Abstract
Background
ImmunoCyt/uCyt (Scimedx, Denville, NJ, USA) is a well-established urinary marker assay with high sensitivity for the diagnosis of urothelial carcinoma (UC) and can function as a second-level test to arbitrate atypical reads of urine cytology.
Objective
To determine the utility of uCyt as a reflex test for atypical cytology in patients undergoing a hematuria evaluation or surveillance with a history of UC.
Design, setting, and participants
The uCyt assay was performed as a second-level reflex test on all voided urine cytology tests read as atypical between January 2007 and June 2010 in an academic medical center. Records were retrospectively reviewed. Three hundred twenty-four patients underwent a total of 506 uCyt assays.
Intervention
Reflex uCyt assay on atypical urine cytology.
Outcome measurements and statistical analysis
The uCyt test characteristics include sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV).
Results and limitations
Reflex uCyt was performed on 506 atypical voided urine samples that were followed by cystoscopy within 90 d. Reflex uCyt with a history of UC showed a sensitivity of 73%, a specificity of 49%, and an NPV of 80%. In those with a history of low-grade UC, reflex uCyt had a sensitivity of 75%, a specificity of 50%, and an NPV of 82%, while in those with a history of high-grade UC, it had a sensitivity of 74%, a specificity of 44%, and an NPV of 79%. Without prior history of UC, reflex uCyt had a sensitivity of 85%, a specificity of 59%, and an NPV of 94%. This study's limitations include its retrospective design and interobserver variability inherent to cystoscopy, which was used as the reference test.
Conclusions
When used as a reflex test on atypical urine cytology, negative uCyt may predict a negative cystoscopy in select patients and modulate the urgency and further work-up in those with no prior history or low-grade disease.
Keywords: ImmunoCyt, Cytology, Bladder cancer, Cystoscopy
1. Introduction
Urothelial carcinoma (UC) of the bladder exhibits a high rate of recurrence that necessitates intensive, invasive, and costly long-term surveillance [1]. Risk-stratified models show 5-yr recurrence rates of 31–78% and 0.8–45% progression rates, increasing from low- to high-risk disease [1,2]. Current guidelines recommend that low-risk patients undergo cystoscopy and cytology at 3 mo, follow-up in 9 mo, and annual cystoscopy and cytology for 5 yr; high-risk patients undergo cystoscopy with urine cytology every 3 mo for the first 2 yr and annually thereafter [3]. Cystoscopy is an invasive procedure that can miss certain lesions, such as the flat lesions of carcinoma in situ (CIS) [4]. In a pooled analysis of 14 studies, the sensitivity and specificity of routine cystoscopy for detection of UC was 65% and 81%, respectively, compared against biopsy [5].
Urine cytology has been used >60 yr for the diagnosis of UC. The test suffers from low sensitivity (38–51%), but its high specificity (94–98%), particularly for high-grade disease, makes it a useful adjunct for cystoscopy [6]. Because of factors such calculi, inflammation, instrumentation, or infection, some samples cannot be categorized as either benign or malignant and are considered “atypical.” Reliability of results depends on sample quality and cytopathologist experience. Urine cytology is diagnosed as atypical in as many as 12% of voided samples and 28% of instrumented samples [6]. Correlation with biopsy reveals that malignancy underlies 23–68% of atypical cytology results [7–10]. Faced with a poorly defined result with a high potential for malignancy, clinicians have sparse data with which to make appropriate and efficient treatment decisions.
Many voided urine biomarker assays, such as UroVysion (Vysis, Downers Grove, IL, USA), NMP22, BTA TRAK (Polymedco, Cortlandt Manor, NY, USA), and ImmunoCyt/uCyt (Scimedx, Denville, NJ, USA) have been developed to improve UC diagnosis but have yet to gain widespread clinical application in the context of atypical urine cytology. A triple immunofluorescent monoclonal antibody assay, uCyt is a US Food and Drug Administration–approved urine test for antigens associated with UC. Fluorescein-labeled antibodies LDQ10 and M334 target cytoplasmic mucins, and Texas Red-labeled antibody 19A211 targets high-molecular-weight carcinoembryonic antigen on exfoliated urothelial cells in voided urine [10]. Early studies using uCyt demonstrated low sensitivity (45–50%) and specificity (52–73%) in surveillance, but this has improved in more recent studies to 77–91% and 68–83%, respectively [5,11].
We assessed whether the use of uCyt as a second-level reflex test performed routinely in all patients with a reading of atypical urine cytology can arbitrate results and improve diagnostic efficiency. The primary objective was to calculate the sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) of the uCyt assay obtained in the context of atypical cytology. We used an abnormal cystoscopy and positive biopsy within 90 d of uCyt as reference standards. We also performed subgroup analyses based on patient disease history.
2. Material and methods
Between January 2007 and June 2010, uCyt assays were performed reflexively on all urine cytology samples at our institution that were read as atypical [12]. Urine cytology was performed as part of a hematuria evaluation or in patients undergoing surveillance for UC. All samples were voided urine taken prior to instrumentation in patients without evidence of urinary tract infection. Patients subsequently underwent work-up and treatment based on current standards of care by their urologist.
After approval by the institutional review board, we retrospectively reviewed the medical records and collected information regarding patient demographics, disease history, uCyt results, and subsequent work-up including white light cystoscopy, cytology, and biopsy. Cystoscopy and biopsy results were considered pertinent to the uCyt assay if performed within 90 d of the assay without a second urine test for bladder cancer (cytology or uCyt) having been performed in the interim. Cystoscopy was considered positive or abnormal if papillary lesions or any areas of erythema distant from recent biopsy sites were identified. ImmunoCyt assays were prepared by certified technicians and read by trained cytopathologists. A test was considered positive if at least one cell exhibiting characteristic red or green fluorescence was visible. Tumors were graded using the 2004 World Health Organization/International Society of Urological Pathology classification system [13]. Statistical tests were performed using Stata statistical software (StataCorp, College Station, TX, USA).
3. Results
Between January 2007 and June 2010, 636 urine cytology specimens from 324 patients were read as atypical. Of these, 41 (6.4%) had inadequate cellularity or volume, and a reflex uCyt assay was not performed. The analysis was limited to 506 uCyt assays that were followed by cystoscopy within 90 d. Figure 1 diagrams the clinical contexts in which these assays were performed. Patient demographic and clinical details are shown in Table 1. The mean age was 68.8 yr (standard deviation [SD]: ±11.4 yr) at the time of testing, with no statistically significant difference in age between men and women. Men made up 88% of the study population. Seventy-eight percent of assays were performed in patients with a history of UC (40% low grade, 56% high grade or CIS). Patients without a history of UC who were undergoing a standard hematuria evaluation made up 36% of the population and underwent 142 (24%) of the reflex uCyt assays. Prior disease history was unavailable for the remaining 2% of patients. Thirty-five percent of patients had more than one uCyt assay performed during the course of the study, with 51 undergoing two assays and 63 having three or more done. Overall disease prevalence was similar in those with and without a history of UC. In 142 patients without a history of UC, 17 (12.0%) were found to have biopsy-proven UC, while in 441 with a history of UC, 55 (12.5%) were found have biopsy-proven recurrent UC at the time of evaluation.
Fig. 1.
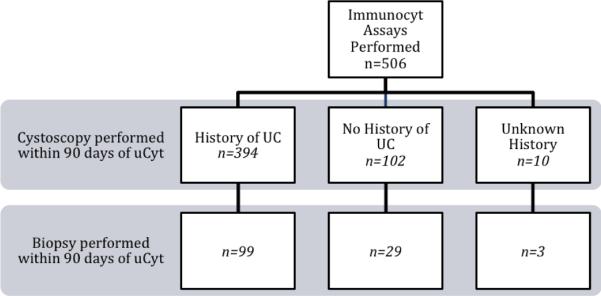
Distribution of clinical contexts in which uCyt was performed. UC = urothelial carcinoma.
Table 1.
Summary of patient characteristics
| Total patients | n = 324 | |
| Male, no. (%) | 277 (85.5) | |
|
| ||
| Female, no. (%) | 47 (14.5) | |
|
| ||
| Mean age | Yr (SD) | |
|
| ||
| Male | 68.7 (±10.6) | p = 0.62 |
|
| ||
| Female | 69.4 (±14.0) | |
|
| ||
| Disease history | No. (%) | |
|
| ||
| History of UC | 201 (62.0) | |
|
| ||
| No prior disease | 115 (35.5) | |
|
| ||
| Unknown | 8 (2.5) | |
SD = standard deviation; UC = urothelial carcinoma
An abnormal cystoscopy within 90 d of ImmunoCyt was used as the reference standard. Of the 506 reflex uCyt test samples obtained, 372 (73.5%) were obtained on the same day as the cystoscopy, and the remaining 132 (26.5%) were obtained at a mean of 33.7 d (SD: ±25.0 d) from cystoscopy. Test characteristics are detailed in Table 2. Overall, reflex uCyt sensitivity was 75.2% and specificity was 49.3%, which led to a PPV of 36.4 and an NPV of 83.7%. In those with no prior history of UC (n = 102), sensitivity and specificity were improved to 85% and 59%, respectively, which led to a higher NPV of 94%. To ensure that the 90-d time frame did not bias results, we further analyzed test characteristics for patients who had the ImmunoCyt test performed on the same day as their cystoscopy (73.5% of all tests). Results (Table 3) revealed test characteristics that mirrored the overall study population.
Table 2.
ImmunoCyt test characteristics with urothelial carcinoma proven by biopsy as the gold standard*
| No. | All positives, no. | True positives, no. | Sensitivity, % | Specificity, % | PPV, % | NPV, % | |
|---|---|---|---|---|---|---|---|
| uCyt with biopsy within 90 d | 131 | 109 | 64 | 86.5 | 21.1 | 58.7 | 54.5 |
|
| |||||||
| With history of UC | 99 | 82 | 47 | 85.5 | 20.5 | 57.3 | 52.9 |
|
| |||||||
| No history of UC | 29 | 24 | 15 | 88.2 | 25.0 | 62.5 | 60.0 |
PPV = positive predictive value; NPV = negative predictive value; UC = urothelial carcinoma.
Three patients with an unknown history of UC were excluded from this analysis.
Table 3.
ImmunoCyt test characteristics with abnormal cystoscopy as the gold standard*
| No. | All positives, no. | True positives, no. | False negatives, no. | Sensitivity, % | Specificity, % | PPV, % | NPV, % | |
|---|---|---|---|---|---|---|---|---|
| uCyt with cystoscopy within 90 d | 506 | 291 | 106 | 35 | 75.2 | 49.3 | 36.4 | 83.7 |
|
| ||||||||
| No history of UC | 102 | 51 | 17 | 3 | 85.0 | 58.5 | 33.3 | 94.1 |
|
| ||||||||
| With history of UC | 394 | 232 | 86 | 32 | 72.9 | 47.1 | 37.1 | 80.2 |
|
| ||||||||
| Low-grade UC | 159 | 91 | 35 | 12 | 74.5 | 50.0 | 38.5 | 82.4 |
|
| ||||||||
| High-grade UC or CIS | 221 | 135 | 50 | 18 | 73.5 | 44.4 | 37.0 | 79.1 |
|
| ||||||||
| uCyt with cystoscopy on the same day | 372 | 206 | 72 | 25 | 74.2 | 51.3 | 35.0 | 84.9 |
PPV = positive predictive value; NPV = negative predictive value; UC = urothelial carcinoma; CIS = carcinoma in situ.
Ten patients with an unknown history of UC were excluded from this analysis.
Subgroup analysis revealed consistent test performance between patients with a history of low-grade (n = 159) and high-grade or CIS (n = 221) disease. Sensitivity was 75% for low-grade and 74% for high-grade or CIS disease, with an NPV of 82% and 79%, respectively. A low specificity (50% and 44%, respectively) led to lower test PPV.
To further characterize reflex uCyt, we evaluated test results in patients who had UC proven by biopsy within 90 d of uCyt testing (Table 2). Of the 131 patients who underwent a biopsy or transurethral resection (TUR) within 90 d of uCyt testing, 99 (76%) had a history of UC, and 29 (24%) underwent random bladder biopsies as part of a comprehensive hematuria evaluation. The mean number of days between ImmunoCyt and biopsy or TUR was 30.1 (SD: ±22.4). Overall sensitivity was 86.5% (85.5% for those with prior history and 88.2% for those without). In this setting, specificity ranged from 20% to 25%, resulting in low PPV (57–63%) and NPV (53–60%).
4. Discussion
ImmunoCyt test performance has been well studied since its introduction by Fradet and Lockhard in 1997 [10]. Recent series have reported a sensitivity of 74–87% and a specificity of 62–78%, with PPVs and NPV of 26–67% and 91–96%, respectively [14–18]. The previously reported values are for uCyt assays performed regardless of underlying cytologic diagnosis and do not focus on test performance in the context of atypical cytology. Tetu and colleagues, using a combination of cystoscopy and biopsy as the reference, performed subgroup analyses focusing on just those patients with atypical cytology. Of the 870 enrolled patients, 109 had a cytology reported as “suspicious.” In this group, uCyt had a sensitivity of 73%, a specificity of 34%, an NPV of 77%, and a PPV of 30%. Although this is a lower specificity than the overall group (62%), leading to a lower NPV, the authors report that there was no statistically significant difference in test performance between the overall group and the “suspicious” subgroup (p = 0.362) [14]. In our study of 506 patients with atypical cytology, uCyt had comparable sensitivity (75%) and increased specificity (49%), with an NPV of 84% and a PPV of 36%. Our larger study, focusing exclusively on analyzing the utility of uCyt to arbitrate atypical cytology, confirms the subgroup analyses from Tetu et al. A sensitivity of 75% for atypical cytology is comparable to overall published uCyt sensitivity. In our study, specificity was noticeably lower than that for overall uCyt. This may be the result of the general ambiguity of the atypical category and the fact that these samples were by definition not clearly differentiable. Work by Yoder and colleagues implementing reflex fluorescence in situ hybridization using UroVysion as a reflex test against all negative and atypical urine cytology results has shown this strategy to be an effective mechanism for improving cytologic diagnosis [19]. UroVysion testing is generally more expensive and requires specialized molecular pathology equipment, whereas uCyt can be performed in a standard cytopathology laboratory utilizing the same specimen, making it easier to implement and more accessible.
Urine cytology is a highly specific test for diagnosis of high-grade UC, routinely reported at >95%. However, it is notoriously unreliable for low-grade disease, which cytologically resembles nonneoplastic urothelium; uCyt was designed to improve urine cytology test performance in those with low-grade disease. In this study, however, subgroup analyses revealed consistent test performance across disease grade. In patients with a history of low-grade disease but atypical cytology, a negative uCyt has a sufficiently high predictive value to safely delay cystoscopy. In work by Millan-Rodriguez, low-risk tumors were shown to recur at 37%, with 0% progression in their cohort, whereas high-risk tumors recurred more often (54%), with a higher rate of progression to invasive disease (15%) [20]. Although some have proposed alternative screening regimens that incorporate urinary markers to decrease cystoscopy frequency, they have yet to be integrated into clinical practice [17,21,22]. Sylvester and colleagues showed that multifactorial analysis of patient characteristics more accurately predicts recurrence and progression risk [2], and incorporation of uCyt and other urinary marker data may improve future nomograms.
Mian and colleagues described 100% sensitivity in detecting recurrent CIS using uCyt in a small group of patients, showing its potential for both low- and high-risk disease [23]. Others have taken patient preferences into account, using patient survey data to determine that any urinary marker assay would need a minimum sensitivity of 75–90% for approximately 90% of patients to prefer it to cystoscopy [24,25].
In those with no prior history of disease, sensitivity was highest at 85%, with an NPV of 94%. uCyt can provide important information in evaluating hematuria, particularly in the patient with negative imaging and cystoscopy but atypical cytology. Schmitz-Dräger and colleagues studied 189 patients being evaluated for microscopic hematuria with ImmunoCyt and noted an NPV of 99.4% [26]. In our study, our lower NPV of 94% is likely the result of patient selection, as our cohort all had an atypical cytology. It is important to note in that even with an atypical cytology, NPV remained high.
There were some limitations in the current study. Approximately 6% of samples were of inadequate quality for uCyt analysis, the result of inadequate collection and processing early in the institutional experience, and the rate rapidly decreased over time. Although all patients with atypical cytology did undergo reflex ImmunoCyt testing, further evaluation and management was at the discretion of the treating urologist. In addition, cystoscopy was used as the reference standard, which is less reliable than a histologically confirmed diagnosis. This method suffers from the inherent variability and subjectivity of cystoscopy but reflects its performance and utilization in practice. A 90-d window within which a cystoscopy was considered relevant was used. There may be a chance of interval development of new lesions, but >74% of uCyt assays were performed on the same day as the cystoscopy. In addition, imaging of the upper tract was not independently evaluated in this study. Although we did analyze data using biopsy as the reference, these data are biased by the fact that only those with positive or suspicious lesions on cystoscopy underwent biopsy, introducing assignment bias. Using biopsy results as a standard, the sensitivity of uCyt was relatively high at 86.5%.
5. Conclusions
When used as a reflex test to arbitrate atypical urine cytology, a negative ImmunoCyt result can be used to predict a negative cystoscopy in select patients. The high NPV can be used to modify the urgency and nature of further urologic work-up, both in those without a history of UC and in those with a history of low-grade UC in which a routine follow-up schedule with fewer cystoscopies can be maintained despite a reading of atypical cytology. A prospective study assessing the utility of second-level marker testing to arbitrate atypical cytology in a standardized pathway will help identify an optimal method of avoiding unnecessary work-up in these patients while ensuring identification of those with disease.
Take-home message.
When used as a reflex test on atypical urine cytology in evaluating urothelial carcinoma, a negative ImmunoCyt assay can effectively predict a negative cystoscopy and may obviate the need for cystoscopy in those with prior low-grade disease.
Acknowledgments
Funding/Support and role of the sponsor: None.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Badrinath R. Konety had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Odisho, Konety, Carroll, Cooperberg.
Acquisition of data: Odisho, Ahmad, Berry.
Analysis and interpretation of data: Odisho, Berry, Cooperberg, Carroll, Konety.
Drafting of the manuscript: Odisho, Konety.
Critical revision of the manuscript for important intellectual content: Odisho, Berry, Cooperberg, Carroll, Konety.
Statistical analysis: Odisho.
Obtaining funding: None.
Administrative, technical, or material support: Konety, Carroll.
Supervision: Cooperberg, Carroll, Konety.
Other (specify): None.
Financial disclosures: Badrinath R. Konety certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/ affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
References
- [1].Cooksley C. Clinical model of cost of bladder cancer in the elderly. Urology. 2008;71:519–25. doi: 10.1016/j.urology.2007.10.056. [DOI] [PubMed] [Google Scholar]
- [2].Sylvester RJ, van der Meijden APM, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–77. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- [3].Oosterlinck W, Lobel B, Jakse G, Malmström PU, Stöckle M, Sternberg C, European Association of Urology (EAU) Working Group on Oncological Urology Guidelines on bladder cancer. Eur Urol. 2002;41:105–12. doi: 10.1016/s0302-2838(01)00026-4. [DOI] [PubMed] [Google Scholar]
- [4].van der Aa MNM, Steyerberg EW, Sen EF, et al. Patients' perceived burden of cystoscopic and urinary surveillance of bladder cancer: a randomized comparison. BJU Int. 2008;101:1106–10. doi: 10.1111/j.1464-410X.2007.07224.x. [DOI] [PubMed] [Google Scholar]
- [5].Mowatt G, Zhu S, Kilonzo M, et al. Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer. Health Technol Assess. 2010;14:1–331. iii–iv. doi: 10.3310/hta14040. [DOI] [PubMed] [Google Scholar]
- [6].Raab S, Grzybicki D, Vrbin C, Geisinger KR. Urine cytology discrepancies: frequency, causes, and outcomes. Am J Clin Pathol. 2007;127:946–53. doi: 10.1309/XUVXFXMFPL7TELCE. [DOI] [PubMed] [Google Scholar]
- [7].Deshpande V, McKee GT. Analysis of atypical urine cytology in a tertiary care center. Cancer. 2005;105:468–75. doi: 10.1002/cncr.21317. [DOI] [PubMed] [Google Scholar]
- [8].Mokhtar G, Al-Dousari M, Al-Ghamedi D. Diagnostic significance of atypical category in the voided urine samples: a retrospective study in a tertiary care center. Urol Ann. 2010;2:100–6. doi: 10.4103/0974-7796.68857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kapur U, Venkataraman G, Wojcik EM. Diagnostic significance of “atypia” in instrumented versus voided urine specimens. Cancer. 2008;114:270–4. doi: 10.1002/cncr.23656. [DOI] [PubMed] [Google Scholar]
- [10].Fradet Y, Lockhard C. Performance characteristics of a new monoclonal antibody test for bladder cancer: ImmunoCyt trade mark. Can J Urol. 1997;4:400–5. [PubMed] [Google Scholar]
- [11].Vriesema J, Atsma F, Kiemeney L, Peelen WP, Witjes JA, Schalken JA. Diagnostic efficacy of the ImmunoCyt test to detect superficial bladder cancer recurrence. Urology. 2001;58:367–71. doi: 10.1016/s0090-4295(01)01217-1. [DOI] [PubMed] [Google Scholar]
- [12].Epstein J, Amin M, Reuter V, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998;22:1435–48. doi: 10.1097/00000478-199812000-00001. [DOI] [PubMed] [Google Scholar]
- [13].Eble J, editor. Pathology and genetics of tumours of the urinary system and male genital organs. ed. 7 IARC Press; Oxford, UK, and Lyon, France: 2004. [Google Scholar]
- [14].Tetu B, Tiguert R, Harel F, Fradet Y. ImmunoCyt/uCyt+ improves the sensitivity of urine cytology in patients followed for urothelial carcinoma. Mod Pathol. 2005;18:83–9. doi: 10.1038/modpathol.3800262. [DOI] [PubMed] [Google Scholar]
- [15].Messing E, Teot L, Korman H, et al. Performance of urine test in patients monitored for recurrence of bladder cancer: a multicenter study in the United States. J Urol. 2005;174:1238–41. doi: 10.1097/01.ju.0000173918.84006.4d. [DOI] [PubMed] [Google Scholar]
- [16].Mian C, Maier K, Comploj E, et al. uCyt+/ImmunoCyt in the detection of recurrent urothelial carcinoma: an update on 1991 analyses. Cancer. 2006;108:60–5. doi: 10.1002/cncr.21712. [DOI] [PubMed] [Google Scholar]
- [17].Lodde M, Mian C, Comploj E, et al. uCyt+ test: alternative to cystoscopy for less-invasive follow-up of patients with low risk of urothelial carcinoma. Urology. 2006;67:950–4. doi: 10.1016/j.urology.2005.11.057. [DOI] [PubMed] [Google Scholar]
- [18].Sullivan PS, Nooraie F, Sanchez H, et al. Comparison of ImmunoCyt, UroVysion, and urine cytology in detection of recurrent urothelial carcinoma. Cancer. 2009;117:167–73. doi: 10.1002/cncy.20026. [DOI] [PubMed] [Google Scholar]
- [19].Yoder B, Skacel M, Hedgepeth R, et al. Reflex UroVysion testing of bladder cancer surveillance patients with equivocal or negative urine cytology: a prospective study with focus on the natural history of anticipatory positive findings. Am J Clin Pathol. 2007;127:295–301. doi: 10.1309/ADJL7E810U1H42BJ. [DOI] [PubMed] [Google Scholar]
- [20].Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, Palou J, Algaba F, Vicente-Rodríguez J. Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J Urol. 2000;164:680–4. doi: 10.1016/s0022-5347(05)67280-1. [DOI] [PubMed] [Google Scholar]
- [21].Lotan Y, Roehrborn CG. Cost-effectiveness of a modified care protocol substituting bladder tumor markers for cystoscopy for the followup of patients with transitional cell carcinoma of the bladder: a decision analytical approach. J Urol. 2002;167:75–9. [PubMed] [Google Scholar]
- [22].Nam RK, Redelmeier DA, Spiess PE, Sampson HA, Fradet Y, Jewett MA. Comparison of molecular and conventional strategies for followup of superficial bladder cancer using decision analysis. J Urol. 2000;163:752–7. [PubMed] [Google Scholar]
- [23].Mian C, Lodde M, Comploj E, et al. The value of the ImmunoCyt/uCyt+ test in the detection and follow-up of carcinoma in situ of the urinary bladder. Anticancer Res. 2005;25:3641–4. [PubMed] [Google Scholar]
- [24].Vriesema JL, Poucki MH, Kiemeney LA, Witjes JA. Patient opinion of urinary tests versus flexible urethrocystoscopy in follow-up examination for superficial bladder cancer: a utility analysis. Urology. 2000;56:793–7. doi: 10.1016/s0090-4295(00)00777-9. [DOI] [PubMed] [Google Scholar]
- [25].Yossepowitch O, Herr H, Donat S. Use of urinary biomarkers for bladder cancer surveillance: patient perspectives. J Urol. 2007;177:1277–82. doi: 10.1016/j.juro.2006.11.066. discussion 1282. [DOI] [PubMed] [Google Scholar]
- [26].Schmitz-Dräger BJ, Beiche B, Tirsar L-A, Schmitz-Dräger C, Bismarck E, Ebert T. Immunocytology in the assessment of patients with asymptomatic microhaematuria. Eur Urol. 2007;51:1582–8. doi: 10.1016/j.eururo.2006.10.046. [DOI] [PubMed] [Google Scholar]


