Abstract
AIMS
To test the null hypothesis that bony pelvis dimensions are similar in women with and without stress urinary incontinence, both in the postpartum and midlife periods.
METHODS
Secondary analyses were performed of two case-control studies comparing women with stress urinary incontinence to asymptomatic controls. One study examined primiparas in the first 9–12 months postpartum; the other study involved middle-aged women. Stress urinary incontinence was confirmed by full-bladder stress test.
All subjects underwent pelvic magnetic resonance imaging. The interspinous and intertuberous diameters, subpubic angle and sacrococcygeal joint-to-the inferior pubic point distance were measured from the images independently by two authors.
RESULTS
In the young cohorts, we compared primiparas with de novo postpartum stress urinary incontinence to both continent primiparas and nulliparas. Postpartum stress urinary incontinence is associated with a wider subpubic angle. There is also a trend towards wider interspinous and intertuberous diameters in the stress-incontinent primiparas as compared to the continent cohorts, although this did not reach statistical significance with our sample sizes. By contrast, no significant differences in bony pelvis dimensions were identified when comparing middle-aged women with stress urinary incontinence and their continent controls.
CONCLUSIONS
Bony pelvis dimensions are different in women with stress urinary incontinence than in matched continent controls. However, these differences are only identified in young primiparas in the postpartum period, not in middle-aged women.
Keywords: Urinary incontinence, Stress; Magnetic Resonance Imaging; Pelvimetry; Postpartum Period; Middle Aged
INTRODUCTION
Urinary incontinence is a common disorder in women with substantial financial and psychosocial consequences.(1) Stress urinary incontinence (SUI) is the most common type of urinary incontinence.(2) Demographic, morphometric and urodynamic characteristics have been associated with female SUI, including age, parity, body mass index (BMI), support of the bladder neck and/or urethra, levator ani injuries and urethral function.
One specific set of morphometric factors that has been correlated with urinary incontinence is bony pelvis dimensions. Pelvic dimensions have long been recognized to impact mechanics of labor and delivery.(3) Two competing hypotheses arising from these birth mechanics that might explain the link between the bony pelvis and continence are: 1) The fetal head may exit the vagina anteriorly in women with a wide pelvis, resulting in urethral compression and damage, whereas 2) a narrow pelvis drives the fetal head dorsally, resulting in greater stretch of the levator ani. The overall effect of these processes is unknown. Furthermore, parity and the mode of childbirth appear to play diminishing roles in urinary continence over the course of women’s lifetimes.(4)
Several studies have demonstrated wider pelvic dimensions in women with urinary incontinence than in continent controls.(5, 6) While these studies are intriguing, they are limited by a lack of demographic matching and/or the use of women seeking healthcare for other reasons rather than healthy volunteers as control subjects. Previous studies have demonstrated that demographic characteristics, such as race, may critically affect pelvis shape and size.(7, 8) The goal of this study is to therefore test the null hypothesis that bony pelvis dimensions are similar in women with stress incontinence and in demographically-matched continent controls. There is also accumulating evidence that the spectrum of factors associated with stress incontinence is different in women soon after birth than in women presenting for care of stress urinary incontinence later in life.(9, 10) Therefore, we examined these differences both in the postpartum and middle-aged periods in order to explore differences in the pathophysiology of SUI during these life periods.
MATERIALS AND METHODS
This is a secondary analysis of two institutional review board-approved case-control studies comparing women with stress urinary incontinence to demographically-matched continent controls. From the Promoting Effective Recovery after Labor II (PERL 2) study (University of Michigan Institutional Review Board IRBMED #1995-0477), we included three cohorts: 1) primiparas in the first 9 – 12 months after vaginal delivery with persistent de novo postpartum SUI, 2) continent primiparas in the first 9 – 12 months postpartum after vaginal delivery, and 3) continent nulliparas. All three cohorts were group matched by age and race.(9) In the Research on Stress Incontinence Etiology (ROSE) study (IRBMED #2002-0636), the incontinent cases were middle-aged women with daily SUI and the continent controls were recruited with group-matching to ensure similar age, race, parity and hysterectomy status.(10) SUI was confirmed for all cases in both studies by full-bladder stress test.
Recruitment strategies were varied for the different studies. In the PERL2 study, eligible primiparous women were identified from delivery records and were sent a letter at 6 months postpartum asking for their willingness to be contacted about the study. Those women who expressed interest were then called to further discuss the study, eligibility and their participation. Healthy, asymptomatic nulliparous subjects were recruited from the community via newspaper advertisements and recruitment posters during the same time period. In the ROSE study, stress incontinent women were recruited both from clinics and from community advertisements. All of the continent controls were healthy, asymptomatic volunteers recruited from community advertisements.
All subjects underwent pelvic magnetic resonance imaging (MRI). Full details of the MRI acquisitions have been previously published (9, 10), but briefly, two-dimensional proton-density images were obtained with an echo time of 15 milliseconds and a repetition time of 4 seconds, using a 1.5 T superconducting magnet (Signa; General Electric Medical Systems, Milwaukee, WI, USA). Slice thickness was 4 mm with a gap of 1 mm, yielding an image spacing of 5 mm.
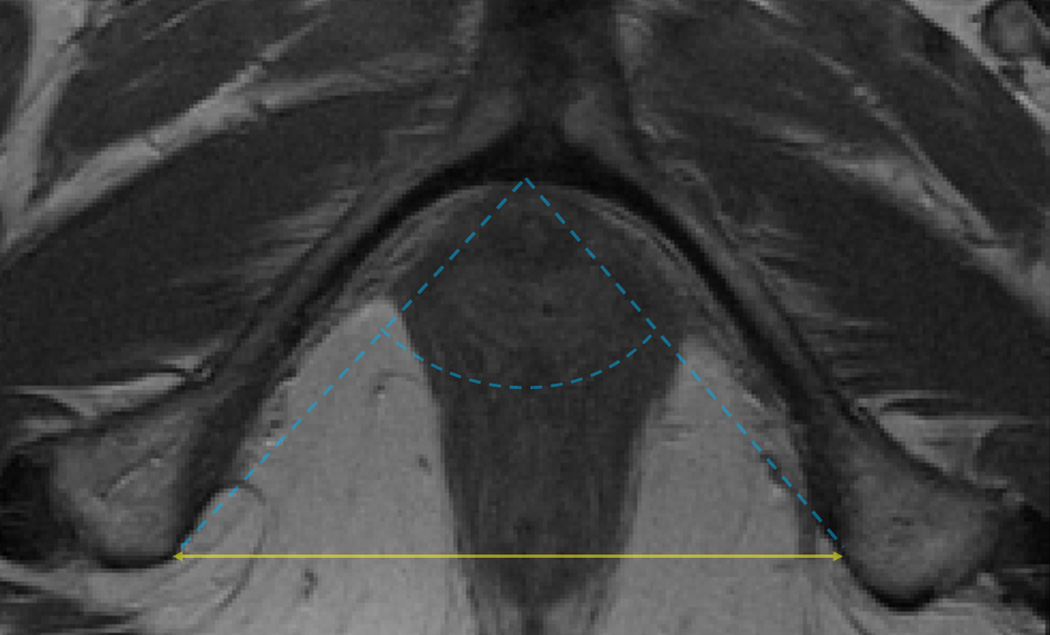
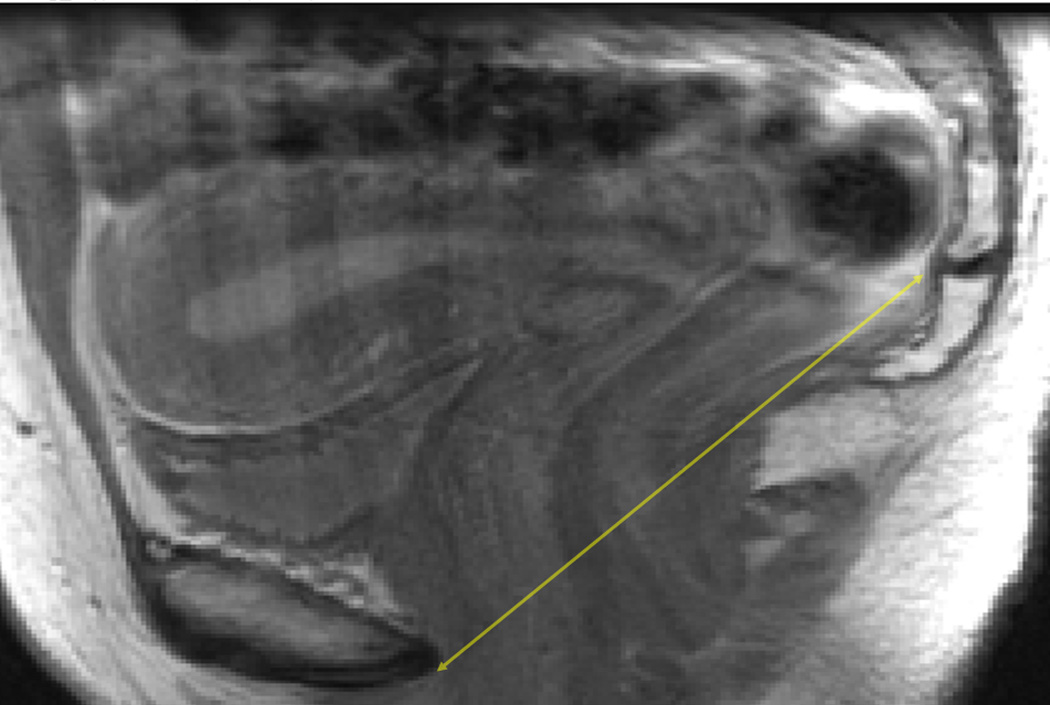
For each subject with digital MRIs available containing the relevant pelvic landmarks, the interspinous diameter (Figure 1a), intertuberous diameter (Figure 1b), subpubic angle (Figure 1b) and the length of the sacrococcygeal joint-to-the inferior pubic point (SCIPP) distance(11) (Figure 1c) were measured independently by two authors (M.B.B. and S.K.D.) using ImageJ 1.42q software (National Institutes of Health, Bethesda, MD, USA). The average value of each measurement was used for final analyses. The (x, y, z) coordinates of each of the following bony pelvis landmarks were collected from axial images where the z coordinate represented the slice number taking into account the fact that the slices had a 5 mm spacing: the infrapubic point, the bilateral ischial tuberosities, and the bilateral ischial spines. For the axial infrapubic point, we selected the midline point at the inferior edge of the arcuate pubic ligament in the first image with left-to-right continuity of the ligament while scrolling through the images in a caudad-to-cephalad direction.(12) The ischial tuberosities were marked at the medial aspect of the periosteum at the medial tip of the insertion site of the sacrotuberous ligament, selecting the first image in which the insertion site appears when scrolling from a cephalad-to-caudad direction. The ischial spines were similarly marked at the medial aspect of the periosteum, using the image with the most readily visualized insertion of the sacrospinous ligaments. Distances were calculated between two sets of coordinate points using the Pythagorean Theorem. This methodology, as opposed to directly measuring distances of lines drawn on a particular MR slice, was used in order to account for the fact that the long axis of a subject is not always perpendicular to the axial plane of an MRI, and that a person’s anatomy may be asymmetric. Measurements using (x, y, z) coordinates and calculation of the distances between them allows for the left and right anatomic points to be selected on different MRI slices when anatomically appropriate. The subpubic angle was defined as the angle between the two lines generated by connecting the infrapubic point and the ischial tuberosity points, and was calculated using the geometric law of cosines.
Figure 1.
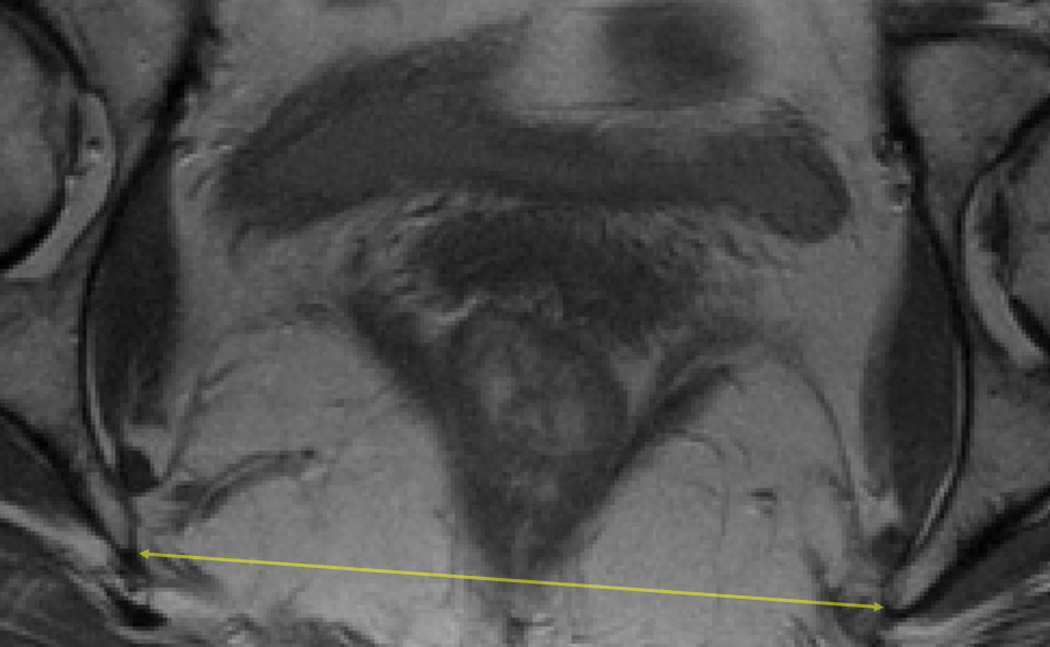
a: Axial pelvic magnetic resonance image (MRI) illustrating the interspinous diameter (solid double-headed arrow).
b: Axial pelvic MRI illustrating the intertuberous diameter (solid double-headed arrow) and the subpubic angle (dashed lines).
c: Sagittal pelvic MRI slice illustrating the sacrococcygeal joint-to-the-inferior pubic point (SCIPP) line (solid double-headed arrow).
The SCIPP lengths were measured on bony mid-sagittal images. For each subject, a line was drawn between the inferior pubic point (at the posterior tip of the inferior surface of the periosteum of the pubic symphysis) and the inferomedial aspect of the periosteum of the sacrococcygeal joint. The distance of this line was calculated using ImageJ software.
A power calculation was based on data from MRI analysis of subjects in the Pelvic Floor Disorders Network’s Childbirth and Pelvic Symptoms (CAPS) study, in which it was previously demonstrated that there was a significantly wider subpubic angle (85.4 ± 6.3 degrees as compared to 83.0 ± 7.1 degrees) and longer intertuberous diameter (124.5 ± 8.7 mm versus 121.0 ± 9.9 mm) in primiparas with postpartum urinary incontinence as compared to those who remained continent (data presented as mean ± standard deviation).(6) Using these results and the number of subjects from our studies available for the analyses described in this paper, we calculate that we have 56.3% power to detect a difference in the subpubic angle and 70.2% power to detect a difference in the length of the intertuberous diameter in the primiparous PERL 2 subjects, with a 95% two-sided confidence interval for each measurement. For the continent PERL 2 subjects, we have 57.9% power to detect a difference in the subpubic angle and 62.1% power to detect a difference in the intertuberous diameter length. Based on the available MRIs for analysis from the ROSE study, we similarly have 73.3% power to detect differences in the subpubic angle and 85.9% power to detect a difference in the intertuberous diameter length, again using a 95% two-sided confidence interval, in middle-aged subjects.
Comparisons were made using independent Student’s t-tests, Mann-Whitney U tests, or analysis of variance (ANOVA) for continuous variables, and chi-squared or Fisher’s exact tests for categorical variables. Correlations were assessed with the Pearson correlation coefficient. Normality of data was tested using the Shapiro-Wilk test. PASW version 18.0 (IBM Corporation, Armonk, NY, USA) was used for statistical analyses, and OpenEpi version 2.3.1(13) was used for power calculations. P values of < 0.05 were considered statistically significant.
RESULTS
From the PERL 2 study, there were 67 primiparas with de novo SUI, 73 continent primiparous controls and 73 continent nulliparas from which pelvic MRIs with the relevant bony landmarks visible were available. Demographics of these subjects were similar, except the incontinent primiparas had a higher BMI than either of the other cohorts (Table I). Comparisons between the individual groups allows for stratified evaluation of birth effects (by comparing the continent primiparas to the nulliparas) and SUI effects (through comparison of stress-incontinent primiparas to the continent primiparous control cohort). There were no significant differences in demographics between the two continent control cohorts, whereas the only significant difference identified in comparison of the two primiparous cohorts was a higher average BMI in the incontinent cohort (Table I).
Table I. Demographics - Young subjects from the PERL 2 study.
Demographics of the primiparous stress-incontinent (PI), primiparous continent (PC) and nulliparous continent (NC) cohorts included from the parent PERL 2 study. Data are presented as mean ± standard deviation or percentage (number of subjects). p values test the null hypothesis that demographic characteristics are similar across all three cohorts, between primiparas with de novo SUI and their primiparous continent controls (incontinence-associated relationships), or between continent primiparas and nulliparas (birth-associated relationships).
| Characteristic | Nulliparous Continent (NC) (n = 73) |
Primiparous Continent (PC) (n = 73) |
Primiparous Incontinent (PI) (n = 67) |
p value - Comparison across all three groups |
p value - “Birth” NC Compared with PC |
p value - “SUI” PC Compared with PI |
|---|---|---|---|---|---|---|
| Age (years) | 29.9 ± 5.5 | 29.9 ± 4.7 | 29.6 ± 5.6 | 0.75 | 0.44 | 0.77 |
| Height (inches) | 65.1 ± 2.5 | 65.4 ± 2.6 | 65.3 ± 2.8 | 0.80 | 0.49 | 0.82 |
| BMI (kg/m2) | 24.2 ± 4.0 | 23.6 ± 4.2 | 26.2 ± 5.8 | 0.004 | 0.36 | 0.003 |
| Race | 0.78 | 0.67 | 0.76 | |||
| Caucasian | 93.2 (68) | 89.0 (65) | 89.6 (60) | |||
| Afro- | 1.4 (1) | 2.7 (2) | 4.5 (3) | |||
| American | ||||||
| Other | 5.5 (4) | 8.2 (6) | 6.0 (4) |
In the ROSE study there were 101 middle-aged subjects with daily SUI and 107 continent controls with appropriate MRIs available. Demographics were similar in the two cohorts from the ROSE study with the exception of significantly higher average BMI in the women with SUI (Table II).
Table II. Demographics - Middle-aged subjects from the ROSE study.
Demographics of middle-aged women with daily SUI and continent controls included from the parent ROSE study. Data are presented as mean ± standard deviation, median (interquartile range), or percentage (number of subjects). p values test the null hypothesis that demographic characteristics are similar between women with daily SUI and their continent controls.
| Characteristic | Continent (n = 107) | SUI(n= 101) | p value |
|---|---|---|---|
| Age (years) | 47.8± 11.4 | 47.8 ± 9.3 | >0.99 |
| Height (inches) | 63.8 ± 3.4 | 64.2 ± 2.2 | 0.25 |
| BMI (kg/m2) | 27.6 ± 5.6 | 30.3 ± 6.6 | 0.002 |
| Parity | 2.0(1.0,3.0) | 2.0 (1.0,3.0) | 0.63 |
| Hysterectomy | 8.4 (9) | 11.9 (12) | 0.41 |
| Race | 0.76 | ||
| Caucasian | 94.4 (101) | 92.1 (93) | |
| Afro-American | 2.8 (3) | 3.0 (3) | |
| Other | 0.9 (1) | 3.0 (3) |
Inter-rater reliability was high for all bony pelvis measurements. For the young subjects from the PERL 2 study, the Pearson correlation coefficients for bony dimensions ranged from 0.84 – 0.99. Similarly, the correlation coefficients for bony pelvis measurements from the middle-aged subjects from the ROSE study ranged from 0.88 – 0.99. P values for all correlation coefficients were < 0.001.
Analysis across all three cohorts from the PERL 2 study shows that the subpubic angle is 2.3 – 3.0 degrees wider in the stress-incontinent primiparas than the continent primiparous and nulliparous cohorts (Table III). Consideration of only the primiparous cohorts (comparing continent with de novo stress-incontinent women) suggests that the subpubic angle is 2.3 degrees wider in primiparas with de novo SUI than in the continent primiparas. There was a similar trend towards 2 mm longer interspinous and intertuberous diameters in the urinary incontinent primiparas, but these comparisons did not reach statistical significance at the sample sizes afforded by the PERL 2 study. There was no significant difference in the length of the SCIPP line between the two cohorts. Comparisons between the continent primiparous and nulliparous cohorts (to distinguish birth-associated variation) reveal no significant differences in any of the bony pelvic dimensions examined (Table III).
Table III. Bony Pelvis Dimensions - Young subjects from the PERL 2 study.
Bony pelvis measurements in young women with and without SUI included from the PERL 2 study. Data are presented as mean ± standard deviation. p values test the null hypothesis that demographic characteristics are similar across all three cohorts (ANOVA), between primiparas with de novo SUI and the primiparous continent controls (incontinence-associated relationships), or between continent primiparas and nulliparas (birth-associated relationships)
| Characteristic | Nulliparous Continent (NC) (n = 73) |
Primiparous Continent (PC) (n = 73) |
Primiparous Incontinent (PI) (n = 67) |
p value – ANOVA |
p value – “Birth” NC Compared with PC |
p value – “SUI” PC Compared with PI |
|---|---|---|---|---|---|---|
| Interspinous Diameter (cm) |
10.3 ± 0.7 | 10.4 ± 0.7 | 10.6 ± 0.7 | 0.06 | 0.60 | 0.08 |
| Intertuberous Diameter (cm) |
11.1 ± 0.8 | 11.2 ± 0.9 | 11.4 ± 0.9 | 0.11 | 0.88 | 0.09 |
| Subpubic Angle (degrees) |
89.0 ± 6.3 | 89.7 ± 6.6 | 92.0 ± 6.8 | 0.02 | 0.50 | 0.04 |
| SCIPP Length (cm) |
11.4 ± 0.8 | 11.5 ± 0.9 | 11.6 ± 1.1 | 0.71 | 0.60 | 0.75 |
It was shown in the parent PERL2 study that primiparous incontinent women were twice as likely to have visible levator ani defects on MRI as the primiparous continent controls.(9) We therefore compared the subpubic angle in primiparas stratified by levator defect type (as previously described).(14) We find no statistically-significant differences in subpubic angle comparing primiparas with normal muscles and primiparas with visible levator defects (data not shown).
Logistic regression was used to identify characteristics that independently associate with the presence of stress urinary incontinence in the primiparous PERL2 subjects. We initially performed bivariate logistic regressions with stress incontinence as the dependent variable and age, race, height, BMI, the presence of levator ani defects, and the subpubic angle as independent variables. The only characteristics that showed statistically-significant associations with stress urinary incontinence were BMI, the presence of levator ani defects, and the subpubic angle. A multivariable model using all of the listed variables (except height, as it has collinearity with BMI) further showed the persistence of these characteristics as statistically-significant predictors of stress urinary incontinence. The odds ratios (OR) and p values associated with each of these variables in the multivariable model are: BMI – OR 1.14 per kilogram/square meter, p = 0.001, levator ani defects – OR 4.00, p = 0.02, and subpubic angle, OR 1.07 per degree, p = 0.001.
In contrast to our findings in young primiparas, we detected no significant differences in bony pelvis dimensions between the middle-aged subjects from the ROSE study with daily stress incontinence or the continent control cohort (Table IV).
Table IV. Bony Pelvis Dimensions – Middle-aged subjects from the ROSE study.
Bony Pelvis measurements in middle-aged women with daily stress incontinence and in continent controls included from the ROSE study. Data are presented as median (25th percentile, 75th percentile) or mean ± standard deviation. p values test the null hypothesis that pelvic dimensions are similar in the stress incontinent and urinary-continent cohorts. For the interspinous and intertuberous diameters, p values are derived from Mann-Whitney U tests, whereas the subpubic angle and SCIPP lengths were compared using independent t-tests.
| Characteristic | Continent (n = 107) | SUI (n = 101) | p value |
|---|---|---|---|
| Interspinous diameter (cm) | 10.8 (10.2, 11.5) | 10.7 (10.3, 11.2) | 0.38 |
| Intertuberous diameter (cm) | 11.5 (10.5, 12.2) | 11.3 (10.7, 12.0) | 0.99 |
| Subpubic angle (degrees) | 91.7 ± 7.5 | 92.6 ± 6.3 | 0.32 |
| SCIPP length (cm) | 11.6 ± 0.9 | 11.7 ± 1.0 | 0.68 |
In order to test whether shape, along with absolute sizes, may be associated with stress urinary incontinence, we compared the bony pelvis length-to-width ratios (using the ratio of the interspinous diameter to the SCIPP length) in both the PERL2 and ROSE studies. No significant differences were identified in either study when comparing the stress-incontinent cases to their respective continent controls, with p values ranging from 0.38 – 0.98.
DISCUSSION
The results of this study suggest that bony pelvis dimensions are indeed different in women with stress incontinence and in matched continent controls. However, these differences are only identified in young primiparas in the postpartum period. By contrast, pelvic dimensions are similar in middle-aged women with and without daily SUI. The subpubic angle is, on average, 2.3 degrees wider in stress-incontinent primiparas than in those who are continent. We also see a trend in the interspinous and intertuberous diameters measuring approximately 2% longer in the primiparas with SUI than in the primiparous continent controls, although these comparisons did not reach statistical significance. The results from the “birth”-related analysis from the PERL 2 study (comparing the continent primiparas to the continent nulliparous cohort) suggests that the bony pelvis differences are associated with stress urinary incontinence, rather than reflecting changes due to pregnancy and/or vaginal delivery.
There are two published studies comparing bony pelvis dimensions in women with and without urinary incontinence. Stav and colleagues used computed tomography (CT) pelvimetry to compare pelvic morphometrics in women (who were, on average, middle-aged) with urinary incontinence (including urgency and mixed urinary incontinence) to those in women undergoing pelvic CT for other clinical reasons.(5) Handa and colleagues used MRI pelvimetry obtained from women involved in the supplementary CAPS study to compare bony pelvis dimensions of young primiparas with pelvic floor disorders to those of asymptomatic women.(6) In both of these studies, women with urinary incontinence were noted to have wider pelvic dimensions at a similar scale to the measurements from our study. However, these studies were also limited by several factors, including lack of demographic matching of the incontinent cases to the continent controls, the use of survey responses rather than physical examination to diagnose urinary incontinence, and the control subjects in Stav’s study were recruited from women having pelvic imaging for clinical purposes, rather than being asymptomatic volunteers. The data from our study support the findings of the CAPS study, i.e., de novo postpartum stress urinary incontinence is associated with a wider pelvic outlet. By contrast, we do not replicate the findings of Stav and colleagues in older subjects. We hypothesize that the differences in bony pelvis dimensions identified in Stav’s study stem from the use of unmatched study cohorts, controls that had unspecified pelvic pathology, and/or the inclusion of women with urinary incontinence other than SUI, and hence, to confounding factors.
It is currently unknown if there is any clinical relevance to the differences identified in the bony pelvis dimensions measured in this study. It is, however, reassuring that the magnitude of these differences is similar to those seen in other studies.(5, 6) Furthermore, the pelvis is not a set of two-dimensional slices, but rather, a dynamic, three-dimensional space. As such, small differences in one region may have a substantial impact on the overall behavior of the pelvis. Additional measurements and biomechanical investigations are needed to quantify the overall effects of these morphologic differences.
We find a higher average body mass index in women with stress urinary incontinence as compared to their controls, both in the young primiparous PERL 2 subjects and the middle-aged ROSE subjects. Similar results were reported in the original analyses of these parent studies.(9, 10) Although it is possible that there are direct associations between bony pelvis dimensions and body mass index, the correlation between higher BMI and stress incontinence in both sets of cohorts is likely due to higher intraabdominal pressures generated rather than skeletal anatomic factors.(15)
Stress urinary incontinence is the result of changes to both urethral supports and to urethral sphincter closure forces. The data from this study contribute to the growing body of research suggesting that although de novo postpartum stress urinary incontinence and midlife SUI share a common outcome, there are differences in the relative contributions of maximum urethral closure pressure (MUCP) and urethral support at different times in a woman’s life. De novo stress urinary incontinence soon after birth is more strongly related to changes in levator ani muscle injury and increased urethral mobility than at middle age, when the age-related decline in urethral pressure dominates. For example, the parent PERL 2 study revealed similar effects of vesical neck support and MUCP in determining continence status of primiparas.(9) By contrast, data from the parent ROSE study suggest that MUCP has an approximately 3-fold greater effect size than vesical neck support in determining continence in middle-aged women.(10) Similarly the PERL 2 study revealed a two-fold higher occurrence of levator ani muscle injury in the stress incontinent cohort while in the ROSE study, levator ani muscle injury occurred with equal frequency in the two groups.
We hypothesize that a wider subpubic angle may be a risk factor for trauma during childbirth that leads to postpartum stress urinary incontinence. The anterior location of the fetal head afforded by a wider pelvis might directly damage the urethra or urethral supports, similar to the vaginal delivery-induced urethral changes seen in previously published animal models.(16, 17) Alternatively, trauma from the delivery may unmask preexisting poor urethral function that, prior to delivery of the newborn, was compensated for by urethral support and/or pelvic floor functioning. Interestingly, despite a two-fold higher rate of visible levator ani defects in the incontinent primiparas than the primiparous controls, we find no association between the rates of these muscle defects and the subpubic angle, suggesting that any birth trauma promoted by a wider subpubic angle is not reflected in the visible levator ani defects noted on MRI. By the middle-aged period, however, bony pelvis dimensions do not seem to associate with the presence of SUI. We hypothesize that other factors dominate such that contributions to continence from birth-related pelvic floor injury are minimized.
The findings in this study are best viewed in the light of hypothesis generation. The aggregate of these data support the conceptual “Lifespan Model” previously published as a means to understand the development of pelvic floor disorders.(18) Essentially, this model suggests that women have baseline pelvic floor function that may be affected by predisposing factors such as genetics, inciting factors such as birth trauma, and intervening factors such as age-related muscle loss. For primiparas, a wide pelvis may lead to birth trauma that is a severe enough inciting factor to induce loss of urethral function and, therefore, SUI. By contrast, a young woman with baseline poor urethral function may be continent due to pelvic floor support that is subsequently weakened by birth trauma, and hence, she develops postpartum stress urinary incontinence. Over the course of their lifetimes, women experience intervening factors such as age-related decline in urethral muscle fiber thickness and number. In addition, they may have undergone further childbirths and/or other traumatic events. As such, by the middle-aged period, these intervening factors predominate such that the influence of inciting factors (including pelvis width) contributes minimally to continence mechanisms.
We must highlight, though, that the results in this study involve two separate cross-sectional studies; the ROSE subjects cannot be viewed simply as older versions of the PERL2 subjects. There are numerous demographic and biologic variables that may be unrecognized confounders, especially when comparing the results of one study to the other. In order to gain a better understanding of how bony pelvis anatomy affects urinary continence, longitudinal studies are needed in which careful pelvimetry and urodynamic measures are collected in large numbers of nulliparas and repeated over several decades, especially after events such as childbirth. A similar approach to obtaining this information would be to restudy the PERL2 subjects with urodynamics and pelvic imaging during the middle aged period, in order to assess the contributions of pelvic floor functioning, pelvimetry, and urodynamic measures to the continence mechanisms.
There are several strengths to this study, including its large sample sizes, demographic matching between cohorts which minimizes confounders, all of the case subjects had clinical evidence of SUI rather than only self-reported symptoms, and the control subjects were all healthy volunteers whose continence status was assessed by questionnaire and urodynamic testing rather than women undergoing imaging for other reasons whose continence was unknown or subjects recruited from pools of healthcare seeking patients. This study is also easily reproducible, especially given the high inter-rater reliability for MRI pelvimetry and the ease of electronic transmission of digital MR images. We must also acknowledge limitations of the study, including the relative homogeneity of the subjects which may not allow generalizability of our findings, the case-control design which precludes determination of causality or monitoring for longitudinal changes, the inherent limitations of image analysis, and the small magnitude of the differences we identified in bony pelvis dimensions. We also recognize that the different recruitment strategies used in the parent studies may influence our results; the data must therefore be interpreted with caution. It is also possible that due to the number of analyses performed, our results regarding the subpubic angle in the PERL2 subjects may simply be due to chance. Although we elected to forgo Bonferroni correction(19), usage of such a construct (utilizing three different group comparisons for four distinct bony pelvis dimensions) would suggest statistical significance would require a p value of < 0.001. It is further possible that this study was underpowered to detect differences in bony pelvis dimensions, although our a priori power calculations suggested a higher power to detect differences in subjects from the ROSE study as compared to the PERL 2 study, whereas we only find significant differences in bony pelvic dimensions in the young primiparous subjects.
CONCLUSIONS
Young primiparas with de novo postpartum stress urinary incontinence have wider bony pelves than continent primiparas or nulliparas. By contrast, there are no significant differences in pelvic dimensions when comparing middle-aged women with and without symptomatic stress incontinence. There is a growing body of literature suggesting that postpartum SUI is not the same disease as midlife incontinence, despite similar disease phenotypes. Although we are only beginning to scratch the surface of understanding this complex system, it is becoming evident that the relative contributions of the different mechanisms promoting urinary continence vary over the course of a woman’s lifetime.
ACKNOWLEDGEMENTS
This research was supported by NIH R01 DK 051405 and the Office for Research on Women’s Health and the National Institute of Child Health and Human Development SCOR 1 P50 HD044406.
REFERENCES
- 1.Farage M, Miller K, Berardesca E, Maibach H. Psychosocial and societal burden of incontinence in the aged population: a review. Arch Gynecol Obstet. 2008;277(4):285–290. doi: 10.1007/s00404-007-0505-3. [DOI] [PubMed] [Google Scholar]
- 2.Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trondelag. J Clin Epidemiol. 2000 Nov;53(11):1150–1157. doi: 10.1016/s0895-4356(00)00232-8. [DOI] [PubMed] [Google Scholar]
- 3.Kilpatrick S, Garrison E. Normal Labor and Delivery. In: Gabbe SG, Niebyl JR, Simpson JL, editors. Obstetrics: Normal and Problem Pregnancies. 5 ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2007. pp. 303–321. [Google Scholar]
- 4.Milsom I, Altman D, Lapitan MC, Nelson R, Sillen U, Thom D. Epidemiology of Urinary (UI) and Faecal (FI) Incontinence and Pelvic Organ Prolapse (POP) In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence 4th International Consultation on Incontinence. Paris, France: 2009. pp. 35–111. [Google Scholar]
- 5.Stav K, Alcalay M, Peleg S, Lindner A, Gayer G, Hershkovitz I. Pelvis architecture and urinary incontinence in women. Eur Urol. 2007 Jul;52(1):239–244. doi: 10.1016/j.eururo.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 6.Handa VL, Lockhart ME, Kenton KS, Bradley CS, Fielding JR, Cundiff GW, et al. Magnetic resonance assessment of pelvic anatomy and pelvic floor disorders after childbirth. Int Urogynecol J Pelvic Floor Dysfunct. 2009 Feb;20(2):133–139. doi: 10.1007/s00192-008-0736-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baragi RV, Delancey JO, Caspari R, Howard DH, Ashton-Miller JA. Differences in pelvic floor area between African American and European American women. Am J Obstet Gynecol. 2002 Jul;187(1):111–115. doi: 10.1067/mob.2002.125703. [DOI] [PubMed] [Google Scholar]
- 8.Hoyte L, Thomas J, Foster RT, Shott S, Jakab M, Weidner AC. Racial differences in pelvic morphology among asymptomatic nulliparous women as seen on three-dimensional magnetic resonance images. Am J Obstet Gynecol. 2005 Decv;193(6):2035–2040. doi: 10.1016/j.ajog.2005.06.060. [DOI] [PubMed] [Google Scholar]
- 9.DeLancey JO, Miller JM, Kearney R, Howard D, Reddy P, Umek W, et al. Vaginal birth and de novo stress incontinence: relative contributions of urethral dysfunction and mobility. Obstet Gynecol. 2007 Aug;110(2 Pt 1):354–362. doi: 10.1097/01.AOG.0000270120.60522.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeLancey JO, Trowbridge ER, Miller JM, Morgan DM, Guire K, Fenner DE, et al. Stress urinary incontinence: relative importance of urethral support and urethral closure pressure. J Urol. 2008 Jun;179(6):2286–2290. doi: 10.1016/j.juro.2008.01.098. discussion 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noll LE, Hutch JA. The SCIPP line--an aid in interpreting the voiding lateral cystourethrogram. Obstet Gynecol. 1969 May;33(5):680–689. [PubMed] [Google Scholar]
- 12.Chou Q, DeLancey JO. A structured system to evaluate urethral support anatomy in magnetic resonance images. Am J Obstet Gynecol. 2001 Jul;185(1):44–50. doi: 10.1067/mob.2001.116368. [DOI] [PubMed] [Google Scholar]
- 13.Dean AG, Sullivan KM, Soe MM. OpenEpi: Open Source Epidemiologic Statistics for Public Health. 2.3.1 ed. 2006. [Google Scholar]
- 14.Morgan DM, Umek W, Stein T, Hsu Y, Guire K, DeLancey JO. Interrater reliability of assessing levator ani muscle defects with magnetic resonance images. Int Urogynecol J Pelvic Floor Dysfunct. 2007 Jul;18(7):773–778. doi: 10.1007/s00192-006-0224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richter HE, Creasman JM, Myers DL, Wheeler TL, Burgio KL, Subak LL. Urodynamic characterization of obese women with urinary incontinence undergoing a weight loss program: the Program to Reduce Incontinence by Diet and Exercise (PRIDE) trial. Int Urogynecol J Pelvic Floor Dysfunct. 2008 Dec;19(12):1653–1658. doi: 10.1007/s00192-008-0694-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocha MA. The impact of pregnancy and childbirth in the urethra of female rats. Int Urogynecol J. 2007;18(6):645–651. doi: 10.1007/s00192-006-0221-8. [DOI] [PubMed] [Google Scholar]
- 17.Rocha MA. Impact of pregnancy and childbirth on female rats' urethral nerve fibers. Int Urogynecol J. 2007;18(12):1453–1458. doi: 10.1007/s00192-007-0355-3. [DOI] [PubMed] [Google Scholar]
- 18.DeLancey JO, Kane Low L, Miller JM, Patel DA, Tumbarello JA. Graphic integration of causal factors of pelvic floor disorders: an integrated life span model. Am J Obstet Gynecol. 2008 Dec;199(6):610 e1–610 e5. doi: 10.1016/j.ajog.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998;316(7139):1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]


