Abstract
Esophageal pressure can be used to approximate pleural pressure and might be clinically useful, particularly in the obese e.g to guide mechanical ventilator settings in critical illness. However, mediastinal artifact (the difference between true pleural pressure and esophageal pressure) may limit acceptance of the measurement, and reproducibility of esophageal pressure measurements remains unknown. Therefore, we aimed to assess the effect of body posture on esophageal pressure in a cohort of obese but healthy subjects, some of whom had multiple measurements, to address the clinical robustness of esophageal manometry. Twenty-five overweight and obese subjects (BMI>25kg/m2) and 11 control lean subjects (BMI<25kg/m2) underwent esophageal manometry with pressures measured seated and supine. Twenty overweight and obese subjects had measurements repeated after ~1-2 weeks. Anthropometric data and sitting and supine spirometry were recorded. The average end-expiratory esophageal pressures sitting and supine were greater in the overweight and obese group than the lean group (sitting −0.1±2.1 vs. −3.3±1.2cmH2O, supine 9.3±3.3 vs. 6.9±2.8cmH2O, respectively). The mean differences between repeated measurements were small (−0.3 ± 1.7cmH2O sitting and −0.1 ± 1.5cmH2O supine). Esophageal pressures correlated with a number of anthropometric and spirometric variables. In conclusion, esophageal pressures are slightly greater in overweight and obese subjects than lean subjects; but changes with position are similar in both groups. These data indicate that mediastinal weight and postural effects on esophageal pressure are within a clinically acceptable range, and suggest that esophageal manometry can be used to inform clinical decision making across wide range of body types.
Keywords: Obesity, mediastinal artifact, pleural pressure, transpulmonary pressure, lung
Introduction
Lung inflation depends on transpulmonary pressure (PL), defined as the airway pressure minus the pleural pressure (Ppl).[1] In certain clinical situations, such as in the care of patients with the Acute Respiratory Distress Syndrome (ARDS), knowledge of pleural pressure can be helpful in preventing lung injury by minimizing over-distension (high PL) or atelectrauma (low or negative PL).[2] However, direct measurements of pleural pressure in humans are impractical, since intrapleural catheters carry risk for bleeding, infection, and pneumothorax. Instead, most clinical practice to date has used plateau pressure (airway pressure minus body surface pressure, usually atmospheric) as a surrogate marker of transpulmonary pressure. Alternatively, we and others have used esophageal manometry as a safe and relatively non-invasive approximation of pleural pressure.[3] A clinical trial using an individualized, physiological approach to mechanical ventilation directed by estimated transpulmonary pressure showed an improvement in oxygenation, and a trend toward improved survival compared to standard ARDS Network management.[4] We found that many patients had a higher than expected pleural pressure, and benefitted from high levels of positive end-expiratory pressure (PEEP) to maintain a positive end-expiratory PL.
However, some experts were concerned that the high esophageal pressures we measured in critical illness did not accurately reflect pleural pressures.[5, 6] Specifically, there has been debate over the magnitude of what is sometimes called the “mediastinal artifact.”[7] Pleural and esophageal pressure increase from the sitting to supine position. Pleural pressure increases due to increased abdominal pressure transmitted across the diaphragm and into the chest; the amount varying by the tension developed across the diaphragm. Functional residual capacity (FRC) decreases as a result. However, esophageal pressure (PEs) increases more with a position change than the directly measured pleural pressure. Measured in a small number of subjects by Mead and Gaensler, PEs increased about 3cmH2O more with position change than the simultaneously measured pleural pressure [3], a finding consistent with subsequent studies.[8] This difference between mid-lung pleural pressure and esophageal pressure when supine is thought to represent the compression of the esophagus by mediastinal structures, such as the heart, and is referred to as the mediastinal artifact. Some have argued that the magnitude of imprecision from these effects may be small in relation to overall utility of PEs in critical illness; however, this assertion is based on data from lean subjects.[9] The mediastinal artifact could be greater in overweight and obese subjects due to mediastinal fat deposits.[10] Additionally, changes in technique (catheter placement, balloon volume, etc) alter the measured pressure. In our prior study, we used a relatively straightforward technique that would be easily reproducible if esophageal manometry is found to be beneficial in the management of critically ill patients.
By measuring the change in PEs with changes in position, we sought to determine whether positional effects were greater in overweight and obese patients than in lean subjects. If changes in PEs are similar in both groups (obese and lean), it would further validate our esophageal manometry technique. Furthermore, to be of clinical value, esophageal manometry must not only be accurate, but also precise, reproducible and simple to measure. Although manometry has been measured in a variety of cohorts, reproducibility has not previously been reported to our knowledge. Therefore, we also sought to determine the reproducibility of esophageal pressure measurements. Finally, with an exploratory intent, we sought to determine whether anthropometric and spirometric measurements could explain differences in PEsbetween subjects or changes that occur with position change. Ultimately, any such relationship(s) could be useful in understanding the relative contribution of physiology (e.g. obesity) and pathophysiology (e.g. pulmonary edema, increased abdominal pressure) in a given patient.
Methods
Subjects
Overweight and obese (BMI>25kg/m2) subjects with and without asthma were recruited as part of an ongoing, two night sleep study. Asthma, if present, had been diagnosed by a physician and was confirmed by provocative concentration of methacholine causing a 20% fall in forced expiratory volume (PC20)<12mg/mL. Asthma subjects were clinically stable, with no medication changes within the month prior to the study, no systemic glucocorticoids, no emergency room visits or hospital admissions. No subject had smoked within 3 months prior to the study, or had >10 pack-year smoking history. Other systemic, respiratory, or esophageal disease, other than snoring or obstructive sleep apnea, was excluded based on thorough history and physical examination. A second group of lean (BMI<25kg/m2) healthy subjects was also recruited, based on the same exclusion criteria, and studied on a single occasion. All subjects gave written, informed consent (approved by local Institutional Review Board).
Measurements
Subject height, weight, and neck, chest, waist and hip circumferences were measured. Spirometry was performed according to ATS criteria using a handheld spirometer (KoKo spirometer, nSpire Health Inc., Longmont, CO) sitting and supine. Forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) are compared to age, gender, and height matched controls to calculate percent predicted values.[11]
Esophageal manometry
Prior to insertion, both nostrils were sprayed with 0.05% oxymetazoline hydrochloride, a decongestant, and the more patent nostril was then anesthetized with 4% lidocaine topical spray. The balloon/catheter (Ackrad Labs Adult Esophageal Balloon Catheter Set, Trumbull, CT) was passed via the nares 40cm and taped in place to prevent movement. The balloon is thin-walled, 9.5cm long and with perimeter approximately 1.5cm, around an 86cm closed-end catheter (inner diameter ~1mm). The balloon was emptied and then inflated with 1mL of air using a double ground glass syringe, and connected to a pressure transducer (DP103-26, Validyne Engineering Corp., Northridge, CA), previously calibrated using a digital manometer (Product #302227, Respironics, Murraysville, PA) to ±20cmH2O relative to atmosphere. Intra-thoracic (rather than intra-abdominal) placement was confirmed by the presence of cardiac oscillations and expected pressure changes with spontaneous breathing (negative esophageal pressure swing with inspiration) using established techniques.[4]
While the subject was seated, esophageal pressure was recorded during quiet breathing for approximately 3 minutes. Measurements were repeated supine, with bed flat and one pillow. Data were acquired on a 1401 plus interface and Spike2 software (Cambridge Electronic Design Ltd, Cambridge, UK).
If well tolerated, esophageal manometry was repeated on the second sleep study night in the overweight and obese group, ~1-2 weeks later. Repeat history and physical examination confirmed no intercurrent illness.
Statistics and Data Analysis
During quiet breathing 10 consecutive breaths free from artifact (e.g. movement, sighs, swallows) were selected for analysis. Esophageal pressure values were taken at end expiration and at the nadir of pressure during inspiration. Reproducibility of measurements was assessed by Pearson correlation coefficient. If data were available on both study nights, these values were averaged prior to linear regression analysis. Linear regression was performed for each dependent variable (sitting end-expiratory PEs, supine end-expiratory PEs, difference between sitting and supine end-expiratory esophageal pressure, and inspiratory pressure swings upright and supine) and independent variables: gender, BMI, height, weight, circumferences, sitting and supine spirometry (FEV1, FVC, FEV1/FVC and percent predicted FEV1 and FVC), and diagnosis of asthma. A multivariate regression was also performed with the same dependent variables, with independent variables chosen using a backwards stepwise approach. . P value <0.05 was considered statistically significant. Values are presented as mean ± standard deviation.
Results
Twenty-five overweight and obese subjects were recruited. Of these, 20 underwent repeat esophageal manometry. Two subjects did not complete the second night of the study, while 3 subjects declined repeat esophageal balloon placement. Second night sitting data were not available in one subject. As designed, average BMI for this group was >30kg/m2. For comparison, 11 normal weight subjects were recruited and had esophageal manometry performed one time. Subject characteristics are listed in Table #1.
Table 1.
Subject characteristics and spirometry
| Overweight and Obese | Normal Weight |
|||
|---|---|---|---|---|
| Total | No Asthma | Asthma | ||
| Sample Size (M/F) |
25 (9/16) | 10 (6/4) | 15 (3/12) | 11 (7/4) |
| Age (years) | 35.6 ± 12.4 | 35.7 ± 12.2 | 35.5 ± 13.0 | 32.5 ± 11.2 |
| BMI (kg/m2) | 33.3 ± 5.7 | 34.1 ± 5.8 | 30.7 ± 5.8 | 22.5 ± 2.1† |
|
| ||||
|
Seated
Spirometry |
||||
| FEV1% predicted | 82.7 ± 17.6 | 96.1 ± 11.7 | 73.7 ± 15.1* | 96.5 ± 11.8† |
| FVC% predicted | 90.6 ± 15.3 | 99.1 ± 12.3 | 84.9 ± 14.6* | 102.7 ± 7.0† |
| FEV1/FVC | 0.77 ± 0.09 | 0.81 ± 0.04 | 0.74 ± 0.10 | 0.79 ± 0.09 |
|
| ||||
|
Supine
Spirometry |
||||
| FEV1% predicted | 76.4 ± 18.1 | 88.7 ± 12.2 | 67.6 ± 16.7* | 88.0 ± 11.1 |
| FVC% predicted | 85.1 ± 16.6 | 93.8 ± 12.0 | 78.9 ± 16.9* | 97.5 ± 8.7† |
| FEV1/FVC | 0.75 ± 0.08 | 0.79 ± 0.05 | 0.73 ± 0.10 | 0.77 ± 0.08 |
p<0.05 compared to No Asthma.
p <0.05 Normal weight compared to Overweight and Obese
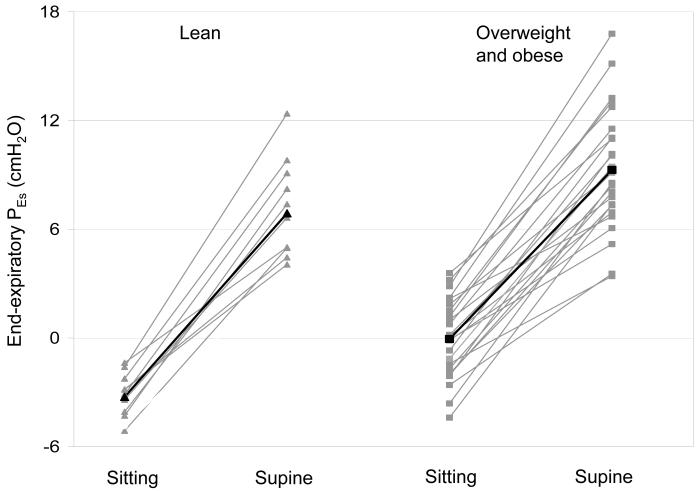
Both sitting and supine PEs were higher in the overweight and obese cohort than in the lean cohort (Figure #1). However, the difference in PEs with change from sitting to supine was similar in both groups. In the overweight and obese group, there were no differences in end-expiratory PEs in those with asthma versus no asthma. However, inspiratory pressures were slightly less positive/more negative in the subjects with asthma (Table #2).
Figure 1.
End expiratory esophageal pressure sitting and supine for each subject. Lean (▲) and overweight and obese (■), with each group mean in black.
Table 2.
Mean esophageal pressures (cmH2O)
| Overweight and Obese | Normal Weight |
||||
|---|---|---|---|---|---|
| Total | No Asthma | Asthma | |||
| Sitting | Expiratory | −0.1 ± 2.1 | 0.7 ± 2.0 | 0.1 ± 2.3 | −3.3 ± 1.2† |
| Inspiratory | −5.8 ± 2.4 | −4.4 ± 1.9 | −6.5 ± 2.4* | −7.0 ± 1.5 | |
| ΔPES | 5.7 ± 2.5 | 5.1 ± 2.0 | 6.6 ± 2.6 | 3.7 ± 1.6 | |
|
| |||||
| Supine | Expiratory | 9.3 ± 3.3 | 10.3 ± 3.6 | 9.4 ± 3.9 | 6.9 ± 2.8† |
| Inspiratory | −1.2 ± 3.6 | 0.3 ± 3.1 | −2.7 ± 3.6* | 0.3 ± 4.1 | |
| ΔPES | 10.4 ± 4.0 | 9.9 ± 4.8 | 12.0 ± 4.0 | 6.6 ± 2.4 | |
|
| |||||
| Supine - | Expiratory | 9.3 ± 2.8 | 9.6 ± 3.0 | 9.2 ± 2.8 | 10.1 ± 2.6 |
| Sitting | Inspiratory | 4.6 ± 3.2 | 4.7 ± 3.7 | 3.8 ± 3.1 | 7.2 ± 3.3 |
p <0.05 Asthma compared to No Asthma.
p <0.05 Normal weight compared to Overweight and Obese
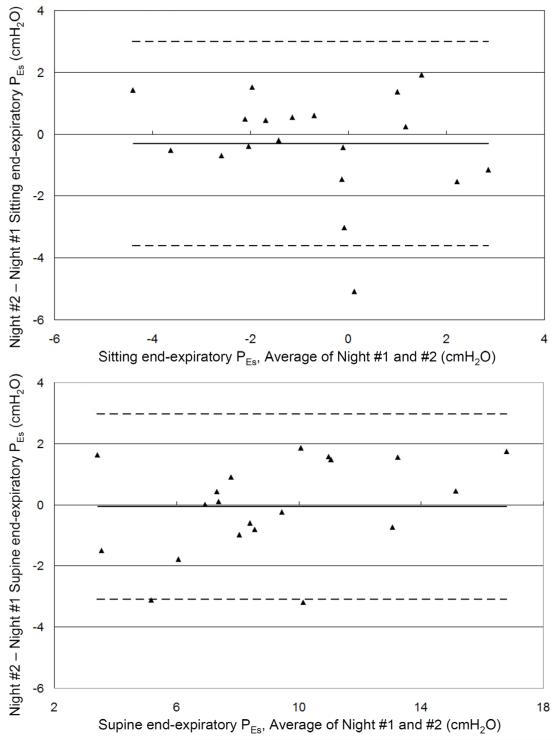
Data from subjects who had two measurements on separate nights were analyzed for reproducibility. Pearson correlation coefficients (r) were 0.69 for sitting (n = 19) and 0.92 for supine (n = 20) esophageal pressures. Bland-Altman analysis showed good agreement between the two measurements with mean difference between first and second measurements −0.3 ± 1.7cmH2O sitting and −0.1 ± 1.5cmH2O supine (Figure #2).
Figure 2.
Bland-Altman plots of end-expiratory esophageal pressures (sitting, A and supine, B) from two separate measurements. Solid line is bias, and dotted lines represent the 95% confidence interval.
Using all 36 subjects, univariate linear regression analysis showed that several factors correlated with sitting and supine esophageal pressures (Table #3). As BMI, weight or body circumference measurements increased, PEs increased. As spirometric measures (such as FEV1% predicted and FVC% predicted) decreased, PEs increased. Sitting and supine esophageal pressures were also correlated with one another (p<0.01, R2=0.36). There was no effect of gender or the diagnosis of asthma on any measurement. Backward stepwise multivariate regression analysis showed that waist circumference and sitting FVC% predicted were most correlated with sitting esophageal pressure, while waist/hip ratio and sitting FVC% predicted were most correlated with supine esophageal pressure. The correlations were stronger with sitting esophageal pressure compared to supine esophageal pressures. The difference in PEs between sitting and supine was not associated with any measured variable.
Table 3.
Univariate regression analysis of end-expiratory esophageal pressures
| Sitting PEs | Supine PEs | ||
|---|---|---|---|
| R2 | R2 | ||
| BMI | 0.49# | 0.19# | |
| Weight | 0.35# | 0.16* | |
| Height | 0.06 | 0.02 | |
| Gender | 0.07 | 0 | |
| Asthma dx | 0.10 | 0.01 | |
|
| |||
|
Body
Measurements |
Neck | 0.27# | 0.13* |
| Chest | 0.39# | 0.22# | |
| Waist | 0.51# | 0.25# | |
| Hip | 0.37# | 0.11* | |
| Waist/Hip | 0.23# | 0.23# | |
|
| |||
|
Spirometry
Sitting |
FEV1 | 0.25# | 0.13* |
| FEV1 %pred | 0.23# | 0.15* | |
| FVC | 0.36# | 0.16* | |
| FVC %pred | 0.48# | 0.24# | |
| FEV1/FVC | 0 | 0 | |
|
| |||
|
Spirometry
Supine |
FEV1 | 0.23# | 0.13* |
| FEV1 %pred | 0.21# | 0.16* | |
| FVC | 0.33# | 0.16* | |
| FVC %pred | 0.41# | 0.22# | |
| Multivariate regression analysis of end-expiratory esophageal pressures | |||
|---|---|---|---|
| Coefficient |
Standard Error of
coefficient |
Standardized coefficient | |
| Sitting | |||
| Waist circumference | 0.067 | 0.016 | 0.50 |
| FVC %predicted, sitting |
−0.071 | 0.021 | −0.42 |
| p = <0.001 | R2 = 0.66 | ||
|
| |||
| Supine | |||
| Waist/hip ratio | 12.64 | 5.97 | 0.337 |
| FVC %predicted, sitting |
−0.08 | 0.037 | −0.346 |
| p = 0.002 | R2 = 0.33 | ||
p<0.05
p<0.01
Univariate linear regression analysis showed that pressure swings sitting and supine with inspiration were correlated with BMI, but were more strongly correlated with predicted and actual spirometric measurements (Table #4). Additionally, multivariate analysis showed that FEV1/FVC ratio and FVC% predicted were most correlated with pressure swings during quiet breathing.
Table 4.
Univariate regression analysis of inspiratory pressure swings (Δ PEs)
| Sitting Δ PEs | Supine Δ PEs | ||
|---|---|---|---|
| R2 | R2 | ||
| BMI | 0.13* | 0.19* | |
| Weight | 0.08 | 0.07 | |
| Height | 0.03 | 0.13* | |
| Gender | 0.04 | 0.06 | |
| Asthma dx | 0.27* | 0.31* | |
|
| |||
|
Body
Measurements |
Neck | 0.14* | 0.22# |
| Chest | 0.09 | 0.17* | |
| Waist | 0.16* | 0.25* | |
| Hip | 0.10 | 0.15* | |
| Waist/Hip | 0.11 | 0.16* | |
|
| |||
|
Spirometry
Sitting |
FEV1 | 0.29* | 0.48* |
| FEV1 %pred | 0.43* | 0.52* | |
| FVC | 0.2* | 0.39* | |
| FVC %pred | 0.29# | 0.42* | |
| FEV1/FVC | 0.2* | 0.17* | |
|
| |||
|
Spirometry
Supine |
FEV1 | 0.25# | 0.43* |
| FEV1 %pred | 0.33# | 0.47* | |
| FVC | 0.19* | 0.37* | |
| FVC %pred | 0.27# | 0.39* | |
| Multivariate regression analysis of inspiratory pressure swings | |||
|---|---|---|---|
| Coefficient |
Standard Error of
coefficient |
Standardized coefficient | |
| Sitting FEV1/FVC | −11.26 | 3.39 | −0.42 |
| FVC %predicted, sitting |
−0.087 | 0.021 | −0.52 |
| p = <0.001 | R2 = 0.47 | ||
|
| |||
| Supine | |||
| FEV1/FVC | −17.19 | 5.05 | −0.39 |
| FVC %predicted, sitting |
−0.08 | 0.0.14 | −0.63 |
| p = <0.001 | R2 = 0.57 | ||
p<0.05
#p<0.01
Discussion
The novel findings of our study are: 1) using our esophageal manometry technique, that although end-expiratory esophageal pressures are slightly higher in overweight and obese subjects than lean subjects, the change in PEs from sitting to supine in obese subjects is not greater than in lean subjects, 2) that repeated PEs measurements show good agreement, and 3) that several anthropometric and spirometric measurements correlate with PEs.
When examined as separate cohorts, PEs was greater in the overweight and obese subjects than in lean subjects, both sitting and supine. The current work compares well with the existing literature, which suggests that PEs increases with increasing BMI (see Table #5). Indeed, across all subjects, BMI was correlated with sitting and supine end-expiratory PEs. Of note, the slightly higher pressures in the lean subjects in this study compared to Washko may reflect the increased balloon volume used (1.0 vs. 0.5mL, respectively).[8] This volume difference will affect the measured pressure slightly[12], increasing the measured pressure by approximately 1-3cmH2O in this catheter-transducer system.[13]
Table 5.
Published esophageal manometry data.
| Study | Subjects | BMI (kg/m2) | PEs | |
|---|---|---|---|---|
| Supine | Sitting | |||
| Washko(6) | Healthy, lean | 24.5 ± 3.7 | 3.3 ± 3.2 | -3.7 ± 2.0 |
| Behazin(15) | Surgical, paralyzed, lean |
25.2 ± 2.8 | 6.9 ± 3.1 | |
|
| ||||
| Healthy, lean | 22.5 ± 2.1 | 6.9 ± 2.8 | -3.3 ± 1.2 | |
| Current study | Healthy, overweight and obese |
33.3 ± 5.7 | 9.3 ± 3.3 | -0.1 ± 2.1 |
|
| ||||
| Healthy, lean | 23.6 ± 3.7 | 5.1 ± 3.5 | -2.0 ± 2.7 | |
| Steier(18) | Healthy, morbidly obese |
42.8 ± 8.6 | 12.8 ± 4.8 | 4.0 ± 4.2 |
| Behazin(15) | Surgical, paralyzed, obese |
48.5 ± 8.9 | 12.5 ± 3.9 | |
|
| ||||
| Talmor(10) | ICU, acute respiratory failure |
31.0 ± 10.0 | 17.5 ± 5.7 | |
Increased esophageal pressure in the overweight and obese likely reflects true increases in pleural pressure, rather than an artifact of esophageal manometry in the overweight and obese. For example, FRC, which represents a balance between lung and chest wall recoil forces, is reduced even at moderate levels of obesity (BMI 30-35kg/m2) such as those studied here, consistent with increased pleural pressures.[14-17] Pleural pressure is probably increased in the obese due to both increased abdominal pressure, which is transmitted into the chest, and increased chest wall mass. Increased abdominal pressure has been observed in the obese[18], and documented to resolve with weight loss.[19] In the work by Babb and colleagues, the increase in abdominal pressure appeared to explain most if not all of a decrease in FRC in obese women.[16] In a cohort of critically ill patients, both gastric pressure and bladder pressure explained some of the variance in end-expiratory esophageal pressure.[9] Similarly, in a morbidly obese group anesthetized and about to undergo surgery, gastric pressure and esophageal pressure were correlated at end expiration.[20] How much of the increased pressure is transmitted to the chest across the diaphragm, and whether acute changes in intra-abdominal pressure (e.g. pancreatitis, trauma) affect the pleural pressures differently from chronic changes (e.g. obesity, ascites) is not known. For example, in some human disease, such as COPD, and in animal models of obesity, diaphragm re-modeling does occur.[21, 22]
Although we anticipated high esophageal pressures in the overweight and obese, we were somewhat surprised that re-positioning subjects from sitting to supine increased PEs by a similar amount in both the overweight and lean groups. Our results are similar to those from Steier and colleagues, who reported a similar change in PEs from seated to supine in an obese and a non-obese control group [8.8 and 7.1cmH2O (p value non-significant), respectively].[23] That our data are similar to those from Steier and colleagues suggests that our straight-forward and simple technique for measurement of esophageal pressure is valid. Our data suggest that mediastinal artifact is not increased with increasing BMI, at least within the mild to moderate range of obesity that we studied. However, an alternative interpretation is that there is an increase in the mediastinal effect or artifact with obesity, but this is offset by a decreased posture-related change in lung volume in the obese. As discussed above, the change in PEs reflects both a change in intra-abdominal pressure and lung volume that occurs with position change, and the weight of the mediastinum now atop the esophageal balloon. In the subjects studied by Washko approximately half of the change in esophageal pressure was attributed to lung volume changes. The relative contribution of these effects may be different in overweight and obese subjects. For example, morbidly obese subjects have much less or even zero lung volume change with position change, with one study reporting that in obese subjects (BMI 44±3kg/m2) FRC decreased only 70mL sitting to supine, compared to 730mL in a leaner (BMI<27kg/m2) control group.[24, 25] Even in overweight and more modestly obese subjects, position change probably has a reduced effect on lung volume.[15] Unfortunately, whether this finding reflects a lower starting position on the pressure-volume curve of the total respiratory system, or reflects a similar smaller change in abdominal pressure with position change in the overweight and obese is not known. Simultaneous measurements of gastric or bladder pressures during our study would have been helpful to answer these questions. Any increase in mediastinal ‘artifact’ in the overweight and obese would presumably be due to increased mediastinal fat. Most thoracic fat is extra-pleural, with the bulkiest deposits of the thorax in the mediastinum.[10]
To our knowledge, the current study is the first to assess reproducibility of esophageal manometry. The standard deviation of the measurements was <2cmH2O, with 95% confidence interval approximately ±3cmH2O. This degree of precision, if similar in other clinical settings including critical illness, should be adequate for repeated measurements, if necessary. Put another way, differences greater than this amount should suggest true changes in (patho-) physiology. Furthermore, esophageal manometry seems as robust as other commonly used ICU parameters, such as pulmonary artery catheter and bladder measurements of intra-abdominal pressure.[26] In terms of clinical use in the ICU, our data provide guidance for ICU practitioners as to a normal value of esophageal pressure in subjects with mild to moderate obesity. Our healthy obese controls had lower esophageal pressures than an ICU cohort with similar BMI.[13] The additional increase in esophageal pressure likely reflects some aspect of critical illness, such as fluid accumulation (e.g. pulmonary edema, which would increase the magnitude of gravitational gradient through the lung) or elevated intra-abdominal pressure, which is common in ICU patients.[27] Overall, we believe esophageal manometry useful in the management of ventilated ICU patients, as it can both identify elevated pleural pressure as a cause or contributor of hypoxemic respiratory failure, and provide reassurance that the correct treatment with increased positive end expiratory pressure (PEEP) does not cause lung over-distension.
Several anthropometric and spirometric measurements correlated with PEs. Although BMI correlated with esophageal pressure, direct measurements of abdominal girth, such as waist circumference and waist/hip ratio were more predictive of esophageal pressures. Again, these findings may further emphasize the importance of intra-abdominal pressure on intra-thoracic pressures, since measurements of abdominal girth, including waist-hip ratio and sagittal abdominal diameter, have been previously found to correlate with intra-abdominal pressure.[28, 29] Along these lines, anthropometric and spirometric measurements explained 66% of the variance in the sitting esophageal pressure. However, these measurements explained less of the variance (only 33%) in the supine position, perhaps because anthropometric measurements were made while subjects were upright. As expected, decreasing FVC and FVC% predicted were associated with increased esophageal pressure. As above, this finding may simply reflect that FVC decreases with obesity. However, as shown in the multivariate regression, the addition of FVC improved the robustness of our model, suggesting that lung volumes do capture additional information about the effects of obesity on the respiratory system. Overall, however, these measurements predict only a portion of the variance of esophageal pressures in healthy controls. Thus, in most clinical situations requiring estimation of pleural pressure at end-expiration, esophageal manometry will likely be required.
At first glance, it seems surprising that many of our healthy overweight subjects appear to have a negative transpulmonary pressure (esophageal pressure greater than airway pressure) at end-expiration in the supine position. Three factors may help explain these findings. First, PEs is a measure of local pleural pressure, which varies with height along the gravitational gradient. Pleural and esophageal pressures from dependent lung regions are greater than the same measurements from non-dependent regions – although this gradient is usually small in healthy lungs, somewhere between 0.2 – 1cmH2O/cm.[30] The average pleural pressure, or the pleural pressure for a large portion of lung, may still be negative. Second, as discussed, the effect of mediastinal structures may overestimate the true local pleural pressure by about 3-5cmH2O when supine. Third, negative transpulmonary pressure could also imply that alveolar and airway pressure have not equilibrated – which could be due to tidal airway closure or expiratory flow limitation. This phenomenon can occur at low lung volumes due to obesity and increases with normal aging.[31, 32] Very high local pleural pressure suggests that airway closure might be occurring in our obese subjects (who presumably breathe at low lung volumes), especially supine. Indeed, intrinsic positive end-expiratory pressure (PEEPi) has been reported in severe obesity (BMI>40kg/m2) ostensibly in the setting of decreased FRC and airway closure.[23, 33] We were able to look for PEEPi when our subjects were supine and flow was measured, by comparing the change in PEs from end-expiratory baseline prior to the start of inspiratory flow. Intrinsic PEEP was observed in 5 of the overweight and obese group, 4 of whom also had asthma. The single subject without asthma (and no obstruction on spirometry) but with PEEPi in the supine position had a BMI of 42.5kg/m2.
Although we were mostly focused on end-expiratory pressures, inspiratory pressure swings increased with increasing BMI (and several other markers of obesity), a finding reported by others as well.[23] However, in this cohort that included asthmatics, pressure swings were also correlated with markers of asthma severity, such as the FEV1/FVC ratio.
Limitations
There are limitations to our study. First, the esophageal balloon was placed according to our established protocol,[4] using cardiac oscillations and inspiratory falls in pleural pressure to guide placement, rather than confirming placement with radiology or the occlusion test. Imaging could have been useful to confirm balloon placement and to assess adjacent areas of lung parenchyma which may be at risk of compression/collapse.[34] However, although differences of a few centimeters in balloon placement in the esophagus will change measured esophageal pressure, the magnitude of this change is negligible.[35] Furthermore, the long partially-inflated balloon used will minimize differences due to placement, as the air bubble will always flow towards the area of least pressure along the entire length of the balloon. We did not perform the occlusion test,[36] instead focusing on an easily reproducible method that could be applied across broad patient populations, including ICU patients who may not be spontaneously breathing. Second, we did not measure abdominal pressure or absolute lung volumes. Both measurements would have been useful to confirm some of the speculations and conclusions we have made from our data. Third, we included subjects with asthma, who were participating in another ongoing study. However, asthma was generally well controlled with minor obstructive abnormalities on spirometry. Again, although the diagnosis of asthma impacted inspiratory pressure swings, it did not appear to affect expiratory esophageal pressure measurements.
Conclusion
These data confirm that esophageal pressures are elevated in the overweight and obese. However, the change in PEs with position change using our esophageal manometry technique is similar in overweight and obese and lean subjects. Repeated measurements show good agreement. Taken together, these results suggest that our easily reproducible esophageal manometry technique can be used to inform clinical decision making across wide range of body types. Some anthropometric and spirometric measurements that may relate to abdominal pressures correlate with esophageal pressure. However, these measurements only explain a portion of the variance of PEs and in clinical situations requiring estimation of pleural pressure, esophageal manometry remains the gold standard.
Acknowledgements
Supported by National Institutes of Health grants F32 HL097578, K24 HL 093218, R01 HL090897, R01 HL085188, 1 P01 HL 095491, K23 HL105542 and American Heart Association 0840159N.
Also supported by the Harvard Clinical and Translational Science Award (UL1 RR 025758 and KL2 RR 025757).
Footnotes
Disclosures
The authors have no conflict of interest related to this manuscript to declare.
References
- (1).Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol. 1970 May;28(5):596–608. doi: 10.1152/jappl.1970.28.5.596. [DOI] [PubMed] [Google Scholar]
- (2).Chu EK, Whitehead T, Slutsky AS. Effects of cyclic opening and closing at low- and high-volume ventilation on bronchoalveolar lavage cytokines. Critical care medicine. 2004 Jan;32(1):168–74. doi: 10.1097/01.CCM.0000104203.20830.AE. [DOI] [PubMed] [Google Scholar]
- (3).Mead J, Gaensler EA. Esophageal and pleural pressures in man, upright and supine. J Appl Physiol. 1959 Jan;14(1):81–3. doi: 10.1152/jappl.1959.14.1.81. [DOI] [PubMed] [Google Scholar]
- (4).Talmor D, Sarge T, Malhotra A, O’Donnell CR, Ritz R, Lisbon A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. The New England journal of medicine. 2008 Nov 13;359(20):2095–104. doi: 10.1056/NEJMoa0708638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Bernard GR. PEEP guided by esophageal pressure--any added value? The New England journal of medicine. 2008 Nov 13;359(20):2166–8. doi: 10.1056/NEJMe0806637. [DOI] [PubMed] [Google Scholar]
- (6).Hager DN, Brower RG. Customizing lung-protective mechanical ventilation strategies. Critical care medicine. 2006 May;34(5):1554–5. doi: 10.1097/01.CCM.0000216183.25478.03. [DOI] [PubMed] [Google Scholar]
- (7).Knowles J, Hong SK, Rahn H. Possible errors using esophageal balloon in determination of pressure-volume characteristics of the lung and thoracic cage. Journal of Applied Physiology. 1959;14:525–30. 1959. [Google Scholar]
- (8).Washko GR, O’Donnell CR, Loring SH. Volume-related and volume-independent effects of posture on esophageal and transpulmonary pressures in healthy subjects. J Appl Physiol. 2006 Mar;100(3):753–8. doi: 10.1152/japplphysiol.00697.2005. [DOI] [PubMed] [Google Scholar]
- (9).Loring SH, O’Donnell CR, Behazin N, Malhotra A, Sarge T, Ritz R, et al. Esophageal pressures in acute lung injury: do they represent artifact or useful information about transpulmonary pressure, chest wall mechanics, and lung stress? J Appl Physiol. Mar;108(3):515–22. doi: 10.1152/japplphysiol.00835.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Fisher ER, Godwin JD. Extrapleural fat collections: pseudotumors and other confusing manifestations. Ajr. 1993 Jul;161(1):47–52. doi: 10.2214/ajr.161.1.8517319. [DOI] [PubMed] [Google Scholar]
- (11).Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. The American review of respiratory disease. 1981 Jun;123(6):659–64. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- (12).Milic-Emili J, Mead J, Turner JM, Glauser EM. Improved Technique for Estimating Pleural Pressure from Esophageal Balloons. J Appl Physiol. 1964 Mar;19:207–11. doi: 10.1152/jappl.1964.19.2.207. [DOI] [PubMed] [Google Scholar]
- (13).Talmor D, Sarge T, O’Donnell CR, Ritz R, Malhotra A, Lisbon A, et al. Esophageal and transpulmonary pressures in acute respiratory failure. Critical care medicine. 2006 May;34(5):1389–94. doi: 10.1097/01.CCM.0000215515.49001.A2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006 Sep;130(3):827–33. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- (15).Benedik PS, Baun MM, Keus L, Jimenez C, Morice R, Bidani A, et al. Effects of body position on resting lung volume in overweight and mildly to moderately obese subjects. Respiratory care. 2009 Mar;54(3):334–9. [PubMed] [Google Scholar]
- (16).Babb TG, DeLorey DS, Wyrick BL, Gardner PP. Mild obesity does not limit change in end-expiratory lung volume during cycling in young women. J Appl Physiol. 2002 Jun;92(6):2483–90. doi: 10.1152/japplphysiol.00235.2001. [DOI] [PubMed] [Google Scholar]
- (17).DeLorey DS, Wyrick BL, Babb TG. Mild-to-moderate obesity: implications for respiratory mechanics at rest and during exercise in young men. International journal of obesity (2005) 2005 Sep;29(9):1039–47. doi: 10.1038/sj.ijo.0803003. [DOI] [PubMed] [Google Scholar]
- (18).Lambert DM, Marceau S, Forse RA. Intra-abdominal pressure in the morbidly obese. Obesity surgery. 2005 Oct;15(9):1225–32. doi: 10.1381/096089205774512546. [DOI] [PubMed] [Google Scholar]
- (19).Sugerman HJ. Effects of increased intra-abdominal pressure in severe obesity. The Surgical clinics of North America. 2001 Oct;81(5):1063–75. vi. doi: 10.1016/s0039-6109(05)70184-5. [DOI] [PubMed] [Google Scholar]
- (20).Behazin N, Jones SB, Cohen RI, Loring SH. Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol. Jan;108(1):212–8. doi: 10.1152/japplphysiol.91356.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Farkas GA, Gosselin LE, Zhan WZ, Schlenker EH, Sieck GC. Histochemical and mechanical properties of diaphragm muscle in morbidly obese Zucker rats. J Appl Physiol. 1994 Nov;77(5):2250–9. doi: 10.1152/jappl.1994.77.5.2250. [DOI] [PubMed] [Google Scholar]
- (22).Testelmans D, Crul T, Maes K, Agten A, Crombach M, Decramer M, et al. Atrophy and hypertrophy signalling in the diaphragm of patients with COPD. Eur Respir J. Mar;35(3):549–56. doi: 10.1183/09031936.00091108. [DOI] [PubMed] [Google Scholar]
- (23).Steier J, Jolley CJ, Seymour J, Roughton M, Polkey MI, Moxham J. Neural respiratory drive in obesity. Thorax. 2009 Aug;64(8):719–25. doi: 10.1136/thx.2008.109728. [DOI] [PubMed] [Google Scholar]
- (24).Watson RA, Pride NB. Postural changes in lung volumes and respiratory resistance in subjects with obesity. J Appl Physiol. 2005 Feb;98(2):512–7. doi: 10.1152/japplphysiol.00430.2004. [DOI] [PubMed] [Google Scholar]
- (25).Yap JC, Watson RA, Gilbey S, Pride NB. Effects of posture on respiratory mechanics in obesity. J Appl Physiol. 1995 Oct;79(4):1199–205. doi: 10.1152/jappl.1995.79.4.1199. [DOI] [PubMed] [Google Scholar]
- (26).Shuster MH, Haines T, Sekula LK, Kern J, Vazquez JA. Reliability of intrabladder pressure measurement in intensive care. Am J Crit Care. Jul;19(4):e29–39. doi: 10.4037/ajcc2010204. quiz e40. [DOI] [PubMed] [Google Scholar]
- (27).Malbrain ML, Chiumello D, Pelosi P, Bihari D, Innes R, Ranieri VM, et al. Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: a multiple-center epidemiological study. Critical care medicine. 2005 Feb;33(2):315–22. doi: 10.1097/01.ccm.0000153408.09806.1b. [DOI] [PubMed] [Google Scholar]
- (28).De Keulenaer BL, De Waele JJ, Powell B, Malbrain ML. What is normal intra-abdominal pressure and how is it affected by positioning, body mass and positive end-expiratory pressure? Intensive care medicine. 2009 Jun;35(6):969–76. doi: 10.1007/s00134-009-1445-0. [DOI] [PubMed] [Google Scholar]
- (29).Sugerman H, Windsor A, Bessos M, Wolfe L. Intra-abdominal pressure, sagittal abdominal diameter and obesity comorbidity. Journal of internal medicine. 1997 Jan;241(1):71–9. doi: 10.1046/j.1365-2796.1997.89104000.x. [DOI] [PubMed] [Google Scholar]
- (30).Lai-Fook SJ. Pleural mechanics and fluid exchange. Physiological reviews. 2004 Apr;84(2):385–410. doi: 10.1152/physrev.00026.2003. [DOI] [PubMed] [Google Scholar]
- (31).Farebrother MJ, McHardy GJ, Munro JF. Relation between pulmonary gas exchange and closing volume before and after substantial weight loss in obese subjects. British medical journal. 1974 Aug 10;3(5927):391–3. doi: 10.1136/bmj.3.5927.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Milic-Emili J, Torchio R, D’Angelo E. Closing volume: a reappraisal (1967-2007) European journal of applied physiology. 2007 Apr;99(6):567–83. doi: 10.1007/s00421-006-0389-0. [DOI] [PubMed] [Google Scholar]
- (33).Pankow W, Podszus T, Gutheil T, Penzel T, Peter J, Von Wichert P. Expiratory flow limitation and intrinsic positive end-expiratory pressure in obesity. J Appl Physiol. 1998 Oct;85(4):1236–43. doi: 10.1152/jappl.1998.85.4.1236. [DOI] [PubMed] [Google Scholar]
- (34).Albert RK, Hubmayr RD. The prone position eliminates compression of the lungs by the heart. American journal of respiratory and critical care medicine. 2000 May;161(5):1660–5. doi: 10.1164/ajrccm.161.5.9901037. [DOI] [PubMed] [Google Scholar]
- (35).Milic-Emili J, Mead J, Turner JM. Topography of Esophageal Pressure as a Function of Posture in Man. J Appl Physiol. 1964 Mar;19:212–6. doi: 10.1152/jappl.1964.19.2.212. [DOI] [PubMed] [Google Scholar]
- (36).Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J. A simple method for assessing the validity of the esophageal balloon technique. The American review of respiratory disease. 1982 Nov;126(5):788–91. doi: 10.1164/arrd.1982.126.5.788. [DOI] [PubMed] [Google Scholar]