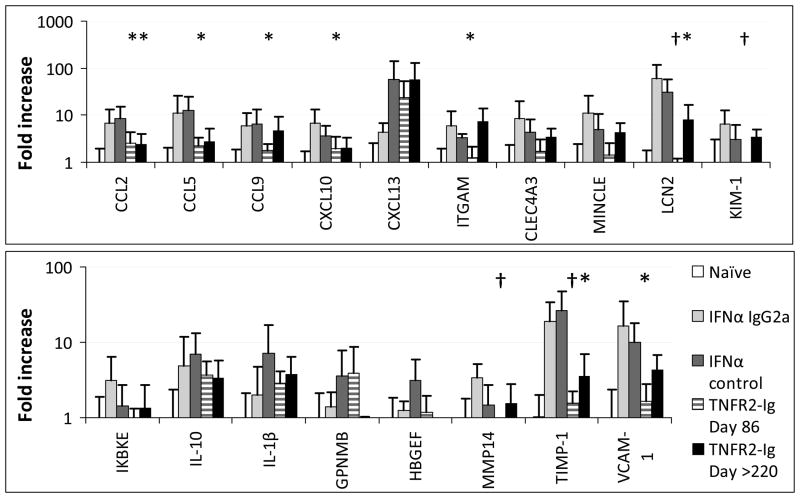
Onset of clinical nephritis in NZB/W mice is associated with the renal expression of a set of inflammatory markers. Some of these are derived from F4/80hi cells, some reflect endothelial activation and some reflect tubular damage and tissue remodeling (10). To determine which of these processes is altered by TNFR2-Ig, we performed real-time PCR for a subset of informative markers (8, 10) (Figure 5). Expression of CXCL13 a chemokine that is expressed early in the disease process before the onset of nephritis and is made by macrophages and dendritic cells predominantly within perivascular infiltrates (8) was not altered by TNFR2-Ig. In contrast the chemokines CCL2, CCL5, CCL9, and CXCL10 were not upregulated in TNFR2-Ig treated mice and CCL2 expression was still decreased even at late stages. Macrophage/DC surface markers ITGAM, Clec4a3 and Clec4e (Mincle) were decreased in TNFR2-Ig treated mice relative to controls at Day 86 but not at Day >220. The endothelial marker VCAM-1 was decreased in TNFR2-Ig treated mice compared with controls even at late stages as were the markers of tissue remodeling, TIMP-1 and MMP14. Markers for tubular damage, lipocalin-2 and KIM-1 (HAVCR1) were decreased compared with controls at Day 86 and lipocalin-2 expression was still inhibited at Day >220. Finally, renal expression of IL-1β was not affected by TNF blockade.
Figure 5.
Real time PCR quantification of expression of informative markers in perfused kidneys from TNFR2-Ig treated mice at Days 86 and >220 and IFN treated controls with or without control IgG2a (harvested at Day 50 or 86) normalized to the mean of naïve controls given an arbitrary value of 1. (5–12 mice per group; * p<0.05; † p<0.01 compared with IFN controls).
To confirm this data at the protein level we performed analysis of renal lysates using semi-quantitative ELISA, quantitative ELISA and Western blotting for inflammatory markers that we have previously shown are significantly upregulated in the late stages of renal disease (8). By cytokine array, lysates from TNFR2-Ig treated mice had lower levels of expression of CCL9 and VCAM-1 (0.79 +/− 0.22 vs. 1.43 +/− 0.65; p <0.05, and 0.35 +/− 0.17 vs. 1.06 +/− 0.37; p <0.01, respectively) and the results for VCAM-1 were confirmed using a high sensitivity ELISA (Figure 6A). Treated mice also had lower levels of lipocalin-2 expression even after 220 days (Figure 6B). To confirm the overall decrease in renal damage in TNFR2-Ig treated mice we performed quantitation of oxidized proteins in the renal lysates. Kidneys from TNFR2-Ig treated mice displayed a decrease in oxidation status as measured by densitometry/area (Figure 6C–F).
Figure 6.
Analysis of renal inflammation. Renal lysates were tested for expression of VCAM-1 (A) and lipocalin-2 (B) by ELISA and Western blot respectively. Day 86 TNFR2-Ig treated mice are shown in black and Day > 220 TNFR2-Ig treated mice are shown in grey. Frozen renal sections were tested for oxidation status. Decreased oxidative reactions were found in kidneys, predominantly in the renal cortex, of TNFR2-Ig treated mice (representative section in C) compared with controls (representative section in D). Panel E shows detection of oxidative reactions with but not without DNPH. Quantitation of renal oxidation by densitometry in shown in F. Day 86 TNFR2-Ig treated mice are shown in black and Day > 220 TNFR2-Ig treated mice are shown in grey (* p < 0.05, † p < 0.01 compared with IFN controls).
DISCUSSION
TNF is a pleiotropic cytokine that has both protective and pathogenic functions in SLE. NZB/W SLE prone mice have a defect in TNFα production and in young mice further depletion of TNFα exacerbates disease whereas TNF replacement is protective (1, 12). The relevance of this mouse model to human biology has been borne out by the finding that up to 50% of patients treated with TNFα inhibitors develop ANAs, 15% develop antibodies to DNA, and 1/500 develops clinical SLE, including SLE nephritis (13–14). The mechanism for the pathogenic role of TNFα blockers in induction of autoimmunity has not been elucidated but it is clearly a class effect of all TNF blockers. Prevailing hypotheses include increased production of Type I interferon (15), blockade of anti-inflammatory effects of TNF, (16), failure to adequately regulate activated T cells including TH17 T cells (17–18) and failure to induce CTL that can kill autoreactive B cells (19).
In NZB/W mice >28 weeks old TNFα replacement is no longer effective and low dose TNFα has even been shown to exacerbate disease in older NZB/W mice that now have high serum levels of TNF (1, 12). TNF levels are similarly elevated in the serum of active SLE patients (20) and TNF is expressed in the kidneys of both mice (6) and humans with SLE nephritis (3). The renal source of this cytokine is not entirely clear, with infiltrating mononuclear phagocytes, T cells and glomerular mesangial cells all having been suggested to produce TNF. TNF receptors, particularly TNFR1, are markedly upregulated in the glomeruli of patients with proliferative SLE nephritis (21) and soluble TNFR1 is shed in the urine (22). Regardless of the precise source of TNF in human lupus glomerulonephritis, a short course of infliximab has had remarkable and long-lasting therapeutic benefit in open-label studies in a small number of SLE patients with refractory nephritis. Nevertheless general enthusiasm for this approach has been tempered by the observation of transient increases in autoantibodies, severe infusion reactions following multiple doses of infliximab and concerns about concomitant treatment with other immunosuppressive agents. An increased rate of bacterial infections and one case of lymphoma were observed in patients under continuous anti-TNF therapy (3).
We show here that treatment with a mouse version of Enbrel in a lupus model in which renal TNF expression is concomitant with nephritis onset, protects the kidneys from damage and prolongs survival. Systemic lymphocyte activation, induction of class switched anti-dsDNA autoantibodies and renal immunoglobulin deposition, all of which occur in the first 4 weeks after IFN induction in this model were not altered by TNF inhibition but the renal inflammatory response to immune complex deposition was dramatically reduced.
The onset of proteinuria in lupus prone mice is associated with the expansion and activation of a dominant population of resident renal mononuclear phagocytes that have variably been referred to as intrinsic renal macrophages or resident renal DCs due to their mixed function as both antigen presenting cells and phagocytic cells (23). At nephritis onset these cells markedly upregulate their expression of CD11b and they accumulate both in the interstitium and in the periglomerular space (10, 24). Proteinuria onset and disease progression in NZB/W mice are also characterized by the renal influx of CD11chi myeloid dendritic cells that accumulate exclusively in large lymphoid aggregates that also contain B cells and T cells and are located in the hilum and perivascular regions (8) In this study we demonstrate that the recruitment of renal F4/80hi macrophages to the periglomerular region and the renal interstitium is TNF dependent whereas the accumulation of myeloid dendritic cells, and B and T cells in perivascular or perihilar lymphoid aggregates and the accumulation of F4/80hi cells in a cuff around these aggregates occurs independently of TNF.
Recruitment of inflammatory cells to the lupus kidney in response to immune complex deposition depends on several factors. In NZB/W mice engagement of Fc receptors on circulating myeloid cells by glomerular immune complexes is required for renal damage to occur (25). Fc receptor crosslinking on monocytes induces TNF production (26), providing a direct link between renal immune complex deposition and TNF production by renal mononuclear phagocytes. Complement activation and consequent activation of inflammatory cascades also contribute to renal damage. Endothelial activation by immune complexes and cytokines results in expression of integrins that help to capture circulating cells. The onset of proteinuria in NZB/W mice is associated with the upregulation of endothelial derived adhesion molecules E-selectin, P-selectin and VCAM-1 (8). Elaboration of chemokines that attract cells expressing the corresponding receptors facilitates transmigration of inflammatory cells into the renal parenchyma and precedes or coincides with clinical nephritis onset. In NZB/W mice we have observed renal expression of chemokines that can attract macrophages and dendritic cells (8). Similarly in MRL/lpr mice expression of a similar set of chemokines in glomeruli is an early feature of SLE nephritis that precedes inflammatory cell infiltration (27). Of these, CCL2 has definitively been shown to be pathogenic (28) and, as shown here, is one of several chemokines whose upregulation is dependent on TNF.
We further show that renal IL-1β production is not decreased by TNF blockade. IL-1β is produced by both intrinsic renal cells and macrophages/DCs and has a pro-inflammatory function in acute immune complex mediated renal inflammatory disease, being involved in crescent formation and inflammatory cell recruitment. In the acute nephrotoxic nephritis model leukocyte derived IL-1β expression is required for the induction of glomerular TNF expression and for the subsequent recruitment of macrophages to the kidneys (29). Our results are consistent with these findings. Similarly CXCL13 that is highly expressed by CD11chi dendritic cells in lymphoid aggregates (8) is not decreased by TNF inhibition.
As shown here, TNF inhibition markedly decreases the recruitment of peri-glomerular and interstitial F4/80hi macrophages that amplify tissue injury (10, 24). Periglomerular macrophages may be attracted by locally produced chemokines or may be activated by inflammatory mediators to proliferate in situ. In addition, because the effluent blood flow from the glomerulus provides the sole blood supply for peritubular capillaries glomerular hypertension and hypertrophy compromise peritubular blood flow resulting in hypoxia, tubular activation (including chemokine secretion) and tubular epithelial cell death. This induces resident macrophage activation and peritubular inflammation and is associated with tissue remodeling, fibrosis (30) and finally, irreversible renal damage. Release of damaged tissue, cytokines, and other inflammatory mediators amplify the inflammatory process. We have previously shown in NZB/W mice that several inflammatory markers are upregulated during renal disease, normalize upon remission induction and remain at low levels even late after remission induction when glomerular damage is recurring but interstitial damage has not yet recurred (8). Of these, we show by protein analysis that CCL9, VCAM-1 and lipocalin-2 are all decreased in TNFR2-Ig treated mice compared with controls. Lipocalin-2 is a highly sensitive marker for renal tubule damage that increases before any rise in serum creatinine and has pro-inflammatory functions (31–32) Furthermore oxidative damage is also decreased in TNFR2-Ig treated kidneys, confirming attenuation of end-stage renal disease. In contrast TNF inhibition does not alter the systemic inflammatory response as evidenced by failure to regulate lymphocyte activation or to prevent the accumulation of renal perivascular and hilar lymphoid infiltrates. These infiltrates may be attracted by soluble circulating mediators and vascular activation/injury, independently of glomerular immune complex deposition and/or the resulting peri-tubular hypoxia and do not appear to be sufficient to mediate end-stage renal failure.
Our findings, in sum, show that TNF is a critical cytokine in the renal effector response to glomerular immune complex deposition and that regulation of this response is highly therapeutic even in the IFN-induced model in which systemic inflammation is difficult to control (6). Judicious inhibition of TNF may be a potential therapeutic option for rapid control of renal damage in active SLE nephritis patients with increased renal expression of TNF and its receptors.
Footnotes
This work was supported by grants from the Lupus Research Institute and NIH DK085241-01 (AD), RO3AR054909 (MR and WH) and by Rheuminations (RB).
References
- 1.Jacob CO, McDevitt HO. Tumour necrosis factor-alpha in murine autoimmune ‘lupus’ nephritis. Nature. 1988;331(6154):356–8. doi: 10.1038/331356a0. [DOI] [PubMed] [Google Scholar]
- 2.Herrera-Esparza R, Barbosa-Cisneros O, Villalobos-Hurtado R, Avalos-Diaz E. Renal expression of IL-6 and TNFalpha genes in lupus nephritis. Lupus. 1998;7(3):154–8. doi: 10.1191/096120398678919949. [DOI] [PubMed] [Google Scholar]
- 3.Aringer M, Smolen JS. Therapeutic blockade of TNF in patients with SLE-Promising or crazy? Autoimmun Rev. 2011 doi: 10.1016/j.autrev.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Liu Z, Bethunaickan R, Huang W, Lodhi U, Solano I, Madaio MP, et al. Interferon-alpha accelerates murine systemic lupus erythematosus in a T cell-dependent manner. Arthritis Rheum. 2011;63(1):219–29. doi: 10.1002/art.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mihara M, Tan I, Chuzhin Y, Reddy B, Budhai L, Holzer A, et al. CTLA4Ig inhibits T cell-dependent B-cell maturation in murine systemic lupus erythematosus. J Clin Invest. 2000;106(1):91–101. doi: 10.1172/JCI9244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Z, Bethunaickan R, Huang W, Ramanujam M, Madaio MP, Davidson A. IFN-{alpha} Confers Resistance of Systemic Lupus Erythematosus Nephritis to Therapy in NZB/W F1 Mice. J Immunol. 2011;187(3):1506–13. doi: 10.4049/jimmunol.1004142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramanujam M, Wang X, Huang W, Schiffer L, Grimaldi C, Akkerman A, et al. Mechanism of action of transmembrane activator and calcium modulator ligand interactor-Ig in murine systemic lupus erythematosus. J Immunol. 2004;173(5):3524–34. doi: 10.4049/jimmunol.173.5.3524. [DOI] [PubMed] [Google Scholar]
- 8.Schiffer L, Bethunaickan R, Ramanujam M, Huang W, Schiffer M, Tao H, et al. Activated renal macrophages are markers of disease onset and disease remission in lupus nephritis. J Immunol. 2008;180(3):1938–47. doi: 10.4049/jimmunol.180.3.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan O, Madaio MP, Shlomchik MJ. The roles of B cells in MRL/lpr murine lupus. Ann N Y Acad Sci. 1997;815:75–87. doi: 10.1111/j.1749-6632.1997.tb52046.x. [DOI] [PubMed] [Google Scholar]
- 10.Bethunaickan R, Berthier CC, Ramanujam M, Sahu R, Zhang W, Sun Y, et al. A unique hybrid renal mononuclear phagocyte activation phenotype in murine systemic lupus erythematosus nephritis. J Immunol. 2011;186(8):4994–5003. doi: 10.4049/jimmunol.1003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiffer L, Sinha J, Wang X, Huang W, von Gersdorff G, Schiffer M, et al. Short term administration of costimulatory blockade and cyclophosphamide induces remission of systemic lupus erythematosus nephritis in NZB/W F1 mice by a mechanism downstream of renal immune complex deposition. J Immunol. 2003;171(1):489–97. doi: 10.4049/jimmunol.171.1.489. [DOI] [PubMed] [Google Scholar]
- 12.Jacob CO, Hwang F, Lewis GD, Stall AM. Tumor necrosis factor alpha in murine systemic lupus erythematosus disease models: implications for genetic predisposition and immune regulation. Cytokine. 1991;3(6):551–61. doi: 10.1016/1043-4666(91)90481-r. [DOI] [PubMed] [Google Scholar]
- 13.Mohan AK, Edwards ET, Cote TR, Siegel JN, Braun MM. Drug-induced systemic lupus erythematosus and TNF-alpha blockers. Lancet. 2002;360(9333):646. doi: 10.1016/S0140-6736(02)09800-8. [DOI] [PubMed] [Google Scholar]
- 14.Stokes MB, Foster K, Markowitz GS, Ebrahimi F, Hines W, Kaufman D, et al. Development of glomerulonephritis during anti-TNF-alpha therapy for rheumatoid arthritis. Nephrol Dial Transplant. 2005;20(7):1400–6. doi: 10.1093/ndt/gfh832. [DOI] [PubMed] [Google Scholar]
- 15.Palucka AK, Blanck JP, Bennett L, Pascual V, Banchereau J. Cross-regulation of TNF and IFN-alpha in autoimmune diseases. Proc Natl Acad Sci U S A. 2005;102(9):3372–7. doi: 10.1073/pnas.0408506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodge-Dufour J, Marino MW, Horton MR, Jungbluth A, Burdick MD, Strieter RM, et al. Inhibition of interferon gamma induced interleukin 12 production: a potential mechanism for the anti-inflammatory activities of tumor necrosis factor. Proc Natl Acad Sci U S A. 1998;95(23):13806–11. doi: 10.1073/pnas.95.23.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kodama S, Davis M, Faustman DL. The therapeutic potential of tumor necrosis factor for autoimmune disease: a mechanistically based hypothesis. Cell Mol Life Sci. 2005;62(16):1850–62. doi: 10.1007/s00018-005-5022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacob N, Yang H, Pricop L, Liu Y, Gao X, Zheng SG, et al. Accelerated pathological and clinical nephritis in systemic lupus erythematosus-prone New Zealand Mixed 2328 mice doubly deficient in TNF receptor 1 and TNF receptor 2 via a Th17-associated pathway. J Immunol. 2009;182(4):2532–41. doi: 10.4049/jimmunol.0802948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Via CS, Shustov A, Rus V, Lang T, Nguyen P, Finkelman FD. In vivo neutralization of TNF-alpha promotes humoral autoimmunity by preventing the induction of CTL. J Immunol. 2001;167(12):6821–6. doi: 10.4049/jimmunol.167.12.6821. [DOI] [PubMed] [Google Scholar]
- 20.Studnicka-Benke A, Steiner G, Petera P, Smolen JS. Tumour necrosis factor alpha and its soluble receptors parallel clinical disease and autoimmune activity in systemic lupus erythematosus. Br J Rheumatol. 1996;35(11):1067–74. doi: 10.1093/rheumatology/35.11.1067. [DOI] [PubMed] [Google Scholar]
- 21.Aten J, Roos A, Claessen N, Schilder-Tol EJ, Ten Berge IJ, Weening JJ. Strong and selective glomerular localization of CD134 ligand and TNF receptor-1 in proliferative lupus nephritis. J Am Soc Nephrol. 2000;11(8):1426–38. doi: 10.1681/ASN.V1181426. [DOI] [PubMed] [Google Scholar]
- 22.Wu T, Xie C, Wang HW, Zhou XJ, Schwartz N, Calixto S, et al. Elevated urinary VCAM-1, P-selectin, soluble TNF receptor-1, and CXC chemokine ligand 16 in multiple murinelupus strains and human lupus nephritis. J Immunol. 2007;179(10):7166–75. doi: 10.4049/jimmunol.179.10.7166. [DOI] [PubMed] [Google Scholar]
- 23.Ferenbach D, Hughes J. Macrophages and dendritic cells: what is the difference? Kidney Int. 2008;74(1):5–7. doi: 10.1038/ki.2008.189. [DOI] [PubMed] [Google Scholar]
- 24.Ramanujam M, Bethunaickan R, Huang W, Tao H, Madaio MP, Davidson A. Selective blockade of BAFF for the prevention and treatment of systemic lupus erythematosus nephritis in NZM2410 mice. Arthritis Rheum. 2010;62(5):1457–68. doi: 10.1002/art.27368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergtold A, Gavhane A, D’Agati V, Madaio M, Clynes R. FcR-bearing myeloid cells are responsible for triggering murine lupus nephritis. J Immunol. 2006;177(10):7287–95. doi: 10.4049/jimmunol.177.10.7287. [DOI] [PubMed] [Google Scholar]
- 26.Mathsson L, Lampa J, Mullazehi M, Ronnelid J. Immune complexes from rheumatoid arthritis synovial fluid induce FcgammaRIIa dependent and rheumatoid factor correlated production of tumour necrosis factor-alpha by peripheral blood mononuclear cells. Arthritis Res Ther. 2006;8(3):R64. doi: 10.1186/ar1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez de Lema G, Maier H, Nieto E, Vielhauer V, Luckow B, Mampaso F, et al. Chemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritis. J Am Soc Nephrol. 2001;12(7):1369–82. doi: 10.1681/ASN.V1271369. [DOI] [PubMed] [Google Scholar]
- 28.Tesch GH, Maifert S, Schwarting A, Rollins BJ, Kelley VR. Monocyte chemoattractant protein 1-dependent leukocytic infiltrates are responsible for autoimmune disease in MRL-Fas(lpr) mice. J Exp Med. 1999;190(12):1813–24. doi: 10.1084/jem.190.12.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Timoshanko JR, Kitching AR, Iwakura Y, Holdsworth SR, Tipping PG. Leukocyte-derived interleukin-1beta interacts with renal interleukin-1 receptor I to promote renal tumor necrosis factor and glomerular injury in murine crescentic glomerulonephritis. Am J Pathol. 2004;164(6):1967–77. doi: 10.1016/s0002-9440(10)63757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlondorff DO. Overview of factors contributing to the pathophysiology of progressive renal disease. Kidney Int. 2008;74(7):860–6. doi: 10.1038/ki.2008.351. [DOI] [PubMed] [Google Scholar]
- 31.Paragas N, Qiu A, Zhang Q, Samstein B, Deng SX, Schmidt-Ott KM, et al. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med. 2011;17(2):216–22. doi: 10.1038/nm.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viau A, El Karoui K, Laouari D, Burtin M, Nguyen C, Mori K, et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest. 2010;120(11):4065–76. doi: 10.1172/JCI42004. [DOI] [PMC free article] [PubMed] [Google Scholar]