Abstract
Familial aggregation of non-diabetic end stage renal disease (ESRD) is found in African Americans and variants in the apolipoprotein L1 gene (APOL1) contribute to this risk. To detect genetic associations with milder forms of nephropathy in high-risk families, analyses were performed using generalized estimating equations to assess relationships between kidney disease phenotypes and APOL1 variants in 786 relatives of 470 families. Adjusting for familial correlations, 23.1, 46.7, and 30.2 percent of genotyped relatives possessed two, one, or no APOL1 risk variants, respectively. Relatives with two compared to one or no risk variants had statistically indistinguishable median systolic blood pressure, urine albumin to creatinine ratio, estimated GFR (MDRD equation) and serum cystatin C levels. After adjusting for age, gender, age at ESRD in families, and African ancestry, significant associations were detected between APOL1 with overt proteinuria and estimated GFR (CKD-EPI equation), with a trend toward significance for quantitative albuminuria. Thus, relatives of African Americans with non-diabetic ESRD are enriched for APOL1 risk variants. After adjustment, two APOL1 risk variants weakly predict mild forms of kidney disease. Second hits appear necessary for the initiation of APOL1-associated nephropathy.
Keywords: African American, APOL1, end-stage renal disease, FSGS, kidney, screening
Introduction
Association between the G1 and G2 coding variants in the apolipoprotein L1 gene (APOL1) on chromosome 22q ranks among the most impressive genetic breakthroughs in complex disease.[1;2] Idiopathic focal segmental glomerulosclerosis (FSGS), hypertension-attributed nephropathy, non-diabetic end-stage renal disease (ESRD), and HIV-associated collapsing glomerulopathy in African Americans are now recognized as one disease spectrum.[3] Significantly shorter kidney allograft survival in African-ancestry deceased donor kidneys also relates to presence of two APOL1 risk variants, independent from donor race.[4] Nephropathy risk variants have been selected for based upon protection from Trypanosoma brucei rhodesiense, a cause of African sleeping sickness.[1]
However, not all individuals inheriting two APOL1 risk variants will develop nephropathy. Additional genetic and/or environmental “second hits” are likely required to initiate kidney disease, interacting with APOL1 risk variants to produce different renal histologic phenotypes.[5] HIV infection is clearly a second hit, typically producing collapsing FSGS with proliferation of podocytes.[6] Podocin gene (NPHS2) variants may also prove to be genetically-mediated second hits.[7]
The mechanism(s) underlying APOL1-associated nephropathy remain under intensive investigation.[8;9] Aggressive blood pressure control and use of angiotensin-converting enzyme inhibitors failed to markedly halt progression of hypertension-attributed nephropathy to ESRD in the African American Study of Kidney Disease and Hypertension (AASK),[10] the kidney disease in AASK has subsequently been shown to be strongly APOL1-associated.[11] Without effective treatments and barring identification of treatable/preventable second hits, widespread screening efforts in the African American population are of unproven benefit. APOL1 risk variants were associated with a higher prevalence of microalbuminuria and higher prevalence of a low glomerular filtration rate (GFR) in African Americans from the population-based Dallas Heart Study.[12] However, proteinuria and estimated GFR did not differ significantly between those with two versus less than two APOL1 nephropathy risk variants. Close relatives of African Americans with non-diabetic forms of ESRD are at exceptionally high risk for developing nephropathy and disparate etiologies of nephropathy are often seen in these families.[13–20] Relatives are often considered for living kidney donation, as well.[21] This study assessed the association between APOL1 nephropathy risk variants and risk of mild nephropathy in first-degree relatives of African Americans with non-diabetic ESRD.
Results
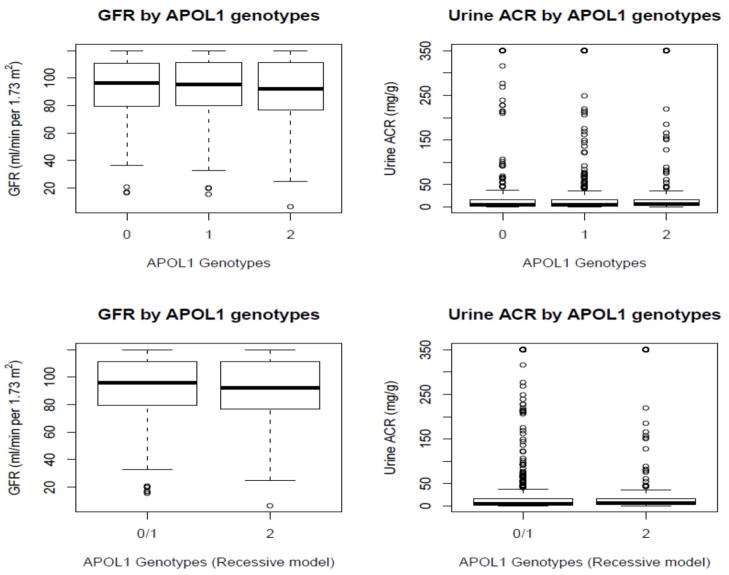
As of December 1, 2011, the Natural History of APOL1-associated Nephropathy Study recruited 826 African American children and siblings of index cases with non-diabetic ESRD and 786 have been genotyped for APOL1 and ancestry informative markers. One individual was recruited in 249 families, two individuals in 137 families (N=274), and three individuals in 101 families (N=303). The mean (SD) median age at ESRD in the proband from families was 47.1 (13.4) 47.0 years. Adjusting for familial correlations, 23.1%, 46.7%, and 30.2% of genotyped relatives possessed two, one, or zero APOL1 risk variants, respectively. Table 1 contains demographic and clinical results in all 786 first degree relatives of ESRD patients, based on the number of APOL1 risk variants. No statistically significant differences in renal or hypertensive phenotypes were observed in an additive model. Figure 1 displays the distribution of MDRD GFR and urine ACR based on genotypes.
Table 1.
Demographic and clinical characteristics of participants, based on the number of APOL1 nephropathy risk variants
| Variable | 0 APOL1 risk variants | 1 APOL1 risk variant | 2 APOL1 risk variants | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean ± SD | Median | IQR | N | Mean ± SD | Median | IQR | N | Mean ± SD | Median | IQR | ||
| African ancestry, % | 231 | 0.78±0.11 | 0.80 | 0.12 | 375 | 0.78±0.11 | 0.80 | 0.13 | 175 | 0.79±0.09 | 0.80 | 0.13 | 0.7587 |
| Age, yrs | 234 | 48.2±12.7 | 49.0 | 18.0 | 377 | 48.0±13.7 | 49.0 | 19.0 | 175 | 45.6±13.5 | 48.0 | 17.0 | 0.1886 |
| Age relative to proband, yrs | 233 | −1.5±9.0 | −1.0 | 15.0 | 374 | 0.8±8.8 | 1.0 | 12.0 | 173 | 0.1±9.2 | 1.0 | 15.0 | 0.0181 |
| BMI, kg/m2 | 233 | 32.3±8.9 | 30.6 | 10.3 | 376 | 33.6±9.7 | 32.1 | 10.9 | 175 | 32.0±9.0 | 30.5 | 10.9 | 0.1070 |
| Cystatin C, mg/L | 234 | 0.8±0.3 | 0.8 | 0.26 | 377 | 0.8±0.4 | 0.8 | 0.22 | 175 | 0.8±0.3 | 0.7 | 0.29 | 0.4652 |
| Diastolic BP, mm Hg | 228 | 88.1±16.3 | 85.0 | 20.3 | 377 | 87.0±15.1 | 85.5 | 17.0 | 175 | 86.9±12.8 | 85.5 | 17.5 | 0.9389 |
| GFR, ml/min/1.73m2 | 234 | 95.3±25.1 | 96.7 | 31.2 | 377 | 96.1±27.0 | 95.5 | 31.5 | 175 | 94.1±26.5 | 92.1 | 35.3 | 0.6755 |
| Systolic BP, mm Hg | 228 | 131.1±26.0 | 128.8 | 27.8 | 377 | 131.1±23.8 | 126.0 | 29.5 | 175 | 128.2±20.7 | 126.0 | 26.5 | 0.5164 |
| Urine ACR, mg/g | 234 | 92.5±559.5 | 5.0 | 14.5 | 377 | 105.4±610.4 | 5.4 | 14.0 | 175 | 62.7±173.7 | 6.4 | 13.8 | 0.3506 |
BMI - body mass index; BP - blood pressure; GFR - MDRD glomerular filtration rate; ACR - albumin: creatinine ratio; IQR – inter-quartile range
Figure 1.
Distribution of GFR and urine ACR by APOL1 genotypes. Bars represent minimum and maximum readings. Bars spanning boxes refer to: 75% (top); median (middle); and 25% (lower)
APOL1 associations with FSGS and non-diabetic ESRD best fit autosomal recessive inheritance. Therefore, unadjusted association analyses with APOL1 nephropathy risk variants in categorical (Table 2) and continuous evaluation of renal phenotypes (Table 3) were performed using a recessive model. No evidence of association was observed in either of these analyses. Categorical classifications of GFR and urine ACR were based upon clinically relevant cut-points (Table 2).
Table 2.
Unadjusted APOL1 associations in the full sample (categorical variables; recessive model)
| Variable, category | 0/1 APOL1 risk variants | 2 APOL1 risk variants | Full Sample | P-value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Kidney disease*, No | 482 | 78.1 | 135 | 21.9 | 617 | 78.5 | 0.6205 |
| Kidney disease*, Yes | 129 | 76.3 | 40 | 23.7 | 169 | 21.5 | |
| Hypertension, No | 346 | 76.9 | 104 | 23.1 | 450 | 57.7 | 0.5976 |
| Hypertension, Yes | 259 | 78.5 | 71 | 21.5 | 330 | 42.3 | |
| Sex, Male | 249 | 79.6 | 64 | 20.4 | 313 | 39.8 | 0.3191 |
| Sex, Female | 362 | 76.5 | 111 | 23.5 | 473 | 60.2 | |
| Age < 20 years | 6 | 46.2 | 7 | 53.8 | 13 | 1.7 | 0.0492 |
| Age 20–30 years | 60 | 74.1 | 21 | 25.9 | 81 | 10.3 | |
| Age 30–40 years | 93 | 80.9 | 22 | 19.1 | 115 | 14.6 | |
| Age 40–50 years | 159 | 76.8 | 48 | 23.2 | 207 | 26.3 | |
| Age 50–60 years | 268 | 78.4 | 74 | 21.6 | 342 | 43.6 | |
| Age ≥ 70 years | 25 | 89.3 | 3 | 10.7 | 28 | 3.6 | |
| Proband-participant age difference: −25 to −15 years | 11 | 64.7 | 6 | 35.3 | 17 | 2.2 | 0.4650 |
| Proband-participant age difference: −15 to −5 years | 162 | 77.1 | 48 | 22.9 | 210 | 26.9 | |
| Proband-participant age difference: −5 to +5 years | 240 | 80.5 | 58 | 19.5 | 298 | 38.2 | |
| Proband-participant age difference: +5 to +15 years | 172 | 75.8 | 55 | 24.2 | 277 | 29.1 | |
| Proband-participant age difference: > +15 years | 22 | 78.6 | 6 | 21.4 | 28 | 3.6 | |
| MDRD GFR <60 ml/min/1.73m2 | 43 | 75.4 | 14 | 24.6 | 57 | 7.3 | 0.8250 |
| MDRD GFR 60–90 ml/min/1.73m2 | 205 | 76.2 | 64 | 23.8 | 269 | 34.2 | |
| MDRD GFR 90–120 ml/min/1.73m2 | 274 | 79.0 | 73 | 21.0 | 347 | 44.1 | |
| MDRD GFR ≥120 ml/min/1.73m2 | 89 | 78.8 | 24 | 21.2 | 113 | 14.4 | |
| Urine ACR 0–30 mg/g | 504 | 78.3 | 140 | 21.7 | 644 | 81.9 | 0.1911 |
| Urine ACR 30–299 mg/g | 82 | 78.8 | 22 | 21.2 | 104 | 13.2 | |
| Urine ACR ≥300 mg/g | 25 | 65.8 | 13 | 34.2 | 38 | 4.8 | |
| Urine ACR <30 mg/g | 504 | 78.3 | 140 | 21.7 | 644 | 81.9 | 0.4508 |
| Urine ACR ≥30 mg/g | 107 | 75.4 | 35 | 24.6 | 142 | 18.1 | |
| Urine ACR 0–17 mg/g (Males only) | 179 | 80.3 | 44 | 19.7 | 223 | 71.3 | 0.6209 |
| Urine ACR ≥17 mg/g (Males only) | 70 | 77.8 | 20 | 22.2 | 90 | 28.7 | |
| Urine ACR 0–25 mg/g (Females only) | 298 | 76.6 | 91 | 23.9 | 389 | 82.2 | 0.9349 |
| Urine ACR ≥25 mg/g (Females only) | 64 | 76.2 | 20 | 23.8 | 84 | 17.8 | |
| Cystatin C <0.96 mg/L | 491 | 77.9 | 139 | 22.1 | 630 | 80.2 | 0.7853 |
| Cystatin C ≥0.96 mg/L | 120 | 76.9 | 36 | 23.1 | 156 | 19.8 | |
• Kidney disease defined as an MDRD GFR <60 ml/min/1.73m2 and/or Urine ACR > 30 mg/g
• GFR – MDRD glomerular filtration rate; ACR – albumin: creatinine ratio
Table 3.
Unadjusted APOL1 associations in the full sample (continuous variables; recessive model)
| Variable | 0/1 APOL1 risk variants (N=611) | 2 APOL1 risk variants (N=175) | P-value | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Mean | SD | Median | ||
| African ancestry, % | 0.78 | 0.11 | 0.80 | 0.79 | 0.09 | 0.80 | 0.2974 |
| Age, yrs | 48.1 | 13.3 | 49.0 | 45.6 | 13.5 | 48.0 | 0.0159 |
| Age relative to proband, yrs | −0.1 | 8.9 | 0.0 | 0.1 | 9.2 | 1.0 | 0.7226 |
| BMI, kg/m2 | 33.1 | 9.4 | 31.5 | 32.0 | 9.0 | 30.5 | 0.1380 |
| Cystatin C, mg/L | 0.8 | 0.3 | 0.8 | 0.8 | 0.3 | 0.7 | 0.6054 |
| Diastolic BP, mm Hg | 87.4 | 15.6 | 85.5 | 86.9 | 12.8 | 85.5 | 0.9140 |
| MDRD GFR, ml/min/1.73m2 | 95.8 | 26.3 | 95.9 | 94.1 | 26.5 | 92.1 | 0.4325 |
| Systolic BP, mm Hg | 131.1 | 24.6 | 127.0 | 128.2 | 20.7 | 126.0 | 0.1525 |
| Urine ACR mg/g | 100.5 | 591.0 | 5.2 | 62.7 | 173.7 | 6.4 | 0.2184 |
BMI - body mass index; BP - blood pressure; GFR - glomerular filtration rate; ACR - albumin: creatinine ratio
APOL1 association analyses were then performed in all 622 relatives without diabetes mellitus (Supplementary Table 1). A trend toward association was seen with urine ACR, but not with other phenotypes. Mean (SD) median urine ACR was 45.5 (257.4) 4.5 mg/g in those with less than two APOL risk variants versus 58.4 (172.8) 6.1 mg/g in those with two risk variants (p=0.1006). Supplementary Table 2 contains the results of unadjusted association analyses limited to the 525 relatives not younger than five years before the age at ESRD in the index case from their family. This analysis was performed since younger patients destined to develop nephropathy may not yet display the phenotype. No significant associations were observed.
A series of multivariate analyses were then computed, adjusting for age, sex, age difference from age at ESRD in probands, and African ancestry. The results in all relatives are shown in Table 4, results limited to those without diabetes mellitus are shown in Supplementary Table 3, and results in participants not younger than five years below the age at onset of ESRD in the index case are shown in Supplementary Table 4. In these analyses, significant association was detected between APOL1 and urine ACR ≥300 mg/g in the full sample (p=0.032, full-adjustment) and non-diabetic sample (p=0.032, full-adjustment); with a trend in the older age sample (p=0.065). Association was also observed with log urine ACR in the non-diabetic sample (p-value 0.050, full-adjustment); with a trend in the full sample (p-value 0.096). Strong and consistent trends toward association were seen with GFR using both MDRD and CKD-EPI equations in the full, non-diabetic, and older samples (p-values ranged from 0.050 to 0.0837, full-adjustment). In contrast, no significant evidence of association was observed for the dichotomous trait of kidney disease (defined as urine ACR ≥30 mg/g and/or MDRD GFR <60 ml/min/1.73m2) or with urine ACR ≥30 mg/g.
Table 4.
Multivariate APOL1 association analyses in the full sample (recessive model)
| Outcome | Model | Estimate | SE | Lower CI | Upper CI | Z | P-value |
|---|---|---|---|---|---|---|---|
| MDRD GFR | 1 | −1.6947 | 2.2773 | −6.1581 | 2.7687 | −0.74 | 0.4568 |
| 2 | −3.7055 | 2.0167 | −7.6581 | 0.2471 | −1.84 | 0.0661 | |
| 3 | −3.8112 | 2.0378 | −7.8052 | 0.1827 | −1.87 | 0.0614 | |
| CKD-EPI GFR | 1 | −1.1306 | 2.1892 | −5.4214 | 3.1601 | −0.52 | 0.6055 |
| 2 | −3.5187 | 1.8506 | −7.1459 | 0.1085 | −1.9 | 0.0573 | |
| 3 | −3.6583 | 1.8696 | −7.3226 | 0.0059 | −1.96 | 0.0504 | |
| Log urine ACR | 1 | 0.163 | 0.1431 | −0.1174 | 0.4433 | 1.14 | 0.2546 |
| 2 | 0.2095 | 0.1434 | −0.0715 | 0.4905 | 1.46 | 0.1439 | |
| 3 | 0.2391 | 0.1435 | −0.0422 | 0.5204 | 1.67 | 0.0957 | |
| Kidney disease | 1 | 0.09 | 0.2132 | −0.328 | 0.5079 | 0.42 | 0.6731 |
| 2 | 0.1733 | 0.2232 | −0.2642 | 0.6107 | 0.78 | 0.4376 | |
| 3 | 0.2144 | 0.2246 | −0.2258 | 0.6545 | 0.95 | 0.3398 | |
| Urine ACR ≥30 mg/g | 1 | 0.143 | 0.2209 | −0.29 | 0.576 | 0.65 | 0.5175 |
| 2 | 0.2021 | 0.2265 | −0.2418 | 0.6461 | 0.89 | 0.3722 | |
| 3 | 0.2422 | 0.2277 | −0.2042 | 0.6886 | 1.06 | 0.2875 | |
| Urine ACR ≥300 mg/g | 1 | 0.7162 | 0.3513 | 0.0277 | 1.4047 | 2.04 | 0.0415 |
| 2 | 0.7191 | 0.3542 | 0.0249 | 1.4132 | 2.03 | 0.0423 | |
| 3 | 0.7607 | 0.3551 | 0.0647 | 1.4568 | 2.14 | 0.0322 |
MDRD GFR - Modification of Diet in Renal Disease estimated glomerular filtration rate;
ACR – albumin: creatinine ratio; Kidney disease reflects either urine ACR ≥30 mg/g and/or MDRD GFR <60 ml/min/1.73 m2 Estimate = β coefficient; Z=(Estimate/SE)
Model 1= Ancestry-adjusted; Model 2= Model 1 + age and sex; Model 3= Model 2 + age difference from ESRD in proband
Finally, suggestive evidence of diabetes by APOL1 interaction effects on cystatin C concentrations was detected. The observed p-values ranged from 0.1321 to 0.1611 for Log (cystatin C), and from 0.0674 to 0.0895 for the dichotomous outcome of high vs. low cystatin C concentrations using a cutoff value of 0.96 mg/L. The interaction effect observed with the dichotomous variable reached statistical significance (p-value=0.0456) in the fully adjusted model after excluding all participants who were more than 5 years younger than their proband. Analyses stratified by diabetes status revealed a trend for a positive association between APOL1 risk and high cystatin C levels in non-diabetic individuals (odds ratio 1.95; p-value=0.0565), and no effect in diabetic individuals (odds ratio 0.51; p-value=0.2927).
Discussion
Marked familial aggregation of non-diabetic ESRD is reproducibly observed in African Americans [13–19] and more than 30% of those initiating renal replacement therapy have close relatives already on dialysis.[20] Variation in the APOL1 gene likely accounts for much of this phenomenon, displaying among the most impressive evidence of association in complex human disease. The present study revealed similar mean and median levels of albuminuria, serum cystatin C concentration, and estimated GFR among relatives of African Americans with non-diabetic ESRD, regardless of the presence of two or less than two APOL1 risk variants. A high proportion of relatives with and without two APOL1 risk variants had subclinical nephropathy. Multi-variable analyses accounting for age, sex, overall African ancestry, and age difference from age at ESRD in index cases revealed weak relationships between APOL1 and urine ACR >300 mg/g and with GFR <60 ml/min per 1.73m2 with p-values ranging from 0.03–0.05, but not with lower levels of albuminuria (urine ACR ≥30 mg/g).
These data are reassuring in that APOL1 associations between heavy proteinuria and reduced GFR were detectable in members of African American families at high risk for nephropathy. The 23% frequency of subclinical nephropathy in these relatives closely resembles frequencies reported in similar families recruited in Alabama.[18] Therefore, our families appear to be representative of such families in the southeastern U.S. The frequencies of urine ACR ≥30 mg/g and ACR ≥300 mg/g in these high risk families greatly exceeds those in the National Health and Nutrition Examination Survey III (NHANES III).[22] However, despite the high prevalence of silent kidney disease, our results suggest there may be limited value in the genetic screening of high risk first-degree relatives of African Americans with non-diabetic ESRD simply to detect those enriched for milder forms of nephropathy. In contrast to albuminuria and mildly reduced GFR, APOL1 risk variants strongly associate with severe forms of nephropathy and ESRD. The majority of relatives with two APOL1 nephropathy risk variants had a normal GFR without elevated urine ACR. In contrast, we previously demonstrated the potential benefit in screening African American deceased kidney donors for these risk variants.[4] It remains possible that APOL1-associated renal diseases may progress more rapidly to overt proteinuria (rendering microalbuminuria less helpful) and/or APOL1-associated microalbuminuria might be hard to identify in a population with more frequent metabolic-syndrome associated microalbuminuria. The families in this study are a unique high genetic/high environmental risk population, likely different from the general population. Longitudinal follow-up is currently underway to determine risk of progressive nephropathy in family members. In addition, 8 relatives with ESRD (<1% of the study population) were excluded, potentially limiting our ability to detect association with severe kidney disease.
The frequencies of two and one APOL1 risk variants were 23.1% and 46.7%, respectively, in this high-risk cohort. This substantially exceeds the 12% and 39% frequencies in the general African American population.[1;12] Current study participants had a mean (SD) age of 47.6 (13.4) years (median 49.0) and 22.9% (40/175) of those with two APOL1 risk variants had kidney disease defined as an MDRD-GFR <60 ml/min per 1.73m2 and/or urine ACR ≥30 mg/dl, compared to 21.1% (129/611) of those with fewer than two risk variants (p=0.63). The mean age at onset of ESRD in African Americans was 59.2 years in the 2009 U.S. Renal Data System report.[23] When limiting our analysis to association with an MDRD estimated GFR <60 ml/min per 1.73m2, low GFR was present in 8% of those with two APOL1 risk variants, compared to 7% with fewer than two risk variants (p=0.66). When considering albuminuria, urine ACR ≥30 mg/g was observed in 20.0% of those with two risk variants relative to 17.5% with fewer than two risk variants (p=0.45). We also observed urine ACR ≥300 mg/g in 7.4% in individuals with 2 risk variants relative to 4.1% in those with 0 or 1 copy of the risk variant (p=0.07). Differences in renal and hypertensive phenotypes were not seen when contrasting those with zero, one or two APOL1 risk variants (Table 1). Accounting for familial relationships and adjusting for participant age, sex, age difference from ESRD onset in probands, and ancestry revealed evidence of association with urine ACR >300 mg/g and GFR <60 ml/min per 1.73m2, but not with low level albuminuria or the dichotomous kidney disease trait defined as urine ACR ≥30 mg/g and/or low MDRD-GFR.
Similar results were reported by Friedman et al. in the large population-based Dallas Heart Study.[12] In the Dallas Heart Study, non-diabetic African American participants had a mean (SD) age of 44.8 (10.3) years. Microalbuminuria and/or MDRD-GFR <60 ml/min per 1.73m2 were observed in 19.2% of non-diabetic subjects with two APOL1 risk alleles, relative to 6.7% of those with fewer than two risk variants. However, mean MDRD-GFR and urine ACR values were similar and within the normal range in participants from both groups. The major difference between our family-based results and the Dallas Heart Study is seen in those with fewer than two APOL1 risk variants; far lower frequencies of kidney disease were detected in Dallas Heart Study samples relative to these high risk relatives of index cases with non-diabetic ESRD.
The present results and those of the Dallas Heart Study strongly support the concept of a second inherited or environmental “hit” required to initiate fall in GFR or development of albuminuria in those with two APOL1 risk variants. Although approximately 70% of cases of idiopathic FSGS in African Americans is APOL1-associated, it appears that the majority of middle-aged African Americans with either one or two APOL1 risk variants do not have kidney disease. Nonetheless, a tenfold increase in risk for FSGS and ESRD is observed in those with two APOL1 risk variants. HIV infection is a strongly associated environmental second hit.[6] Fifty percent of those with two APOL1 risk variants and untreated HIV infection will develop HIVAN, in contrast to the 18% explained fraction (or proportion of total risk) of idiopathic FSGS cases attributable to APOL1. Accounting for the recessive APOL1 genetic effect is predicted to reduce the prevalence of HIV-associated collapsing glomerulopathy and idiopathic FSGS by 67% in the African American population.[6]
Based on the results in this high risk family-based study and the Dallas Heart Study, we suggest caution before embarking on widespread APOL1 screening to detect mild nephropathy in the African American community. At present, it appears that recording a family history of ESRD and routinely measuring urine ACR and estimated GFR may be more effective tools for detecting asymptomatic mild nephropathy. This recommendation will change upon identification of novel treatments and/or remediable second hits that initiate nephropathy. The Natural History of APOL1-associated Nephropathy Study is now following these participants longitudinally. Those with two APOL1 risk variants, with and without nephropathy, will be contrasted to detect interactive genetic risk variants and evidence of environmental exposures (e.g., non-HIV viral infections) that may trigger progressive nephropathy in genetically susceptible individuals.
There are limitations to this report. By design, many of our participants were related and eight relatives who had ESRD were not included due to the study design (788/796 or 99% of available relatives were recruited). Most have had only a single visit and they are relatively young. The current cross-sectional data will give way to longitudinal data, and follow-up has begun. Some unaffected relatives will likely develop future nephropathy, while low level albuminuria may regress in others. These phenomena will occur in both the APOL1 risk and non-risk groups. The Dallas Heart Study report also utilized cross-sectional data and a follow-up visit is planned. Familial data were addressed by applying GEE. We expected to see reduced GFR and/or albuminuria in the majority of subjects with two APOL1 risk variants destined to develop ESRD by the age of 5 years before the age at ESRD in the index case from their family. An earlier age at ESRD has been reported in those with two, relative to one, relative to zero copies of the APOL1 G1 variant (49±14.9 v 55.9±16.7 v 61.8±17.1 years, respectively; p=1×10−6).[24] The mean age of our participants approaches the age at ESRD in the G1 homozygous group from the Kanji et al. report. Additional strengths of our study were adjustment for genome-wide African ancestry, consideration of the age at ESRD in the index case from each family, and a relatively large sample size. Asymptomatic first-degree relatives of ESRD index cases are a unique resource, more difficult to recruit than prevalent dialysis patients.
In this era of personalized medicine, geneticists are understandably rushing to translate genetic association results in the clinical setting. APOL1 demonstrates among the highest odds ratio for genetic association with severe kidney disease and ESRD seen in any complex disease. Nonetheless, the results of this report in African Americans multiplex ESRD family members at high risk for nephropathy, as well as in a large population-based study, support that the majority of middle-aged individuals with two APOL1 nephropathy risk variants will not manifest clinical nephropathy. Efforts to detect second hits that facilitate the development and progression of nephropathy, particularly treatable environmental risk factors and gene-gene interactions, should aggressively be pursued in order to further our understanding of the pathogenesis of APOL1-associated nephropathy.[5;7] There appears to be a role for screening African-ancestry donor kidneys and living donors for APOL1 risk variants in order to gauge likelihood of long term allograft survival; however, additional studies on the effect of APOL1 on allograft outcome and allograft or recipient selection are necessary.[21] APOL1 has a strong effect on a wide range of non-diabetic forms of severe kidney disease in AAs. However, it has only marginal effects on mild perturbations in renal function and low level albuminuria. This observation may provide important clues to mechanisms of action. At present, caution should be exercised before screening African Americans for APOL1 genotypes to predict risk of mild nephropathy. We note that 21% of the first-degree relatives of high risk African American ESRD families possessing zero or one APOL1 risk variants also had subclinical nephropathy, a finding that reflects other, as yet unidentified, risk factors.
Materials and Methods
Study Participants
The “Natural History of APOL1-associated Nephropathy Study” recruited up to three siblings and/or children without ESRD (first degree relatives) per family of self-described African Americans with non-diabetic forms of ESRD, including nephropathy attributed to hypertension, FSGS, HIV-infection, or unknown cause in non-diabetic subjects.[8] Parents were not recruited, nor were siblings or children who had ESRD (on dialysis or a functioning kidney transplant). We excluded 8 participants who were diagnosed with ESRD during the screening visit. Eligible relatives who reported having diabetes mellitus were included. Siblings and offspring were eligible if they were no more than 15 years below the age at ESRD in the proband from their family (relatives older than the age at ESRD of their proband were included). Relatives were genotyped for APOL1 nephropathy risk variants and genome-wide ancestry informative markers, which were used for individual ancestry proportion estimation. Fasting serum, ethylenediaminetetraacetic acid plasma, urine, buffy coat and DNA samples were collected and phenotyping performed for sub-clinical kidney disease and associated risk factors (e.g., blood pressure, fasting blood sugar, body mass index [BMI]). Participants will be longitudinally evaluated in an attempt to detect the natural history of, and triggers for, APOL1-associated nephropathy.
Genotype Analysis
DNA extraction from whole blood was performed using the PureGene system (Gentra Systems, Minneapolis, MN). Two SNPs in the APOL1 G1 nephropathy risk variant (rs73885319; rs60910145) and an indel for the G2 risk variant (rs71785313) were genotyped on the Sequenom (San Diego, California). In addition, 106 di-allelic ancestry informative markers (AIMs) were genotyped to determine population substructure. Samples included 44 Yoruba (YRI), 39 European American controls and the 786 African American study participants using Illumina Inc. Custom Genotyping Services (San Diego, CA) or the Sequenom Mass Array (San Diego, CA).
Laboratory Evaluation
Serum creatinine concentrations were measured using creatinase enzymatic spectrophotometry, blood urea nitrogen using the urease enzymatic assay, cystatin C using an immunoassay, and urine albumin: creatinine ratio (ACR) by microalbumin immunoturbidimetric methods at Laboratory Corporation of America (LabCorp; Burlington, North Carolina; www.labcorp.com). Urine ACR values ≥30 mg/g were considered elevated (≥300 mg/g was considered overt proteinuria). Estimated GFR was computed using both the four-variable MDRD [25] and CKD-EPI [26] equations; MDRD GFR values <60 ml/minute per 1.73m2 were considered abnormally low. A variable reflecting subclinical kidney disease based on urine ACR ≥30 mg/g and/or MDRD GFR <60 ml/minute per 1.73m2 was employed, as in Friedman et al [12]. Normal values for cystatin C were <0.95 mg/L.
Statistical Methods
Genotype frequency estimates were adjusted for familial correlation using McPeek’s best linear unbiased estimator of allele frequency for pedigree data.[27] This estimate is given by (1TK−11)−11TK−1X where 1 is vector of ones, K is the expected kinship coefficient matrix, and X is the observed genotype at the G2 six base pair insertion deletion marker and rs73885319, one of the two G1 SNPs that are in perfect linkage disequilibrium. This estimate was multiplied by a factor of 0.5 to account for the fact that APOL1 genotype is effectively a combination of the genotypes observed at these 2 markers.
We used the Wilcoxon two-sample test for comparing the distribution of all continuous outcomes between individuals who carry two copies of the APOL1 risk variant and those who have zero or one copy (recessive coding), and its generalization, the Kruskal-Wallis test for comparing the observed distributions in the three genotypes (additive coding). Association with categorical outcomes was tested using Chi-square tests with the appropriate degree of freedom.
A series of generalized estimating equations (GEE) assuming exchangeable correlation and using the empirical estimate of the variance to adjust for familial correlation was computed to test for associations between renal phenotypes and APOL1 risk genotypes.[28] This approach could lead to type I error inflation for markers with low minor allele frequency in more complex pedigrees. However, this approach is expected to perform well in this case, given the observed allele frequencies and study design including only first degree relatives. The Box-Cox method was applied to identify the appropriate transformation of each outcome variable that would best approximate the distributional assumptions of conditional normality and homogeneity of variance of the residuals.[29] Age, sex, age relative to age at ESRD in probands, and ancestry adjustments were incorporated, after an initial unadjusted analysis. These models were fitted using GFR, urine ACR, and cystatin C, respectively, as the outcome variable defined both on the continuous scale, as well as categorical variables using clinically meaningful cutoff values. Standard regression diagnostics for colinearity and influence were computed for each model reported. Finally, we tested for interaction effects between gender and APOL1 status, and between diabetes status in relatives and APOL1 on these outcome variables using the same set of covariates.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01 HL56266, RO1 DK070941, and RO1 DK084149 (BIF).
Footnotes
The authors report no conflicts of interest.
Reference List
- 1.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tzur S, Rosset S, Shemer R, et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010;128:345–350. doi: 10.1007/s00439-010-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedman BI, Kopp JB, Langefeld CD, et al. The Apolipoprotein L1 (APOL1) Gene and Nondiabetic Nephropathy in African Americans. J Am Soc Nephrol. 2010;21:1422–1426. doi: 10.1681/ASN.2010070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reeves-Daniel AM, Depalma JA, Bleyer AJ, et al. The APOL1 Gene and Allograft Survival after Kidney Transplantation. Am J Transplant. 2011;11:1025–1030. doi: 10.1111/j.1600-6143.2011.03513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bostrom MA, Freedman BI. The spectrum of MYH9-associated nephropathy. Clin J Am Soc Nephrol. 2010;5:1107–1113. doi: 10.2215/CJN.08721209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopp JB, Nelson GW, Sampath K, et al. APOL1 Genetic Variants in Focal Segmental Glomerulosclerosis and HIV-Associated Nephropathy. Journal of the American Society of Nephrology. 2011;22:2129–2137. doi: 10.1681/ASN.2011040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bostrom MA, Kao WH, Li M, et al. Genetic Association and Gene-Gene Interaction Analyses in African American Dialysis Patients With Nondiabetic Nephropathy. Am J Kidney Dis. 2011 doi: 10.1053/j.ajkd.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freedman BI, Langefeld CD, Murea M, et al. Apolipoprotein L1 nephropathy risk variants associate with HDL subfraction concentration in African Americans. Nephrol Dial Transplant. 2011;26:3805–3810. doi: 10.1093/ndt/gfr542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madhavan SM, O’Toole JF, Konieczkowski M, et al. APOL1 localization in normal kidney and nondiabetic kidney disease. J Am Soc Nephrol. 2011;22:2119–2128. doi: 10.1681/ASN.2011010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appel LJ, Wright JT, Jr, Greene T, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363:918–929. doi: 10.1056/NEJMoa0910975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopp JB, Freedman BI, Winkler CA, et al. APOL1 and MYH9 genetic variants are independently associated with kidney diseaes risk in African Americans. (abstract) J Am Soc Nephol. 2011;FR-PO1501 [Google Scholar]
- 12.Friedman DJ, Kozlitina J, Genovese G, et al. Population-based risk assessment of APOL1 on renal disease. J Am Soc Nephrol. 2011;22:2098–2105. doi: 10.1681/ASN.2011050519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson R, Grim CE, Opgenorth TJ. A familial risk of chronic renal failure among blacks on dialysis? J Clin Epidemiol. 1988;41(12):1189–1196. doi: 10.1016/0895-4356(88)90023-6. [DOI] [PubMed] [Google Scholar]
- 14.Freedman BI, Spray BJ, Tuttle AB, Buckalew VM., Jr The familial risk of end-stage renal disease in African Americans. Am J Kidney Dis. 1993;21:387–393. doi: 10.1016/s0272-6386(12)80266-6. [DOI] [PubMed] [Google Scholar]
- 15.Freedman BI, Tuttle AB, Spray BJ. Familial predisposition to nephropathy in African-Americans with non-insulin-dependent diabetes mellitus. Am J Kidney Dis. 1995;25(5):710–713. doi: 10.1016/0272-6386(95)90546-4. [DOI] [PubMed] [Google Scholar]
- 16.Freedman BI, Wilson CH, Spray BJ, et al. Familial clustering of end-stage renal disease in blacks with lupus nephritis. Am J Kidney Dis. 1997;29(5):729–732. doi: 10.1016/s0272-6386(97)90126-8. [DOI] [PubMed] [Google Scholar]
- 17.Freedman BI, Soucie JM, Stone SM, Pegram S. Familial clustering of end-stage renal disease in blacks with HIV-associated nephropathy. Am J Kidney Dis. 1999;34(2):254–258. doi: 10.1016/s0272-6386(99)70352-5. [DOI] [PubMed] [Google Scholar]
- 18.Bergman S, Key BO, Kirk KA, et al. Kidney disease in the first-degree relatives of African-Americans with hypertensive end-stage renal disease. Am J Kidney Dis. 1996;27(3):341–346. doi: 10.1016/s0272-6386(96)90356-x. [DOI] [PubMed] [Google Scholar]
- 19.Freedman BI, Soucie JM, McClellan WM. Family history of end-stage renal disease among incident dialysis patients. J Am Soc Nephrol. 1997;8:1942–1945. doi: 10.1681/ASN.V8121942. [DOI] [PubMed] [Google Scholar]
- 20.Freedman BI, Volkova NV, Satko SG, et al. Population-based screening for family history of end-stage renal disease among incident dialysis patients. Am J Nephrol. 2005;25:529–535. doi: 10.1159/000088491. [DOI] [PubMed] [Google Scholar]
- 21.Reeves-Daniel A, Bailey A, Assimos D, et al. Donor-recipient relationships in African American vs. Caucasian live kidney donors. Clin Transplant. 2011 doi: 10.1111/j.1399-0012.2011.01468.x. [DOI] [PubMed] [Google Scholar]
- 22.Bryson CL, Ross HJ, Boyko EJ, Young BA. Racial and ethnic variations in albuminuria in the US Third National Health and Nutrition Examination Survey (NHANES III) population: associations with diabetes and level of CKD. Am J Kidney Dis. 2006;48:720–726. doi: 10.1053/j.ajkd.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Renal Data System. Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Vol. 1. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2009. USRDS 2009 Annual Data Report. Ref Type: Generic. [Google Scholar]
- 24.Kanji Z, Powe CE, Wenger JB, et al. Genetic Variation in APOL1 Associates with Younger Age at Hemodialysis Initiation. Journal of the American Society of Nephrology. 2011;22:2091–2097. doi: 10.1681/ASN.2010121234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, et al. A New Equation to Estimate Glomerular Filtration Rate. Annals of Internal Medicine. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McPeek MS, Wu X, Ober C. Best linear unbiased allele-frequency estimation in complex pedigrees. Biometrics. 2004;60:359–367. doi: 10.1111/j.0006-341X.2004.00180.x. [DOI] [PubMed] [Google Scholar]
- 28.Liang K-Y, Zeger SL. Longitudinal data anlysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 29.Box GEP, Cox DR. An analysis of tranformations. Journal of the Royal Statistical Society, Series B. 1964;26:211–246. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.