Abstract
BACKGROUND
Many disease-specific, quality-of-life (QOL) instruments exist for chronic rhinosinusitis (CRS), producing confusion about the best application and use of each instrument. We hypothesized that the most prevalently utilized instruments do not strongly correlate in all domains, but rather act in complementary fashion for QOL assessment.
METHODS
A systematic literature review (MEDLINE) was performed to identify the type and frequency of available CRS-specific QOL instruments. Univariate analyses of the three most common instruments (Rhinosinusitis Disability Index (RSDI), Chronic Sinusitis Survey (CSS) and Sinonasal Outcomes Test (SNOT-22)) were performed using a multi-institutional prospective cohort of sinusitis patients.
RESULTS
Systematic literature review found that the SNOT-20 (and its derivatives), RSDI, and CSS are the most commonly utilized CRS-specific QOL instruments. The majority of RSDI domains were weakly or not correlated with the CSS domains (r=0.097–0.501; p<0.001). In contrast, the RSDI was highly correlated with the SNOT-22 (r≥0.666; p<0.001). Patients with asthma and/or allergies reported significantly worse CSS scores (p<0.001). Comorbidities had no significant impact on RSDI or SNOT-22 responses.
CONCLUSIONS
Different disease-specific CRS QOL instruments measure different aspects of the patient’s experience. The RSDI and SNOT-22 are more sensitive to measuring the emotional impact of CRS, whereas the CSS examines medication use and symptoms. These instruments play complementary roles in the evaluation of CRS treatment outcomes.
Index Medicus: sinusitis, surgery, evidence based medicine, endoscopy, quality of life
INTRODUCTION
Quality-of-life (QOL) research has gained significant importance in recent years in the evaluation and treatment of chronic rhinosinusitis (CRS). Today, multiple disease-specific QOL instruments exist for the evaluation of CRS. QOL instruments are typically composed of multiple questions and utilize either Likert scales or continuous measures of disease severity across various patient domains or disease-specific symptoms. Not all available methods and instruments have been validated and whether clinically relevant associations exist between instruments has not yet been completely elucidated. This can lead to confusion about the potential role and application of each instrument as well as its relationship to other disease-specific QOL instruments. Furthermore, it is not uncommon for multiple QOL surveys to be used in the same study population in a single investigation. While this practice can produce complimentary information about a study population, it may also introduce a level of redundancy and/or lead to difficulty interpreting results.
The purpose of this study was two-fold. First, we identified the type and frequency of the most common validated, disease-specific QOL instruments in the current CRS literature. Secondly, we analyzed the relationship between these instruments: the Rhinosinusitis Disability Index (RSDI), the Chronic Sinusitis Survey (CSS), and the 22-item Sinonasal Outcomes Test (SNOT-22). We hypothesized that these instruments would not strongly correlate in all domains but rather act as complementary tools for more comprehensive QOL assessment.
MATERIALS & METHODS
Literature Review
A systematic review of the CRS literature was performed using MEDLINE (January 1st, 1950 to February 6th, 2012) using keywords to identify the types and frequencies of disease-specific QOL instruments currently available. Initial search was performed using “chronic”, “sinusitis” and “quality-of-life” as MeSH headings (n=321). The search was limited to human subjects, adult patients, and English publications (n=169). Abstracts were reviewed for appropriate content. Each abstract was reviewed for use of disease-specific QOL instruments and included if it involved patients with CRS. Studies addressing other patient populations such as those with acute rhinosinusitis and/or granulomatous disease were excluded unless a portion of patients also had CRS. Articles that did not use a validated QOL instrument (such as review articles) were also excluded. If the information was unavailable or incomplete, the full article was reviewed. Each instrument was reviewed for validity, reliability and sensitivity to change.
Study Population and Data Collection
A total of 792 study subjects with CRS were recruited from three tertiary care centers between September, 2000 and August, 2009 as part of a multi-institutional prospective cohort study. Overall initial results from these study subjects have been previously reported elsewhere.1,2 Additional study subjects (n=132) were newly enrolled from the senior author’s institution between April, 2011 and February, 2012, and have not been reported elsewhere. All study patients had a diagnosis of CRS based on criteria endorsed by the American Academy of Otolaryngology–Head and Neck Surgery.3 Adult patients (≥18 yrs old) were enrolled in the study after having failed medical management and elected to proceed with sinus surgery. Demographic and clinical data, including a medical history and physical exam, were collected prospectively. All study protocols and informed consent were collected and approved by the Institutional Review Boards at each study enrollment site.
Survey Instrumentation
Study subjects with CRS were asked to respond to a host of disease-specific QOL surveys during an initial study enrollment meeting. The Rhinosinusitis Disability Index (RSDI) is a 30-item, Likert-scale survey consisting of three individual subscales that include the physical, functional, and emotional domains. Total scores range between 0–120. Higher RSDI total and domain scores imply a higher impact of disease. The Chronic Sinusitis Survey (CSS) is a duration based 6-item survey that measures sinusitis-specific symptoms and medication use during the preceding 8 weeks. Standardized total scores range between 0–100. In the case of CSS, lower total and subscale scores signify a greater impact of disease on the patient.4 The 31-question Rhinosinusitis Outcome Measure (RSOM-31) is a broad-based, validated outcomes tool that includes both rhinosinusitis disease-specific and general QOL measurements. The RSOM-31 was compacted into a set of 20 questions to become the SNOT-20 followed by the addition of two other questions to finalize as the SNOT-22. The two additions consist of the measurement of nasal blockage and loss of sense of taste and smell. Scoring for the SNOT-22 has also been simplified. Total scores range between 0–120, with higher scores indicating a higher impact of disease.5
Psychometric Measures of QOL Instruments
The following criteria were examined for each QOL instrument identified during literature review: validity, reliability, responsiveness, and interpretability/ease of use.
Validity is the extent to which an instrument measures what it was intended to measure. Multiple types of validity exist including content, criterion, construct, and discriminant validity. Content-related validity is a measure of the “appropriateness and redundancy of items and scales of the instrument”.6 Criterion-related validity is the extent to which the instrument relates to a gold-standard; this is difficult to test when generally accepted criteria are not widely accepted. Construct-related validity is the degree to which the condition gets worse (reflected in the score), which should ideally correlate with general health measures. Discriminant validity implies that the instrument is able to distinguish between disease-affected patients and those that do not have the specific disease process.7
Reliability is a reflection of internal consistency, which is a measure of how individual items within the survey instrument relate to each other. Adequate reliability would suggest homogeneity within the instrument and that items that reflect the same construct yield similar results.6,8 Internal consistency can be estimated by calculating Cronbach’s alpha (range 0–1). An alpha ≥0.7 is satisfactory for group comparison whereas an alpha ≥0.9 is appropriate for individual comparison in a clinical setting.8 Test-retest reliability is a measure of the stability of the instrument with repeated testing at different time points.
Responsiveness implies sensitivity to clinical change in the patient over a relatively short period of time. This can reflect confounding variables beyond the nature of the disease, including an altered perception of the disorder due to social dynamics or changes related to treatment. Responsiveness can be measured by standardized response mean (SRM), defined as the mean change score divided by its standard deviation. An SRM<0.2 is insensitive to change and SRM>0.8 is highly sensitive to change.6
Additionally, instruments should be easy to administer, interpret, and score. The patient burden should be limited, including the time it takes to complete the questions. The survey answers should be easily interpretable when obtaining an overall score.
Validation of CSS, RSDI, and SNOT-22 instruments
The RSDI was developed using a methodology with proven effectiveness in the validation of disease-specific patient perception outcomes inventories. The questions are written in the first person, with the thought of allowing the patient to individualize the impact of the disease on their physical, emotional, and functional limitations. Test-retest validity and reproducibility over time have been validated for the RSDI. It also has internal consistency and is able to distinguish between patients with and without rhinosinusitis. Lastly, the RSDI is simple to deliver for the physician, and the average time burden is 10 minutes.7
The CSS has demonstrated good test-retest reliability for individual test questions and total score. It has also shown considerable sensitivity to longitudinal change in symptoms. When initially validated, the CSS was compared to a general health survey (Medical Outcome Study Short Form-36; SF-36) and found to correlate well in three subscores: physical functioning, physical role, and bodily pain, suggesting convergent validity (when compared to a standard measure).9 Given the brevity of the survey with six straightforward questions, it proves to have good interpretability for both the physician and the patient.
The ability of the SNOT-20 to detect clinical improvement and a response to treatment has been questioned in its validation study, though this may have been attributable to the time elapsed between repeated samples.10 Concerns also arose regarding content validity (ability to adequately measure all important aspects of the disease) of the SNOT-20, leading to the addition of two items regarding nasal blockage and loss of sense of taste and smell, to form the newer SNOT-22. The latter has been validated recently in terms of reliability, validity, and the ability to detect significant clinical change over time.5
Statistical Analyses
All statistical analyses were performed using SPSS v.19.0 statistical software (SPSS Inc., Chicago, IL). Descriptive statistics (means, standard deviations (SD), and frequency) were calculated and reported for all cohort measures. Pearson’s correlation coefficients (rP) were calculated to assess the relationship between RSDI, CSS, and SNOT-22 responses. The CSS symptom subscale was the only outcome measure that was not normally distributed; therefore, Spearman’s correlation coefficient (rS) was used for this parameter.11 With regard to clinical significance, comparisons with r-value ≤0.3 were weakly or not correlated; r-values 0.4–0.6 were moderately correlated, while r-values ≥0.7 were highly correlated.12 We compared survey responses for patients with and without certain comorbid conditions (e.g. asthma, allergies, aspirin intolerance, and nasal polyposis) to assess significant differences in QOL responses using an independent samples t-test. Correlation and t-testing was considered significant at the 0.05 level (two-tailed).
RESULTS
Literature Review
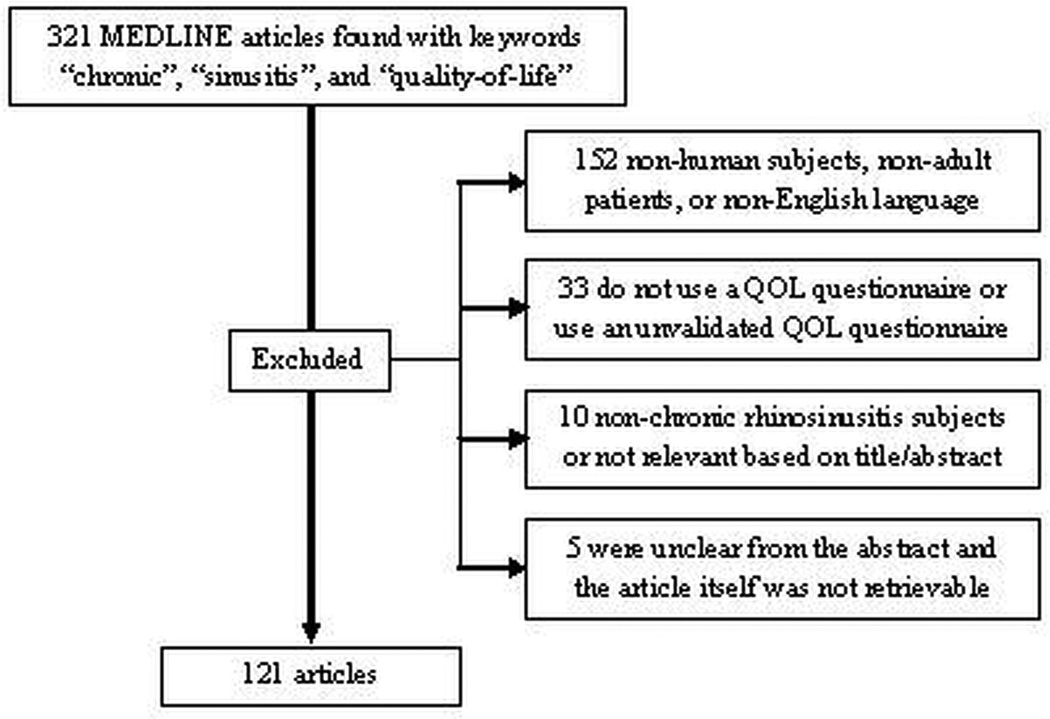
A total of 121 articles were considered for the literature review, based on the criteria mentioned previously (Figure 1). There were five articles whose QOL instrument data was not retrievable from the abstract and the full text was inaccessible. The most commonly used validated disease-specific QOL questionnaires were CSS (37/122), SNOT-20 (32/121), RSDI (29/121), and SNOT-22 (8/121). The validity, reliability, responsiveness, and interpretability of each of these most commonly used CRS-specific QOL instruments is displayed along with the results from the literature review in Table 1. The most commonly used general health questionnaire was the short form survey-36 (SF-36) (30/121). Approximately 48% (58/121) of the studies that met inclusion criteria used more than one questionnaire.
Figure 1.
Requirements for literature search and results.
Table 1.
Literature review results and description of the most commonly used CRS-specific QOL instruments.
| Instrument | Domains or subscales | Number of items in survey | Validity | Reliability | Responsiveness to change | Interpre- tation |
Number of studies using instrument arranged by country |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Content | Criterion | Construct | Cron- bach’s α |
Test- retest |
||||||
| RSDI7,13,14 | Functional, emotional, physical |
30 | Authors’ experience13 |
No significant association with CT score13 |
Discriminant: sinus disease patients have different scores for all items compared to controls13 |
0.9513 | r=0.60– 0.92 for each of 3 domains 13 |
Change in score over 24 months correlated with change in symptom severity, physical health, mood, and perceived control of symptoms14 |
Understan- dable and short (<5 minutes to complete)7 |
USA - 26 United Kingdom - 1 Switzerland - 1 Turkey - 1 TOTAL: 29 |
| CSS13 | Symptoms, medica- tions |
6 | Literature review, authors’ experience |
No correla- tion with CT score |
Convergent: correlates with 3/8 SF-36 subscales; CSS and SF-36 scores improved with surgery |
0.73 | r=0.86 at 14–60 days |
Effect size: 1.12 after surgery SRM: 0.33–0.82 after medical/surgical treatment |
Not assessed | USA - 32 Australia - 2 Canada - 1 Nova Scotia - 1 Taiwan - 1 TOTAL: 37 |
| SNOT- 2010,13 |
Nasal, paranasal, sleep, social, emotional |
20 | Derived from RSOM-31 |
No correla- tion with computed tomo- graphy (CT) score13 |
Convergent: SNOT-20 and SF-12 scores improved13 Discriminant: significantly different scores between patients with rhinosinusitis and controls10 |
0.910 | r=0.910 | SRM: 0.37 at 2 months after surgery13; 0.4 at 6 months10; 0.4 at 1 year10; 38% (95% CI, 28% to 49%) improvement in score with surgery13; change scores significantly different between patients with improved symptoms vs no improvement at 6 months, but not at 1 year10 |
Time burden: 10 minutes easy to interpret: score is the average of the 20 items10 |
USA - 13 Australia - 3 China - 2 Germany - 2 Egypt - 2 New Zealand - 1 United Kingdom - 1 Scotland - 1 Spain - 1 Italy - 1 Greece - 1 Thailand - 1 Turkey - 1 Vietnam - 1 Japan - 1 TOTAL: 32 |
| SNOT-225 | Same as SNOT-20, plus questions regarding nasal blockage and loss of sense of taste and smell |
22 | Derived from SNOT-20 |
Not assessed |
Discriminant: significantly different scores between patients with CRS and healthy controls, and between sub- groups of patients with CRS |
0.91 | r=0.93 | Effect size at 3 months: 0.81; statistically significant decrease in patient-reported scores at 3 months |
Respondent burden decreased compared to SNOT-20 (impor-tance rating removed) |
United Kingdom - 5 Denmark - 1 Czech Republic - 1 Belgium - 1 TOTAL: 8 |
CRS, chronic rhinosinusitis; QOL, quality of life; RSDI, Rhinosinusitis Disability Index; CSS, Chronic Sinusitis Survey; CT, computed tomography; SF-36, Medical Outcomes Study Short Form Survey-36; SNOT, Sinonasal Outcomes Test; SF-12, Medical Outcomes Study Short Form Survey-12; RSOM, RhinoSinusitis Outcome Measure; CI, confidence interval; SRM, standardized response mean.
Bivariate Correlation between RSDI and CSS
Comparisons between the RSDI and CSS were performed using a multi-institutional cohort of patients with sinusitis (n=792). The mean total preoperative RSDI score was 47.9(standard deviation (SD) 20.6); the physical, functional and emotional subscales were 19.1(SD 7.8), 15.5(SD 7.3), and 13.3(SD 8.0), respectively. The mean total preoperative CSS score was 37.4(SD 20.4); the mean symptom and medication scale scores were 29.3(SD 26.5) and 45.6(SD 26.4), respectively. In this study population of 792 patients, 220(28%) had nasal polyposis, 226(29%) had asthma, 177(22%) had allergic rhinitis and 68(9%) had aspirin intolerance.
Physical RSDI subscale scores moderately correlated with CSS symptom score and with CSS total score (r= −0.501 and r= −0.420 respectively; both p<0.001) (Table 2). Total RSDI score was weakly to moderately correlated with CSS symptom score and with CSS total score (r= −0.394 and r= −0.359 respectively; both p<0.001). Other domains were either weakly or not correlated (r= −0.097 to −0.318).
Table 2.
Correlation coefficients for baseline QOL scores from RSDI and CSS surveys (n=792)
| CSS Symptom | CSS Medication | Total CSS | |
|---|---|---|---|
| RSDI Physical RSDI Functional RSDI Emotional Total RSDI |
−0.501* −0.318* −0.236* −0.394* |
−0.129* −0.151* −0.097 −0.139* |
−0.420* −0.318* −0.227* −0.359* |
Single asterisk (*) indicates significant p-values <0.001. Negative values reflect the inverse relationship between survey scores. CSS, Chronic Sinusitis Survey; RSDI, Rhinosinusitis Disability Index.QOL, quality of life.
RSDI and SNOT-22 comparison
Comparisons between the RSDI and SNOT-22 were performed using a prospective cohort of sinusitis patients surveyed at the senior author’s home institution (n=132). All total and subscale scores of the RSDI were highly correlated with SNOT-22 scores (r=0.675–0.839, all p<0.001) (Table 3).
Table 3.
Correlation coefficients for baseline QOL scores from RSDI and SNOT-22 surveys (n=132)
Single asterisk (*) indicates significant p-values <0.001. SNOT-22, Sinonasal Outcomes Test-22; RSDI, Rhinosinusitis Disability Index. QOL, quality of life.
Role of Comorbid Conditions on Disease-Specific QOL
Patients with asthma, allergy, aspirin intolerance and/or nasal polyposis reported similar RSDI and SNOT-22 scores to patients without each of these comorbidities. Patients with aspirin intolerance and/or nasal polyposis had similar CSS scores to patients without these conditions. In contrast, patients with asthma and/or allergies had significantly worse mean scores than patients without asthma or allergies on the total CSS (p<0.001). (Table 4)
Table 4.
QOL score averages in study population with and without asthma and/or allergies
| Total RSDI Score | Total CSS Score | Total SNOT-22 Score | |||||||
|---|---|---|---|---|---|---|---|---|---|
| With | Without | With | Without | With | Without | ||||
| Mean(SD) | Mean(SD) | p value | Mean(SD) | Mean(SD) | p value | Mean(SD) | Mean(SD) | p value | |
| Asthma | 49.0(20.2) | 47.4(20.9) | 0.286 | 33.9(19.2) | 39.6(20.7) | <0.001 | 57.3(19.5) | 50.8(19.4) | 0.172 |
| Allergies | 47.7(19.3) | 48.2(21.2) | 0.757 | 32.9(18.3) | 39.6 (21.0) | <0.001 | 54.8(20.3) | 52.3(19.4) | 0.628 |
QOL, Quality of Life; RSDI, Rhinosinusitis Disability Index; CSS, Chronic Sinusitis Survey; SNOT-22, Sinonasal Outcomes Test-22; SD, standard deviation.
The analysis was continued by exploring the correlation between instruments for patients with and without each comorbidity. The RSDI was weakly to moderately correlated with the CSS symptom subscale (r=0.182–0.564) and CSS total score (r=0.159–0.433). The RSDI was not correlated with the CSS medication subscale (r=0.016–0.190). The RSDI and the SNOT-22 were highly correlated (r=0.604–0.924). Comorbidities did not alter the correlations between any of the scales.
DISCUSSION
Clinicians who wish to quantify the extent of disease impact on their patients’ lives find themselves with a multitude of QOL instruments at their disposal. Many of these have been used extensively in the literature, making it a challenge to select an instrument to use in one’s own practice. This study sought to identify and compare some of the most frequently used CRS-specific QOL instruments in the literature. In general, the RSDI and SNOT-22 were highly correlated with correlation coefficients approximating 0.8 whereas the RSDI was only moderately correlated with CSS symptom scale and not correlated with the CSS medication subscale. These associations generally held true among chronic sinusitis patients whether or not they had asthma, allergies, nasal polyposis or ASA intolerance.
The CSS is a duration-based evaluation of CRS-related symptoms and medication use composed of 6 questions. It is convenient for practitioners to use in that it is very brief (in the authors’ experience, time burden for patients is 2–3 minutes). In addition, unlike the other two instruments, it captures data about recent medication use. One limitation to the survey is that is does not measure the impact of sinusitis on patients’ sense of taste or smell. Since the development of the survey, this has become part of the American Academy of Otolaryngology-Head and Neck Surgery Task Force on Rhinosinusitis (RSTF) criteria for the diagnosis of CRS.3 Scoring requires a conversion which can be cumbersome in fast-paced clinical activities. As seen in this study, it complements data collected by the RSDI.
The RSDI moves beyond patients’ symptoms to capture more sophisticated and unique aspects of the patient’s physical, functional, and emotional experience. The trade-off for this additional information is that it increases patient burden. In the authors’ experience, surveys take approximately 5–6 minutes to complete, although Benninger and Senior reported a time burden of less than 5 minutes.7 In contrast to the CSS, medication use is not measured.
The SNOT-22 is similar to the RSDI in that it covers a broad range of health problems, including physical issues, functional limitations, and emotional effects, and does not measure medication use.5 With the addition of the two items in the SNOT-22, it now includes all four symptom measures of the diagnostic criteria laid out by the RSTF. The time burden is less than the RSDI, but greater than the CSS (approximately 3–4 minutes in the authors’ experience). The RSDI and the SNOT-22 are similar in composition and highly correlated with each other. Therefore, it would seem redundant to use both the RSDI and the SNOT-22 to evaluate CRS-specific QOL in the same patient population.
Other patient-based survey instruments have been used to evaluate patients with CRS. The most common have been the Medical Outcomes Study Short-Form (SF) health surveys (most commonly SF-36) and the visual analog scale (VAS). The SF-36 has been used as a comprehensive measure sensitive to the full range of illness and at the same time, relatively brief and easy to use.15 Function is measured in eight separate domains: physical functioning, social functioning, role functioning, bodily pain, general health, vitality, mental health, and health transition. An advantage of using such a widespread instrument is that it can enable us to compare the QOL of patients with CRS to patients with other disease processes. Notably, in one classic CRS study using the SF-36, patients with sinusitis scored lower in measures of bodily pain and social functioning than patients with congestive heart failure or chronic obstructive pulmonary disease.16 The SF-12 was derived from the SF-36 with the purpose of creating a survey with similar objectives that was shorter. The 12 items in the SF-12 provide a representative sampling of the eight domains of its longer predecessor. The shorter SF-12 is generally adequate for studies with large sample sizes having constraints on questionnaire length and time burden.17
The VAS is a visually based instrument and has been validated based on the diagnostic criteria for CRS as described by the RSTF.3 Symptoms are assessed by instructing each study subject to indicate current symptom severity on a continuous scale of 0–10cm, where 0cm represents an absence of the symptom and 10cm signifies the most severe symptom imaginable.18 Ling and Kountakis evaluated the RSTF symptoms as scored by a VAS system and SNOT-20 scores. They found that the scores from both instruments were moderately correlated at baseline and at 1 year postoperatively(r=0.36 and r=0.37, respectively).19
As noted previously, 48% of the articles examined in our literature review used more than one survey. Many validation studies for disease-specific surveys used a general health tool (such as the SF-36) to determine validity.9 Others used both a general and a disease-specific instrument in order to obtain a more integral evaluation of the patient, including more general health measures such as mental health and social functioning, in addition to questions centered on the disease process itself. It is also common for studies to use more than one disease-specific survey, such as the RSDI and the CSS. It has been recognized that the advantage of the RSDI over other surveys lies in its measurement of more general QOL domains related to social and emotional functioning, thereby reducing the need for an additional general health tool such as the SF-36.7 Per our findings, a comprehensive evaluation of a patient’s QOL can be obtained at the expense of added survey burden by using two complementary QOL surveys that assess different facets of the disease (such as CSS with RSDI, or CSS with SNOT-22).
Our comorbidity analysis suggests that asthma, nasal polyps, allergy, and aspirin intolerance are not factors that will influence average scores on the RSDI or SNOT-22 surveys when evaluating patients with CRS. Patients with asthma and allergies on average scored 6–7 points (on a 100-point scale) lower on the CSS instrument. Whether this translates into a clinically important difference is unclear. It was surprising that comorbidities lacked significant impact on the RSDI or SNOT-22, given that these surveys evaluate a wide range of comprehensive health measures. In the setting of a chronic condition such as asthma, one might expect various aspects of a patient’s life to be affected in a more significant manner. One study showed that increasing severity of asthma is associated with advancing radiological severity of CRS.20 Patients with CRS and comorbid asthma constitute ~29% of our multi-institutional cohort. If asthma severity correlates with CRS, patients with asthma could be experiencing a significantly greater duration of sinusitis symptoms and/or medications as reflected on responses to the CSS instrument. It may be interesting to explore whether the severity of asthma, confirmed through forced expiratory volume in 1 second (FEV1) testing, is associated with CRS-specific QOL scores as well. A study by Emanuel and Shah concluded that there was a relatively lower percentage of allergy in their cohort of patients with the most severe sinus disease on CT scan.21 If our data is clinically relevant, it would appear to conflict with this finding, since allergy may imply worse QOL as measured through total CSS scores. The question of whether subjective measures of disease can predict disease severity on CT scan has been explored in the literature. A study by Nair reports that symptom severity (not determined by a QOL survey) correlates to specific sinus involvement on CT scan.22 Some studies have shown that the radiological severity of CRS disease does not correlate to many QOL instruments such as SNOT-20 or CSS in a general CRS population, though there may be correlation in certain sub-groups such as CRS with nasal polyposis or smokers.23,24
It is interesting to see the correlation between surveys when the patient population is broken down into sub-groups according to comorbidities. With this analysis, it was evident that the medication subscale of the CSS is not correlated with the RSDI, for all comorbidities. This provides further evidence that these two surveys measure different aspects of the disease process, and is something to keep in mind when deciding which surveys to use in a heterogeneous population.
This article has several minor limitations that should be acknowledged. The patient population with CRS was obtained from a tertiary care center, making external generalizations to other surgical or non-surgical patient populations challenging. Some articles (5/126) were excluded from the literature review due to inaccessibility of the text. Additionally, correlation studies are limited in explanatory ability and cannot imply direct cause-and-effect associations. Lastly, due to the number of QOL subscale comparisons, there is an increased likelihood of committing a type I error and an erroneous report of significant findings. However, these comparisons were very significant (p<0.001) and likely to meet the criteria for significance with any test used to adjust for multiple comparisons.
CONCLUSION
One may need to consider several factors when choosing a QOL questionnaire for a professional practice. QOL instruments may be weighted towards different dimensions of a patient’s QOL (i.e. physical and objective versus emotional and subjective). The comorbidities present in the population studied may influence outcomes on some instruments. It is noteworthy that different surveys, such as the RSDI and the CSS, can complement each other. Regardless of the instrument chosen, all three described in this paper have been validated and used extensively in the CRS literature. They have consistently shown the ability to provide valuable information about the disease impact on a patient’s life and the outcome after treatment.
ACKNOWLEDGMENT
This investigation was made possible by grant funding (2R01DC005805) from the National Institute on Deafness and Other Communication Disorders, one of the National Institutes of Health.
Footnotes
Potential Conflict of Interest: None
Financial Disclosure: Timothy L Smith, MD, is a consultant for Intersect ENT (Palo Alto, CA.) which provided no financial support for this work. Both Timothy L. Smith, MD, and Jess C. Mace receive grant support from the National Institute on Deafness and Other Communication Disorders, one of the National Institutes of Health (R02DC005805; PI: Smith).
The Institutional Review Board at Oregon Health & Science University provided approval for this human subjects investigation.
Accepted for oral presentation at the American Rhinologic Society meeting at the 115th annual Combined Otolaryngologic Spring Meeting (COSM) in San Diego, CA., April, 2012.
REFERENCES
- 1.Litvack JR, Fong K, Mace J, et al. Predictors of olfactory dysfunction in patients with chronic rhinosinusitis. Laryngoscope. 2008;18:2225–2230. doi: 10.1097/MLG.0b013e318184e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litvack JR, Mace JC, Smith TL. Olfactory function and disease severity in chronic rhinosinusitis. Am J Rhinol Allergy. 2009;23(2):139–144. doi: 10.2500/ajra.2009.23.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: Definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surgery. 2003;129(suppl):S1–S32. doi: 10.1016/s0194-5998(03)01397-4. [DOI] [PubMed] [Google Scholar]
- 4.Smith TL, Kern RC, Palmer JN, et al. Medical therapy vs surgery for chronic rhinosinusitis: a prospective, multi-institutional study. Int Forum Allergy Rhinol. 2011;1:235–241. doi: 10.1002/alr.20063. [DOI] [PubMed] [Google Scholar]
- 5.Hopkins C, Gillett S, Slack R, et al. Psychometric validity of the 22-item Sinonasal Outcome Test. Clinical Otolaryngology. 2009;34:447–454. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 6.Morley AD, Sharp HR. A review of sinonasal outcome scoring systems – which is best? Clinical Otolaryngology. 2006;31(2):103–109. doi: 10.1111/j.1749-4486.2006.01155.x. [DOI] [PubMed] [Google Scholar]
- 7.Benninger MS, Senior BA. The development of the rhinosinusitis disability index. Arch Otolaryngol Head Neck Surg. 1997;123:1175–1179. doi: 10.1001/archotol.1997.01900110025004. [DOI] [PubMed] [Google Scholar]
- 8.Alobid I, Bernal-Sprekelsen M, Mullol J. Chronic rhinosinusitis and nasal polyps: the role of generic and specific questionnaires on assessing its impact on patient’s quality of life. Allergy. 2008;63:1267–1279. doi: 10.1111/j.1398-9995.2008.01828.x. [DOI] [PubMed] [Google Scholar]
- 9.Gliklich RE, Metson R. Techniques for Outcomes Research in Chronic Sinusitis. Laryngoscope. 1995;105:387–390. doi: 10.1288/00005537-199504000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Piccirillo JF, Merritt MG, Richards ML. Psychometric and clinimetric validity of the 20-item Sino-0Nasal Outcome Test (SNOT-20) Otolaryngol Head Neck Surg. 2002;126:41–47. doi: 10.1067/mhn.2002.121022. [DOI] [PubMed] [Google Scholar]
- 11.Pagano M, Gauvreau K. Principles of Biostatistics. 2nd ed. Pacific Grove, CA: Duxbury Thompson Learning; 2000. Correlation; pp. 404–407. [Google Scholar]
- 12.Hinkle DE, Wiersma W, Jurs SG, editors. Applied Sciences for the Behavioral Sciences. 2nd ed. Boston, MA: Houghton Mifflin Co.; 1988. Correlation: A measurement of relationship; pp. 252–285. [Google Scholar]
- 13.Linder JA, Singer DE, van den Ancker M, et al. Measures of Health-related Quality of Life for Adults with Acute Sinusitis. J Gen Intern Med. 2003;18:390–401. doi: 10.1046/j.1525-1497.2003.20744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen H, Katz PP, Shiboski S, et al. Evaluating change in health-related quality of life in adult rhinitis: Responsiveness of the Rhinosinusitis Disability Index. Health Qual Life Outcomes. 2005;3:68. doi: 10.1186/1477-7525-3-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brazier JE, Harper R, Jones NMB, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160–164. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khalid AN, Quraishi SA, Kennedy DW. Long-term quality of life measures after functional endoscopic sinus surgery. Am J Rhinol. 2004;18:131–136. [PubMed] [Google Scholar]
- 17.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Walker FDL, White PS. Sinus symptom scores: what is the range in healthy individuals? Clinical Otolaryngology & Allied Sciences. 2000;25(6):482–484. doi: 10.1046/j.1365-2273.2000.00349.x. [DOI] [PubMed] [Google Scholar]
- 19.Ling FTK, Kountakis SE. Rhinosinusitis Task Force symptoms versus the Sinonasal Outcomes Test in patients evaluated for chronic rhinosinusitis. Am J Rhinol. 2007;21:495–498. doi: 10.2500/ajr.2007.21.3052. [DOI] [PubMed] [Google Scholar]
- 20.Lin DC, Chandra RK, Tan BK, et al. Association between severity of asthma and degree of chronic rhinosinusitis. Am J Rhinol Allergy. 2011;25(4):205–208. doi: 10.2500/ajra.2011.25.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emanuel IA, Shah SB. Chronic rhinosinusitis: allergy and sinus computed tomography relationships. Otolaryngol Head Neck Surg. 2000;123(6):687–691. doi: 10.1067/mhn.2000.110961. [DOI] [PubMed] [Google Scholar]
- 22.Nair S. Correlation between symptoms and radiological findings in patients of chronic rhinosinusitis: a modified radiological typing system. Rhinology. 2009;47:181–186. [PubMed] [Google Scholar]
- 23.Zheng Y, Zhao Y, Lv D, et al. Correlation between computed tomography staging and quality of life instruments in patients with chronic rhinosinusitis. Am J Rhinol Allergy. 2010;24(1):e41–e45. doi: 10.2500/ajra.2010.24.3430. [DOI] [PubMed] [Google Scholar]
- 24.Wabnitz DAM, Nair S, Wormald PJ. Correlation between Preoperative Symptom Scores, Quality-of-Life Questionnaires, and Staging with Computed Tomography in Patients with Chronic Rhinosinusitis. Am J Rhinol. 2005;19:91–96. [PubMed] [Google Scholar]