Abstract
Emerging evidence supporting the activation of the Akt-mTOR signaling network in head and neck squamous cell carcinoma (HNSCC) progression has provided the rationale for exploring the therapeutic potential of inhibiting this pathway for HNSCC treatment. Indeed, rapamycin, a clinically relevant mTOR inhibitor, promotes the rapid regression of HNSCC-tumor xenografts in mice. However, rapamycin does not affect the growth of HNSCC cells in vitro, thus raising the possibility that, as for other cancer types, rapamycin may not target cancer cells directly but may instead act on a component of the tumor microenvioronment, such as tumor-associated vasculature. Here, we utilized a retro-inhibition approach to assess the contribution of cancer cell-autonomous actions of rapamycin to its antitumor activity in HNSCC. A rapamycin-resistant form of mTOR (mTOR-RR) was expressed in HNSCC cells, while retaining the wild-type (rapamycin-sensitive) mTOR alleles in host-derived endothelial and stromal cells. Expression of mTOR-RR prevented the decrease in phospho-S6 levels caused by rapamycin through mTOR in HNSCC cells but not in stromal cells, and rendered HNSCC xenografts completely resistant to the antitumoral activity of rapamycin. This reverse-pharmacology strategy also enabled monitoring the direct consequences of inhibiting mTOR in cancer cells within the complex tumor micro-environment, which revealed that mTOR controls the accumulation of HIF-1α and the consequent expression of VEGF and a glucose transporter, Glut-1, in HNSCC cells. These findings indicate that HNSCC cells are the primary target of rapamycin in vivo, and provide evidence that its anti-angiogenic effects may represent a downstream consequence of mTOR inhibition in HNSCC cells.
Keywords: mTOR, xenograft, signal transduction, human squamous cell carcinoma, drug discovery, rapamycin, lentivirus
Introduction
A better understanding of the molecular basis of cancer has provided the rationale for the development of novel therapeutic strategies to prevent and treat human malignancies. In this regard, emerging evidence suggests that the aberrant activity of the Akt-mTOR signaling pathway is a frequent event in some of the most prevalent human cancers (1, 2). Indeed, most tumors display overactivity of growth factor receptors or mutations in the GTPase Ras that cause the activation of PI3K, a lipid kinase that acts upstream of Akt and mTOR (3). Amplification and mutations of the PI3K p110α catalytic subunit and mutations of its p85 regulatory subunit, which results in the activation of Akt, occur frequently in colon and ovarian cancer, as well as in squamous carcinomas of the head and neck (HNSCC) (4-10). Genetic alterations that inactivate PTEN, a phosphatase that degrades PIP3, drives chronic hyperactivation of the PI3K signaling cascade, which includes the protein kinases, Akt and mTOR. Germline PTEN mutations trigger tissue overgrowth syndromes, Cowden's disease, in which patients develop multiple gastrointestinal polyps, which occasionally progress to frank malignancies (11, 12). A high incidence of PTEN mutations is also observed in endometrial carcinoma and glioblastoma . In fact, the importance of PTEN as a tumor suppressor gene in human cancer is rivaled only by that of p53. Surprisingly, three other tumor suppressor genes, tsc1 and tsc2, involved in tuberous sclerosis syndrome, and LKB1, which is associated with Peutz-Jeghers syndrome (13, 14), encode proteins that downregulate mTOR activity (15). Thus, while hereditary mutations in genes leading to overactivity of Akt-mTOR increase cancer susceptibility, the acquisition of sporadic mutations or altered expression and activity of molecules regulating the Akt-mTOR pathway are frequent events in human malignancies, underscoring the importance of this signaling route in cancer development.
Because chemically-induced animal models of squamous carcinogenesis display elevated Akt activity (16), we focused our attention on the role of Akt-mTOR pathway in HNSCC, a cancer affecting primarily the oral cavity and pharynx that is diagnosed in approximately 31,000 patients each year in the United States alone (17). Surprisingly, we found that the activation of Akt and mTOR is highly prevalent in HNSCC, as judged by the accumulation of the phosphorylated-active-forms of Akt and ribosomal protein S6, the latter a downstream target for mTOR (18). These findings and emerging data indicating that the activation of Akt-mTOR is associated with a poor prognosis in HNSCC (19), prompted us to explore whether this signaling pathway is a suitable clinical target in HNSCC. We observed that inhibition of PDK1, a kinase acting upstream of Akt, by a novel chemotherapeutic agent, UCN-01, prevents the growth of human HNSCC-derived cell lines in vitro, and effectively inhibits tumor growth in xenografted, immunodeficient mice (6, 20). However, PDK1 performs numerous tissue specific functions, including the regulation of glucose metabolism in response to insulin (21), which may limit the clinical benefits of blocking the activation of Akt by targeting PDK1 in HNSCC.
Thus, we next focused our attention on rapamycin, which inhibits mTOR specifically and has already shown promising results in both preclinical and clinical trials in different solid tumors (22, 23). Using a number of HNSCC xenograft models, we observed that rapamycin exerts a potent antitumoral effect, leading to a rapid decrease in tumor vascularity and the death of cancer cells, thus provoking tumor regression (18). Rapamycin derivatives also diminish microscopic residual disease and enhance the effectiveness of EGFR inhibitors in experimental SCC models (24, 25). Unexpectedly, we show here that rapamycin does not exert growth suppressive or pro-apoptotic activities in HNSCC cells in vitro, thus raising the possibility that the antitumoral effects of rapamycin may result from its effects on the stromal cells within the tumor microenvironment rather than by acting on HNSCC cells directly.
To address this possibility, we utilized a reverse-pharmacology approach, which involved the lentiviral expression of a rapamycin-insensitive form of mTOR in HNSCC cells. This experimental strategy revealed that cancer cells are the primary targets of rapamycin in vivo, and also enabled us to monitor the direct consequences of inhibiting mTOR in cancer cells. Indeed, we now provide evidence that the blockade of mTOR in HNSCC cells prevents the accumulation of the transcription factor HIF-1α, thereby reducing the expression of key molecules involved in cellular glucose metabolism, such as the cell surface expression of glucose transporter, Glut-1 and the maintenance of the tumor vascularity, the latter by decreasing the expression of proangiogenic mediators such as VEGF.
Material and Methods
Cells culture and reagents
The HNSCC cell lines HN12 and CAL27 were maintained as described (18, 26). For in vitro studies, HNSCC cells were grown to 60-70% confluence, serum-starved for 24h, and treated with rapamycin or LY294002 (Calbiochem; San Diego, CA) for 24-48h. Tumor necrosis factor alpha (TNF-α) and interleukin 1β (IL-1β) were obtained from Sigma (St. Louis, MO). For in vivo experiments, rapamycin was provided by the Developmental Therapeutics Program (NCI), and diluted in an aqueous solution as reported (18, 27).
Antibodies
Rabbit monoclonal anti-phospho-threonine 308-Akt (pT308-Akt), and rabbit polyclonal antisera against S6 and phospho-S6 (p-S6), Akt, phospho-serine 473-Akt (pS473-Akt), phospho-ERK1/2 (p-ERK1/2), 4E-BP1, phospho-4E-BP1 (pT37/46-4E-BP1), and cleaved caspase-3 were purchased from Cell Signaling Technology (Beverly, MA). Rabbit polyclonal antisera against VEGF was purchased from Santa Cruz Biothecnology (Santa Cruz, CA); rat monoclonal anti-CD31, mouse monoclonal anti-HIF-1α and anti-E-cadherin from BD Pharmingen (San Diego, CA); and mouse monoclonal anti-Glut-1 from Abcam (Cambridge, MA). All antibodies were used for Western blot analysis or immunohistochemistry (IHC) at a dilution of 1:1000 and 1:100, respectively.
Lentiviral-gene expression
The cDNAs encoding AU1 tagged-mTOR wild-type and its rapamycin resistant mutant (mTOR-RR) (28) and GFP control were subcloned into the lentiviral expression vector pWPIGW, and recombinant lentiviruses were packaged using Vesicular Stomatitis Virus envelope glycoprotein (VSV-G) and used to infect HNSCC cells (29).
Treatment of tumor xenografts in athymic nu/nu mice
All animal studies were carried out according to NIH approved protocols, in compliance with the Guide for the Care and Use of Laboratory Animals. Female athymic (nu/nu) nude mice (Harlan Sprague Dawley, Frederick, MD), 5–6 weeks of age and weighing 18–20 g, were used in the study, and housed in appropriate sterile filter-capped cages, fed and watered ad libitum. Two million control or lentiviral-infected HN12 and CAL27 cells were used to induce HNSCC tumor xenografts, and tumor growth analysis was performed as described (30). Drug treatment was initiated when tumor volume reached approximately 100-150 mm3 (31). For each experiment, tumor bearing animals were randomly divided into two groups of 20 animals and injected intraperitoneally with rapamycin (10 mg/kg/day) or diluent for 5 consecutive days (27), and monitored daily thereafter for tumor growth and body weight. These experiments were repeated three independent times. At the indicated time points, animals were sacrificed and tissues collected. Tissues were either lysed in protein lysis buffer, fixed and paraffin embedded, or frozen and embedded in OCT compound (Optimal cutting temperature, Tissue-Tek, Sakura Finetek, CA) (18).
Immunohistochemistry (IHC) and immunofluorescence (IF) double staining
For IHC, paraffin-embedded tissues slides were deparaffinized, hydrated, and rinsed with PBS. Antigen retrieval was performed using a citrate buffer (pH 6) in a microwave for 20 min and processed as described (18). Primary antibodies were diluted in blocking solution (2% BSA in PBS-0.1% Tween20), using a biotinylated secondary antibody (Vector Laboratories, 1:400) followed by the reaction with the ABC complex (Vector Stain Elite, ABC kit, Vector Laboratories), and color development using 3,3- diaminobenzidine (Sigma FASTDAB tablet, Sigma Chemical, St. Louis, MO, USA) under microscopic observation. Tissues were counterstained with Mayer's hematoxylin, dehydrated and mounted.
For IF staining, OCT embedded frozen tissues were cut (15μm) onto silanated glass slides, air-dried and stored at -80°C. Cryosections were thawed at room temperature, hydrated, washed with PBS, and incubated in blocking solution (5% heated-inactivated goat serum in 0.1% triton X-100 PBS) for 1h followed by incubation with the first primary antibody diluted 1:100 in blocking solutions at 4°C, overnight. After washing, slides were sequentially incubated with the biotinylated secondary antibody (Vector Laboratories, 1:300) for 1h, followed by the fluorophore-labeled avidin antibody (Vector Stain Elite, ABC kit, Vector Laboratories) for 30 min at room temperature. The slides were washed and incubated in avidin/biotin blocking kit (Vector Laboratories), before repeating the staining process with the second primary antibody. The tissues were then mounted in Vectashield mounting medium with DAPI (Vector Laboratories), and images captured and analyzed using a Carl Zeiss Axioplan 2 microscope.
Western Blotting
Cells or small pieces of tissues were sensed with PBS and rapidly lysed with protein lysis buffer (62.5mM Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 50 mM DTT), and transferred immediately to microcentrifuge tubes and sonicated for 20 sec. Protein yield was quantified using the DC protein assay kit (Bio-Rad, Hercules, CA). Equivalent amounts protein (50 μg) were separated by SDS-PAGE, transferred to PVDF membranes, and immunodetection performed as described (18).
Cell Proliferation assay and MTT assay
[3H] thymidine (ICN Pharmaceuticals, Inc., Costa Mesa, CA) uptake was performed as described (20) using HNSCC cells (1–2×104 per well) seeded in 24-well plates. For MTT assays, cells (~2,000 cells per well) were seeded in 96-well plates, cultured for 12h, serum starved overnight, and treated with rapamycin or LY294002. Cells were also kept in serum containing media and left untreated or treated with IL-1β (30 ng/ml) or TNF-α (50 ng/ml) in the absence or presence of rapamycin (100 nM), monitoring their growth for up to 4 days. Cell growth was assessed by the methyl thiazole tetrazolium (MTT) assay (Promega, Madison, WI), as described by the manufacturer. Each experiment consisted of 3-4 replicate wells for each dose of treatment, and each experiment was performed at least 3 times.
Cell Cycle Analysis
Cell cycle analysis was performed as described (30). Briefly, cells were harvested after 24h incubation with vehicle or rapamycin in DMEM with 1% FBS, fixed in 70% ethanol, and stained with 50 μg/ml of propidium iodide and 0.1 μg /ml of RNase A in PBS. DNA content of cells was quantified on a Becton Dickinson FACScan and analyzed using Cell Quest software (Immunocytometry system; Becton Dickinson).
Microvessel density analysis
Microvessels were identified by CD31 IF staining. Images were captured from different areas in each section and morphometric analysis was performed using the MetaMorph 4.0 Imaging system (Molecular Devices Corporation, Sunnydale, CA). Microvessel density was calculated by dividing the total perimeter of microvessels by the number area counted. Values reported for each experimental condition correspond to the average values obtained from seven individual images.
Active caspase-3 immunofluorescent analysis
The images of positive immunofluorescent detection from cleaved caspase-3 antibody were captured from at least 5 different areas in each tissue slide and saved in TIFF format. After adjusting the fluorescent signal-noise threshold of the images, the total area presenting fluorescent signal was measured using the ImageJ program (NIH, Bethesda, MD). Values reported correspond to the mean ± SEM of values obtained from 4 samples for each experimental condition.
Statistical Analysis
ANOVA followed by Bonferroni's multiple comparison tests were used to analyze the differences of tumor mass volume between experimental groups. Data analysis was performed with using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA); p values < 0.05 were considered statistically significant.
Results
Inhibition of mTOR by rapamycin alone does not affect the proliferation of HNSCC cells in vitro
Human HNSCC and their derived cell lines exhibit high activity of the EGFR/Akt/mTOR intracellular signaling axis (6-8, 26, 32, 33) which provided the rationale for exploring the therapeutic potential of inhibiting key molecular components of this signaling pathway. We have recently observed that rapamycin exerts a potent antitumoral effect in a variety of HNSCC xenograft models (18). Surprisingly, the results obtained from in vitro studies in the same HNSCC cell lines used in these xenograft models did not reflect the effect of rapamycin in vivo. For example, the effect of rapamycin on a typical HNSCC cell line, HN12 (18), was characterized by the decrease phosphorylation of the downstream targets of mTOR, ribosomal protein S6 (phospho-S6, pS6) and eukaryotic translation initiation factor 4E binding protein 1 (phospho-4EBP1) in a dose-dependent manner (Fig. 1A). In contrast, rapamycin did not have any demonstrable effect on DNA-synthesis, as determined by using [3H] thymidine incorporation, in spite of the escalation of rapamycin concentration up to 1 μM (not shown). Similar results were obtained from cell cycle analysis, which showed that HNSCC cells treated with rapamycin do not display any significant changes in any of the phases of the cell cycle (Fig 1B). In parallel experiments, we also failed to observe an increase in apoptotic cell death upon rapamycin treatment, as judged by TUNEL assays and the accumulation of active caspase-3 (data not shown). Furthermore, rapamycin did not affect HNSCC cell proliferation as judged by MTT assays, even when the cells were challenged by prolonged (48h) exposure to this drug (Fig. 1C). In contrast, an inhibitor that targets PI3K, LY294002 (25 μM), when used as positive control, caused more than 50 % inhibition in both DNA-synthesis and cell proliferation under identical experiment condition. Similar results were obtained in all other HNSCC cell lines tested such as HN13, Hep-2 and CAL27 (not shown) (18). Furthermore, rapamycin did not suppress the growth of these cells in serum (10% fetal calf serum)-containing medium (Supp. Fig. 1A and data not shown). Rapamycin modestly diminished the proliferation of HNSCC cells when exposed to inflammatory cytokines often found in tumor microenvironment, such as IL-1β and TNF-α, which alone did not exert any antiproliferative effect (Fig. 1 D and Supp. Fig. 1A). Thus, in contrast to the remarkable results obtained from HNSCC-bearing mice in vivo (18), rapamycin alone does not reduce HNSCC cell proliferation or promote their death in vitro, even after prolonged treatment.
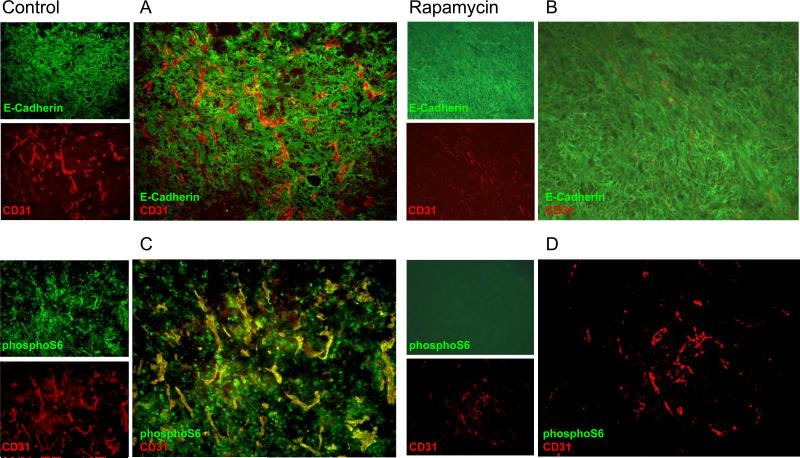
Figure 1.
Rapamycin inhibits the mTOR signaling pathway in HNSCC cells but does not affect cell proliferation in vitro. A, Inhibition of constitutively activate mTOR downstream targets, pS6 and p4E-BP1 by rapamycin (RP) is dose dependent in HNSCC cells without affecting the expression levels of S6, 4E-BP1, and Akt, or the detection of the phosphorylated species of Akt, as judged by Western blotting (WB) with the indicated antibodies. B, Cell cycle distribution represented as the fraction of HNSCC cells in G1, S, and G2/M phase upon rapamycin treatment for 24 h at the indicated concentrations was evaluated by FACS analysis after nuclear labeling of cells with propidium iodide. C and D Cytotoxic effects of rapamycin in HNSCC cell lines were determined by a colorimetric assay, MTT. C, After serum starvation for 24 h, cells were exposed to rapamycin at the indicated dose for 48 h before performing this assay. LY294002 (25 μM, LY) was used throughout as a positive control for PI3K/Akt/mTOR pathway inhibition. D, cells cultured in serum containing media were treated with interleukin 1β (IL-1β; 30 ng/ml) and rapamycin (100 nM) or vehicle for the indicated days. These assays were performed in triplicate. Similar results were obtained in multiple repetitions of these experiments (at least three times). *** p< 0.001
Rapamycin inhibits the mTOR pathway in both tumor and endothelial cells in vivo
The administration of rapamycin in vivo resulted in a reduction in intratumoral blood vessel density (18), consistent with the antiangiogenic effect of rapamycin in tumor angiogenesis models (34). This observation raised the possibility that the antitumor activity of rapamycin reflects a significant, perhaps dominant, effect on the tumor microenvironment, especially the surrounding stromal and endothelial cells. A dominant role for drug-induced alterations in the tumor microenvironment could explain the above observations that rapamycin does not exert a strong, direct antiproliferative effect on the HNSCC cells in tissue culture. To explore this possibility, we developed an IF double staining procedures that enabled monitoring the changes of the status of the mTOR pathway and the onset of apoptosis in response to rapamycin in both HNSCC and endothelial cells.
Initially, immunostaining for the endothelial cell marker CD31 in tumor tissues from control HN12 xenografts revealed the presence of an abundant vascular supply in the HNSCC tumor-bearing areas (Fig. 2A, red). The HNSCC tumor cells were recognized by E-cadherin immunostaining (Fig. 2A, green). As shown in Figure 2B, the level of CD31 staining was markedly diminished in tumors from rapamycin-treated mice after 3-4 days of drug treatment, indicating that rapamycin disrupts the formation of blood vessels that support the growth of these tumors. The molecular consequences of mTOR inhibition in the tumor cells were clearly observed upon examination of the expression of the phosphorylated form of S6 (pS6). High levels of pS6 were observed in both tumors and endothelial cells (Fig. 2C, green). The treatment with rapamycin caused a dramatic reduction in pS6 expression in both tumor cells, and the tumor-associated endothelial cells (Fig. 2D).
Figure 2.
Rapamycin treatment inhibits mTOR activity in both tumor and stromal cells in HNSCC xenografts. A and B, Double IF staining of anti- E-cadherin (in green) and anti- CD31, the endothelial cell marker (in red) were performed to demonstrate the architecture and relationship of tumor cells and blood vessels, respectively, in control (A) and rapamycin treated group (B). C and D, Expression of phosphorylated S6 in tumor tissues in control (C) and rapamycin treated mice (D) were detected by IF against pS6 antibody (in green) after two days of rapamycin treatment (10 mg/kg/day). CD31 (in red), was used for double IF labeling. Vehicle was used for injection in the control group. Representative IF experiments are depicted.
Rapid appearance of apoptotic tumor but not endothelial cells upon rapamycin treatment
In general, apoptosis leading to cell death is initiated by the activation of a family of intracellular cysteine proteases, known as caspases, among which caspase-3 is thought to play a critical role in executing the apoptotic process (35). Caspase-3 is synthesized as a pro-enzyme, and its activation by upstream caspases involves its cleavage at an aspartic acid residue (35). Thus, we utilized an antibody recognizing only the cleaved form of caspase-3 to specifically identify cells undergoing apoptosis by the dual labeling of active caspase and tumor and endothelial cells. Clusters of cleaved caspase-3 positive stained cells accumulated as early as two days of rapamycin treatment. These cleaved caspase-3 positive cells did not exhibit overlapping immnunostaining with CD31 positive (endothelial) cells, but a complete overlap was instead observed with E-cadherin, which stains HNSCC cells, as judged by the yellowish to orange color in the merged photomicrographs. Parallel quantitative analysis of blood vessels in the xenograft tissue demonstrated a progressive reduction of blood vessel density in response to rapamycin (Fig. 3B). These observations suggested that rapamycin directly affects the viability of HNSCCcells, rather than causing tumor cell death secondary to its anti-angiogenic effects.
Figure 3.
Induction of apoptosis and anti-angiogenic effects of rapamycin in HNSCC xenograft model in vivo. A, Apoptosis was determined in tumor tissues by the activation of caspase cascade as detected by IF against the cleaved form of caspase-3. Double IF labeling with anti-CD31 and anti-E-cadherin were performed to discriminate between endothelial cells and tumor cells, respectively. Note the overlapping immunodetection of cleaved caspase-3 and tumor cells (lower panels) but not with the endothelial cells (upper panels). B, Quantitative analysis of blood vessel density in tumor tissues obtained from rapamycin treated animals during the course of the treatment. Tissues were collected at the indicated time after initiating the rapamycin treatment and processed for fluorescent labeling of endothelial cells with CD31 antibody. The images from different 8 microscopic fields were taken and the blood vessel density was analyzed by Metamorph 4.0 as described in detail in Materials and Methods. Column, means blood vessel density in arbitrary unit; bars, SE
A rapamycin-resistant mTOR mutant defines the cellular target of rapamycin in HNSCC
To address whether rapamycin acts primarily on HNSCC cells in vivo, we used previously described mTOR mutants that retain signaling functions in the presence of rapamycin (36). The rapamycin-resistant mTOR mutant used in these studies contains a Ser→Ile substitution in the FKBP12-rapamycin-binding (FRB), which greatly decreases the binding affinity of the inhibitory immunophilin-drug complex to mTOR (37)(Fig. 4A) (28). To generate stable HNSCC cells overexpressing wild-type or rapamycin-resistant mTOR (mTOR-RR) above endogenous mTOR levels we used lentiviral gene delivery, which overcomes the low transfection and selection efficiency of human HNSCC cells (Fig 4B). Western blot analysis of pS6 in rapamycin-treated HN12 cells revealed that, indeed, expression of mTOR-RR but not wild-type mTOR or GFP, as a control, rescues HNSCC from the inhibitory effect of rapamycin on the mTOR pathway (Fig. 4B).
Figure 4.
The lentiviral expression of the rapamycin-insensitive mTOR mutant (mTOR-RR) reverts the biochemical effects of rapamycin in HNSCC cells, and reveals that HNSCC cells are the primary targets of rapamycin in HNSCC xenografts. A, Cartoon shows the domain structure of mTOR. The rapamycin-insensitive mTOR mutant was obtained by mutating the amino acid at position Ser 2035 located in the FRB domain for Ile, which disrupts the binding of rapamycin-FKBP12 complex to wild type (wt) mTOR. B, The mTOR-RR was expressed in HNSCC cell lines by lentiviral gene delivery, and cells were left untreated (-) or exposed to rapamycin (RP)(+) for 30 min. The expression of mTOR and the levels of total and phosphorylated S6 were analyzed Western blotting. C, Cartoon depicting the experimental design. HNSCC cell lines expressing mTOR-RR and GFP or mTOR-WT as controls were injected into the flanks of nude mice. After tumor development, rapamycin treatment was initiated for 5 consecutive days. The tissues were collected at every day of treatment for further analysis. D, Top panel; Double IF staining of pS6 (in green) and CD31 (in red) were performed after two days of rapamycin treatment. Note the reduction of pS6 in the tissues from the control group expressing GFP (a), but not after expressing the mTOR-RR (b). The architecture of cancer and endothelial cells within the tumor mass was documented by the fluorophore labeling by E-cadherin (in green) and CD31 (in red), respectively, as shown in panels c and d. In parallel, the apoptotic response to rapamycin treatment detected by the IF staining of cleaved caspase-3 (in red) was found to overlap with the E-cadherin staining (in green) of tumor cells (e) in the control group but not in xenografts of mTOR-RR expressing cells (f).
Rapamycin induces apoptosis in HNSCC tumor cells but not in tumor cells expressing mTOR-RR
We subsequently performed xenograft experiments with the mTOR-RR-expressing HNSCC cell lines in order to determine whether the in vivo antitumor effect of rapamycin was tumor cell autonomous, or due to the disruption of tumor cell extrinsic signals delivered from the microenvironment. The experimental design is depicted Fig. 4C. HN12 cells expressing GFP, mTOR-WT and mTOR-RR form were transplanted into nude mice, and the animals were treated with rapamycin or vehicle and sacrificed at each day of treatment. Immunostaining of CD31 and pS6 demonstrated that rapamycin reduces the levels of pS6 and the vascular supply in control animals bearing xenografts of HN12 wild-type mTOR cells (Fig 4D). In contrast, the pS6 staining the mTOR-RR-expressing HN12 tumors remained elevated in rapamycin-treated animals (Fig 5B). The density of blood vessels in these mTOR-RR-expressing tumors was also higher than in the control group (Fig. 4D and 5A). Moreover, the massive accumulation of apoptotic cells caused by rapamycin treatment defined by the positive immuno-labeling of active caspase-3 in tumors from HN12-control cells was nearly absent in tumors from HN12-mTORRR cells (Fig 4D and 5C). Thus, the pro-apoptotic effect of rapamycin in HNSCC tumor cells in vivo is virtually abrogated by expression of a rapamycin-resistant mTOR mutant exclusively in the xenografted cancer cells.
Figure 5.
The mTOR mutant reverts the anti-tumoral effect of rapamycin in HNSCC xenografts. A, The quantitative analysis of blood vessel density for each group was performed at the indicated days upon initiation of the rapamycin treatment as described above. Column, means blood vessel density in arbitrary unit; bars, SE. B, Tissue lysates extracted from HNSCC xenograft expressing mTOR-RR and control constructs (GFP) after treatment with vehicle control or rapamycin for 4 consecutive days were examined by Western blot analysis with antibodies against HIF-1α, S6, and its phosphorylated form, as indicated. C, Active caspase-3 immunofluorescence staining from GFP (control) and mTOR-RR group at the indicated days as described in A. The images from 8 different microscopic fields were taken and the fluorescence intensity analyzed by Metamorph 4.0 as detail described in Materials and Methods. Column, means fluorescence intensity in arbitrary unit; bars, SEM. D&E, HNSCC cell lines (HN12 and Cal27) expressing the mTOR-WT, mTOR-RR and GFP were injected subcutaneously into nude mice, and animals were treated with rapamycin or vehicle control. Tumor volumes were determined as described in Materials and Methods. Column, mean tumor volume of HN12 and CAL27 xenografts from the GFP, mTOR-WT and mTOR-RR groups at day 23. The mean tumor volume of the mTOR-RR groups at day 30 and 33 for HN12 and CAL27 HNSCC cells, respectively, when they achieve a volume similar to that of the their control xenografts, were also included; bars, SEM. The significance of the differences between each group was analyzed by ANOVA and Bonferroni's multiple comparison tests. Statistically significance at the indicated p value in each vehicle and rapamycin treated mice are shown, NS represents no significant difference between group.
Rapamycin reduces the tumor volume in HNSCC xenografts by acting directly on cancer cells
The resistance to the pro-apoptotic and anti-angiogenic effects caused by rapamycin treatment in tumors expressing mTOR-RR prompted us to assess the long-term effects of rapamycin in this xenograft model. We observed a dramatic reduction in tumor volume in the rapamycin-treated animals bearing HN12-GFP or HN12-mTOR-WT tumor xenografts (Fig. 5D). In contrast, rapamycin had little effect on the tumor volume in HN12-mTOR-RR-engrafted mice. After 23 days of treatment, the difference in rapamycin sensitivity between the HN12-mTORRR-derived tumors and the HN12-GFP- or HN12-mTOR-WT-derived tumors was highly significant (p<0.0001) after 23 days of drug treatment. In fact, tumors generated by mTOR-RR-expressing cells showed no significant response to rapamycin therapy at the same or later time points when tumors achieve a volume comparable to those of the control cells expressing GFP and mTOR wild-type, in spite of the slower intrinsic growth rate of the tumors expressing mTOR-RR (Fig. 5D). The reversion of the antitumoral effect of rapamycin by the expression of mTOR-RR was also observed in additional HNSCC tumor xenografts tested, as shown in Fig. 5E for Cal27 cells. Collectively, these results provide strong support for a cell-autonomous mechanism of action for rapamycin in HNSCC xenografts. They also suggest that whereas in other cancer types rapamycin acts primarily by affecting tumor endothelial cells (34), in HNSCC the disruptive effect of rapamycin treatment on host responses, such as on the tumor vascularization, appear to be a downstream consequence of the direct actions of this drug on the tumor cells.
Rapamycin may cause indirect effects on the tumor microenvironment through HIF-1α
During the progression of solid tumors, growth factors and hypoxia complement each other in the regulation of the angiogenic process. When the expansion of tumor mass gradually distances tumor cells from the blood supply, and thus from oxygen and nutrients, tumor cells initiate the formation of new blood vessels by a paracrine mechanism (38, 39). Although many molecules and receptors have been implicated in the angiogenic response, the hypoxia-inducible factor-1α (HIF-1α) seems critical for initiating the angiogenesis process through the transcriptional control of the expression of VEGF and other pro-angiogenic factors (40). In HNSCC, several reports indicated that elevated expression of HIF-1α and VEGF correlate with tumor aggressiveness and a poor prognosis (41, 42). Indeed, Western blot analysis of tumor xenograft lysates demonstrated high level of HIF-1α expression in HNSCC tumors, which was dependent on mTOR signaling, as judged by its reduction after rapamycin treatment (Fig 5B). Furthermore, mTOR-RR-expressing HNSCC xenografts displayed persistent expression of pS6 and HIF-1α in rapamycin-treated host animals (Fig 5B).
Aligned with these results and the emerging evidence that expression of HIF-1α and VEGF in developing tumors is highly dependent on the PI3K-AKT-mTOR pathway (43, 44), immunostaining of control HNSCC xenografts revealed that the nuclear localization of HIF-1α in the tumor cells was clearly diminished after rapamycin treatment. Once again, however, the mTOR-RR-expressing tumor cells showed little or no response to the rapamycin treatment regimen (Fig 6). The transcriptional activity of HIF-1 was also resistant to rapamycin treatment in the HN12-mTOR-RR-derived tumors, as suggested by the expression of Glut-1 and VEGF, two direct transcriptional targets of HIF-1α (45) in the drug-exposed tumor tissues. In contrast, control tumors treated with rapamycin showed clear reductions in Glut-1 and VEGF expression. These findings suggest that the tumor cell-autonomous effects of rapamycin on the mTOR-HIF-1α axis may play central roles in the metabolic and pro-angiogenic responses of hypoxic tumor tissues to microenvironmental stress.
Figure 6.
Immunodetection of mTOR/HIF-1α downstream targets in control and mTOR-RR expressing HNSCC xenografts following rapamycin treatment. Immunohistochemistry was performed on tissue sections using antibodies against pS6, HIF-1α, Glut-1 and VEGF to determine the effects of rapamycin. pS6 and VEGF protein were mainly localized in cytoplasm. HIF-1α was localized in nuclei of tumor cells. Glut-1 is visible at the cell membrane and cytoplasm.
Discussion
Rapamycin is a selective inhibitor of the mTOR pathway that has been approved by the Food and Drug Administration for nearly 10 years based on its immunosuppressive and anti-restenosis properties (23). Rapamycin and its analogues, including CCI-779, RAD001, and AP23573, collective known as rapalogs, have been recently shown to exhibit promising antitumor effects in several cancer types (23). Rapamycin forms a molecular complex with FKBP12 and mTOR, leading to the durable inhibition of this atypical serine/threonine kinase, and, in turn, diminishes the translation of mRNA transcripts whose protein products drive cell mass accumulation and cell-cycle progression (46). Despite the remarkable pharmacological activity of rapamycin as in a variety of experimental cancer models, its antiproliferative effect on cancer cells in vitro is often limited (23). For example, we observed that exposure of HNSCC cells to rapamycin does not affect their survival or growth, thus raising the possibility that, as for other cancer types, rapamycin may not target the cancer cells directly, but it may instead act based on its anti-angiogenic effects (34). However, by rescuing HNSCC cells from the growth-suppressive activity of rapamycin by the expression of a rapamycin-insensitive mutant of mTOR, we now show that HNSCC cells are the primary targets of rapamycin in vivo. Furthermore, this approach enabled the examination of the direct consequences of inhibiting mTOR in HNSCC-derived tumors, which revealed that in HNSCC, mTOR controls the expression of HIF-1α, a key transcription factor that orchestrates the cellular response to hypoxic stress, including the regulation of the expression of angiogenic factors (44), thus providing a likely mechanism by which rapamycin exerts its antiangiogenic effects. The emerging results suggest that rapamycin may ultimately promote the rapid regression of HNSCC lesions by promoting the apoptotic death of HNSCC by a still not fully understood mechanism, while concomitantly inhibiting the production of VEGF and other HIF-1α-dependent pro-angiogenic cytokines in cancer cells.
Based on previously published reports in other cancer types (34, 47, 48) and the lack of growth inhibitory effects of rapamycin on HNSCC cells in vitro, we initially considered the possibility that rapamycin might act primarily on the tumor microenvironment, particularly the tumor-associated vasculature, thereby promoting tumor stasis and/or cell death through disruption of oxygen and nutrient delivery, as well as other host-derived supportive signals, to the growing tumor mass. The results obtained by double IF staining of endothelial and epithelial cells and early apoptotic markers were not consistent with this possibility, as we observed the accumulation of tumor cells displaying the apoptotic marker, active caspase-3, as early as two days after rapamycin treatment, a time at which we could still observe intact capillary networks of CD31-positive endothelial cells that themselves showed no evidence of apoptotic death. While the demise of endothelial cells may occur by another, non-apoptotic process, such as necrosis, autophagy or senescence (49), the overall reduction in vessel density appears to occur after the apoptosis of HNSCC cells rather than preceding it, suggesting that the effects of rapamycin on the tumor vasculature are an indirect outcome of the disruption of tumor cell functions by this drug.
We next addressed the possibility that rapamycin acts directly on tumor cells in vivo, thus triggering growth suppressive and pro-apoptotic outcomes that cannot be mimicked in in vitro cell culture systems. Accordingly, we hypothesized that the generation of HNSCC lines that were resistant to the rapamycin should allow a direct assessment of the contribution of cancer cell-autonomous actions of the drug to its overall antitumoral effects. In this model, host-derived endothelial and stromal cells retain wild-type mTOR alleles and hence their normal level of sensitivity to rapamycin. The HNSCC cells were rendered insensitive to rapamycin by expressing a catalytically active mTOR-RR mutant, which exhibits several orders of magnitude lower binding affinity for the FKBP12-rapamycin complex (36). Although this mTOR-RR mutant exhibits reduce ability to phosphorylate 4E-BP1 in immune complex kinase assays (36), it is capable of complementing the defects in mTOR-dependent signaling observed in rapamycin-treated cells (36). Consistent with the hypomorphic behavior observed in previous studies (28, 50), HN12 cells that constitutively expressed the mTOR-RR allele displayed a reduced growth rate as xenograft tumors in nude mice; nonetheless, this strategy provided a unique opportunity to study the effects of rapamycin on tumor growth when the tumor cells themselves were engineered to express high-level resistance to this drug. Indeed, by this reverse-pharmacology strategy, we obtained strong evidence that the potent antitumor effect of rapamycin in preclinical models of HNSCC is largely if not entirely attributable to the inhibition of mTOR signaling in the cancer cells themselves, rather than in vascular and other host-derived elements that help to support tumor growth.
The expression of wild-type mTOR did not increase the in vivo growth rate of HNSCC cells, and tumor xenografts arising from cells overexpressing mTOR-RR grew slower than GFP-expressing cells, suggesting that the basal mTOR expression in HN12 cells is not limiting for the growth of tumors in immunodeficient mice. On the other hand, expression of mTOR-RR generated HNSCC cells exquisitely resistant to all biochemical and biological effects of rapamycin in vitro and in vivo. Besides supporting the selectivity of rapamycin as an mTOR-specific inhibitor, these findings provided an opportunity to monitor the precise changes provoked by mTOR inhibition in HNSCC in the context of the tumor microenvironment. This led to the observation that rapamycin causes a reduction in the expression of pro-angiogenic and metabolic mediators that are regulated by HIF-1α and the direct apoptotic death of HNSCC cancer cells.
These observations may have important implications for the treatment of HNSCC and other solid tumors, and may explain the nature of the anti-proliferative and pro-apoptotic effects of rapamycin, as the expression of HIF-1α is required for developing tumors to stimulate angiogenesis, and other metabolic and microenvironmental responses that allow continued expansion of tumor tissue mass. This process involves the enhanced release of pro-angiogenic mediators, such as VEGF, whose expression is tightly regulated by HIF-1α (43), as well as the increased expression of glucose transporters and a myriad of cytosolic and mitochondrial proteins involved in the appropriate balance between aerobic and anaerobic glucose metabolism (43, 45). In this scenario, it is tempting to speculate that by interfering with the mTOR-dependent regulation of HIF-1α expression, rapamycin may deprive HNSCC cells of an essential stress response network, thus resulting in tumor cell stasis and/or death. This exciting possibility, as well as the precise mechanism by which rapamycin interferes with the expression of HIF-1α warrant s further investigation.
There is an urgent need for new treatment options for HNSCC patients, considering that their overall 5-year survival is relatively low (~50%) and has not improved much over the past 3 decades. The emerging wealth of information on the nature of the deregulated molecular mechanisms responsible for HNSCC progression has provided the possibility of exploring new mechanisms-based therapeutic approaches for HNSCC (32). In this regard, our present results indicate that the direct inhibition of mTOR in cancer cells is responsible for the potent antitumoral effects of rapamycin in HNSCC models, thus providing the rationale for the early evaluation of the clinical benefits of inhibiting mTOR in HNSCC patients that exhibit elevated activity of the Akt-mTOR pathway. Furthermore, the observation that rapamycin causes decreased expression of HIF-1α, VEGF, and the Glut1 glucose transporter in cancer cells suggests that the impact of rapamycin on glucose and nutrient metabolism, together with the inhibition of the expression of pro-angiogenic factors may now help explain the potent antitumoral effect of rapamycin in vivo. These findings may also provide a battery of readily available molecular end-points to monitor the effectiveness of rapamycin and its analogs as potential anti-cancer agents for the treatment of HNSCC.
Supplementary Material
Acknowledgements
This work was supported by the Intramural Research Program, National Institute of Dental and Craniofacial Research, National Institutes of Health
Bibliography
- 1.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–34. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 2.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 3.Manning BD, Cantley LC. Rheb fills a GAP between TSC and TOR. Trends Biochem Sci. 2003;28:573–6. doi: 10.1016/j.tibs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Bader AG, Kang S, Zhao L, et al. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–29. doi: 10.1038/nrc1753. [DOI] [PubMed] [Google Scholar]
- 5.Philp AJ, Campbell IG, Leet C, et al. The Phosphatidylinositol 3'-kinase p85{alpha} Gene Is an Oncogene in Human Ovarian and Colon Tumors. Cancer Res. 2001;61:7426–29. [PubMed] [Google Scholar]
- 6.Amornphimoltham P, Sriuranpong V, Patel V, et al. Persistent activation of the Akt pathway in head and neck squamous cell carcinoma: a potential target for UCN-01. Clin Cancer Res. 2004;10:4029–37. doi: 10.1158/1078-0432.CCR-03-0249. [DOI] [PubMed] [Google Scholar]
- 7.Pomerantz RG, Grandis JR. The role of epidermal growth factor receptor in head and neck squamous cell carcinoma. Curr Oncol Rep. 2003;5:140–6. doi: 10.1007/s11912-003-0101-z. [DOI] [PubMed] [Google Scholar]
- 8.Mao L, Hong WK, Papadimitrakopoulou VA. Focus on head and neck cancer. Cancer Cell. 2004;5:311–6. doi: 10.1016/s1535-6108(04)00090-x. [DOI] [PubMed] [Google Scholar]
- 9.Qiu W, Schonleben F, Li X, et al. PIK3CA mutations in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12:1441–6. doi: 10.1158/1078-0432.CCR-05-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozaki K, Imoto I, Pimkhaokham A, et al. PIK3CA mutation is an oncogenic aberration at advanced stages of oral squamous cell carcinoma. Cancer Sci. 2006;97:1351–8. doi: 10.1111/j.1349-7006.2006.00343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sulis ML, Parsons R. PTEN: from pathology to biology. Trends Cell Biol. 2003;13:478–83. doi: 10.1016/s0962-8924(03)00175-2. [DOI] [PubMed] [Google Scholar]
- 12.Zbuk KM, Eng C. Cancer phenomics: RET and PTEN as illustrative models. Nat Rev Cancer. 2007;7:35–45. doi: 10.1038/nrc2037. [DOI] [PubMed] [Google Scholar]
- 13.Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- 14.Hemminki A, Markie D, Tomlinson I, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–87. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 15.Corradetti MN, Inoki K, Bardeesy N, et al. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev. 2004;18:1533–38. doi: 10.1101/gad.1199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segrelles C, Ruiz S, Perez P, et al. Functional roles of Akt signaling in mouse skin tumorigenesis. Oncogene. 2002;21:53–64. doi: 10.1038/sj.onc.1205032. [DOI] [PubMed] [Google Scholar]
- 17.Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 18.Amornphimoltham P, Patel V, Sodhi A, et al. Mammalian Target of Rapamycin, a Molecular Target in Squamous Cell Carcinomas of the Head and Neck. Cancer Res. 2005;65:9953–61. doi: 10.1158/0008-5472.CAN-05-0921. [DOI] [PubMed] [Google Scholar]
- 19.Massarelli E, Liu DD, Lee JJ, et al. Akt activation correlates with adverse outcome in tongue cancer. Cancer. 2005;104:2430–6. doi: 10.1002/cncr.21476. [DOI] [PubMed] [Google Scholar]
- 20.Patel V, Lahusen T, Leethanakul C, et al. Antitumor activity of UCN-01 in carcinomas of the head and neck is associated with altered expression of cyclin D3 and p27(KIP1). Clin Cancer Res. 2002;8:3549–60. [PubMed] [Google Scholar]
- 21.Cohen P, Alessi DR, Cross DAE. PDK1, one of the missing links in insulin signal transduction? FEBS Letters. 1997;410:3–10. doi: 10.1016/s0014-5793(97)00490-0. [DOI] [PubMed] [Google Scholar]
- 22.Easton JB, Houghton PJ. mTOR and cancer therapy. Oncogene. 2006;25:6436–46. doi: 10.1038/sj.onc.1209886. [DOI] [PubMed] [Google Scholar]
- 23.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–88. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 24.Nathan CO, Amirghahari N, Rong X, et al. Mammalian target of rapamycin inhibitors as possible adjuvant therapy for microscopic residual disease in head and neck squamous cell cancer. Cancer Res. 2007;67:2160–8. doi: 10.1158/0008-5472.CAN-06-2449. [DOI] [PubMed] [Google Scholar]
- 25.Jimeno A, Kulesza P, Wheelhouse J, et al. Dual EGFR and mTOR targeting in squamous cell carcinoma models, and development of early markers of efficacy. Br J Cancer. 2007;96:952–9. doi: 10.1038/sj.bjc.6603656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon GA, Lee JS, Patel V, et al. Global gene expression profiles of human head and neck squamous carcinoma cell lines. Int J Cancer. 2004;112:249–58. doi: 10.1002/ijc.20399. [DOI] [PubMed] [Google Scholar]
- 27.Wendel HG, De Stanchina E, Fridman JS, et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–7. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 28.McMahon LP, Choi KM, Lin TA, et al. The rapamycin-binding domain governs substrate selectivity by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7428–38. doi: 10.1128/MCB.22.21.7428-7438.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basile JR, Castilho RM, Williams VP, et al. Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. Proc Natl Acad Sci U S A. 2006;103:9017–22. doi: 10.1073/pnas.0508825103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel V, Senderowicz AM, Pinto D, Jr., et al. Flavopiridol, a novel cyclin-dependent kinase inhibitor, suppresses the growth of head and neck squamous cell carcinomas by inducing apoptosis. J Clin Invest. 1998;102:1674–81. doi: 10.1172/JCI3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiebig HH, Berger DP, Winterhalter BR, et al. In vitro and in vivo evaluation of US-NCI compounds in human tumor xenografts. Cancer Treat Rev. 1990;17:109–17. doi: 10.1016/0305-7372(90)90034-d. [DOI] [PubMed] [Google Scholar]
- 32.Forastiere A, Koch W, Trotti A, et al. Head and neck cancer. N Engl J Med. 2001;345:1890–900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 33.Cardinali M, Pietraszkiewicz H, Ensley JF, et al. Tyrosine phosphorylation as a marker for aberrantly regulated growth-promoting pathways in cell lines derived from head and neck malignancies. Int J Cancer. 1995;61:98–103. doi: 10.1002/ijc.2910610117. [DOI] [PubMed] [Google Scholar]
- 34.Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–35. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 35.Yuan J. Divergence from a Dedicated Cellular Suicide Mechanism: Exploring the Evolution of Cell Death. Molecular Cell. 2006;23:1–12. doi: 10.1016/j.molcel.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Zheng XF, Brown EJ, et al. Identification of an 11-kDa FKBP12-rapamycin-binding domain within the 289-kDa FKBP12-rapamycin-associated protein and characterization of a critical serine residue. Proc Natl Acad Sci U S A. 1995;92:4947–51. doi: 10.1073/pnas.92.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abraham RT. TOR Signaling: An Odyssey from Cellular Stress to the Cell Growth Machinery. Curr Biol. 2005;15:R139–41. doi: 10.1016/j.cub.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 38.Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007 doi: 10.1038/nrc2067. advanced online publication. [DOI] [PubMed] [Google Scholar]
- 39.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 40.Blagosklonny MV. Antiangiogenic therapy and tumor progression. Cancer Cell. 2004;5:13–17. doi: 10.1016/s1535-6108(03)00336-2. [DOI] [PubMed] [Google Scholar]
- 41.Slomiany MG, Black LA, Kibbey MM, et al. IGF-1 induced vascular endothelial growth factor secretion in head and neck squamous cell carcinoma. Biochem Biophys Res Commun. 2006;342:851–8. doi: 10.1016/j.bbrc.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 42.Beasley NJ, Leek R, Alam M, et al. Hypoxia-inducible factors HIF-1alpha and HIF-2alpha in head and neck cancer: relationship to tumor biology and treatment outcome in surgically resected patients. Cancer Res. 2002;62:2493–7. [PubMed] [Google Scholar]
- 43.Semenza GL. Targeting HIF-1 for cancer therapy. Nature Reviews Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 44.Abraham RT. mTOR as a positive regulator of tumor cell responses to hypoxia. Curr Top Microbiol Immunol. 2004;279:299–319. doi: 10.1007/978-3-642-18930-2_18. [DOI] [PubMed] [Google Scholar]
- 45.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nature Reviews Cancer. 2004;4:891–99. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 46.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–45. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 47.Thomas GV, Tran C, Mellinghoff IK, et al. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med. 2006;12:122–7. doi: 10.1038/nm1337. [DOI] [PubMed] [Google Scholar]
- 48.Phung TL, Ziv K, Dabydeen D, et al. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006;10:159–70. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okada H, Mak TW. Pathways of apoptotic and non-apoptotic death in tumour cells. Nature Reviews Cancer. 2004;4:592–603. doi: 10.1038/nrc1412. [DOI] [PubMed] [Google Scholar]
- 50.Edinger AL, Linardic CM, Chiang GG, et al. Differential effects of rapamycin on mammalian target of rapamycin signaling functions in mammalian cells. Cancer Res. 2003;63:8451–60. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.