Abstract
Renal ischemia reperfusion injury is a major cause of acute kidney injury. We previously found that renal A1 adenosine receptor (A1AR) activation attenuated multiple cell death pathways including necrosis, apoptosis and inflammation. Here, we tested whether induction of cytoprotective sphingosine kinase (SK)-1 and sphingosine-1 phosphate (S1P) synthesis might be the mechanism of protection. A selective A1AR agonist (CCPA) increased the synthesis of S1P and selectively induced SK-1 in mouse kidney and HK-2 cells. This agonist failed to protect SK1-knockout but protected SK2-knockout mice against renal ischemia reperfusion injury indicating a critical role of SK1 in A1AR-mediated renal protection. Inhibition of SK prevented A1AR-mediated defense against necrosis and apoptosis in HK-2 cells. A selective S1P1R antagonist (W146) and global in vivo gene knockdown of S1P1Rs with small interfering RNA completely abolished the renal protection provided by CCPA. Mice selectively deficient in renal proximal tubule S1P1Rs (S1P1Rflox/flox PEPCKCre/−) were not protected against renal ischemia reperfusion injury by CCPA. Mechanistically, CCPA increased nuclear translocation of hypoxia inducible factor-1α in HK-2 cells and selective hypoxia inducible factor-1α inhibition blocked A1AR-mediated induction of SK1. Thus, proximal tubule SK-1 has a critical role in A1AR-mediated protection against renal ischemia reperfusion injury.
Keywords: apoptosis, hypoxia inducible factor-1α, inflammation, necrosis, sphingosine 1-phosphate
Introduction
Renal ischemia-reperfusion (IR) injury is a frequent cause of acute kidney injury (AKI) [1]. AKI a major clinical problem often leading to multi-organ dysfunction and systemic inflammation with extremely high mortality [2]. Unfortunately, the severity and incidence of AKI has been increasing without any improvements in therapy or patient survival over the past 50 years [3]. Currently, the incidence of renal dysfunction after major surgery in high risk patients has been reported to be as high as 70–80% [4–6]. Despite continued research on renal protective strategies, there are no proven therapies to reduce AKI in the perioperative setting.
We have previously demonstrated that A1 adenosine receptor (AR) activation reduced necrosis and apoptosis in cultured proximal tubule cells [7,8]. Moreover, A1AR activation immediately before renal ischemia protected against renal IR injury in rats and mice in vivo [9,10]. In addition to reduction in renal necrosis and apoptosis [9,11], we also observed a surprising reduction in renal inflammation (reduced leukocyte influx and pro-inflammatory cytokine upregulation) as an important component of renal protection with A1AR activation. Unlike the better characterized and traditionally understood anti-inflammatory mechanisms of A2aARs, the anti-inflammatory mechanisms of A1ARs remain unclear. Therefore, we hypothesized that A1AR activation may induce additional mediator(s) that produce direct anti-inflammatory effects to protect against renal IR.
Phosphorylation of sphingosine by 2 subtypes of sphingosine kinase (SK1 and SK2) leads to the formation of sphingosine-1-phosphate (S1P), a lysophospholipid targeting G-protein coupled receptors with diverse extracellular as well as intracellular effects [12–14]. In particular, renal tubular SK1 activation has been shown to produce renoprotection after IR [15–17]. Moreover, of 5 G-protein coupled S1P receptors (S1PR), S1P1R activation has been shown to counteract against cardiac [18,19] and renal IR injury [17,20] and attenuates T-lymphocytes-mediated tissue inflammation [13,21,22]. Furthermore, S1P1R agonists produce renal protection via direct activation of S1P1Rs in renal proximal tubules [23].
In this study, we tested the hypothesis that renal tubular A1AR activation produces anti-inflammatory and cytoprotective S1P via activation of renal proximal tubule SK. We utilized genetically modified strains of mice lacking SK1 (SK1−/−) and SK2 (SK2−/−) enzyme. In addition, we tested that activation of S1P1R via A1AR-mediated S1P generation is critical in renal protection utilizing mice treated with a selective S1P1R antagonist (W146) as well as mice treated with siRNA designed for in vivo targeting of S1P1R. To investigate the direct involvement of proximal tubule S1P1R in A1AR agonist-mediated renal protection in vivo, we generated mice with proximal tubule specific deletion of S1P1R by crossing mice carrying the floxed S1P1R gene [12] with mice expressing Cre recombinase under the control of the phosphoenolpyruvate carboxykinase promoter [24]. Finally, we examined the mechanisms of A1AR-mediated renal proximal tubule SK activation. Utilizing cultured human proximal tubule cells in vitro, we tested the hypothesis that hypoxia inducible factor-1α (HIF-1α) plays a critical role in mediating A1AR-mediated induction of SK1.
Results
A1AR activation or overexpression increases SK1 synthesis and induces SK activity in mouse kidney
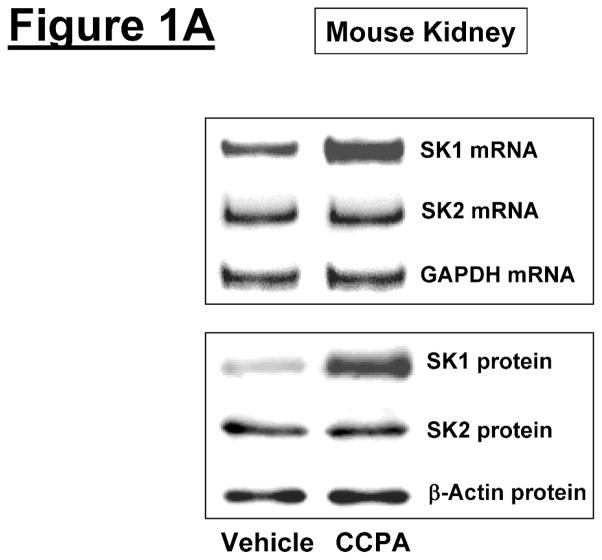
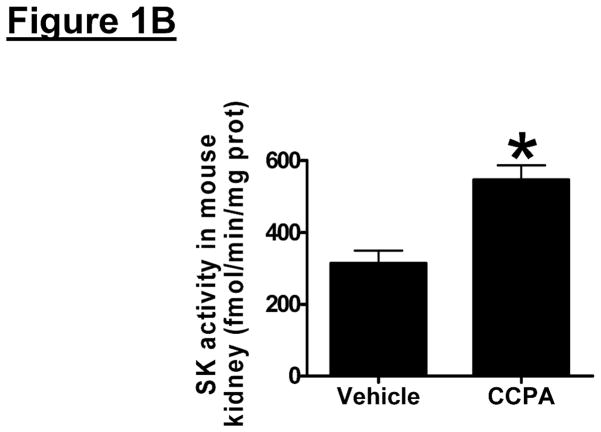
We initially tested whether A1AR activation increased SK1 expression and activity in mouse kidney (cortex and cortico-medullary junction). Figure 1A and 1B show that a selective A1AR agonist CCPA (0.1 mg/kg, i.p.) increased SK1 mRNA (measured at 6 hr) and protein expression (measured 16 hr) and upregulated SK activity (measured at 6 hr) in mouse kidney. In contrast, the A1AR agonist CCPA failed to increase SK2 synthesis in mouse kidney. We measured preferentially SK1 activity by adding Triton x-100 in our SK activity assay as described by Vessey et al. [25].
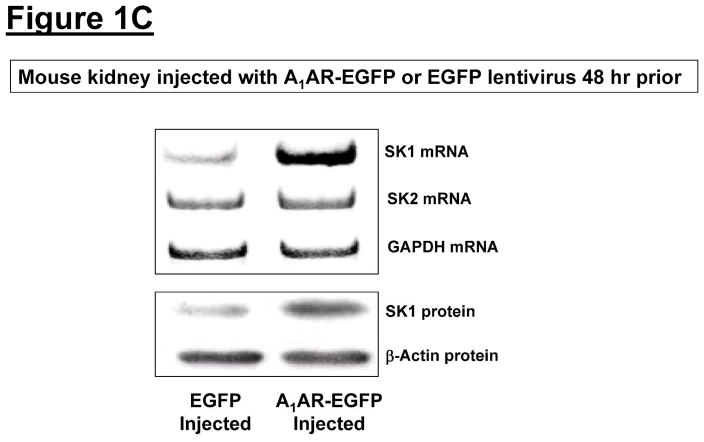
Figure 1. A1AR activation or overexpression increases sphingosine kinase (SK)-1 expression and activity in mouse kidney.
A. Representative bands for SK1 and SK2 mRNA (RT-PCR, 6 hr) and protein (immunoblotting, 16 hr) expression in mouse kidney treated with vehicle (0.4% DMSO in saline) or CCPA (0.1 mg/kg, i.p.) (N=4 for each group). B. Mouse kidney SK activity 6 hr after vehicle (0.4% DMSO) or CCPA (0.1 mg/kg, i.p.) treatment (N=4 for each group). Data are presented as means ± SEM. *P<0.05 vs. vehicle-treated mice. C. Representative bands for SK1 and SK2 mRNA (RT-PCR) and SK1 protein (immunoblotting) expression in mouse kidney injected with EGFP or A1AR-EGFP lentivirus 48 hr prior (N=4 for each group).
Next we aimed to determine whether overexpression of renal A1ARs increased SK1 expression in mouse kidney. We previously demonstrated that intrarenal A1AR-EGFP lentivirus transduction resulted in a selective and robust A1AR overexpression in the injected kidneys within 48 hrs [26]. In this study, we show that kidneys injected with A1AR-EGFP lentivirus 48 hrs prior showed increased SK1 mRNA and protein expression compared to EGFP lentivirus injected kidneys (Figure 1C). SK2 mRNA expression did not change with A1AR-EGFP lentivirus injection.
SK1 activation is critical for A1AR-mediated protection against renal IR
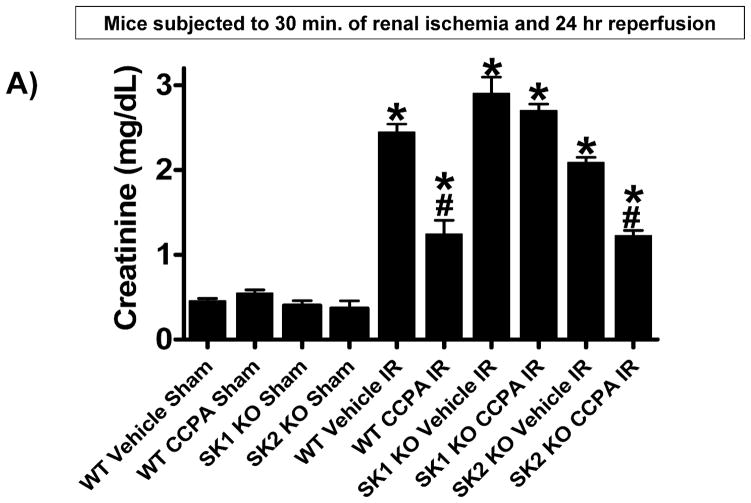
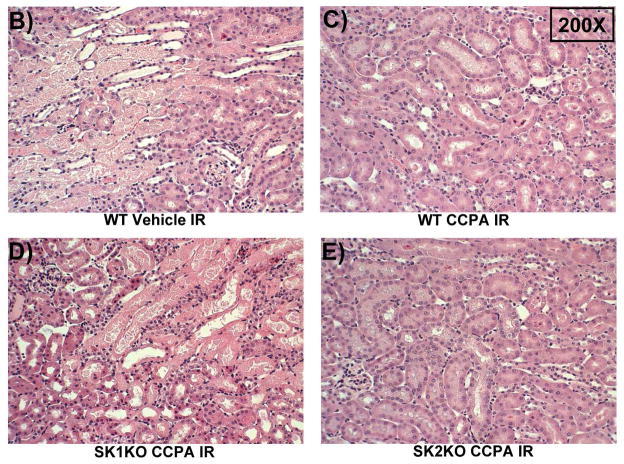
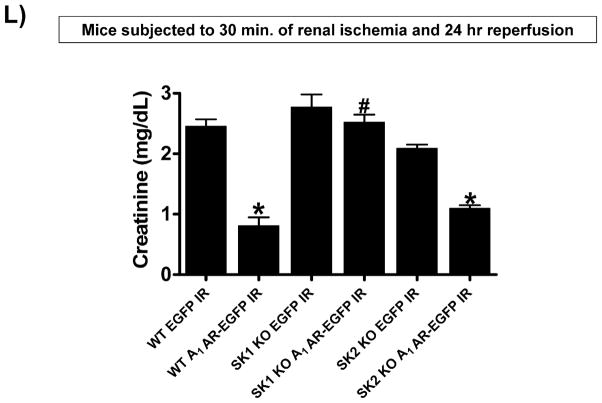
Next, we tested whether SK1 activation is critical in A1AR-mediated protection against renal IR. Plasma creatinine values were similar between sham-operated (anesthesia, laparotomy, right nephrectomy and recovery) WT (Cr=0.4±0.03 mg/dL, N=3), SK1−/− (SK1 KO, Cr=0.4±0.06 mg/dL, N=3), and SK2−/− (SK2 KO, Cr=0.4±0.09 mg/dL, N=3) mice. Plasma creatinine increased in WT, SK1−/−, and SK2−/− mice subjected to 30 min. renal IR compared to sham-operated mice (Figure 2A). As we described previously, SK1−/− and SK2−/− mice had slightly increased and decreased renal injury, respectively, compared to WT mice [17]. Treatment with CCPA significantly attenuated the increases in plasma creatinine in WT and SK2−/− mice but not in SK1−/− mice (Figure 2A). Figure 2B demonstrates increased histological injury in WT mice 24 hr after renal IR. Compared to sham-operated mice (not shown), the kidneys of WT mice subjected to renal IR showed significant tubular vacuolization, proteinaceous casts with increased tubular dilatation, congestion and simplification (Figure 2B). Consistent with the plasma creatinine data, WT mice or SK2−/− mice treated with CCPA had reduced renal necrosis and tubular injury (Figure 2C and Figure 2E). In contrast, SK1−/− mice were not protected with CCPA and continued to show histological signs of severe renal tubular injury (Figure 2D).
Figure 2. SK1 is critical for A1AR-mediated renal protection against ischemic AKI.
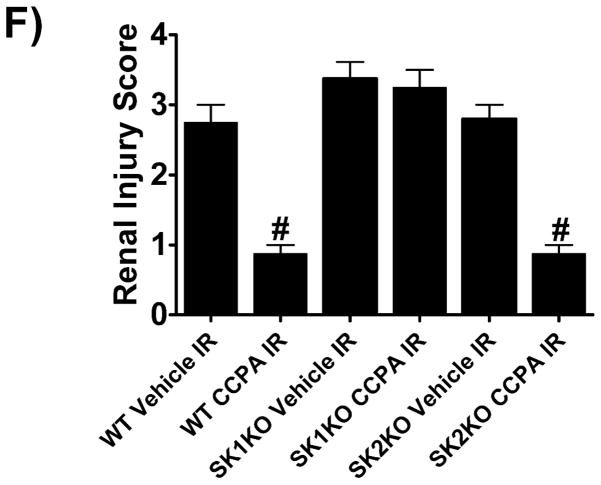
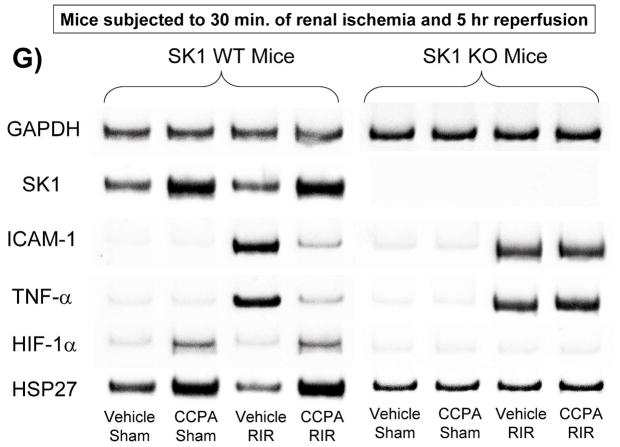
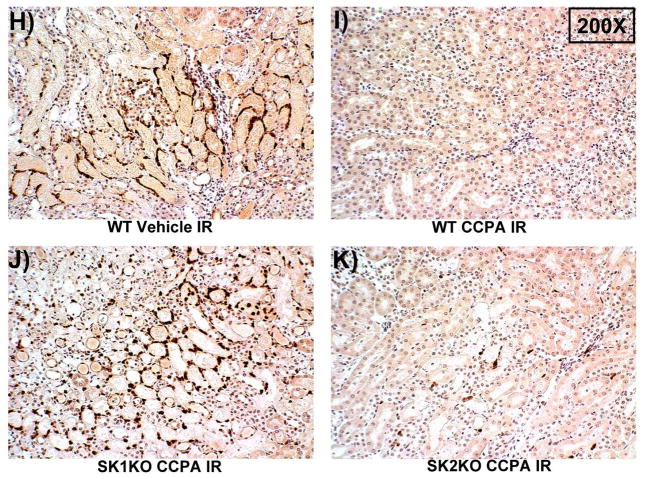
A. Plasma creatinine levels (24 hrs after surgery) from wild type (WT), SK1−/− (SK1 KO) or SK2−/− (SK2 KO) mice subjected to sham-surgery (N=4) or to renal ischemia and reperfusion (IR) after vehicle (0.4% DMSO in saline) or a selective A1AR agonist treatment (CCPA, 0.1 mg/kg, i.p. 15 min before renal ischemia) treatment (N=6 for each group). Treatment with CCPA significantly attenuated the increases in plasma creatinine in WT and SK2−/− mice but not in SK1−/− mice. *P<0.05 vs. vehicle-treated mice subjected to sham-surgery. #P<0.05 vs. vehicle-treated mice subjected to renal IR. B–E. Representative photomicrographs (magnification 200X) hematoxylin and eosin staining of kidney sections of mice subjected to 30 min of renal ischemia followed by 24 hr of reperfusion. Photographs are representative of 4 independent experiments. Vehicle-treated wild-type mice (WT Vehicle IR) showed severe renal tubular necrosis after renal IR (B). CCPA treatment significantly attenuated renal IR injury in WT (C) and SK2−/− mice (E) but not in SK1−/− (D) mice. F. Summary of Jablonski scale renal injury scores (scale 0–4) for mice subjected to sham-operation or renal IR. #P<0.05 vs. vehicle-treated mice subjected to renal IR. Error bars represent 1 SEM. G. Representative RT-PCR bands of TNF-α, ICAM-1, SK1, HIF-1α and HSP27 mRNA in the kidney tissues from SK1+/+ (WT) or SK1−/− (KO) mice (N=4 for each group). Mice were treated with vehicle (0.4% DMSO in saline) or with a selective A1AR agonist (CCPA, 0.1 mg/kg, i.p. 15 min before renal ischemia or sham-operation) and then subjected to sham operation or to 30 min of renal ischemia and 5 hr of reperfusion. Treatment with CCPA induced SK1 and HSP27 mRNA and reduced induction of pro-inflammatory TNF-α and ICAM-1 mRNA expression after renal IR in SK1+/+ mice but not in SK1−/− mice. H-K. Representative immunohistochemistry (magnification 200X) for neutrophil infiltration of kidney sections of mice subjected to 30 min of renal ischemia followed by 24 hr of reperfusion. Photographs are representative of 4 independent experiments. Vehicle-treated wild-type mice (WT Vehicle IR, H) showed many neutrophils after renal IR. CCPA treatment attenuated neutrophil infiltration in WT (I) and SK2−/− mice (K) but not in SK1−/− (J) mice. Secondary antibody conjugated to horseradish peroxidase was developed with diaminobenzidine to stain neutrophils dark brown. L. WT and mice deficient in SK1 or SK2 were renally injected with lentivirus endocing EGFP or A1AR-EGFP 48 hrs prior. WT mice renally injected with A1AR-EGFP lentivirus were protected against renal IR injury (N=4) when compared to EGFP injected WT mice (N=4). In contrast, SK1−/− mice (SK1 KO) renally injected with A1AR-EGFP lentivirus were not protected against renal IR (N=4) when compared to EGFP injected SK1−/− mice (N=4). In contrast, SK2−/− (SK2 KO) mice were protected against renal IR after A1AR-EGFP lentivirus injection (N=4). *P<0.05 vs. EGFP-injected subjected to renal IR. #P<0.05 vs. A1AR-EGFP-injected mice subjected to renal IR
The Jablonski scale [27] renal injury score (RIS) histology grading was used to grade renal tubular necrosis 24 hr after renal IR (Figure 2F). Thirty minutes of renal ischemia and 24 hr of reperfusion resulted in severe acute tubular necrosis in vehicle-treated WT, SK1−/− or SK2−/− mice. In contrast, WT or SK2−/− mice treated with CCPA had significantly lower renal injury scores compared to vehicle-treated mice subjected to renal IR. Consistent with plasma creatinine and histology data, SK1−/− mice treated with CCPA failed to improve the RIS (Figure 2F).
Our data also demonstrate that a selective A1AR agonist CCPA induced SK1, HIF-1α as well as HSP27 and reduced pro-inflammatory mRNA (ICAM-1 and TNF-α) expression in kidneys from WT mice but not in kidneys from SK1−/− mice (SK1 KO, Figure 2G). Finally, we show that CCPA reduced neutrophil infiltration in kidneys from WT and SK2−/− mice but not in kidneys from SK1−/− mice (Figure 2H–K). Therefore, CCPA-mediated reduction in renal inflammation (pro-inflammatory ICAM-1 and TNF-α mRNA expression and neutrophil infiltration) requires SK1.
Finally, WT mice renally injected with A1AR-EGFP lentivirus 48 hrs prior were protected against renal IR injury (Figure 2L) when compared to EGFP injected WT mice. Consistent with the hypothesis that A1AR-mediated induction of SK1 mediates the renal protection in A1AR-EGFP renally injected mice, SK1−/− renally injected with A1AR-EGFP lentivirus were not protected against renal IR when compared to EGFP injected SK1−/− mice. In contrast, SK2−/− mice were protected against renal IR after A1AR-EGFP lentivirus injection (Figure 2L).
Critical role of S1P1R in A1AR-mediated protection against renal IR
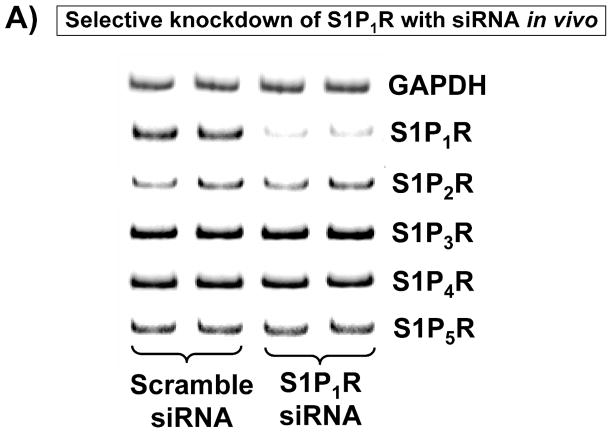
We first treated WT mice with a selective S1P1R antagonist W146 or with an siRNA designed for in vivo targeting of S1P1R. Figure 3A demonstrates significantly reduced S1P1R mRNA expression in the kidneys of mice treated with siRNA targeting S1P1R compared to scrambled control siRNA-treated mice. Other S1PR mRNAs (S1P2R, S1P3R, S1P4R and S1P5R) in the kidney were not affected by S1P1R siRNA treatment.
Figure 3. Critical role of S1P1R in A1AR-mediated protection against renal IR.
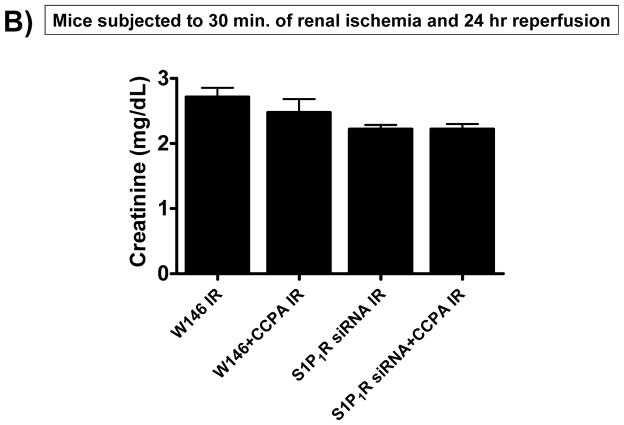
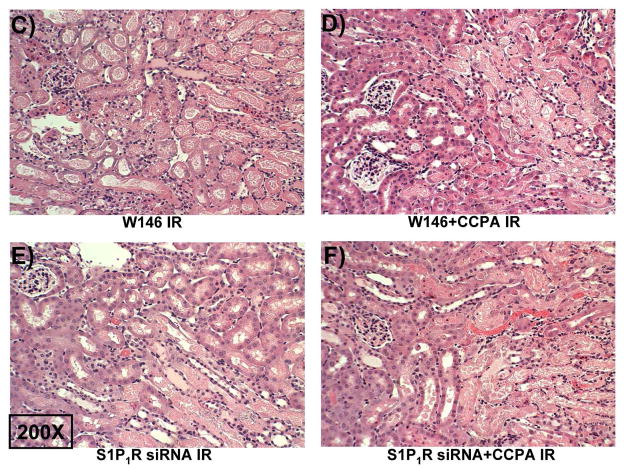
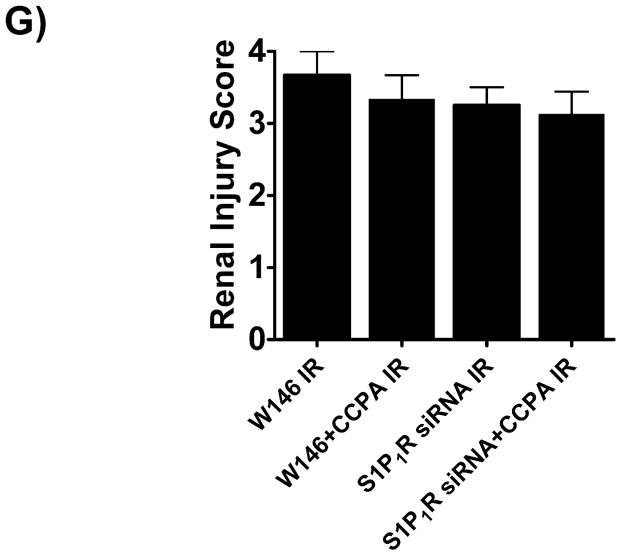
A. Evidence for in vivo knockdown of the S1P1R with siRNA treatment. Representative RT-PCR bands of S1PR subtype mRNA (normalized to GAPDH) in mouse kidney treated with scramble siRNA or siSTABLE™ targeting S1P1R. Mice were injected with scrambled control siRNA or siRNA targeting the S1P1R 48 hr prior. Mice injected with siSTABLE™ targeting S1P1R show selective reduction in S1P1R mRNA without affecting S1P2–5R mRNAs. B. Plasma creatinine levels from mice treated with vehicle (0.4% DMSO in saline) or with a selective A1AR agonist (CCPA, 0.1 mg/kg, i.p.) and subjected to sham operation or to 30 min of renal ischemia and 24 hr of reperfusion. A selective S1P1R antagonist (W146, 0.1 mg/kg, i.p., 15 min prior to renal ischemia) or siRNA for S1P1R (50 μg i.v., 48 hr before renal ischemia) prevented renal protection achieved with CCPA (N=4–6 for each group). C–G. Representative photomicrographs (C–F, N=4 for each group, magnification 200X) and renal injury scores (G) of hematoxylin and eosin staining of kidney sections of mice treated with W146 (C, D) or with siRNA for S1P1R (E, F). Blockade of S1P1R with W146 or in vivo knock down of S1P1R prevented CCPA-mediated protection against necrosis. Data are presented as means ± SEM. *P<0.05 vs. vehicle-treated sham operated mice. #P<0.05 vs. vehicle-treated mice subjected to renal IR.
A selective A1AR agonist (CCPA) failed to protect against renal IR injury in mice treated with an antagonist for S1P1R (W146) or with S1P1R siRNA (Figure 3B). Significant renal injury was evident in mice treated with W146 or S1P1R siRNA and subjected to renal IR (Figures 3C and 3E) with severe tubular necrosis, corticomedullary congestion and hemorrhage, and development of proteinaceous casts (30 min. renal ischemia and 24 hr reperfusion). Importantly, both W146 as well as S1P1R siRNA prevented the renal protection provided by CCPA (Figures 3D and 3F). The Jablonski scale renal injury score (RIS) histology grading also confirmed lack of CCPA-mediated renal protection in W146 or S1P1R siRNA treated mice (Figure 3G).
Critical role of renal proximal tubule S1P1R in A1AR-mediated renal protection
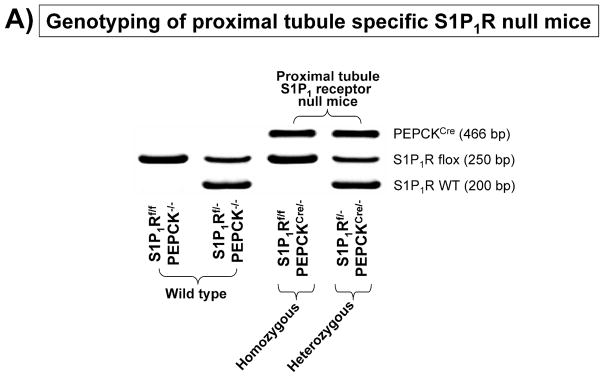
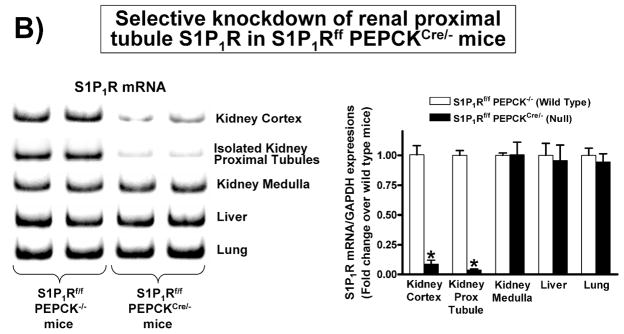
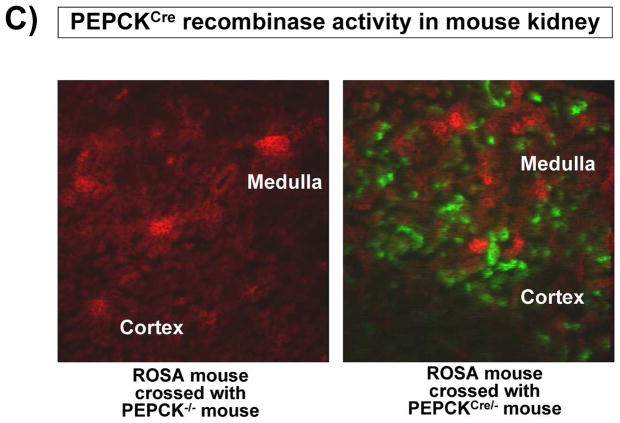
To investigate the direct involvement of proximal tubule S1P1R in A1AR agonist-mediated renal protection after IR, we generated mice with proximal tubule specific deletion of S1P1R by crossing mice carrying the floxed S1P1R gene (S1P1Rf/f mice, provided by Dr. Richard Proia (NIH) [12]) with PEPCKCre recombinase mice (Cre recombinase under the control of the phosphoenolpyruvate carboxykinase promoter, provided by Dr. Volker Haase, Vanderbilt University [24]). The PEPCKCre transgenic mice have ~10-fold increase in PEPCK expression in renal proximal tubules [24]. Mating S1P1Rf/f mice with PEPCKCre/− mice resulted in mice carrying floxed S1P1R with (null) or without (wild type) PEPCK Cre recombinase. Tail PCR confirmed the genotypes of proximal tubule specific S1P1R null mice (S1P1Rf/f PEPCKCre/−) and the control transgenic mice (S1P1Rf/f or PEPCKCre/−) generated (Figure 4A). To confirm selective deletion of S1P1R in renal proximal tubules from S1P1Rf/f PEPCKCre/− mice, S1P1R mRNA levels in freshly isolated proximal tubules (with percoll gradient separation [28]) as well as in kidney cortex, inner medulla, liver and lung were measured with conventional and real time RT-PCR. A representative gel image of PCR products and the quantified mRNA levels (Figure 4B) show a drastically decreased S1P1R mRNA (>90%) in renal cortex and proximal tubules from proximal tubule specific S1P1R null mice (S1P1Rf/f PEPCKCre/−) compared to the wild type control (S1P1Rf/f PEPCK −/−) mice. S1P1R mRNA levels in the kidney inner medulla, liver, and lung did not change with proximal tubule specific S1P1R deletion. Other S1PR subtypes (S1P2-5R) also did not change with proximal tubule specific S1P1R deletion (data not shown). We also crossed PEPCKCre mice with ROSA26Sortm4(ACTB-tdTomato,-EGFP)Luo/J mice to confirm specific Cre recombinase activity. Figure 4C shows kidney EGFP reporter immunofluorescence expression indicative of Cre recombinase activity in ROSA26Sortm4(ACTB-tdTomato,-EGFP)Luo/J mouse crossed with PEPCKCre/− mouse.
Figure 4. Critical role of renal proximal tubule S1P1R in A1AR-mediated protection against renal IR.
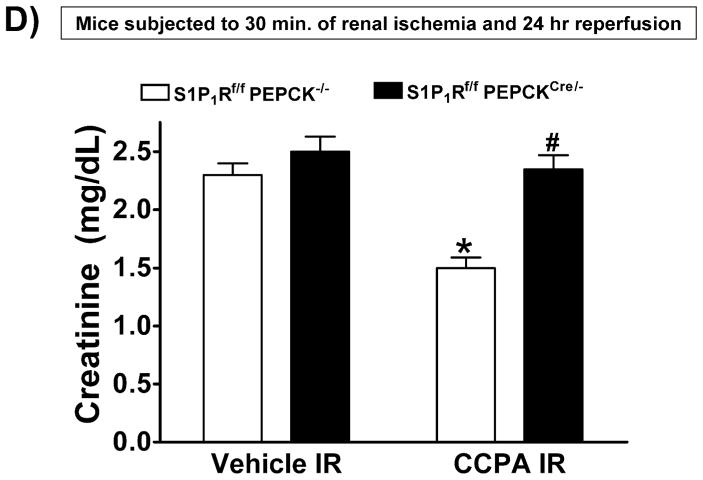
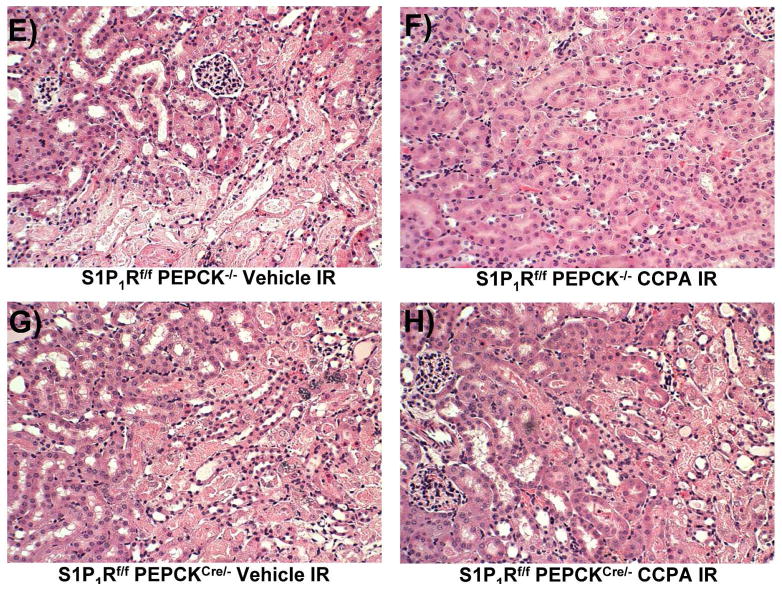
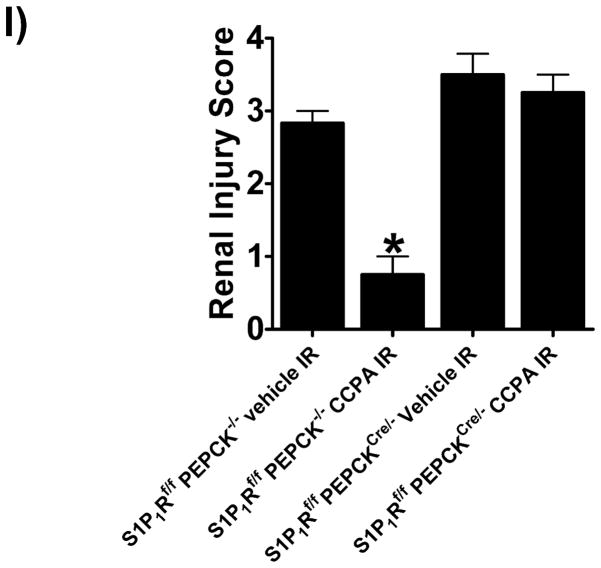
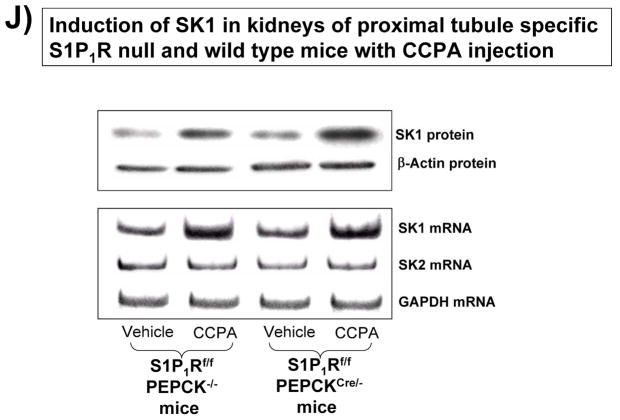
A. Tail PCR confirming the genotypes of proximal tubule specific S1P1R null mice (S1P1Rf/f PEPCKCre/− or S1P1Rf/− PEPCKCre/−) and the control transgenic mice (S1P1Rf/f or PEPCKCre/−). B. Selective deletion of S1P1R in renal proximal tubules in S1P1Rf/f PEPCKCre/− mice. S1P1R mRNA levels in freshly isolated proximal tubules as well as in kidney cortex, inner medulla, liver and lung were measured with conventional (left panel, representative gel image of PCR products) and real time (right panel) RT-PCR. Proximal tubule specific S1P1R null mice (S1P1Rf/f PEPCKCre/−) show a drastically decreased S1P1R mRNA (>90%) in renal cortex and proximal tubules from compared to the wild type control (S1P1Rf/f PEPCK −/−) mice. S1P1R mRNA levels in the kidney inner medulla, liver, and lung did not change with proximal tubule specific S1P1R deletion. *P<0.05 vs. S1P1Rf/f PEPCK−/− mice. C. Kidney EGFP reporter expression indicative of Cre recombinase activity (green fluorescence) in ROSA26Sortm4(ACTB-tdTomato,-EGFP)Luo/J mouse (red fluorescence) crossed with PEPCKCre/− mouse. Representative of 3 mice generated. D. Plasma creatinine from wild type (S1P1Rf/f PEPCK−/−) or from proximal tubule specific S1P1R null mice (S1P1Rf/f PEPCKCre/−) subjected to 30 min of renal ischemia and 24 hr of reperfusion. Treatment with a selective A1AR agonist (CCPA, 0.1 mg/kg, i.p. 15 min before renal ischemia) failed to reduce plasma creatinine level in S1P1R null mice subjected to renal IR. *P<0.05 vs. vehicle-treated S1P1Rf/f PEPCK−/− mice. #P<0.05 vs. CCPA-treated S1P1Rf/f PEPCK−/− mice. E–I. Representative photomicrographs (E–H, N=4 for each group, magnification 200X) and renal injury scores (K) of hematoxylin and eosin staining of kidney sections from wild type (S1P1Rf/f PEPCK−/−) or from proximal tubule specific S1P1R null mice (S1P1Rf/f PEPCKCre/−) subjected to 30 min of renal ischemia and 24 hr of reperfusion. Vehicle-treated S1P1Rf/f PEPCK−/− mice show severe renal tubular necrosis after renal IR (E, I). CCPA treatment significantly attenuated renal IR injury in S1P1Rf/f PEPCK−/− mice (F, I) but not in S1P1Rf/f PEPCKCre/− mice (H, I). *P<0.05 vs. vehicle-treated S1P1Rf/f PEPCK−/− mice. All data are presented as means ± SEM. J. Induction of SK1 in kidneys from wild type (S1P1Rf/f PEPCK−/−) or from proximal tubule specific S1P1R null mice (S1P1Rf/f PEPCKCre/−) injected with a selective A1AR agonist (CCPA). Representative of 3 experiments.
Figure 4D shows plasma creatinine from wild type (S1P1Rf/f PEPCK−/−) or from proximal tubule specific S1P1R null mice (S1P1Rf/f PEPCKCre/−) subjected to 30 min of renal ischemia and 24 hr of reperfusion. Treatment with a selective A1AR agonist (CCPA, 0.1 mg/kg, i.p. 15 min before renal ischemia) did not protect against ischemic AKI in proximal tubule S1P1R null mice subjected to renal IR. Lack of A1AR-mediated renal protection is again demonstrated in representative hematoxylin and eosin stains (Figure 4E–H, magnification 200X) and renal injury scores (K). Kidneys from wild type (S1P1Rf/f PEPCK−/−) mice were protected against renal IR after CCPA treatment. However, kidneys from proximal tubule specific S1P1R null mice (S1P1Rf/f PEPCKCre/−) treated with CCPA showed severe renal necrosis and renal injury scores after IR. Finally, we demonstrate induction of SK1 in kidneys from wild type (S1P1Rf/f PEPCK−/−) or from proximal tubule specific S1P1R null mice (S1P1Rf/f PEPCKCre/−) injected with a selective A1AR agonist (CCPA, Figure 4J).
Critical role of ABCC1 transporters in A1AR-mediated renal protection
Next, we tested the hypothesis that induction of SK1 and subsequent generation of S1P require export of intracellular S1P to bind to extracellular S1P1R. Previous studies implicated an important role of ABCC1 transporters (MRP1) in translocating intracellular S1P to the extracellular space in several cell types [29–31]. We determined that a specific ABCC1 transporter inhibitor (MK571 [29,32,33]) completely inhibited (Cr=2.24±0.2 mg/dL, N=4) the CCPA-mediated renal protection (Cr=1.24±0.2 mg/dL, N=6) in mice. MK571 itself had no effect on renal function after renal IR injury (Cr=2.25±0.2 mg/dL, N=4).
A1AR activation increases SK1 synthesis and induces SK activity in human proximal tubule (HK-2) cells
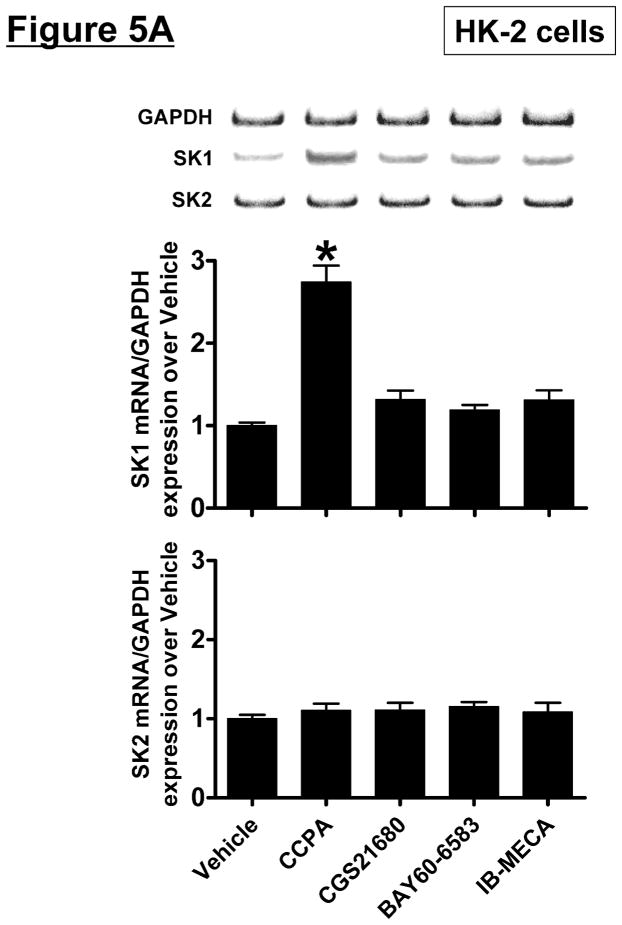
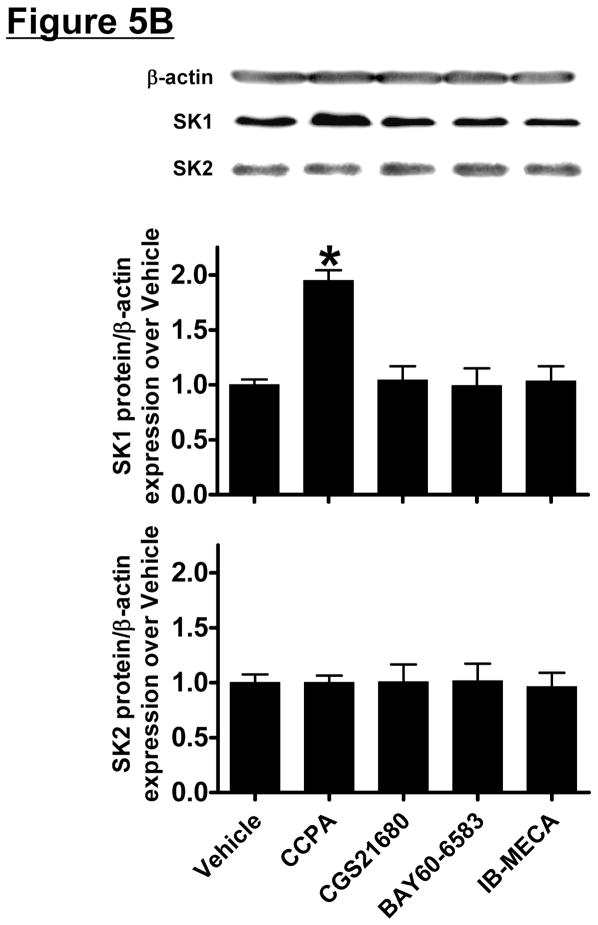
HK-2 cells were treated with selective AR agonists (CCPA for A1AR, CGS21680 for A2aAR, BAY60-6583 for A2bAR or IB-MECA for A3AR – all 1 μM) for 5 hr (mRNA analysis) or 16 hr (protein analysis). We determined that only the A1AR agonist (CCPA) increased SK1 mRNA and protein expression and SK activity in HK-2 cells (Figure 5). SK2 mRNA or protein expression did not change after AR agonist treatment. Again, we preferentially measured SK1 activity by adding Triton x-100 in our SK activity assay.
Figure 5. A1AR activation increases sphingosine kinase (SK)-1 expression and activity in human proximal tubule (HK-2) cells.
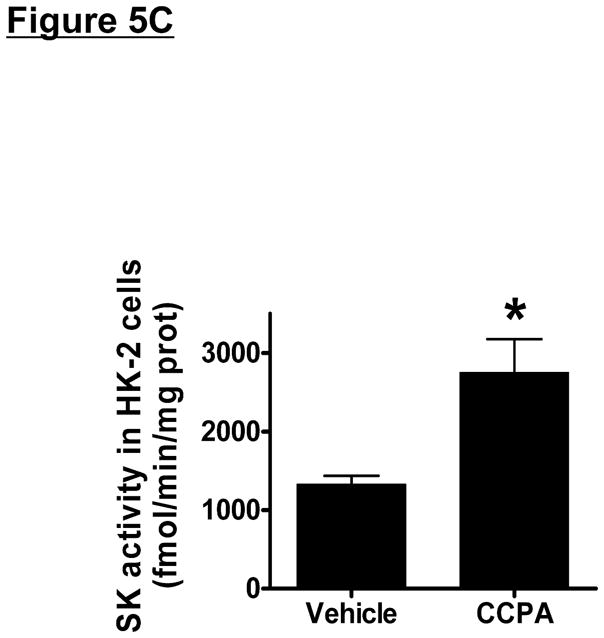
A and B. Representative bands and densitometric quantification of sphingosine kinase (SK) mRNA (A, RT-PCR) and protein (B, immunoblotting) expression in HK-2 cells treated with vehicle (0.00003% DMSO) or with selective adenosine receptor (AR) agonists (N=4 for each group). HK-2 cells were treated with 1 μM of selective AR agonist (CCPA for A1AR, CGS21680 for A2aAR, BAY60-6583 for A2bAR or IB-MECA for A3AR) for 5 hr (mRNA analysis) or 16 hr (protein analysis). Note that only the A1AR agonist (CCPA) selective increased SK1 mRNA and protein expression in HK-2 cells without changing SK2 expression. Data are presented as means ± SEM. *P<0.05 vs. vehicle treatment. C. SK activity in HK-2 cells treated with vehicle (0.00003% DMSO) or with CCPA (1 μM) for 16 hr (N=4 for each group). Data are presented as means ± SEM. *P<0.05 vs. vehicle treatment.
SK and S1P1R signaling is critical for the cytoprotective effects of A1AR activation in HK-2 cells
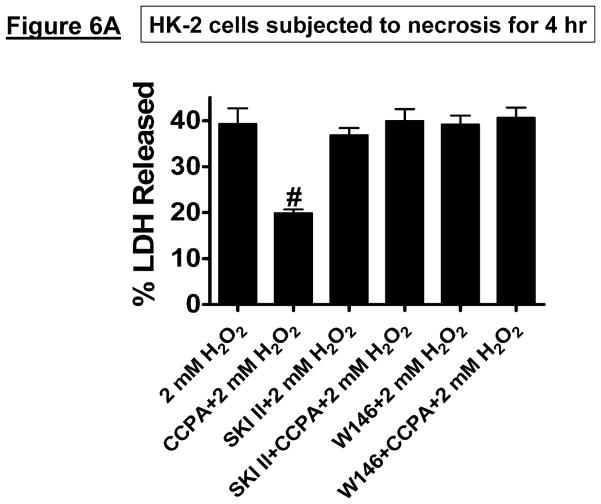
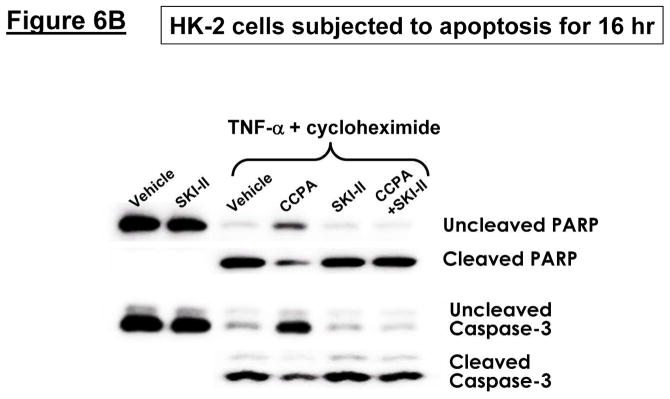
HK-2 cells pretreated with the selective A1R agonist CCPA were protected against H2O2-induced necrosis (2 mM H2O2 for 4 hr, Figure 6A) and apoptosis (20 ng/ml TNF-α and 10 μg/ml cycloheximide treatment for 16 hr, Figure 6B). We determined that A1AR-mediate protection against necrosis was blocked by inhibition of SK1 (with SKI-II treatment) or by blockade of S1P1R (with W146 treatment) (Figure 6A). Moreover, CCPA-mediated protection against apoptosis was inhibited by pretreatment with a selective inhibitor of SK activity (SKI-II, Figure 6B).
Figure 6. Inhibition of SK signaling blocks the cytoprotective effects of A1AR activation in HK-2 cells.
A. Lactate dehydrogenase (LDH) release as a marker of hydrogen peroxide (H2O2)-induced necrosis in HK-2 cells (N=6 for each group, expressed as a percentage of total LDH released). Vehicle (0.00003% DMSO) or CCPA (1 μM) were applied 6 hr before H2O2 treatment, and a selective SK1 inhibitor (SKI-II, 10 μM) or a selective S1P1R inhibitor (W146, 1 μM) was given 30 min before vehicle or CCPA. A1AR activation significantly attenuated H2O2-induced necrosis in HK-2 cells and this protective effect was blocked by the inhibition of SK1 or S1P1R signaling. Data are presented as means ± SEM. #P<0.05 vs. vehicle treatment. B. Representative poly(adenosine diphosphate-ribose) polymerase (PARP) and Caspase-3 fragmentation (N=4 for each group) as indices of HK-2 cell apoptosis. HK-2 cell apoptosis was induced by TNF-α (20 ng/mL) and cycloheximide (10 μg/mL), and vehicle (0.00003% DMSO) or CCPA (1 μM) were applied 30 min before induction of apoptosis. A selective SK1 inhibitor (SKI-II, 1 μM given 30 min before vehicle or CCPA treatment) reduced the anti-apoptotic effect of A1AR activation in HK-2 cells. Representative of 4 independent experiments.
Hypoxia-inducible factor (HIF)-1α plays a critical role in A1AR mediated SK1 induction
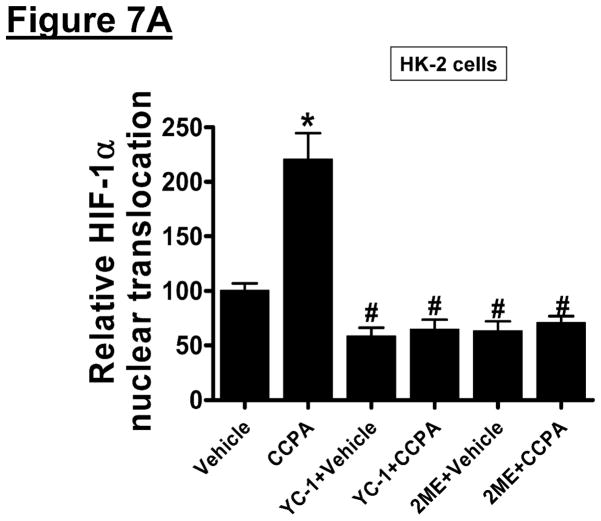
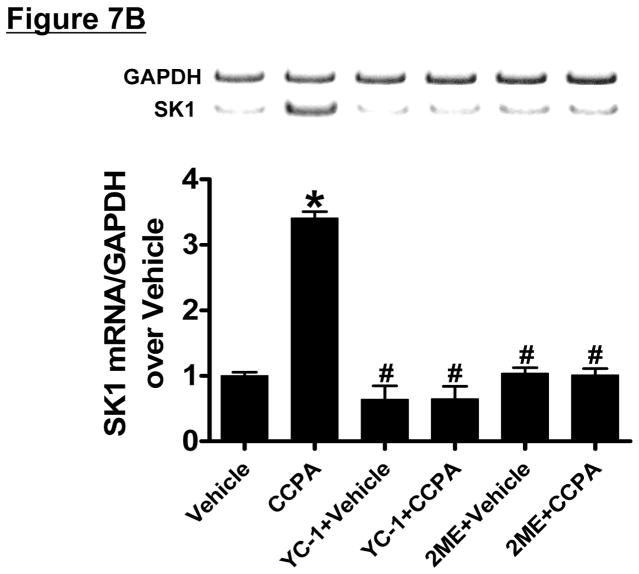
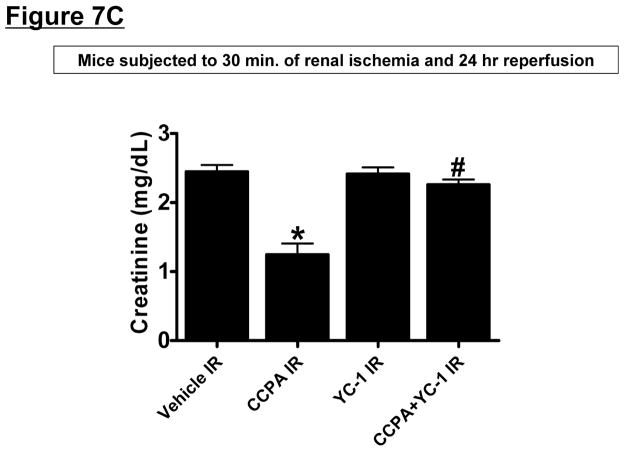
Since HIF-1α signaling can induce SK1 [34], we tested the hypothesis that A1AR agonist CCPA induces SK1 via a HIF-1α dependent mechanism in HK-2 cells. First, we tested whether CCPA directly increases nuclear HIF-1α translocation and subsequent DNA binding in HK-2 cells (Figure 7A). In HK-2 cells, a selective A1AR agonist CCPA (1 μM) increased nuclear HIF-1α and DNA binding which was completely inhibited by 2ME (10 μM) or YC-1 (25 μM) given 30 min before CCPA treatment, Figure 7A). Furthermore, when HK-2 cells were pretreated with inhibitors of HIF-1 signaling (2-ME or YC-1) before exposure to CCPA (1 μM for 6 hr), induction of SK1 mRNA was significantly attenuated (Figure 7B) without affecting the SK2 expression (data not shown). Finally, we showed that mice pretreated with a selective HIF-1α inhibitor, YC-1 (50 mg/kg i.p.) were not protected against renal IR injury with CCPA treatment (Figure 7C). Taken together, these data demonstrate a critical role of increased nuclear HIF-1α in mediating SK1 induction as well as in vivo renal protection with A1AR activation.
Figure 7. Hypoxia-inducible factor (HIF)-1α plays a critical role in A1AR mediated SK1 induction.
A. HIF-1α DNA binding activity in nuclear extracts from HK-2 cells treated with vehicle (0.00003% DMSO) or CCPA (1 μM) for 6 hr (N=6 for each group). CCPA significantly increased the nuclear translocation of HIF-1α in HK-2 cells. Treatment with selective HIF-1α inhibitors [YC-1 (inhibitor of HIF-1α activity, 25 μM given 30 min before CCPA treatment) or 2-methoxyestradiol (2ME, posttranscriptional down-regulator, 10 μM given 30 min before CCPA treatment)] blocked the increase in nuclear translocation of HIF-1α. *P<0.05 vs. vehicle-treated group. #P<0.05 vs. CCPA-treated group. B. Representative bands and densitometric quantification of relative sphingosine kinase (SK)-1 mRNA normalized to GAPDH from RT-PCR reactions in HK-2 cells treated with vehicle (0.00003% DMSO) or CCPA (1 μM) for 5 hr (N=6 for each group). CCPA significantly increased SK1 mRNA expression in HK-2 cells, which was blocked by selective HIF-1α inhibitors (YC-1 and 2ME) given 30 min before CCPA treatment. *P<0.05 vs. vehicle-treated group. #P<0.05 vs. CCPA-treated group. C. YC-1 (50 mg/kg intraperitoneally) prevented renal protection with CCPA (N=4–6 in each group). Plasma creatinine was measured from 24 hours after reperfusion. *P<0.05 vs. vehicle IR. #P<0.05 vs. CCPA IR.
Discussion
AKI is a frequent and disastrous clinical complication with high mortality, morbidity and cost [2,35]. Renal ischemia and reperfusion (IR) injury is a major cause of AKI for patients subjected to surgical procedures involving the kidney, heart, liver or aorta [5,6]. Although incompletely understood, renal tubular necrosis, apoptosis and inflammation during and after renal IR contribute significantly to the pathogenesis of ischemic AKI [36]. Cell surface adenosine receptor (AR) activation can attenuate cell death by reducing necrosis, apoptosis and inflammation [37]. In particular, activation of A1ARs reduces necrosis and apoptosis whereas A2aAR stimulation leads to tissue protection via modulation of leukocyte-mediated inflammation. In the kidney, previous studies showed powerful reno-protective effects of cell surface AR modulation against renal IR injury. In particular, activation of A1AR, A2aAR or A2bAR was found to be renal protective whereas administration of a selective A3AR antagonist or a deficiency in A3AR led to renal protection against IR [9,11,38,39].
We previously demonstrated that activation of renal A1ARs improved plasma creatinine in mice and rats after renal IR with dramatically reduced proximal tubular necrosis and apoptosis [9,10]. Anti-necrotic and anti-apoptotic effects of renal A1AR involve activation of extracellular signal-regulated kinase (ERK), Akt and heat shock protein 27 (HSP27) through a pertussis-toxin sensitive G-protein pathway [26,40]. Surprisingly, we also identified a significant renal anti-inflammatory effect of A1AR activation in vivo [9,11]. Unlike the better characterized and traditionally understood anti-inflammatory mechanisms of A2aAR activation, the anti-inflammatory mechanisms of A1AR activation remain unclear. Our data (pro-inflammatory mRNA expression and neutrophil infiltration, Figure 2G–K) suggest that A1AR activation may specifically produce anti-inflammatory effects (in addition to modulating necrosis and apoptosis) via direct induction and activation of sphingosine kinase-1 (SK1) and sphingosine 1-phosphate (S1P) synthesis. A1AR-mediated induction of SK1 and S1P synthesis has never before described in any organ system and represents major and novel developments in the understanding of cytoprotective mechanisms of A1AR activation.
SK is a conserved lipid kinase that catalyzes the formation of S1P from sphingosine [41–43]. Of the two forms of SK, SK1 is a well known mediator of tissue protection (including protection against IR injury), growth and survival [19,43,44]. In acute lung injury, overexpression of SK1 was protective [45]. Furthermore, in cardiac IR injury, SK1 activation plays a potent cytoprotective role and SK1 deficient myocytes had increased injury after ischemia [19]. We and others previously demonstrated the protective role of SK1 activation or overexpression against renal IR injury [15,16,23,46].
Our studies also show that A1AR-mediated renal protective effects are dependent on SK1 signaling as mice deficient in the SK1 enzyme were not protected against renal IR injury (necrosis and inflammation) with A1AR activation. We propose that A1AR-mediated SK1 induction would increase the synthesis of endogenous S1P in renal cortex and in proximal tubules. S1P is a potent lipid signaling molecule that activates five S1P receptors (S1PR) to regulate diverse biological effects including cell growth, survival and modulation of inflammation [21,47,48]. S1P1R activation in particular has been shown to produce tissue protection via reducing T-lymphocyte mediated inflammation [13]. Activation of the S1P1Rs on endothelial cells also reduces vascular permeability, hence maintaining the integrity of the vascular endothelial cell barrier function [49].
Direct S1P1R activation with a selective agonist [23] or enhanced activation of S1P1R secondary to increased SK1 activity [15] produces protection against renal IR in mice. All 5 S1PR subtypes can bind to S1P with similar nanomolar affinities [50]. However, since the S1P1R is the most heavily expressed S1PR subtype in the kidney [51], we propose that increased SK1 mediated S1P synthesis with A1AR activation directly mediates renal protection via S1P1R activation Our results support this concept as a selective S1P1R antagonist (W146) or siRNA-mediated deletion of S1P1R completely blocked the renal protective effects of A1AR activation.
Renal proximal tubules in the corticomedullary junction (mainly of S3 segments) are highly susceptible to IR injury [52] and our studies confirm severe proximal tubule necrosis in mice subjected to renal IR. A1AR-mediated renal protection as associated with significantly preserved corticomedullary junction architecture and reduction in proximal tubule necrosis. Therefore, we tested the hypothesis that A1AR-mediated SK1 induction and S1P generation protect against renal IR by targeting proximal tubular S1P1R. We confirmed the specific contribution of renal proximal tubule cell S1P1R in A1AR-mediated renal protection by creating mice with cell type specific deletion of S1P1R (with S1P1Rff PEPCKCre mice). We also confirmed that induction of renal SK1 was preserved in mice deficient in proximal tubule S1P1R. Renal tubular S1P produced from A1AR-SK1 signaling may limit leukocyte-mediated renal inflammation after IR. Moreover, A1AR-medated SK1 induction and S1P1R activation may also produce direct anti-necrotic and anti-apoptotic effects in renal proximal tubules as inhibition of SK1 or S1P1R blocked the cytoprotective effects of CCPA in HK-2 cells in vitro.
S1P has been shown to produce cytoprotection via activation of cell surface S1PRs or via intracellular mechanisms targeting mitochondria, intracellular calcium or nitric oxide signaling [53,54]. Our data show that either selective S1P1R blockade (with W146) or renal S1P1R depletion (with siRNA or with cre-loxP mediated proximal tubule specific deletion) prevented CCPA-mediated renal protection. Furthermore, blocking export of intracellular S1P to extracellular space (with MK571-mediated selective ABCC1 transporter inhibition) abolished CCPA-mediated renal protection in vivo. Collectively, these data suggest that increased SK1 activity with A1AR activation produces renal protection via export of intracellular S1P with subsequent activation of extracellular S1P1R.
We previously demonstrated that renal tubular A1AR activation resulted in rapid phosphorylation of ERK and Akt [7,8]. A recent study showed that the direct renal tubular protective effects of S1P1R activation are mediated by activation of the Akt/ERK pathways [23]. S1P also has been shown to induce HSP27 via the p38 MAP-kinase and the PI3-kinase/Akt pathways in osteoblasts and aortic smooth muscle cells [55,56]. We previously demonstrated that HSP27 plays an important role in A1AR-mediated renal protection [40,57]. Specifically, we showed that renal tubular A1AR activation led to induction of HSP27 and a selective inhibitor of HSP27 synthesis attenuated the renal tubular protective effects of A1AR activation. Moreover, overexpression of renal HSP27 mimicked the protection conferred by A1AR agonist in vivo renal IR injury [58]. Our current study indicates that the induction of renal tubular HSP27 is dependent on A1AR-mediated induction of SK1 as a selective A1AR agonist (CCPA) failed to induce HSP27 in SK1 deficient mice. Our findings of A1AR-mediated induction of SK1 with subsequent activation of S1P1R explain the similarities in cytoprotective signaling pathways (ERK, Akt and HSP27) generated with S1P1R or with A1AR activation.
Previous studies have demonstrated that HIF-1α activation protects against renal IR injury [59]. HIF-1 is a heterodimeric transcription factor composed of an α and a β subunit [59,60]. Under normoxic conditions, prolyl-hydroxylation followed by ubiquitination of the oxygen-dependent degradation domain of HIF-1α results in HIF-1α degradation. With hypoxia or ischemia, HIF-1α stabilizes and interacts with HIF-1β; forming the HIF-1 heterodimer. Nuclear translocation of HIF-1 allows binding to the hypoxia-responsive element with subsequent induction of several cytoprotective genes.
Our findings implicate an important role for HIF-1α in mediating the induction of SK1 with A1AR activation. We showed that a selective A1AR agonist caused increased HIF-1α mRNA production, increased HIF-1α nuclear translocation and SK1 induction with subsequently enhanced SK1 activity. We propose that A1AR activation increases binding of hypoxia inducible factor-1α (HIF-1α) to hypoxia response elements of the SK1 promoter leading to increase in SK1 protein synthesis and activity. Consistent with this hypothesis, we showed that selective HIF-1α blockers [2-methoxyestradiol (2-ME) or 3-(5′-Hydroxymethyl-2′-furyl)-1-benzylindazole (YC-1)] prevented CCPA-mediated HIF-1α nuclear translocation as well as induction of SK1 in HK-2 cells. 2-ME is a natural metabolite of estrogen that is known to inhibit HIF-1α at the level of translation independent of effects of estrogen receptors [61]. YC-1 is a selective inhibitor of HIF-1α transcriptional activity [62].
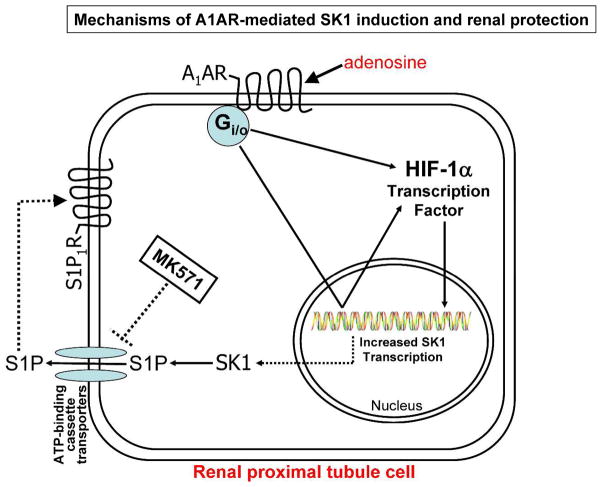
Figure 8 summarizes the potential mechanisms of A1AR-mediated renal protection involving HIF-1α mediated SK1 induction, S1P synthesis and S1P1R activation. Our findings that A1AR-mediated SK1 induction and S1P release lead to reduced renal injury represent a significant paradigm shift and a new direction in A1AR biology. Modulation of an endogenous tissue protectant to decrease renal IR injury has important clinical significance with a high therapeutic potential. Our studies may lead to new therapeutic approaches with a drug that can reduce all 3 pathways of renal cell death (necrosis, apoptosis and inflammation) to lessen the clinical perils from AKI and have implications in organ protection strategies beyond the kidney. Furthermore, SK1-based renal protection would avoid some of the systemic side effects (e.g., bradycardia, hypotension, hypothermia, sedation) of A1AR agonist therapy. Sphingolipid based clinical trials are already ongoing to attenuate organ damage due to inflammation in renal transplantation and multiple sclerosis [63].
Figure 8. A schematic proposed cellular mechanisms of renal protection with A1AR.
Our data suggest that selective A1AR activation results in increased HIF-1α mRNA synthesis, increased HIF-1α nuclear translocation and SK1 induction with subsequent S1P1R activation. Consistent with this hypothesis, selective blockade of SK1 or S1P1R inhibits the renal protective effects of A1AR activation. Furthermore, a selective ABCC1 transporter inhibitor (MK571) would prevent the export of intracellular S1P to bind to extracellular S1P1R. Indeed, MK571 prevented the renal protective effects of an A1AR selective agonist (CCPA).
Methods
Materials
Full systematic chemical names for compounds used in this study are listed in the Supplementary File. BAY 60-6583 (a selective A2bAR agonist) as well as Solutol® HS 15 (vehicle for BAY 60-6583) were kind gifts from Bayer AG (Leverkusen, Germany). Hypoxia-inducible factor (HIF)-1α inhibitors [3-(5′-Hydroxymethyl-2′-furyl)-1-benzylindazole (YC-1, an inhibitor of HIF-1α transcriptional activity) and (17β)-2-Methoxyestra-1,3,5(10)-triene-3,17-diol (2ME, a posttranscriptional inhibitor of HIF-1α expression)] were purchased from Enzo Biochem (New York, NY). W146 (a selective S1P1R antagonist) was obtained from Avanti Polar Lipids, Inc (Alabaster, AL) and a potent SK inhibitor (SKI-II, 4-[[4-(4-chlorophenyl)-2-thiazolyl]amino]-phenol) was obtained from Cayman Chemicals (Ann Arbor, Michigan). Selective A1AR, A2aAR and A3AR agonist ligands [2-Chloro-N6-cyclopentyladenosine (CCPA), 2-p-(2-Carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine (CGS21680), N6-(3-Iodobenzyl)adenosine-5′-N-methyluronamide (IB-MECA), respectively] were obtained from Tocris (Ellisville, MO). Unless otherwise specified, all other reagents were purchased from Sigma (St. Louis, MO). All drugs were dissolved in DMSO and diluted for in vivo (range=2.5–10%) and in vitro (range=0.001–0.2%) use.
Murine model of renal IR injury
After Institutional Animal Care and Use Committee approval, we subjected male C57BL/6 (Harlan, Indianapolis, IN; 20 to 25 g, referred to as wild type (WT) mice) as well as SK1−/− and SK2−/− mice (kindly provided by Dr. R. L. Proia, NIH, Bethesda, MD; 20 to 25g) to 30 min renal ischemia and reperfusion as described [26,58]. To test the effects of selective A1AR activation on modulation of renal function after IR, we treated wild type mice with a selective A1AR agonist (N6-2-chloro-cyclopentyladenosine (CCPA), 0.1 mg/kg i.p.) 15 min prior to renal ischemia or sham-operation as described [9,11]. Some mice were injected with CCPA without being subjected to surgery to determine the induction of SK1 mRNA and protein. To test the effects of S1P1R inhibition, mice were treated with W146 (0.1 mg/kg i.p.) 15 min prior to CCPA or vehicle injection as described previously [64]. For in vivo inhibition of HIF-1α transcriptional activity, some mice were treated with YC-1 (50 mg/kg i.p. 24 hrs and 15 minutes before renal ischemia). To inhibit S1P export via ABCC1 transporter (MRP1), mice were pretreated with 1 mg/kg MK571 (an ABCC1 specific inhibitor [29,32,33]) i.p. 30 min. before renal ischemia. We collected kidney (cortex and cortico-medullary junction) and plasma 5 (RT-PCR) and 24 hrs (immunoblotting, plasma creatinine and renal necrosis) after renal IR injury to examine the severity of renal dysfunction.
In Vivo intrarenal Lentivirus Delivery
Lentivirus encoding EGFP or EGFP-huA1AR was generated as described previously [26]. In vivo virus transduction to express human A1ARs in kidneys of WT, SK1−/− or SK2−/− mice was performed as described by Nakamura et al. [65] with slight modifications as described previously [26]. Forty eight hrs after lentivirus injection, we confirmed transgene expression and processed the kidney for SK mRNA and protein expression analysis. Some mice were also subjected to 30 min. of renal ischemia and 24 hrs of reperfusion 48 hrs after EGFP or EGFP-huA1AR lentivirus injection.
siRNA preparation and delivery to mice in vivo
A chemically synthesized 21 nucleotide siSTABLE™ (Stability enhanced siRNA) sequences specific for mouse S1P1R were custom made and purchased from Dharmacon Research (Lafayette, CO) in 2′-hydroxyl, annealed, desalted and dialyzed duplex form for in vivo use. The siSTABLE™ is a modified siRNA with improved resistance against nuclease degradation and enhanced silencing duration in vivo. The double stranded sequence for S1P1R siRNA was 5′-CCTGTGACATCCTGTACAA-3′. Mice were injected with 50 μg of siRNA (100 μl) i.v. 48 hrs prior to surgery.
Generation and genotyping of mice with proximal tubule specific deletion of S1P1R
We crossed mice carrying the floxed S1P1R gene (S1P1Rff mice, provided by Dr. R. L. Proia (NIH) [12]) with PEPCK-Cre recombinase mice (provided by Dr. Volker Haase, Vanderbilt University, [24]). S1P1R-floxed mice carrying the PEPCK-Cre gene (S1P1Rf/f PEPCKCre/−) and control mice (S1P1Rf/f PEPCK−/−) were genotyped by tail biopsy PCR using Cre transgene and S1P1RloxP specific primers (Table 1). PEPCK-Cre primers generated a 466-bp fragment. Flox primers amplified a 250-bp fragment for the S1P1R floxed allele and a 200-bp fragment for the S1P1R WT allele. To assess the specificity of Cre-recombinase expression, PEPCK-Cre mice were crossed with ROSA26Sortm4(ACTB-tdTomato,-EGFP)Luo/J mice (Jackson Labs) [66]. The Rosa mice express red fluorescence prior to, and green fluorescence following, Cre-mediated recombination.
Table 1.
Primers used to amplify mRNAs based on published GenBank sequences for mice.
| Primers | Accession Number | Sequence (Sense/Antisense) | Product Size (bp) | Cycle Number | Annealing Temp (°C) |
|---|---|---|---|---|---|
| Mouse S1P1R | NM_007901 | 5′-CGGTGTAGACCCAGAGTCCT-3′ 5′-AGCAGCAGATGAGAATGAAC-3′ |
393 | 20 | 64 |
| Mouse S1P2R | NM_010333 | 5′-AAAACCAACCACTGGCTGTC-3′ 5′-GAGTGGAACTTGCTGTT-3′ |
317 | 25 | 60 |
| Mouse S1P3R | NM_010101 | 5′-AAGCCTAGCGGGAGAGAAAC-3′ 5′-GGCAATCAAAACCATCAGGT-3′ |
452 | 22 | 64 |
| Mouse S1P4R | NM_010102 | 5′-GCAGAAGTCTCCACGTCCTC-3′ 5′-GCTGAGTGACCGAGAAGTCC-3′ |
403 | 23 | 62 |
| Mouse S1P5R | NM_053190 | 5′-ACACCAAATGCCCAGCTTAC-3′ 5′-ACCAAGAGCACAGCCAAGTTC-3′ |
335 | 32 | 62 |
| Mouse ICAM-1 | X52264 | 5′-TGTTTCCTGCCTCTGAAGC-3′ 5′-CTTCGTTTGTGATCCTCCG-3′ |
409 | 21 | 60 |
| Mouse TNF-α | X02611 | 5′-TACTGAACTTCGGGGTGATTGGTCC-3′ 5′-CAGCCTTGTCCCTTGAAGAGAACC-3′ |
290 | 24 | 65 |
| Mouse HSP27 | NM_024441 | 5′-CCTAAGGTCTGGCATGGTA-3′ 5′-AGGAAGCTCGTTGTTGAAGC-3′ |
373 | 25 | 66 |
| Mouse SK1 |
NM_011451 (variant 1) NM_025367 (variant 2) |
5′-GATGCATGAGGTGGTGAATG-3′ 5′-GCCCACTGTGAAACGAATC-3′ |
337 | 22 | 64 |
| Human SK1 |
NM_021972 (variant 1) NM_182965 (variant 2) NM_001142601 (variant 3) NM_001142602 (variant 4) |
5′-ATCTCCTTCACGCTGATGC-3′ 5′-GTGCAGAGACAGCAGGTTCA-3′ |
330 | 26 | 66 |
| Human SK2 |
NM_020126 (variant 1) NM_001204158 (variant 2) NM_001204159 (variant 3) NM_001204160 (variant 4) |
5′-GGAGGAAGCTGTGAAGATGC-3′ 5′-GCAGGTCAGACACAGAACGA-3′ |
482 | 22 | 66 |
| Mouse and human GAPDH | M32599 | 5′-ACCACAGTCCATGCCATCAC-3′ 5′-CACCACCCTGTTGCTGTAGCC-3′ |
450 | 15 | 65 |
| Cre transgene | 5′-TGGGCGGCATGGTGCAAGTT-3′ 5′-CGGTGCTAACCAGCGTTTTC-3′ |
466 | 30 | 61 | |
| S1P1RloxP | 5′-GAGCGGAGGAAGTTAAAAGTG-3′ 5′-CCTCCTAAGAGATTGCAGCAA-3′ |
WT: 200 LOX: 250 |
45 | 56 |
bp, base pairs; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HSP27, heat shock protein 27; ICAM-1, intercellular adhesion molecule-1; S1PR, sphingosine 1-phosphate receptor; SK, sphingosine kinase; TNF-α, tumor necrosis factor alpha.
Measurement of Renal Function
Plasma creatinine was measured by an enzymatic creatinine reagent kit according to the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA). This method of creatinine measurement largely eliminates the interferences from mouse plasma chromagens well known to the Jaffe method [67].
Histologic detection of necrosis
Morphological assessment was performed by an experienced renal pathologist (V.DA.) who was unaware of the treatment that each animal had received. An established grading scale of necrotic injury (0–4, Renal Injury Score) to the proximal tubules was used for the histopathological assessment of IR-induced damage as outlined by Jablonski et al. [27] and as described previously in our studies [9,11].
Reverse transcription polymerase chain reaction (RT-PCR) analyses
We measured mRNA encoding markers of inflammation [intercellular adhesion molecule-1 (ICAM-1) and tumor necrosis factor-α (TNF-α)] as well as SK1 and HSP27 5 hr after renal IR as described [58,64,68] (Table 1).
Immunohistochemistry for kidney neutrophil infiltration
We measured renal neutrophil infiltration with immunohistochemistry 24 hrs after renal IR as described previously [69].
HK-2 cell culture and induction of necrosis and apoptosis
Necrotic injury in HK-2 cells was induced with exposure to 2 mM H2O2 for 4 hrs and LDH released into cell culture media was measured as described [7]. To induce apoptosis, HK-2 cells were exposed to TNF-α (10 ng/ml) plus cycloheximide (5 μg/ml) for 16 hrs as described previously [57]. Some HK-2 cells were pretreated with 1 μM CCPA 6 hrs before induction of necrosis or apoptosis. Separate cohorts of HK-2 cells were treated with selective AR agonists (CCPA, CGS-21680, BAY60-6583 or IB-MECA all 1 μM) for 6 hrs to test for the induction of SK mRNA and protein. To inhibit HIF-1α, some HK-2 cells were pretreated with 10 μM 2-ME and 25 μM YC-1 30 min before CCPA treatment.
Immunoblotting analyses of kidney tissue and HK-2 cells
Mouse kidney cortical tissues (including corticomedullary junction) and HK-2 cell lysates were collected for immunoblotting analyses of SK1, SK2 and β-actin (internal protein loading control) as described previously [64,68]. The primary antibodies for human and mouse SK2 were obtained from Abcam (Cambridge, MA). The primary antibody for human and mouse β-actin was obtained from Sigma (St. Louis, MO). The secondary antibody (goat anti-rabbit and anti-mouse IgG conjugated to horseradish peroxidase used at 1:5000 dilution) was detected with enhanced chemiluminescence immunoblotting detection reagents (Amersham, Piscataway, NJ), with subsequent exposure to a CCD camera coupled to a UVP Bio-imaging System (Upland, CA) and a personal computer.
HK-2 cell HIF-1αDNA binding assay
Nuclear extracts from HK-2 cells were prepared using the Transfactor Extraction Kit (BD Biosciences Clontech, Palo Alto, CA) according to the manufacturer’s instructions. HIF-1α DNA-binding activity in nuclear extracts was determined using a TransFactor Family Colorimetric kit specific for HIF-1α (BD Biosciences Clontech) according to the manufacturer’s instructions. HK-2 cells were treated with 1 μM CCPA for 6 hrs. To inhibit HIF-1α, some HK-2 cells were pretreated with 10 μM 2-ME and 25 μM YC-1 30 min before CCPA treatment.
SK activity assay
SK activity was measured as described previously [15]. Briefly, kidney tissues from mice or HK-2 cells were collected and homogenized in cell lysate buffer [100 mM sucrose, 1 mM EGTA, 20 mM MOPS, pH 7.4, 5% Percoll, 0.01% digitonin, protease (Calbiochem, La Jolla, CA) and phosphatase inhibitors] on ice. After a 1,000 × g spin for 15 min to pellet cellular debris, protein concentrations were determined and SK activity was measured as described by Vessey et al. [25]. To preferentially measure SK1 activity, we supplemented the assay buffer with 0.5% Triton X-100.
Isolation of mouse kidney proximal tubules cells
Mouse kidneys were removed, rinsed in PBS containing 0.5% BSA and 2 mM EDTA (GIBCO), minced and digested in collagenase A (1 mg/mL, Sigma) at 37°C for 45 minutes with occasional agitation. The cellular digest was filtered through a nylon mesh, centrifuged at 600g for 10 minutes, and washed twice. Mouse kidney proximal tubules were isolated according to the method of Vinay et al. using Percoll density gradient separation [28].
Statistical analysis
The data were analyzed with Student’s t-test when comparing means between two groups. One-way ANOVA plus Tukey’s post hoc multiple comparison test was used when comparing multiple groups. Two-way ANOVA plus Bonferroni posttest was used to test the effects of sham operation or renal IR injury on different mouse strains or treatment groups. The ordinal values of the renal injury scores were analyzed by the Mann–Whitney nonparametric test. In all cases, a probability statistic < 0.05 was taken to indicate significance. All data are expressed throughout the text as means ± SEM.
Acknowledgments
We thank R. L. Proia for providing the floxed S1P1 receptor, SK1−/− and SK2−/− mice.
Funding: This work was supported by National Institutes of Health Grants R01 DK-058547
Footnotes
Disclosures: No conflict of interest exists for each author. An abstract form of this work was presented at the American Society of Nephrology meeting in Philadelphia, PA, 2011.
References
- 1.Ikeda M, Prachasilchai W, Burne-Taney MJ, et al. Ischemic acute tubular necrosis models and drug discovery: a focus on cellular inflammation. Drug Discov Today. 2006;11:364–370. doi: 10.1016/j.drudis.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Jones DR, Lee HT. Perioperative renal protection. Best Pract Res Clin Anaesthesiol. 2008;22:193–208. doi: 10.1016/j.bpa.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury: why drugs haven’t worked and what is on the horizon. Clin J Am Soc Nephrol. 2007;2:356–365. doi: 10.2215/CJN.03280906. [DOI] [PubMed] [Google Scholar]
- 4.Jones dr, Lee HT. Protecting the kidney during critical illness. Curr Opin Anaesthesiol. 2007;20:106–112. doi: 10.1097/ACO.0b013e328013f83c. [DOI] [PubMed] [Google Scholar]
- 5.Aronson S, Blumenthal R. Perioperative renal dysfunction and cardiovascular anesthesia: concerns and controversies. J Cardiothorac Vasc Anesth. 1998;17:117–130. doi: 10.1016/s1053-0770(98)90106-9. [DOI] [PubMed] [Google Scholar]
- 6.Star RA. Treatment of acute renal failure. Kidney Int. 1998;54:1817–1831. doi: 10.1046/j.1523-1755.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee HT, Emala CW. Adenosine attenuates oxidant injury in human kidney proximal tubular cells via A1 and A2a adenosine receptor activation. Am J Physiol Renal Physiol. 2002;282(5):F844–F852. doi: 10.1152/ajprenal.00195.2001. [DOI] [PubMed] [Google Scholar]
- 8.Lee HT, Emala CW. Preconditioning and adenosine protect human proximal tubule cells in an in vitro model of ischemic injury. J Am Soc Nephrol. 2002;13:2753–2761. doi: 10.1097/01.asn.0000032421.79225.6e. [DOI] [PubMed] [Google Scholar]
- 9.Lee HT, Gallos G, Nasr SH, Emala CW. A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2004;15:102–111. doi: 10.1097/01.asn.0000102474.68613.ae. [DOI] [PubMed] [Google Scholar]
- 10.Lee HT, Emala CW. Protective effects of renal ischemic preconditioning and adenosine pretreatment: role of A(1) and A(3) receptors. Am J Physiol Renal Physiol. 2000;278:F380–F387. doi: 10.1152/ajprenal.2000.278.3.F380. [DOI] [PubMed] [Google Scholar]
- 11.Lee HT, Xu H, Nasr SH, et al. A1 adenosine receptor knockout mice exhibit increased renal injury following ischemia and reperfusion. Am J Physiol Renal Physiol. 2004;286:F298–F306. doi: 10.1152/ajprenal.00185.2003. [DOI] [PubMed] [Google Scholar]
- 12.Allende ML, Yamashita T, Proia RL. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood. 2003;102:3665–3667. doi: 10.1182/blood-2003-02-0460. [DOI] [PubMed] [Google Scholar]
- 13.Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 14.Chun J, Hla T, Lynch KR, et al. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2010;62:579–587. doi: 10.1124/pr.110.003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim M, Kim M, Kim N, et al. Isoflurane mediates protection from renal ischemia-reperfusion injury via sphingosine kinase and sphingosine-1-phosphate-dependent pathways. Am J Physiol Renal Physiol. 2007;293:F1827–F1835. doi: 10.1152/ajprenal.00290.2007. [DOI] [PubMed] [Google Scholar]
- 16.Kim M, Kim M, Park SW, et al. Isoflurane protects human kidney proximal tubule cells against necrosis via sphingosine kinase and sphingosine-1-phosphate generation. Am J Nephrol. 2010;31:353–362. doi: 10.1159/000298339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SW, Kim M, Kim M, et al. Sphingosine kinase 1 protects against renal ischemia-reperfusion injury in mice via S1P1 receptor activation. Kidney Int. 2011 doi: 10.1038/ki.2011.281. In Press. [DOI] [PubMed] [Google Scholar]
- 18.Karliner JS. Mechanisms of cardioprotection by lysophospholipids. J Cell Biochem. 2004;92:1095–1103. doi: 10.1002/jcb.20129. [DOI] [PubMed] [Google Scholar]
- 19.Vessey DA, Kelley M, Li L, et al. Role of sphingosine kinase activity in protection of heart against ischemia reperfusion injury. Med Sci Monit. 2006;12:BR318–BR324. [PubMed] [Google Scholar]
- 20.Lien YH, Yong KC, Cho C, et al. S1P(1)-selective agonist, SEW2871, ameliorates ischemic acute renal failure. Kidney Int. 2006;69:1601–1608. doi: 10.1038/sj.ki.5000360. [DOI] [PubMed] [Google Scholar]
- 21.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem. 2004;279:15396–15401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- 22.Sanna MG, Liao J, Jo E, et al. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279:13839–13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- 23.Bajwa A, Jo SK, Ye H, et al. Activation of sphingosine-1-phosphate 1 receptor in the proximal tubule protects against ischemia-reperfusion injury. J Am Soc Nephrol. 2010;21:955–965. doi: 10.1681/ASN.2009060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rankin EB, Tomaszewski JE, Haase VH. Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res. 2006;66:2576–2583. doi: 10.1158/0008-5472.CAN-05-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vessey DA, Kelley M, Karliner JS. A rapid radioassay for sphingosine kinase. Anal Biochem. 2005;337:136–142. doi: 10.1016/j.ab.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 26.Kim M, Chen SW, Park SW, et al. Kidney-specific reconstitution of the A1 adenosine receptor in A1 adenosine receptor knockout mice reduces renal ischemia-reperfusion injury. Kidney Int. 2009;75:809–823. doi: 10.1038/ki.2008.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jablonski P, Howden BO, Rae DA, et al. An experimental model for assessment of renal recovery from warm ischemia. Transplantation. 1983;35:198–204. doi: 10.1097/00007890-198303000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Vinay P, Gougoux A, Lemieux G. Isolation of a pure suspension of rat proximal tubules. Am J Physiol. 1981;241:F403–F411. doi: 10.1152/ajprenal.1981.241.4.F403. [DOI] [PubMed] [Google Scholar]
- 29.Mitra P, Oskeritzian CA, Payne SG, et al. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nieuwenhuis B, Luth A, Chun J, et al. Involvement of the ABC-transporter ABCC1 and the sphingosine 1-phosphate receptor subtype S1P(3) in the cytoprotection of human fibroblasts by the glucocorticoid dexamethasone. J Mol Med (Berl) 2009;87:645–657. doi: 10.1007/s00109-009-0468-x. [DOI] [PubMed] [Google Scholar]
- 31.Lee YM, Venkataraman K, Hwang SI, et al. A novel method to quantify sphingosine 1-phosphate by immobilized metal affinity chromatography (IMAC) Prostaglandins Other Lipid Mediat. 2007;84:154–162. doi: 10.1016/j.prostaglandins.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dallas S, Zhu X, Baruchel S, et al. Functional expression of the multidrug resistance protein 1 in microglia. J Pharmacol Exp Ther. 2003;307:282–290. doi: 10.1124/jpet.103.054304. [DOI] [PubMed] [Google Scholar]
- 33.Prechtl S, Roellinghoff M, Scheper R, et al. The multidrug resistance protein 1: a functionally important activation marker for murine Th1 cells. J Immunol. 2000;164:754–761. doi: 10.4049/jimmunol.164.2.754. [DOI] [PubMed] [Google Scholar]
- 34.Schwalm S, Doll F, Romer I, et al. Sphingosine kinase-1 is a hypoxia-regulated gene that stimulates migration of human endothelial cells. Biochem Biophys Res Commun. 2008;368:1020–1025. doi: 10.1016/j.bbrc.2008.01.132. [DOI] [PubMed] [Google Scholar]
- 35.Faubel S. Acute kidney injury and multiple organ dysfunction sindrome. Minerva Urol Nefrol. 2009;61:171–188. [PubMed] [Google Scholar]
- 36.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199–2210. doi: 10.1097/01.asn.0000079785.13922.f6. [DOI] [PubMed] [Google Scholar]
- 37.Vallon V, Osswald H. Adenosine receptors and the kidney. Handb Exp Pharmacol. 2009:443–470. doi: 10.1007/978-3-540-89615-9_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okusa MD, Linden J, Macdonald T, Huang L. Selective A2A adenosine receptor activation reduces ischemia- reperfusion injury in rat kidney. Am J Physiol. 1999;277:F404–F412. doi: 10.1152/ajprenal.1999.277.3.F404. [DOI] [PubMed] [Google Scholar]
- 39.Grenz A, Osswald H, Eckle T, et al. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 2008;5:e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joo JD, Kim M, Horst P, et al. Acute and delayed renal protection against renal ischemia and reperfusion injury with A1 adenosine receptors. Am J Physiol Renal Physiol. 2007;293:F1847–F1857. doi: 10.1152/ajprenal.00336.2007. [DOI] [PubMed] [Google Scholar]
- 41.Hait NC, Oskeritzian CA, Paugh SW, et al. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta. 2006;1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Liu H, Chakravarty D, Maceyka M, et al. Sphingosine kinases: a novel family of lipid kinases. Prog Nucleic Acid Res Mol Biol. 2002;71:493–511. doi: 10.1016/s0079-6603(02)71049-0. [DOI] [PubMed] [Google Scholar]
- 43.Maceyka M, Payne SG, Milstien S, Spiegel S. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim Biophys Acta. 2002;1585:193–201. doi: 10.1016/s1388-1981(02)00341-4. [DOI] [PubMed] [Google Scholar]
- 44.Goparaju SK, Jolly PS, Watterson KR, et al. The S1P2 receptor negatively regulates platelet-derived growth factor-induced motility and proliferation. Mol Cell Biol. 2005;25:4237–4249. doi: 10.1128/MCB.25.10.4237-4249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wadgaonkar R, Patel V, Grinkina N, et al. Differential regulation of sphingosine kinases 1 and 2 in lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;296:L603–L613. doi: 10.1152/ajplung.90357.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jo SK, Bajwa A, Ye H, et al. Divergent roles of sphingosine kinases in kidney ischemia-reperfusion injury. Kidney Int. 2009;75:167–175. doi: 10.1038/ki.2008.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venkataraman K, Thangada S, Michaud J, et al. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem J. 2006;397:461–471. doi: 10.1042/BJ20060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chae SS, Proia RL, Hla T. Constitutive expression of the S1P1 receptor in adult tissues. Prostaglandins Other Lipid Mediat. 2004;73:141–150. doi: 10.1016/j.prostaglandins.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Jo SK, Bajwa A, Awad AS, et al. Sphingosine-1-phosphate receptors: biology and therapeutic potential in kidney disease. Kidney Int. 2008;73:1220–1230. doi: 10.1038/ki.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Young N, Van B., Jr Signal transduction of sphingosine-1-phosphate G protein-coupled receptors. ScientificWorldJournal. 2006;6:946–966. doi: 10.1100/tsw.2006.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Awad AS, Ye H, Huang L, et al. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2006;290:F1516–F1524. doi: 10.1152/ajprenal.00311.2005. [DOI] [PubMed] [Google Scholar]
- 52.Molitoris BA, Dahl R, Geerdes A. Cytoskeleton disruption and apical redistribution of proximal tubule Na(+)-K(+)-ATPase during ischemia. Am J Physiol. 1992;263:F488–F495. doi: 10.1152/ajprenal.1992.263.3.F488. [DOI] [PubMed] [Google Scholar]
- 53.Zheng DM, Kitamura T, Ikejima K, et al. Sphingosine 1-phosphate protects rat liver sinusoidal endothelial cells from ethanol-induced apoptosis: Role of intracellular calcium and nitric oxide. Hepatology. 2006;44:1278–1287. doi: 10.1002/hep.21384. [DOI] [PubMed] [Google Scholar]
- 54.gudo-Lopez A, Miguel BG, Fernandez I, Martinez AM. Involvement of mitochondria on neuroprotective effect of sphingosine-1-phosphate in cell death in an in vitro model of brain ischemia. Neurosci Lett. 2010;470:130–133. doi: 10.1016/j.neulet.2009.12.070. [DOI] [PubMed] [Google Scholar]
- 55.Kozawa O, Niwa M, Matsuno H, et al. Sphingosine 1-phosphate induces heat shock protein 27 via p38 mitogen-activated protein kinase activation in osteoblasts. J Bone Miner Res. 1999;14:1761–1767. doi: 10.1359/jbmr.1999.14.10.1761. [DOI] [PubMed] [Google Scholar]
- 56.Takai S, Tokuda H, Matsushima-Nishiwaki R, et al. Phosphatidylinositol 3-kinase/Akt plays a role in sphingosine 1-phosphate-stimulated HSP27 induction in osteoblasts. J Cell Biochem. 2006;98:1249–1256. doi: 10.1002/jcb.20846. [DOI] [PubMed] [Google Scholar]
- 57.Lee HT, Kim M, Jan M, et al. Renal tubule necrosis and apoptosis modulation by A1 adenosine receptor expression. Kidney Int. 2007;71(12):1249–1261. doi: 10.1038/sj.ki.5002227. [DOI] [PubMed] [Google Scholar]
- 58.Kim M, Park SW, Kim M, et al. Selective Renal Over-Expression of Human Heat Shock Protein 27 Reduces Renal Ischemia-Reperfusion Injury in Mice. Am J Physiol Renal Physiol. 2010 doi: 10.1152/ajprenal.00194.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schodel J, Klanke B, Weidemann A, et al. HIF-prolyl hydroxylases in the rat kidney: physiologic expression patterns and regulation in acute kidney injury. Am J Pathol. 2009;174:1663–1674. doi: 10.2353/ajpath.2009.080687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosenberger C, Rosen S, Shina A, et al. Activation of hypoxia-inducible factors ameliorates hypoxic distal tubular injury in the isolated perfused rat kidney. Nephrol Dial Transplant. 2008;23:3472–3478. doi: 10.1093/ndt/gfn276. [DOI] [PubMed] [Google Scholar]
- 61.Mooberry SL. Mechanism of action of 2-methoxyestradiol: new developments. Drug Resist Updat. 2003;6:355–361. doi: 10.1016/j.drup.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 62.Yeo EJ, Chun YS, Cho YS, et al. YC-1: a potential anticancer drug targeting hypoxia-inducible factor 1. J Natl Cancer Inst. 2003;95:516–525. doi: 10.1093/jnci/95.7.516. [DOI] [PubMed] [Google Scholar]
- 63.Mansoor M, Melendez AJ. Recent trials for FTY720 (fingolimod): a new generation of immunomodulators structurally similar to sphingosine. Rev Recent Clin Trials. 2008;3:62–69. doi: 10.2174/157488708783330486. [DOI] [PubMed] [Google Scholar]
- 64.Park SW, Kim M, Chen SW, et al. Sphinganine-1-phosphate protects kidney and liver after hepatic ischemia and reperfusion in mice through S1P(1) receptor activation. Lab Invest. 2010;90:1209–1224. doi: 10.1038/labinvest.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakamura A, Imaizumi A, Yanagawa Y, et al. beta(2)-Adrenoceptor activation attenuates endotoxin-induced acute renal failure. J Am Soc Nephrol. 2004;15:316–325. doi: 10.1097/01.asn.0000111247.76908.59. [DOI] [PubMed] [Google Scholar]
- 66.Muzumdar MD, Tasic B, Miyamichi K, et al. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 67.SLOT C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest. 1965;17:381–387. doi: 10.3109/00365516509077065. [DOI] [PubMed] [Google Scholar]
- 68.Park SW, Kim M, Chen SW, et al. Sphinganine-1-phosphate attenuates both hepatic and renal injury induced by hepatic ischemia and reperfusion in mice. Shock. 2010;33:31–42. doi: 10.1097/SHK.0b013e3181c02c1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen SW, Kim M, Kim M, et al. Mice that overexpress human heat shock protein 27 have increased renal injury following ischemia reperfusion. Kidney Int. 2008 doi: 10.1038/ki.2008.572. [DOI] [PMC free article] [PubMed] [Google Scholar]