Abstract
Although high serum levels of galactose-deficient-IgA1 (an important biomarker of IgA nephropathy (IgAN)), are found in most patients with IgAN, their relationship to disease severity and progression remains unclear. To help clarify this we prospectively enrolled 275 patients with IgAN and followed them for a median of 47 months (range 12–96 months). Serum galactose-deficient-IgA1 was measured at the time of diagnosis using a lectin-based ELISA and renal survival was modeled using the Cox proportional hazards method. The serum levels of galactose-deficient-IgA1 were higher in patients with IgAN compared to those in healthy controls. Importantly, in adjusted analysis, higher levels of galactose-deficient-IgA1 were independently associated with a greater risk of deterioration in renal function with a hazard ratio of 1.44 per standard deviation of the natural log-transformed galactose-deficient-IgA1 concentration. In reference to the first quartile, the risk of kidney failure increased such that the hazard ratio for the second quartile was 2.47, 3.86 for the third, and 4.76 for the fourth quartile of the galactose-deficient-IgA1 concentration. Hence, elevated serum levels of galactose-deficient-IgA1 are associated with a poor prognosis in IgAN.
Keywords: Galactose-deficient IgA1, kidney disease progression, IgA nephropathy
Introduction
IgA nephropathy (IgAN) is the most common form of primary glomerulonephritis worldwide 1 and the leading cause of end-stage renal disease (ESRD) in young adults.2 The pattern of glomerular immunoglobulin deposits is suggestive of an immune complex-mediated mechanism.3–7 Multiple studies have established the contribution of aberrantly glycosylated IgA1 in the pathogenesis of IgAN.8–12 IgA1 is one of the very few serum proteins with O-glycosylation. The O-glycans in the hinge-region of normal IgA1 consist of N-acetylgalactosamine (GalNAc), galactose, and sialic acid. In patients with IgAN, some of the O-glycans of circulating IgA1 are deficient in galactose. Similarly, mesangial immune deposits eluted directly from glomeruli of IgAN patients contained galactose-deficient IgA1 (Gd-IgA1).7,13,14 Based on the potential pathogenic role of Gd-IgA1 in the development of IgAN, a quantitative assay for serum Gd-IgA1 holds promise as a non-invasive diagnostic tool. We previously reported an increased binding of a GalNAc -specific lectin from Helix aspersa (HAA) to desialylated serum Gd-IgA1 in Caucasian patients with IgAN.15 Our earlier study demonstrated that Gd-IgA1 was also closely associated with the pathologic phenotype of IgAN.16 However, it is not known whether elevated levels of Gd-IgA1 in sera of IgAN patients are associated with accelerated disease progression, or a worse prognosis.17
We have previously established a large prospective cohort of patients with IgAN followed regularly at the Peking University Institute of Nephrology. This cohort has been assembled and followed as part of the Peking University IgAN database project since 2003 (http://www.renal-online.org), with blood and urine samples collected at the time of kidney biopsy, and clinical data collected prospectively for all enrolled patients. In this study, we utilize the above resource to examine the prognostic utility of serum levels Gd-IgA1.
Results
Baseline clinical, laboratory and pathological data
Among the 275 IgAN patients, there were 147 (53.5%) males and 128 (46.5%) females with mean age at the time of kidney biopsy of 32.7 ± 10.7 years. On biopsy, average proteinuria level was 1.92±1.89 g/24 h (range 0.01–13.72 g/24 h) and average eGFR was 82.65 ± 27.44 ml/min/1.73 m2 (range 6.3–164.9 ml/min/1.73 m2). Systolic blood pressure was 124±16 mmHg, diastolic blood pressure 79±12mmHg, with 131 patients (47.6%) being hypertensive at baseline. The distribution by Haas grade I, II, III, IV and V was 10.9%, 0.4%, 32.4%, 42.9%, and 13.5%, respectively. The median follow-up time was 47 months (range 12–96 months) (Table 1). During the follow-up period, 266 (96.7%) patients received ACE inhibitors or ARBs therapy, 127 (46.2%) of received oral corticosteroids alone or combined with other immunosuppressive agents. In total, 42 patients reached the composite endpoint of 50% decline in eGFR (n=39), ESRD (n=3) or death (n=2; both had a 50% decline in eGFR before death).
Table 1.
Baseline clinical and laboratory data and levels of serum Gd-IgA1 in 275 patients with IgAN
| Characteristics | Mean±SD (range or Percentage) |
|---|---|
| Age (year) | 32.7±10.7 (15–76) |
| Gender (Male) | 147/275 (53.5%) |
| SBP (mm Hg) | 124±16 |
| DBP (mm Hg) | 79±12 |
| Hypertension (%)a | 131/275 (47.6%) |
| Initial proteinuria (g/d) | 1.92±1.89 |
| <0.3(%) | 15/275(5.5%) |
| 0.3–0.99(%) | 84/275(30.5%) |
| 1.0–2.99(%) | 130/275(47.3%) |
| ≥3.0 | 46/275(16.7%) |
| eGFR (ml/min/1.73m2) | 82.65±27.44 |
| Stage 1,2,3,4CKD (K/DOQI)b | 125(45.7%),92(33.3%),50(18.0%),8(3%) |
| Follow-up interval (month, median, IQR) | 47 (30–60) |
| Gd-IgA1 (U/ml, median, IQR) | 312.5 (236.5–407.8) |
| Total IgA (ug/ml, median, IQR) | 2630.0 (2125.0–3510.0) |
| Plasma IgA1 (ug/ml, median, IQR) | 1709.9 (1376.2–2159.7) |
| Therapy (%) | |
| ACE inhibitors or ARBs | 96.7% |
| Prednisone | 46.2% |
| Any other immunosuppressive agents (cyclophosphamide, MMF or others) |
29.8% |
| Histological grading (%)c | |
| I, II, III, IV, V | 30(10.9%), 1(0.36%), 89(32.4%), 118(42.9%), 37(13.5%) |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; eGFR, estimate glomerular filtration rate; IQR, interquartile range
Hypertension was defined as systolic blood pressure of greater than 140 mm Hg, and/or diastolic blood pressure of greater than 90 mm Hg at resting, or use of anti-hypertension medication.
CKD stage 1, 2, 3, 4 were divided by eGFR ≥ 90, 60 to 89, 30 to 59, 15 to 29.
Histological grading was classified according to pathologic scheme proposed by Haas.45
Serum Gd-IgA1 levels
HAA-based ELISA for Gd-IgA1 was highly reproducibile (r=0.903, p<0.0001). The level of Gd-IgA1 in IgAN patients (median: 312.5 U/ml, IQR 236.5–407.8 U/ml) was significantly higher compared to that of the healthy controls (148.3 U/ml, IQR 106.1–209.1 U/ml, P < 0.001). The patients were next divided into four equal groups according to the quartiles of the Gd-IgA1 distribution; Group 1: serum Gd-IgA1 levels <236.5 U/ml; Group 2: serum Gd-IgA1 between 236.5 and 312.5 U/ml; Group 3: serum Gd-IgA1 between 312.5 and 407.8 U/ml; and Group 4: serum Gd-IgA1 >407.8 U/ml.
Among cases, we observed no significant association of Gd-IgA1 levels with age, gender, or body mass index (BMI) (Table 2). There was a trend for lower eGFR, higher proteinuria, and increased use of immunosuppressives in groups 3 and 4 compared with groups 1 or 2, albeit none of these differences were statistically significant. There were also no significant differences between the groups in the blood pressure control (P=0.96) or proteinuria during follow-up (P=0.94).
Table 2.
Baseline data of clinical features and levels of serum Gd-IgA1 of four groups defined by quartiles of serum Gd-IgA1
| 1 | 2 | 3 | 4 | ||
|---|---|---|---|---|---|
| Group | U/ml, median (IQR) 193.9(158.4, 213.8) |
U/ml, median (IQR) 272.5(247.2, 289.0) |
U/ml, median (IQR) 345.7(324.2, 369.9) |
U/ml, median (IQR) 487.4(438.8, 540.3) |
P value |
| Number | 69 | 69 | 68 | 69 | |
| Gender (M) | 41/69 | 37/69 | 33/68 | 36/69 | 0.638 |
| Age (year) | 32.78±11.43 | 33.87±10.63 | 32.47±11.3 | 31.80±9.34 | 0.718 |
| Hypertension (%) | 34 (49.3%) | 34 (49.3%) | 27 (39.7%) | 36 (52.2%) | 0.488 |
| Proteinuria (%, >1g/d) | 42/69(60.9%) | 39/69(56.5%) | 50/68(73.5%) | 45/69(65.2%) | 0.196 |
| eGFR (ml/min/1.73 m2) Stage 1,2,3,4 CKD(KDOQI) | 29/28/10/2 | 34/18/16/1 | 29/24/13/2 | 30/22/13/4 | 0.715 |
| Histological grading | 0.525 | ||||
| (Mild lesions/Severe lesions) | 26/43 | 29/40 | 34/34 | 31/38 | |
| Prednisone | 27 (39.13%) | 29 (42.03%) | 37 (54.41%) | 34 (49.28%) | 0.264 |
| Any other immunosuppressive agents | 21 (30.4%) | 18 (26.1%) | 16 (23.5%) | 24 (34.7%) | 0.481 |
| ACE inhibitors or ARBs | 68 (98.60%) | 69 (100.0%) | 66 (97.10%) | 64 (94.10%) | 0.157 |
| Total IgA (ug/ml, median, IQR) | 2026(1566.5,2411) | 2479(2309.5,2849) | 2880(2362.5,3669) | 3483(2855,4012) | <0.001 |
| Plasma IgA1 (ug/ml, median, IQR) | 1352.2(1138.3,1699.7) | 1574.7(1325.9,1827.6) | 1868.8(1556.8,2289.6) | 2109.5(1765.5,2558.6) | <0.001 |
| Kidney Failure events (%) | 6(8.69%) | 6/69(8.69%) | 15/68(22.1%) | 15/69(21.7%) | 0.026 |
Abbreviation: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; eGFR, estimate glomerular filtration rate; IQR, interquartile range.
Histological grading was divided into mild lesions and severe lesions group. The former group included Haas I, II and III, the latter group included IV and V. Continuous data were compared by One-Way ANOVA test; dichotomous or categorical data were analyzed by Chi-square test.
Association of Gd-IgA1 with kidney disease progression
Baseline clinical and pathological variables were tested for association with the primary progression outcome using Cox proportional hazards model. In univariate analyses, lower baseline eGFR, higher histological grading, greater degree of proteinuria, use of steroids, and higher levels of Gd-IgA1 were all significantly associated with a progression risk, but age, gender, hypertension, hemoglobin or albumin levels were not (Supplementary Table 1).
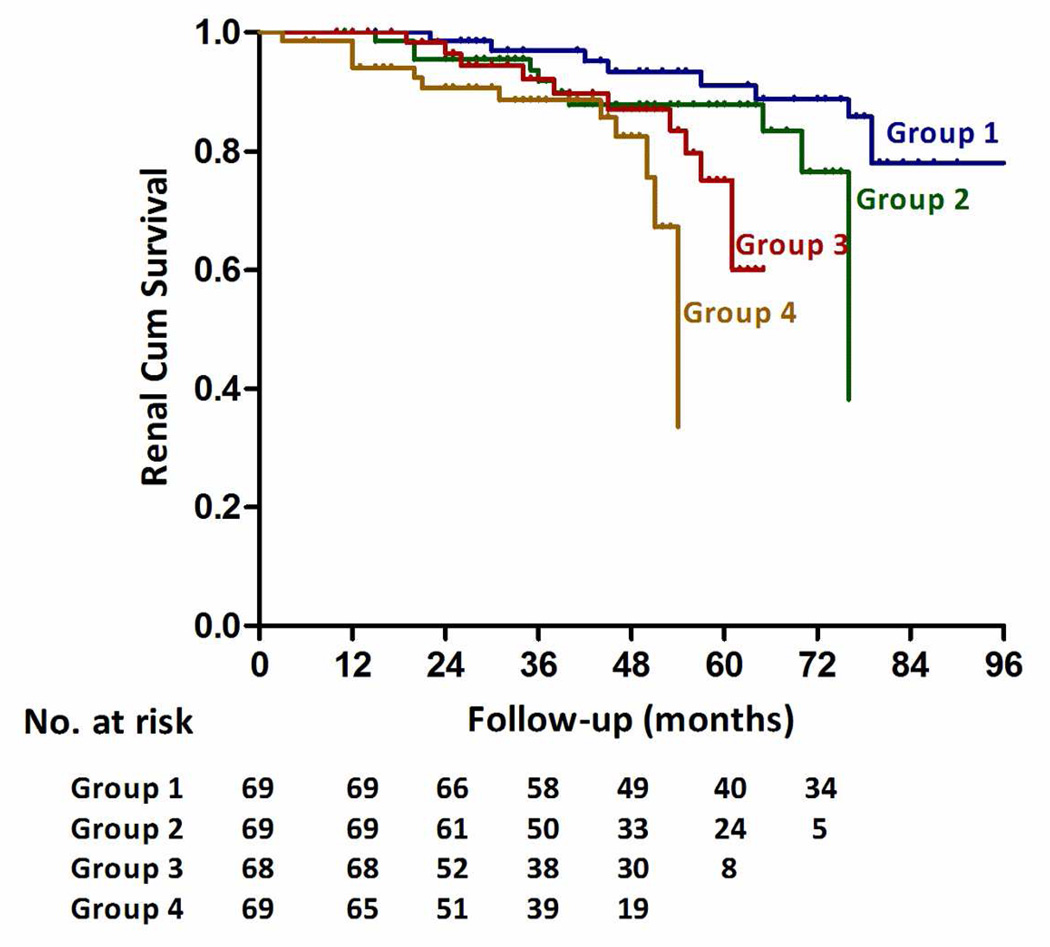
Unadjusted and multivariable adjusted HRs for composite of end-point are presented in Table 3 according to baseline Gd-IgA1, expressed as a continuous variable or in quartiles. In adjusted analysis including baseline proteinuria, hypertension, eGFR, Haas classification, and steroid therapy, higher levels of Gd-IgA1 were independently associated with a greater risk of kidney failure (hazard ratio [HR], per SD of natural log-transformed Gd-IgA1 1.44; 95% confidence interval [CI], 1.11–1.88, p=0.006). Gd-IgA1 level remained strongly associated with progression even after adjustment for time-average proteinuria and other baseline risk factors including hypertension and eGFR (HR=1.39, 95%CI 1.24–1.56, P=1.15×10−8). Compared to the first quartile of Gd-IgA1 (reference), the risk of kidney failure increased by quartile of Gd-IgA1 level: the HRs were 2.47 (95% CI, 0.91–6.72) for the second quartile, 3.86 (95% CI, 1.33–11.33) for the third quartile, 4.76 (95% CI, 1.61–14.09) for the fourth quartile As shown in Figure 1, the renal survival deteriorated by the quartile of serum Gd-IgA1 level. The renal survival at 1st and 3rd year in each group of patients was 100.0% and 96.9%; 100.0% and 91.8%; 100.0% and 92.2%; 98.6% and 88.6% respectively. (Log Rank test, p=0.004). We also analyzed Gd-IgA1/IgA1 ratio and Gd-IgA1/total IgA ratio instead of Gd-IgA1. Gd-IgA1/IgA1 and Gd-IgA1/IgA ratios also represented risk factors for kidney disease progression in the univariate analysis (unadjusted HR=1.42, 95%CI 1.05–1.92, p=0.021; and HR=1.56, 95%CI 1.152.12, p=0.005, respectively) but were not significantly associated after adjustment for other covariates in the multivariate model (p=0.2 and 0.34, respectively).
Table 3.
Risks of composite end-point natural Log-transformed Gd-IgA1 and ascending quartiles
| Hazard Ration (95% Confidence Interval) & p value | |||||||
|---|---|---|---|---|---|---|---|
| Gd-IgA1,median (Range), U/ml |
Unadjusted | Model 1 a | Model 2 b | Model 3 c | |||
| Composite endpoint | |||||||
| Per 1SD lnGd-IgA1 | 312.5 (89.0–1442.0) | 2.07 (1.53–2.78) 1.68×10−6 |
1.51 (1.16–1.97) 0.002 |
1.50 (1.15–1.96) 0.003 |
1.44 (1.11–1.88) 0.006 |
||
| Gd-IgA1 quartiles | |||||||
| 1 | 193.88 (89.0–237.0) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | ||
| 2 | 272.51 (239.0–312.0) | 2.63 (0.94–7.36) 0.066 |
2.71 (0.99–7.39) 0.052 |
2.73 (0.99–7.45) 0.051 |
2.47 (0.91–6.72) 0.077 |
||
| 3 | 345.67 (313.0–406.0) | 4.03(1.36–11.96) 0.012 |
3.74 (1.28–10.93) 0.016 |
3.72 (1.27–10.89) 0.016 |
3.86 (1.33–11.33) 0.013 |
||
| 4 | 487.36 (408.0–1442.0) | 6.76 (2.23–20.19) 0.001 |
5.18 (1.75–15.34) 0.003 |
5.29 (1.78–15.73) 0.003 |
4.76 (1.61–14.09) 0.005 |
||
Composite endpoint was defined as 50% decline of eGFR(n=29), ESRD(n=3) or death(n=2). The 2 deaths also had 50% decline of eGFR.
Abbreviation: LnGd-IgA1, Natural Log-transformed galactose-deficient IgA1. Unadjusted Model analyzed Gd-IgA1 as continuous data.
Model 1 adjusted for eGFR, proteinuria and hypertension (yes or no). Hypertension (yes or no) was analyzed as dichotomous data.
Model 2 adjusted for covariates in model 1 plus histological grading (mild and severe lesion group).The latter variable was analyzed as categorical data.
Model 3 adjusted for covariates in model 2 plus steroid use (yes or no). The latter variable was analyzed as dichotomous data.
Figure 1. Renal survival in IgAN patients with four quartile serum Gd-IgA1 levels.
The renal survival deteriorated by the quartile of serum Gd-IgA1 level. The time zero was kidney biopsy. The division between the four groups of patients was based on quartile of Gd-IgA1 level expressed as 236.5 U/ml, 312.5 U/ml, and 407.8 U/ml. The renal survival at the 1st and 3rd year in each group of patients was 100.0% and 96.9%; 100.0% and 91.8%; 100.0% and 92.2%; 98.6% and 88.6% respectively. (Log Rank test, p=0.004).
Discussion
IgAN can have a highly variable course. Some patients progress rapidly to ESRD, whereas others have a rather benign disease trajectory.17, 18 The individual outcomes of IgAN remain difficult to predict. Previous longitudinal studies have associated proteinuria, hypertension, and impaired renal function at the time of diagnosis with IgAN progression.19 In this study, we hypothesize that the increased serum level of Gd-IgA1, the key pathogenic feature in IgAN, causes accelerated disease progression in IgAN.
Based on the comparison of Gd-IgA1 levels between the IgAN patients and a large group of healthy population controls, our study confirmed that serum Gd-IgA1 levels are elevated in IgAN. Most importantly, our study also demonstrated that high levels of Gd-IgA1 were predictive of renal function decline in IgAN. Based on our prospective follow-up data, we found that elevated level of serum Gd-IgA1 was independently associated with increased risk of renal disease progression. Among cases with biopsy-based diagnosis of IgAN, participants with the highest vs. the lowest quartile demonstrated a 4.76-fold greater risk of kidney progression. Thus our data suggest that high levels of Gd-IgA1 may have prognostic utility in IgAN. Our observations that individuals with higher levels of Gd-IgA1 are more prone to disease progression may justify closer follow-up and possibly more aggressive treatment of such individuals.
A recent study from Japan found that Gd-IgA1 was also significantly elevated in Japanese IgAN patients,20 however, this study did not examine its impact on kidney disease progression. Another study investigated the impact of Gd-IgA1 levels on the renal function decline rate among 62 Caucasian patients with IgAN.21 Gd-IgA1 was independently associated with proteinuria level during the follow-up. Although Gd-IgA1 by itself was not associated with the rate of GFR decline, the combination of a high level of Gd-IgA1 and advanced oxidation protein products contributed to a more rapid loss of renal function. However, the small sample size was the major limitation of the prior studies. Taken together, these studies suggest that aberrant glycosylation of IgA1 contributes to the risk of nephropathy in both Chinese and Caucasian populations.
Both in vivo and in vitro studies have now firmly established that altered O-glycosylation of serum IgA1 plays a central role in the development of IgAN.22–24 Novak et al. have demonstrated that B cell abnormal expression/activity of enzymes involved in sialylation and/or galactosylation of the IgA1 hinge-region O-linked glycans underlie this defect. Furthermore, anti-glycan antibodies (IgG and/or IgA1) recognize Gd-IgA1 (auto-antigen) and trigger the formation of IgA1-containing immune complexes.5 In addition, it appears that the defect in the glycosylation of IgA1 is, to a large degree, genetically determined. 25–27 However, this IgA1 O-glycosylation aberrancy (Hit 1) itself is not sufficient to induce renal injury.25 Synthesis and binding of antibodies directed against Gd-IgA1 are required for formation of immune complexes that accumulate in the glomerular mesangium (Hits 2).28 Based on the results of this study, we speculate that a higher level of circulating Gd-IgA1, when exposed to the second hit, may result in more severe kidney injury. The limitations of our study include the relatively short follow-up, with a median of approximately 4 years. This reduces the study power, with fewer endpoints observed during this time period. We used a composite end-point of 50% decline from baseline eGFR, ESRD, or death, which is a robust outcome used in many clinical trials.29 Future extension of this study to a follow-up period that exceeds 10 years would enable examination of harder endpoints (such as ESRD alone) and provide more accurate effect estimates. Other limitations stem from the complicated multi-step ELISA assay used in this study to quantify Gd-IgA1 levels. This method may not be ideal for accurate detection of small differences in Gd-IgA1 levels. Mass spectrometry-based methods, such as those 30–33 developed recently may be more appropriate future approaches. In addition, the observed distribution of serum Gd-IgA1 levels deviates from normality and is positively skewed. To circumvent this problem, we used quartiles of Gd-IgA1 levels in our regression analyses. Finally, we recognize that our results may not be applicable to all patient populations, as our study cohort consists of only Han Chinese patients followed at a single tertiary care medical center. Additional longitudinal studies of more diverse patient cohorts are needed to validate our findings.
Patients with IgAN are characterized by a highly variable clinical course ranging from a totally benign incidental condition to rapidly progressive renal failure. Many studies have focus on predicting outcomes in IgAN from the baseline clinical or pathological variables. 19, 20, 34–37 In the French cohort with subjects followed over an average of 13 years, Berthoux et al proposed a comprehensive scoring system based on the number of risk factors as hypertension, proteinuria and severe pathological lesions to predict the long-term dialysis or death in patients with IgAN. 35 Another scoring system that was developed from a large Japanese multicenter study with more than 2,000 patients and 10-year followed-up, the risk factors for predict long-term renal outcomes included age, sex, family history of kidney disease, proteinuria, hypoalbuminuria, mild hematuria, low glomerular filtration rate and a high histological grade. 36 The recent Oxford classification of IgAN identified pathologic variables of prognostic significance for disease progression.37 A set of four kidney lesions, the MEST score (M, mesangial hypercellularity; E, endocapillary proliferation; S, segmental glomerulosclerosis/adhesion; T, tubular atrophy/interstitial fibrosis) was closely associated with kidney failure independent of clinical assessment which was also validated in other studies.38–40 Our study has suggested that measurement of baseline Gd-IgA1 can be used as an additional variable and improve power of these prediction systems in IgAN.
As in other studies, baseline proteinuria, kidney function and pathological lesions are also risk factors for disease progression. However, in this study we did not find baseline hypertension associated with kidney failure. This may be due to the small sample size or few end-point events to detect the difference. Another possible explanation is that these data are from a prospective database and blood pressure was well controlled. Several prospective studies have also observed the same phenomenon. 41–43
In summary, this is the first study to demonstrate that elevated Gd-IgA1 levels in Asian patients with IgAN may affect disease progression. Further studies are needed to determine if Gd-IgA1 levels change in the course of disease or respond to specific treatment regimens, and if these factors alter the overall prognosis in IgAN.
Materials and Methods
Patients and Healthy Controls
This study includes 275 patients with IgAN diagnosed from renal biopsy. All enrolled patients had been followed for a minimum of 12 months (range 12 to 96 months). The diagnosis was based upon the demonstration, by immunofluorescence, of IgA as the dominant or co-dominant immunoglobulin in mesangial deposits, as well as the lack of clinical or serological evidence of other inflammatory conditions, such as systemic lupus erythematosus, vasculitis, or Henoch–Schoenlein purpura. Complete clinical data, including age, gender, systolic/diastolic blood pressure, serum creatinine, total serum IgA, and 24-h total protein excretion were collected at the time of renal biopsy. The composite endpoint in this study was defined by a 50% decline in eGFR from the time of diagnosis ESRD or death, whichever occurred first. For the purpose of this study, ESRD was defined as eGFR < 15 ml/min/ 1.73 m2 or need for renal replacement therapy (such as hemodialysis, peritoneal dialysis, or renal transplantation). The eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) formula.44 All renal biopsy specimens were reviewed and graded by an independent pathologist who was blinded to patients’ outcomes. The histological lesions were classified as grade I to V as proposed by Haas when patients participated in this study.45 Haas grading I to III were classified as mild lesions, and IV and V as severe lesions. Hypertension was defined as systolic blood pressure of greater than 140 mm Hg, and/or diastolic blood pressure of greater than 90 mm Hg at resting, or use of anti-hypertension medication. All the patients receive a same therapy regimen including optimal blood pressure control target to less than 130/80mm Hg, RAS inhibition and steroids or other immunosuppressive agents for patients with persistent proteinuria. The blood pressure and proteinuria control were expressed as time-average mean artery pressure or time-average proteinuria. 43
The healthy population controls consisted of 499 residents of the same geographic area matched by age and gender with the IgAN patients. The healthy controls consisted of 342 males and 157 females. The mean age was 32.4±8.7 years. All individuals were at least 18 years old (range 19 to 55 years). The Institutional Review Boards at the Peking University First Hospital approved this study. Written informed consent was obtained from all study participants.
Detection of serum IgA and Gd-IgA1 by ELISA
Sera from patients, collected before renal biopsy, and from healthy controls were aliquoted and stored at −20°C until the time of assays. Serum IgA and Gd-IgA1 were quantified by ELISA, according to a protocol established earlier.15 Briefly, high-binding MaxiSorp 96-well plates (Nalge-Nunc, Rochester, NY) were coated overnight with 2.5 ug/ml F(ab’)2 fragment of goat anti-human IgA (Jackson Immuno-Research Labs., West Grove, PA) in phosphate-buffered saline (PBS). Coated plates were blocked with 1% bovine serum albumin (Sigma Chemical Company, St Louis, MO) in PBS-0.05%-Tween 20. Duplicates of two-fold dilutions of serum samples and standards were added and the plates were incubated overnight at room temperature. The sample dilutions ranged from 1:80,000 to 1:2,560,000 for total IgA, from 1:10,000 to 1:40,000 for plasma IgA1 and from 1:2,000 to 1:16,000 for Gd-IgA1. To quantitate the total IgA, a standard consisting of a pool of normal human sera previously calibrated for immunoglobulin isotype concentration (Binding Site, Birmingham, UK), was used at concentrations between 25 ng/ ml and 0.195 ng/ ml. For measurement of plasma IgA1, a standard consisting of native IgA1 purified by normal human plasma (EMD Chemicals Inc., Gibbstown, US) was used at concentrations between 500 ng/ ml and 0.686 ng/ ml. For measurement of Gd-IgA1, the standard consisting of a polymeric Gd-IgA1 protein 12 isolated from a patient with multiple myeloma was used. To determine the galactose-deficient glycans on IgA1, the terminal sialic acid from O-linked GalNAc on bound samples and the standard IgA1 myeloma protein were removed by incubation with 1 mU/well neuraminidase (Roche Diagnostic Corp., Indianapolis, IN) in 0.01 M acetate buffer pH 5 for 3h at 37°C. 6 After washing, 100 ul biotin-labeled HAA (1:500 dilution; Sigma) was added to each well and incubated for 3-h at 37°C. The total IgA was detected by incubation with biotin-labeled F(ab’)2 fragment of goat IgG anti-human IgA (BioSource, Camarillo, CA) for 3 h at 37°C. Total level of IgA1 was determined by incubation with affinipure F(ab’)2 fragment goat anti-human serum IgA (Jacksonimmuno, Baltimore Pike, West Grove, PA) at 4°C over night. After washing, 100 ul mouse anti-human monoclonal antibody (1:50,000 dilution; Sigma) was added to each well and incubated for 1 h at 37 °C. For IgA, total IgA1, and Gd-IgA1, the plates were further incubated with horseradish peroxidase-ExtrAvidin (Sigma) for 1 h at 37°C. The plates were then developed with the peroxidase chromogenic substrate o-phenylenediamine-H2O2 (Sigma). The color reaction was stopped with 1 M sulfuric acid and the absorbance was measured at 490 nm with an EL312 Bio-Kinetics microplate reader (Bio-Tek Instruments Inc., Winooski, VT). The amounts of total IgA and Gd-IgA1 in the tested samples were calculated with the DeltaSoft II program (BioMettalics Inc., Princeton, NJ) by interpolating the optical densities on calibration curves, constructed using serum IgA and a Gd-IgA1 myeloma protein. The results for total IgA were expressed as ug/ml. As the standard IgA1 myeloma protein is not entirely devoid of galactose, the expression of results in ug/ml does not precisely reflect the concentration of Gd-IgA1 in the sera. Therefore, we expressed the results in U/ml. One unit of Gd-IgA1 was de.ned as 1 ug of this standard Gd-IgA1 myeloma protein.15 To assess the degree of intra-assay variability, the HAA-ELISA was repeated with 108 randomly selected patients samples.
Statistical analyses
Summary statistics for normally distributed quantitative variables were expressed as means and standard deviations. For non-normally distributed variables, we used median and IQR, categorical data were summarized by ratios and percentages. Differences in means for continuous variables were compared using Student’s t-test (2 groups) or ANOVA (multiple groups), and differences in proportions were tested by a Chi-square test. Cox proportional hazards models were used to analyze the association of serum Gd-IgA1 levels and the primary outcome. Gd-IgA1 was highly skewed to the right in this group patients and natural log-transformation was used. Serum Gd-IgA1 was first analyzed as a continuous variable with hazard ratios (HRs) calculated per SD increment of natural log-transformed Gd-IgA1, and the Gd-IgA1 quartile as a categorical variable, with the lowest quartile defined as the reference group. The relationship between Gd-IgA1 and risk of endpoint was examined in unadjusted and multivariable adjusted Cox models. Proportional hazards assumption were verified by testing the interaction of survival time and lnGd-IgA1 and quartiles of Gd-IgA1 (p =0.23 and p =0.17, respectively) and by inspecting parallelism of estimated hazard functions. A two-sided p-value <0.05 was considered statistically significant. All statistical tests were performed using SPSS version 16.0.
Supplementary Material
Acknowledgements
This work was supported by the National Natural Science Foundation for Distinguished Young Scholars of China (Grant No. 30825021) to H.Z. and Peking University First Hospital Clinical Research Funds (2011) and by the National Natural Science Foundation for Innovative Research Groups of China (Grant No. 81021004) to M.Z. We appreciate the work of all colleagues and we are grateful for the participation of all of the patients and healthy control subjects. ZM, KK, AGG, and JN were supported in part by grants DK082753, DK078244, and DK083663 from the National Institutes of Health, USA.
Footnotes
Disclosure
None of the authors declared competing interests.
Conflict of Interest: None to declare
References
- 1.Levy M, Berger J. Worldwide perspective of IgA nephropathy. Am J Kidney Dis. 1988;12:340–347. doi: 10.1016/s0272-6386(88)80021-0. [DOI] [PubMed] [Google Scholar]
- 2.Nair R, Walker PD. Is IgA nephropathy the commonest primary glomerulopathy among young adults in the USA? Kidney Int. 2006;69:1455–1458. doi: 10.1038/sj.ki.5000292. [DOI] [PubMed] [Google Scholar]
- 3.Donadio JV, Grande JP. IgA nephropathy. N Engl J Med. 2002;347:738–748. doi: 10.1056/NEJMra020109. [DOI] [PubMed] [Google Scholar]
- 4.Smith AC, Molyneux K, Feehally J, et al. O-glycosylation of serum IgA1 antibodies against mucosal and systemic antigens in IgA nephropathy. J Am Soc Nephrol. 2006;17:3520–3528. doi: 10.1681/ASN.2006060658. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki H, Fan R, Zhang Z, et al. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest. 2009;119:1668–1677. doi: 10.1172/JCI38468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomana M, Matousovic K, Julian BA, et al. Galactose-deficient IgA1 in sera of IgA nephropathy patients is present in complexes with IgG. Kidney Int. 1997;52:509–516. doi: 10.1038/ki.1997.361. [DOI] [PubMed] [Google Scholar]
- 7.Novak J, Julian BA, Tomana M, et al. IgA glycosylation and IgA immune complexes in the pathogenesis of IgA nephropathy. Semin Nephrol. 2008;28:78–87. doi: 10.1016/j.semnephrol.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amore A, Cirina P, Conti G, et al. Glycosylation of circulating IgA in patients with IgA nephropathy modulates proliferation and apoptosis of mesangial cells. J Am Soc Nephrol. 2001;12:1862–1871. doi: 10.1681/ASN.V1291862. [DOI] [PubMed] [Google Scholar]
- 9.Barratt J, Feehally J, Smith AC. Pathogenesis of IgA nephropathy. Semin Nephrol. 2004;24:197–217. doi: 10.1016/j.semnephrol.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Barratt J, Feehally J. IgA nephropathy. J Am Soc Nephrol. 2005;16:2088–2097. doi: 10.1681/ASN.2005020134. [DOI] [PubMed] [Google Scholar]
- 11.Smith AC, de Wolff JF, Molyneux K, et al. O-glycosylation of serum IgD in IgA nephropathy. J Am Soc Nephrol. 2006;17:1192–1199. doi: 10.1681/ASN.2005101115. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki H, Moldoveanu Z, Hall S, et al. IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest. 2008;118:629–639. doi: 10.1172/JCI33189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen AC, Bailey EM, Brenchley PE, et al. Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: observations in three patients. Kidney Int. 2001;60:969–973. doi: 10.1046/j.1523-1755.2001.060003969.x. [DOI] [PubMed] [Google Scholar]
- 14.Hiki Y, Odani H, Takahashi M, et al. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int. 2001;59:1077–1085. doi: 10.1046/j.1523-1755.2001.0590031077.x. [DOI] [PubMed] [Google Scholar]
- 15.Moldoveanu Z, Wyatt RJ, Lee JY, et al. Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int. 2007;71:1148–1154. doi: 10.1038/sj.ki.5002185. [DOI] [PubMed] [Google Scholar]
- 16.Xu LX, Zhao MH. Aberrantly glycosylated serum IgA1 are closely associated with pathologic phenotypes of IgA nephropathy. Kidney Int. 2005;68:167–172. doi: 10.1111/j.1523-1755.2005.00390.x. [DOI] [PubMed] [Google Scholar]
- 17.Coppo R, Amore A. Aberrant glycosylation in IgA nephropathy (IgAN) Kidney Int. 2004;65:1544–1547. doi: 10.1111/j.1523-1755.2004.05407.x. [DOI] [PubMed] [Google Scholar]
- 18.Novak J, Tomana M, Kilian M, et al. Heterogeneity of O-glycosylation in the hinge region of human IgA1. Mol Immunol. 2000;37:1047–1056. doi: 10.1016/s0161-5890(01)00019-0. [DOI] [PubMed] [Google Scholar]
- 19.Bartosik LP, Lajoie G, Sugar L, et al. Predicting progression in IgA nephropathy. Am J Kidney Dis. 2001;38:728–735. doi: 10.1053/ajkd.2001.27689. [DOI] [PubMed] [Google Scholar]
- 20.Shimozato S, Hiki Y, Odani H, et al. Serum under-galactosylated IgA1 is increased in Japanese patients with IgA nephropathy. Nephrol Dial Transplant. 2008;23:1931–1939. doi: 10.1093/ndt/gfm913. [DOI] [PubMed] [Google Scholar]
- 21.Camilla R, Suzuki H, Dapra V, et al. Oxidative stress and galactose-deficient IgA1 as markers of progression in IgA nephropathy. Clin J Am Soc Nephrol. 2011;6:1903–1911. doi: 10.2215/CJN.11571210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barratt J, Smith AC, Feehally J. The pathogenic role of IgA1 O-linked glycosylation in the pathogenesis of IgA nephropathy. Nephrology (Carlton) 2007;12:275–284. doi: 10.1111/j.1440-1797.2007.00797.x. [DOI] [PubMed] [Google Scholar]
- 23.Tam KY, Leung JC, Chan LY, et al. Macromolecular IgA1 taken from patients with familial IgA nephropathy or their asymptomatic relatives have higher reactivity to mesangial cells in vitro. Kidney Int. 2009;75:1330–1339. doi: 10.1038/ki.2009.71. [DOI] [PubMed] [Google Scholar]
- 24.Novak J, Tomana M, Matousovic K, et al. IgA1-containing immune complexes in IgA nephropathy differentially affect proliferation of mesangial cells. Kidney Int. 2005;67:504–513. doi: 10.1111/j.1523-1755.2005.67107.x. [DOI] [PubMed] [Google Scholar]
- 25.Gharavi AG, Moldoveanu Z, Wyatt RJ, et al. Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. J Am Soc Nephrol. 2008;19:1008–1014. doi: 10.1681/ASN.2007091052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiryluk K, Moldoveanu Z, Sanders JT, et al. Aberrant glycosylation of IgA1 is inherited in both pediatric IgA nephropathy and Henoch-Schonlein purpura nephritis. Kidney Int. 2011;80:79–87. doi: 10.1038/ki.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin X, Ding J, Zhu L, et al. Aberrant galactosylation of IgA1 is involved in the genetic susceptibility of Chinese patients with IgA nephropathy. Nephrol Dial Transplant. 2009;24:3372–3375. doi: 10.1093/ndt/gfp294. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki H, Kiryluk K, Novak J, et al. The pathophysiology of IgA nephropathy. J Am Soc Nephrol. 2011;22:1795–1803. doi: 10.1681/ASN.2011050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manno C, Torres DD, Rossini M, et al. Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol Dial Transplant. 2009;24:3694–3701. doi: 10.1093/ndt/gfp356. [DOI] [PubMed] [Google Scholar]
- 30.Wada Y, Dell A, Haslam SM, et al. Comparison of methods for profiling O-glycosylation: Human Proteome Organisation Human Disease Glycomics/Proteome Initiative multi-institutional study of IgA1. Mol Cell Proteomics. 2010;9:719–727. doi: 10.1074/mcp.M900450-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi K, Wall SB, Suzuki H, et al. Clustered O-glycans of IgA1: defining macro- and microheterogeneity by use of electron capture/transfer dissociation. Mol Cell Proteomics. 2010;9:2545–2557. doi: 10.1074/mcp.M110.001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi K, Smith AD, Poulsen K, et al. Naturally ocurring structural isomers in serum IgA1 Hinge-region O-glycosylation. J Proteome Res. 2011 doi: 10.1021/pr200608q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wada Y, Tajiri M, Ohshima S. Quantitation of saccharide compositions of O-glycans by mass spectrometry of glycopeptides and its application to rheumatoid arthritis. J Proteome Res. 2010;9:1367–1373. doi: 10.1021/pr900913k. [DOI] [PubMed] [Google Scholar]
- 34.Berthoux F, Mohey H, Laurent B, et al. Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol. 2011;22:752–761. doi: 10.1681/ASN.2010040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goto M, Wakai K, Kawamura T, et al. A scoring system to predict renal outcome in IgA nephropathy: a nationwide 10-year prospective cohort study. Nephrol Dial Transplant. 2009;24:3068–3074. doi: 10.1093/ndt/gfp273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manno C, Strippoli GF, D'Altri C, et al. A novel simpler histological classification for renal survival in IgA nephropathy: a retrospective study. Am J Kidney Dis. 2007;49:763–775. doi: 10.1053/j.ajkd.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Cattran DC, Coppo R, Cook HT, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–545. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 38.Herzenberg AM, Fogo AB, Reich HN, et al. Validation of the Oxford classification of IgA nephropathy. Kidney Int. 2011;80:310–317. doi: 10.1038/ki.2011.126. [DOI] [PubMed] [Google Scholar]
- 39.Edstrom HS, Soderberg MP, Berg UB. Predictors of outcome in paediatric IgA nephropathy with regard to clinical and histopathological variables (Oxford classification) Nephrol Dial Transplant. 2011 doi: 10.1093/ndt/gfr339. [DOI] [PubMed] [Google Scholar]
- 40.Shi SF, Wang SX, Jiang L, et al. Pathologic predictors of renal outcome and therapeutic efficacy in IgA nephropathy: validation of the oxford classification. Clin J Am Soc Nephrol. 2011;6:2175–2184. doi: 10.2215/CJN.11521210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coppo R, Peruzzi L, Amore A, et al. IgACE: a placebo-controlled, randomized trial of angiotensin-converting enzyme inhibitors in children and young people with IgA nephropathy and moderate proteinuria. J Am Soc Nephrol. 2007;18:1880–1888. doi: 10.1681/ASN.2006040347. [DOI] [PubMed] [Google Scholar]
- 42.Praga M, Gutierrez E, Gonzalez E, et al. Treatment of IgA nephropathy with ACE inhibitors: a randomized and controlled trial. J Am Soc Nephrol. 2003;14:1578–1583. doi: 10.1097/01.asn.0000068460.37369.dc. [DOI] [PubMed] [Google Scholar]
- 43.Reich HN, Troyanov S, Scholey JW, et al. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol. 2007;18:3177–3183. doi: 10.1681/ASN.2007050526. [DOI] [PubMed] [Google Scholar]
- 44.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 45.Haas M. Histologic subclassification of IgA nephropathy: a clinicopathologic study of 244 cases. Am J Kidney Dis. 1997;29:829–842. doi: 10.1016/s0272-6386(97)90456-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.