Abstract
Sphingosine 1-phosphate (S1P) is a signaling molecule involved in a host of cellular and physiological functions – most notably cell survival and migration. S1P, which signals via a set of five G protein coupled receptors (S1P1–5), is formed by the action of two sphingosine kinases (SphKs) from sphingosine. Interfering RNA strategies and SphK1 null mouse studies implicate SphK1 in multiple signaling cascades, yet there is a paucity of potent and selective SphK1 inhibitors necessary to evaluate the effects of rapid onset inhibition of this enzyme. We have identified a set of sub-micromolar, amidine-based SphK1 inhibitors and herein report using a pair of these compounds to probe the cellular and physiological functions of SphK1. In doing so, we demonstrate that our inhibitors effectively lower S1P levels in cell based assays, but we have been unable to correlate SphK1 inhibition with changes in cell survival. However, SphK1 inhibition did diminish epidermal growth factor-driven increases in S1P levels and Akt/ERK phosphorylation. Finally, administration of the SphK1 inhibitor to wild type, but not Sphk1−/−, mice resulted in a rapid decrease in blood S1P levels indicating that circulating S1P is rapidly turning over.
Keywords: Sphingosine kinase (SphK), Sphingosine 1-phosphate (S1P), SphK inhibitor
INTRODUCTION
Sphingosine 1-phosphate (S1P) is a bioactive lipid implicated in a host of biologic functions, including cell migration [1–3] and survival [4, 5] as well as angiogenesis [6]. S1P exerts its effects via five G-protein coupled receptors – S1P1–5 [7] and perhaps through less well-defined intracellular targets. Due in part to the remarkable clinical success of the S1P receptor agonist and immunomodulatory pro-drug, fingolimod (FTY720) [8], S1P signaling pathways are currently the subject of many investigations.
Two sphingosine kinases (SphK1 & 2) are solely responsible for S1P synthesis by catalyzing the phosphorylation of sphingosine (Sph) [9, 10]. Studies with SphK1 null mice reveal that SphK1 is responsible for a substantial fraction of circulating S1P [11]. These kinases have come under increasing scrutiny as drug targets due to their role in the production of S1P and the SphK-S1P axis has been linked to cell growth, survival, angiogenesis and metastasis [12, 13].
SphK1 & 2 play a role in controlling the relative concentrations of S1P and its precursor, sphingosine, and this proposed homeostatic mechanism has been referred to as the ‘sphingolipid rheostat’ [14]. The implication of this concept is that SphK inhibition would simultaneously decrease S1P levels and increase concentrations of sphingosine and its precursor, ceramide. The concept of a sphingolipid homeostasis, reinforced by numerous studies using interfering RNA strategies, suggests SphKs are important drug targets for the treatment of conditions characterized by hyper-proliferation such as fibrosis and cancer. The relative importance of SphK1 versus SphK2 as a drug target remains a topic of debate with some studies indicating SphK1 [15–18], while other studies point towards SphK2 [19, 20]. Our present work is focused on SphK1.
Previously described SphK inhibitors are relatively low potency (Ki values > 1 µM) [21] and for most of these compounds their influence on the Sph : S1P ratio in either cells in culture or in whole animals has not been reported [22–25]. Further, many of the existing inhibitors are long chain bases that, like sphingosine, are toxic to cells at micromolar concentrations [26]. We recently discovered an amidine-based scaffold of SphK inhibitors that has allowed us to generate potent (Ki < 0.2 µM) and selective SphK1 inhibitors [27, 28]. In this report, we describe the use of an enantiomeric pair of these inhibitors. We observed that acute SphK1 inhibition results in a pronounced, rapid decrease in S1P levels in both cultured cells and mice. In agreement with the mitogenic effects of S1P, we found that EGF-induced phosphorylation of Akt and ERK in SKOV3 cells was antagonized by our inhibitor. We failed to observe, however, a direct effect of our inhibitor on the survival of U937 and Jurkat T cells.
EXPERIMENTAL PROCEDURES
Materials
Compounds 1a and 1b were synthesized as described [28]. Sphk1−/− mice were a gift from Dr. R. Proia (NIH/NIDDK, Bethesda, MD). Compound SKI-II was purchased from Sigma Aldrich (St Louis, MO). C57BL/6j mice were from Jackson Laboratories (Bar Harbor, ME). Antibodies to ERK, p-ERK, Akt, p-Akt, PARP and caspase 3 were purchased from Cell Signaling Technology (Danvers, MA). Plasmids encoding diacylglycerol kinase alpha and diacylglycerol kinase zeta were gifts from Dr. Kaoru Goto (Yamagata University School of Medicine, Yamagata, Japan) and Dr. Matthew Topham (University of Utah, Salt Lake City, UT), respectively. C17 S1P and C17 sphingosine were purchased from Avanti Polar Lipids (Alabaster, AL).
Kinase assays
SphK activity was measured by a scintillation proximity assay as described by us previously [21]. Briefly, recombinant SphK1 or SphK2 were incubated in 96 well FlashPlates (Perkin-Elmer) with D-erythro-sphingosine and γ-[33P]ATP and the [33P]S1P product, which adheres to the plate wall, was quantified by scintillation counting. To assay ceramide kinase and diacylglycerol kinases, the recombinant proteins were incubated with γ-[32P]ATP and substrate (C6 ceramide and 1-O-hexadecyl-2-acetyl-sn-glycerol, respectively) and the lipid product, after recovery by organic extraction, was resolved by thin layer chromatography, detected by autoradiography and quantified by liquid scintillation counting. These assays were performed with and without a fixed concentration of inhibitor and the effect on Km and Vmax determined.
Lipid extraction
Extraction protocols and LC/MS procedures were from Shaner et al. [29] with minor modifications. Cell pellets (approximately 5 million cells) or whole blood (20 µL) were mixed with 2 mL of a 3:1 methanol : chloroform mixture and transferred to a capped glass vial. To this suspension was added 10 µL of internal standard solution containing 1 µM C17 S1P, 1 µM C17 sphingosine and 1 µM of an undecyl analogue of 1a and 1b (dodecyl). The mixture was homogenized in a bath sonicator for 10 minutes and incubated at 48°C for 16 hours. The mixture was then cooled to ambient temperature and mixed with 200 µL of 1M KOH in methanol. The samples were again sonicated and incubated at 37°C for 2 hours. After this time, the samples were neutralized through the addition of 20 µL of glacial acetic acid and transferred to 2 mL microcentrifuge tubes. Samples were then centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatant fluid was collected in a separate glass vial and the pellets discarded. The resulting solution was evaporated under a stream of nitrogen gas. Immediately prior to LC/MS analysis, the material was dissolved in 300 µL of methanol and centrifuged at 12,000 × g for 12 minutes at 4°C. Fifty µL of the resulting supernatant fluid were analyzed by LC/MS.
LC/MS protocol
Analyses were performed by Liquid Chromatography/ESI Mass Spectrometry (LC/MS) using a triple quadrupole mass spectrometer (Sciex 4000 Q-Trap) coupled to a Shimadzu LC-20AD LC system. A binary solvent gradient with a flow rate of 1 mL/min was used to separate sphingolipids and drugs by reverse phase chromatography using a Supelco Discovery C18 column (50mm × 2.1 mm, 5 µm bead size). Mobile phase A consisted of water : methanol : formic acid (79:20:1) while mobile phase B was methanol : formic acid (99:1). The run started with 100% A for 0.5 minutes. Solvent B was then increased linearly to 100% B in 5.1 minutes and held at 100% for 4.3 minutes. The column was finally re-equilibrated to 100% A for 1 min. Natural sphingolipids were detected using multiple reaction monitoring (MRM) methods previously described [29] as follows: C17S1P (366.4 → 250.4); S1P (380.4 → 264.4); dihydroS1P (382.4 → 266.4); C17sphingosine (286.4 → 250.3); sphingosine (300.5 → 264.4); sphinganine (302.5 → 260.0). Fragmentation of compounds 1a and 1b was analyzed by direct infusion of 1 µM solutions in methanol : formic acid (99:1) and the following transitions were found to produce the most intense product ions in positive mode: C12-proline-amidines 1a, 1b (386.3 → 273.1); VPC96077 (C11-proline-amidine) (372.5 → 259.2). All analytes were analyzed simultaneously using the afore mentioned MRMs. Voltages (DP, EP, CE and CXP) for 1a and 1b were: 35, 10, 25, 6; and 156, 10, 25, 14 volts, respectively. Retention times for all analytes under our experimental conditions were between 5.1 and 5.6 min. The following ceramides were measured by monitoring the m/z 264.4 product ion as previously described [29] using a Supelco Supelcosil LC-NH2 column (50mm × 2.1 mm, 3 µm bead size) : 12:0, 16:0, 18:1, and 24:1. Quantification was carried out by measuring peak areas using commercial software (Analyst 1.5.1).
Cell culture
U937 and Jurkat T cells were grown as described [22]. Briefly, cells were grown in RPMI 1640 media supplemented with L-glutamate, 10% fetal bovine serum (FBS) and penicillin/streptomycin at 37°C in an atmosphere containing 5% CO2. SKOV3 cells were grown in MyCoy’s 5a media supplemented with 10% FBS and penicillin/streptomycin at 37°C in an atmosphere containing 5% CO2. Twenty-four hours before adding inhibitors, the growth media was replaced with media containing 0.5% or 2% FBS.
Western blot analysis
Cells were incubated with various concentrations of inhibitor for the times indicated (usually 2 h). After incubation, cells were washed with phosphate-buffered saline and lysed using a Dounce homogenizer. Equal amounts of protein were resolved by SDS-PAGE analysis using 10% polyacrylamide gels and resolved proteins transferred to a nitrocellulose membrane. Membranes were blocked with 5% non-fat milk in Tris-buffered saline (TBS, pH 7.4) containing 0.1% Tween 20 for 1 hour at room temperature. After rinsing, membranes were incubated with antibodies (diluted 1:1000 in TBS) against ERK, p-ERK, Akt, p-Akt, caspase-3, PARP or β-actin for 1 hour. After washing three times in TBS buffer, the nitrocellulose membrane was incubated with a 1:2000 dilution (in TBS) of HRP-conjugated anti-IgG antibody. Detection was accomplished by chemiluminesence using a commercial kit (Perkin Elmer Western Lightning).
MTT cell viability assay
U937 and separately Jurkat T cells were plated in a 96 well plate at a density of 50–60,000 cells per well. Cells were grown overnight in 2% FBS and then treated with the indicated concentration of compound overnight. The TACS™ MTT assay was performed according to the manufacturer's protocol (R&D Systems, Minneapolis, MN). Briefly, MTT reagent (10 µL per 100 µL of cell culture medium) was added to each well and the plate was incubated at 37°C for 4 h, followed by incubation with Detergent Reagent at room temperature for 2 h. Absorbance was measured at a wavelength of 570 nm.
Pharmacokinetic analysis
Groups of 8–12 week old mice (strain: C57BL/6j) were anesthetized with methoxyflurane and injected into the tail vein with either 1a, 1b (dose: 10 mg/kg) or and equal volume of vehicle. The vehicle was a 2% solution of hydroxypropyl-β-cyclodextrin (Cargill Cavitron 82004) in water. After injection, animals were lightly anesthetized and bled from the retro-orbital sinus at the specified time points. (ASAP time points were 1–2 minutes after dosing). Blood was extracted immediately as described above for lipid and drug analysis. Animal protocols were approved prior to experimentation by the University of Virginia’s School of Medicine Animal Care and Use Committee.
RESULTS
Inhibitor design strategy
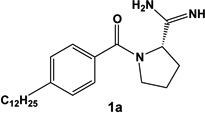
We have described previously a set of SphK inhibitors with an amidine ‘warhead’ [27]. An homology model suggests that the amidine group interacts directly with ATP via a bidentate chelation of the γ-phosphate [28]. In the design of biologically active small molecules, rigid analogues are a structural motif often used to improve selectivity among related targets. For example, we found that restricting the rotatable bonds of FTY720 analogues has a significant impact on their rate of phosphorylation by SphK1 & 2 [30]. To increase selectivity and potency for SphK1, we designed a rigid analogue of our previously reported amidine-based SphK inhibitors. The limited rotational degrees of freedom of rigid analogues were expected to provide greater structural differences between stereoisomers. Indeed, the enantiomers of our proline analogue exaggerated the differences in activity at SphK1 over those described in our initial study [27]. Our structure activity relationship studies also identified the 12 carbon alkyl tail length analogues as the most potent for SphK inhibition [27, 28]. Taking these structural considerations into account, we selected enantiomers 1a and 1b (structures in Table 1) for in depth characterization.
Table 1.
Chemical structure and inhibitory properties of compounds 1a and 1b. The chemical structures of compounds 1a and 1b along with their inhibitory constants (Ki) for recombinant SphK1 1 and SphK2 are shown. Inhibitory constants were obtained by kinetic analysis of S1P production using variable concentrations of sphingosine and a fixed concentration of ATP in presence and absence of compounds 1a and 1b. These compounds exhibit a pattern of competitive inhibition therefore Ki’s were calculated as Ki = [I] / (Km’/Km-1), where [I] is the concentration of inhibitor, and Km’ and Km are the Michaelis constants obtained in presence and absence of inhibitor. Measurements were carried out using [33P]-ATP as a tracer and a microplate-based scintillation proximity assay for the detection of [33P]-S1P as previously described [21].
| Compound | SphK1 Ki | SphK2 Ki |
|---|---|---|
 |
0.1 µM | 1.5 µM |
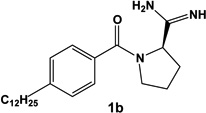 |
16 µM | 5 µM |
Evaluation of 1a and 1b in vitro
We first determined the Ki values of 1a and 1b at SphK isotypes by measuring the synthesis of [33P]-S1P catalyzed by recombinant SphK1 and SphK2 [21]. In agreement with our previous findings [27], the s-enantiomer (1a), derived from L-proline, was significantly more potent at SphK1 than its r-counterpart (1b). Due to their enantiomeric nature, comparing these compounds in biological systems is useful in establishing the target selectivity of the inhibitors. Importantly, there is a >100-fold difference in potency between 1a (Ki 0.1 µM) and 1b (Ki 16 µM) at SphK1, thus differences in biologic responses to these compounds can reasonably be assigned to activity at SphK1. Our amidine-based SphK1 inhibitors, including 1a and 1b, are competitive with sphingosine, but not with ATP. Therefore, as a further assessment of selectivity, we tested 1a at related lipid kinases including ceramide kinase and diacylglycerol kinase isotypes alpha and zeta. At a concentration of 3 µM, there was no inhibition of these lipid kinases (data not shown).
Evaluation of 1a and 1b on cultured cells
We next determined whether the inhibitors were capable of penetrating cells and inhibiting SphK1 therein. Due to high levels of SphK1 expression and ease of culture, we choose human monocyte U937 cells as our primary model system for the evaluation of SphK1 inhibitors [31]. Spiegel and co-workers had also evaluated their mono-methylated sphingosine analogue SKI-1 using U937 cells thus affording comparison of SphK1 inhibitors while recognizing possible differences in strains of U937 cells [22].
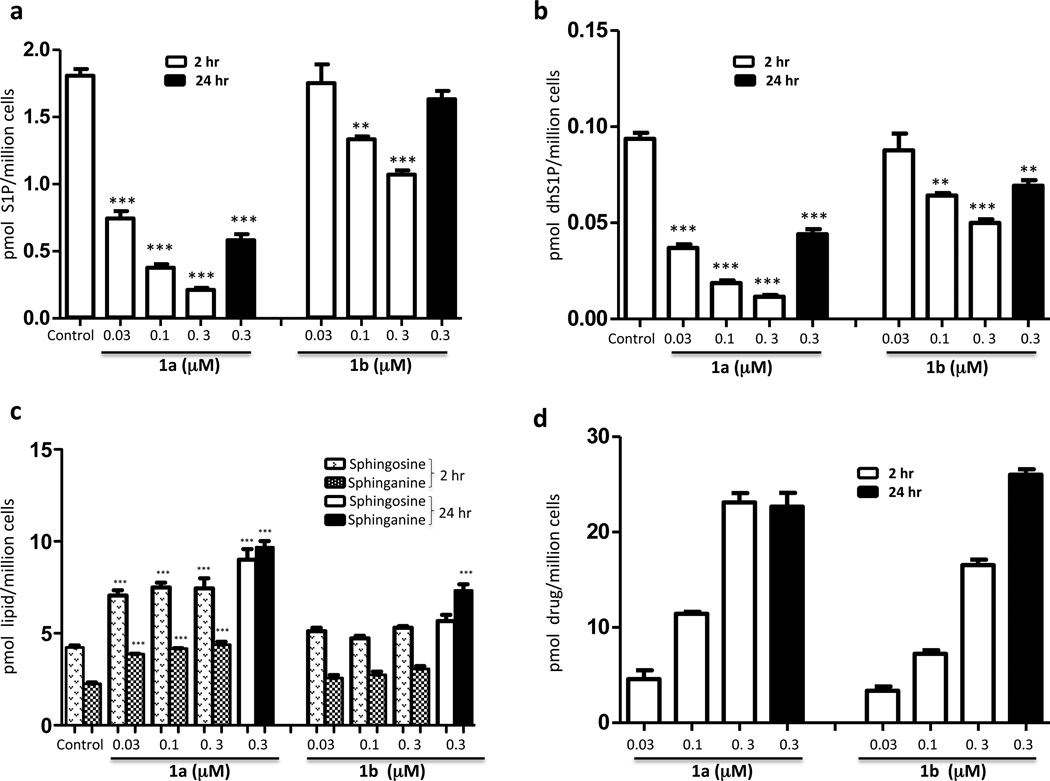
We treated U937 cultures with either enantiomer and quantified the accumulation of cell-associated S1P, dhS1P, sphingosine (Sph), sphinganine (dhSph) and 1a or 1b. As depicted in Figure 1, treatment of cultures with 1a or 1b for 2 hours resulted in diminished S1P (Figure 1a) and dhS1P (Figure 1b) in a drug concentration-dependent manner. As predicted from assessment at recombinant SphK1, enantiomer 1a was significantly more potent than 1b; the IC50 value for 1a was about 0.03 µM while the IC90 value was about 0.3 µM. Concomitant with the decrease in the phosphorylated species, both sphingosine and sphinganine levels rose on treatment with 1a, but the extent of the increase was 2-fold or less (Figure 1c). The decrease in S1P was observed as soon as 10 minutes after inhibitor addition (not shown) and persisted for at least 24 hours (Figure 1a). The amount of cell-associated 1a and 1b increased proportionate to added compound and significant amounts of compound remain cell-associated after 24 hours (Figure 1d). One abundant ceramide species, C16:0 ceramide, was also slightly increased after 2 hr and significantly elevated after 24 hr of 1a, but not 1b, treatment (Figure 1e). A similar increase, albeit to a lesser extent, was also observed with C18:1 and C24:1 ceramides (data not shown). These data are consistent with the observations reported by Paugh et al. [22]. Treatment of another cell line, human T cell leukemia Jurkat T cells, for 2 hours with 1a (but not 1b) also resulted in decreased S1P and increased sphingosine levels (Figure 1f–1g), although the magnitude of the changes were less than with U937 cells.
Fig. 1.
Levels of sphingolipids and compounds 1a and 1b in cell cultures treated with various concentrations of compounds 1a and 1b. Cultured U937 and Jurkat T cells were exposed to different concentrations of compounds 1a and 1b as indicated in the figure. After a 2 or 24 h period of exposure, cells were harvested, lysed and the amounts of sphingolipids and compounds 1a and 1b in the lysates were measured by LC/MS as described in the Methods section. a: S1P in U937 cells; b: dihydroS1P in U937 cells; c: sphingosine and sphinganine in U937 cells; d: 1a and 1b in U937 cells; e: C16:0 ceramide level in U937 cells; f: S1P in Jurkat T cells; g: sphingosine in Jurkat T cells. Amounts in cells are expressed as the number of pmoles per million cells. Drug concentrations refer to the concentration of drugs in the culture medium. Data are means ± SD of three independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001 (1 way ANOVA, and Dunnett's Multiple Comparison Post Test, compared to Control).
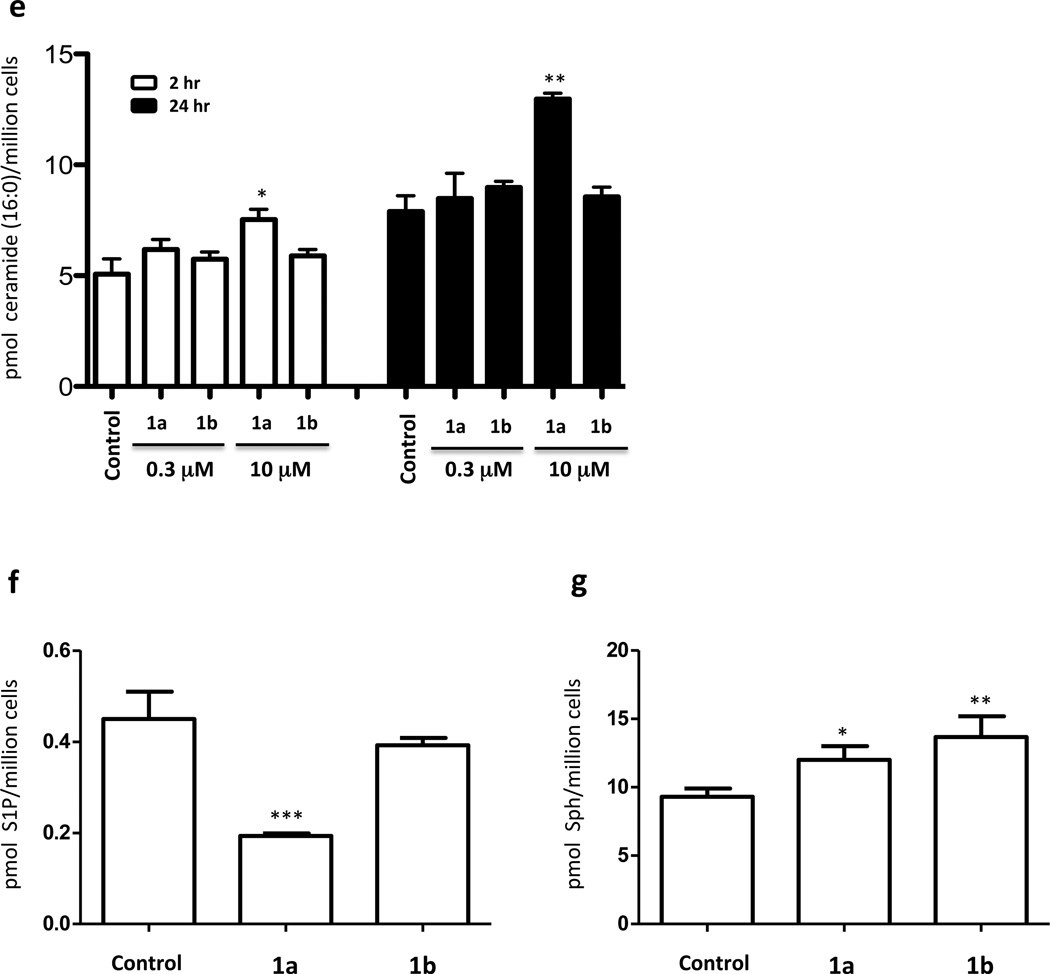
To confirm that the observed decrease in S1P accumulation in response to 1a was the result of decreased synthesis (as opposed to increased degradation / export), we added exogenous sphingosine and measured S1P in U937 cells with or without 1a in the culture medium. Cells supplemented with sphingosine to 0.3 or 1 µM exhibited pronounced increases in both sphingosine and S1P after two hours (Figure 2). The concomitant addition of 1a to 0.3 µM largely blocked the appearance of S1P (Figure 2a) while exaggerating the accumulation of sphingosine (Figure 2b). These results indicate that the decrease in S1P levels observed in U937 cells treated with 1a is primarily the result of blockade of SphK1 activity. Presumably, the decreased S1P levels observed as a consequence of 1a treatment (Figure 1a) occur because S1P metabolism by phosphatases and/or S1P lyase, and/or S1P export proceeds unimpeded while synthesis is blocked. These results also document that the inhibitors are readily taken up by U937 and Jurkat T cells.
Fig. 2.
Levels of S1P and sphingosine in U397 cells treated with sphingosine and compound 1a. Cultured U937 cells were exposed to different concentrations of sphingosine and 0.3 µM 1a as indicated in the figure. After 2 hours, cells were harvested by centrifugation and the amounts of S1P and sphingosine associated with the cell pellet were measured by LC/MS. a: S1P; b: sphingosine. Amounts are expressed as the number of pmoles per million cells. Drug and sphingosine concentrations refer to the concentration of these molecules in the culture medium. Data are means ± SD of three independent experiments. ** p < 0.01, *** p < 0.001 (Unpaired two-tailed T-test, comparing 0.3 µM sphingosine treatment to 0.3 µM sphingosine + 0.3 µM 1a treatment; separately comparing 1 µM sphingosine treatment to 1 µM sphingosine + 0.3 µM 1a treatment.)
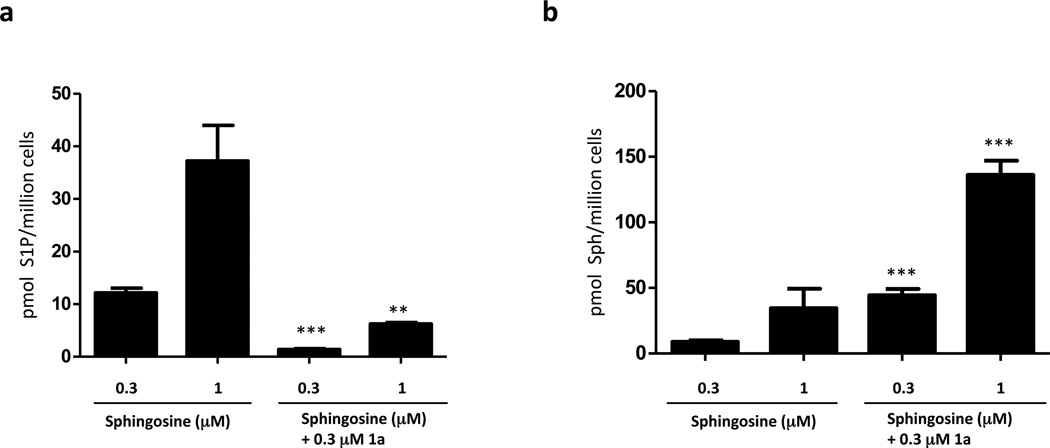
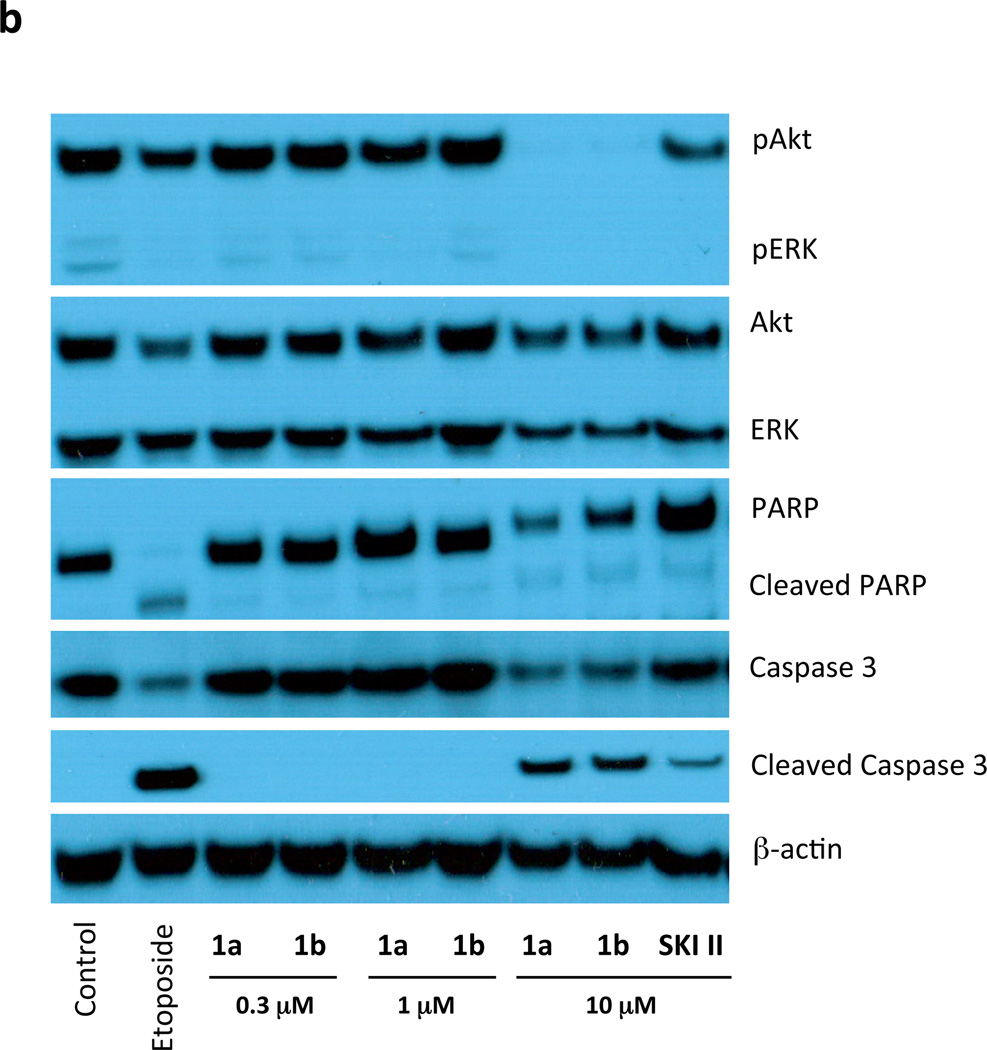
The ability to block SphK activity in U937 cells enabled an examination of cell signaling and survival by these cells in response to the blockade. A previous report documenting the effects of another SphK1 inhibitor, SKI-1 (SphK1 Ki 10 µM), on U937 cells ascribed decreased cell survival to the blockade of S1P biosynthesis [22]. Specifically, treatment with 20 µM of SKI-1 blocked the constitutive phosphorylation of ERK and Akt that is characteristic of U937 cells [22]. Therefore, we asked whether treatment with 1a (Ki 0.1 µM) might have similar effects on ERK and Akt phosphorylation by U937 cells. As depicted in Figure 3, we did not detect a change in ERK phosphorylation at 1a applied at 0.3 µM – a concentration that results in a substantial blockade of S1P synthesis (see Figure 1 and Figure 2). Effects on ERK phosphorylation were observed only at high 1a concentration (10 µM) or after prolonged exposure times (> 2h). We observed a similar pattern for Akt phosphorylation, although even longer exposure times (16 hours) were required to observe an effect. Paugh et al. [22] also reported activation of PARP cleavage after treating Jurkat T cells with 10 µM SKI-1. We observed this activity after 1a treatment, but only if the inhibitor was present for 16 hours at a concentration (10 µM) far in excess of that required to inhibit SphK1 effectively (Figure 3b). Since SKI-1 was not available to us for direct comparison, we tested the widely used inhibitor, SKI-II [32], a low affinity, non-selective SphK inhibitor (Ki 12–40 µM) [21] that we have found previously lowers S1P levels 4-fold in U937 cells when added at 10 µM for 2 hours [28]. In the present study, SKI-II inhibited ERK phosphorylation but, like 1a, only when added for > 2 h to U-937 cells. Likewise, cleavage of PARP in response to SKI-II treatment required extended (16 hour) treatment of Jurkat T cells.
Fig. 3.
Expression of proteins related to cell viability in U937 and Jurkat T cells treated with compounds 1a and 1b. a: Cultured U937 cells were treated with the indicated concentrations of compounds 1a and 1b (or 10 µM SKI-II) for the indicated times. Cells were then lysed and samples containing the same amount of protein (40 µg) were analyzed by SDS PAGE/Western blotting using primary antibodies against the phosphorylated (pERK and pAkt) and de-phosphorylated forms of ERK and Akt. PD098059 (10 µM) was used as a positive control for inhibition of ERK phosphorylation. b: Cultured Jurkat T cells were treated with the indicated concentrations of compounds 1a and 1b, or 10 µM SKI-II, for 16 h. Cells were then lysed and analyzed as described for panel using primary antibodies against the phosphorylated (pAkt/pERK) and de-phosphorylated forms of Akt/ERK, and the cleaved and un-cleaved forms of Caspase 3 and PARP. Etoposide (5 µM) was used as a positive control for caspase activation. In both panels β-actin was used as a loading control.
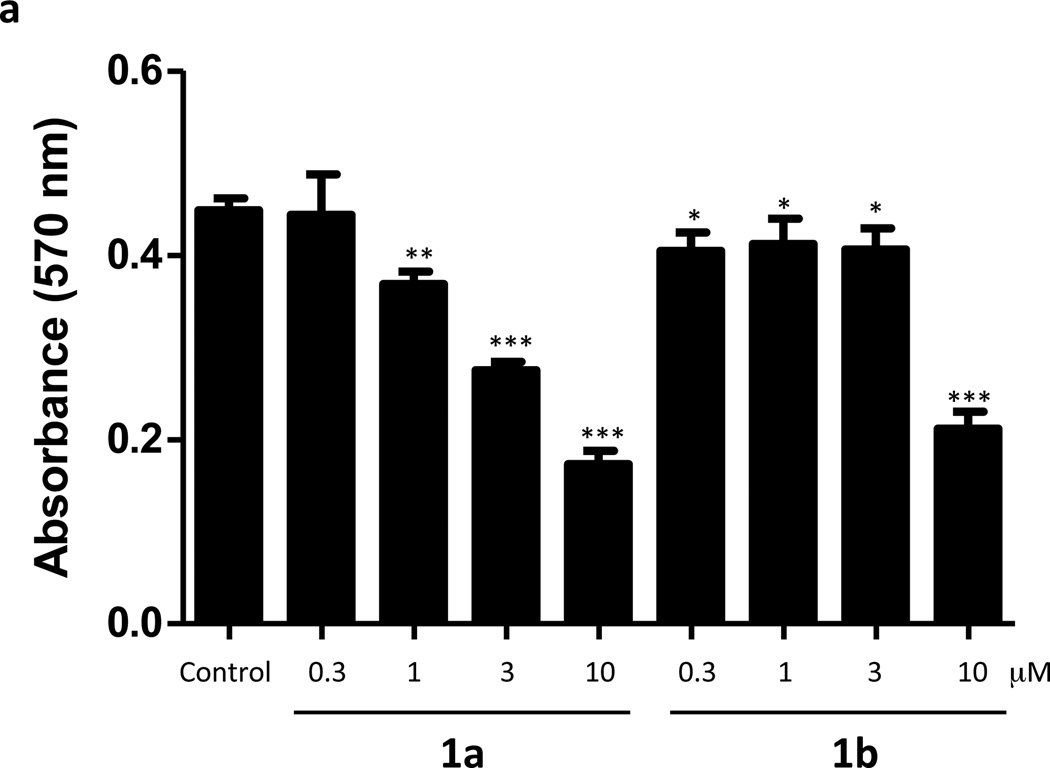
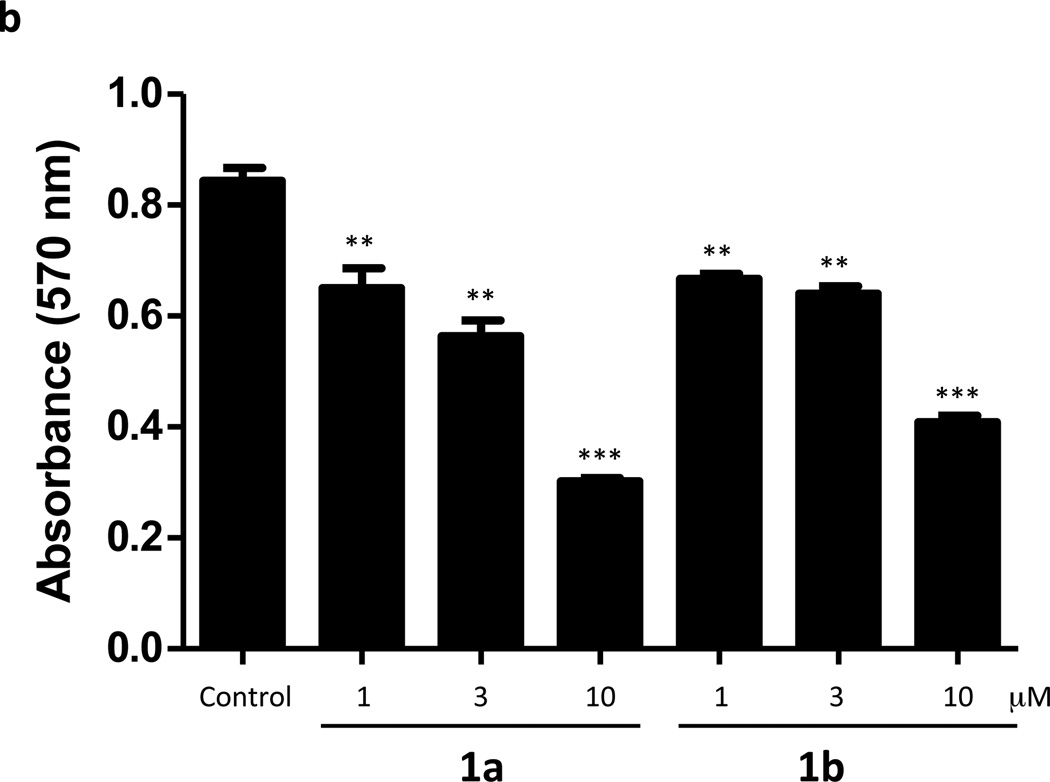
To ascertain whether inhibition of SphK correlated with cytotoxicity, we treated cultures of U937 (Figure 4a) and Jurkat T (Figure 4b) cells with 0.3–10 µM 1a or 1b for 24 hours and assessed cell viability with an MTT assay. As documented in Figure 4, both 1a and 1b exhibit cytotoxic effects on the cells, but only at concentrations far greater than those required to inhibit S1P synthesis. The threshold for cytotoxicity was about 1 µM, which is a value 10-fold higher than is required to significantly reduce S1P levels in these cells. Finally, we observed no change in the growth of U937 cells cultured in the presence of 1a at concentrations of up to 1.0 µM (not shown).
Fig. 4.
Viability of U937 and Jurkat T cells treated with compounds 1a and 1b. Cultured U937 and Jurkat T cells were exposed to different concentrations of compounds 1a and 1b as indicated in the figure. After a 24 h period of exposure, the viability of cells was measured as their ability to reduce a tetrazole compound to the corresponding purple formazan dye (MTT assay). Viability is directly proportional to the amount of formazan dye produced as measured by light absorbance at 570 nm. a: U937 cells, b: Jurkat T cells. Data are means ± SD of six independent experiments. * p < 0.05, *** p < 0.001 (1 way ANOVA, and Dunnett's Multiple Comparison Post Test, compared to Control).
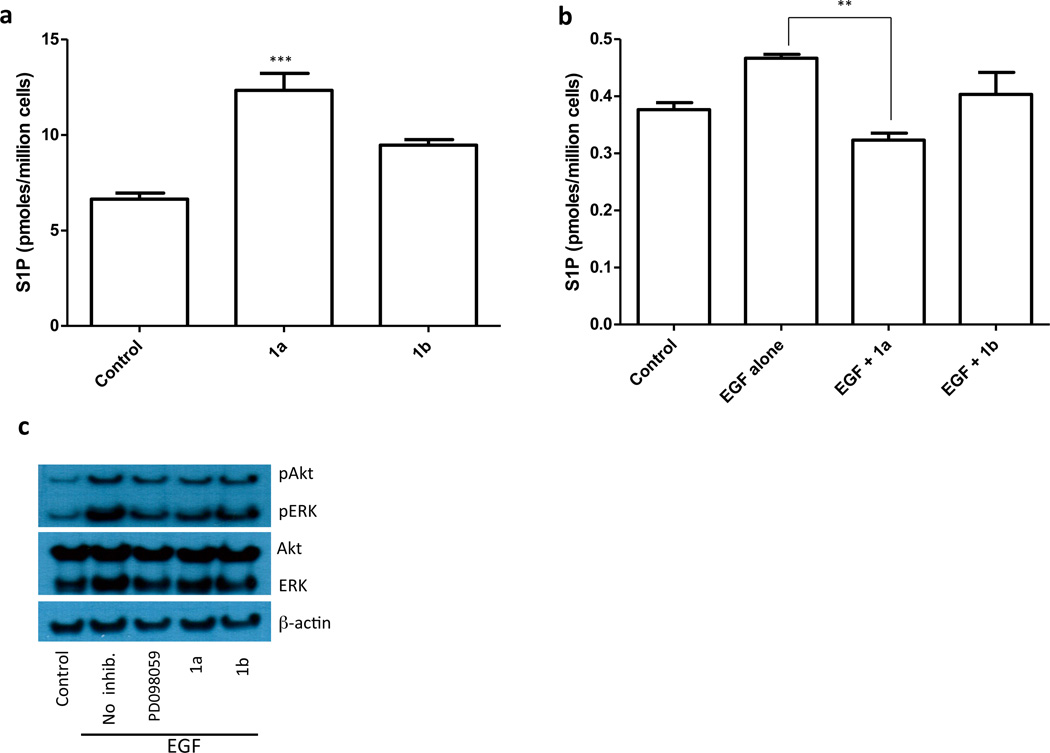
Our results indicate that inhibition of SphK1 in U937 cells does not correlate with inhibition of Akt or ERK phosphorylation. Because U937 cells are unusual in their constitutively high levels of pAkt and pERK, we considered the possibility that SphK1 may play a role in growth factor-mediated phosphorylation of Akt and ERK, a hypothesis that has been supported in several experimental models [23]. To test this hypothesis we used cultured human ovarian cancer SKOV3 cells. Unlike U937 cells, SKOV3 cells have low basal levels of pAkt and pERK, and pAkt and pERK can be stimulated by epidermal growth factor (EGF) treatment. SKOV3 cells, similar to U937 and Jurkat T cells, are sensitive to inhibitor 1a as revealed by the reduction of S1P levels observed after treatment with 0.3 µM 1a (Figure 5a). We found that S1P levels in SKOV3 cells rose in response to EGF treatment and that this increase in S1P was antagonized by inhibitor 1a, but not 1b, treatment (Figure 5b). We observed a similar pattern regarding EGF-evoked increases in pAkt and pERK levels (Figure 5c).
Fig. 5.
Effect of SphK1 inhibition on S1P levels and Akt/ERK phosphorylation by SKOV3 cells. a: Cultured SKOV3 cells were treated with 0.3 µM 1a or 1b for 1 hour. Cells were then, harvested and the amount of cell-associated S1P was measured by LC/MS. b: SKOV3 cells were treated for 1 hour with 0.3 µM 1a or 1b and then stimulated with EGF (10 ng/ml) for 10 min. Cells were then harvested, lysed and the amount S1P was measured by LC/MS. c: Cultured SKOV3 cells treated as described in panel b were analyzed by Western blotting using primary antibodies against the phosphorylated Akt/ERK, total Akt/ERK and β-actin (loading control). The MAP kinase inhibitor PD098059 was used as a positive control.
In vivo evaluation of 1a
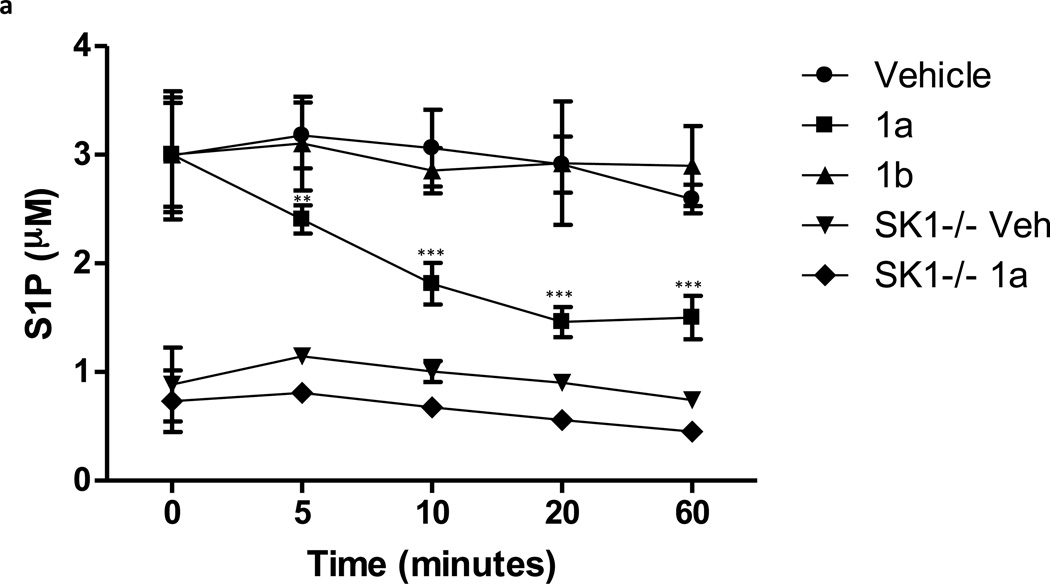
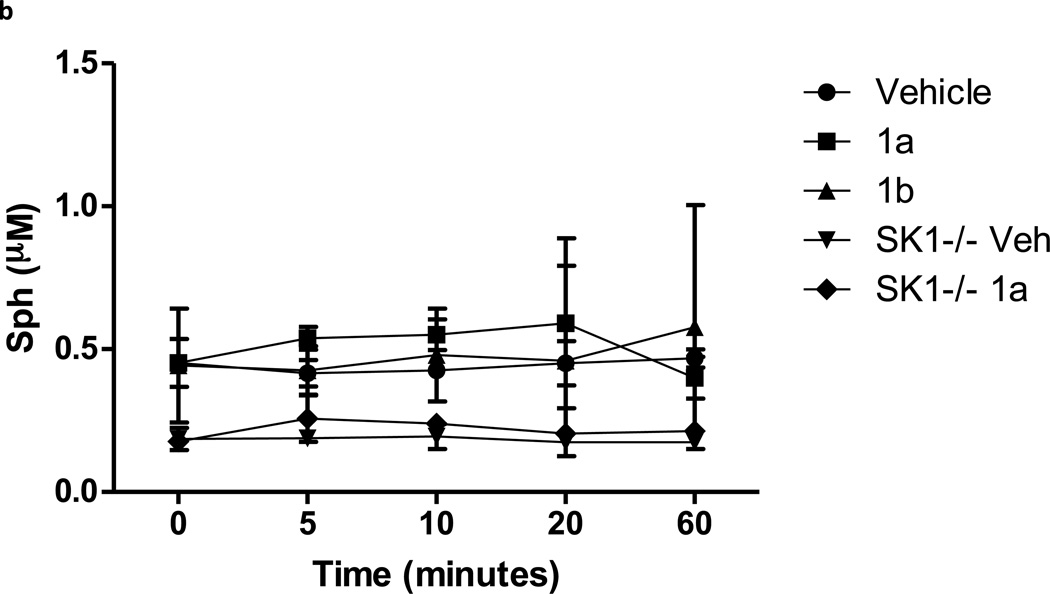
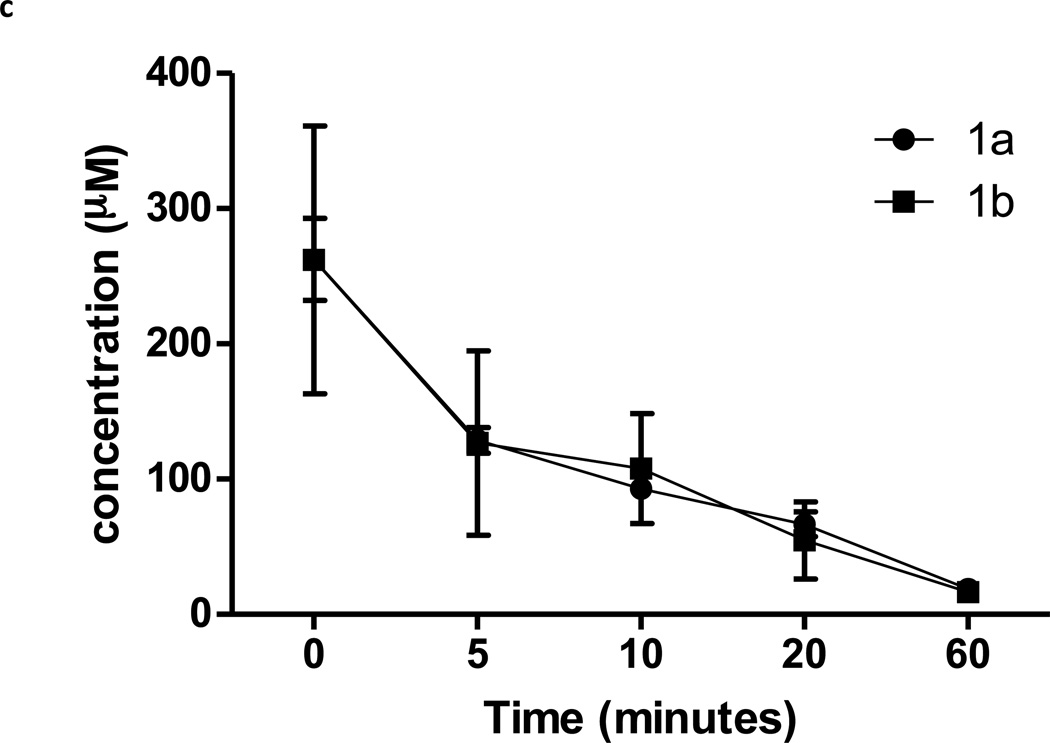
Lastly we determined whether 1a blocked SphK1 in animals. Hla and colleagues reported that intraveneously (IV) administered C17-S1P was cleared from mouse circulation with a 15 minute half life [33]. Therefore we reasoned that an effective SphK1 blockade in mice would likewise result in a rapid diminution of endogenous circulating S1P. To this end we determined the levels of sphingosine and S1P in the blood of mice that were injected IV with 1a or 1b and the levels of these sphingolipids as well as 1a or 1b were measured in blood samples obtained at various times after injection. We found that whole blood S1P levels dropped rapidly to about one-half of control levels shortly after injection of 1a. In contrast, administration of the same dose (10 mg/kg) of the less potent enantiomer, 1b, or vehicle, did not change blood S1P (Figure 6a). As an additional assessment of target specificity, we injected 1a or vehicle into SphK1 null (Sphk1−/−) mice. In these mice, the same dose of compound 1a did not change blood S1P levels (Figure 6a). There was no significant change in blood sphingosine levels in either type of mouse (Figure 6b). Finally, Figure 6c documents that both 1a and 1b are rapidly cleared from circulation, i.e. > 90% of the compound was cleared from the bloodstream by 60 minutes after injection. We performed a parallel study in rats and observed the same result – a rapid decrease in blood S1P levels and rapid clearance of the test compounds. In the rat study, where blood was drawn over a 4 hr time course, we found that blood S1P level returned to pre-dose levels at 2 hr post injection (not shown).
Fig. 6.
S1P, sphingosine and compounds 1a and 1b levels in the blood of mice injected with compounds 1a and 1b. Wild type or Sphk1 null mice were dosed with 10 mg/kg of compounds 1a or 1b or vehicle via the tail vein. Blood samples were obtained after injection at the times indicated in the figure. Levels of S1P, sphingosine and compounds 1a or 1b in blood samples were measured by LC/MS. a: S1P; b: sphingosine, c: compounds 1a and 1b. The lower blood levels of S1P observed in SphK1 null mice are consistent with literature reports [47, 48]. Data are means ± SD of three measurements in different animals. Sphk1 null mice are referred to in the figure as ‘SK1 −/−‘. ** p < 0.01, *** p < 0.001 (repeated measures two way ANOVA, and Bonferroni’s Multiple Comparison Post Test).
DISCUSSION
The pro-survival, pro-migratory and mitogenic effects of S1P coupled with the inducibility of SphK1 by a wide variety of stimuli have generated repeated suggestions that SphK1 could be a viable therapeutic target for small molecule inhibition in diseases characterized by hyper-proliferation, particularly cancer [34, 35]. Numerous studies deploying interfering RNA strategies implicate SphK1 in a variety of signaling cascades, which lends credence to SphK1 as a drug target. However, the SphK1 inhibitors necessary to learn whether rapidly inhibiting enzyme activity recapitulates the effects observed after slowly decreasing protein levels are largely lacking. With few exceptions [36], the inhibitors described heretofore are low potency (Ki values are on the order of 10 µM), non-isotype selective [21] and have not been documented as inhibiting SphK1 enzymatic activity in cells or animals. Further, most of these inhibitors are long chain bases that tend to be cytotoxic to cells, making their use at micromolar concentrations problematic.
In this report, we describe an inhibitor that selectively blocks SphK1. Our claim for selectivity is based on a rank order of IC50 values in cells that matches the Ki values (1a more potent that its enantiomer, 1b) and the lack of an effect of 1a on circulating S1P levels in SphK1 null mice. Although these results build confidence, we cannot rule out off-target effects on, for example, other proteins that bind sphingosine, by these long chain bases particularly at micromolar concentrations. Concern about the non-specific cytotoxicity of long chain bases is addressed to some extent by the use of 1b. Ultimately, the specificity of SphK1 inhibition by small molecules is best established by comparison of multiple inhibitors with a variety of structures.
Compound 1a decreased S1P in cultured U937, Jurkat T and SKOV3 cells and prevents the conversion of Sph to S1P in these cells. Further, we observed no evidence of cell toxicity at concentrations (0.3 µM) of 1a that effectively inhibit SphK1 for treatment periods up to 24 hours. The cellular toxicity that we did observe occurred at concentrations that far exceeded those required to block S1P production. Moreover, equivalent cytotoxicity was observed at equal concentrations of 1a and 1b despite their >100-fold difference in KI values at SphK1.
Our data do not support the contention of Paugh et al. [22] that a SphK1 blockade affects growth, viability or signaling in U937 within a 24 hour time window. Indeed, we were unable to detect any effect on U937 cells despite a nearly complete blockade of the conversion of sphingosine to S1P by SphK1. Only when inhibitor was present at high concentrations (10 µM) could we evoke a response from these cells, and at these concentrations the enantiomers were equipotent, which suggests to us an off-target mechanism. However, we found that SphK1 blockade with 1a did inhibit EGF stimulated accumulation of pAkt and pERK in SKOV3 cells. At this juncture, we do not know whether the S1P produced in response to EGF is acting through cell surface S1P receptors in an autocrine fashion or through some other intracellular mechanism that is independent of canonical S1P receptors [37].
The concept of sphingolipid homeostasis – cells balancing the relative levels of sphingosine / ceramide and S1P – is intriguing. An implication of this idea is that blockade of the conversion of sphingosine to S1P will both lower S1P and raise sphingosine and ceramide levels. Reportedly, sphingosine levels are approximately 10-fold higher than S1P in cells and ceramide levels are another log order higher [38]. Given this 100-fold difference between cellular levels of ceramide and S1P, we thought it unlikely that SphK inhibition would increase ceramide substantially per se. Indeed, when SphK1 is inhibited by 1a, S1P levels decrease more than 10-fold, while sphingosine levels rise barely 2-fold in U937 cells while changes in ceramide levels are undetectable (Figure 1).
The rapid decrease in circulating S1P in response to SphK1 inhibition by 1a observed in vivo is intriguing. In addition to providing compelling evidence of the selectivity of 1a for the SphK1 isotype, this result indicates that blood S1P levels can serve as a biomarker of SphK1 inhibition. We do not know the mechanism(s) whereby S1P is cleared from blood, but an obvious potential route is de-phosphorylation catalyzed by the ubiquitous lipid phosphate phosphohydrolases; another is by S1P lyase. We found that the S1P concentration in whole mouse blood is about 3 µM (Figure 6a) while that in mouse plasma is about 1o-fold lower (not shown). Such a difference is expected in view of the observation that erythrocytes have high S1P levels [39, 40]. It will be interesting to learn whether, following SphK1 inhibition, plasma S1P levels remain constant while whole blood S1P stores are depleted. Regardless of the mechanism(s), our data suggest that the S1P pool in blood is in a state of rapid flux. Interestingly, a structurally similar bioactive lysophospholipid, lysophosphatidic acid, also turns over rapidly in circulation [41, 42].
The existence of S1P in the bloodstream at low micromolar levels and in plasma at high nanomolar levels suggests a physiologic role for this molecule. Studies with the S1P receptor agonist pro-drug, FTY720 (fingolimod), led to the realization that S1P is involved in egress of lymphocytes from secondary lymphoid tissues and implicated S1P in controlling heart rate [43]. A third role for circulating S1P was suggested by administration of S1P1/3 receptor antagonists, which elicit vascular leakage acutely in mice [44, 45]. The decrease in blood S1P in response to SphK1 inhibition provides, in theory, a method to test whether acute changes in circulating S1P levels influence blood lymphocyte levels, heart rate and/or capillary integrity.
Signaling at the S1P1 receptor is critical for proper lymphocyte egress from lymphoid tissues [46]. Therefore we considered using 1a to induce lymphopenia in animals. However, the short half-life of 1a in vivo coupled with the observation that normal lymphocyte trafficking is maintained until plasma S1P becomes very low [46] makes detecting changes in circulating lymphocytes problematic with this particular compound. Injected S1P slows heart rate in anesthetized rats [47], but we do not yet know whether the 50% reduction in blood S1P achieved after 1a injection increases heart rate in rodents. We have measured pulmonary leakage of Evans blue dye in mice injected with 1a to assess the effects of a decreased S1P circulatory tone on the endothelial barrier. However, our results to date have been inconsistent. In future studies, we will attempt to magnify the effect of SphK1-selective inhibitors by using SphK2 null (Sphk2−/−) mice and analogs that persist longer in vivo.
In sum, administration of compound 1a mounts a sustained, selective blockade of SphK1 in cultured cells and a transient, blockade in vivo. This inhibition neither induces a cell stress response nor limits proliferation by U937 cells, but the inhibition does blunt an EGF response in SKOV3 cells. Although too short lived in vivo to be useful in animal models of disease, 1a is adequate in establishing persistent SphK1 inhibition in cultured cells. We will be particularly interested to learn how well the results obtained by the slow onset, partial reduction in SphK1 protein levels correlate with the rapid inhibition of SphK1 catalytic activity achieved by compounds such as 1a.
ACKNOWLEDGEMENTS
The authors thank Dr. Jeremy Allegood (Department of Biochemistry, Virginia Commonwealth University, Richmond, VA) for help in adapting the protocol described in ref. 29, Dr. Richard Proia (NIH/NIDDK, Bethesda, MD) for the gift of Sphk1−/− mice, Dr. Kaoru Goto (Yamagata University School of Medicine, Yamagata, Japan) and Dr. Matthew Topham (University of Utah, Salt Lake City, UT) for their gifts of diacylglycerol kinase plasmids and Gina Wimer (Department of Comparative Medicine, University of Virginia) for help with animal handling.
FUNDING
This work was supported by grants by the National Institutes of Health (R01 GM067958, T32 GM008715) and the Ivy Foundation for Biomedical Research.
Abbreviations used in this text
- S1P
sphingosine 1-phosphate
- Sph
sphingosine
- SphK1
sphingosine kinase type 1
- SphK2
sphingosine kinase type 2
- LC/MS
liquid chromatography / mass spectrometry
- HPCD
hydroxypropyl-β-cyclodextrin
- dhS1P
dihydrosphingosine 1-phosphate
- dhSph
dihydrosphingosine (sphinganine)
Footnotes
Author Contributions: Y.K., T.P.M. and K.R.L. designed the experiments, T.P.M., M.L.M. and T.L.M. designed and synthesized the inhibitors, Y.K., T.P.M. and J.L.T. performed LC/MS sphingolipid analyses. Y.K. and P.C.K. performed the in vivo studies, T.P.M. and K.R.L. co-wrote the manuscript, all of the authors participated in editing the manuscript.
Conflict of Interest Statement: Y.K., T.P.M., T.L.M. and K.R.L. are inventors on a patent application claiming amidine-based sphingosine kinase inhibitors including 1a and 1b. This application has been licensed by the University of Virginia to a commercial entity in which both T.L.M. and K.R.L. have equity positions.
REFERENCES
- 1.Ishii M, Egen JG, Klauschen F, Meier-Schellersheim M, Saeki Y, Vacher J, Proia RL, Germain RN. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 2009;458:524–528. doi: 10.1038/nature07713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kai M, Heisenberg CP, Tada M. Sphingosine-1-phosphate receptors regulate individual cell behaviours underlying the directed migration of prechordal plate progenitor cells during zebrafish gastrulation. Development. 2008;135:3043–3051. doi: 10.1242/dev.020396. [DOI] [PubMed] [Google Scholar]
- 3.Czeloth N, Bernhardt G, Hofmann F, Genth H, Förster R. Sphingosine-1-phosphate mediates migration of mature dendritic cells. J. Immunology. 2005;175:2960–2967. doi: 10.4049/jimmunol.175.5.2960. [DOI] [PubMed] [Google Scholar]
- 4.Shah MV, Zhang R, Irby R, Kothapalli R, Liu X, Arrington T, Frank B, Lee NH, Loghran TP., Jr Molecular profiling of LGL leukemia reveals role of sphingolipid signaling in survival of cytotoxic lymphocytes. Blood. 2008;112:770–781. doi: 10.1182/blood-2007-11-121871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J, Honbo N, Goetzl EJ, Chatterfee K, Karliner JS, Gray MO. Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am. J Physiology. 2007;293:H3150–H3158. doi: 10.1152/ajpheart.00587.2006. [DOI] [PubMed] [Google Scholar]
- 6.Oyama O, Sugimoto N, Qi X, Takuwa N, Mizugishi K, Koizumi J, Takuwa Y. The lysophospholipid mediator sphingosine-1-phosphate promotes angiogenesis in vivo in ischaemic hindlimbs of mice. Cardiovascular Res. 2008;78:301–307. doi: 10.1093/cvr/cvn002. [DOI] [PubMed] [Google Scholar]
- 7.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: Mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacology & Therapeutics. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Brinkmann V. FTY720 (fingolimod) in multiple sclerosis: therapeutic effects in the immune and the central nervous system. Br. J. Pharmacology. 2009;158:1173–1182. doi: 10.1111/j.1476-5381.2009.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olivera A, Kohama T, Tu Z, Milstien S, Spiegel S. Purification and characterization of rat kidney sphingosine kinase. J. Biol. Chem. 1998;273:12576–12583. doi: 10.1074/jbc.273.20.12576. [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Sugiura M, Nava VE, Edsall LC, Kono K, Poulton S, Milstien S, Kohama T, Spiegel S. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J. Biol. Chem. 2000;275:19513–19520. doi: 10.1074/jbc.M002759200. [DOI] [PubMed] [Google Scholar]
- 11.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol. Cell. Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia P, Gamble JR, Wang L, Pitson SM, Moretti PA, Wattenberg BW, D'Andrea RJ, Vadas MA. An oncogenic role of sphingosine kinase. Curr. Biology. 2000;10:1527–1530. doi: 10.1016/s0960-9822(00)00834-4. [DOI] [PubMed] [Google Scholar]
- 13.Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim. Biophys. Acta. 2006;1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Mandala SM, Thornton R, Tu Z, Kurtz MB, Nickels J, Broach J, Menzeleev R, Spiegel S. Sphingoid base 1-phosphate phosphatase: a key regulator of sphingolipid metabolism and stress response. Proc. Natl. Acad. Sci. USA. 1998;95:1150–1155. doi: 10.1073/pnas.95.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nava VE, Hobson JP, Murthy S, Milstien S, Spiegel S. Sphingosine kinase type 1 promotes estrogen-dependent tumorigenesis of breast cancer MCF-7 cells. Exp. Cell Res. 2002;281:115–127. doi: 10.1006/excr.2002.5658. [DOI] [PubMed] [Google Scholar]
- 16.Pchejetski D, Golzio M, Bonhoure E, Calvet C, Doumerc N, Garcia V, Mazerolles C, Rischmann P, Teissié J, Malavaud B, Cuvillier O. Sphingosine kinase-1 as a chemotherapy sensor in prostate adenocarcinoma cell and mouse models. Cancer Res. 2005;65:11667–11675. doi: 10.1158/0008-5472.CAN-05-2702. [DOI] [PubMed] [Google Scholar]
- 17.Sauer L, Nunes J, Salunkhe V, Skalska L, Kohama T, Cuvillier O, Waxman J, Pchejetski D. Sphingosine kinase 1 inhibition sensitizes hormone-resistant prostate cancer to docetaxel. Int. J. Cancer. 2009;125:2728–2736. doi: 10.1002/ijc.24640. [DOI] [PubMed] [Google Scholar]
- 18.Young N, Pearl DK, van Brocklyn JR. Sphingosine-1-phosphate regulates glioblastoma cell invasiveness through the urokinase plasminogen activator system and CCN1/Cyr61. Mol. Cancer Res. 2009;7:23–32. doi: 10.1158/1541-7786.MCR-08-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weigert A, Schiffmann S, Sekar D, Ley S, Menrad H, Werno C, Grosch S, Geisslinger G, Brüne B. Sphingosine kinase 2 deficient tumor xenografts show impaired growth and fail to polarize macrophages towards an anti-inflammatory phenotype. Int. J. Cancer. 2009;125:2114–2121. doi: 10.1002/ijc.24594. [DOI] [PubMed] [Google Scholar]
- 21.Kharel Y, Mathews TP, Kennedy AJ, Macdonald TL, Lynch KR. A rapid assay for assessment of sphingosine kinase inhibitors and substrates. Anal. Biochem. 2011;411:230–235. doi: 10.1016/j.ab.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paugh SW, Paugh BS, Rahmani M, Kapitonov D, Almenara JA, Kordula T, Milstien S, Adams JK, Zipkin RE, Grant S, Spiegel S. A selective sphingosine kinase 1 inhibitor integrates multiple molecular therapeutic targets in human leukemia. Blood. 2008;112:1382–1391. doi: 10.1182/blood-2008-02-138958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapitonov D, Allegood JC, Mitchell C, Hait NC, Almenara JA, Adams JK, Zipkin RE, Dent P, Kordula T, Milstien S, Spiegel S. Targeting sphingosine kinase 1 inhibits Akt signaling, induces apoptosis, and suppresses growth of human glioblastoma cells and xenografts. Cancer Res. 2009;69:6915–6923. doi: 10.1158/0008-5472.CAN-09-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong L, Tan SS, Lam Y, Melendez AJ. Synthesis and evaluation of sphingosine analogues as inhibitors of sphingosine kinases. J. Med. Chem. 2009;52:3618–3626. doi: 10.1021/jm900121d. [DOI] [PubMed] [Google Scholar]
- 25.Puneet P, Yap C, Wong L, Yulin L, Koh D, Moochhala S, Pfeilschifter J, Huwiler A, Melendez A. SphK1 regulates proinflammatory responses associated with endotoxin and polymicrobial sepsis. Science. 2010;328:1290–1294. doi: 10.1126/science.1188635. [DOI] [PubMed] [Google Scholar]
- 26.Kim HL, Sacket SJ, Han M, Im DS. Characterization of N,N, dimethyl-D-erythro-sphingosine-induced apoptosis and signaling in U937 cells: independence of sphingosine kinase inhibition. Prostaglandins & Other Lipid Mediators. 2008;86:18–25. doi: 10.1016/j.prostaglandins.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Mathews TP, Kennedy AJ, Kharel Y, Kennedy PC, Nicoara O, Sunkara M, Morris AJ, Wamhoff BR, Lynch KR, Macdonald TL. Discovery, biological evaluation, and structure-activity relationship of amidine based sphingosine kinase inhibitors. J. Med. Chem. 2010;53:2766–2778. doi: 10.1021/jm901860h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy AJ, Mathews TP, Kharel Y, Field SD, Moyer JL, East JE, Houck JD, Lynch KR, Macdonald TL. Development of amidine-based sphingosine kinase 1 nanomolar inhibitors and reduction of sphingosine 1-phosphate in human leukemia cells. J. Med. Chem. 2011;54:3524–3548. doi: 10.1021/jm2001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaner RL, Allegood JC, Park H, Wang E, Kelly S, Haynes CA, Sullards MC, Merrill AH. Quantitative analysis of sphingolipids for lipidomics using triple quadrupole and quadrupole linear ion trap mass spectrometers. J. Lipid Res. 2009;50:1692–1707. doi: 10.1194/jlr.D800051-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu R, Snyder AH, Kharel Y, Schaffter L, Sun Q, Kennedy PC, Lynch KR, Macdonald TL. Asymmetric synthesis of conformationally constrained fingolimod analogues--discovery of an orally active sphingosine 1-phosphate receptor type-1 agonist and receptor type-3 antagonist. J. Med. Chem. 2007;50:6428–6435. doi: 10.1021/jm7010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edsall LC, van Brocklyn JR, Cuvillier O, Kleuser B, Spiegel S. N,N Dimethylsphingosine is a potent competitive inhibitor of sphingosine kinase but not of protein kinase C: modulation of cellular levels of sphingosine 1-phosphate and ceramide. Biochemistry. 1998;37:12892–12898. doi: 10.1021/bi980744d. [DOI] [PubMed] [Google Scholar]
- 32.French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Everly JL, Yun JK, Smith CD. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003;63:5962–5969. [PubMed] [Google Scholar]
- 33.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circulation Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pitman MR, Pitson SM. Inhibitors of the sphingosine kinase pathway as potential therapeutics. Curr. Cancer Drug Targets. 2010;10:354–367. doi: 10.2174/156800910791208599. [DOI] [PubMed] [Google Scholar]
- 35.Ader I, Malavaud B, Cuvillier O. When the sphingosine kinase 1/sphingosine 1-phosphate pathway meets hypoxia signaling: new targets for cancer therapy. Cancer Res. 2009;69:3723–3726. doi: 10.1158/0008-5472.CAN-09-0389. [DOI] [PubMed] [Google Scholar]
- 36.Xiang Y, Asmussen G, Booker M, Hirth B, Kane JL, Jr, Liao J, Noson KD, Yee C. Discovery of novel sphingosine kinase 1 inhibitors. Bioorg. Med. Chem. Lett. 2009;19:6119–6121. doi: 10.1016/j.bmcl.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 37.Shu X, Wu W, Mosteller RD, Broek D. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases. Mol. Cell. Biol. 2002;22:7758–7768. doi: 10.1128/MCB.22.22.7758-7768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hannun Y, Obeid L. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 39.Hänel P, andréami P, Gräler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 40.Bode C, Sensken S, Peest U, Beutel G, Thol F, Levkau B, Li Z, Bittman R, Huang T, Tölle M, Van Der Giet M, Gräler MH. Erythrocytes serve as a reservoir for cellular and extracellular sphingosine 1-phosphate. J. Cell. Biochem. 2010;109:1232–1243. doi: 10.1002/jcb.22507. [DOI] [PubMed] [Google Scholar]
- 41.Tomsig JL, Snyder AH, Berdyshev EV, Skobeleva A, Mataya C, Natarajan V, Brindley DN, Lynch KR. Lipid phosphate phosphohydrolase type 1 (LPP1) degrades extracellular lysophosphatidic acid in vivo. Biochemical J. 2009;419:611–618. doi: 10.1042/BJ20081888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albers HMHG, Dong A, van Meeteren LA, Egan DA, Sunkara M, van Tilburg EW, Schuurman D, van Telingen O, Morris AJ, Smyth SS, Moolenaar WH, Ovaa H. Boronic acid-based inhibitor of autotaxin reveals rapid turnover of LPA in the circulation. Proc. Natl. Acad. Sci. USA. 2010;107:7257–7262. doi: 10.1073/pnas.1001529107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forrest M, Sun SY, Hajdu R, Bergstrom J, Card D, Doherty G, Hale J, Keohane C, Meyers C, Milligan J, Mills S, Nomura N, Rosen H, Rosenbach M, Shei GJ, Singer II, Tian M, West S, White V, Xie J, Proia RL, Mandala S. Immune cell regulation and cardiovascular effects of sphingosine 1-phosphate receptor agonists in rodents are mediated via distinct receptor subtypes. J. Pharmacology Experimental & Therapeutics. 2004;309:758–768. doi: 10.1124/jpet.103.062828. [DOI] [PubMed] [Google Scholar]
- 44.Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, Wei SH, Parker I, Jo E, Cheng WC, Cahalan MD, Wong CH, Rosen H. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nature Chem. Biol. 2006;2:434–441. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- 45.Foss FW, Snyder AH, Davis MD, Rouse M, Okusa MD, Lynch KR, Macdonald TL. Synthesis and biological evaluation of gammaaminophosphonates as potent, subtype-selective sphingosine 1-phosphate receptor agonists and antagonists. Bioorg. Med. Chem. 2007;15:663–677. doi: 10.1016/j.bmc.2006.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 47.Sugiyama A, Aye NN, Yatomi Y, Ozaki Y, Hashimoto K. Effects of sphingosine 1-phosphate, a naturally occurring biologically active lysophospholipid, on the rat cardiovascular system. Jpn. J. Pharmacol. 2000;82:338–342. doi: 10.1254/jjp.82.338. [DOI] [PubMed] [Google Scholar]
- 48.Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Dekert G, Hajdu R, Rosenbach M, Keohane CA, Mandala S, Spiegel S, Proia RL. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J. Biol. Chem. 2004;279:52478–52492. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- 49.Zenmann B, Kinzel B, Müller M, Reuschel R, Mechtcheriakova D, Urtz N, Bornacin R, Baumruker T, Billich A. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood. 2006;107:1454–1458. doi: 10.1182/blood-2005-07-2628. [DOI] [PubMed] [Google Scholar]