Abstract
Purpose
To evaluate the effect of glucocorticoid (triamcinolone acetonide injectable suspension) pre-treatment on corneal neovascularization, lymphangiogenesis, and inflammation in a murine penetrating keratoplasty (PK) and corneal suture model.
Methods
For the PK model, BALB/c mice were used as recipients and C57BL/6 mice were used as donors. A group pre-treated with subconjunctival glucocorticoid and a combination of post-subconjunctival and topical glucocorticoids (Group I) was compared to two groups that did not receive glucocorticoid pre-treatment (one group received a combination of subconjunctival and topical glucocorticoids post-op (Group II), the other group received only topical glucocorticoid treatment post-op (Group III)). All groups were treated with subconjunctival glucocorticoid on the day of surgery. For the corneal suture model BALB/c mice were used. A group receiving only pre-suture glucocorticoid treatment (Group A) and a group receiving only post-suture glucocorticoid treatment (Group C) were compared to a control group that did not receive glucocorticoid therapy (Group B). The degree of neovascularization, lymphangiogenesis, and inflammatory infiltration was compared in each of these models.
Results
In the PK model, the group receiving glucocorticoid pre-treatment (Group I) showed less neovascularization compared to the post-treatment only groups (Group II: p=0.043, Group III: p=0.020) and less lymphangiogenesis compared to Group III (p=0.005). In the corneal suture model, the glucocorticoid pre-treatment group showed a similar level of neovascularization, lymphangiogenesis, and inflammatory infiltration as the post-treatment only groups (p>0.05).
Conclusion
Glucocorticoid pretreatment prior to penetrating keratoplasty decreases neovascularization and lymphangiogenesis compared to post-transplant glucocorticoid treatment alone.
Keywords: penetrating keratoplasty, glucocorticoid pre-treatment, angiogenesis, triamcinolone acetonide
Introduction
Penetrating keratoplasty (PK) is one of the most successful organ transplant procedures worldwide with 2-year survival rates as high as 90% in uncomplicated cases.1–3 Conversely, even with current immunosuppression techniques, corneal graft rejection rates in high-risk patients can exceed 70%.4 Key risk factors for graft rejection include active inflammation and corneal vascularization.5,6 Corticosteroids are the current standard of care for prevention of graft rejection after PK due to their strong anti-inflammatory effects.1,7–11
The anti-inflammatory effects of corticosteroids occur through the inhibition of chemotaxis and phagocytosis and by blocking the synthesis of prostaglandins through the phospholipase A2 pathway.7,8,12 They also inhibit vascularization and have immunosuppressive action by reducing the number of circulating T lymphocytes. The mechanisms by which glucocorticoids inhibit angiogenesis include direct effects on vascular endothelial cells and indirect effects on other cells, resulting in the inhibition of migration and activation of macrophages, mast cells, and other cell type that release agents contributing to angiogenesis.13–17 Glucocorticoids also exert an anti-lymphangiogenic effect. 13,18,19
Corneal transplantation has a two year success rate of over 90% for initial grafts into an avascular cornea.1,3 However, in patients with high risk characteristics such as a vascularized corneal recipient bed, graft failure rates are between 60% and 90%.1,2,20,21 Vascularized and inflamed corneal tissue provides a conduit for both the blood and lymphatic systems to bring immune cells to and from the cornea, contributing to subsequent immunologic graft rejection.1,2,10,20–22 As a result, the graft rejection cascade can be initiated or accelerated. Perioperative suppression of neovascularization, lymphangiogenesis, and inflammation could be a key factor in improving the outcomes of ocular surgeries, including keratoplasty and wound healing.19,20
Blood and lymphatic vessels grow into the cornea as early as the second day after an angiogenic inflammatory insult.14,18,19,23 In murine PK models, lymphatic vessels can reach the graft-host interface within 6 days of surgery.19,24 As a result, starting anti-inflammatory treatment after the insult occurs may not be sufficient to prevent or slow angiogenesis. Therefore, starting anti-inflammatory therapy before the insult may decrease angiogenesis and improve outcomes.
Currently, the most common immunosuppressive regimen is the combination of subconjunctival glucocorticoid injection at the time of surgery followed by continued topical glucocorticoid application.9,10 While it is generally accepted that glucocorticoids exert a protective effect against corneal graft rejection at the time of and after surgery, their potential use prior to PK has not been thoroughly examined. The aim of this study was to evaluate the impact of glucocorticoid pre-treatment on corneal neovascularization, lymphangiogenesis, and inflammation in murine PK and corneal suture models.
Materials and Methods
All experiments were performed in accordance with the regulations of ARVO (Association for Research in Vision and Ophthalmology) and were approved by the IACUC (Institutional Animal Care and Use Committee) of the University of Utah.
PK Model
Orthotopic corneal transplantation
Penetrating corneal transplantation was done using BALB/c mice as recipients (12 mice in Group I, 13 mice in Group II, 10 mice in Group III) and C57BL/6 mice as donors (The Jackson Laboratory, JAX® Mice and Services, Bar Harbor, ME, USA). All mice were male and 8 to 12 weeks old. Mice were anesthetized by intramuscular injection with ketamine (100 mg/kg) and xylazine (20 mg/kg). The donor cornea was marked with a 2 mm trephine, the anterior chamber was penetrated using a knife (ClearCut™, Alcon, Inc) and the cornea was cut with Vannas scissors and placed in Balanced Salt Solution (BSS® Alcon Laboratories, Inc, Fort Worth, USA). The recipient mouse was anesthetized as described previously. To dilate and anesthetize the pupil, 1% tropicamide ophthalmic solution and 0.5% proparacaine ophthalmic solution was used. The recipient’s right cornea was marked with 1.5 mm trephine and removed by the same method as the donor cornea. The donor graft was sutured into the recipient bed using 6–8 interrupted sutures (11-0 nylon, CS160-6, ETHICON, Inc). After the transplantation, the eye was covered with 0.5% erythromycin ophthalmic ointment and the lid was sutured with 8-0 coated vicryl sutures (BV130-5, ETHICON, Inc). Sutures were removed after one week.
Glucocorticoid treatment
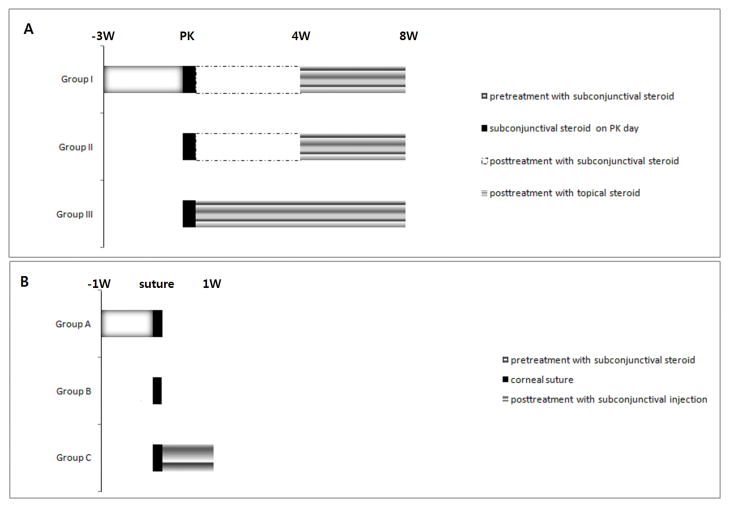
KENALOG®-40 injection (triamcinolone acetonide injectable suspension, USP, 40 μg/μl) was used as the glucocorticoid pre-treatment in this study. Figure 1 details the timing of glucocorticoid application in the PK model. The long-acting glucocorticoid (triamcinolone) was given by subconjunctival injection (10 μl). In Group I, glucocorticoid injections were given twice a week for 3 weeks prior to PK, on the day of PK, and weekly for 4 weeks after PK. Topical glucocorticoid drops (2 μl of triamcinolone acetonide injectable suspension, 40 μg/μl) were then given twice a week during weeks 4 to 8 (post-PK). In Group II, subconjunctival glucocorticoid injection was started on the day of PK and given weekly through week 4 after PK, followed by topical glucocorticoid drops twice weekly during weeks 4 to 8. In Group III, glucocorticoid subconjunctival injection was given on the day of PK followed by topical glucocorticoid drops twice a week for 8 weeks.
Figure 1.
A. Schedule of glucocorticoid application in the penetrating keratoplasty model.
B. Schedule of glucocorticoid application in the corneal suture model.
Clinical evaluation of rejection
The mice were examined every week using an operating microscope (SZX7, Olympus, Japan). Corneal opacity was graded (from 0 to 5) to determine graft rejection.25 Opacity of grade 3 or higher was considered to be a graft rejection. Mice with severe inflammation and hemorrhage were excluded from the study. Mice with significant cataract were excluded from opacity grading but were included in flat mounting for the calculation of angiogenesis and lymphangiogenesis.
Analysis of Angiogenesis and Lymphangiogenesis
After planned treatment injections and observations, mice eyes were harvested (9 corneas in Group I, 11 corneas in Group II, and 9 corneas in Group III). The cornea was trimmed of remaining limbus and iris. Immunohistochemical staining for vascular and lymphatic endothelial cells was performed on corneal flat mounts. Corneas were dissected, rinsed in phosphate-buffered saline (PBS) for 30 minutes, and fixed in 100% acetone (Sigma) for 20 minutes. After washing in PBST (0.1% Tween®20/PBS), nonspecific binding was blocked with 3% bovine serum albumin (BSA)/PBS for 3 nights at 4°C. Incubation with fluorescein isothiocyanate (FITC)–conjugated monoclonal anti-mouse CD31 antibody (558738, BD Pharmingen) at a dilution of 1:500 and rabbit anti-LYVE-1 (ab 14917, Abcam Inc, Cambridge, MA, USA) at a dilution 1:200 in 3% BSA/PBS at 4°C overnight was followed by 1:1000 goat anti rabbit antibody-Alexa Fluor® 546 (A11071, Invitrogen Corporation, Carlsbad, CA, USA) for 1 hour and subsequent washes in PBST at room temperature. Corneas were mounted with the antifading agent Gelmount (Biomeda, San Francisco, CA). After immunochemical staining and flat mounting, images of the corneal vasculature were captured using a fluorescent microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY, USA). The neovascularization and lymphangiogenesis was quantified by setting a threshold level of fluorescence above which only vessels were captured and processed using Image J (National Institutes of Health). The total corneal area was outlined using the innermost vessel of the limbal (rim of the cornea) arcade as the border and the graft area was outlined using the scar line between recipient and donor graft. The total area of neovascularization and lymphangiogenesis was normalized to the total corneal area. The graft neovascularization and lymphangiogenesis were normalized to the graft area. The 3 groups were compared by graft survival, neovascularization (NV) (total corneal NV = total NV area/total corneal area), graft NV (graft NV area/graft area), lymphangiogenesis (LY) (total corneal LY = total LY area/ total corneal area) and graft LY (graft LY area/graft area).
Intraocular pressure
Intraocular pressure (IOP) was monitored by averaging 5 tonometer (TonoLab® tv 02, ICARE, Finland) measurements for each eye. The IOP of eyes pretreated with glucocorticoids (N=12) and the fellow eyes of Group I (N=12) were compared prior to PK on the day of surgery. The IOPs of Group II (N=13) were measured prior to PK on the day of surgery.
Corneal Suture Model
Animals
Balb/c mice (The Jackson Laboratory, JAX® Mice and Services, Bar Harbor, ME, USA) aged from 8 to 10 weeks were used. Before the corneal suture was placed, mice were anesthetized with intramuscular tribromoethanol (Avertin) and 0.5% proparacaine ophthalmic solution. Two corneal sutures were placed with 11-0 nylon between the corneal center and the limbus at the 12 and 6 o’clock positions. After suture placement, 0.5% topical erythromycin ointment was applied to the cornea.
Preoperative and post operative injection of glucocorticoids
Figure 1 shows the schedule of glucocorticoid application in the corneal suture model. In Group A, subconjunctival glucocorticoid was injected twice during the week prior to suture placement (on day 7 and 4 prior to suture placement), then corneal sutures were placed (day 0) and corneas were harvested 1 week after suture placement (day 7). In Group B, corneal sutures were placed without any pretreatment on day 0 and corneas were harvested 1 week after suture placement (day 7). In Group C, corneal sutures were placed (day 0), afterwhich we injected subconjunctival glucocorticoid on days 2 and 4 and harvested the cornea 1 week after suture placement (day 7).
Analysis of angiogenesis, lymphangiogenesis, and inflammatory infiltration
After planned injections and observations, the eyes were harvested (6 corneas in Group A, 6 corneas in Group B, and 5 corneas in Group C). The cornea was trimmed of remaining limbus and iris. Immunohistochemical staining for vascular endothelial cells, lymphatic endothelial cell and monocyte and macrophage was performed as previously described. Image analysis of fluorescent microscopic picture was the same as above (in the PK model).
To quantify volume of inflammatory infiltration, we used confocal microscope (Olympus FluoView FV1000, Olympus, Tokyo). A total of 12 suture sites of Group A, 12 suture sites in Group B, and 10 suture sites in Group C were quantified. Horizontal sections (objective magnification ×10) were obtained from the surface of the suture to the deepest focal plane of neovascularized area around suture in 5 μm steps. A total of 22 to 30 slices of two dimensional horizontal section images were stacked to form three dimensional volume of each suture area. The number of pixels stained with CD11b were used to calculate volume. Scaling factor (μm/pixel) for each x, y, and z dimension was 1.242, 1.242, and 5.
Statistical Analysis
For statistical analysis, we used SPSS 11.5 for Windows. To compare graft survival, we used the Kaplan-Meier analysis and log-rank test. To compare neovascularization, lymphangiogenesis, inflammatory infiltration, and intraocular pressure (IOP), unpaired two-tailed t tests and Mann-Whitney U tests were used.
Results
PK Model
Graft survival
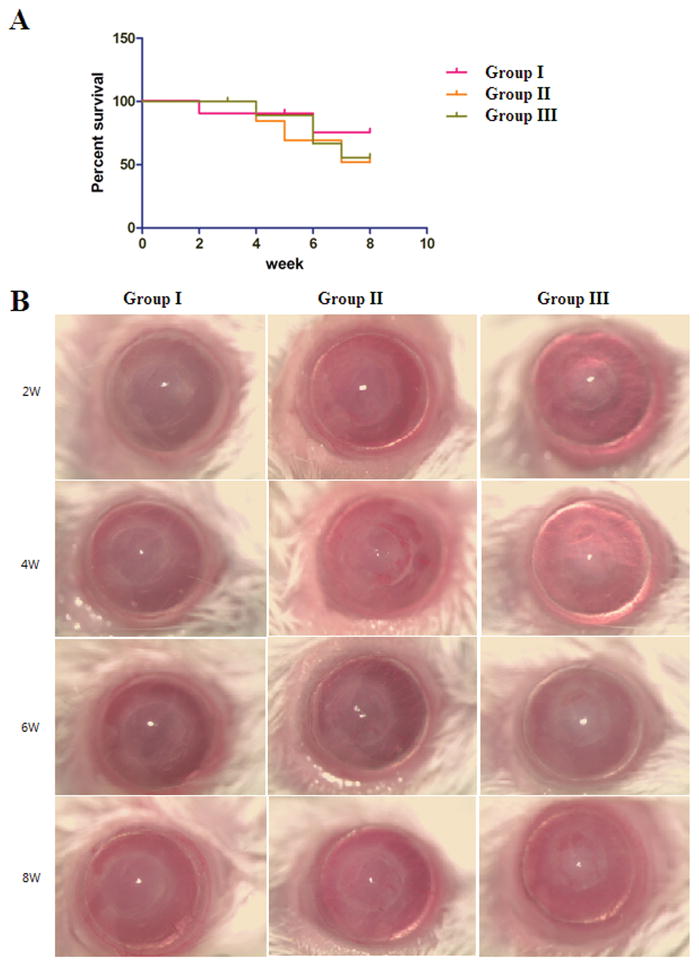
Group I (75.0%) showed a trend towards increased survival compared to Groups II (51.9%) or III (55.5%) based on opacity grading, but this difference did not reach statistical significance (Figure 2) (p=0.408, p=0.560, respectively).
Figure 2.
(A) Kaplan-Meier survival graph of Groups I, II, and III in the PK model. Group I showed improved graft survival compared to Groups II and III, but this did not reach statistical significance (p=0.408, p=0.560).
(B) Representative pictures of Group I, II, and III.
Neovascularization
For total corneal neovascularization, Group I (0.062±0.008, Mean±SEM, N=9) demonstrated significantly less total neovascularization than Group II (0.112±0.019, p=0.043, N=11) or Group III (0.139±0.028 p=0.020, N=9). The difference in total neovascularization between group I and II is likely due to addition of glucocorticoid pretreatment before PK in Group I. Combination glucocorticoid treatment before and after PK has the potential to decrease corneal neovascularization compared to only postoperative treatment. The difference between Group I and III was larger than that of Group I and II, likely due to Group II receiving additional postoperative subconjunctival glucocorticoid therapy after corneal transplantation. There was no significant difference in graft neovascularization between the groups (Figure 3).
Figure 3.
(A) & (B) - In the PK model, Group I showed significantly less total neovascularization compared to Groups II and III (p=0.043, p=0.020, respectively) and less total lymphangiogenesis (p=0.005) and less graft lymphangiogenesis (p=0.020) compared to Group III.
(C) Representative pictures of Group I, II, and III. Upper row: CD31staining, Lower row: LYVE-1 staining
Lymphangiogenesis
Group I (0.033±0.008) showed significantly less total lymphangiogenesis compared to Group III (0.098±0.018, p=0.005). Group I (0.035±0.008) also showed significantly less graft lymphangiogenesis compared to Group III (0.166±0.050, p=0.020). Figure 3 shows comparisons of lymphangiogenesis and their representative pictures. Group II (0.040±0.006) showed significantly less total lymphangiogenesis than Group III (0.098±0.018, p=0.007). Pre-treatment with subconjunctival glucocorticoids suppressed lymphangiogenesis. Further, when comparing the two post treatment groups, combined subconjunctival injection post treatment was more effective in decreasing lymphangiogenesis than topical post treatment alone.
Intraocular Pressure
Prior to PK, there was no significant difference in intraocular pressure between the PK scheduled eye (9.90±0.67, Mean±SEM, mmHg) and the fellow eye of Group I (10.30±0.74) or the PK scheduled eye of group I and Group II (9.61±0.41). In our study, glucocorticoid pre-treatment twice a week for 3 weeks prior to PK (total 2.4 mg of triamcinolone acetonide per eye) did not increase IOP compared to eyes not receiving glucocorticoid treatment.
Corneal Suture Model
Neovascularization and Lymphangiogenesis
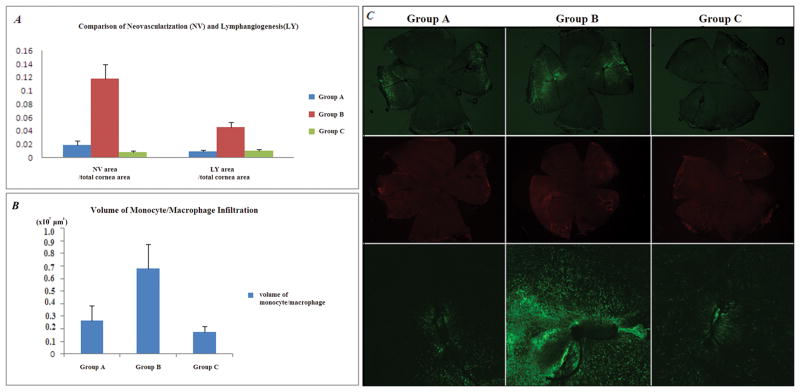
Figure 4 presents the result from the corneal suture model. Groups A (0.018±0.007) and C (0.008±0.002) showed significantly less neovascularization compared to Group B (0.117±0.022) (p=0.002, p=0.004, respectively). Groups A (0.009±0.002) and C (0.010±0.002) showed significantly less lymphangiogenesis than Group B (0.045±0.008) (p=0.004, p=0.009, respectively). There was no significant difference in neovascularization or lymphangiogenesis between Groups A and C. Both glucocorticoid pre-treatment and post-treatment groups showed less neovascularization and lymphangiogenesis than the group without glucocorticoid treatment. We found glucocorticoid pre-treatment showed a similar effect to post-treatment in decreasing neovascularization and lymphangiogenesis.
Figure 4.
(A) & (B) In the corneal suture model, Groups A and C showed significantly less neovascularization and lymphangiogenesis compared to Group B (p<0.05). There was no significant difference of neovascularization and lymphangiogenesis between Groups A and C (p>0.05).
(C) Representative pictures of Group A, B, and C. Upper row: Fluorescent microscopic picture of CD31 staining (green) in flat mount of whole cornea. Middle row: Fluorescent microscopic picture of LYVE-1 staining (red) in flat mount of whole cornea. Bottom row: Confocal microscopic picture of CD11b staining cells (green) around suture area.
Volume of monocyte/macrophage infiltration in Confocal Microscope
Figure 4 shows the comparison of volume of CD11b+ cell infiltration of each suture area and their representative pictures. Groups A (0.266×107±0.115×107, μm3) a n d C (0.172×107±0.049×107) demonstrated significantly less infiltration of CD11b+ cells than Group B (0.680×107±0.191×107) (p=0.028, p=0.030, respectively). There was no difference in infiltration of CD11b+ cells between Groups A and C. Both glucocorticoid pre-treatment and post-treatment groups showed less inflammatory infiltration than groups without glucocorticoid treatment. We found glucocorticoid pre-treatment showed a similar effect to post-treatment in decreasing inflammatory infiltration.
Discussion
Corneal transplantation often represents one of the last options available for restoring sight for patients with blindness due to corneal opacification. While several decades have passed since the initial development of corneal transplantation, the mainstay of treatment to increase transplant survival continues to be glucocorticoid therapy.7–11,22,26 In high risk PK, other immunosuppressive agents have been used but are typically combined with glucocorticoid treatment.9,11 We hypothesized that fostering a good milieu in the recipient bed by suppressing inflammation and vascularization through steroid pre-treatment would improve corneal engraftment through decreased neovascularization, lymphangiogenesis, and inflammation and result in improved graft survival. While our PK model results showed a decrease in neovascularization and lymphangiogenesis, unfortunately there was no increase in graft survival compared to current treatment regimens.
VEGF gene expression that is induced within 24 hours after corneal injury is stimulated by proinflammatory cytokines27,28 These cytokines are expressed as early as 1 hour after corneal injury.28 As glucocorticoid treatment can suppress proinflammatory cytokines and inhibit cell chemotaxis, the maximum concentration of glucocorticoid in the cornea should be maintained during the early wound healing period. As time is required for the glucocorticoid to distribute in the cornea and anterior chamber, pre-treatment with the glucocorticoid can increase the concentration at the time of corneal injury to allow for the maximal anti-inflammatory effect. Therefore, starting glucocorticoid treatment after the insult may not be optimal and beginning treatment prior the insult may be beneficial. Published reports reviewing the glucocorticoid treatment found they are most effective in angiogenesis suppression when they are applied before or immediately after the corneal injury.29–31
Blood and lymphatic vessels can grow into the cornea as early as day 2 after an angiogenic, inflammatory insult.14,18,19,23 In murine PK, lymphatic vessel can reach the graft-host interface within 6 days of surgery.19,32 When glucocorticoids are applied by subconjunctival injection at the time of PK, the peak concentration in the anterior chamber via transcorneal delivery occurs after the expression of the cytokines induced by the injury. 29,31,33 Thus, maintaining the highest concentration of anti-inflammatory treatment at the time of injury is expected to prevent or slow cytokine expression. As a result, starting anti-angiogenic and anti-inflammatory treatment after the insult may not be sufficient and starting treatment before the insult may be required to minimize angiogenesis.
Another issue is that angiogenesis and lymphangiogenesis peak after a stimulus and then stabilize or regressed as the lesion heals and the stimulus is removed18,23 If the stimulus were transient and easily dissipated, such as a thermal corneal injury, early perioperative treatment for preventing neovascularziation and lymphangiogenesis near the time of stimulus could be effective. But if the stimulus were to be prolonged, sustained treatment would be more important than early perioperative treatment. Keratoplasty can be a transient angiogenic stimulus or prolonged angiogenic stimulus depending on individual risk factors. Blood vessels and lymphatic vessels reaching the graft are essential for delivery of antigen presenting cells and alloreactive T lymphocytes to the graft.1,3 If sensitized T cells do not occur until 14 to 21 days after grafting, they must compete with the regulatory T cells of anterior chamber associated immune deviation (ACAID), which begin to emerge at that time. 3,24,25,34 Therefore, in PK if the growth of afferent rim (lymphatics) can be slowed in the early perioperative period, keratoplasty could be a transient stimulus under the control of ACAID. Thus, if we can reduce or shorten the stimulus causing antigen presentation to the immune system, then there would not be additional stimulus to activate the efferent arm, thereby limiting the immune reaction.
Since glucocorticoid pre-treatment showed a trend towards increased graft survival (which did not reach statistical significance) in this trial, it suggests that there may be other factors that contribute to graft survival beyond neovascularization and lymphangiogenesis. On the other hand, optimizing the pre-treatment regimen by taking into account factors such as glucocorticoid duration of action and potency could yield improved survival. Therefore, pre-treatment using longer acting and increased potency glucocorticoids may improve PK outcomes and should be investigated.7,13,35
It is widely accepted that topical glucocorticoid treatment can protect against immunologic graft rejections in normal risk PK. In our study, Group II (group receiving a combination of subconjunctival and topical glucocorticoids post-op) showed less total lymphangiogenesis than Group III (group received only topical glucocorticoids treatment post-op) (p=0.007). As a result, the mode of delivery should be a consideration when designing treatment regimens aimed at reducing neovascularization and lymphangiogensis, especially in high risk keratoplasty where topical glucocorticoid treatment may not be enough to protect against graft rejection.1,2,7,21,35,36
One consideration that must be taken into account with any glucocorticoids treatment is the potential for an elevation of IOP. 7,37 While this was a concern for our model, our study found no increase in IOP in the eyes pre-treated with glucocorticoids. This suggests that the potential benefits of using glucocorticoids before an angiogenic stimulus may outweigh the risk of increased IOP and should be further investigated. Cataractogenesis is another known risk associated with glucocorticoid use. Unlike Group II and III which used glucocorticoids only after the postoperative period (for a total of 8 weeks), Group I was exposed to glucocorticoid both preoperatively and postoperatively (for a total of 11 weeks) and had cataract development in 2 eyes. This is likely related to the increased glucocorticoid exposure of Group I. Another risk associated with glucocorticoid use is eruption of a nascent corneal infection, especially with long term use. We did not find any signs of corneal infection during the course of our study, although this may, in part, be due to using 5% erythromycin ophthalmic ointment at the time of surgery.
One limitation of our study is that we did not compare the effectiveness of topically administered glucocorticoid pretreatment to subconjunctival glucocorticoid pretreatment, although based on published findings we would not expect the topically administered pre-treatment to be more effective than subconjunctival delivery.38,39 A second limitation is that we focused on the clinical markers of efficacy such as corneal opacification. Further research could utilize biochemical markers such as CD11b or F4/80 to determine the effectiveness of glucocorticoid pre-treatment of decreased immune reaction.
In conclusion, glucocorticoid therapy has been the standard anti-inflammatory and anti-angiogenic treatment for diseases associated with corneal neovascularization and lymphangiogenesis, including corneal transplantation. This study found that glucocorticoids delivery before an angiogenic stimulus such as PK or a corneal suture is effective in reducing angiogenesis, lymphangiogenesis, and inflammatory infiltration. Therefore, glucocorticoid pre-treatment should be considered in the optimization of anti-angiogenesis, anti-lymphangiogenesis, and transplant survival protocols.
Acknowledgments
Funding support provided by the National Institutes of Health: NIH 5R01EY017950.
Footnotes
Conflicts of Interest and Source of Funding
Funding Source: NIH 5R01EY017950
Dr. Ambati has received grant funding from the National Institutes of Health (NIH), is an employee of the University of Utah, and is on the speakers bureau for Alcon. All the authors are employees of University of Utah.
References
- 1.Lam H, Dana MR. Corneal graft rejection. Int Ophthalmol Clin. 2009 Winter;49(1):31–41. doi: 10.1097/IIO.0b013e3181924e23. [DOI] [PubMed] [Google Scholar]
- 2.The collaborative corneal transplantation studies (CCTS) Effectiveness of histocompatibility matching in high-risk corneal transplantation. The Collaborative Corneal Transplantation Studies Research Group. Arch Ophthalmol. 1992 Oct;110(10):1392–1403. [PubMed] [Google Scholar]
- 3.Streilein JW, Yamada J, Dana MR, Ksander BR. Anterior chamber-associated immune deviation, ocular immune privilege, and orthotopic corneal allografts. Transplant Proc. 1999 May;31(3):1472–1475. doi: 10.1016/s0041-1345(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 4.Santos LN, de Moura LR, Fernandes BF, Cheema DP, Burnier MN., Jr Histopathological study of delayed regraft after corneal graft failure. Cornea. 2011 Feb;30(2):167–170. doi: 10.1097/ICO.0b013e3181e843e5. [DOI] [PubMed] [Google Scholar]
- 5.Baradaran-Rafii A, Karimian F, Javadi M, et al. Corneal Graft Rejection: Incidence and Risk Factors. Iran J Ophthalmic Res. 2007;2(1):7–14. [Google Scholar]
- 6.Weisbrod DJ, Sit M, Naor J, Slomovic AR. Outcomes of repeat penetrating keratoplasty and risk factors for graft failure. Cornea. 2003 Jul;22(5):429–434. doi: 10.1097/00003226-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Athanasiadis I, De Wit D, Tsatsos M, Patel A, Sharma A. Subconjunctival Injection of Triamcinolone Acetonide in the Management of Corneal Graft Rejection and New Vessels. J Clin Pharmacol. 2011 Apr 19; doi: 10.1177/0091270011400073. [DOI] [PubMed] [Google Scholar]
- 8.Cursiefen C, Wenkel H, Martus P, et al. Impact of short-term versus long-term topical steroids on corneal neovascularization after non-high-risk keratoplasty. Graefes Arch Clin Exp Ophthalmol. 2001 Jul;239(7):514–521. doi: 10.1007/s004170100313. [DOI] [PubMed] [Google Scholar]
- 9.Randleman JB, Stulting RD. Prevention and treatment of corneal graft rejection: current practice patterns (2004) Cornea. 2006 Apr;25(3):286–290. doi: 10.1097/01.ico.0000178731.42187.46. [DOI] [PubMed] [Google Scholar]
- 10.Tabbara KF. Pharmacologic strategies in the prevention and treatment of corneal transplant rejection. Int Ophthalmol. 2008 Jun;28(3):223–232. doi: 10.1007/s10792-007-9100-7. [DOI] [PubMed] [Google Scholar]
- 11.Unal M, Yucel I. Evaluation of topical ciclosporin 0.05% for prevention of rejection in high-risk corneal grafts. Br J Ophthalmol. 2008 Oct;92(10):1411–1414. doi: 10.1136/bjo.2008.143024. [DOI] [PubMed] [Google Scholar]
- 12.Haynes WL, Proia AD, Klintworth GK. Effect of inhibitors of arachidonic acid metabolism on corneal neovascularization in the rat. Invest Ophthalmol Vis Sci. 1989 Jul;30(7):1588–1593. [PubMed] [Google Scholar]
- 13.Hos D, Saban DR, Bock F, et al. Suppression of inflammatory corneal lymphangiogenesis by application of topical corticosteroids. Arch Ophthalmol. 2011 Apr;129(4):445–452. doi: 10.1001/archophthalmol.2011.42. [DOI] [PubMed] [Google Scholar]
- 14.Nakao S, Hata Y, Miura M, et al. Dexamethasone inhibits interleukin-1beta-induced corneal neovascularization: role of nuclear factor-kappaB-activated stromal cells in inflammatory angiogenesis. Am J Pathol. 2007 Sep;171(3):1058–1065. doi: 10.2353/ajpath.2007.070172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niederkorn JY. Immune mechanisms of corneal allograft rejection. Curr Eye Res. 2007 Dec;32(12):1005–1016. doi: 10.1080/02713680701767884. [DOI] [PubMed] [Google Scholar]
- 16.Murata M, Shimizu S, Horiuchi S, Taira M. Inhibitory effect of triamcinolone acetonide on corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2006 Feb;244(2):205–209. doi: 10.1007/s00417-005-0036-1. [DOI] [PubMed] [Google Scholar]
- 17.Nauck M, Karakiulakis G, Perruchoud AP, Papakonstantinou E, Roth M. Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur J Pharmacol. 1998 Jan 12;341(2–3):309–315. doi: 10.1016/s0014-2999(97)01464-7. [DOI] [PubMed] [Google Scholar]
- 18.Boneham GC, Collin HB. Steroid inhibition of limbal blood and lymphatic vascular cell growth. Curr Eye Res. 1995 Jan;14(1):1–10. doi: 10.3109/02713689508999908. [DOI] [PubMed] [Google Scholar]
- 19.Flynn TH, Ohbayashi M, Dawson M, Larkin DF, Ono SJ. The effect of perioperative allergic conjunctivitis on corneal lymphangiogenesis after corneal transplantation. Br J Ophthalmol. 2011 Jun 7; doi: 10.1136/bjo.2010.201939. [DOI] [PubMed] [Google Scholar]
- 20.Niederkorn JY. High-risk corneal allografts and why they lose their immune privilege. Curr Opin Allergy Clin Immunol. 2010 Oct;10(5):493–497. doi: 10.1097/ACI.0b013e32833dfa11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu H, Larkin DF, George AJ. Immune modulation in corneal transplantation. Transplant Rev (Orlando) 2008 Apr;22(2):105–115. doi: 10.1016/j.trre.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Dana R. Comparison of topical interleukin-1 vs tumor necrosis factor-alpha blockade with corticosteroid therapy on murine corneal inflammation, neovascularization, and transplant survival (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2007;105:330–343. [PMC free article] [PubMed] [Google Scholar]
- 23.Cursiefen C, Maruyama K, Jackson DG, Streilein JW, Kruse FE. Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation. Cornea. 2006 May;25(4):443–447. doi: 10.1097/01.ico.0000183485.85636.ff. [DOI] [PubMed] [Google Scholar]
- 24.Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004 Apr;113(7):1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonoda Y, Streilein JW. Orthotopic corneal transplantation in mice--evidence that the immunogenetic rules of rejection do not apply. Transplantation. 1992 Oct;54(4):694–704. doi: 10.1097/00007890-199210000-00026. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen NX, Seitz B, Martus P, Langenbucher A, Cursiefen C. Long-term topical steroid treatment improves graft survival following normal-risk penetrating keratoplasty. Am J Ophthalmol. 2007 Aug;144(2):318–319. doi: 10.1016/j.ajo.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 27.Frank SHS, Breier G, Longaker MT, Greenhalgh DG, Werner S. Regulation of vascular endothelial growth factor expression in culturedkeratinocytes. Journal of biological chemistry. 1995;270:12607–12613. doi: 10.1074/jbc.270.21.12607. [DOI] [PubMed] [Google Scholar]
- 28.Planck SRRL, Ansel JC, Huang XN, Rosenbaum JT. Trauma and alkai burns distinct patterns of cytokine gene expression in the rat cornea. Ocular Immunology and Inflammation. 1997;5:95–100. doi: 10.3109/09273949709085057. [DOI] [PubMed] [Google Scholar]
- 29.GKK . Corneal Angiogenesis: A comprehensive critical review. Vol. 16. New York, NY: Springer Verlag; 1991. [Google Scholar]
- 30.Gupta DIC. Treatment for Corneal Neovascularization: A Review. Cornea. 2011;30:927–938. doi: 10.1097/ICO.0b013e318201405a. [DOI] [PubMed] [Google Scholar]
- 31.Fromer CHKG. An evaluation of the role of leukocytes in the pathogenesis of experimentally induced corneal vascularization. II. Studies on the effect of leukocytic elimination on corneal vascularization. Am J Pathol. 1975;81:531–544. [PMC free article] [PubMed] [Google Scholar]
- 32.Cursiefen C, Cao J, Chen L, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci. 2004 Aug;45(8):2666–2673. doi: 10.1167/iovs.03-1380. [DOI] [PubMed] [Google Scholar]
- 33.Weijtens OFE, Schoemaker RC, Cohen AF, Lentjes EGWM, Romijn FPHTM, van Meurs JC. High Concentration of Dexamethasone in Aqueous and Vitreous After Subconjunctival injection. Am J Ophthalmol. 1999;128:192–197. doi: 10.1016/s0002-9394(99)00129-4. [DOI] [PubMed] [Google Scholar]
- 34.Dietrich T, Bock F, Yuen D, et al. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol. 2010 Jan 15;184(2):535–539. doi: 10.4049/jimmunol.0903180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kagaya F, Usui T, Kamiya K, et al. Intraocular dexamethasone delivery system for corneal transplantation in an animal model. Cornea. 2002 Mar;21(2):200–202. doi: 10.1097/00003226-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Dastjerdi MH, Saban DR, Okanobo A, et al. Effects of topical and subconjunctival bevacizumab in high-risk corneal transplant survival. Invest Ophthalmol Vis Sci. 2010 May;51(5):2411–2417. doi: 10.1167/iovs.09-3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edelman JL. Differentiating intraocular glucocorticoids. Ophthalmologica. 2010;224( Suppl 1):25–30. doi: 10.1159/000315158. [DOI] [PubMed] [Google Scholar]
- 38.Negi AKBA, Vernon SA. Single perioperative triamcinolone injection versus standard postoperative steroid drops after uneventful phacoemulsification surgery. J Cataract Refract Surg. 2006;32:468–474. doi: 10.1016/j.jcrs.2005.12.102. [DOI] [PubMed] [Google Scholar]
- 39.Dieleman MWR, van Kooten-Noordzij M, de Waard PET. Single perioperative subconjunctival steroid depot versus postoperative steroid eyedrops to prevent intraocular inflammation and macular edema after cataract surgery. J Cataract Refract Surg. 2011;37:1589–1597. doi: 10.1016/j.jcrs.2011.03.049. [DOI] [PubMed] [Google Scholar]