Abstract
OSI-930, a dual c-Kit and KDR tyrosine kinase inhibitor, is reported to have undergone a Phase I dose escalation study in patients with advanced solid tumors. A series of fifteen pyridyl and phenyl analogues of OSI-930 were designed and synthesized. Extensive screening of these compounds led to the discovery that nitropyridyl and ortho-nitrophenyl analogues, VKJP1 and VKJP3, were effective in reversing ABC subfamily G member 2 (ABCG2) transporter-mediated multidrug resistance (MDR). VKJP1 and VKJP3 significantly sensitized ABCG2-expressing cells to established substrates of ABCG2 including mitoxantrone, SN-38, and doxorubicin in a concentration-dependent manner, but not to the non-ABCG2 substrate cisplatin. However, they were unable to reverse ABCB1- or ABCC1-mediated MDR indicating their selectivity for ABCG2. Western blotting analysis was performed to evaluate ABCG2 expression and it was found that neither VKJP1 nor VKJP3 significantly altered ABCG2 protein expression for up to 72 h. [3H]-mitoxantrone accumulation study demonstrated that VKJP1 and VKJP3 increased the intracellular accumulation of [3H]-mitoxantrone, a substrate of ABCG2. VKJP1 and VKJP3 also remarkably inhibited the transport of [3H]-methotrexate by ABCG2 membrane vesicles. Importantly, both VKJP1 and VKJP3 were efficacious in stimulating the activity of ATPase of ABCG2 and inhibited the photoaffinity labeling of this transporter by its substrate [125I]-iodoarylazidoprazosin. The results suggested that VKJP1 and VKJP3, specifically inhibit the function of ABCG2 through direct interaction with its substrate binding site(s). Thus VKJP1 and VKJP3 represent a new class of drugs for reducing MDR in ABCG2 over-expressing tumors.
Keywords: OSI-930, ABC transporters, ABCG2, multidrug resistance
1. Introduction
Multidrug resistance (MDR) is a major obstacle to successful chemotherapy in cancer treatment. It is a phenomenon in which cancer cells become resistant to a wide spectrum of anti cancer drugs that belong to different chemical or pharmacological classes and results in decreased cytotoxic activity of these anticancer drugs [1, 2]. The mechanisms of MDR are multifactorial and include alteration in the permeability of lipid bilayer membrane, increased DNA repair in cancer cells, increased inactivation or detoxification of drugs, or inhibition of apoptosis [3, 4]. Over-expression of multiple ATP binding cassette (ABC) transporters in cancer cells still remains the leading cause of MDR [5–7]. The term ABC transporter, is based on the fact that almost all members of the ABC superfamily, from yeast and bacteria to man, share a conserved consensus sequence of 90–110 amino acids, where ATP binds and is hydrolyzed by the ATPase enzyme. This consensus has Walker A and B motifs and another C region called ABC cassette. ABC transporters is the largest trans-membrane protein family encoded in the human genome, which are divided into 7 subfamilies ABC-A to G and subdivided into sub-subfamilies depending on their structural similarity or difference in their trans-membrane domain. The prominent ABC transporters playing a role in MDR development are ABCB1, also called P-glycoprotein (P-gp) [8, 9], multidrug resistance proteins (MRPs, ABCCs) [10], and ABCG2 (BCRP/MXR/ABCP) [11, 12]. This ABC cassette is involved in transportation of not only toxic metabolites or xenobiotics out of the cell, but also a multitude of anticancer drugs across cell membrane, thus decreasing their intracellular concentration and cytotoxicity. Therefore, their expression is believed to be the major mechanism by which MDR to anticancer drugs develops. Initially, verapamil was found by Tsuruo et. al. to be the first modulator which could inhibit the function of ABCB1 and increase drug accumulation in the cancer cells [13]. Later, cyclosporine A (CsA), an immunosuppressant, was discovered to inhibit the function of ABCB1 and was used as another modulator of ABCB1 [14]. After that, many other compounds have been studied for their inhibitory effects to other members of ABC transporter family [15–18], raising hopes that ABC transporter mediated MDR could be reversed. At present, as the newly synthesized molecule-targeting agents such as tyrosine kinase inhibitors (TKIs) are being introduced into the clinic, research is being conducted to determine whether these novel compounds could represent a new class of ABC transporter inhibitors. TKIs such as imatinib, nilotinib, erlotinib, dasatinib and lapatinib have been extensively investigated as modulators of ABC transporters-mediated MDR, which most likely act as competitive inhibitors [19–23]. More recently, pre-clinical research and clinical trials have been conducted to study the efficacy of the combination of some TKIs with other anticancer drugs in improving the therapeutic outcome of cancer patients. Therefore, discovering novel inhibitors of ABC transporters to reverse the drug resistance can lead to improvement in the use of currently available anticancer drugs.
OSI-930 {N-(4-trifluoromethoxyphenyl) 3-((quinolin-4-ylmethyl) amino) thiophene -2-carboxamide} is a heterocyclic anthranilamide analogue synthesized by OSI Pharmaceuticals Inc. It is a potent and selective dual inhibitor of the closely related receptor tyrosine kinases (RTKs) Kit (c-Kit, a type III RTK over-expressed in seminoma, acute myeloid leukemia and in gastrointestinal stromal tumor) and kinase insert domain receptor (KDR, a type V RTK as a regulator of tumor angiogenesis) [24,25]. OSI-930 acts by these two distinct mechanisms in appropriate tumor types: i.e., direct effects on the tumor cell phenotype through inhibition of c-Kit and indirect effects via disruption of endothelial cell function by inhibition of KDR and is reported to be under phase I clinical trials. The quinoline domain of OSI-930 was modified with heteroatom substituted pyridyl and phenyl ring systems by our group to obtain a series of fifteen compounds (Figure 1) [26]. The effects of these changes on their activities and the binding site characteristics of c-Kit and KDR were evaluated using a kinase assay. In the present study, we report the screening of our newly designed and synthesized OSI 930 analogues to determine their ability to reverse ABC transporter-mediated MDR. The reversal mechanisms of the most efficacious analogues VKJP1 and VKJP3 were further evaluated by membrane vesicle transport assay, photolabeling affinity assay, intracellular drug accumulation assay and ATPase assay.
Fig. 1. Chemical structures of OSI-930 and VKJP compounds 1–15.
2. Materials and Methods
2.1 Chemicals and reagents
[3H]-mitoxantrone (4 Ci/mmol) and [3H]-methotrexate (23 Ci/mmol) were purchased from Moravek Biochemicals, Inc. (Brea, CA). [125I]-iodoarylazidoprazosin (IAAP, 2,200 Ci/mmol) were obtained from Perkin-Elmer Life Sciences. (Boston, MA). BXP-21 (against ABCG2) was acquired from Signet Laboratories, Inc. (Ded-Ham, MA). Anti-actin monoclonal antibody was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). OSI-930 was gift from Selleck Chemical. (Houston, TX) OSI-930 analogues were successfully designed and synthesized by our group at St. John’s University. (New York, NY). PAK104P was a kind gift from Prof. Shin-Ichi Akiyama (Kagoshima University, Kagoshima, Japan) from Nissan Chemical Ind. Co. Ltd (Chiba, Japan). Fumitremorgin C (FTC) was synthesized by Thomas McCloud Developmental Therapeutics Program, Natural Products Extraction Laboratory, National Cancer Institute, NIH. (Bethesda, MD). Mitoxantrone, SN-38, doxorubicin, cisplatin, colchicine, vincristine, verapamil, Penicillin/streptomycin, 3-(4, 5-dimethylthiazol -2-yl)-2, 5-diphenyltetrazolium bromide (MTT), dimethylsulfoxide (DMSO), ATP, AMP and other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO).
2.2 Cell lines and cell culture
HEK293/pcDNA3.1, ABCG2-482-R2, and ABCG2-482-T7 cells were established by selection with G418 after transfecting HEK293 with either the empty pcDNA3.1 vector or pcDNA3.1 vector containing the full length ABCG2 coding either arginine (R) or threonine (T) at amino acid 482, respectively [27]. They were cultured in medium with 2 mg/ml of G418 [27]. The parental human epidermoid carcinoma cell line KB-3-1 was selected in a stepwise manner using increasing concentration of colchicine to establish the ABCB1-overexpressing drug resistant cell line KB-C2 and was cultured in the medium with 2 µg/ml of colchicine [28]. An ABCC1- overexpressing MDR cell line, KB-CV60, was also cloned from KB-3-1 cells and was maintained in medium with 1 µg/ml cepharanthine and 60 ng/ml vincristine [29]. All the cell lines were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% bovine serum, 100 units/ml penicillin, and 100 mg/ml streptomycin in a humidified incubator containing 5% CO2 at 37°C.
2.3 Cell sensitivity to drugs by MTT assay
Cell sensitivity to drugs was analyzed using an MTT colorimetric assay [19]. Cells were harvested with trypsin and resuspended in a final concentration 8 × 103 cells/ml for KB-C2 and 5 × 103 for all the other cell lines. Cells were seeded evenly into (160 µl/well) 96-well multiplates. After 24 h of incubation, the test VKJP compound (20 µl/well) was added 1 h prior to different concentrations of chemotherapeutic drugs (20 µl/well) into designated wells. FTC, verapamil or PAK-104 (20 µl/well) was added as positive control inhibitors of ABCG2, ABCB1 or ABCC1, respectively. After 72 h of incubation, 20 µl of MTT solution (4 mg/ml) was added to each well, further incubated for 4 h, the medium was discarded, and 100 µl of DMSO was added into each well to dissolve the formazan crystals. The absorbance was determined at 570 nm by an OPSYS microplate Reader from DYNEX Technologies, Inc. (Chantilly, VA). The concentrations required to inhibit growth by 50% (IC50) were calculated from survival curves. The degree of resistance was calculated by dividing the IC50 of the MDR cells by that of the parental sensitive cells.
2.4 Preparation of cell lysates
Cells in T-25 flask treated with 10 µM VKJP1 or VKJP3 for different time periods (0, 36, 72 h), then were harvested and rinsed twice with cold PBS. The cell extracts were prepared by incubating the cells with the Radioimmunoprecipitation assay (RIPA) buffer [1 × PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 100 µM p-aminophenylmethylsulfonyl fluoride (p-APMSF), 10 µM leupeptin, and 10 µM aprotinin] for 30 min on ice with occasional rocking, followed by centrifugation at 12,000 × g at 4°C for 15 min. The supernatant containing total cell lysates were collected and stored at −80°C until future experiments. The protein concentration was determined by bicinchoninic acid (BCA™)-based protein assay (Thermo Scientific, Rockford, IL).
2.5 Western blotting
Equal amounts of total cell lysates (40 µg) were resolved by 4–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred onto PVDF membrane, then immersed in blocking solution (5% skim milk in Tris-Buffered Saline (TBST: 0.3% Tris, 0.8% NaCl, 0.02% KCl, 0.05% Tween 20) for 1 h at room temperature. The membrane was then immunoblotted overnight with monoclonal anti-ABCG2 antibody (BXP-21) at 1:500 dilution at 4°C. The following day, the membrane was washed with TBST buffer three times and followed by 3 h incubation with horseradish peroxide (HRP)-conjugated secondary anti-mouse IgG at 1:1000 for 3 h. Proteins were detected by enhanced chemiluminescence detection system (Amersham, NJ). β-Actin was used to confirm equal loading in each lane in the samples prepared from cell lysates.
2.6 [3H]-mitoxantrone accumulation
The effect of VKJP1 or VKJP3 on the intracellular accumulation of [3H]-mitoxantrone in HEK293/pcDNA3.1, ABCG2-482-R2, and ABCG2-482-T7 cells was determined as previously described [30]. Confluent cells in 24-well plates were preincubated with or without VKJP1, VKJP3 (FTC as positive control) for 1 h at 37°C. Intracellular drug accumulation was measured by incubating cells with 0.2 µM [3H]-mitoxantrone for another 2 h. The cells were washed three times with ice-cold PBS, trypsinized and lysed in 10 mM lysis buffer (pH 7.4, containing 1% Triton X-100 and 0.2% SDS). Each sample was placed in scintillation fluid and radioactivity was measured in a Packard TRI-CARB® 1900CA liquid scintillation analyzer from Packard Instrument Company, Inc (Downers Grove, IL).
2.7 Preparation of membrane vesicles and in vitro transport assays
Membrane vesicles were prepared by using the nitrogen cavitation method [31]. Cells were rinsed twice with PBS and then scraped into PBS containing 1% aprotinin. Cells were then washed at 4°C in PBS, collected by centrifugation (4000 × g for 10 min), suspended in buffer A (10 mM Tris-HCl, pH 7.4, 0.25 M sucrose, 1 mM p-APMSF, and 0.2 mM CaCl2) and equilibrated at 4°C for 15 min under a nitrogen pressure of 500 psi. EDTA was added to the suspension of lysed cells to make a final concentration of 1 mM, and the suspension was then diluted 1:4 with buffer B (10 mM Tris-HCl, pH 7.4, 0.25 M sucrose, and 1 mM p-APMSF) and centrifuged at 4000 × g for 10 min at 4°C to remove nuclei and unlysed cells. The supernatant was layered onto a sucrose cushion (35% sucrose, 10 mM Tris-HCl, pH 7.4, and 1 mM EDTA) and centrifuged for 30 min at 16,000 × g at 4°C. The interface was collected and centrifuged for 45 min at 100,000 × g at 4°C. The pellet was resuspended in buffer B by repeated passage through a 25-gauge needle.
Transport assays were performed using the rapid filtration method as previously described [19]. Membrane vesicles were incubated with VKJP1 or VKJP3 (FTC as positive control) for 1 h on ice, and then transport experiments were carried out at 37°C for 10 min in a total volume of 50 µl medium (membrane vesicles 10 µg, 0.25 M sucrose, 10 mM Tris-HCl, pH 7.4, 10 mM MgCl2, 4 mM ATP or 4 mM AMP, 10 mM phosphocreatine, 100 µg/ml creatine phosphokinase, and 0.5 µM [3H]-methotrexate). Reactions were stopped by the addition of 3 ml of ice-cold stop solution (0.25 M sucrose, 100 mM NaCl, and 10 mM Tris-HCl, pH 7.4). During the rapid filtration step, samples were passed through 0.22 µM GVWP filters (Millipore Corporation, Billerica, MA) that were presoaked in the stop solution. The filters were washed three times with 3 ml of ice-cold stop solution. Radioactivity was measured by the use of a liquid scintillation analyzer from Packard Instrument Company, Inc (Downers Grove, IL).
2.8 Effect of VKJP1 and VKJP3 on ATPase activity of ABCG2
ATPase activities of ABCG2 in High Five insect cell crude membranes were measured by endpoint inorganic phosphate Pi assay as described previously with minor modifications [32]. ABCG2-specific ATPase activity was recorded as BeFx-sensitive ATPase activity. Briefly, The membrane vesicles (100 µg protein/ml) were incubated in ATPase assay buffer (50 mM MES-Tris, pH 6.8, 50 mM KCl, 5 mM NaN3, 1 mM EGTA, 1 mM ouabain, 2 mM dithiothreitol and 10 mM MgCl2) for 20 min at 37°C in the absence or presence of 0.2 mM beryllium sulfate and 2.5 mM sodium fluoride (BeFX), then incubated with different concentrations of VKJP1 or VKJP3 at 37°C for 3 min. The reaction was initiated by the addition of 5 mM ATP and terminated by the addition of SDS (2.5% final concentration). The amount of Pi released was quantified using a colorimetric method.
2.9 Effect of VKJP1 and VKJP3 on photoaffinity labeling with [125I]-iodoarylazidoprazosin (IAAP) of ABCG2
Crude membranes of High Five insect cells (250 µg protein/mL) were incubated with increasing concentrations of VKJP1 or VKJP3 at room temperature in 50 mM Tris-HCl, pH 7.5, for 3 min. [125I]-IAAP (3–6 nM) was added and further incubated for additional 5 min under subdued light. The samples were exposed to a UV lamp (365 nm) for 10 min at room temperature and processed as described previously [33]. The samples were separated on a 7 % Tris-acetate gel at a constant voltage. Gel was dried and exposed to Bio-Max MR film (Eastman Kodak, Rochester, NY) for 9 hours at −80 °C. The incorporation of [125I]-IAAP into ABCG2 band was quantified by estimating the radioactivity of this band using the STORM 860 phosphorimager (Molecular Dynamics, Sunnyvale, CA) and ImageQuaNT.
Statistical analysis
Unless otherwise indicated, all experiments were repeated at least three times and the differences were determined by the two-tailed Student's t-test. When statistical differences among more than 2 groups were analyzed, one-way ANOVA followed by Tukey’s multiple comparison tests were performed. Results were presented as mean ± standard deviations (SD). The statistical significance was determined to be P < 0.05.
3. Results
3.1 Structure of VKJP1 and VKJP3
A series of OSI-930 analogues 1–15 (Figure 1) were successfully designed, synthesized, and evaluated for their reversal effect of ABC tranpsorter-mediated MDR.
3.2 The effect of VKJP compounds on sensitivity of ABC-overexpressing cells to chemotherapeutic drugs
Prior to determining the effects of VKJP1 and VKJP3 in reversing the MDR, the effect of OSI-930 and its analogues on cell viability was examined. The IC50 values of these compounds in ABC–overexpressing cells were in the range of 100 µM (data not shown). In order to avoid cellular toxicity, the highest non-toxic concentration of the test compounds used in subsequent reversal experiments was 10 µM.
Subsequently, we screened the 15 compounds in the test series and determined whether they could sensitize the transfected wild-type ABCG2-overexpressing MDR cells to certain chemotherapeutic drugs. VKJP1 and VKJP3 were the first two in the rank order of potencies for increasing the cytotoxicity of mitoxantrone among all of the members of the test series. For this reason, VKJP1 and VKJP3 were chosen for further study. Compound 13 had no significant reversing ability, while compound 14 was found to protect cells from cytotoxicity by mitoxantrone (Table 1). As shown in Table 2, VKJP1 or VKJP3 at 1, 3 and 10 µM, concentration-dependently decreased the IC50 values of ABCG2 substrates including mitoxantrone, SN-38, and doxorubicin of the transfected wild-type (R2) or mutant (T7) ABCG2-overexpressing cells. VKJP1 was shown to be the most potent inhibitor of ABCG2 than VKJP3 at all concentrations. The magnitude of the sensitization produced by 10 µM VKJP1 was similar to that induced by the known specific ABCG2 inhibitor FTC at 2.5 µM. In addition, 10 µM VKJP1 could significantly sensitize the parental HEK293-pcDNA3.1 cells to all the aforementioned chemotherapeutic drugs. However, this effect was significantly lower than measured in both ABCG2-482-R2 and ABCG2-482-T7 cells. In contrast, there was no significant effect in the IC50 values for all the cell lines when cisplatin (which is not a substrate of ABCG2) was incubated with VKJP1, VKJP3 or FTC (Table 2). Besides, VKJP1 or VKJP3 at 10 µM did not significantly alter the cytotoxicity of colchicine (a substrate of ABCB1) on KB-C2 cells which is a drug seletced cell line overexpressing ABCB1 (Supplemental Table 1) or vincristine (a substrate of ABCC1) on KB-CV60 cells which is a drug seletced cell line overexpresses ABCC1 (Supplemental Table 2). These results suggest that VKJP1 and VKJP3 selectively sensitize chemotherapeutic drugs that are the substrates of ABCG2 but not ABCB1 or ABCC1.
Table 1.
Effects of the test compounds in reversing ABCG2-mediated resistance to mitoxantrone
| Treatment | HEK293/pcDNA3.1 | ABCG2-482-R2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| IC50(nM) | FR a | IC50(nM) | FR a | ||||||
| Mitoxantrone | 17.77 | ± | 1.34 | 1.00 | 227.40 | ± | 14.95 | 12.80 | |
| +OSI-930 | 10 µM | 23.17 | ± | 3.37 | 1.30 | 42.24 | ± | 17.56 | 2.38 |
| +VKJP1 | 10 µM | 11.28 | ± | 1.23 | 0.63 | 14.90 | ± | 8.70 | 0.84 |
| +VKJP2 | 10 µM | 18.95 | ± | 7.45 | 1.07 | 61.81 | ± | 14.77 | 3.48 |
| +VKJP3 | 10 µM | 10.68 | ± | 0.84 | 0.60 | 35.15 | ± | 11.96 | 1.98 |
| +VKJP4 | 10 µM | 17.24 | ± | 2.96 | 0.97 | 58.95 | ± | 0.94 | 3.32 |
| +VKJP5 | 10 µM | 14.93 | ± | 0.92 | 0.84 | 50.59 | ± | 5.57 | 2.85 |
| +VKJP6 | 10 µM | 20.42 | ± | 0.69 | 1.15 | 61.88 | ± | 9.23 | 3.48 |
| +VKJP7 | 10 µM | 15.64 | ± | 4.01 | 0.88 | 67.21 | ± | 11.78 | 3.78 |
| +VKJP8 | 10 µM | 16.40 | ± | 3.35 | 0.92 | 79.48 | ± | 20.81 | 4.47 |
| +VKJP9 | 10 µM | 18.32 | ± | 8.31 | 1.03 | 54.82 | ± | 24.55 | 3.08 |
| +VKJP10 | 10 µM | 17.35 | ± | 8.45 | 0.98 | 53.93 | ± | 25.04 | 3.03 |
| +VKJP11 | 10 µM | 15.72 | ± | 1.94 | 0.88 | 65.34 | ± | 25.35 | 3.68 |
| +VKJP12 | 10 µM | 22.36 | ± | 6.90 | 1.26 | 54.66 | ± | 17.06 | 3.08 |
| +VKJP13 | 10 µM | 17.96 | ± | 1.40 | 1.01 | 217.52 | ± | 18.65 | 12.20 |
| +VKJP14 | 10 µM | 19.97 | ± | 5.74 | 1.12 | 257.63 | ± | 35.88 | 14.50 |
| +VKJP15 | 10 µM | 14.51 | ± | 2.49 | 0.82 | 57.05 | ± | 26.69 | 3.21 |
| +FTC | 2.5 µM | 16.15 | ± | 1.19 | 0.91 | 20.17 | ± | 2.38 | 1.14 |
Fold-resistance was determined by dividing the IC50 value for mitoxantrone of ABCG2-482-R2 cells by IC50 value of HEK293/pcDNA3.1 cells in the absence or presence of the test compounds or FTC.
Table 2.
Effect of VKJP1 and VKJP3 in reversing ABCG2-mediated MDR
| Treatment | HEK293/pcDNA3.1 | ABCG2-482-R2 | ABCG2-482-T7 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50(nM) | FR a | IC50(nM) | FR a | IC50(nM) | FR a | |||||||
| Mitoxantrone | 24.78 | ± | 0.68 | 1 | 277.91 | ± | 35.02 | 11.21 | 814.61 | ± | 3.05 | 32.87 |
| +VKJP1 1 µM | 21.34 | ± | 4.65 | 0.86 | 109.68 | ± | 95.05 | 4.43 | 235.57 | ± | 97.66 | 9.51 |
| +VKJP1 3 µM | 23.91 | ± | 0.93 | 0.96 | 28.52 | ± | 3.59 | 1.15 | 118.47 | ± | 67.94 | 4.78 |
| +VKJP1 10 µM | 18.97 | ± | 5.41 | 0.77 | 16.75 | ± | 9.1 | 0.68 | 24.06 | ± | 0.49 | 0.97 |
| +VKJP3 1 µM | 21.84 | ± | 7.17 | 0.88 | 108.58 | ± | 1.7 | 4.38 | 313.45 | ± | 194.86 | 12.65 |
| +VKJP3 3 µM | 21.43 | ± | 7.75 | 0.86 | 46.94 | ± | 11.55 | 1.89 | 163.69 | ± | 37.21 | 6.6 |
| +VKJP3 10 µM | 17.78 | ± | 6.88 | 0.72 | 53.19 | ± | 30.81 | 2.15 | 68.19 | ± | 0.08 | 2.75 |
| +FTC 2.5 µM | 27.16 | ± | 3.43 | 1.1 | 9.03 | ± | 0.09 | 0.36 | 28.19 | ± | 1.97 | 1.14 |
| SN-38 | 63.08 | ± | 0.94 | 1 | 2456.15 | ± | 376.77 | 38.94 | 2750.44 | ± | 180.13 | 43.6 |
| +VKJP1 1 µM | 64.28 | ± | 7.45 | 1.02 | 641.69 | ± | 405.07 | 10.17 | 684.43 | ± | 40.57 | 10.85 |
| +VKJP1 3 µM | 58.59 | ± | 21.93 | 0.93 | 310.36 | ± | 177.14 | 4.92 | 172.93 | ± | 16.78 | 2.74 |
| +VKJP1 10 µM | 45.1 | ± | 18.13 | 0.72 | 213.17 | ± | 120.53 | 3.38 | 67.4 | ± | 5.01 | 1.07 |
| +VKJP3 1 µM | 76.81 | ± | 17 | 1.22 | 931.17 | ± | 814.32 | 14.76 | 1164.95 | ± | 61.44 | 18.47 |
| +VKJP3 3 µM | 73.12 | ± | 7.24 | 1.16 | 229.58 | ± | 23.21 | 3.64 | 403.42 | ± | 38.39 | 6.4 |
| +VKJP3 10 µM | 68.49 | ± | 13.5 | 1.09 | 137.06 | ± | 39 | 2.17 | 191.8 | ± | 16.45 | 3.04 |
| +FTC 2.5 µM | 61.88 | ± | 13.4 | 0.98 | 106.03 | ± | 63.17 | 1.68 | 61.33 | ± | 3.64 | 0.97 |
| Doxorubicin | 4.39 | ± | 0.21 | 1 | 15.02 | ± | 0.08 | 3.42 | 80.52 | ± | 5.96 | 18.33 |
| +VKJP1 1 µM | 4.56 | ± | 0.69 | 1.04 | 10.5 | ± | 2.57 | 2.39 | 29.72 | ± | 3.35 | 6.77 |
| +VKJP1 3 µM | 3.59 | ± | 0.12 | 0.82 | 4.78 | ± | 0.44 | 1.09 | 23.75 | ± | 7.78 | 5.41 |
| +VKJP1 10 µM | 1.97 | ± | 0.89 | 0.45 | 4.9 | ± | 2.87 | 1.12 | 12.71 | ± | 6.55 | 2.89 |
| +VKJP3 1 µM | 4.56 | ± | 0.2 | 1.04 | 13.85 | ± | 5.58 | 3.15 | 85.21 | ± | 34.22 | 19.4 |
| +VKJP3 3 µM | 3.2 | ± | 0.46 | 0.73 | 9.35 | ± | 5.5 | 2.13 | 24.92 | ± | 10.13 | 5.67 |
| +VKJP3 10 µM | 2.81 | ± | 0.2 | 0.64 | 6.51 | ± | 0.92 | 1.48 | 17.22 | ± | 8.58 | 3.92 |
| +FTC 2.5 µM | 4.53 | ± | 0.57 | 1.03 | 5.05 | ± | 0.99 | 1.15 | 6.6 | ± | 3.92 | 1.5 |
| Cisplatin | 2.38 | ± | 0.04 | 1 | 2.41 | ± | 0.3 | 1.01 | 2.46 | ± | 0.16 | 1.04 |
| +VKJP1 10 µM | 2.11 | ± | 0.24 | 0.89 | 2.3 | ± | 0.53 | 0.97 | 2.29 | ± | 0.17 | 0.96 |
| +VKJP3 10 µM | 2.33 | ± | 0.17 | 0.98 | 2.61 | ± | 0.31 | 1.1 | 2.57 | ± | 0.36 | 1.08 |
| +FTC 2.5 µM | 2.21 | ± | 0.05 | 0.93 | 2.61 | ± | 0.41 | 1.1 | 2.37 | ± | 0.26 | 1 |
Fold-resistance was determined by dividing the IC50 value for mitoxantrone, SN-38, doxorubicin, and cisplatin of ABCG2-482-R2 and ABCG2-482-T7 cells by IC50 value of HEK293/pcDNA3.1 cells in the absence or presence of VKJP1, VKJP3 or FTC.
3.3 Effects of VKJP1 and VKJP3 on the expression of ABCG2 in ABCG2-overexpressing MDR cell
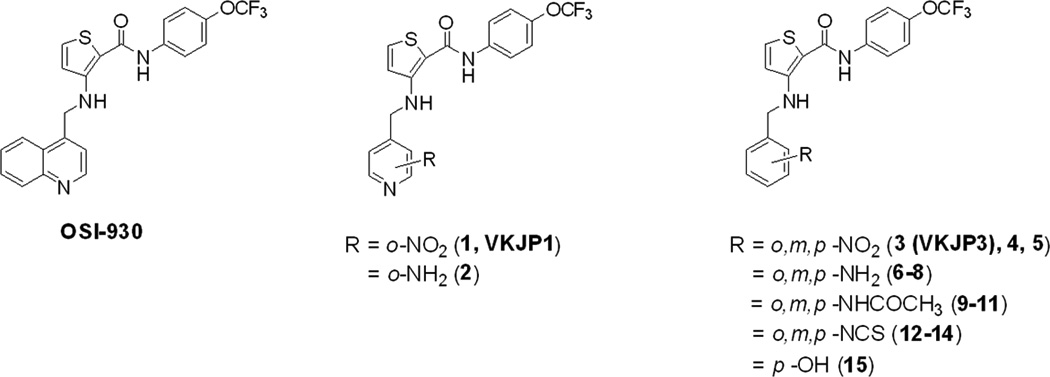
The reversal of ABCG2-mediated MDR can be achieved either by decreasing ABCG2 expression or by inhibiting its efflux function. To study the effects of VKJP1 and VKJP3 on ABCG2 expression, ABCG2-482-R2 and ABCG2-482-T7 cells were treated with VKJP1 or VKJP3 at 10 µM for different time periods (0, 36, 72 h), and ABCG2 expression levels were examined by Western blotting analysis. As shown in Figs. 2B & C, the protein levels of ABCG2 in both cell lines were not significantly altered by VKJP1 or VKJP3 treatment, suggesting that the regulatory mechanisms of VKJP1 and VKJP3 are other than alteration of ABCG2 expression.
Fig. 2. Western blot detection of ABCG2 in cells.
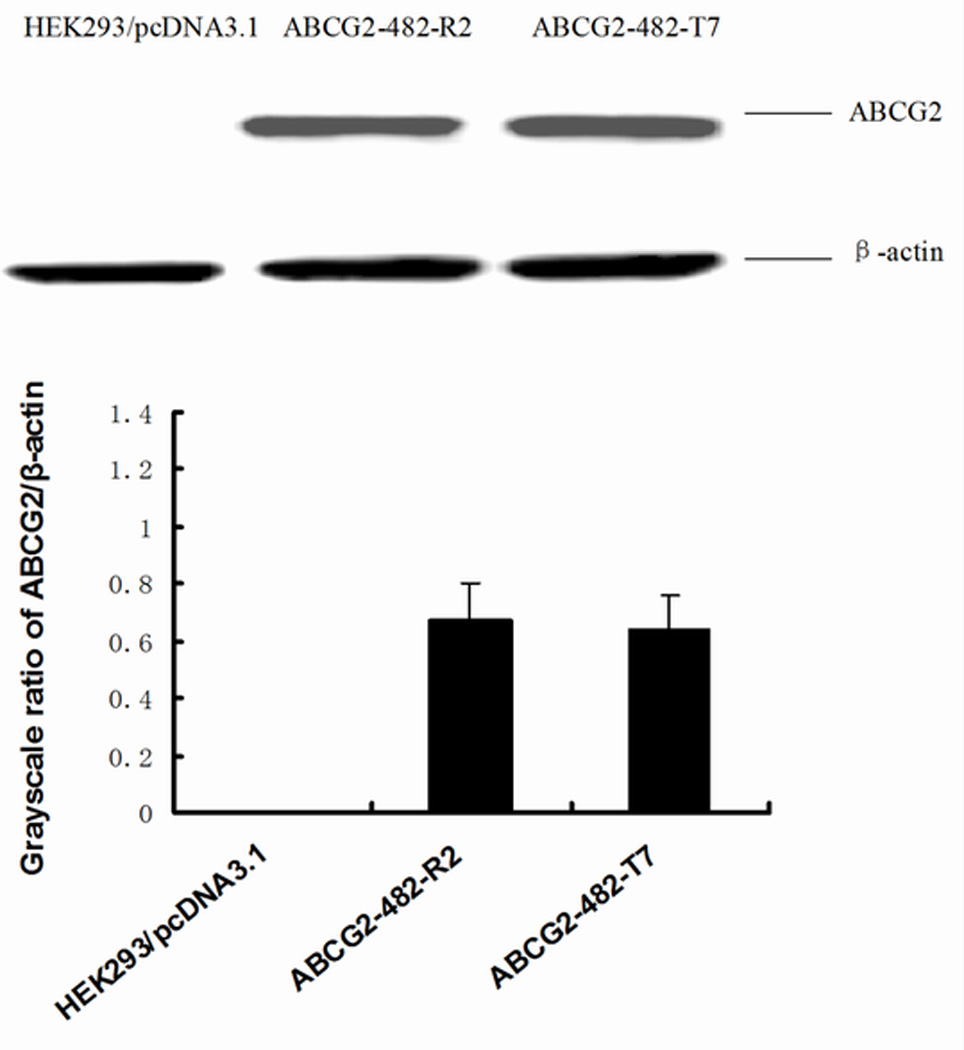
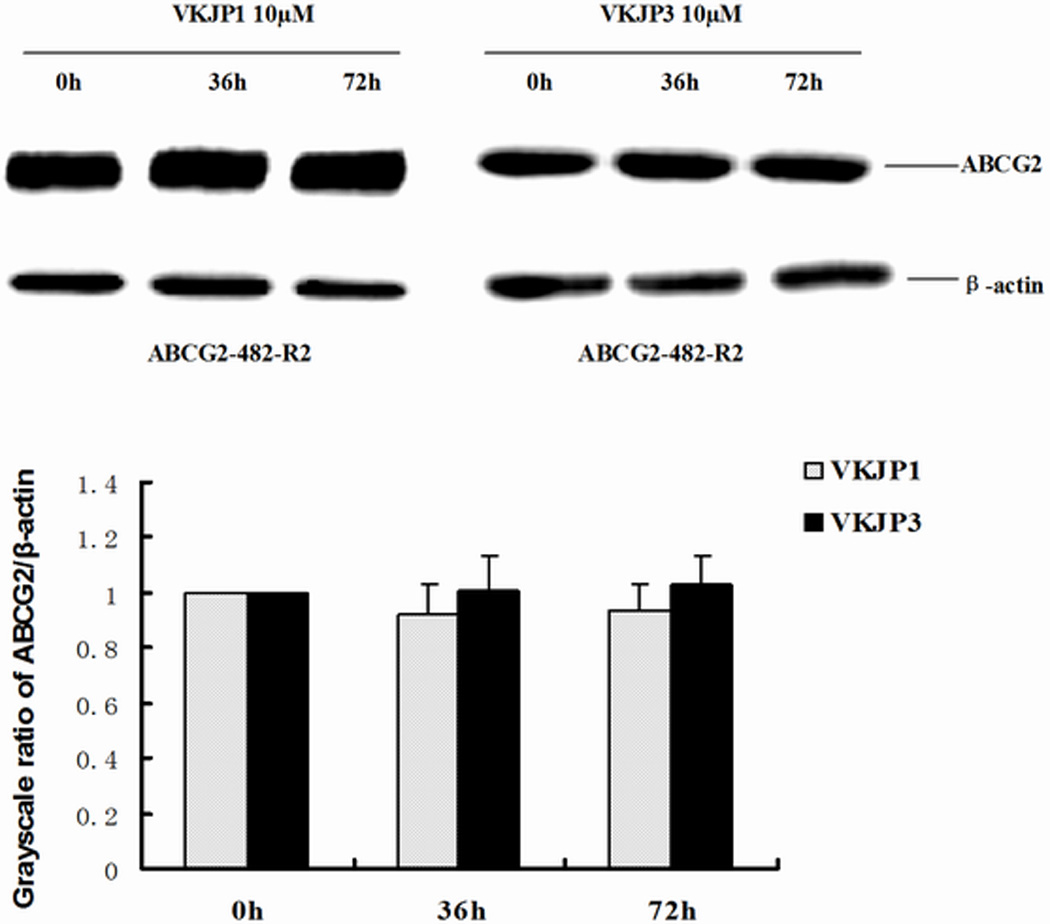
A: The expression of ABCG2 in HEK293/pcDNA3.1, ABCG2-482-R2, ABCG2-482-T7 cells. Equal amounts (40 µg protein) of total cell lysates were used for each sample. The membranes were immunoblotted with primary antibody against ABCG2 at 1:500 dilutions at 4°C overnight, then incubated with HRP-conjugated secondary antibody at 1:1000 dilutions at room temperature for 3 h. Proteins were detected by enhanced chemoluminescence detection system (Amersham, NJ). β-Actin was used to confirm equal loading in each lane in the samples prepared from cell lysates. Representative result is shown out of three experiments. B: Representative result showed the ABCG2 expression of ABCG2-482-R2 cells when treated with VKJP1 or VKJP3 (10 µM) at 0, 36, and 72 h. C: Representative result showed the ABCG2 expression of ABCG2-482-T7 cells when treated with VKJP1 or VKJP3 (10 µM) at 0, 36, and 72 h.
3.4 Effect of [3H]-mitoxantrone intracellular accumulation and [3H]-methotrexate transport by VKJP1 and VKJP3
In order to ascertain the mechanisms of VKJP1 and VKJP3 on the modulation of ABCG2 function, the intracellular levels of [3H]-mitoxantrone, a known substrate of ABCG2 was measured in the presence or absence of VKJP1 or VKJP3 in HEK293/pcDNA3.1, ABCG2-482-R2 and ABCG2-482-T7 cells (Fig. 3A). Both VKJP1 and VKJP3 at 10 µM, were found to produce a significant increase in the intracellular levels of [3H]-mitoxantrone in both wild-type and mutant ABCG2 transfected cell lines. VKJP1 and VKJP3 at 10 µM possess almost equal potency compared to the specific ABCG2 inhibitor FTC at 2.5 µM. There was no significant change in the intracellular levels of [3H]-mitoxantrone in parental HEK293/pcDNA3.1 cells incubated either with VKJP1 or VKJP3. Due to the short time course, we did not see any difference between the potency of VKJP1 and VKJP3 on [3H]-mitoxantrone accumulation.
Fig. 3. Effect of VKJP1 and VKJP3 on the intracellular accumulation of [3H]-mitoxantrone and ABCG2-mediated transport of [3H]-methotrexate.
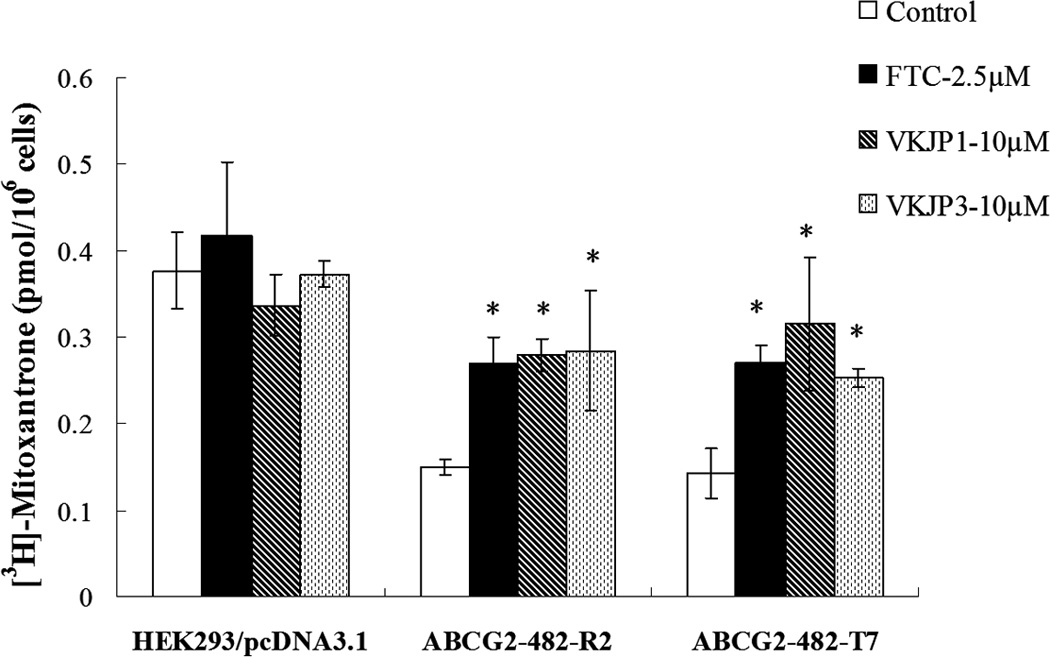
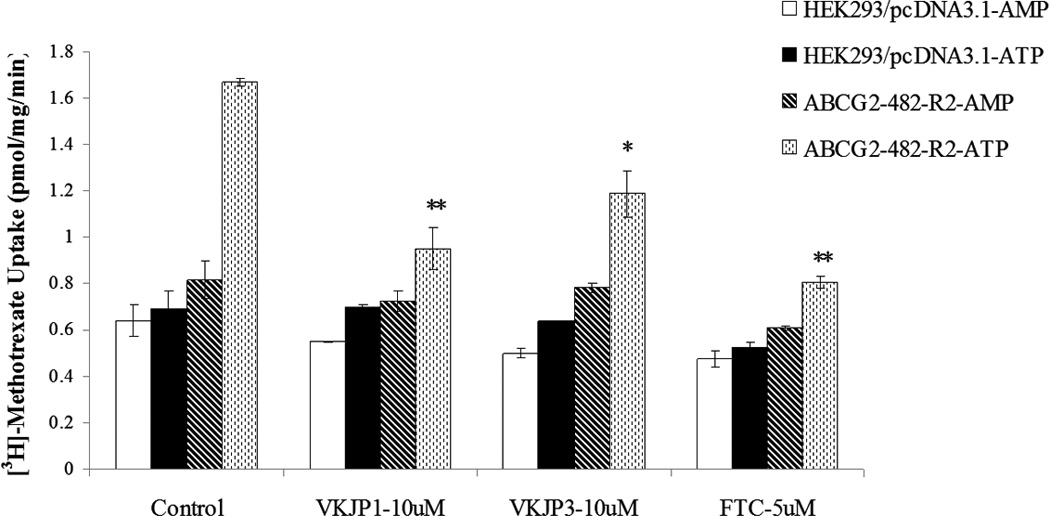
A: The accumulation of [3H]-mitoxantrone was measured after cells were preincubated with or without VKJP1, VKJP3 (10 µM), or FTC (2.5 µM) for 1 h at 37°C and then incubated with 0.2 µM [3H]-mitoxantrone for another 2 h at 37°C. Columns, mean of triplicate determinations; bars, SD; *, P < 0.05 versus the control group. Experiments were repeated at least three times and a representative experiment is shown. B: Membrane vesicles (10 µg) were prepared from HEK293/pcDNA3.1 and ABCG2-482-R2 cells. The uptake rates of [3H]-methotrexate into membrane vesicles were measured for 10 min at 37°C in uptake medium containing 4 mM of ATP or AMP. For inhibition experiments, membrane vesicles were incubated with VKJP1, VKJP3, or FTC for 1 h on ice, and then transport reactions were carried out for 10 min at 37°C in uptake medium containing 4 mM ATP. Columns, mean of triplicate determinations; bars, SD; *, P < 0.05; **, P < 0.01, versus the control group. Experiments were repeated at least three times and a representative experiment is shown.
To further validate the ability of VKJP1 and VKJP3 to inhibit the transport activity of ABCG2, we performed transport experiments using membrane vesicles to determine the rate of ATP-dependent uptake of [3H]-methotrexate, another substrate of ABCG2. Previously, we have reported that only wild-type ABCG2 was able to robustly transport [3H]-methotrexate in the in vitro transport system [26,15]. Thus, in this study, we only used the membrane vesicles prepared from HEK293/pcDNA3.1 and ABCG2-482-R2 cell lines. Our data showed that VKJP1 and VKJP3 at 10 µM produced a significant inhibition of the ATP-energized uptake rate of [3H]-methotrexate in ABCG2-482-R2 membrane vesicles (Fig. 3B), confirming that both VKJP1 and VKJP3 are able to directly inhibit the transport function of ABCG2-overexpressing membrane vesicles.
3.5 Effect of VKJP1 and VKJP3 on the ATPase activity of ABCG2
Since ABCG2 is an ATP-dependent membrane efflux pump which posses ATPase activity, we next measured the ABCG2-mediated ATP hydrolysis to investigate the effect of VKJP1 and VKJP3 on its ATPase activity. As shown in Figs. 4A & B, VKJP1 and VKJP3 enhanced the ATPase activity of ABCG2 in a concentration-dependent manner. The concentrations of VKJP1 or VKJP3 required for 50% stimulation of ATPase activity of ABCG2 were 0.024 ± 0.004 and 0.057 ± 0.016 µM, respectively, indicating that VKJP1 and VKJP3 may be the substrates of ABCG2.
Fig. 4. Effect of VKJP1 and VKJP3 on the ATPase activity of ABCG2.
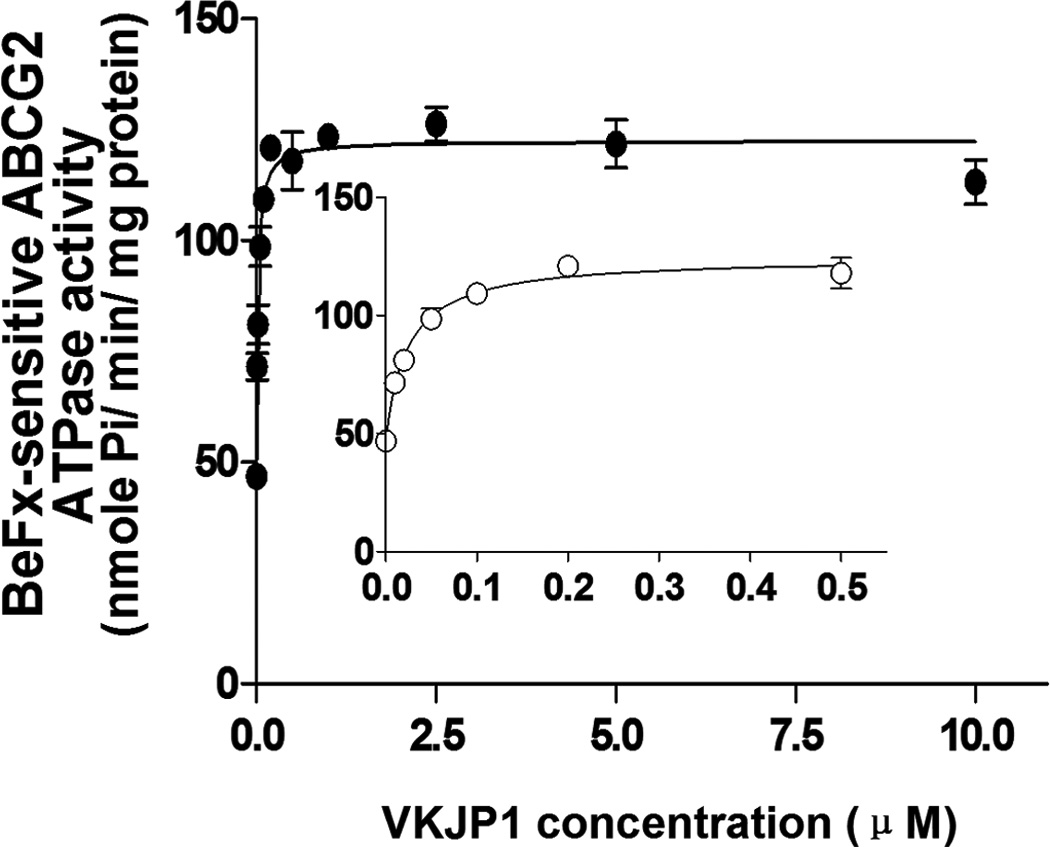
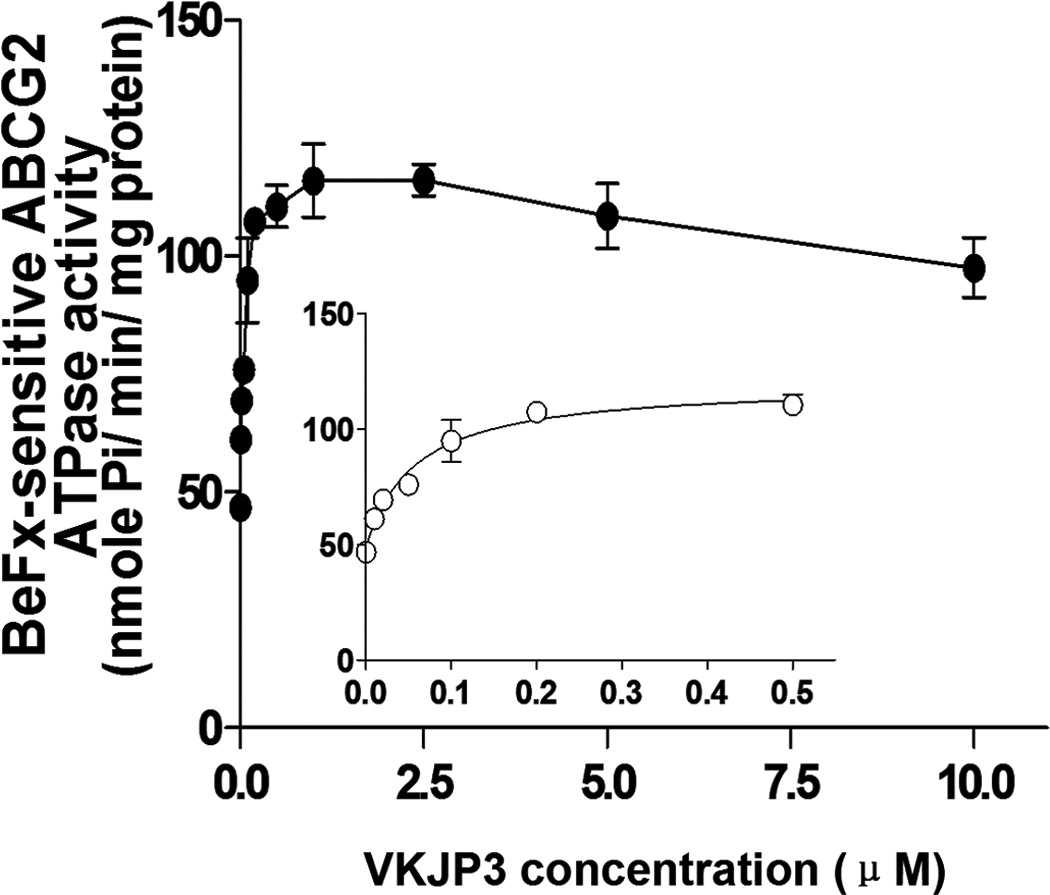
ATPase activity of ABCG2 in membrane vesicles was measured with different concentrations of VKJP1 (panel A) and VKJP3 (panel B) as described in “Experimental Procedures”. The lines represent the best fit for the data either by linear or non-linear leastsquares regression analysis using GraphPad Prism version 2.0. Spot, mean of triplicate determinations; bars, SD. In both panels A and B, inset shows ATP hydrolysis in the presence of 0 to 0.5 µM VKJP1 and VKJP3. Experiments were repeated at least three times and a representative experiment is shown.
3.6 Effect of VKJP1 and VKJP3 on photoaffinity labeling of ABCG2 with [125I]-IAAP
ABCG2 is able to be photo-labeled by its substrate [125I]-IAAP, and its substrates as well as inhibitors can compete with [125I]-IAAP for binding to ABCG2. To understand whether there is physical interaction of VKJP1 or VKJP3 with the substrate interaction sites of ABCG2, we examined the photoaffinity labeling of ABCG2 with [125I]-IAAP by using the membranes in the presence of various concentrations of VKJP1 and VKJP3. As indicated in Fig. 5A & B, VKJP1 and VKJP3 significantly inhibited the photoaffinity labeling of ABCG2 with [125I]-IAAP in a concentration-dependent manner. The concentrations of VKJP1 and VKJP3 required for 50% inhibition of photoaffinity labeling were 6.89 ± 0.12 µM and 9.32 ± 2.64 µM, respectively. These results suggest that VKJP1 and VKJP3 could bind to ABCG2 substrate-binding sites with different affinity.
Fig. 5. Effect of VKJP1 and VKJP3 on the photoaffinity labeling of ABCG2 by [125I]-IAAP.
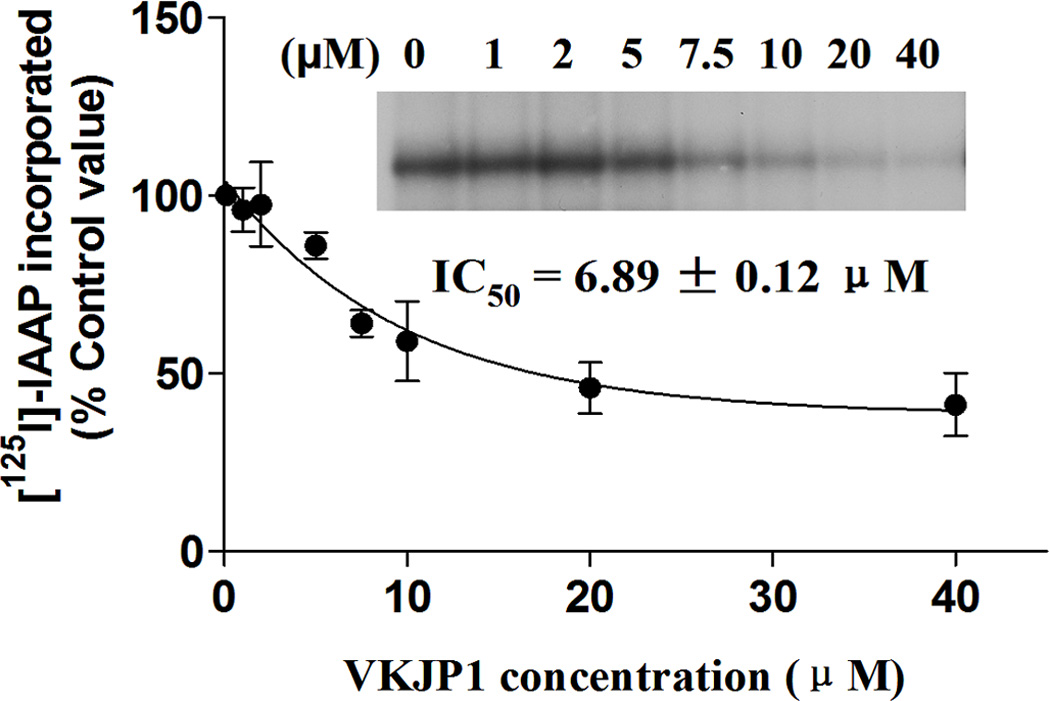
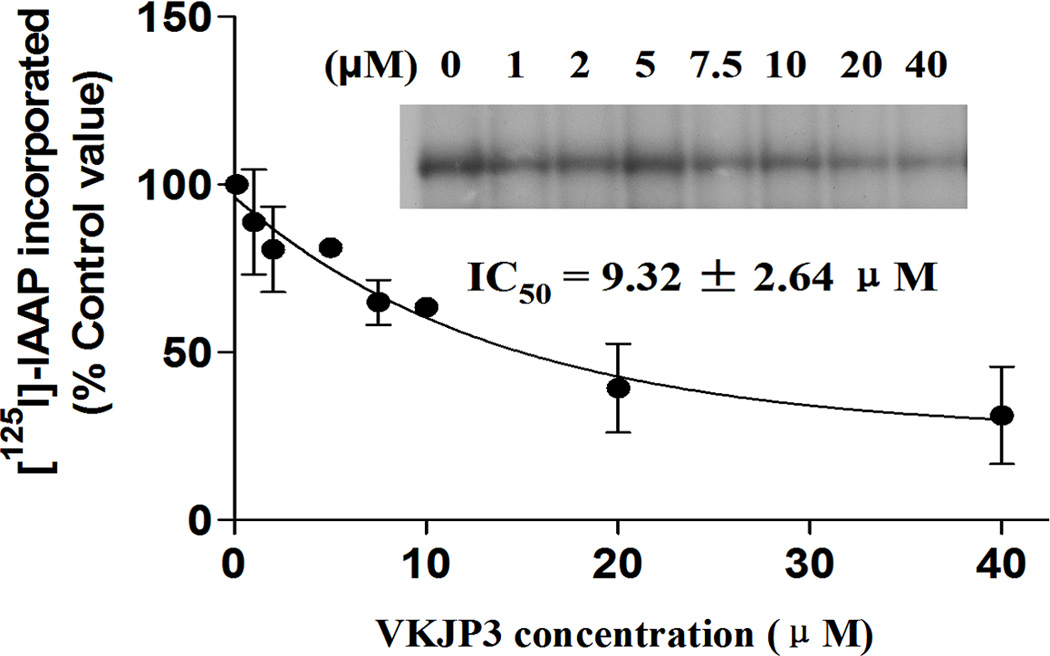
The photoaffinity labeling of ABCG2 with [125I]-IAAP was performed with different concentration of VKJP1 or VKJP3. The radioactivity incorporated into ABCG2 was determined by exposing the gel to an X-ray film at −70°C. An autoradiogram and quantification of incorporation of IAAP into the ABCG2 band from at least three independent experiments.
4. Discussion
ABCG2 is a 655-amino acid membrane protein, located on 4q22 chromosome, with a molecular weight of 72 KDa. It is a half transporter in since it has one single N-terminal nucleotide binding domain and one trans-membrane domain. Its unique structure indicates that it might have to dimerize with itself (homodimerize) or other members of ABC-G subfamily (hetrodimerize) to function as an efflux transporter [34, 35]. ABCG2 is expressed endogenously in many tissues, including placental syncytiotrophoblast cells, epithelial cells of gastrointestinal tract and liver, and endothelial cells of blood brain barrier [36]. It is also over-expressed in many kinds of MDR cancer tissues or established MDR cell lines. In recent years, ABCG2 has been shown to confer resistance to a diverse group of chemotherapeutic drugs, including nucleoside analogues, anthracyclines, camptothecin-derived indolocarbazole topoisomerase I inhibitors, methotrexate, and flavopiridols [37]. Several compounds have been identified that serve as ABCG2 inhibitors and have been shown to overcome ABCG2-mediated MDR [38]. Additionally, many newly designed compounds are under investigation for their inhibitory effects in in vitro experiments. Several tyrosine kinase inhibitors (TKIs), including imatinib (Gleevec, STI571), AG1478, erlotinib (Tarceva, OSI-774), and lapatinib (Tykerb, GW572016) [19, 20–23] have been identified as novel ABCG2 reversal agents by our group and others. These TKIs interact with ABCG2 and block its efflux function; increasing the intracellular accumulation of anticancer drugs and reverse ABCG2- mediated MDR.
In the current study, we found that nearly all the OSI-930 and its pyridyl and phenyl analogues were shown to reverse drug resistance in wild-type ABCG2 transfected MDR ABCG2-482-R2 cells. Among which, VKJP1 and VKJP3 had the most potency for increasing the cytotoxicity of ABCG2 substrate mitoxantrone. For this reason, we chose to conduct further mechanistic studies on these analogues. We found that meta-isothiocyanate derivative 13 had no significant reversing ability, while the para isothiocyanate derivative 14 interestingly had an opposite effect of protecting the cells from cytotoxicity by mitoxantrone (Table. 1). We assume that these 15 OSI-930 analogues resulted in variant reversal or protective effects due to their structural differences. The isothiocyanate analogues in phenyl series were less potent in reversing the MDR. Based on the present results, OSI-930 analogues such as VKJP1, VKJP3 also showed the enhancement of cytotoxicity of mitoxantrone in the parental HEK293/pcDNA3.1 cells. One explanation could be that the parental cells might also have other endogenous drug transporters that we could not detect, or some other mechanisms, which were interfered by the compounds, we tested.
Our previous data showed that amino acid residues at position 482 of ABCG2 is essential for substrate recognition [30]. In order to further study whether the reversal abilities of VKJP1 and VKJP3 is related to the amino acid residue, we used wild-type and mutant transfectants, ABCG2-482-R2 and ABCG2-482-T7 cells, in our experiments. Our results showed that VKJP1 and VKJP3 markedly increased the cytotoxicity of several ABCG2 anticancer substrates including mitoxantrone, SN-38, and doxorubicin in a concentration-dependent manner in both cell lines implying that the amino acid residues at ABCG2 482 position did not play an important role in the reversal abilities of VKJP1 and VKJP3. The reversal effect of VKJP1 on ABCG2-mediated MDR is more potent than that of VKJP3. The sensitization produced by 10 µM VKJP1 was similar to that induced by the known specific ABCG2 inhibitor FTC at 2.5 µM, showing that the use of VKJP1 as an ABCG2 modulator is very promising. Besides, both VKJP1 and VKJP3 were found to be ABCG2 selective inhibitors as demonstrated by their inability to significantly increase the sensitivity of all cell lines to a non ABCG2 substrate, cisplatin (Table. 2) and inability to reverse ABCB1 or ABCC1 mediated drug resistance (Supplemental Table. 1& 2).
Neither VKJP1 nor VKJP3 at 10 µM altered the expression levels of ABCG2 in the ABCG2-482-R2 and ABCG2-482-T7 cells suggesting that VKJP1 and VKJP3 exerted their inhibitory abilities by directly interacting with ABCG2 transporter. Consistent with the above hypothesis, we found that when ABCG2-482-R2 and ABCG2-482-T7 MDR cells were incubated concomitantly with [3H]-mitoxantrone and VKJP1 or VKJP3 at 10 µM, a higher intracellular drug accumulation was seen than when the cells were incubated with [3H]-mitoxantrone alone (Fig. 3A). A similar result was obtained when we examined the transport of [3H]-methotrexate using wild-type ABCG2 overexpressing membrane vesicles, the rates of [3H]-methotrexate uptake were significantly inhibited by VKJP1 or VKJP3 (Fig. 3B). These results were similar to some of the ABCG2 inhibitors that were previously reported by our research group. For example, we previously discovered that several TKIs such as AG1478, erlotinib, lapatinib could selectively modulate MDR protein-ATPase activity, by competitively inhibiting the efflux function of ABCG2 and ABCB1 and thus increase the intracellular accumulation of certain substrates into the MDR cells19, 22–23. We then examined whether VKJP1 and VKJP3 have the ability to interact with the ABCG2 transporter substrate binding sites and stimulate ATP hydrolysis. Indeed this was confirmed by the finding that VKJP1 and VKJP3 significantly stimulated the ATP hydrolysis and inhibited the binding of [125I]-IAAP to the substrate binding sites of ABCG2 (Figs. 4 & 5).
In conclusion, our study shows that VKJP1 and VKJP3 have specific and the most potential reversal activity on ABCG2-mediated MDR by directly inhibiting the drug efflux function, resulting in an increase in the intracellular drug accumulation. The mechanistic study found that VKJP1 and VKJP3 stimulated the activity of ATPase and inhibited the photoaffinity labeling of ABCG2 with [125I]-IAAP. The pyridyl and phenyl analogues of OSI-930 are novel reversal agents of ABCG2 transporter with a potential to increase clinical response when combined with conventional chemotherapeutic agents.
Supplementary Material
Acknowledgements
We thank Drs. S.E. Bates and R.W. Robey (National Cancer Institute, NIH) for the ABCB1, ABCG2 and ABCC1 transfectant cell lines and FTC; Drs. Michael M. Gottesman (NCI, NIH, Bethesda, USA) for KB-3-1 cells; Shin-ichi Akiyama (Kagoshima University, Japan) for KB-C2 and KB-CV60 cell lines and PAK104P and Selleck Chemical for OSI-930 (http://www.selleckchem.com/). This work was supported by funds from NIH R15 No. 1R15CA143701 (Z. S. Chen), St. John’s University Seed Grant No.579-1110, (Z.S. Chen), the National Natural Science Foundation of China No. 81000690 (YH. Kuang), the Millions of Strategic Project of Xiang Ya hospital (YH. Kuang), the Freedom Explore Program of Central South University (YH. Kuang).
Abbreviations
- OSI-930
{N-(4-trifluoromethoxyphenyl) 3-((quinolin-4-ylmethyl) amino) thiophene -2-carboxamide}
- ABC
ATP binding cassette
- ABCG2 (BCRP/MXR/ABCP)
ABC subfamily G member 2
- MDR
Multidrug resistance
- P-gp
P-glycoprotein
- ABCCs (MRPs)
ABC subfamily C member
- RTK
receptor tyrosine kinases
- KDR
kinase insert domain receptor
- TKIs
tyrosine kinase inhibitors
- IAAP
[125I]-iodoarylazidoprazosin
- FTC
Fumitremorgin C
- MTT
3-(4, 5-dimethylthiazol -2-yl)-2, 5-diphenyltetrazolium bromide
- DMSO
dimethylsulfoxide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pratt WB, Ruddon RW, editors. The anticancer drugs. New York: Oxford University Press; 1979. [Google Scholar]
- 2.Zhou J. Multidrug Resistance in Cancer. Humana Press; 2010. [Google Scholar]
- 3.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 4.Beck WT. Mechanisms of multidrug resistance in human tumor cells. The roles of P-glycoprotein, DNA topoisomerase II, other factors. Cancer Treat Rev. 1990;17(Suppl A):11–20. doi: 10.1016/0305-7372(90)90011-4. [DOI] [PubMed] [Google Scholar]
- 5.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 6.Wu CP, Hsieh CH, Wu YS. The emergence of drug transporter-mediated multidrug resistance to cancer chemotherapy. Mol Pharm. 2011;8:1996–2011. doi: 10.1021/mp200261n. [DOI] [PubMed] [Google Scholar]
- 7.Dean M, Annilo T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu Rev Genomics Hum Genet. 2005;6:123–142. doi: 10.1146/annurev.genom.6.080604.162122. [DOI] [PubMed] [Google Scholar]
- 8.Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 9.Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001 Jul;11:1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 10.Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22:7537–7552. doi: 10.1038/sj.onc.1206953. [DOI] [PubMed] [Google Scholar]
- 11.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maliepaard M, van Gastelen MA, de Jong LA, Pluim D, van Waardenburg RC, Ruevekamp-Helmers MC, et al. Overexpression of the BCRP/MXR/ABCP gene in a topotecan-selected ovarian tumor cell line. Cancer Res. 1999;59:4559–4563. [PubMed] [Google Scholar]
- 13.Tsuruo T, Iida H, Tsukagoshi S, Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981;41:1967–1972. [PubMed] [Google Scholar]
- 14.Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2003;55:3–29. doi: 10.1016/s0169-409x(02)00169-2. [DOI] [PubMed] [Google Scholar]
- 15.Tan B, Piwnica-Worms D, Ratner L. Multidrug resistance transporters and modulation. Curr Opin Oncol. 2000;12:450–458. doi: 10.1097/00001622-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Robert J, Jarry C. Multidrug resistance reversal agents. J Med Chem. 2003 Nov 6;46:4805–4817. doi: 10.1021/jm030183a. [DOI] [PubMed] [Google Scholar]
- 17.Dantzig AH, de Alwis DP, Burgess M. Considerations in the design and development of transport inhibitors as adjuncts to drug therapy. Adv Drug Deliv Rev. 2003;55:133–150. doi: 10.1016/s0169-409x(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 18.Chen ZS, Kawabe T, Ono M, Aoki S, Sumizawa T, Furukawa T, et al. Effect of multidrug resistance-reversing agents on transporting activity of human canalicular multispecific organic anion transporter. Mol Pharmacol. 1999;56:1219–1228. doi: 10.1124/mol.56.6.1219. [DOI] [PubMed] [Google Scholar]
- 19.Shi Z, Peng XX, Kim IW, Shukla S, Si QS, Robey RW, et al. Erlotinib (Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B member 1 and ATP-binding cassette subfamily G member 2-mediated drug resistance. Cancer Res. 2007;67:11012–11020. doi: 10.1158/0008-5472.CAN-07-2686. [DOI] [PubMed] [Google Scholar]
- 20.Liu W, Baer MR, Bowman MJ, Pera P, Zheng X, Morgan J, et al. The tyrosine kinase inhibitor imatinib mesylate enhances the efficacy of photodynamic therapy by inhibiting ABCG2. Clin Cancer Res. 2007;13:2463–2470. doi: 10.1158/1078-0432.CCR-06-1599. [DOI] [PubMed] [Google Scholar]
- 21.Houghton PJ, Germain GS, Harwood FC, Schuetz JD, Stewart CF, Buchdunger E, et al. Imatinib mesylate is a potent inhibitor of the ABCG2 (BCRP) transporter and reverses resistance to topotecan and SN-38 in vitro. Cancer Res. 2004;64:2333–2337. doi: 10.1158/0008-5472.can-03-3344. [DOI] [PubMed] [Google Scholar]
- 22.Shi Z, Tiwari AK, Shukla S, Robey RW, Kim IW, Parmar S, et al. Inhibiting the function of ABCB1 and ABCG2 by the EGFR tyrosine kinase inhibitor AG1478. Biochem Pharmacol. 2009;77:781–793. doi: 10.1016/j.bcp.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai CL, Tiwari AK, Wu CP, Su XD, Wang SR, Liu DG, et al. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res. 2008;68:7905–7914. doi: 10.1158/0008-5472.CAN-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garton AJ, Crew AP, Franklin M, Cooke AR, Wynne GM, Castaldo L, et al. OSI-930: a novel selective inhibitor of Kit and kinase insert domain receptor tyrosine kinases with antitumor activity in mouse xenograft models. Cancer Res. 2006;66:1015–1024. doi: 10.1158/0008-5472.CAN-05-2873. [DOI] [PubMed] [Google Scholar]
- 25.Petti F, Thelemann A, Kahler J, McCormack S, Castaldo L, Hunt T, et al. Temporal quantitation of mutant Kit tyrosine kinase signaling attenuated by a novel thiophene kinase inhibitor OSI-930. Mol Cancer Ther. 2005;4:1186–1197. doi: 10.1158/1535-7163.MCT-05-0114. [DOI] [PubMed] [Google Scholar]
- 26.Patel JP, Kuang YH, Chen ZS, Korlipara VL. Inhibition of c-Kit, VEGFR-2 (KDR), and ABCG2 by analogues of OSI-930. Bioorg Med Chem Lett. 2011;21(21):6495–6499. doi: 10.1016/j.bmcl.2011.08.070. [DOI] [PubMed] [Google Scholar]
- 27.Robey RW, Honjo Y, Morisaki K, Nadjem TA, Runge S, Risbood M, et al. Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br J Cancer. 2003;89:1971–1978. doi: 10.1038/sj.bjc.6601370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akiyama S, Fojo A, Hanover JA, Pastan I, Gottesman MM. Isolation and genetic characterization of human KB cell lines resistant to multiple drugs. Somat Cell Mol Genet. 1985;11:117–126. doi: 10.1007/BF01534700. [DOI] [PubMed] [Google Scholar]
- 29.Nagayama S, Chen ZS, Kitazono M, Takebayashi Y, Niwa K, Yamada K, et al. Increased sensitivity to vincristine of MDR cells by the leukotriene D4 receptor antagonist, ONO-1078. Cancer Lett. 1998;130:175–182. doi: 10.1016/s0304-3835(98)00132-3. [DOI] [PubMed] [Google Scholar]
- 30.Chen ZS, Robey RW, Belinsky MG, Shchaveleva I, Ren XQ, Sugimoto Y. Transport of methotrexate, methotrexate polyglutamates, and 17beta-estradiol 17-(beta-D-glucuronide) by ABCG2: effects of acquired mutations at R482 on methotrexate transport. Cancer Res. 2003;63:4048–4054. [PubMed] [Google Scholar]
- Cornwell MM, Gottesman MM, Pastan IH. Increased vinblastine binding to membrane vesicles from multidrug-resistant KB cells. J Biol Chem. 1986;261:7921–7928. [PubMed] [Google Scholar]
- 32.Ambudkar SV. Drug-stimulatable ATPase activity in crude membranes of human MDR1-transfected mammalian cells. Meth Enzymol. 1998;292:504–514. doi: 10.1016/s0076-6879(98)92039-0. [DOI] [PubMed] [Google Scholar]
- 33.Sauna ZE, Ambudkar SV. Evidence for a requirement for ATP hydrolysis at two distinct steps during a single turnover of the catalytic cycle of human P-glycoprotein. Proc Natl Acad Sci USA. 2000;97:2515–2520. doi: 10.1073/pnas.97.6.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ejendal KF, Hrycyna CA. Multidrug resistance and cancer: The role of the human ABC transporter ABCG2. Curr Prot Pep Sci. 2002;503:503–511. doi: 10.2174/1389203023380521. [DOI] [PubMed] [Google Scholar]
- 35.Mao Q, Unadkat JD. Role of the Breast Cancer Resistance Protein (ABCG2) in Drug Transport. AAPS J. 2005;7:E118–E133. doi: 10.1208/aapsj070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86:849–899. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- 37.Mao Q, Unadkat JD. Role of the breast cancer resistance protein (ABCG2) in drug transport. AAPS J. 2005;7:E118–E133. doi: 10.1208/aapsj070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, et al. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002;1:417–425. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


