Abstract
Human antigen R (HuR) is a post-transcriptional regulator of gene expression that plays a key role in stabilizing mRNAs during cellular stress, leading to enhanced survival. HuR expression is tightly regulated through multiple transcription and post-transcriptional controls. Although HuR is known to stabilize a subset of mRNAs involved in cell survival, its role in the survival pathway of PI3-kinase/Akt signaling is unclear. Here, we show that in renal proximal tubule cells, HuR performs a central role in cell survival by amplifying Akt signaling in a positive feedback loop. Key to this feedback loop is HuR-mediated stabilization of mRNA encoding Grb10, an adaptor protein whose expression is critical for Akt activation. Stimulation of Akt by interaction with Grb10 then activates NF-κB, which further enhances HuR mRNA and protein expression. This feedback loop is active in unstressed cells, but its effects are increased during stress. Therefore, this study demonstrates a central role for HuR in Akt signaling and reveals a mechanism by which modest changes in HuR levels below or above normal may be amplified, potentially resulting in cell death or cellular transformation.
Keywords: HuR, Akt, Grb10, Apoptosis, Cell stress
INTRODUCTION
HuR is a ubiquitously expressed regulator of post-transcriptional processing, with effects on pre-mRNA splicing, mRNA stability, and translation (Lebedeva et al., 2011; Mukherjee et al., 2011). HuR has been estimated to regulate ~8% of the human transcriptome, with broad effects on cell cycle, apoptosis, and inflammatory responses (Khabar, 2005). Its best studied effects are on mRNA stability, in which it binds to uridine- or adenine + uridine-rich elements in the 3’ untranslated regions of mature mRNAs and slows their rates of degradation. Regulation of HuR expression must be very tightly controlled, as loss of HuR expression results in rapid cell death (Ghosh et al., 2009; Katsanou et al., 2009), while overexpression by only a few-fold can result in tumorigenicity (Danilin et al., 2010; Denkert et al., 2004a; Denkert et al., 2004b; Lopez de Silanes et al., 2003; Lopez de Silanes et al., 2005; Mazan-Mamczarz et al., 2008; Nabors et al., 2001). We have demonstrated that in cultured renal proximal tubule cells, HuR stabilizes mRNAs and prevents apoptosis resulting from energy depletion (Ayupova et al., 2009; Jeyaraj et al., 2005). We also showed that regulation of HuR expression is tightly regulated at both transcriptional and post-transcriptional levels in vitro and in vivo (Ayupova et al., 2009; Jeyaraj et al., 2006; Jeyaraj et al., 2010). This is in part due to the expression of two alternate HuR mRNAs with different translational properties, whose relative levels change following cell stress. In rat kidneys, ischemia-reperfusion injury results in overall increases in HuR mRNA throughout the nephron, but HuR protein levels are increased only in proximal tubules (Ayupova et al., 2009). This distinction is notable, as proximal tubule cells are highly sensitive to ischemic injury.
HuR’s role as a mediator of cell survival is well-established (Abdelmohsen et al., 2007). Indeed, HuR’s capacity to bind mRNAs is enhanced during cell stress, when it switches from a typical nuclear steady-state location to the cytoplasm (Atasoy et al., 1998; Fan and Steitz, 1998). Stresses that trigger HuR activation include energy depletion (Jeyaraj et al., 2005), ultraviolet irradiation (Wang et al., 2000), hypoxia (Levy et al., 1998), nutrient deprivation (Yaman et al., 2002), and heat shock (Gallouzi et al., 2001), among others. HuR has been shown to promote the mRNA stability and/or translation of several anti-apoptotic proteins including Bcl-2, Mcl-1, prothymosin-α, and XIAP (Abdelmohsen et al., 2007). In addition, it was demonstrated that HuR promotes alternative splicing of the mRNA for the death receptor Fas such that the resulting protein no longer retains its pro-apoptotic functions (Izquierdo, 2008).
The PI3K/Akt pathway has been demonstrated to be critical to cell survival and repair in multiple tissue injury models, including the kidney. Renal ischemia/reperfusion (I/R) was shown to transiently increase Akt activation (Andreucci et al., 2003), which was further demonstrated to play a protective role in I/R-injured kidney function (Satake et al., 2008). The PI3K/Akt pathway also has been shown to protect against acute kidney injury induced by cisplatin toxicity (Kuwana et al., 2008). Akt, a family of highly related serine/threonine kinases, protects against apoptosis through phosphorylation of multiple proteins involved in regulating cell survival, including Bcl-2 family members, caspases and caspase inhibitors, the mTOR signaling pathway, and FoxO transcription factors (Datta et al., 1999). Previous studies have suggested interaction between Akt signaling and HuR function. In gastric tumor cells, it was shown that Akt activation could promote HuR expression through stimulation of an NF-κB element in the HuR promoter (Kang et al., 2008). Conversely, it was suggested that HuR was required for Akt activation in renal tubule cells (Danilin et al., 2010). Therefore, the precise interaction of HuR with the PI3K/Akt pathway is unclear.
Grb10 is a member of a family of adaptor proteins (including Grb7 and Grb14) that functions downstream of multiple receptor tyrosine kinases such as the insulin and insulin-like growth factor-I receptor (Liu and Roth, 1995; Morrione et al., 1996), growth hormone receptor (Moutoussamy et al., 1998), and the Ret receptor tyrosine kinase (Pandey et al., 1995), among others. Grb10 may also interact with a number of non-receptor kinases, including Akt. It has been proposed that in some cell types, Grb10 stimulates Akt function by translocating Akt to the plasma membrane where it is phosphorylated and activated by PI3 kinase (Jahn et al., 2002). Here we show that in renal proximal tubule cells, HuR binds Grb10 mRNA and promotes its expression, leading to their participation in a positive feedback loop that amplifies the pro-survival functions of Akt signaling.
MATERIALS AND METHODS
Cell culture and RNA interference
The human proximal tubule cell line HK-2 and the porcine proximal tubule cell line LLC-PK1 were cultured in Dulbecco’s modified Eagle medium (DMEM) containing fetal bovine serum (10%) and penicillin/streptomycin at 37°C in a 5% CO2 environment. ATP depletion of these cells were performed as previously described (Ayupova et al., 2009; Jeyaraj et al., 2005; Jeyaraj et al., 2006). Briefly, cells were grown to confluence and cultured overnight in fresh medium. The cells were rinsed twice with phosphate-buffered saline and the growth medium was replaced with pre-warmed DMEM base supplemented with L-glucose, sodium bicarbonate, and 0.1 µM antimycin A. RNAi-mediated knockdown of HuR was performed as reported using either a previously described siRNA with sequence identity to both human and porcine HuR (Jeyaraj et al., 2005) or a commercially available human siRNA (Santa Cruz Biotechnology). RNAi-mediated knockdown of Grb10 was performed by transfecting 100 nM commercially available siRNA (Santa Cruz Biotechnology) into HK-2 cells using Lipofectamine and Plus reagent (Invitrogen). The PI3 kinase inhibitor LY-294002 (Cell Signaling) was added to cultures, where appropriate, at a concentration of 50 µM for 16–18 hours prior to ATP depletion experiments. The NF-κB inhibitor BAY11-7082 (Sigma) was used at a concentration of 2 µM.
Plasmids
Plasmids encoding wild-type and constitutively active Akt were a kind gift of Dr. Michael Ostrowski (The Ohio State University). The cDNA encoding mouse Grb10 eta was an I.M.A.G.E. clone obtained from the American Type Culture Collection (accession number BC016111). Restriction endonuclease XbaI was used to remove 2434 bases (82.4%) of the Grb10 3’ untranslated region for experiments testing the role of the 3’ UTR in Grb10 expression.
Antibodies and Western analysis
HuR was detected using a mouse monoclonal antibody (3A2) obtained from Santa Cruz Biotechnology. Rabbit antibodies reacting with total Akt and phospho-Akt were obtained from Cell Signaling Technology. Antibodies detecting human Grb10 were purchased from Cell Signaling and Santa Cruz, while a rabbit antibody specific to mouse Grb10 was purchased from Santa Cruz. A mouse antibody reactive with β-actin was purchased from Abcam. Secondary antibodies coupled to horseradish peroxidase were purchased from GE Healthcare. Western analysis was performed by standard methodologies. Proteins were separated by SDS-PAGE (Bio-Rad), transferred to Hybond P membrane (GE Healthcare), and after incubations with primary and secondary antibodies, were detected using a SuperPico detection kit (Pierce).
RT-PCR and immunoRT-PCR
An internal standard for competitive RT-PCR of HuR was previously synthesized (Jeyaraj et al., 2005). An internal standard for Grb10 was synthesized using the same methodology. Total RNA from HK-2 or LLC-PK1 cells was isolated using Trizol (Invitrogen) following the manufacturer’s instructions. A mixture of internal standard RNA and cellular RNA was reverse transcribed using the Super Script cDNA synthesis system (Invitrogen). The resulting cDNA was subjected to PCR using Platinum Taq DNA polymerase (Invitrogen). For HuR, PCR primers were of the sequences 5’-GGTTATGAAGACCACATGGCCG-3’ (sense) and 5’-AAGCCATAGCCCAAGCTGT-3’ (antisense). Human Grb10 primers were of the sequences 5’-ACAAGGTGGAGCAGACACCTC-3’ (sense) and 5’-GTAAAGAACCCGGCGGTGAGC-3’ (antisense), while mouse Grb10 primers were of the sequences 5’- GAATCCAGTGAACTTCTTCCC -3’ (sense) and 5’- GCGTTGTACTGCTTCTTTCC -3’ (antisense).
Immunoprecipitation of HuR followed by RT-PCR of associated mRNAs was performed as previously described (Jeyaraj et al., 2005; Yeap et al., 2002). Briefly, HK-2 cells were lysed in CEB buffer containing protease and RNAse inhibitors, followed by immunoprecipitation of HuR using mouse monoclonal 3A2 (Santa Cruz Biotechnology) and Protein A/G (Thermo Scientific). The resulting precipitate was washed with CEB buffer, and RNA was extracted using Trizol. Following RNA isolation and cDNA synthesis, the presence of Grb10 was assessed using the PCR primers described above.
PCR arrays
RT2 Profiler PCR arrays and associated reagents were purchased from SABiosciences/Qiagen. To compare gene expression between control and HuR-suppressed HK-2 cells, total RNA was harvested from cells treated with control oligonucleotide or HuR siRNA 48 hours post-transfection, then subjected to ATP depletion for 6 hours. cDNA synthesis was performed using an RT2 First Strand kit. PCR arrays were analyzed using a Bio-Rad iCycler iQ Real-time Detection System in the Plant-Microbe Genomics Facility at The Ohio State University. Analysis of resulting PCR products was performed using RT2 Profiler PCR Array Data Analysis software according to SABiosciences/Qiagen protocols.
Apoptosis assays
Assays for apoptosis were performed as previously described (Ayupova et al., 2009), using the Caspase Glo 3/7 Assay kit (Promega Corp.). To test the role of PI3 kinase signaling, cells were treated with 50 µM LY-294002 or vehicle for 16–18 hours prior to ATP depletion and harvesting for caspase assays. In other experiments, Grb10 expression was suppressed by siRNA for 48 hours prior to ATP depletion and harvesting for caspase assays. Each treatment was performed in triplicate, and three such independent experiments were performed.
Immunocytochemistry
For immunolocalization of total Akt, pAkt and Grb10, cells were seeded on glass coverslips and grown to confluence, at which time the cells were given fresh medium and cultured overnight prior to use. Following the necessary treatments, cells were fixed and permeabilized in 2% formaldehyde in stabilization buffer. Cells were then probed with anti-Akt, anti-pAkt (phosphoserine 473) or anti-Grb10 antibody, and Alexa 488-or Alexa 568-conjugated goat anti-rabbit secondary antibodies from Molecular Probes. Cells were visualized with a Nikon Eclipse 80i epifluorescent microscope with SPOT software (Diagnostic Instruments).
RESULTS
HuR expression is regulated by PI3K/Akt activity in proximal tubule cells
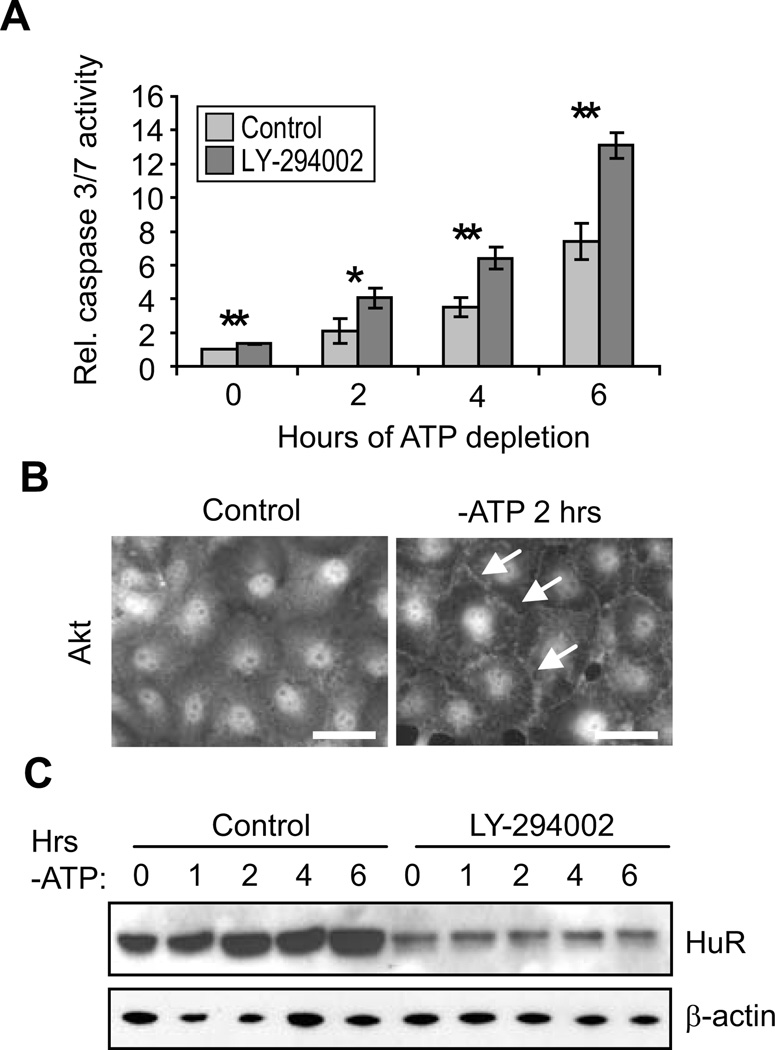
Numerous previous reports have established the importance of PI3K/Akt activity in cell survival in energy-depleted renal epithelia (Andreucci et al., 2003; Chen et al., 2008; Joo et al., 2006; Loverre et al., 2004; Sharples et al., 2004; Xie et al., 2006). To confirm these findings in the HK-2 proximal tubule cell line, cells were subjected to ATP depletion in the presence or absence of PI3K inhibitor LY-294002, and apoptosis was measured through assessment of caspase 3/7 activity. Figure 1A demonstrates that over the course of 6 hours, activity of these caspases increased approximately 7-fold and that the magnitude of this response was almost doubled by inhibition of PI3K signaling. Similar results were obtained in the porcine proximal tubule line LLC-PK1 (Supplementary Fig. S1A). Activation of Akt signaling is also demonstrated in Figure 1B, which shows that ATP depletion resulted in changes in the distribution of total Akt. Although Akt is most typically a cytosolic protein, control HK-2 cells demonstrated a primarily nuclear distribution, as has been demonstrated for a number of cell types including HEK293, HeLa, PC12, and cardiomyocytes (Martelli et al., 2006; Miyamoto et al., 2009; Wang and Brattain, 2006). In contrast, ATP-depleted cells demonstrated an accumulation of Akt at the cell periphery (arrows), indicative of Akt activation at the plasma membrane. ATP depletion also induced an increased in pAkt levels, as assessed by Western blots (discussed below). These results confirm a role for PI3K/Akt signaling in cell survival in HK-2 cells undergoing energy depletion.
Figure 1. HuR levels are regulated by PI3K/Akt signaling.
(A) Apoptosis levels were assessed by measurements of caspase 3/7 activity in HK-2 cells treated either with vehicle or PI3K inhibitor LY-294002. *, P< 0.05; **, P<0.005. (B) Control or ATP-depleted HK-2 cells were immunolabeled with an antibody against total Akt. Arrows indicate plasma membrane distribution of Akt, which is indicative of its activation. Scale bars = 20 µm. (C) The effects of PI3K inhibition on HuR levels were measured by Western blot. β-actin was detected as a loading control.
PI3K/Akt signaling was previously shown to increase HuR expression in gastric tumor cells (Kang et al., 2008). Therefore, we performed experiments to determine whether PI3K/Akt signaling enhanced HuR expression in HK-2 cells. Cells were ATP depleted in the presence or absence of LY-294002 and HuR expression levels were determined. Figure 1C shows that by 2 hours of ATP depletion of HK-2 cells there is an approximately 2-fold increase in HuR protein, as we previously demonstrated in LLC-PK1 cells (Jeyaraj et al., 2006). Inhibition of PI3K strongly suppressed HuR levels (by 76 ± 6% over all timepoints in three independent experiments). These results demonstrate that PI3K activity regulates HuR expression in proximal tubule cells under both normal (timepoint 0) and energy-depleted conditions.
NF-κB activity is critical for HuR expression
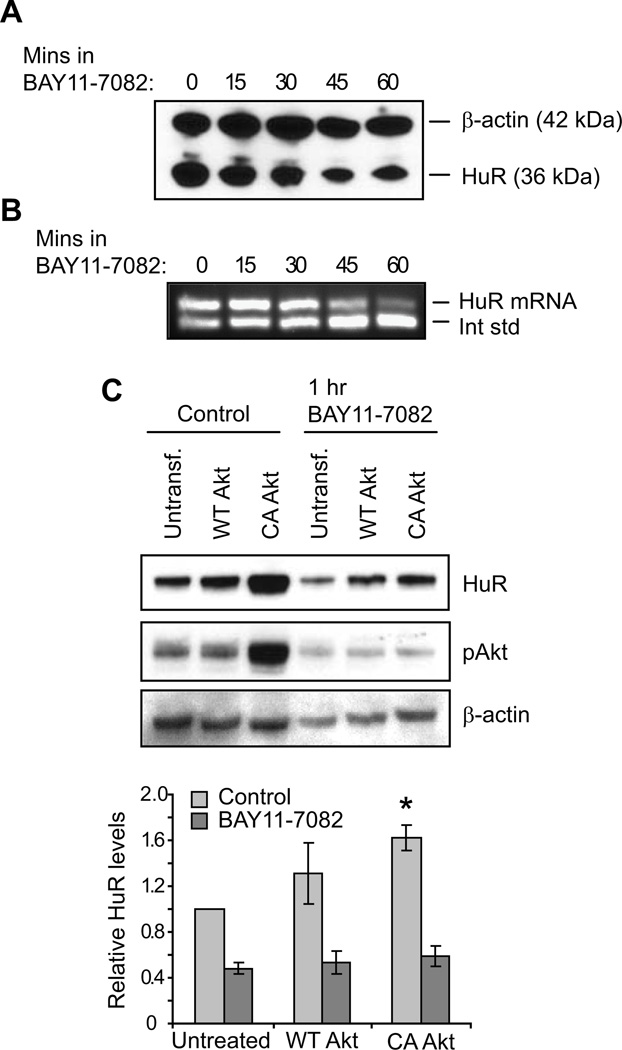
To determine whether PI3K/Akt signaling regulates HuR expression through the Akt downstream effector NF-κB, normally growing HK-2 cells were treated with NF-κB inhibitor BAY11-7082, and HuR levels were assessed. Both Western blot (Figure 2A) and competitive RT-PCR (Figure 2B) demonstrate that inhibition of NF-κB activity in normally growing cells results in decreased HuR protein and mRNA levels (by 31–41% after 60 minutes; n = 3). Inhibition of NF-κB also resulted in decreased HuR expression in LLC-PK1 cells (Supplementary Figure S1B). To demonstrate the relationship between Akt and NF-κB relative to HuR expression, HK-2 cells were transfected with either wild-type (WT) or constitutively active (CA) Akt (Figure 2C). Overexpression of WT-Akt, which did not significantly alter activated Akt levels, induced a mild increase in HuR, although this change did not reach statistical significance. In contrast, overexpression of CA-Akt increased HuR (by 62 ± 11%; n = 3). These data confirm that Akt and NF-κB are regulators of HuR expression. Surprisingly, inhibition of NF-κB also resulted in diminished pAkt in all cells, including those expressing CA-Akt (Figure 2C, middle panel). NF-κB is a known downstream effector of Akt signaling; however, these results suggest that NF-κB signaling may also induce Akt activation, indicative of a regulatory feedback loop.
Figure 2. HuR levels are regulated by NF-κB signaling.
The effects of NF-κB inhibition on HuR expression were demonstrated by treatment of HK-2 cells with BAY11-7082. (A) HuR protein levels were assessed by Western analysis. A representative blot is shown that also detects β-actin as a loading control. (B) Competitive RT-PCR demonstrates that inhibition of NF-κB suppresses HuR mRNA levels. (C) HK-2 cells overexpressing wild-type (WT) or constitutively active (CA) Akt1 were treated with vehicle or BAY11-7082, as were untransfected controls. HuR levels were assessed by Western analysis, along with β-actin as a loading control. A Western of pAkt levels indicates Akt activation levels. Below, data from three independent experiments are represented graphically. *, P= 0.01.
Akt activity but not Akt expression is regulated by HuR levels in proximal tubule cells
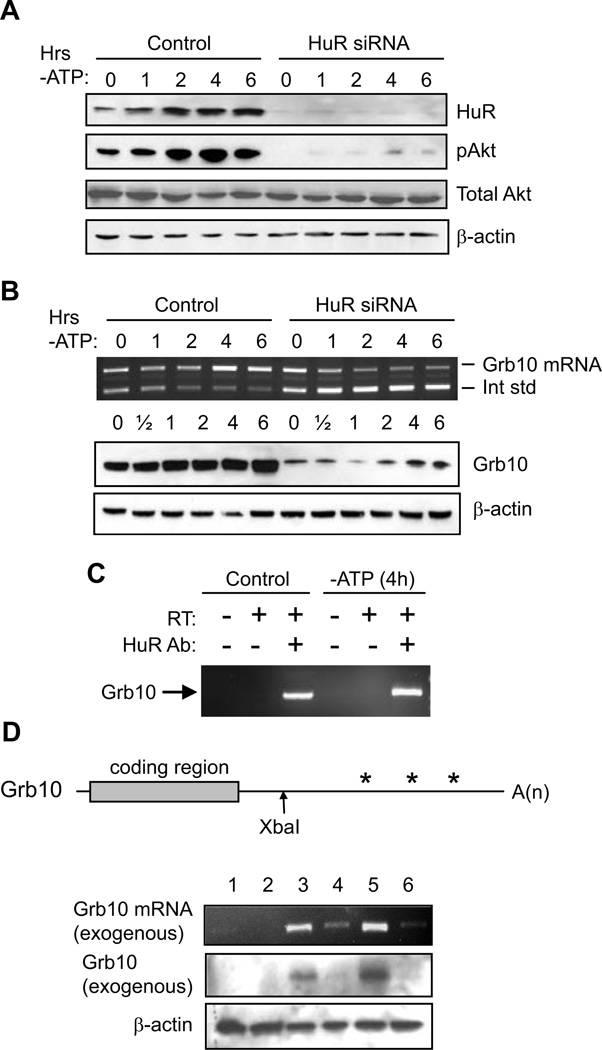
Our data demonstrate that HuR levels are increased by PI3K/Akt signaling in proximal tubule cells. Conversely, other studies have suggested that this relationship may be reversed; that is, that HuR modulates Akt activity (Danilin et al., 2010; Meng et al., 2002). Therefore, we performed Western analysis in our cell model to determine whether siRNA-mediated suppression of HuR affected Akt expression or activity in HK-2 cells. For these experiments, we utilized an siRNA that was previously shown to give specific knockdown in LLC-PK1 cells (Jeyaraj et al., 2005) by binding to an mRNA sequence that is identical between the human and porcine forms. Figure 3A, top row, illustrates that HuR protein expression, which increases during ATP depletion, can be strongly inhibited by HuR siRNA in HK-2 cells (92 ± 7% inhibition over all timepoints in three independent experiments). Similar results were obtained with a second, independent siRNA against human HuR (Supplementary Figure S2A). Figure 3A also demonstrates that Akt is activated during ATP depletion, to 2.0 ± 0.1-fold greater than normal levels at 6 hours (n = 3). Further, HuR knockdown strongly suppresses this activation, by 72 ± 6% over all timepoints. In contrast, total Akt expression is unaffected by the knockdown, demonstrating that HuR modulates activation, rather than the expression, of Akt. Similar results were obtained in LLC-PK1 cells (Supplementary Figure S1C). These results and those in previous figures show that in proximal tubule cells, HuR levels are regulated by Akt and conversely, HuR regulates Akt activation.
Figure 3. HuR regulates Akt activation and Grb10 expression.
(A) Western analysis demonstrates that ATP depletion increases HuR protein levels and Akt phosphorylation. siRNA-mediated knockdown of HuR strongly inhibits Akt activation without affecting total Akt levels. β-actin is shown as a loading control. (B) Competitive RT-PCR (top row) demonstrates that Grb10 mRNA levels increase during ATP depletion, but are diminished by HuR knockdown. Western analysis, below, shows similar results for Grb10 protein. β-actin is shown as a loading control. (C) HuR was immunoprecipitated from ATP-depleted or control cells, and associated RNA was purified. The presence of Grb10 mRNA was assessed by RT-PCR. Controls demonstrate specificity by showing that a lack of HuR antibody or reverse transcriptase (RT) in the procedure fails to produce Grb10 cDNA. (D) (top) A murine Grb10 cDNA used for overexpression is shown schematically, including the 174 bp 5’-UTR, 1626 bp coding region, and the 2954 bp 3’ UTR. For generation of a mutant cDNA, 3’ UTR sequences downstream of the XbaI site were deleted. Asterisks indicate potential regions of HuR binding, based on sequence analysis. (bottom) The role of the Grb10 3’ UTR in controlling its expression was demonstrated by both RT-PCR and Western blot. β-actin levels are included as a loading control for the Western blot. Lane 1: untransfected; lane 2: empty expression vector; lane 3: full-length murine Grb10; lane 4: murine Grb10 lacking 3’ UTR sequences; lane 5: full-length murine Grb10 + HuR; lane 6: murine Grb10 lacking 3’ UTR sequences + HuR.
Expression of adaptor protein Grb10 is regulated by HuR levels in proximal tubule cells
HuR is a post-transcriptional regulator of gene expression and not a kinase, therefore HuR’s effects on Akt activity are likely to be a result of alteration in expression of an upstream activator of Akt. To determine what members of the PI3K/Akt signaling pathway might be regulated by HuR, PCR array analysis was performed on control HK-2 cells and those in which HuR expression was suppressed by RNAi. HuR suppression resulted in reduced expression of 39 out of 84 mRNAs involved in PI3K/Akt signaling (Supplementary Table SI). In two independent experiments, the mediator of Akt signaling most strongly affected by HuR suppression was Grb10 (growth factor receptor-bound protein 10), a member of the Grb7 family of adaptor proteins that was shown to be a key regulator of PI3 kinase activity downstream of the insulin and insulin-like growth factor-1 receptor tyrosine kinases (Lim et al., 2004; Riedel, 2004). To confirm the PCR array analysis, Grb10 mRNA and protein levels were compared in control cells and cells in which HuR was suppressed by RNAi. Figure 3B demonstrates that Grb10 mRNA and protein levels increase by less than 2-fold in control cells during ATP depletion. However, knockdown of HuR strongly inhibited expression of Grb10 (protein levels decreased by 65 ± 4% over all timepoints in two experiments). Similar results were obtained in LLC-PK1 cells (Supplementary Figure S1C).
This result suggests that Grb10 mRNA may be a target of HuR-mediated post-transcriptional control. To determine whether HuR binds Grb10 mRNA, HuR from control or ATP-depleted cells was immunoprecipitated, the co-precipitating mRNAs were isolated, and the presence of Grb10 mRNA was assessed by RT-PCR. As shown in Figure 3C, under both normal and stressed conditions, HuR was capable of co-precipitating Grb10 mRNA. An increase in HuR-associated Grb10 was noted in stressed cells, as is consistent with HuR’s protective role (in 3 independent experiments, an average increase of 66 ± 21% over control cells). Further, the 3’ UTR of Grb10 contains multiple regions that conform to potential HuR binding sites (Lebedeva et al., 2011; Lopez de Silanes et al., 2004; Mukherjee et al., 2011). To determine whether the 3’ UTR does indeed contain a functional binding site, full-length mouse Grb10 or a mutant lacking most of the 3’ UTR was transfected into HK-2 cells in the presence or absence of exogenous HuR. As shown in Figure 3D, the exogenous mouse Grb10 mRNA and protein were then detected by RT-PCR (top row) or in Western blots using an antibody specific to the mouse protein (middle row). These experiments show that intense bands were detected in cells overexpressing wild-type Grb10 (lanes 3 and 5) but not truncated Grb10 (lanes 4 and 6). This result is consistent with a positive role for the Grb10 3’ UTR in its expression. Additionally, when WT Grb10 was co-transfected with HuR (lane 5), Grb10 expression was 2.5 ± 0.6-fold higher than when expressed alone (lane 3, n = 3). These results, coupled with the previous figure showing HuR’s ability to bind Grb10 mRNA, demonstrate that HuR plays a positive role in promoting Grb10 expression.
Grb10 stimulates Akt activation and HuR levels
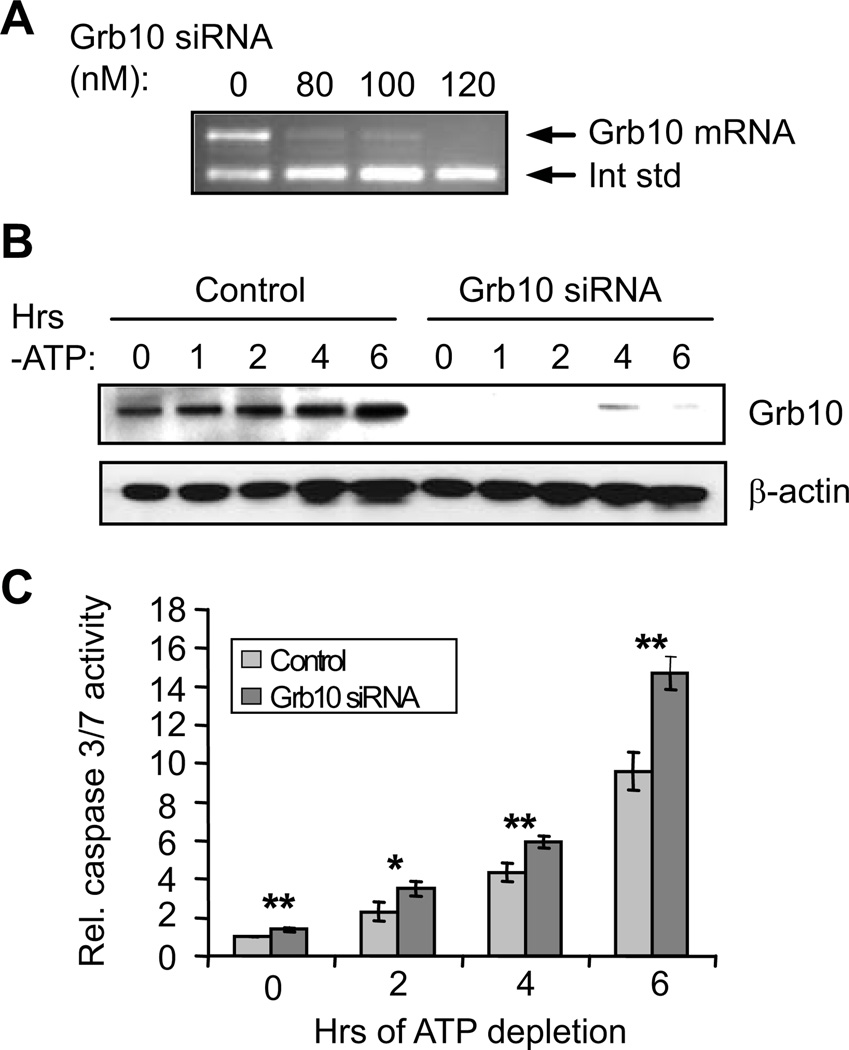
As described above, Grb10 has been implicated in some cell types as a positive regulator of survival (Lim et al., 2004; Riedel, 2004). To determine whether Grb10 has any effects on cell survival and the PI3K/Akt signaling pathway in proximal tubule cells, RNAi was used to knock down its expression. Four alternately spliced Grb10 isoforms with yet unknown specific functions are present in human cells (Lim et al., 2004; Riedel, 2004). The siRNAs used in this study were designed to knock down all isoforms. Figure 4A demonstrates that Grb10 mRNA can be strongly suppressed in HK-2 cells using nanomolar levels of siRNA. This loss of Grb10 mRNA results in a corresponding loss of Grb10 protein (knockdown of 95 ± 2% over all time points in two experiments; Figure 4B). To determine whether Grb10 has effects on cell survival, HK-2 cells treated with either Grb10 siRNA or a control oligonucleotide underwent ATP depletion, and apoptosis was measured by assays for caspase 3/7 activation. As shown in Figure 4C, ATP depletion in control cells resulted in a ~10-fold increase in caspase 3/7 activity over 6 hours. However, suppression of Grb10 expression increased this response over time, and after 6 hours, caspase 3/7 activity was ~50% higher in the siRNA-treated cells than the control cells. Thus, Grb10 plays an important role in cell survival during energy depletion.
Figure 4. siRNA-mediated knockdown of Grb10 increases apoptosis in ATP-depleted HK-2 cells.
(A) Competitive RT-PCR demonstrates efficient knockdown of Grb10 mRNA with nanomolar levels of siRNA. (B) Western analysis demonstrates efficient knockdown of Grb10 protein during ATP depletion. β-actin levels are assessed as a loading control. (C) Caspase 3/7 assays show that knockdown of Grb10 increases apoptosis during ATP depletion. *, P < 0.05; **, P< 0.01.
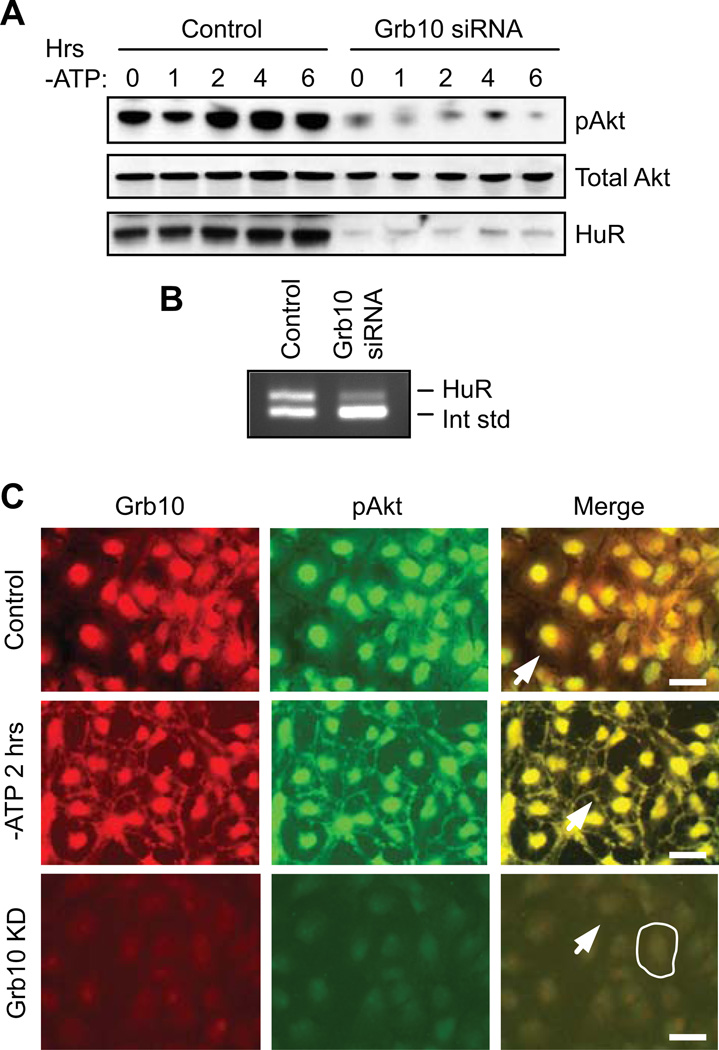
Grb10 was previously shown to be a positive regulator of Akt signaling downstream of PI3 kinase (Jahn et al., 2002). To test whether Grb10 has a similar effect in proximal tubule cells, Grb10 was knocked down by RNAi in cells undergoing ATP depletion, and total and activated Akt levels were assessed by Western blot. As shown in the top row of Figure 5A, ATP depletion of control cells induced Akt phosphorylation, as previously demonstrated in Figure 3A. The same blot shows that suppression of Grb10 resulted in a dramatic decrease in Akt activation at the 0 timepoint and prevented any increase in activation during ATP depletion (reduction of pAkt by 88 ± 4% over all time points). This was not due to alterations in total Akt levels, however, as these were unchanged by loss of Grb10 (Figure 5A). Interestingly, siRNA-mediated knockdown of Grb10 also resulted in severe loss of HuR (by 85 ± 3% over all timepoints; Figure 5A). This siRNA-mediated loss of HuR, as well as the decrease in Akt activation, could be reversed by rescuing Grb10 levels with expression of an exogenous cDNA (Supplementary Figure S2B). This result further suggests that HuR participates in Akt signaling in a positive feedback loop. To determine whether knockdown of Grb10 diminishes HuR expression at the level of mRNA, control cells or those treated with Grb10 siRNA were assessed for HuR mRNA levels by competitive RT-PCR. As shown in Figure 5B, suppression of Grb10 did indeed result in the loss of HuR mRNA, suggesting that Akt and NF-κB signaling are likely to regulate HuR transcription, as previously described.
Figure 5. Knockdown of Grb10 inhibits Akt activation and HuR expression.
(A) Western analysis of HK-2 cells treated with control oligonucleotides or Grb10 siRNAs show that suppression of Grb10 results in strongly diminished Akt activation and HuR protein expression. (B) Competitive RT-PCR demonstrates that knockdown of Grb10 decreases HuR expression at the mRNA level. (C) Under normal growth conditions (top row), Grb10 and pAkt demonstrate almost complete overlap in the nucleus but show no discernable distribution at the plasma membrane (arrow). ATP depletion (middle row) results in movement of a subset of both proteins to the plasma membrane (arrow). SiRNA-mediated knockdown of Grb10 (bottom row) results in a marked loss of pAkt and no clear plasma membrane distribution of either protein. The periphery of a single cell is outlined and the plasma membrane of a second cell is indicated with an arrow. Scale bars = 20 µm.
Finally, we examined the relative distributions of Grb10 and phosphorylated Akt, as Grb10 has been suggested to aid in Akt activation through their association (Jahn et al., 2002). Figure 5C demonstrates an almost complete overlap of pAkt and Grb10 in the nucleus under normal growth conditions. In addition, ATP depletion results in Grb10, along with pAkt, being re-distributed to the cell periphery. Similar effects were seen in LLC-PK1 cells (Supplementary Figure S1D). As expected from the findings in Figure 5A, knockdown of Grb10 resulted in an almost complete loss of pAkt along with an inability of pAkt to translocate to the cell periphery (Figure 5C). These results are consistent with a role for Grb10 in assisting activation of pAkt in renal epithelia.
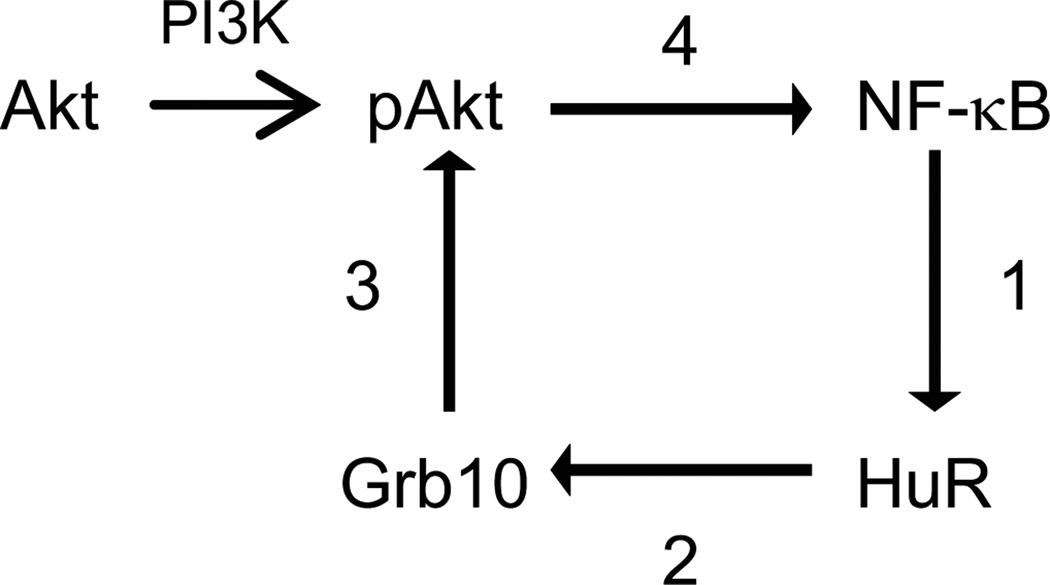
In summary, the studies described here suggest a central role for HuR within a positive feedback loop that amplifies Akt signaling in proximal tubule cells. A schematic of this proposed feedback loop, which is based both on the data presented here as well as work published in the literature, is shown in Figure 6. We hypothesize that this feedback loop plays a key role in maintaining pAkt activity under both normal growth conditions and during stress.
Figure 6. Schematic of HuR’s role in promoting a positive feedback loop of Akt signaling.
Based on data presented here and in the literature, we propose the following positive feedback loop for HuR in Akt signaling. (1) NF-κB promotes HuR expression, most likely through transcriptional control; (2) HuR binds to and stabilizes Grb10 mRNA, resulting in increased Grb10 expression; (3) Grb10 activates Akt by aiding in its transport to the plasma membrane where it is activated by PI3K; and (4) Akt activation stimulates NF-kB activity.
DISCUSSION
Regulation of HuR expression must necessarily be under close cellular control to permit cell survival while preventing cellular transformation. Yet, our previous results suggest that cells must maintain the ability to temporarily upregulate HuR levels in order to survive certain injuries and prepare for future stresses (Ayupova et al., 2009; Jeyaraj et al., 2005; Jeyaraj et al., 2006; Jeyaraj et al., 2010). The necessity for precision in HuR expression is reflected in the existence of numerous levels of regulation, including transcriptional and translational control of HuR during cell stress (Ayupova et al., 2009; Jeyaraj et al., 2010). Sequences in the 3’ untranslated region of HuR mRNA are also critical for its expression. HuR has been shown to be translationally repressed by multiple microRNAs whose suppression appears to lead to tumor growth and resistance to chemotherapy (Abdelmohsen et al., 2010; Guo et al., 2009; Kojima et al., 2010; Xu et al., 2010). Interestingly, HuR mRNA is also expressed as alternate polyadenylation variants that contain or lack HuR binding sites, allowing for auto-regulation of expression (Al-Ahmadi et al., 2009; Dai et al., 2011). Here we introduce another level of regulation, in which HuR joins with Akt signaling pathways in a positive feedback loop.
HuR’s central role in PI3K/Akt signaling is evident in the results of our PCR array analysis, in which HuR knockdown resulted in decreased expression of almost half of the tested genes that contribute to this signaling. Indeed, our initial examinations of HuR’s role in PCR arrays of genes involved in general apoptosis revealed the PI3K/Akt pathways to be particularly influenced by HuR levels relative to other apoptotic pathways (not shown). We have identified the adapter protein Grb10 as a target of HuR regulation and have shown its importance in mediating cell survival during stress. Consistent with this study, two very recent transcriptome-wide analyses of HuR targets identified Grb10 as containing HuR binding sites with both 3’ UTR and intronic sequences (Lebedeva et al., 2011; Mukherjee et al., 2011). Interestingly, both HK-2 and LLC-PK1 cells under normal conditions demonstrate a primarily nuclear localization of pAkt and Grb10, which have more typically been considered cytoplasmic proteins. Energy depletion results in the appearance of these proteins at the plasma membrane, although the nuclear versions still remain the great majority. The consequences of this distribution are unclear. Nuclear pAkt (and PI3K) has been demonstrated in multiple cell types (Martelli et al., 2006), and indeed, the nuclear form was shown to be as effective as the plasma membrane form in protecting cardiomyocytes from stress-induce apoptosis (Shiraishi et al., 2004). It seems likely that alterations in distribution would allow Akt access to different downstream targets, which include both cytoplasmic (e.g. BAD, caspase 9) and nuclear (e.g. transcription factor FoxO3a) proteins (Datta et al., 1999). Nonetheless, our results show that Grb10 is critical for Akt activation in renal epithelia, both under normal and stressed conditions.
Although our studies indicate that Grb10 promotes cell survival in renal epithelia, it is a negative regulator of growth, as its disruption in mice leads to overgrowth and heightened signaling in insulin-sensitive tissues (Charalambous et al., 2003; Wang et al., 2007). Very recently, this was shown to be due to Grb10’s role as a downstream target of serine/threonine kinase mTOR complex 1 (mTORC1), a potent negative regulator of insulin signaling. Briefly, it was shown that Grb10 is phosphorylated by mTORC1, which increases Grb10’s stability, allowing it to negatively regulate insulin signaling and subsequent PI3K/Akt activation (Hsu et al., 2011; Yu et al., 2011). This scenario is in distinct opposition to our findings in renal epithelia, where we find that Grb10 is a positive regulator of cell survival and Akt activation. These discrepancies may be attributable in part to tissue- and cell-specific differences, such as cellular expression of insulin and insulin-like growth factor receptors or other regulators of PI3K signaling. An anti-apoptotic role for Grb10 has been demonstrated in several other cell models (Hu et al., 2010; Jahn et al., 2002; Kebache et al., 2007). Further, Grb10 expression has been shown to be upregulated in some tumor types (Casas et al., 2003; Mirmohammadsadegh et al., 2004; Okino et al., 2005) and downregulated in others (Yu et al., 2011). Perhaps relevant to these studies, disruption of a maternally-imprinted allele of Grb10 resulted in a general overgrowth of resulting mice, although the kidney was spared (Garfield et al., 2011). These results suggest that Grb10’s effects vary within different cellular contexts. In addition, Grb10 mRNA is expressed as multiple alternately spliced transcripts (3 in mice, 4 in humans) whose functions may or may not differ. Whether alternately spliced forms play unique roles in activation of Akt signaling or other pathways remains to be determined. Finally, the subcellular distribution of Grb10 and pAkt may play a role, as we have shown that their presence is highest in the nuclear compartment in proximal tubule cells.
These studies provide evidence to show that HuR plays a central role in Akt signaling in renal epithelia, as its expression is both enhanced by Akt activation and is required for Akt activation through its effects on Grb10. These findings therefore reveal another level of control to HuR expression, and elucidate its role in a key pathway that has implications not only for cell survival under stress, but also for HuR’s role in promoting tumor growth.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Dina Ayupova and Dr. Brooke McMichael for helpful discussion and technical advice. They also thank Dr. Michael Ostrowski for providing the Akt plasmids used in this study, the Plant-Microbe Genomics Facility at The Ohio State University for aid in PCR array analysis, and Dr. Christopher Pelloski for author assistance. This work was supported by grants from the National Institutes of Health, R01 DK052131 and R01 DK052131-14S1 (to B.S.L.).
Contract grant sponsor: NIH/NIDDK
LITERATURE CITED
- Abdelmohsen K, Kim MM, Srikantan S, Mercken EM, Brennan SE, Wilson GM, Cabo R, Gorospe M. miR-519 suppresses tumor growth by reducing HuR levels. Cell Cycle. 2010;9(7):1354–1359. doi: 10.4161/cc.9.7.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelmohsen K, Lal A, Kim HH, Gorospe M. Posttranscriptional orchestration of an anti-apoptotic program by HuR. Cell Cycle. 2007;6(11):1288–1292. doi: 10.4161/cc.6.11.4299. [DOI] [PubMed] [Google Scholar]
- Al-Ahmadi W, Al-Ghamdi M, Al-Haj L, Al-Saif M, Khabar KS. Alternative polyadenylation variants of the RNA binding protein, HuR: abundance, role of AU-rich elements and auto-Regulation. Nucleic Acids Res. 2009;37(11):3612–3624. doi: 10.1093/nar/gkp223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreucci M, Michael A, Kramers C, Park KM, Chen A, Matthaeus T, Alessandrini A, Haq S, Force T, Bonventre JV. Renal ischemia/reperfusion and ATP depletion/repletion in LLC-PK(1) cells result in phosphorylation of FKHR and FKHRL1. Kidney Int. 2003;64(4):1189–1198. doi: 10.1046/j.1523-1755.2003.00204.x. [DOI] [PubMed] [Google Scholar]
- Atasoy U, Watson J, Patel D, Keene JD. ELAV protein HuA (HuR) can redistribute between nucleus and cytoplasm and is upregulated during serum stimulation and T cell activation. J Cell Sci. 1998;111(Pt 21):3145–3156. doi: 10.1242/jcs.111.21.3145. [DOI] [PubMed] [Google Scholar]
- Ayupova DA, Singh M, Leonard EC, Basile DP, Lee BS. Expression of the RNA-stabilizing protein HuR in ischemia-reperfusion injury of rat kidney. Am J Physiol Renal Physiol. 2009;297(1):F95–F105. doi: 10.1152/ajprenal.90632.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas S, Ollila J, Aventin A, Vihinen M, Sierra J, Knuutila S. Changes in apoptosis-related pathways in acute myelocytic leukemia. Cancer Genet Cytogenet. 2003;146(2):89–101. doi: 10.1016/s0165-4608(03)00102-x. [DOI] [PubMed] [Google Scholar]
- Charalambous M, Smith FM, Bennett WR, Crew TE, Mackenzie F, Ward A. Disruption of the imprinted Grb10 gene leads to disproportionate overgrowth by an Igf2-independent mechanism. Proc Natl Acad Sci U S A. 2003;100(14):8292–8297. doi: 10.1073/pnas.1532175100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Xing B, Liu X, Zhan B, Zhou J, Zhu H, Chen Z. Ischemic postconditioning inhibits apoptosis after renal ischemia/reperfusion injury in rat. Transpl Int. 2008;21(4):364–371. doi: 10.1111/j.1432-2277.2007.00606.x. [DOI] [PubMed] [Google Scholar]
- Dai W, Zhang G, Makeyev EV. RNA-binding protein HuR autoregulates its expression by promoting alternative polyadenylation site usage. Nucleic Acids Res. 2011 doi: 10.1093/nar/gkr783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilin S, Sourbier C, Thomas L, Lindner V, Rothhut S, Dormoy V, Helwig JJ, Jacqmin D, Lang H, Massfelder T. Role of the RNA-binding protein HuR in human renal cell carcinoma. Carcinogenesis. 2010;31(6):1018–1026. doi: 10.1093/carcin/bgq052. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13(22):2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Denkert C, Weichert W, Pest S, Koch I, Licht D, Kobel M, Reles A, Sehouli J, Dietel M, Hauptmann S. Overexpression of the embryonic-lethal abnormal vision-like protein HuR in ovarian carcinoma is a prognostic factor and is associated with increased cyclooxygenase 2 expression. Cancer Res. 2004a;64(1):189–195. doi: 10.1158/0008-5472.can-03-1987. [DOI] [PubMed] [Google Scholar]
- Denkert C, Weichert W, Winzer KJ, Muller BM, Noske A, Niesporek S, Kristiansen G, Guski H, Dietel M, Hauptmann S. Expression of the ELAV-like protein HuR is associated with higher tumor grade and increased cyclooxygenase-2 expression in human breast carcinoma. Clin Cancer Res. 2004b;10(16):5580–5586. doi: 10.1158/1078-0432.CCR-04-0070. [DOI] [PubMed] [Google Scholar]
- Fan XC, Steitz JA. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J. 1998;17(12):3448–3460. doi: 10.1093/emboj/17.12.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallouzi IE, Brennan CM, Steitz JA. Protein ligands mediate the CRM1-dependent export of HuR in response to heat shock. Rna. 2001;7(9):1348–1361. doi: 10.1017/s1355838201016089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield AS, Cowley M, Smith FM, Moorwood K, Stewart-Cox JE, Gilroy K, Baker S, Xia J, Dalley JW, Hurst LD, Wilkinson LS, Isles AR, Ward A. Distinct physiological and behavioural functions for parental alleles of imprinted Grb10. Nature. 2011;469(7331):534–538. doi: 10.1038/nature09651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Aguila HL, Michaud J, Ai Y, Wu MT, Hemmes A, Ristimaki A, Guo C, Furneaux H, Hla T. Essential role of the RNA-binding protein HuR in progenitor cell survival in mice. J Clin Invest. 2009;119(12):3530–3543. doi: 10.1172/JCI38263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Wu Y, Hartley RS. MicroRNA-125a represses cell growth by targeting HuR in breast cancer. RNA Biol. 2009;6(5):575–583. doi: 10.4161/rna.6.5.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, Peterson TR, Choi Y, Gray NS, Yaffe MB, Marto JA, Sabatini DM. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011;332(6035):1317–1322. doi: 10.1126/science.1199498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu ZQ, Zhang JY, Ji CN, Xie Y, Chen JZ, Mao YM. Grb10 interacts with Bim L and inhibits apoptosis. Mol Biol Rep. 2010;37(7):3547–3552. doi: 10.1007/s11033-010-0002-9. [DOI] [PubMed] [Google Scholar]
- Izquierdo JM. Hu antigen R (HuR) functions as an alternative pre-mRNA splicing regulator of Fas apoptosis-promoting receptor on exon definition. J Biol Chem. 2008;283(27):19077–19084. doi: 10.1074/jbc.M800017200. [DOI] [PubMed] [Google Scholar]
- Jahn T, Seipel P, Urschel S, Peschel C, Duyster J. Role for the adaptor protein Grb10 in the activation of Akt. Mol Cell Biol. 2002;22(4):979–991. doi: 10.1128/MCB.22.4.979-991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaraj S, Dakhlallah D, Hill SR, Lee BS. HuR stabilizes vacuolar H+-translocating ATPase mRNA during cellular energy depletion. J Biol Chem. 2005;280(45):37957–37964. doi: 10.1074/jbc.M502883200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaraj SC, Dakhlallah D, Hill SR, Lee BS. Expression and distribution of HuR during ATP depletion and recovery in proximal tubule cells. Am J Physiol Renal Physiol. 2006;291(6):F1255–F1263. doi: 10.1152/ajprenal.00440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaraj SC, Singh M, Ayupova DA, Govindaraju S, Lee BS. Transcriptional control of human antigen R by bone morphogenetic protein. J Biol Chem. 2010;285(7):4432–4440. doi: 10.1074/jbc.M109.062216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo JD, Kim M, D'Agati VD, Lee HT. Ischemic preconditioning provides both acute and delayed protection against renal ischemia and reperfusion injury in mice. J Am Soc Nephrol. 2006;17(11):3115–3123. doi: 10.1681/ASN.2006050424. [DOI] [PubMed] [Google Scholar]
- Kang MJ, Ryu BK, Lee MG, Han J, Lee JH, Ha TK, Byun DS, Chae KS, Lee BH, Chun HS, Lee KY, Kim HJ, Chi SG. NF-kappaB activates transcription of the RNA-binding factor HuR, via PI3K-AKT signaling, to promote gastric tumorigenesis. Gastroenterology. 2008;135(6):2030–2042. 2042, e2031–e2033. doi: 10.1053/j.gastro.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Katsanou V, Milatos S, Yiakouvaki A, Sgantzis N, Kotsoni A, Alexiou M, Harokopos V, Aidinis V, Hemberger M, Kontoyiannis DL. The RNA-binding protein Elavl1/HuR is essential for placental branching morphogenesis and embryonic development. Mol Cell Biol. 2009;29(10):2762–2776. doi: 10.1128/MCB.01393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebache S, Ash J, Annis MG, Hagan J, Huber M, Hassard J, Stewart CL, Whiteway M, Nantel A. Grb10 and active Raf-1 kinase promote Bad-dependent cell survival. J Biol Chem. 2007;282(30):21873–21883. doi: 10.1074/jbc.M611066200. [DOI] [PubMed] [Google Scholar]
- Khabar KS. The AU-rich transcriptome: more than interferons and cytokines, and its role in disease. J Interferon Cytokine Res. 2005;25(1):1–10. doi: 10.1089/jir.2005.25.1. [DOI] [PubMed] [Google Scholar]
- Kojima K, Fujita Y, Nozawa Y, Deguchi T, Ito M. MiR-34a attenuates paclitaxel-resistance of hormone-refractory prostate cancer PC3 cells through direct and indirect mechanisms. Prostate. 2010;70(14):1501–1512. doi: 10.1002/pros.21185. [DOI] [PubMed] [Google Scholar]
- Kuwana H, Terada Y, Kobayashi T, Okado T, Penninger JM, Irie-Sasaki J, Sasaki T, Sasaki S. The phosphoinositide-3 kinase gamma-Akt pathway mediates renal tubular injury in cisplatin nephrotoxicity. Kidney Int. 2008;73(4):430–445. doi: 10.1038/sj.ki.5002702. [DOI] [PubMed] [Google Scholar]
- Lebedeva S, Jens M, Theil K, Schwanhausser B, Selbach M, Landthaler M, Rajewsky N. Transcriptome-wide analysis of regulatory interactions of the RNA-binding protein HuR. Mol Cell. 2011;43(3):340–352. doi: 10.1016/j.molcel.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Levy NS, Chung S, Furneaux H, Levy AP. Hypoxic stabilization of vascular endothelial growth factor mRNA by the RNA-binding protein HuR. J Biol Chem. 1998;273(11):6417–6423. doi: 10.1074/jbc.273.11.6417. [DOI] [PubMed] [Google Scholar]
- Lim MA, Riedel H, Liu F. Grb10: more than a simple adaptor protein. Front Biosci. 2004;9:387–403. doi: 10.2741/1226. [DOI] [PubMed] [Google Scholar]
- Liu F, Roth RA. Grb-IR: a SH2-domain-containing protein that binds to the insulin receptor and inhibits its function. Proc Natl Acad Sci U S A. 1995;92(22):10287–10291. doi: 10.1073/pnas.92.22.10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez de Silanes I, Fan J, Yang X, Zonderman AB, Potapova O, Pizer ES, Gorospe M. Role of the RNA-binding protein HuR in colon carcinogenesis. Oncogene. 2003;22(46):7146–7154. doi: 10.1038/sj.onc.1206862. [DOI] [PubMed] [Google Scholar]
- Lopez de Silanes I, Lal A, Gorospe M. HuR: post-transcriptional paths to malignancy. RNA Biol. 2005;2(1):11–13. doi: 10.4161/rna.2.1.1552. [DOI] [PubMed] [Google Scholar]
- Lopez de Silanes I, Zhan M, Lal A, Yang X, Gorospe M. Identification of a target RNA motif for RNA-binding protein HuR. Proc Natl Acad Sci U S A. 2004;101(9):2987–2992. doi: 10.1073/pnas.0306453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loverre A, Ditonno P, Crovace A, Gesualdo L, Ranieri E, Pontrelli P, Stallone G, Infante B, Schena A, Di Paolo S, Capobianco C, Ursi M, Palazzo S, Battaglia M, Selvaggi FP, Schena FP, Grandaliano G. Ischemia-reperfusion induces glomerular and tubular activation of proinflammatory and antiapoptotic pathways: differential modulation by rapamycin. J Am Soc Nephrol. 2004;15(10):2675–2686. doi: 10.1097/01.ASN.0000139932.00971.E4. [DOI] [PubMed] [Google Scholar]
- Martelli AM, Faenza I, Billi AM, Manzoli L, Evangelisti C, Fala F, Cocco L. Intranuclear 3'-phosphoinositide metabolism and Akt signaling: new mechanisms for tumorigenesis and protection against apoptosis? Cell Signal. 2006;18(8):1101–1107. doi: 10.1016/j.cellsig.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Mazan-Mamczarz K, Hagner PR, Corl S, Srikantan S, Wood WH, Becker KG, Gorospe M, Keene JD, Levenson AS, Gartenhaus RB. Post-transcriptional gene regulation by HuR promotes a more tumorigenic phenotype. Oncogene. 2008;27(47):6151–6163. doi: 10.1038/onc.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, Liu L, Chin PC, D'Mello SR. Akt is a downstream target of NF-kappa B. J Biol Chem. 2002;277(33):29674–29680. doi: 10.1074/jbc.M112464200. [DOI] [PubMed] [Google Scholar]
- Mirmohammadsadegh A, Baer A, Nambiar S, Bardenheuer W, Hengge UR. Rapid identification of dysregulated genes in cutaneous malignant melanoma metastases using cDNA technology. Cells Tissues Organs. 2004;177(3):119–123. doi: 10.1159/000079985. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Rubio M, Sussman MA. Nuclear and mitochondrial signalling Akts in cardiomyocytes. Cardiovasc Res. 2009;82(2):272–285. doi: 10.1093/cvr/cvp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrione A, Valentinis B, Li S, Ooi JY, Margolis B, Baserga R. Grb10: A new substrate of the insulin-like growth factor I receptor. Cancer Res. 1996;56(14):3165–3167. [PubMed] [Google Scholar]
- Moutoussamy S, Renaudie F, Lago F, Kelly PA, Finidori J. Grb10 identified as a potential regulator of growth hormone (GH) signaling by cloning of GH receptor target proteins. J Biol Chem. 1998;273(26):15906–15912. doi: 10.1074/jbc.273.26.15906. [DOI] [PubMed] [Google Scholar]
- Mukherjee N, Corcoran DL, Nusbaum JD, Reid DW, Georgiev S, Hafner M, Ascano M, Jr, Tuschl T, Ohler U, Keene JD. Integrative regulatory mapping indicates that the RNA-binding protein HuR couples pre-mRNA processing and mRNA stability. Mol Cell. 2011;43(3):327–339. doi: 10.1016/j.molcel.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabors LB, Gillespie GY, Harkins L, King PH. HuR, a RNA stability factor, is expressed in malignant brain tumors and binds to adenine- and uridine-rich elements within the 3' untranslated regions of cytokine and angiogenic factor mRNAs. Cancer Res. 2001;61(5):2154–2161. [PubMed] [Google Scholar]
- Okino K, Konishi H, Doi D, Yoneyama K, Ota Y, Jin E, Kawanami O, Takeshita T. Up-regulation of growth factor receptor-bound protein 10 in cervical squamous cell carcinoma. Oncol Rep. 2005;13(6):1069–1074. [PubMed] [Google Scholar]
- Pandey A, Duan H, Di Fiore PP, Dixit VM. The Ret receptor protein tyrosine kinase associates with the SH2-containing adapter protein Grb10. J Biol Chem. 1995;270(37):21461–21463. doi: 10.1074/jbc.270.37.21461. [DOI] [PubMed] [Google Scholar]
- Riedel H. Grb10 exceeding the boundaries of a common signaling adapter. Front Biosci. 2004;9:603–618. doi: 10.2741/1227. [DOI] [PubMed] [Google Scholar]
- Satake A, Takaoka M, Nishikawa M, Yuba M, Shibata Y, Okumura K, Kitano K, Tsutsui H, Fujii K, Kobuchi S, Ohkita M, Matsumura Y. Protective effect of 17beta-estradiol on ischemic acute renal failure through the PI3K/Akt/eNOS pathway. Kidney Int. 2008;73(3):308–317. doi: 10.1038/sj.ki.5002690. [DOI] [PubMed] [Google Scholar]
- Sharples EJ, Patel N, Brown P, Stewart K, Mota-Philipe H, Sheaff M, Kieswich J, Allen D, Harwood S, Raftery M, Thiemermann C, Yaqoob MM. Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion. J Am Soc Nephrol. 2004;15(8):2115–2124. doi: 10.1097/01.ASN.0000135059.67385.5D. [DOI] [PubMed] [Google Scholar]
- Shiraishi I, Melendez J, Ahn Y, Skavdahl M, Murphy E, Welch S, Schaefer E, Walsh K, Rosenzweig A, Torella D, Nurzynska D, Kajstura J, Leri A, Anversa P, Sussman MA. Nuclear targeting of Akt enhances kinase activity and survival of cardiomyocytes. Circ Res. 2004;94(7):884–891. doi: 10.1161/01.RES.0000124394.01180.BE. [DOI] [PubMed] [Google Scholar]
- Wang L, Balas B, Christ-Roberts CY, Kim RY, Ramos FJ, Kikani CK, Li C, Deng C, Reyna S, Musi N, Dong LQ, DeFronzo RA, Liu F. Peripheral disruption of the Grb10 gene enhances insulin signaling and sensitivity in vivo. Mol Cell Biol. 2007;27(18):6497–6505. doi: 10.1128/MCB.00679-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Brattain MG. AKT can be activated in the nucleus. Cell Signal. 2006;18(10):1722–1731. doi: 10.1016/j.cellsig.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Wang W, Furneaux H, Cheng H, Caldwell MC, Hutter D, Liu Y, Holbrook N, Gorospe M. HuR regulates p21 mRNA stabilization by UV light. Mol Cell Biol. 2000;20(3):760–769. doi: 10.1128/mcb.20.3.760-769.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Zheng X, Qin J, Chen Z, Jin Y, Ding W. Role of PI3-kinase/Akt signalling pathway in renal function and cell proliferation after renal ischaemia/reperfusion injury in mice. Nephrology (Carlton) 2006;11(3):207–212. doi: 10.1111/j.1440-1797.2006.00558.x. [DOI] [PubMed] [Google Scholar]
- Xu F, Zhang X, Lei Y, Liu X, Liu Z, Tong T, Wang W. Loss of repression of HuR translation by miR-16 may be responsible for the elevation of HuR in human breast carcinoma. J Cell Biochem. 2010;111(3):727–734. doi: 10.1002/jcb.22762. [DOI] [PubMed] [Google Scholar]
- Yaman I, Fernandez J, Sarkar B, Schneider RJ, Snider MD, Nagy LE, Hatzoglou M. Nutritional control of mRNA stability is mediated by a conserved AU-rich element that binds the cytoplasmic shuttling protein HuR. J Biol Chem. 2002;277(44):41539–41546. doi: 10.1074/jbc.M204850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeap BB, Voon DC, Vivian JP, McCulloch RK, Thomson AM, Giles KM, Czyzyk-Krzeska MF, Furneaux H, Wilce MC, Wilce JA, Leedman PJ. Novel binding of HuR and poly(C)-binding protein to a conserved UC-rich motif within the 3'-untranslated region of the androgen receptor messenger RNA. J Biol Chem. 2002;277(30):27183–27192. doi: 10.1074/jbc.M202883200. [DOI] [PubMed] [Google Scholar]
- Yu Y, Yoon SO, Poulogiannis G, Yang Q, Ma XM, Villen J, Kubica N, Hoffman GR, Cantley LC, Gygi SP, Blenis J. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science. 2011;332(6035):1322–1326. doi: 10.1126/science.1199484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.