Abstract
Transdermal nicotine almost doubles tobacco cessation rates; however little is known about what happens to smokers during the quit process when they are wearing the nicotine patch and confronted with high-risk smoking triggers. This is particularly important for smokers with psychological disorders who disproportionately represent today’s smokers and have more trouble quitting. Using a mixed between- and within-subjects design, smokers with anxiety disorders (n = 61) and smokers without any current Axis I disorders (n = 38) received transdermal nicotine (21 mg) or a placebo patch over two assessment days separated by 48 hours. Urge to smoke was evaluated during a 5-hour patch absorption period (reflecting general smoking deprivation) and during imaginal exposure to theoretically high-risk triggers containing smoking cues, anxiety cues, both, or neutral cues. No differences were observed between smokers with and without anxiety disorders. Significant Patch X Time and Patch X Cue Content interactions were found. Both patch conditions experienced an increase in urge during the deprivation period, but post-absorption urge was significantly higher in the placebo condition, suggesting that transdermal nicotine attenuated the degree to which urge to smoke increased over time. During the cue reactivity trials, when participants received the nicotine patch, they experienced significantly lower urge in response to both smoking-only and neutral cues, but not when anxiety cues were present (alone or in combination with smoking cues). These data suggest that transdermal nicotine alleviates urge only under certain circumstances, and that adjunctive interventions are likely necessary to address smoking urges in response to spikes in distress among smokers trying to quit.
Every year, approximately 443,000 people die from diseases related to cigarette smoking, accounting for $193 billion annually in health-care expenditures and lost productivity (Dube, McClave, James, Caraballo, Kaufmann & Pechacek, 2010). Despite widespread public health education and prevention efforts, the prevalence of smoking has reached a plateau with 19.3% of the U.S. population still smoking (King, Dube, Kaufman, Shaw & Pechacek, 2011). However, rates of smoking are higher among those with psychological disorders (e.g., Lasser et al., 2000; Kalman, Morissette & George, 2005), and data are accumulating to suggest that those with anxiety disorders are disproportionately represented among smokers (Morissette, Tull, Gulliver, Kamholz & Zimering, 2007; Zvolensky, Schmidt & Stewart, 2003). This is notable given that individuals with histories of anxiety disorders experience more severe withdrawal symptoms after quitting smoking (Breslau, Kilbey & Andreski, 1992; Weinberger, Desai & McKee, 2010) and have more difficulty quitting (Piper et al., 2010a). Thus, in a group already challenged by mental health symptoms, cigarette smoking may lead to additional health-care expenses and associated costs in social, health, and occupational functioning. Efforts to assist those with anxiety disorders to quit smoking and manage symptoms of withdrawal are greatly needed.
Nicotine replacement therapy (NRT) is often recommended for smoking cessation with the rationale of slowly tapering nicotine and curbing physiological withdrawal symptoms that smokers experience during their quit attempt (Tonnensen, 1997). NRT might be particularly important for patients with anxiety disorders who continue to smoke to avoid nicotine withdrawal symptoms that are uncomfortable and mimic their anxiety symptoms. Common nicotine withdrawal symptoms include nervousness, restlessness, somatic complaints, insomnia, and difficulty concentrating (West, Ussher, Evans & Rashid, 2006) and are notably worse in those with anxiety disorders (Breslau et al., 1992). Evidence suggests that transdermal nicotine can double quit rates (Fiore, Smith, Jorenby & Baker, 1994; Rose, Herskovic, Behm & Westman, 2009). However, despite these positive outcomes and the common use of NRT products, relatively little is known about the immediate effects of transdermal nicotine on urge and anxiety responses when smokers are faced with high-risk triggers to smoke, and no studies have been published to date that explore these effects among smokers with anxiety disorders.
One means of evaluating whether transdermal nicotine attenuates self-reported craving is through cue reactivity paradigms. Cue reactivity paradigms assume that drug users will react to stimuli that are associated with drug use, and that elevation in urge is a laboratory-induced proxy for high-risk-for-relapse situations (e.g., Drummond, Tiffany, Glautier & Remington, 1995). Cue reactivity paradigms allow for the systematic examination of craving in response to internal or external drug cues, and elicit robust increases in self-reported craving (Carter & Tiffany, 1999). Elevated cue reactivity is particularly relevant in light of findings by Waters et al. (2004) in which cue-provoked craving in response to cigarette stimuli predicted relapse to tobacco use during smoking cessation treatment using high-dose transdermal nicotine, but not when using a placebo patch. Although craving can operate independently of drug use behavior, these data suggest the importance of evaluating cue reactivity during transdermal nicotine use among smokers who are sensitive to tobacco- and mood-related cues, especially smokers with anxiety disorders. The interested reader is referred to Tiffany and Wray (in press) for additional discussion on the clinical significance of drug craving.
Studies have examined the influence of transdermal nicotine during cue reactivity procedures among smokers without psychological disorders. In a placebo-controlled laboratory assessment study, Tiffany, Cox and Elash (2000) found that, regardless of patch type, general craving ratings increased across all participants over the course of the assessment procedures (6 hours in duration), but more so in the placebo condition than with transdermal nicotine. In contrast, negative affect increased in the placebo condition, but remained stable in the nicotine condition, suggesting a benefit of transdermal nicotine in attenuating general negative affect. With respect to cue reactivity, as expected, cigarette stimuli elicited greater craving and negative affect than neutral stimuli. However, compared to placebo, transdermal nicotine did not reduce craving in response to smoking cues. Thus, transdermal nicotine appeared to only assist craving related to general deprivation.
Expanding on findings by Tiffany et al. (2000), Morissette, Palfai, Gulliver, Spiegel and Barlow (2005) examined the contribution of imaginal mood cues (alone and in combination with smoking cues) on both craving and anxiety during transdermal nicotine. Consistent with other studies (e.g. Maude-Griffin & Tiffany, 1996), mood cues elicited similar levels of craving as explicit smoking cues, highlighting the importance of understanding the impact of other internal and external cues in eliciting craving beyond direct smoking stimuli. Consistent with Tiffany et al. (2000), transdermal nicotine attenuated craving during general deprivation. Although a significant Patch X Smoking Cues X Anxiety Cues was statistically significant, follow-up analyses by cue type revealed that there was no benefit of nicotine over placebo in alleviating craving during the cue reactivity procedures. Moreover, transdermal nicotine did not alleviate anxiety during general deprivation or during the cue reactivity trials. Notably, this study included college smokers without psychological disorders, and it is not known whether smokers with anxiety disorders would respond differently to smoking and mood cues during transdermal nicotine. Findings among smokers without psychological disorders could be nonsignificant with respect to transdermal nicotine’s ability to alleviate anxiety because smokers without psychological disorders might have a restricted range in anxious mood. Furthermore, the subjective experience of cue reactivity and/or withdrawal may be different among clinically-anxious smokers, compared to their counterparts without psychological disorders.
The current study aimed to determine whether smokers with and without anxiety disorders responded differentially to transdermal nicotine during both general deprivation (i.e., in the absence of explicit smoking/mood cues) and when faced with theoretically high-risk triggers to smoke (i.e., during cue reactivity trials). With respect to general deprivation, it was hypothesized that, in keeping with prior studies, nicotine would have a beneficial effect over placebo. However, it was anticipated that smokers with anxiety disorders would have a reduced response to transdermal nicotine (cf. Piper, Cook, Schlam, Jorenby and Baker, 2010b), as withdrawal symptoms were anticipated to be worse in the anxiety disorders group than in the nonpsychiatric group, particularly when receiving placebo. With respect to the cue reactivity trials, although Morissette et al. (2005) found a significant Patch X Cue Content interaction, they did not find absolute differences between nicotine and placebo when evaluating differences by cue type. Thus, we anticipated that no differences would be observed by patch type with respect to cues. However, we hypothesized that different patterns would be observed by diagnosis such that those with anxiety disorders would respond more strongly to the mood cues. Moreover, although nicotine was expected to alleviate withdrawal symptoms in both groups, it was not anticipated that nicotine would alleviate anxiety symptoms, which would contribute to greater urge during both general smoking deprivation and the cue reactivity trials. A better understanding of these complex relationships may help in the development of more effective treatments and relapse prevention programs for the disproportionate number of smokers with anxiety disorders.
Method
Participants and Design
Smokers with and without anxiety disorders were recruited through advertisements in local newspapers and websites in the Boston area. Participants were screened by telephone to determine initial eligibility. Final eligibility was determined during the first assessment day. Eligibility criteria were as follows: 1) 18 years of age or older; 2) English speaking; 3) daily moderate to heavy smoking (10–40 cigarettes per day [cpd]) for at least one year; 4) CO level 8 ppm or higher; 5) willingness to wear the nicotine patch; 6) willingness to deprive themselves of smoking for the duration of the assessment (about 7 hours); 7) diagnosis of an anxiety disorder or free of current mental health diagnoses; and 8) any participants with anxiety disorders who were taking anxiolytics or antidepressant medications were required to be on a stable dose (i.e., for a period of 3 months; see also exclusion #5), and still meet full diagnostic criteria.
Participants were excluded based on the following criteria: 1) use of the nicotine patch within the last year (to protect the medication blind); 2) current plan to quit or change their smoking, or change in their amount of smoking in the past month (to minimize fluctuation in daily smoking); 3) current psychosis, severe depression, or suicidality; 4) patch contraindications (history of skin problems or an allergy to adhesive tape; diagnoses of heart disease, a recent heart attack, irregular heartbeat, or high blood pressure [defined as 140/90 or higher]); 5) current use of medications to control smoking craving, short acting “as needed” anxiety medications (e.g., Wellbutrin, PRN benzodiazepines or beta blockers), or over the counter diet medications; or 6) currently pregnant or breastfeeding, or not using a reliable form of birth control. In addition, because smokers with polysubstance dependence may have different smoking patterns and urge reactions than smokers without additional addictive behaviors, participants were excluded if: 1) they engaged in daily heavy drinking (5+ drinks/day for men; 4+ drinks/day for women) or drug use (aside from smoking) in the past year; 2) were currently in treatment for substance abuse/dependence; 3) scored 8 or higher on the Alcohol Use Disorders Identification Test (AUDIT; Babor et al., 1992); or 4) scored 10 or more on the Drug Abuse Screening Test (DAST; Skinner, 1982). Aside from the exclusionary diagnoses described above, participants in the anxiety disorders group were allowed to have other psychological disorders to foster generalizablity of the sample to patients with anxiety disorders.
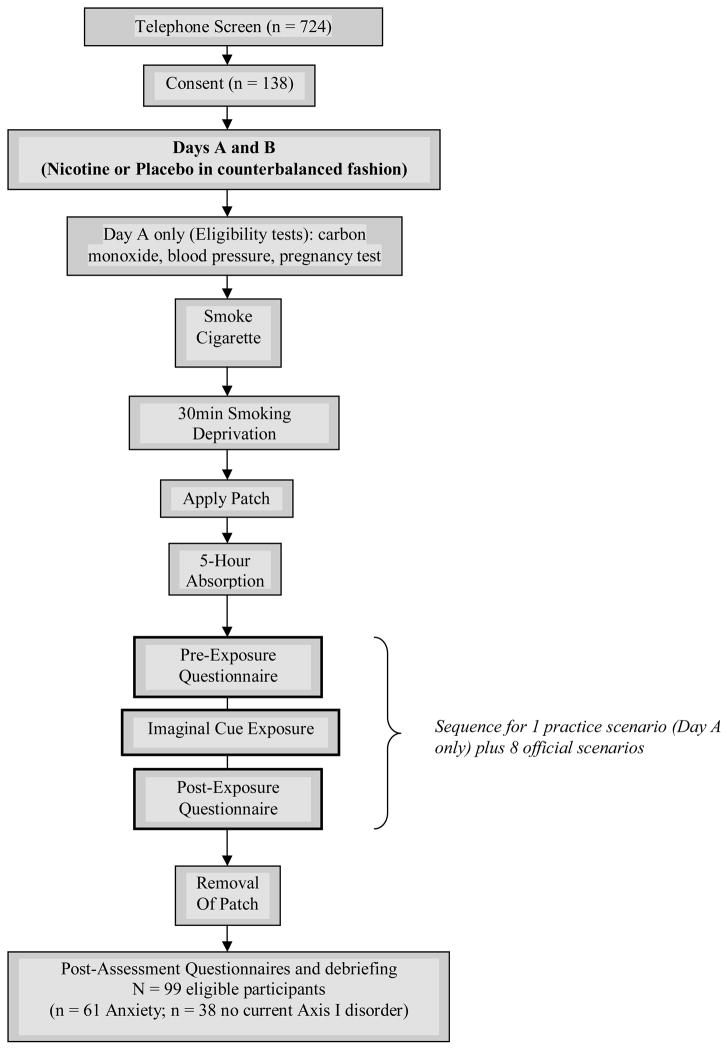
A total of 724 smokers were screened by telephone. Of these, 184 callers were deemed initially eligible, of whom 138 were consented. The most common reasons for ineligibility at the telephone screen level were: elevated score on AUDIT/DAST, smoking less than 10 cpd, use of the nicotine patch within the last year, and currently considering quitting/changing smoking. Final eligibility was determined during the course of the assessment by carbon monoxide (CO) breath sample, blood pressure reading, and diagnostic interview (see Procedure). A total of 99 participants met final eligibility criteria (61 with an anxiety disorder; 38 without any current Axis I psychological disorder) and are included in the current analyses. The most common reasons for ineligibility after the informed consent process were: high blood pressure (n = 13), carbon monoxide level less than 8 ppm (n = 10), and current diagnosis of a substance disorder (n = 9).
Procedure
All study procedures were approved by local Institutional Review Boards. Figure 1 depicts the study procedures. Following verbal informed consent, potential participants were screened by telephone to determine initial eligibility using a Medical and Smoking History Form (MSHF), the AUDIT (Babor et al., 1992) and the DAST (Skinner, 1982). Based on the telephone screen, those who were deemed initially eligible were scheduled for two assessment sessions. They were asked to abstain from alcohol for 12 hours prior to each assessment day, and instructed to drink caffeine and smoke as usual.
Figure 1.
Outline of Experimental Procedures During Days A and B.
Written informed consent was obtained at the first visit. Each participant engaged in two exposure sessions scheduled two days apart. Each assessment day lasted approximately 7 hours and began at 8:30 a.m. Each day was identical, except for the diagnostic interview or standardized measures, which were completed on opposite days during the 5-hour patch absorption period in a counterbalanced fashion (see below).
At the beginning of the first assessment day, smoking status was verified by a carbon monoxide (CO) monitor and blood pressure was taken. Average CO on Day A was 16.48 (SD=8.47); no significant differences were observed between CO on Day A versus B (M=16.80, SD=8.28). Female participants were given a pregnancy test. Participants then smoked one cigarette and waited a standardized deprivation period of 30 minutes, during which pre-patch absorption measures were completed. Following the 30-minute deprivation period, participants were randomly given either the nicotine (21 mg) or placebo patch. Whichever patch was given during the first day, the alternate was assigned on the second. The randomization was preassigned so that patch status was double-blind between the investigator and participants. The patch was applied to the upper portion of the dominant smoking arm. The placebo patch looked identical to the nicotine patch. Because the placebo patch did not smell like the nicotine patch, study personnel applied all patches, and covered them with gauze and tape so participants could not manipulate the patch or get the smell on their fingers. Participants then waited a 5-hour patch absorption (nicotine) or deprivation (placebo) period (hence forth referred to as the patch absorption period). A period of 2–4 hours is needed for nicotine levels to become constant (personal communication, Jenny Flannigan, M.D., December 14, 1999). The 5-hour absorption period was chosen to ensure that nicotine fully absorbed across participants. During the 5-hour absorption period, either an abbreviated version of the Anxiety Disorders Interview Schedule for DSM-IV (ADIS-IV, non-lifetime version; Brown, DiNardo & Barlow, 1994) was administered to evaluate current diagnoses of anxiety and related disorders versus current status of no mental health disorders, or participants completed several trait questionnaires about their smoking and anxiety, as described below. During the remaining time of the 5-hour absorption periods, participants were allowed to read a magazine, book, or watch a movie (containing no smoking cues) to minimize boredom. During the last 15 minutes of each 5-hour absorption period, participants completed identical measures to those completed prior to the absorption period (i.e., during the 30-minute deprivation period).
The cue exposure scenarios were those validated previously by Morissette et al. (2005). Following the 5-hour absorption period, participants were seated in a reclining chair. A white noise machine was used to minimize outside noise. Participants listened to and imagined each of the scenarios with their eyes closed. For demonstration purposes, a practice imaginal scenario was first presented over headphones along with post exposure measures. Eight standardized imagery scenarios were then presented in the same manner. Four types of scenarios were presented that varied in cue content: smoking plus anxiety cues, anxiety cues alone, smoking cues alone, and neutral cues. Two scripts of each type were used for a total of 8 scripts. Script presentation was counterbalanced. Based on procedures used by Tiffany and colleagues (e.g., Tiffany et al., 2000), each script sequence consisted of a 30-second baseline period, a 50-second script presentation period, and a 30-second active imagery by the participant terminated with the word “stop”. The participant was then asked to open his/her eyes and complete post-exposure trial questionnaires.
Following presentation of the imaginal scenarios, the patch was removed and participants completed post-assessment measures. Participants were not informed whether they received a nicotine or placebo patch after Day 1 in order to maintain the blind; as a safety precaution, all participants were instructed not to smoke for a period of 4 hours following removal of the patch. At the end of Assessment Day 2, participants were debriefed about the nature of the study, the blind was broken, and they were paid up to $150 for completing both assessment days.
Experimental Measures
Screening Instruments
The MSHF was designed for the current study and included questions about patch contraindications and diagnostic status designed to determine initial eligibility. The AUDIT is a 10-item measure of alcohol intake, dependence, and adverse consequences. A standard cut-off of 8 is indicative of potential alcohol problems. The AUDIT has demonstrated good sensitivity and specificity, test-retest reliability and internal consistency (Reinert & Allen, 2002). The DAST is a 28-item measure that quantifies drug misuse (Skinner, 1982). Approximately 92% of individuals with drug abuse scored greater than 10. The DAST has good internal consistency (alpha = 0.92) and concurrent validity with frequency of drug use over 12 months.
Pre- and Post-Patch Absorption Measures
Participants completed pre- and post-patch absorption questionnaires during the 30-minute deprivation period and during the last 15 minutes of the 5-hour absorption period. These questionnaires were administered to gain a baseline measure of symptoms prior to patch administration and prior to the exposures. The absorption period also represented a period of general tobacco deprivation (i.e. in the absence of cues) when participants were either nicotine deprived (placebo condition) or not (nicotine condition). The Questionnaire of Smoking Urges (QSU-32; Tiffany & Drobes, 1991) is a 32-item measure with two factors that demonstrate good internal consistency and are moderately intercorrelated: 1) desire to smoke/anticipation of pleasure; and 2) anticipation of relief of negative affect/withdrawal and urgent desire to smoke. Chronbach’s alphas for the current study for Factors 1 and 2 were all above .90 across Days A and B and pre- and post-absorption. The Brief Questionnaire of Smoking Urges (QSU-Brief; Cox, Tiffany & Christen, 2001) was administered along with the QSU-32 so that there was a consistent measure of urge across the study, as the QSU-32 would not have been feasible to use during the cue reactivity trials. The QSU-Brief is a 10-item, brief version of the QSU-32, and was also administered for comparability with the cue-reactivity ratings (see below). The QSU-Brief has high internal consistency and a similar factor structure as the QSU-32 (Tiffany & Drobes, 1991). Chronbach’s alphas for the current study were all above .93 across Days A and B and pre- and post-absorption. The Shiffman-Jarvik Withdrawal Scale-Short Version (SJWS; Shiffman & Jarvik, 1976) is a 15-item scale that has four factors capturing smoking withdrawal symptoms: craving, physical symptoms, psychological symptoms, and stimulation/sedation. Chronbach’s alphas for the current study for the craving subscale were all above .80 across Days A and B and pre- and post-absorption. The physical symptom subscale performed less well with respect to internal consistency, likely due to this scale only having 3 items (Chronbach’s alphas ranging from .66 to .78 across Days A and B and pre- and post-absorption). Likewise, Chronbach’s alphas for the psychological subscale ranged from .67 to .81. Chronbach’s alphas were not calculated for the stimulation/sedation subscale as it only includes one item. Despite low internal consistency, reporting on certain subscales the SJWS was retained for comparability across studies, as this measure is commonly used in the literature.
Measures Completed During the 5-Hour Absorption Period
Clinician Assessments
The ADIS-IV, non-lifetime version (Brown et al., 1994) was administered by the first author or trained research assistant to assess for current diagnoses, including the full range of anxiety and mood disorders, substance disorders, psychosis, and other related disorders (e.g., somatoform, hypochondriasis, etc) based on diagnostic criteria from the DSM-IV (APA, 1994). Research assistants were trained to diagnostic criterion and were required to perfectly match diagnoses with a licensed clinical psychologist on three interviews. After completion of training, all interviews continued to be reviewed and supervised by a licensed clinical psychologist (the first author). The ADIS-IV has good to excellent diagnostic agreement (Brown, DiNardo, Lehman & Campbell, 2001).
Self-Report Indices
Several self-report indices were administered at the beginning of the 5-hour period to assess smoking characteristics and anxiety/mood symptomatology. These measures were not likely to be influenced by deprivation during the 5-hour period, as they were completed at the beginning of the deprivation period when smokers would not have been significantly deprived, and the measures did not assess momentary smoking or mood states, but rather ongoing characteristics. The Fagerström Test for Nicotine Dependence FTND (Heatherton et al., 1991) is a commonly-used 6-item instrument to quantify nicotine dependence, and has satisfactory internal consistency (α=.61), as well as convergent validity with biochemical indices of heaviness of smoking (Heatherton et al., 1991). The Affect Intensity Measure (AIM; Larsen & Diener, 1987; 40 items) measures the strength of affective reactions and has very good test-retest reliability and good convergent validity with daily mood intensity, suggesting its utility for measuring emotional response intensity. Chronbach’s alpha in the current study was .91. The Anxiety Sensitivity Index (ASI; Reiss, Peterson, Gursky & McNally, 1986; 16 items) assesses beliefs that anxiety-related symptoms will lead to negative somatic, mental, or social consequences (Reiss, 1991) and has high internal consistency and test-retest reliability. Chronbach’s alpha in the current study was .91. The Questionnaire Upon Mental Imagery (QMI; Sheehan, 1967; 35 items) evaluates a person’s ability to engage in imagery in seven sensory modalities: visual, auditory, cutaneous, kinaesthetic, gustatory, offactory, organic. Chronbach’s alphas in the current study ranged from .83 to .94 across subscales.
Pre- and Post-Exposure Trial Measures
Following each of the 8 exposure trials, participants completed the QSU-Brief and subjective Likert ratings of Anxiety and Vividness of Imagery to assess response to the exposure trials. For all Likert scales, participants circled a number between 0–100, listed in 10-point intervals (0, 10, 20, etc.). Single-item Likert variables were chosen due to the repetitive trials and need for brevity. Notably, single-item ratings of anxiety are common in the anxiety literature and have practical value in that they are relatively intuitive in nature (Burisch, 1984; Carver, Meyer & Antoni, 2000).
Post Experimental Session Measures
To evaluate post-assessment withdrawal symptoms, participants completed the SJWS at the end of each assessment day. In addition, following Assessment Day 2, participants completed ratings to assess maintenance of the double-blind.
Data Analytic Procedure and Power Analysis
Cue reactivity ratings from the eight scenarios were combined and averaged to form dependent measures of responses to the four cue types (smoking plus anxiety, anxiety, smoking, neutral). The primary data analytic strategy focused on the effects of patch type on cue reactivity measures in smokers with and without anxiety disorders. A series of 2 X 2 X 4 (Diagnosis X Patch X Cue Content) ANOVAs were conducted with diagnostic group (anxiety/no anxiety disorder) as a between-subjects factor, and patch condition (nicotine/placebo) and cue content (smoking plus anxiety, anxiety, smoking, neutral) as within subjects factors. As appropriate, degrees of freedom were adjusted using Greenhouse-Geisser estimates of sphericity. A modified Bonferroni technique was used to control for error related to conducting multiple follow-up contrasts, which involves a progressive loosening of the critical alpha value that is less stringent than the Bonferroni (Holland & Copenhaver, 1988).
Previous studies using meta-analysis to examine the effect of drug cues versus neutral cues on urge response have consistently demonstrated large effect sizes in smokers (Carter & Tiffany, 1999). Because of lack of research in this area, the study was powered to detect a medium effect size. Accounting for a medium effect size (f = .25 and power of .80), a total sample size of 80 was required for a mixed between- and within-subjects ANOVA.
Results
Between Groups Differences
No significant differences were observed between diagnostic groups on demographic variables. Females represented 40.4% of the sample. Participants were largely not of Hispanic/Latino origin (90.9%). With respect to race, 65.3% were Caucasian, 24.5% African American, 1% Native Hawaiian or other Pacific Islander, 1% American Indian or Alaskan Native, 5.1% more than one race, and 3.1% of other racial background. On average, participants were 39.4 years old (SD = 11.8) and had completed 13.8 years of education (SD = 2.2).
Smokers had the following anxiety disorder diagnoses: 60.7% (37/61) social anxiety disorder, 34.4% (21/61) obsessive compulsive disorder, 31.1% (19/61) specific phobia, 29.5% (18/61) panic disorder with and without agoraphobia, 19.7% (12/61) generalized anxiety disorder, 14.8% (9/61) post-traumatic stress disorder, and 8.2% (5/61) anxiety disorder not otherwise specified. Numbers do not add to 100% as it was common for participants to have more than one disorder: 23.7% had one diagnosis, 33.9% had two diagnoses, 18.6% had three diagnoses, and 23.8% had four or more diagnoses. In particular, depressive disorders commonly co-occurred (32.8%; 20/61).
No group differences were observed between smokers with and without anxiety disorders. Participants smoked an average of 19.1 cpd (SD = 6.5) for 13.6 years (SD = 10.2), and had made 3.9 quit attempts (SD = 11.2). Average level of nicotine dependence from the FTND was 3.9 (SD = 1.4).
Manipulation Checks
Double blind
Percent accuracy scores were calculated for participants and interviewers for both Days A and B to determine whether or not they were able to accurately guess whether the participant had received a nicotine or placebo patch. Participants and interviewers were also asked to rate their confidence in guessing which patch the participant received on a scale from 0 (no confidence) to 100 (absolutely confident). Participants accurately guessed the patch type 52.5% of the time on Day A and 68.4% of the time on Day B. However, participants were only moderately confident in their ability to guess (average of 58.8 and 63.1 confidence on a scale from 0–100 for Days A and B, respectively). Interviewers accurately guessed patch type 52.5% of the time on Day A and 51.6% on Day B. Interviewers were less confident in ability to guess than participants (43.1 and 41.6 for Days A and B, respectively). These data suggest that the blind was fairly well maintained, particularly on Day A in which participants only had slightly more than a 50/50 chance of correctly guessing the patch. Participants were more successful at guessing the blind on Day B, highlighting the importance of the counterbalance.
Vividness and Mental Imagery Ability
A Diagnosis X Patch X Cue Content interaction was conducted to ensure that results could not be attributed to how clearly participants were able to imagine the scenarios (i.e., ability to vividly imagine the scenarios did not vary by diagnostic status, patch type, or script type). The overall 3-way interaction was nonsignificant [F (2.9, 262.4) = .12, p = .94]. The Patch X Cue Content interaction was also nonsignificant [F (2.9, 262.4) = 1.50, p = .22], suggesting equivalence of imagery across scenarios by patch type. Thus, vividness was not used as a covariate in subsequent analyses. Vividness scores across scripts ranged from 68.03 to 73.30 (SD range = 20.28 – 25.31) on a scale from 0–100, suggesting good levels of vividness. Smokers with and without anxiety disorders did not differ on ability to engage in imaginal imagery as measured by the QMI (all p’s > .22 for total and subscales).
Anxiety Manipulation
To ensure the anxiety scenarios elicited sufficient anxiety, the anxiety and neutral scripts for both Day A and Day B were compared. On both days, the anxiety scenario elicited greater anxiety than neutral [t(98) = 12.72, p < .001 for Day A; t (94) = 8.67, p < .001 for Day B]. Generally, the anxiety scenarios elicited moderate levels of anxiety for Day A (mean = 48.4, SD = 26.4) and Day B (mean = 41.4, SD = 28.2), as measured by the Likert anxiety scale (0–100). Notably, responses to the anxiety script during Day B were significantly lower than Day A [t(94) = 2.72, p < .01], suggesting some habituation in response to the anxiety content over time and highlighting the importance of the counterbalance procedure used.
Between-Groups Differences
Table 1 depicts smoking characteristics and relevant measures of anxiety and affect intensity. Comparisons were made between those with and without anxiety disorders on relevant smoking characteristics. Contrary to expectations, smokers with anxiety disorders did not smoke significantly more cigarettes per day than smokers without anxiety disorders or have greater nicotine dependence levels. Smokers with anxiety disorders did not differ with respect to reasons for smoking or ability to engage in mental imagery compared to their counterparts without anxiety disorders; however, as expected, smokers with anxiety disorders endorsed significantly greater affect intensity and anxiety sensitivity compared to smokers without anxiety disorders.
Table 1.
Means (Standard Deviations) of Smoking Characteristics and Other Measures by Diagnostic Group Status
| Index | Anxiety (n=59) | No Axis I disorders (n=38) |
|---|---|---|
| CPD | 18.8 (6.06) | 19.42 (7.14) |
| FTND | 3.90 (1.26) | 3.82 (1.45) |
| AIM * | 3.81 (0.59) | 3.54 (0.58) |
| ASI** | 29.20 (12.87) | 17.92 (9.60) |
| QMI total | 2.88 (1.22) | 2.69 (1.19) |
Note: cpd = cigarettes per day; FTND = Fagerstrom Test for Nicotine Dependence (Heatherton et al., 1991; range of possible scores = 0 to 10; higher scores indicate more physical dependence); AIM = Affect Intensity Measure (Larsen & Diener, 1987; range of possible scores = 1 to 6; higher scores indicate greater intensity of emotions); ASI = Anxiety Sensitivity Index (Reiss et al., 1986; range of possible scores = 0 to 64; higher scores indicate greater intensity of emotions); QMI = Questionnaire of Mental Imagery (Sheehan, 1967; range of possible scores = 1 to 7; lower scores indicate more imagery vividness).
p < .05;
p < .001. N = 59 for anxiety disorders group due to missing data.
Patch Absorption/Deprivation Period
Table 2 presents means and standard deviations of measures completed prior to and following the patch absorption period to evaluate the effects of transdermal nicotine across the 5-hour period of general tobacco deprivation, as well as at the post-experimental time point after all cue reactivity trials had been completed. Between and within subjects ANOVAs (Diagnosis X Patch X Time) were conducted. For the QSU-Brief and QSU32, there were two levels of time (pre/post absorption). For the SJWS, there were three levels of time (pre/post absorption and post-experiment). None of the Diagnosis X Patch X Time interactions were significant, suggesting that the presence of an anxiety disorder did not alter how participants responded to transdermal nicotine versus placebo over time. However, significant Patch X Time (pre/post absorption) interactions were observed for QSU-Brief [F (1, 92) = 15.25, p < .001, partial eta squared = .14], and QSU32 Factor 1 [F (1,93) = 11.38, p = .001, partial eta squared = .11] and Factor 2 [F (1, 9) = 18.69, p < .001, partial eta squared = .17]. In all cases, nicotine and placebo conditions significantly increased from pre- to post-absorption (all p’s < .01). However, the nicotine condition experienced significantly lower urge at the post-absorption time point compared to placebo (all p’s < .01).
Table 2.
Means (Standard Deviations) of Measures Completed Prior to and Following the 5-Hour Patch Absorption Phase and at the Final Post-Experimental Assessment (N = 96) by Patch Status.
| Measure | Nicotine | Placebo | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Final | Pre | Post | Final | |
| QSU – Brief*** | 16.55 (23.55) | 34.81 (24.84) | --- | 12.40 (17.44) | 42.44 (23.81) | --- |
| QSU – 32 | ||||||
| Factor 1** | 3.48 (1.48) | 4.55 (1.59) | --- | 3.32 (1.38) | 5.16 (1.47) | --- |
| Factor 2*** | 2.07 (1.25) | 2.47 (1.32) | --- | 1.96 (1.09) | 3.05 (1.39) | --- |
| SJWS | ||||||
| Craving*** | 2.73 (1.60) | 4.16 (1.74) | 4.11 (1.81) | 2.36 (1.32) | 5.00 (1.63) | 5.14 (1.58) |
| Psych. Discomfort*** | 3.04 (1.16) | 3.02 (1.27) | 3.08 (1.36) | 2.76 (1.06) | 3.56 (1.38) | 3.62 (1.42) |
| Physical Symptoms | 1.78 (1.12) | 1.58 (0.90) | 1.73 (1.13) | 1.59 (0.99) | 1.76 (1.12) | 1.77 (1.15) |
| Stimulation/Sedation | 2.81 (1.82) | 3.26 (1.86) | 2.84 (1.86) | 2.65 (1.76) | 3.57 (2.01) | 3.16 (1.88) |
Note: Pre = pre-absorption; QSU-32 = Questionnaire of Smoking Urges (Tiffany & Drobes, 1991; Factor 1 = intention/desire to smoke and anticipation of pleasure; Factor 2 = anticipation of relief of negative affect/withdrawal and urgent desire to smoke; range of possible scores for each factor 1 to 7; higher scores indicate greater levels of Factors 1 and 2); SJWS = Shiffman-Jarvik Withdrawal Scale-Short Version (Shiffman & Jarvik, 1976; range of possible scores 0 – 7; higher scores signify more withdrawal symptoms present, 4 = neutral). Anxiety ratings (0 – 100; 0 = none; 100 = as much as imaginable).
= significant Patch X Time interaction at p <.01;
= significant Patch X Time interaction at p <.001.
Withdrawal symptoms (as measured by the SJWS) were also assessed at the close of the experiment (in addition to pre/post absorption) to determine how symptomatic participants were following the cue reactivity trials. As with urge, the 3-way interactions were not significant, but the Patch X Time interactions were significant for the SJWS craving [F (1.6, 148.2) = 27.45, p < .001, partial eta squared = .23] and psychological symptoms [F (1.8, 170.1) = 15.18, p < .001, partial eta squared = .14]. A tendency was observed for a significant Patch X Time interaction for physical symptoms [F (1.6, 153.3) = 3.21, p = .053, partial eta squared = .03]. For the craving subscale, both nicotine and placebo conditions significantly increased from pre- to post-absorption (all p’s < .001), but did not significantly increase from post-absorption to post-experiment (all p’s > .16), suggesting that craving reached a plateau in both conditions. However, nicotine and placebo conditions were significantly different at each time point (pre-and post-absorption, and post-experimental). As can be seen in Table 2, when participants received nicotine, the nicotine condition started with significantly greater craving than placebo at pre-absorption (p < .05), but had significantly lower craving at both post-absorption (p < .001) and post-experiment (p < .001), suggesting that although craving reached plateau in both conditions, it did not rise as high when participants received nicotine compared to placebo.
In contrast, psychological symptoms remained stable in the nicotine condition across all time points (p’s > .52). Within the placebo condition, symptoms significantly increased from pre-to post-absorption (p < .001), but stabilized from post-absorption to post-experiment (p = .29). When comparing nicotine versus placebo over time, findings mirrored those with craving. The nicotine condition had significantly higher psychological discomfort at pre-absorption (p < .05), but significantly lower psychological discomfort at both post-absorption and post-experiment (p’s < .01). These data suggest an added benefit in attenuating psychological discomfort when participants used transdermal nicotine during general deprivation and after being exposed to multiple high-risk cues.
Cue Reactivity Indices
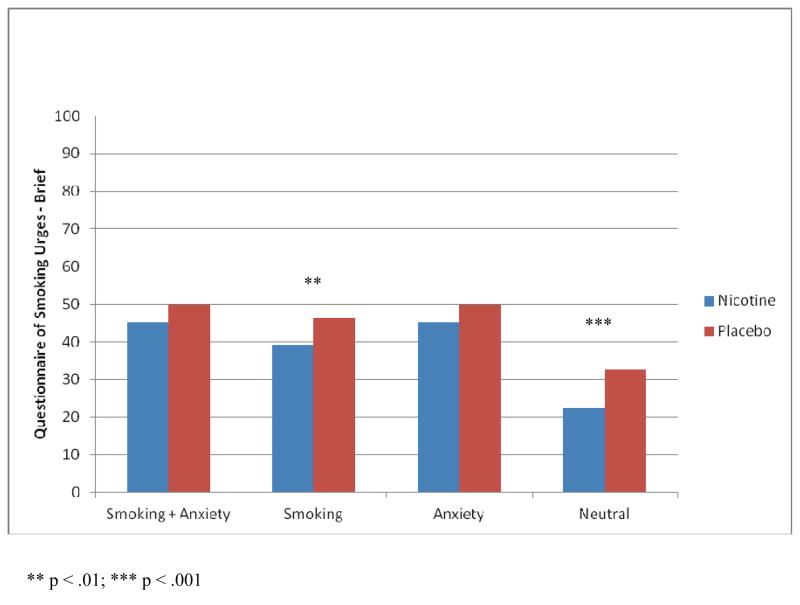
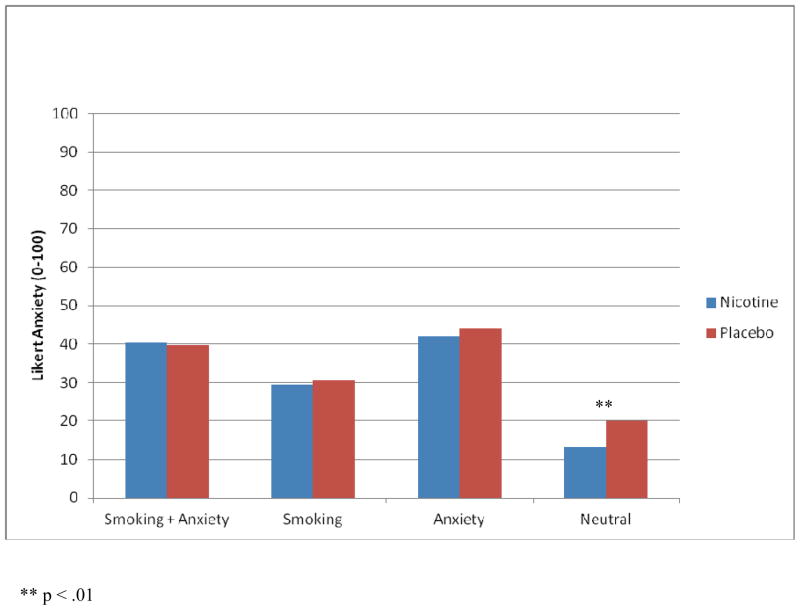
Three-way ANOVAs were conducted to determine the influence of transdermal nicotine on smoking urge and anxiety during the cue reactivity trials. Notably, post-absorption period urge was not used as a covariate when analyzing urge because this would violate the assumptions of ANCOVA. Average QSU-Brief and average anxiety scores are depicted by patch condition and cue content in Figures 2 and 3, respectively.
Figure 2.
Average QSU-Brief Scores by Patch Condition and Cue Content (All Smokers).
Figure 3.
Average Anxiety Scores by Patch Condition and Cue Content (All Smokers).
Smoking Urge
A 2 X 2 X 4 (Diagnosis X Patch X Cue Content) ANOVA using the QSU-Brief as the primary dependent variable was non-significant. However, the Patch X Cue Content interaction was significant [F (3, 276) = 3.11, p < .05, partial eta squared = .03], suggesting that urge response to cues differed based on whether the participant received a nicotine or placebo patch. Follow-up comparisons were conducted to examine the nature of the two-way interaction. Compared to placebo, when participants received nicotine they described significantly lower urge in response to smoking and neutral cues (but not anxiety plus smoking or anxiety-only cues; p < .01 and p < .001, respectively). Thus, transdermal nicotine had some benefit in that urges were lower in response to smoking and neutral cues, but not with respect to mood cues.
Within each patch condition, patterns were identical. Overall, all cue-content scenarios elicited greater urge than neutral content, and scenarios containing anxiety cues (alone or in combination with smoking cues) elicited greater urge than smoking cues alone (all p’s < .01 for nicotine and < .05 for placebo).
Notably, to evaluate whether anxiety sensitivity (vs. anxiety disorder) influenced urge response to the cues by patch type, the three-way interaction was also conducted using high/low anxiety sensititivity (Hi/low ASI X Patch X Cue Content). Anxiety sensitivity has theoretical relevance given the potential overlap in anxiety and withdrawal symptoms and associated fear of physical symptoms. The three-way interaction was nonsignificant (p = .93), suggesting that anxiety sensitivity did not moderate the effect of patch type during the cue reactivity trials.
Likert Anxiety
A 2 X 2 X 4 (Diagnosis X Patch X Cue Content) ANOVA using average Likert anxiety as the primary dependent variable was conducted. As with smoking urge, the 3-way interaction was not significant, but the Patch X Cue Content interaction was significant [F (3, 276) = 3.96, p < .01, partial eta squared = .04]. Follow-up comparisons indicated that nicotine and placebo conditions only differed in response to the neutral script (p = .001), suggesting that nicotine did not alleviate anxiety in response to anxiety or smoking cue content alone or in combination. Within both patch conditions cue-content scenarios elicited stronger anxiety than neutral content, and scenarios containing anxiety cues (alone or in combination with smoking cues) elicited stronger anxiety than smoking cues only (all p’s < .001). In the nicotine condition, scenarios containing anxiety plus smoking cues elicited equivalent levels of anxiety as anxiety cues only (p = .39); however, unexpectedly, in the placebo condition, anxiety plus smoking cues elicited less anxiety than anxiety-only cues (p < .05).
To further explore the relationship between diagnostic status and cue exposure, Diagnosis (Anxiety/nonpsychiatric) X Cue (Anxiety/Neutral) interactions were conducted for both Days A and B (i.e., collapsed across patch status). Both were nonsignificant, suggesting that people with anxiety disorders responded with similar levels of urge in response to the anxiety cues as those without psychiatric disorders. For both groups, moderate urge levels were elicited by the anxiety cues (Day A: 56.3 vs. 43.1 for anxiety and nonpsychiatric, respectively; Day B: 47.3 vs. 44.2 for anxiety and nonpsychiatric, respectively). Although statistically nonsignificant, urge response was in the expected direction (i.e., higher in the anxiety group).
As with urge, we evaluated whether anxiety sensitivity (vs. anxiety disorder) influenced state-anxiety response to cue content by patch type. The three-way interaction (Hi/low ASI X Patch X Cue Content) was nonsignificant (p = .66).
Discussion
The current study evaluated whether smokers with and without anxiety disorders responded differently to transdermal nicotine during general tobacco deprivation as well as in response to cue reactivity trials. A main strength of the study was the within-subjects element of the design (i.e., comparing the same individuals when receiving nicotine and placebo). Although findings were not moderated by anxiety disorder status, patch condition significantly influenced craving and withdrawal symptoms over time and in response to the cue reactivity trials. With respect to general tobacco deprivation (i.e., in the absence of specific smoking triggers), both patch conditions experienced an increase in urge (QSU-Brief, QSU Factors 1 and 2) from pre- to post-absorption, but post-absorption urge was higher in the placebo condition, suggesting that transdermal nicotine attenuated the degree to which urge to smoke increased over time. Effect sizes were generally large. These findings are consistent with both Tiffany et al. (2000) and Morissette et al. (2005).
Withdrawal symptoms were evaluated over time, not only prior to and following the general tobacco deprivation period, but also at the end of the experiment after participants had completed the cue reactivity trials. These assessment intervals served as a proxy for understanding the early stages of patch administration, first in an environment designed not to have explicit tobacco cues, and then following a period of protracted cue exposure. Findings differed depending on the measure used. With respect to craving, both patch conditions increased from pre- to post-absorption and leveled off from post-absorption to post experiment. However, compared to placebo, the nicotine condition started with significantly higher craving and ended with significantly lower craving at both post-absorption and post-experiment (large effect), suggesting that when receiving nicotine participants were at an advantage both going into and coming out of the cue reactivity trials.
Nicotine also appeared to benefit psychological symptoms. Whereas psychological discomfort increased from pre- to post-absorption and stabilized by post-experiment within the placebo condition, it remained stable across time in the nicotine condition and significantly lower than placebo (large effect). In contrast, nicotine did not appear to any beneficial effect over placebo on physical symptoms, and both conditions reported low levels of physical symptoms. The absence of differences in physical symptoms is notable for an anxiety-disorders sample, given that anxiety patients are often highly sensitive to physical withdrawal (or side effect) sensations that are reminiscent of their anxiety symptoms. These data suggest that the patch was well tolerated physically by both smokers with and without anxiety disorders.
With respect to the cue reactivity trials, when receiving the nicotine patch participants experienced significantly lower urge in response to smoking-only and neutral cues, but not when anxiety cues were present (alone or in combination with smoking cues). Although the effect size was small, this finding contradicts Morissette et al. (2005) in which transdermal nicotine did not alleviate urge during cue reactivity whatsoever. This discrepancy could be due to differences in the sample (college smokers in Morissette et al., 2005), although notably, both samples had similar levels of nicotine dependence as measured by the FTND. Nonetheless, consistent across both studies and with the cue reactivity literature, scenarios containing anxiety cue content elicited moderate levels of smoking urge. These data suggest that, in addition to a platform of NRT, clinicians may need to incorporate other strategies to aid smokers when they are confronted with mood-related cues. Although patterns in response to the cue reactivity trials did not differ by anxiety disorder status, such strategies may be particularly important for smokers with anxiety disorders.
Finally, smokers with and without anxiety disorders did not differ with respect to anxiety response during the cue reactivity trials. As with urge, a significant Patch X Cue Content interaction was observed, although the effect size was small. Follow-up comparisons indicated that the nicotine condition experienced significantly lower anxiety in response to the neutral scenario (but not other scenarios) compared to the placebo condition. This finding is consistent with the literature on nicotine only being anxiolytic in the context of a benign distractor (Kassel & Unrod, 2000). In the current study, the neutral script could have served as a benign distractor thereby enabling transdermal nicotine to have an anxiolytic effect. Again, although not moderated by anxiety disorder status, this anxiolytic effect (likely occurring on a variable ratio schedule in daily life) may be particularly reinforcing for smokers with anxiety disorders who are looking for any relief (even if momentary and only in the context of neutral cues) of their anxiety. If true, this will require careful coordination between smoking cessation and anxiety disorder treatments, as smokers with anxiety disorders may have a harder time tapering off of transdermal nicotine due to these reinforcing effects. Future research is needed to understand the conditions under which different forms of NRT are anxiolytic, and how such affects might affect smoking cessation treatments for smokers with and without psychological disorders.
The lack of differences in cue reactivity between smokers with and without anxiety disorders is inconsistent with recent findings by Piper et al. (2010b). Specifically, in a randomized, placebo-controlled clinical trial comparing placebo, buproprion SR, nicotine patch, nicotine lozenge, buproprion SR + nicotine lozenge, or nicotine patch + nicotine lozenge, Piper et al. found that smokers with a history of anxiety disorders had a reduced benefit from smoking cessation pharmacotherapies compared to smokers without anxiety disorders. However, a key difference between these studies is that the current study evaluated smokers with current anxiety disorder diagnoses (versus lifetime history). Future studies are needed to examine pharmacotherapy for smokers with current anxiety disorders who have notoriously worse withdrawal symptoms. This should include studies examining the influence of high-dose transdermal nicotine in those with anxiety disorders. High dose nicotine (i.e., 42-mg) during initial abstinence has been demonstrated to increase comfort and alleviate withdrawal and urge to smoke (Rohsenow et al., 2007). Studies should also investigate the effects of different routes of nicotine administration in smokers with anxiety disorders, including but not limited to faster acting products such as rapid-release nicotine gum (Shiffman et al. 2009; Niaura et al., 2005). Finally, smokers with anxiety disorders may require alternative strategies beyond NRT to improve success rates in quit outcomes, and this should be an aim of future research.
Of interest, although the blind was generally well maintained on Day A (52.5%), as anticipated, participants were better at guessing the blind on Day B (68.4%). These data raise the question as to whether nicotine expectancies could have played a role in response to the scenarios. Prior studies have demonstrated that smokers hold expectancies for the efficacy of NRT in reducing craving (Juliano & Brandon, 2004); future research is needed to evaluate nicotine expectancies among clinical samples.
Several limitations should be noted regarding the findings of the current study. First, although a strength of the study is the comprehensive and precise psychological assessment of anxiety and related disorders, we did not systematically assess past history of all psychological disorders. Consequently, differences between smokers with and without current anxiety disorders may be clouded by a past history of those disorders within the group without current anxiety disorders. Although the study initially attempted to recruit participants with a never-history of a psychological disorder, the feasibility of such a recruitment strategy was hampered by the fact that the majority of current smokers have past or present psychological disorders. The fact that the smokers without anxiety disorders were not a never-history of psychological disorders group may explain the unexpected absence of differences between smokers with and without anxiety disorders on cigarettes smoked and nicotine dependence.
Second, because there were a significant number of necessary inclusion/exclusion criteria, a large number of smokers were screened to identify the final sample, which affects generalizability. The most common reason for ineligibility at the telephone screen level was elevated AUDIT/DAST score, followed by those who were smoking less than 10 cpd. Thus, participants were included who were smoking at a level appropriate to the patch prescription (21 mg). Moreover, although other substance disorders co-occur at high rates with both tobacco use (e.g., Monti, Rohsenow, Colby & Abrams, 1995) and anxiety disorders (Thyer, Parrish, Himle, Cameron, Curtis & Nesse, 1986), smokers with other current alcohol or substance disorders were excluded because poly-substance users may have different smoking topography and potentially different responses to the cue reactivity trials. Therefore, in this first study of cue reactivity among a sample of participants with clinical anxiety disorders, we aimed to make the sample as homogeneous as possible with respect to substance diagnoses, while focusing on a broad range of anxiety disorders. Notably, the literature at the time of the design of this study was in its infancy, and thus a broad range of anxiety disorders was targeted versus specific anxiety disorders now known to be strongly associated with smoking. Sample size limitations precluded the analysis of effects by specific anxiety disorder groups, but this should be an aim of future research given the preponderance of data suggesting the strongest relationship between tobacco use/dependence is among those with panic disorder (Morissette, Brown, Kamholz & Gulliver, 2005; Zvolensky et al., 2003) and PTSD (Beckham et al., 1997; Feldner et al., 2007).
Third, cue reactivity response was assessed following a relatively brief period of abstinence. By design, this was to evaluate the experiences of smokers during the early stages of a smoking quit attempt when using NRT and while being confronted with multiple cues. It is unclear whether differences would emerge with longer periods of abstinence when those with anxiety disorder might have a more severe withdrawal response compared to their counterparts without psychological disorders. Alternatively, quitting smoking is known to alleviate mental health symptoms (Parrott, 1995). Thus, future studies should evaluate cue reactivity throughout a quit attempt to better understand why transdermal nicotine improves quit rates. With respect to the repeated measurement of urge response to multiple cues, due to concerns about fatigue and demand effects, the QSU-Brief was not administered prior to starting each exposure. Instead, it was assumed that one cue might influence the next and scenarios were counterbalanced for order and sequence accordingly. This procedure was also designed to be reflective of real world cue exposure during an early quit attempt, in which cue reactivity does not always have a chance to return to baseline between exposures.
Finally, it should be noted that the anxiety scripts elicited only moderate anxiety. Anxiety appeared to habituate over the experimental days, highlighting the importance of the counterbalance procedure. This could be due to the fact that participants with anxiety disorders who were taking anxiolytics or antidepressant medications were not excluded from the study (although they were required to be on a stable dose and still meet current criteria for an anxiety disorder). This was to ensure generalizability of the sample, but could have minimized reactivity to the cues making it harder to observe between-groups differences. Furthermore, although some studies have demonstrated that imaginal and in vivo smoking cues elicit comparable levels of urge reactivity (Drobes & Tiffany, 1997), others have found more robust findings with in vivo cues (Heishman, Lee, Taylor, & Singleton, 2010). Thus, findings might have differed if in vivo smoking and anxiety cues were used. Future research might also intensify anxiety cues by tailoring scenarios to the individual.
In summary, anxiety disorder status did not moderate the relationship between patch condition and cue content on urge or anxiety reactivity. Despite the lack of significant differences, findings have clinical importance for those with anxiety disorders who smoke at higher rates and have more trouble quitting. Findings are encouraging in that those with anxiety disorders tolerated transdermal nicotine well and responded similarly during general deprivation and during cue reactivity as those without current psychological disorders. Still, the findings suggest that transdermal nicotine may only be part of a successful smoking cessation plan for smokers with anxiety disorders. The nicotine patch may be insufficient to meet the needs of smokers attempting to quit, who may benefit from additional treatment strategies that address mood-related triggers (e.g., emotional tolerance and coping skills, reduction in interoceptive sensitivity). Consistently, Zvolensky and colleagues (2008) present promising case series data regarding the development of an interoceptive exposure-based smoking cessation program for smokers high in anxiety sensitivity. Moreover, McFall et al. (2010) demonstrated that, among military veterans with PTSD, integrating smoking cessation treatment into mental health care resulted in higher prolonged absence rates as compared to those referred to specialized cessation treatment. Additional research is clearly needed to determine best practices for smokers with anxiety disorders who are attempting to quit.
Acknowledgments
This research was supported by a NIDA grant awarded to the first author (DA016376). We would like to acknowledge the contributions of those who helped conduct the current research including: Dr. John Renner, Dr. Michael Angelini, Ms. Kirsten Johnson, and Ms. Dorothy Nelson Plumb.
References
- Babor TF, De La Fuente JR, Saunders J, Grant M. AUDIT: The Alcohol Use Disorder Identification Test. Guidelines for use in primary health care. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- Beckham JC, Kirby AC, Feldman ME, Hertzberg MA, Moore SD, Crawford AL, Davidson JR, Fairbank JA. Prevalence and correlates of heavy smoking in Vietnam veterans with chronic post-traumatic stress disorder. Addictive Behavior. 1997;22:637–647. doi: 10.1016/S0306-4603(96)00071-8. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kilbey M, Andreski P. Nicotine withdrawal symptoms and psychiatric disorders: Findings from an epidemiologic study of young adults. American Journal of Psychiatry. 1992;149:464–469. doi: 10.1176/ajp.149.4.464. [DOI] [PubMed] [Google Scholar]
- Brown TA, DiNardo P, Barlow DH. Anxiety Disorders Interview Schedule for DSM-IV. San Antonio, TX: Graywind Publication Incorporated/The Psychological Corporation; 1994. [Google Scholar]
- Brown TA, DiNardo P, Lehman CL, Campbell LA. Reliability of DSM-IV anxiety and mood disorders: Implications for the classification of emotional disorders. Journal of Abnormal Psychology. 2001;110:49–58. doi: 10.1037/0021-843X.110.1.49. [DOI] [PubMed] [Google Scholar]
- Burisch M. Approaches to personality inventory construction: A comparison of merits. American Psychologist. 1984;39:214–227. doi: 10.1037/003-066X.39.3.214. [DOI] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. doi: 10.1046/j.1360-0443.1999.9433273.x. [DOI] [PubMed] [Google Scholar]
- Carver CS, Meyer B, Antoni MH. Responsiveness to threats and incentives, expectancy of recurrence, and distress and disengagement: Moderator effects in women with early stage breast cancer. Journal of Consulting and Clinical Psychology. 2000;68:965–975. doi: 10.1037/0022-006X.68.6.965. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the Brief Questionnaire of Smoking Urges (QSU-Brief) in laboratory and clinical settings. Nicotine and Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Tiffany ST. Induction of smoking urge through imaginal and in vivo procedures: Physiological and self-report manifestations. Journal of Abnormal Psychology. 1997;106:15–25. doi: 10.1037//0021-843x.106.1.15. [DOI] [PubMed] [Google Scholar]
- Drummond D, Tiffany ST, Glautier S, Remington B. Cue exposure in understanding and treating addictive behaviours. In: Drummond D, Tiffany ST, Glautier S, Remington B, Drummond D, Tiffany ST, Remington B, editors. Addictive behaviour: Cue exposure theory and practice. Oxford England: John Wiley & Sons; 1995. pp. 1–17. [Google Scholar]
- Dube SR, McClave A, James C, Caraballo R, Kaufmann R, Pechacek T. Vital signs: Current cigarette smoking among adults aged ≥ 18 years --- United States, 2009. Morbidity and Mortality Weekly Report. 2010;59 (35):1135–1140. [PubMed] [Google Scholar]
- Feldner MT, Babson KA, Zvolensky MJ, Vujanovic AA, Lewis SF, Gibson LE, Bernstein A. Posttraumatic stress symptoms and smoking to reduce negative affect: An investigation of trauma-exposed daily smokers. Addictive Behaviors. 2007;32(2):214–227. doi: 10.1016/j.addbeh.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Fiore M, Smith S, Jorenby D, Baker T. The effectiveness of the nicotine patch for smoking cessation: a meta-analysis. JAMA: Journal of the American Medical Association. 1994;271(24):1940–1947. [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of the Addictions. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Lee DC, Taylor RC, Singleton EG. Prolonged duration of craving, mood, and autonomic responses elicited by cues and imagery in smokers: Effects of Tobacco deprivation and sex. Experimental and Clinical Psychopharmacology. 2010;18:245–256. doi: 10.1037/a0019401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland BS, Copenhaver M. Improved Bonferroni-type multiple testing procedures. Psychological Bulletin. 1988;104:145–149. doi: 10.1037/0033-2909.104.1.145. [DOI] [Google Scholar]
- Juliano LM, Brandon TH. Smokers’ expectancies for nicotine replacement therapy vs. cigarettes. Nicotine & Tobacco Research. 2004;6:569–574. doi: 10.1080/14622200410001696574. [DOI] [PubMed] [Google Scholar]
- Kalman D, Morissette S, George TP. Co-Morbidity of Smoking in Patients with Psychiatric and Substance Use Disorders. The American Journal on Addictions. 2005;14(2):106–123. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassel JD, Unrod M. Smoking, anxiety, and attention: Support for the role of nicotine in attentionally mediated anxiolysis. Journal of Abnormal Psychology. 2000;109:161–166. doi: 10.1037/0021-843X.109.1.161. [DOI] [PubMed] [Google Scholar]
- Larsen RJ, Diener E. Affect intensity as an individual difference characteristic: A review. Journal of Research in Personality. 1987;21:1–39. doi: 10.1016/0092-6566(87)90023-7. [DOI] [Google Scholar]
- Lasser K, Boyd J, Woolhandler S, Himmelstein D, McCormick D, Bor D. Smoking and mental illness: a population-based prevalence study. JAMA: Journal of the American Medical Association. 2000;284(20):2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Maude-Griffin PM, Tiffany ST. Production of smoking urges through imagery: The impact of affect and smoking abstinence. Experimental and Clinical Psychopharmacology. 1996;4:198–208. doi: 10.1037/1064-1297.4.2.198. [DOI] [Google Scholar]
- McFall M, Sazon AJ, Malte CA, Chow B, Bailey S, Baker DG, Beckham JC, Boardman KD, Carmody TP, Joseph AM, Smith MW, Shih M, Lu Y, Holodniy M, Lavori PW. Integrating tobacco cessation into mental health care for posttraumatic stress disorder. Journal of the American Medical Association. 2010;304:2485–2493. doi: 10.1080/10550490600859892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Colby SM, Abrams DB. Smoking among alcoholics during and after treatment: implications for models, treatment strategies and policy. In: Fertig JB, Allen JP, editors. Alcohol and Tobacco: From Basic Science to Clinical Practice (NIAAA Research Monograph No 30) National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 1995. pp. 187–206. [Google Scholar]
- King B, Dube S, Kaufman R, Shaw L, Pechacek T. Vital Signs: Current cigarette smoking among adults aged ≥ 18 Years --- United States, 2005–2010. Morbidity and Mortality Weekly Report. 2011;60:1207–1212. [PubMed] [Google Scholar]
- Morissette SB, Brown TA, Kamholz BW, Gulliver SB. Differences between smokers and nonsmokers with anxiety disorders. Journal of Anxiety Disorders. 2005;20:597–613. doi: 10.1016/j.janxdis.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Morissette S, Palfai TP, Gulliver S, Spiegel DA, Barlow DH. Effects of Transdermal Nicotine During Imaginal Exposure to Anxiety and Smoking Cues in College Smokers. Psychology of Addictive Behaviors. 2005;19(2):192–198. doi: 10.1037/0893-164X.19.2.192. [DOI] [PubMed] [Google Scholar]
- Morissette S, Tull MT, Gulliver S, Kamholz B, Zimering RT. Anxiety, anxiety disorders, tobacco use, and nicotine: A critical review of interrelationships. Psychological Bulletin. 2007;133(2):245–272. doi: 10.1037/0033-2909.133.2.245. [DOI] [PubMed] [Google Scholar]
- Niaura R, Sayette M, Shiffman S, Glover ED, Nides M, Shelanski M, Shadel W, Koslo R, Robbins B, Sorrentino J. Comparative efficacy of rapid-release nicotine gum versus nicotine polacrilex gum in relieving smoking cue-provoked craving. Addiction. 2005;100:1720–1730. doi: 10.1111/j.1360-0443.2005.01218.x. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Smoking cessation leads to reduced stress, but why? International Journal of the Addictions. 1995;30:1509–1516. doi: 10.3109/10826089509055846. [DOI] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Baker TB. Anxiety diagnoses in smokers seeking cessation treatment: relations with tobacco dependence, withdrawal, outcome and response to treatment. Addiction. 2010b;106:418–427. doi: 10.1111/j.1360-0443.2010.0317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Fleming MF, Bittrich AA, Brown JL, Leitzke CJ, Zehner ME, Fiore MC, Baker TB. Psychiatric disorders in smokers seeking treatment for tobacco dependence: prevalence and relations with tobacco dependence and cessation. Journal of Consulting and Clinical Psychology. 2010a;78(1):13–23. doi: 10.1037/a0018065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert DF, Allen JP. The Alcohol Use Disorders Identification Test (AUDIT): A review of recent research. Alcoholism: Clinical and Experimental Research. 2002;26(2):272–279. doi: 10.1097/00000374-200202000-00016. [DOI] [PubMed] [Google Scholar]
- Reiss S. Expectancy model of fear, anxiety, and panic. Clinical Psychology Review. 1991;11(2):141–153. doi: 10.1016/0272-7358(91)90092-9. [DOI] [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behaviour Research and Therapy. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Hutchison KE, Swift RM, MacKinnon SV, Sirota AD, Kaplan GB. High-dose transdermal nicotine and naltrexone: Effects on nicotine withdrawal, urges, smoking, and effects of smoking. Experimental and Clinical Psychopharmacology. 2007;15(1):81–92. doi: 10.1037/1064-1297.15.1.81. [DOI] [PubMed] [Google Scholar]
- Rose JE, Herskovic JE, Behm FM, Westman EC. Precessation treatment with nicotine patch significantly increases abstinence rates relative to conventional treatment. Nicotine & Tobacco Research. 2009;11(9):1067–1075. doi: 10.1093/ntr/ntp103. [DOI] [PubMed] [Google Scholar]
- Sheehan PW. A shortened form of Betts’ questionnaire upon mental imagery. Journal of Clinical Psychology. 1967;23:386–389. doi: 10.1002/1097-4679(196707)23:3<386::AID-JCLP2270230328>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Assessing smoking patterns and motives. Journal of Consulting and Clinical Psychology. 1993;61:732–742. doi: 10.1037/0022-006X.61.5.732. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Cone EJ, Buchhalter AR, Henningfield JE, Rohay JM, Gitchell JG, Pinney JM, Chau T. Rapid absorption of nicotine from new nicotine gum formulations. Pharmacology Biochemistry and Behavior. 2009;91:380–385. doi: 10.1016/j.pbb.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Shiffman SM, Jarvik ME. Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology. 1976;50:35–39. doi: 10.1007/BF00634151. [DOI] [PubMed] [Google Scholar]
- Skinner HA. The Drug Abuse Screening Test. Addictive Behaviors. 1982;7:363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Thyer BA, Parrish RT, Himle J, Cameron OG, Curtis GC, Nesse RM. Alcohol abuse among clinically anxious patients. Behaviour Research and Therapy. 1986;24:357–359. doi: 10.1016/0005-7967(86)90195-6. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Cox LS, Elash CA. Effects of the transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. Journal of Consulting and Clinical Psychology. 2000;68:233–240. doi: 10.1037/0022-006X.68.2.233. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. British Journal of Addictions. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Wray JM. The clinical significance of drug craving. The Annals of the New York Academy of Sciences. doi: 10.1111/j.1749-6632.2011.06298.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnesen P. Nicotine replacement and other drugs in smoking cessation. In: Bolliger CT, Fagerström KO, editors. The tobacco epidemic. Progress in respiratory research. Vol. 28. Basel, Switzerland: Karger; 1997. pp. 178–189. [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Cue-provoked craving and nicotine replacement therapy in smoking cessation. Journal of Consulting and Clinical Psychology. 2004;72:1136–1143. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Desai RA, McKee SA. Nicotine withdrawal in U.S. smokers with current mood, anxiety, alcohol use, and substance use disorders. Drug and Alcohol Dependence. 2010;108:7–12. doi: 10.1016/j.drugalcdep.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Ussher M, Evans M, Rashid M. Assessing DSM-IV nicotine withdrawal symptoms: A comparison and evaluation of five different scales. Psychopharmacology. 2006;184:619–627. doi: 10.1007/s00213-005-0216-z. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Schmidt NB, Stewart SH. Panic disorder and smoking. Clinical Psychology: Science and Practice. 2003;10:29–51. doi: 10.1093/clipsy/10.1.29. [DOI] [Google Scholar]
- Zvolensky MJ, Yartz AR, Gregor K, Gonzalez A, Bernstein A. Interoceptive exposure-based cessation intervention for smokers high in anxiety sensitivity: A case series. Journal of Cognitive Psychotherapy. 2008;22:346–365. doi: 10.1891/0889-8391.22.4.346. [DOI] [Google Scholar]