Abstract
The immune and hematopoietic systems play an important role in the normal homeostasis of blood and blood cells and for immune responses to endogenous and exogenous processes and insults. In order to interpret histopathologic changes in the immune and hematopoietic systems, it is important to understand the normal anatomy and histology of the thymus, spleen, lymph nodes, bone marrow, and other tissues. The thymus, spleen, and lymph nodes can be categorized by anatomical compartments, each of which contributes to specific immune functions. Lesions may be diagnosed by interpretive or descriptive (semiquantitative) methods. The interpretation of these tissues by lesion in anatomical compartments should allow for better understanding of these reactions and more definitive pathologic findings. Proliferative lesions may be difficult to differentiate from lymphomas and leukemias. The use of immunohistochemistry, compartmental pathology, and methods for the evaluation of clonality will make interpretation easier.
Keywords: clonality, mice, hematopoietic system, immune system, immunohistochemistry, lymph nodes, lymphoid, lymphoid hyperplasia, lymphoma, lymphoproliferative disease, rats, spleen, Thymus
Introduction
Pathologists who are not familiar with the histology and pathology of the immune and hematopoietic systems may find challenges in the diagnoses of lesions in these tissues (Germolec, Kashon et al. 2004; Germolec, Nyska et al. 2004). The background variation of lesions in rodent immune and hematopoietic tissues of controls also makes it more difficult to diagnose and interpret induced lesions. It is the aim of this manuscript to review the histology and histopathology of these tissues by compartment and with interpretative diagnoses.
Normal Anatomy and Histology
The normal anatomy and histology of the rodent hematopoietic and lymphoid systems has been described (Cesta 2006; Elmore 2006a; Linden et al. in press; Mebius and Kraal 2005; Ohtani and Ohtani 2008; Pearse 2006a; Suttie 2006; van den Broeck et al. 2006; Ward et al. 1999; Willard-Mack 2006). The lymph nodes of mice are generally very small, and routine sections can be at any angle through the node producing appearances of lesions when the node is actually normal. To avoid misdiagnosing normal tissue as a lesion, one should be familiar with the many possible cross- and tangential sections of the normal mouse node (Linden et al. in press). In rats, the nodes are larger, but the same problem can occur. Familiarity with normal histological variation in controls is essential. The rat spleen has a wide normal marginal zone, whereas the mouse marginal zone is narrower and varies in width by strain, stock, and line.
Reactive Lesions
Reactive lesions are any histological responses of cells and tissue to any insult or even a change in normal physiological processes. They can be categorized histologically by anatomical (compartmental) location in the spleen, lymph nodes, thymus, and Peyer’s patches. For lymph nodes, the compartments include the cortex (follicles, germinal centers, interfollicular areas), paracortex (T-cell–rich deep cortical unit), and medulla (medullary cords and sinuses; Willard-Mack 2006). For the spleen, the compartments include white pulp (B-cell–rich follicles, germinal centers and mantle zone, T-cell–rich periarteriolar lymphoid sheath, and macrophage rich marginal zone) and red pulp (sinuses and cords). The thymus is composed of the cortex and the medulla. Each compartment has its own types of cells, which may be unique for that zone or may be found elsewhere. Reactive lesions may be either hyperplastic or nonhyperplastic but rather composed of infiltrative cells. Cellular antigens are shown by immunohistochemistry to be expressed by the specific cell types (Rehg et al. in press; Ward 2006). The spleen, lymph nodes, and thymus also have capsules that may show reactive lesions. Bone marrow anatomy and histopathology have been described and will not be discussed in this paper (Travlos 2006.
The anatomical compartments of these tissues and their cellular components may react individually or collectively in response to insults to cells and tissues (Tables 1 and 2). The use of interpretative diagnoses (Ward 1990a; Ward 1990b; Ward et al. 1993) and of descriptive (semi-quantitative) terminology Elmore 2006a; Elmore 2006b; Elmore 2006c; Elmore 2010; Elmore, 2012; Haley et al. 2005; Kuper et al. 2000) are examples of the histopathologic evaluation of anatomical compartments for diagnoses. Interpretative diagnoses include terms such as atrophy and hyperplasia, whereas descriptive diagnoses indicate an increase or decrease of cellularity or area within a specific anatomic compartment (Elmore 2010); Elmore, in press). The descriptive diagnoses are especially helpful for immunotoxicity studies, which include various doses of the test chemical agent and time course sacrifices. Both methods can be graded for severity (Elmore, in press). Computerized image analysis may be used for more accurate interpretation of changes in lymphoid anatomy, morphology, and antigen expression. A good example of chemically induced lesions categorized by semiquantitative descriptive histopathological analysis has recently been published (Pearse et al. 2009).
TABLE 1.
Types of reactive lesions in lymph nodes of rodents.
| Anatomical region | Sub-anatomical region/cell type/compartment |
Cell loss | Increase in cells (hyperplasia) |
Neoplasia |
|---|---|---|---|---|
| Entire LN | All | Atrophy | Lymphoid hyperplasia | Lymphoma |
| Cortex | Subcapsular sinuses | N/A | Histiocytic hyperplasia | Histiocytic sarcoma |
| Follicles | Atrophy | Hyperplasia | Follicular or lymphoblastic lymphoma | |
| Germinal centers | Atrophy | Hyperplasia | Follicular or lymphoblastic lymphoma | |
| Interfollicular areas | Atrophy | Hyperplasia | N/A | |
| Paracortex | Lymphocytes | Atrophy | Hyperplasia | Lymphoma |
| Interdigitating cells (IDC) | N/A | Hyperplasia | IDC Sarcoma | |
| High endothelial venules | N/A | Hypertrophy or Hyperplasia | N/A | |
| Medulla | Medullary cords | Atrophy | Hyperplasia | N/A |
| Sinuses | Atrophy | hyperplasia | Histiocytic sarcoma |
N/A, not applicable or not usually observed.
TABLE 2.
Types of reactive lesions in the spleen of rodents.
| Anatomical region | Sub-anatomical region/cell type/compartment |
Cell loss | Increase in cells | Neoplasia |
|---|---|---|---|---|
| White pulp | Whole unit | Atrophy | Lymphoid hyperplasia | Follicular lymphoma |
| Follicle (B-cells) | Atrophy | Hyperplasia | Follicular or lymphoblastic lymphoma | |
| Germinal center (B-cells) | Atrophy | Hyperplasia | Follicular or lymphoblastic lymphoma | |
| Mantle zone (B-cells) | Atrophy | Hyperplasia | Mantle cell lymphoma | |
| PALS (T-cells) | Atrophy | Hyperplasia | Metastases of T or B cell lymphomas | |
| Marginal zone (macrophages, B-cells) | Atrophy | Hyperplasia | Marginal zone lymphoma | |
| Red pulp | Whole unit | Atrophy | ||
| Sinusoids/cords | Angiectasis | Littoral cell angioma | ||
| Blood vessels | Angiectasis | Hemangioma and hemangiosarcoma | ||
| Myeloid cells | Myeloid hyperplasia | Leukemia | ||
| Erythroid cells | Erythroid hyperplasia | Leukemia | ||
| Megakaryocytes | Hyperplasia | Leukemia | ||
| Macrophages | Histiocytosis | Histiocytic sarcoma |
Age-associated changes in the lymphoid system may also be considered reactive to changes in the aging endogenous environment (hormonal, biochemical, molecular, genetic) and exogenous interactions (diet, air, water). Age-related lesions are common in the thymus and include atrophy (involution) in all species, glandular/epithelial hyperplasia in aging rats, and medullary and nodular hyperplasia in aging mice.
In humans, patterns of reaction in lymph nodes have been described as follicular and nodular, predominantly sinus, interfollicular, or mixed and diffuse (Jaffe et al. 2011). These patterns are well characterized for specific disease conditions in humans that are caused by genetic abnormalities, infectious agents, acquired disorders, and response to exogenous substances. The tissues of the rodent lymphoid system also respond in a similar manner, although the preferred terminology is slightly different.
Stepwise Histological Examination of Lymphoid Tissues
Optimal interpretation of a lymph node or spleen is best initiated with a review of the tissue at low magnification to observe the anatomical compartments and relationships to adjacent tissues (Table 3). All observations should be compared with those in normal or control tissues, and questions to be considered while reviewing the tissues are as follows. Are the normal anatomical structures in their usual order and of their usual sizes in relationship to each other, or is one compartment larger in comparison to others? Is more than one compartment abnormal in size or appearance? Is the node or spleen associated with local or systemic lesions? Can the histological appearance of the node be explained?
TABLE 3.
Stepwise histopathological analysis of the lymph nodes, spleen, and thymus.
| 1. Note gross appearances of all lymph nodes, spleen, thymus, and other lymphoid tissues. |
| 2. Review histologically, at low magnification, the anatomy and histology. |
| 3. Compare the anatomy and histology with normal tissues. |
| 4. Review anatomical compartments for size compared to normal and relationships to other compartments. |
| 5. Review cytology of cells in each compartment, comparing to normal cell types, and increase or decrease in compartment cellularity. |
| 6. Use immunohistochemistry as an adjunct. |
| 7. Compare with immunology techniques for interpretation. |
Immunohistochemistry can aid in the interpretation of histological changes in lymph node, spleen, Peyer’s patch, thymus, or bone marrow. Each organ or tissue has unique normal antigen expression patterns because of its normal or abnormal cell populations. The normal cellular compartments in each organ can be compared with the affected tissue. For rodents, panels of antibodies have been validated for routine use in identifying specific cell types (Kunder et al. 2007; Mikaelian et al. 2004; Rehg and Sundberg 2008; Rehg et al. in press; Ward et al. 1993; Ward et al. 2006; http://tumor.informatics.jax.org/html/antibodies.html; http://www.niehs.nih.gov/research/atniehs/labs/lep/path-support/immuno/protocols.cfm; http://icg.cpmc.columbia.edu/cattoretti/Protocol/Mouse_IHC/Antibodies_for_mouse_IHC.html).
Proliferative Lesions of the Thymus
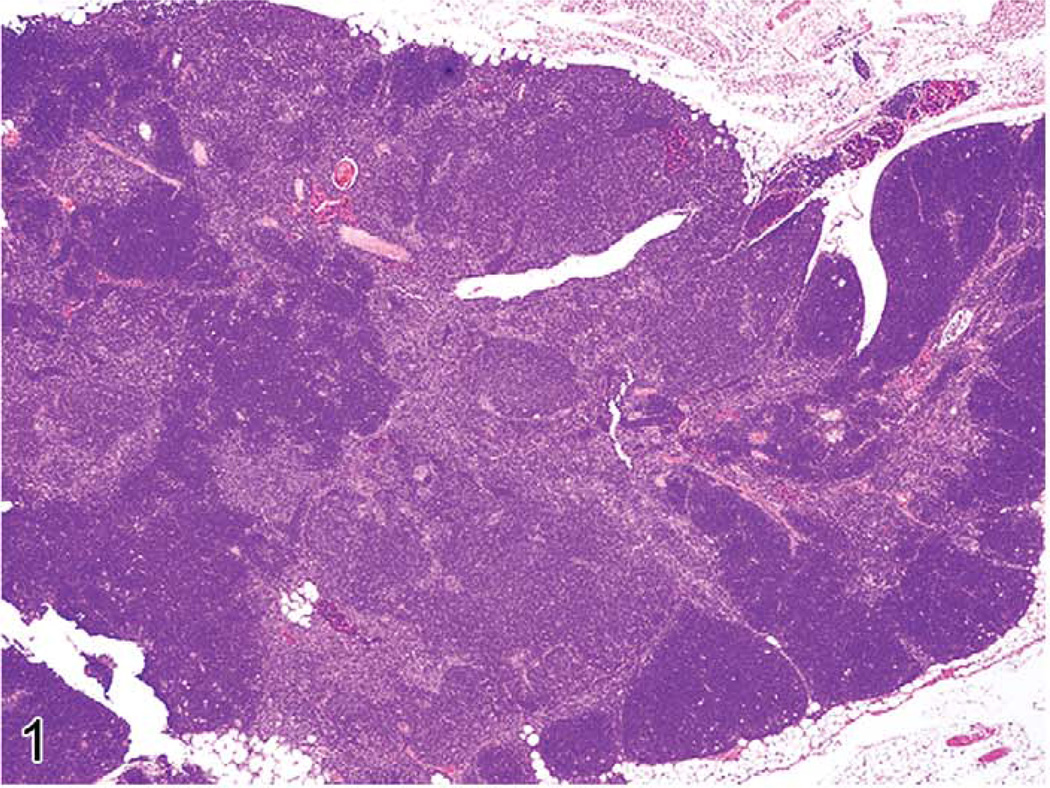
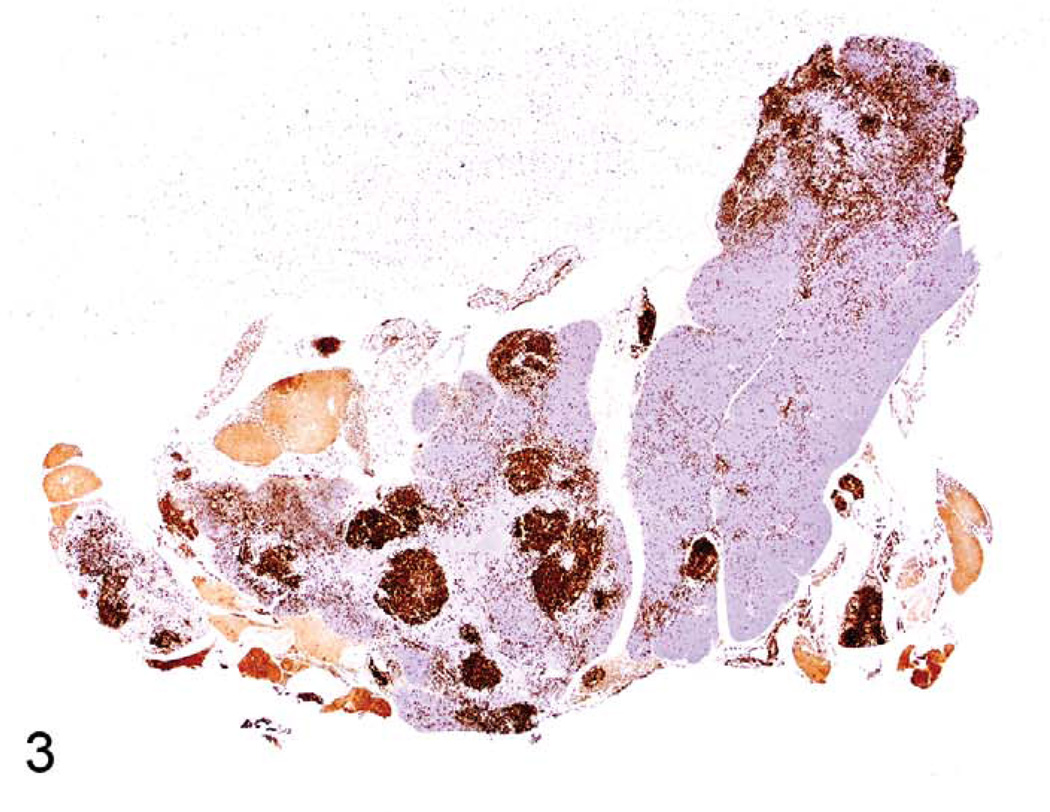
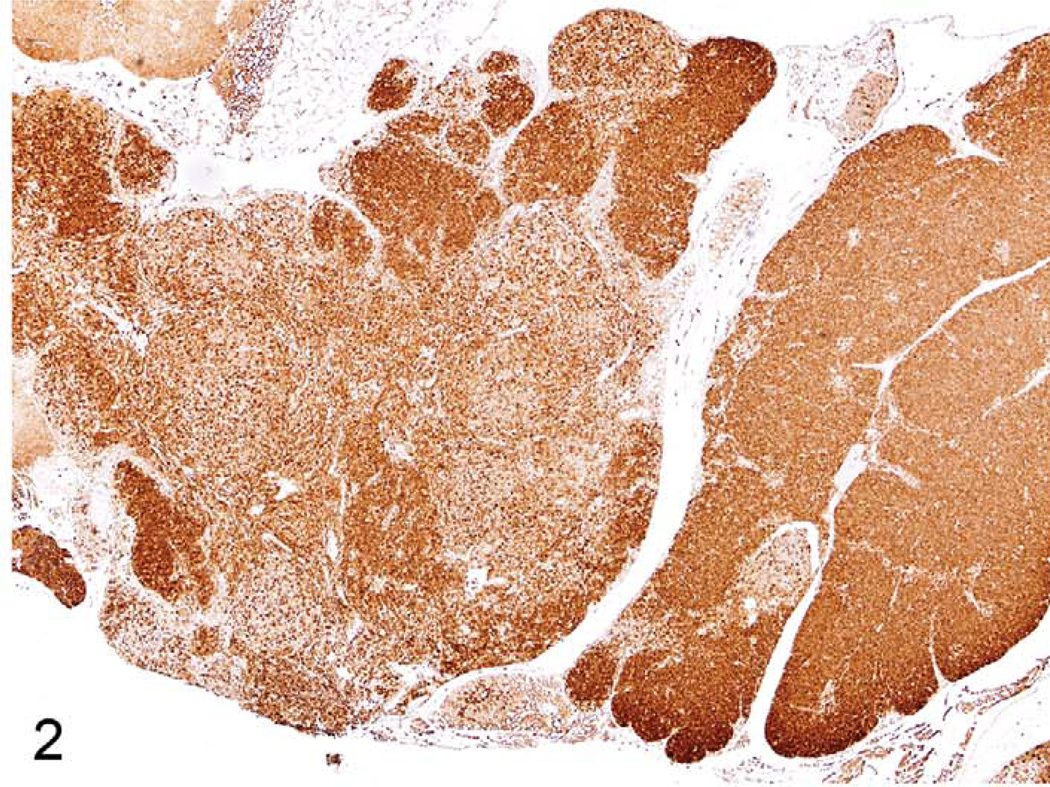
The thymus reacts in various ways to stress, toxins, and infectious agents (Pearse et al. 2006a,b). Atrophy (aging involution) and hyperplasia of anatomical and cellular compartments of the thymus are seen in aging rats and mice (Elmore 2006a; Pearse et al. 2006b). Epithelial cell and glandular hyperplasia are more commonly seen in aging rats, whereas aging mice develop medullary hyperplasia. When pronounced, thymic medullary hyperplasia, sometimes nodular, can mimic neoplasia in aging mice and in some lines of mice with autoimmune disorders. These hyperplastic lesions are not as large as tumor masses seen in thymic lymphomas, however, even when they are nodular (Figures 1–3). These hyperplastic foci may be composed of B-cells, which can form germinal centers and plasma cells (Figures 2 and 3).
FIGURE 1.
Thymus of an eighteen-month-old B6,129 mouse showing nodular medullary hyperplasia.
FIGURE 3.
Thymus of an eighteen-month-old B6,129 mouse with medullary hyperplasia showing CD45R expression. Note the increase in CD45R+ B cells in several areas.
FIGURE 2.
Thymus of another eighteen-month-old B6,129 mouse with medullary hyperplasia and CD3 expression in T-cells. Note that there is loss of CD3+ lymphocytes in the medullary hyperplasia.
Genetically engineered mice with various altered genes may develop lymphomas that pass through histopathologic stages of unilateral or bilateral thymic cortical lymphocyte depletion, as well as medullary atypical hyperplasia (Dunnick et al. 1997; Ward et al. 1999). Atypical thymic medullary hyperplasia is characterized by unilateral or bilateral thymic lobular lesions and the appearance of immature lymphoid cells, including blasts with large nuclei. These lesions often progress into lymphoma with a grossly enlarged thymic lobe or lobes and metastases to the spleen, liver, and other tissues (Ward et al. 1999). The same histopathologic sequence can occur for thymic lymphomas induced by chemicals in mice and rats (Konishi et al. 1988), and by irradiation or viruses in mice.
Proliferative Lesions of Lymph Nodes
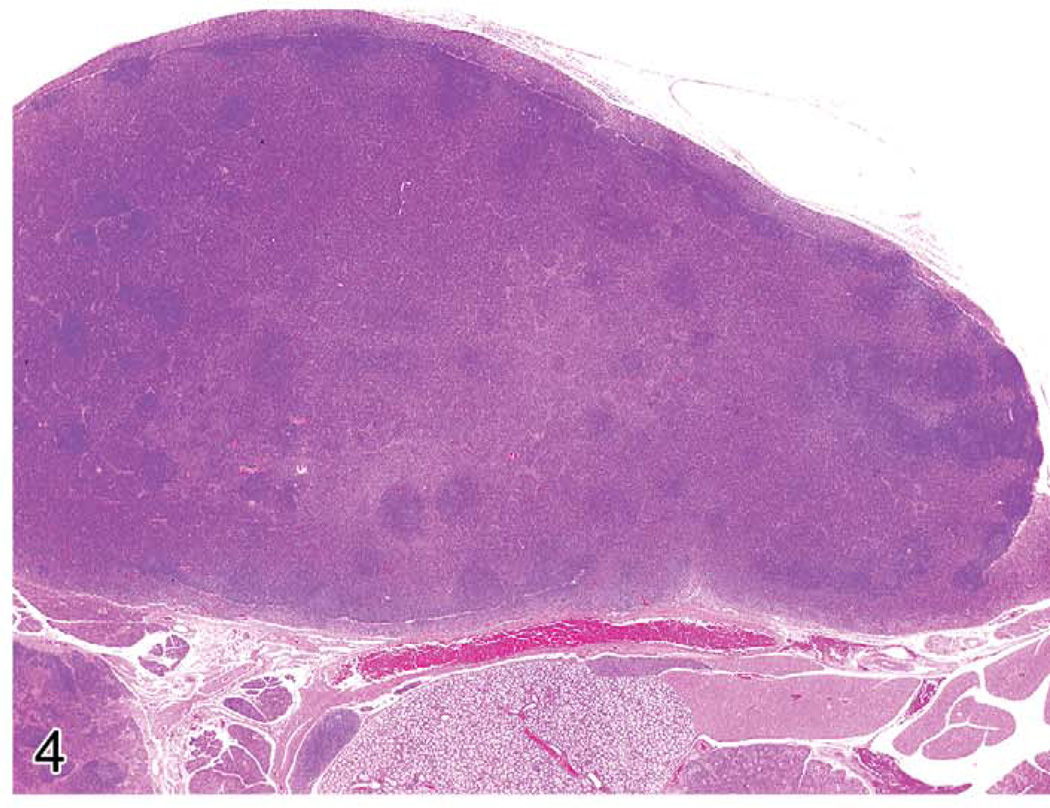
Lymph nodes often react to regional or systemic insults. The increase in lymph node size is often a result of enlargement of the various compartments. For example, the tissues affected by skin inflammation and ulceration drain via the lymphatics to the regional lymph nodes. The nodal responses may include inflammation with neutrophils, mast cells, germinal center hyperplasia, and the production of many plasma cells. Plasma cells may be abundant in chronic lesions, and the enlarged lymph node may be composed primarily of plasma cells and their precursors (Figure 4). Differentiation between plasma cell hyperplasia and a plasma cell lymphoma can be difficult. If only the regional node at the site of inflammation is involved, the change can readily be diagnosed as reactive hyperplasia. If generalized lymph node enlargement is seen, with many plasma cells in each enlarged node and no obvious etiology, assays for clonality can be performed (see below). Other reactive lesions in lymph nodes include germinal center hyperplasia associated with infectious diseases or other antigenic stimuli. This lesion is often accompanied by plasma cell hyperplasia in the medullary cords and cortex. T-cell hyperplasias in the paracortex may occur after viral infections. Unique lymphoproliferative disorders that involve the lymph nodes are also discussed below.
FIGURE 4.
A much enlarged mandibular lymph node of a mouse with ulcerative skin lesions. There is loss of normal nodal architecture. Most of the node contains mature plasma cells.
Proliferative Lesions of the Spleen
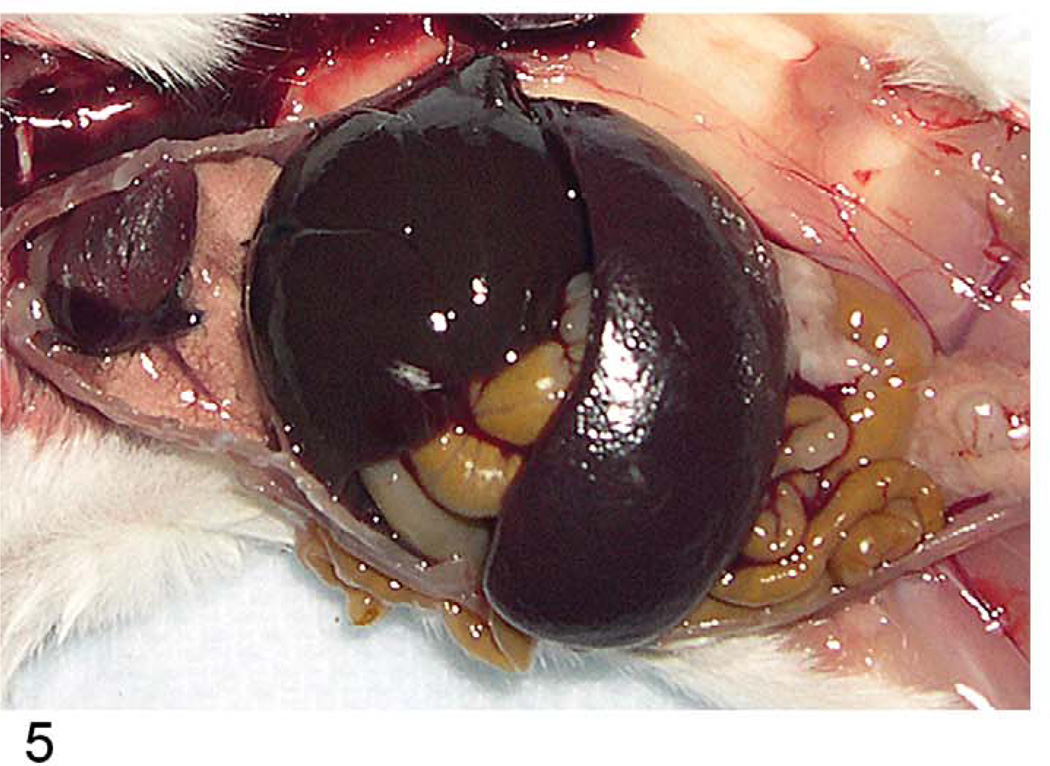
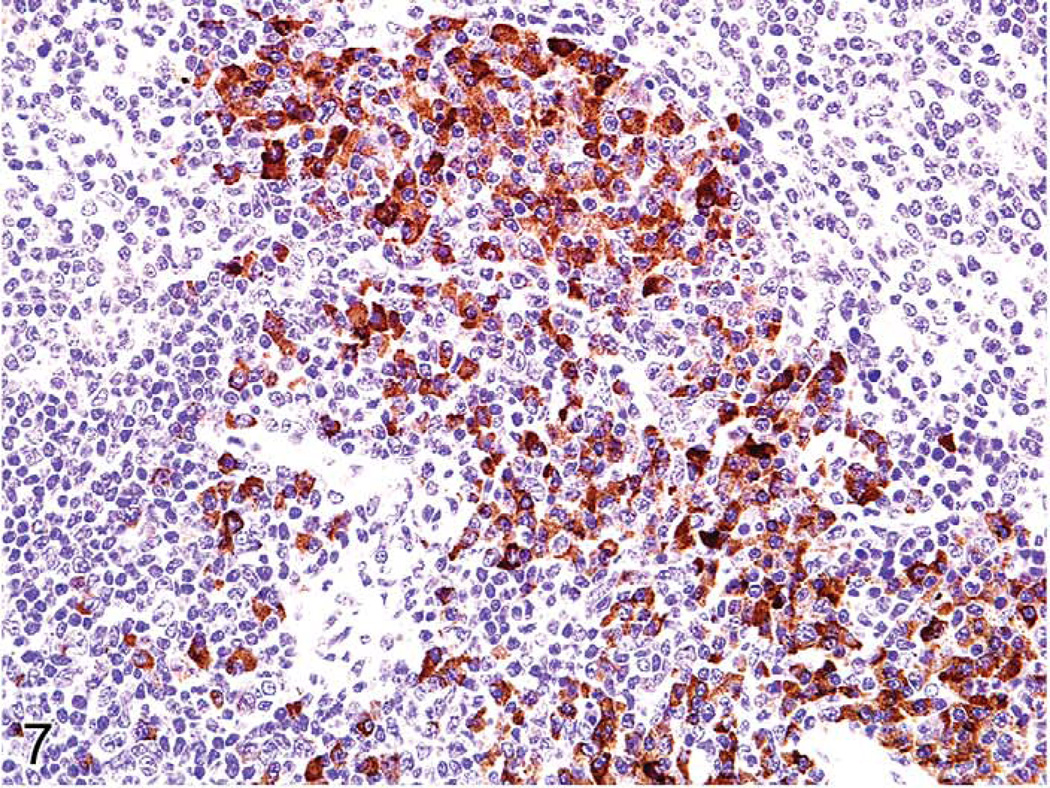
Proliferative splenic lesions can involve the white or red pulp, or both compartments at the same time. Lymphoid hyperplasia in the white pulp occurs after infections and inflammatory lesions in other tissues or as responses to tumors in any tissue. The hyperplasia may include germinal center hyperplasia, plasma cell hyperplasia, and general lymphoid hyperplasia of the many cell types that compose the white pulp. In experimental malaria infection of mice, spleens may attain 5 g in weight after seven to twelve days of infection because of a marked immune response in the spleen accompanied by erythroid hyperplasia (Waisberg et al. 2011). The white pulp is much enlarged, mostly because of germinal center and plasma cell hyperplasia (Figures 5–7). The red pulp is expanded by many erythroid precursors.
FIGURE 5.
Greatly enlarged spleen of a mouse infected with Plasmodium berghei ten days previously.
FIGURE 7.
Spleen in a malaria-infected mouse with numerous plasma cells. Immunohistochemistry for human κ light chains expressed mostly in the plasma cells.
Splenic Myeloid and Erythroid Hyperplasia
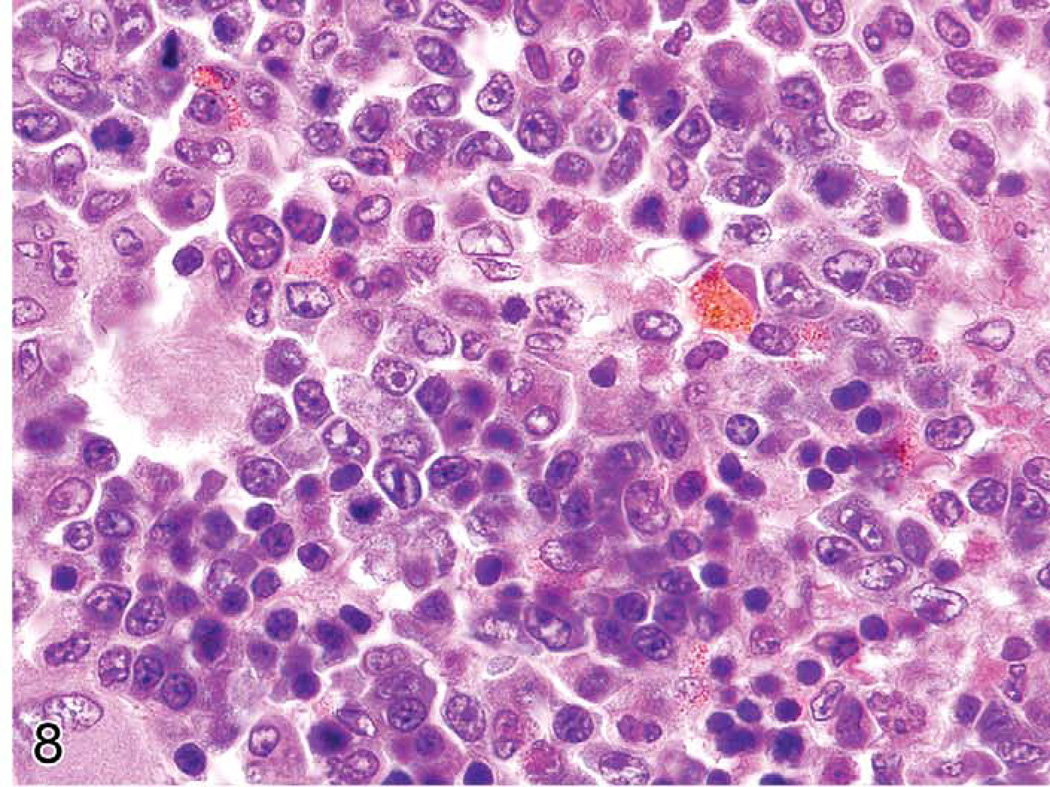
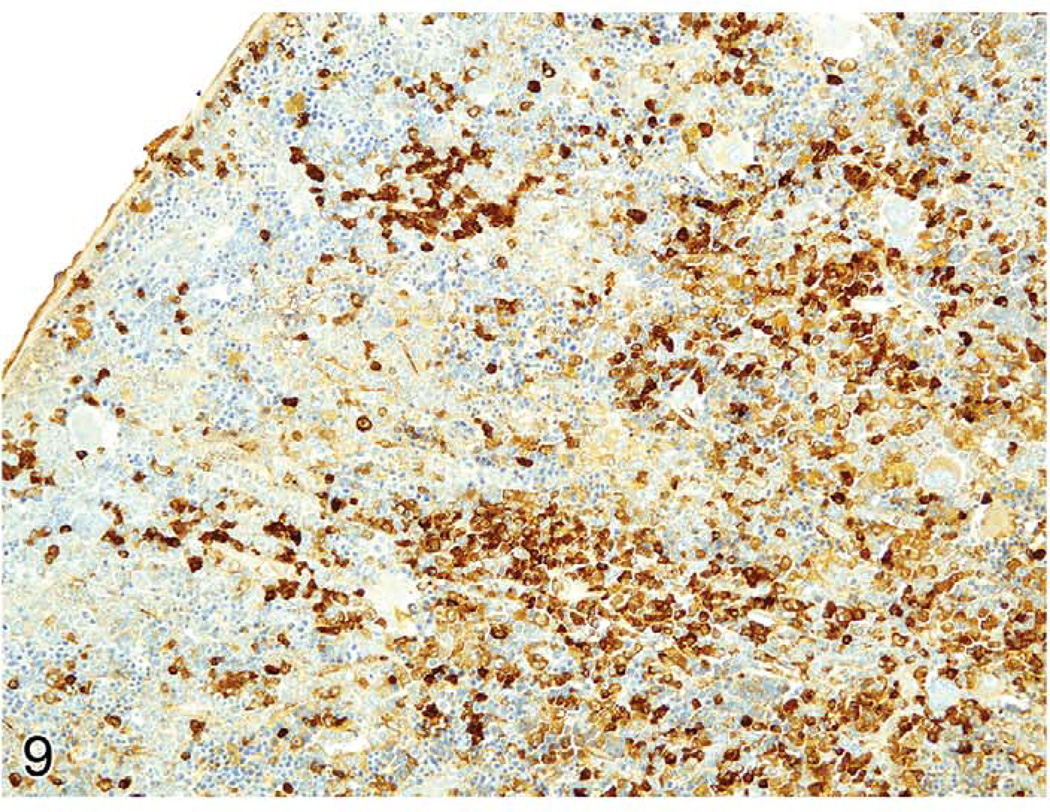
The spleens of rats and mice are highly reactive to endogenous lesions, including inflammation and neoplasia. Inflammatory lesions in various organs, especially in the skin, lung, and intestine, can stimulate myelopoiesis in the bone marrow, spleen, and other tissues. The spleen is particularly susceptible to enlargement caused by myeloid and erythroid hyperplasia, which are often accompanied by megakaryocyte hyperplasia. Immature myeloid and erythroid precursors can predominate in such reactions, mimicking leukemia. These spleens often exhibit lymphoid hyperplasia in the white pulp, in response to antigens expressed by tumors or infectious agents. Evaluation of the anatomical appearance of the spleen and its compartments at low magnification is a first step in histological evaluation. The appearance under high magnification of mature and immature hematopoietic cell types may reveal differences in the ratio of the most immature cells to mature cells (Figure 8). These lesions are often accompanied by plasma cell hyperplasia (Figure 9). If a majority of the cells in a compartment are of a similar immature cell type, this finding may be indicative of neoplasia. If the cause of a splenic response (inflammatory and ulcerative skin lesions, tumor, or internal inflammatory lesions in other tissues) is found, the splenic hyperplastic state can be explained as a response to those lesions. Sometimes, a lesion cannot be found to explain the splenic reaction. Detailed suggestions for histopathological differentiation of hyperplasia versus leukemia have been reported (Long et al. 1986; Ward 1990). One should be conservative and not diagnose leukemia unless there is overwhelming evidence to support the diagnosis.
FIGURE 8.
Enlarged rat spleen with myeloid and erythroid hyperplasia.
FIGURE 9.
Enlarged rat spleen with myeloid and erythroid hyperplasia also has many plasma cells expressing IgG. Immunohistochemistry for rat IgG.
Lymphoproliferative Disorders
Lymphoproliferative disorders (LPD) have been described in humans (Swerdlow et al. 2008), monkeys (Schmidtko et al. 2002), and less often, in mice. In humans and monkeys, they may occur naturally (of genetic or other origin) or from inadvertently induced immune disorders (herpes viral infections or after drug-induced immunosuppression during organ transplants). In mice, they are of genetic origin or induced by experimental procedures such as viral infection. Lymphoproliferative disorders are characterized by a non-neoplastic proliferation of lymphocytes in one or more lymphocyte lineages in the various lymphoid and other tissues. Their non-neoplastic nature may be shown by laboratory assays demonstrating lack of clonality or other characteristics. These conditions may progress to lymphomas, especially in monkeys and humans, when caused by drug-induced immunosuppression and herpes virus infection (Swerdlow et al. 2008; Schmidtko et al. 2002). Inmice, LPD has been described in the SJL/J strain (Tang et al. 1998) and in mice with naturally occurring mutations in Fas (lpr) and Fasl (gld; Cohen and Eisenberg 1991; Davidson et al. 1998), and it has also been induced by viruses including a mutant retrovirus, LP-BM5 murine leukemia virus (MuLV), which causes murine acquired immunodeficiency syndrome (MAIDS) (Hartley et al. 1989; Hartley et al. 2000; Klinken et al. 1988), the γ herpes virus, MHV68 (Barton et al. 2011), and mouse cytomegalovirus (CMV; Karupiah et al. 1998). Lymphoproliferative disorders have also been described in genetically engineered mice, including mice bearing transgenes for IL6 (Kovalchuk et al. 2002), Myc (Adams et al. 1985; Park et al. 2005), BCL6 (Cattoretti et al. 2005), and BCL10 (Li et al. 2009).
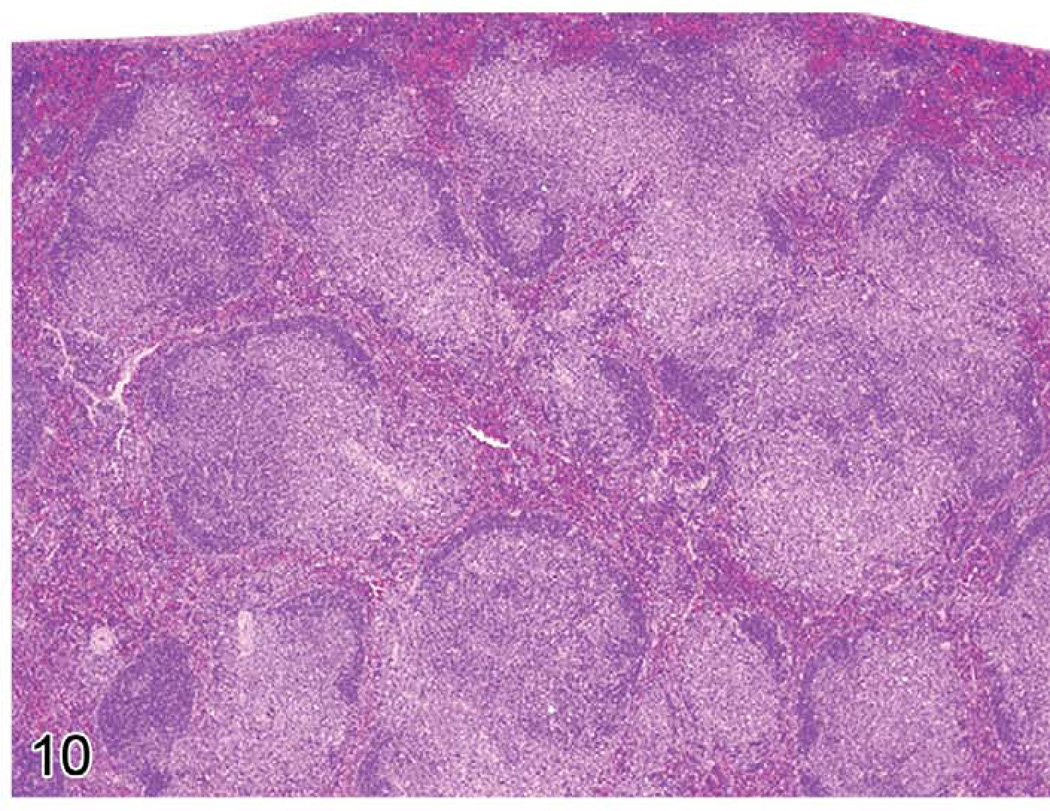
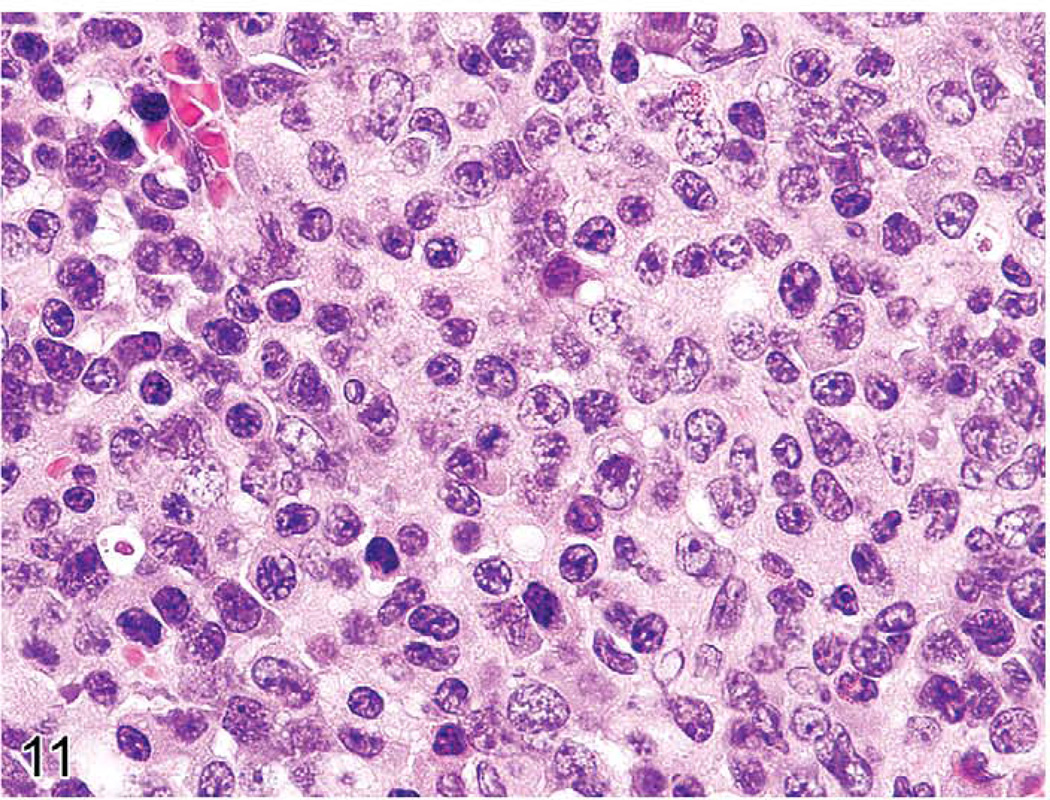
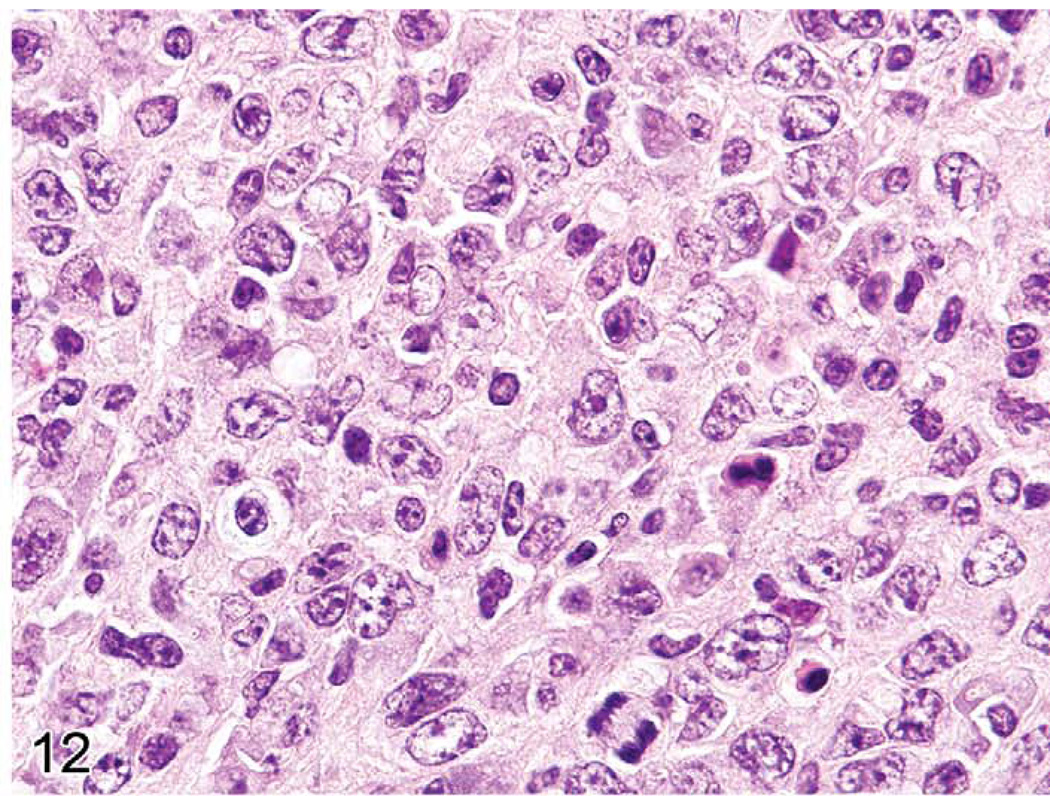
The gross lesions of lymphoproliferative disorders in mice vary from a massive lymph node enlargement to moderate enlargements of the lymph nodes and spleen. In the spleen, the white pulp usually expands (Figure 10) as a result of proliferation of a uniform population of mature lymphoid cells of a single lineage (T-cells, as in gld or lpr mice) or, more often, of a mixed population of mature B- and T-cells and plasma cells (Figure 11), especially in the early stages of MAIDS and the other disorders. This early stage can be followed by the appearance of a neoplastic population of blastic lymphoma cells with many mitotic figures (Figure 12). Although nonlymphoid organs can be involved, they need not be. The morphology and antigen expression patterns of the lymphoid cells can help differentiate the lesions from lymphomas.
FIGURE 10.
Enlarged spleen of a mouse with early stage murine acquired immunodeficiency syndrome showing hyperplasia of the white pulp.
FIGURE 11.
High magnification of previous figure of a mouse with murine acquired immunodeficiency syndrome showing a heterogeneous population of plasmacytoid cells, immature and mature plasma cells.
FIGURE 12.
Spleen of a mouse with later stage murine acquired immunodeficiency syndrome showing many centroblasts with mitotic figures.
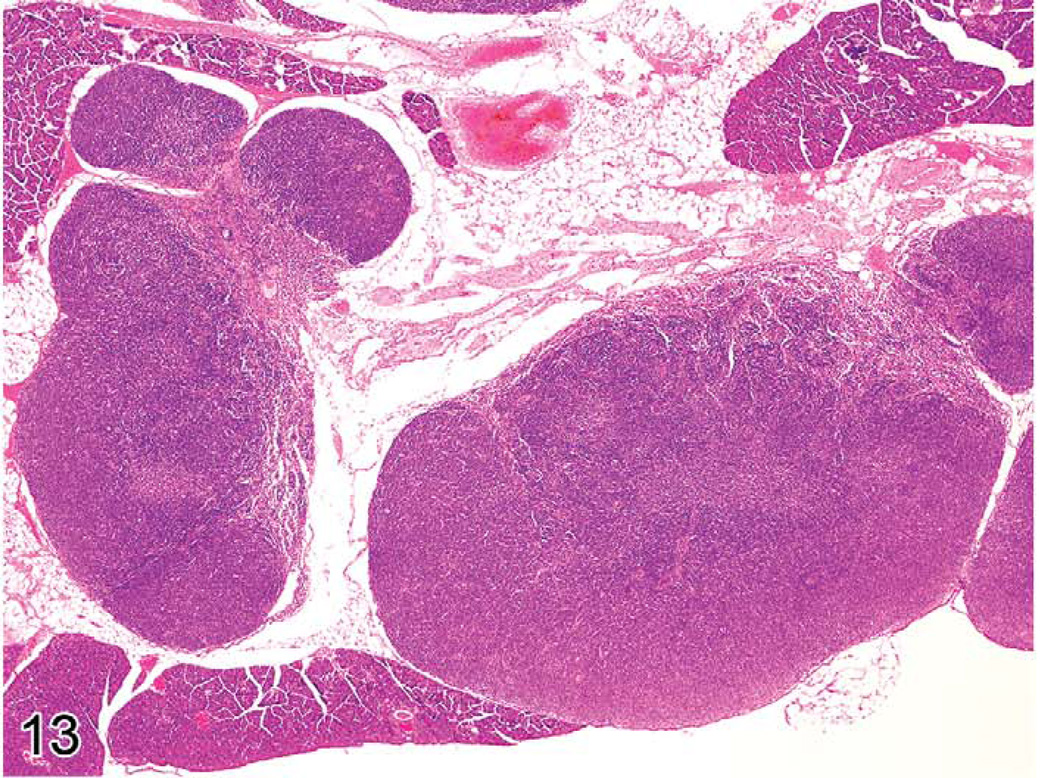
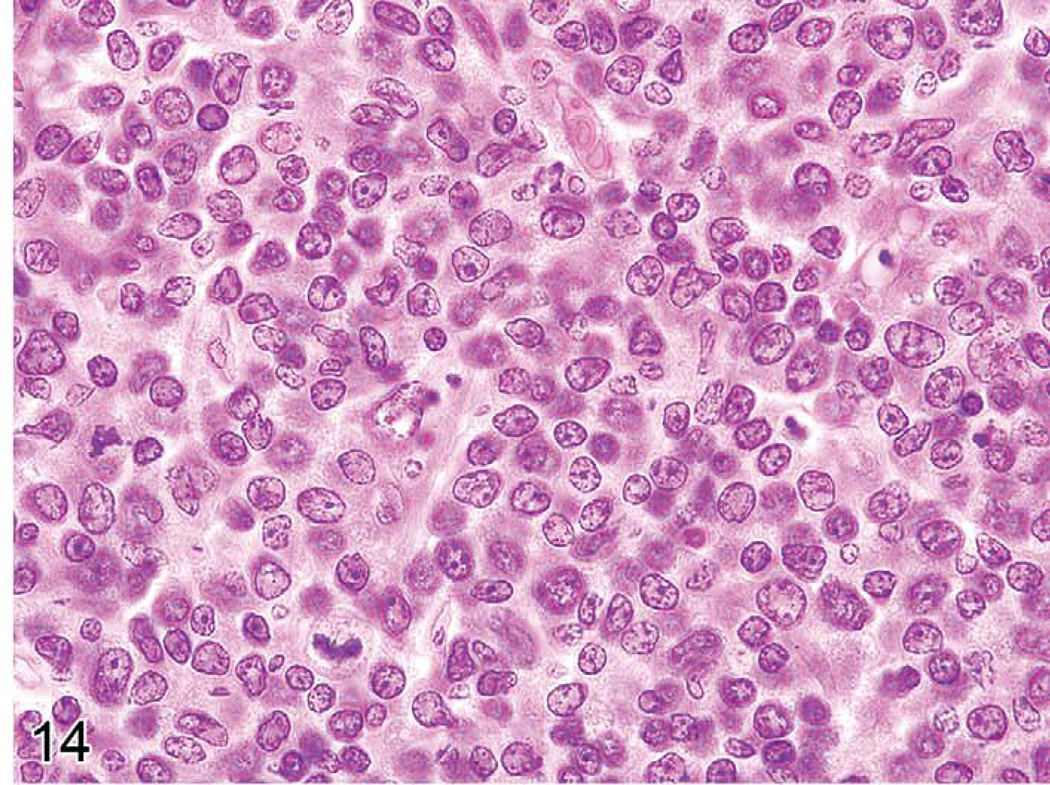
Unique genetic disorders in mice, which result from the gld or lpr mutation, involve genes similar to those in humans causing similar LPD. They have an initial proliferative non-neoplastic stage composed of a uniform population of T-cells (Figures 13 and 14). The lesion appears morphologically as a lymphoma of medium-sized lymphocytes that express CD3 and CD45R/B220 but that are not clonal. With time, the lesions progress into a plasmacytoma composed of a pleomorphic population of B-cells producing immunoglobulins (Davidson et al. 1998).
FIGURE 13.
Greatly enlarged lymph nodes of a six-month-old C3H/lpr mouse with lymphoproliferative disease.
FIGURE 14.
C3H/lpr mouse with lymphoproliferative disease. A monomorphic population of medium-sized T-cells is seen.
If a chemical induces a lymphoproliferative lesion that does not appear to be a lymphoma, one should try to characterize the nature and cause of the lesions. For example, in monkeys and humans, post-transplant LPD often occur one to six months after the initial immunosuppressive treatment. In many cases, herpes viruses have been shown to be responsible (Swerdlow et al. 2008; Schmidtko et al. 2002). The herpes virus– and retrovirus-induced LPD of mice are generally composed of several cellular components and can be differentiated from typical mouse lymphomas (Klinken et al. 1988).
With time, several of the LPD of mice that are initially polyclonal have been shown to evolve in the same lymphoid tissues into clonal B-cell lineage lymphomas, primarily of germinal center and postgerminal center origin. These include follicular and diffuse large B-cell lymphomas (SJL/J) and immature to fully differentiated plasma cell neoplasms (SJL/J, MAIDS, lpr, gld) and marginal-zone lymphomas (p53, BCL10).
Early Lymphomas in the Lymph Nodes, Spleen, and Peyer’s Patches
In aging control mice of various strains, stocks, and lines, lymphomas often arise in the splenic white pulp, mesenteric lymph node cortex, and/or Peyer’s patches (Ward 2006. These lesions can be seen in some genetically engineered mice, that develop B-cell lymphomas. These anatomical locations are also the sites of reactive lesions that can be similar to prelymphomatous lesions. The classification and nomenclature of mouse hematopoietic neoplasms is well described (Dunn 1954; Kogan et al. 2002; Morse et al. 2002).
It is important to differentiate the type of lymphomas that develop in controls from those that appear in treated mice (Ward 2006. The age of the mouse and the anatomical locations of the primary and metastatic or multicentric lymphomas are important, as well as phenotype (T-cell, B-cell, etc.). For example, it is unusual for lymphomas to arise in the thymuses of young mice, except for specific mouse strains such as AKR. Young CD1 mice and rats appear to have a low incidence of thymic lymphomas. Tumors induced in genetically engineered mice and by chemicals or irradiation often arise in the thymus (Ward et al. 1999). If lymphomas are disseminated at the time of illness or death, it may be difficult to determine the origin of the lymphomas.
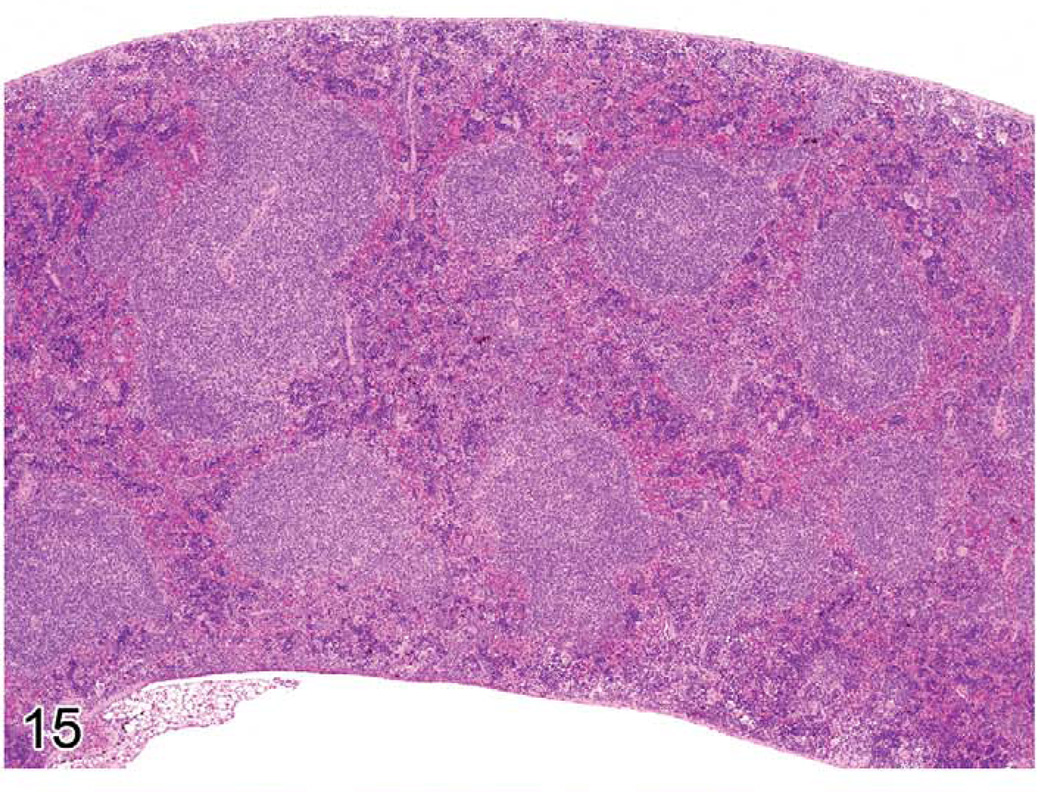
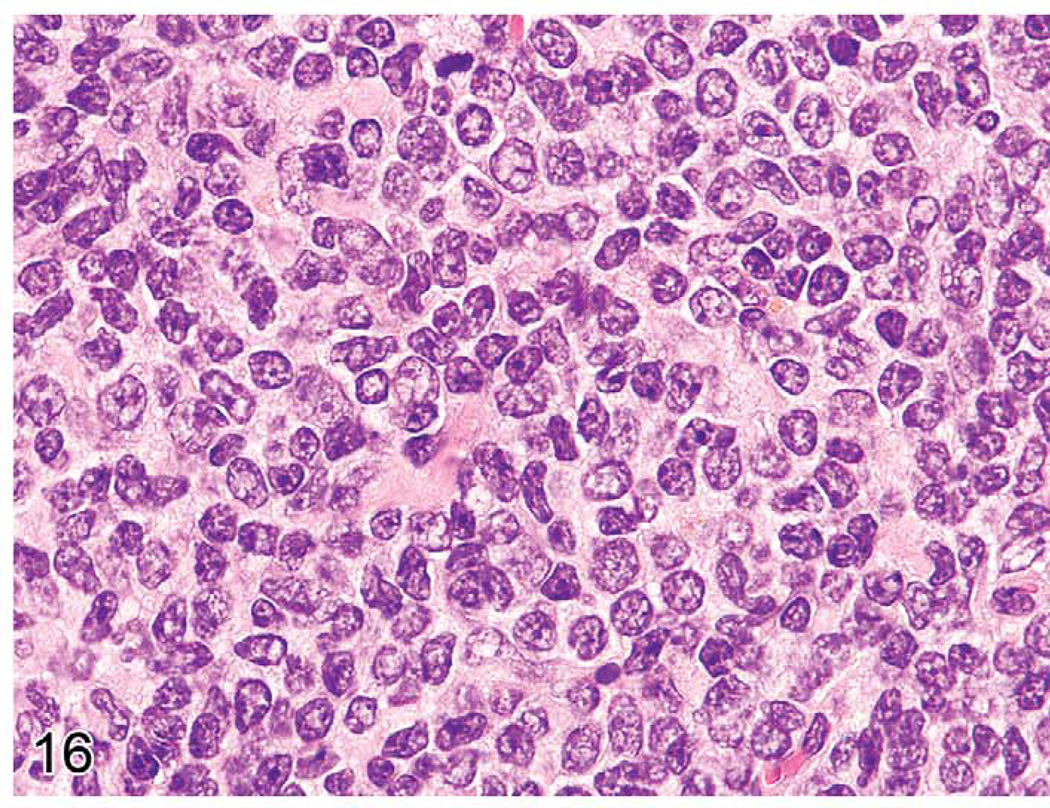
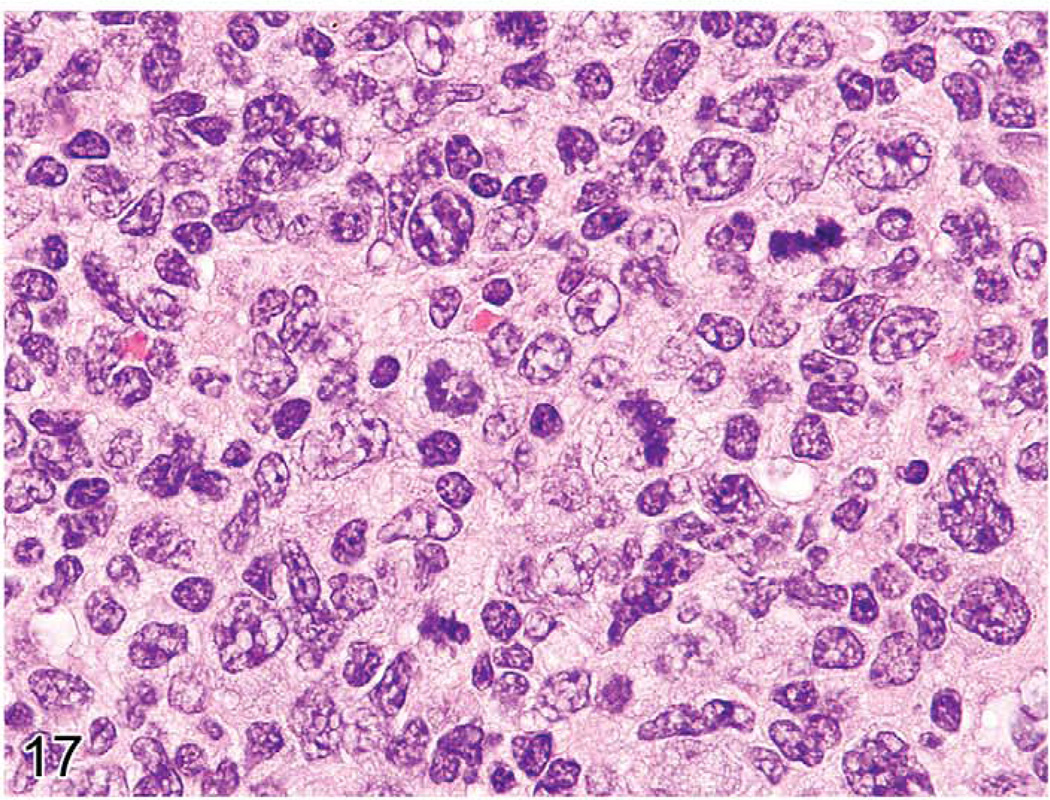
Early lymphomas in the splenic white pulp may be focal, multifocal, or diffuse (Ward 1990c). The occurrence of early lymphomas in the spleen, mesenteric or other lymph nodes, and Peyer’s patches may also be restricted to one organ or be multifocal or diffuse in several organs (multicentric). At low magnification, the splenic white pulp enlarges and appears less basophilic than the normal white pulp areas (Figure 15). The normal white pulp has areas with small lymphocytes (Figure 16), and in the germinal center, the centroblasts and centrocytes occupy different zones (polarization) and tingible body macrophages are present. Early lymphoma lesions in splenic white pulp may be seen within follicular areas or even in the periarteriolar lymphoid sheath. Although enlargement of white pulp is often seen in reactive splenic lymphoid hyperplasia, the morphology of immature lymphoid cells in early follicular lymphomas (often centroblasts and centrocytes) is unique (Figure 17). The early lymphoma cells can be blasts with round large nuclei, cells with cleaved (folded) nuclei, and other immature lymphoid cells in various proportions. Tingible body macrophages, a characteristic of reactive hyperplasia, are absent, and the centroblasts and centrocytes are randomly distributed. If plasmacytoid cells are present, they have to be differentiated from a plasma cell reaction.
FIGURE 15.
Spleen of a twenty-four-month-old C57BL/6NCr mouse with one enlarged white pulp area (upper left), which represents an early follicular lymphoma.
FIGURE 16.
Normal splenic white pulp with many mature lymphocytes (centrocytes) in the eighteen-month-old C57BL/6NCr mouse from Figure 15.
FIGURE 17.
White pulp of the mouse in Figure 15 showing early follicular lymphoma containing a mixed population of centroblasts and centrocytes, including cells with cleaved nuceli.
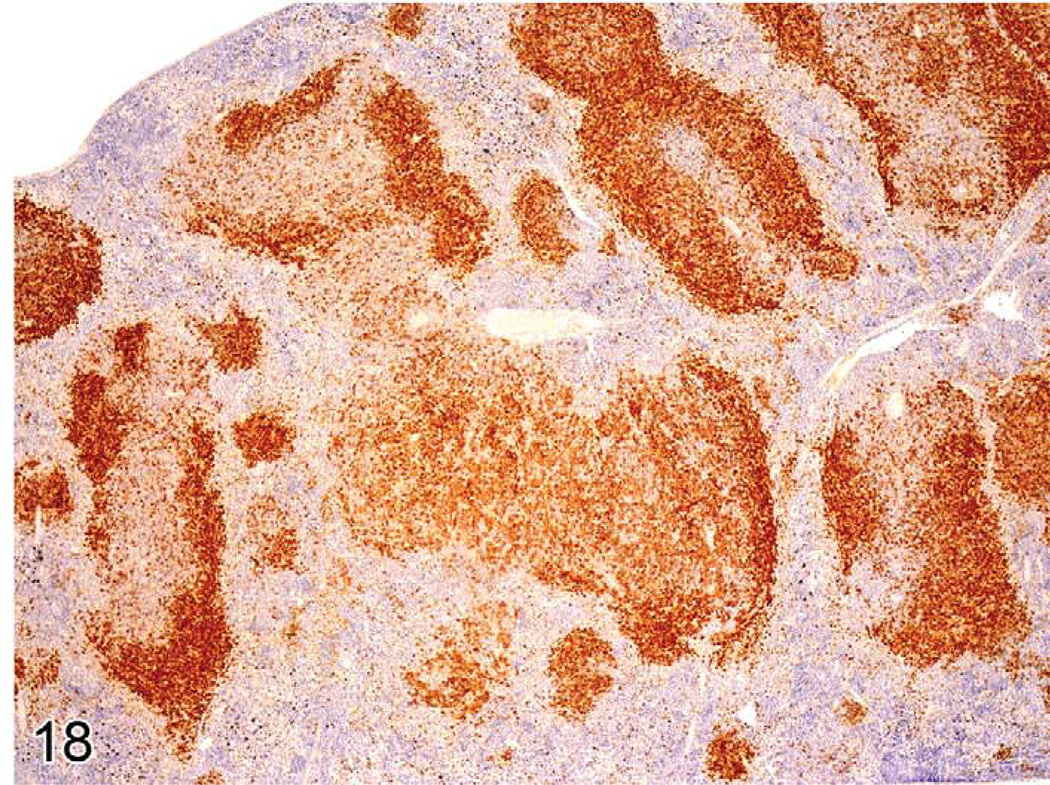
Immunohistochemistry can identify different gene expression patterns for early lymphomas in the white pulp areas (Ward et al. 1999). The areas are larger than normal white pulp areas and often express more or less antigen (Figure 18). In lymph nodes and Peyer’s patches, spontaneous lymphomas may arise in the cortex from follicular areas and may also show a change in the normal B-cell antigen (CD45R, PAX5) expression pattern. Induced lymphomas of the spleen, Peyer’s patches, and lymph nodes may also less commonly arise from germinal centers.
FIGURE 18.
Spleen of a B6C3F1 mouse with early follicular lymphoma in one large white pulp area. There is a loss of dense cellular CD45R expression in the early lymphoma.
Proof of Clonality (neoplasia)
There are several methods for determining whether presumed tumor populations are clonal. Proof of clonality would strongly suggest neoplasia rather than hyperplasia. For lymphoid disorders, analyses of DNA for the genomic organization of immunoglobulin heavy chain (IgH) and κ light chain (IgK) and T-cell receptor β chain (TCRβ) loci using Southern blot hybridization with locus-specific probes have been the gold standard (Davidson et al. 1998; Fredrickson et al. 1993; Hao et al. 2010; Hartley et al. 2000; Klinken et al. 1988). Infectious ecotropic murine leukemia viruses (MuLV) can contribute to transformation by insertional mutagenesis. Clonality can also be demonstrated by the presence of new somatically acquired proviral integrations identified by Southern blot hybridization of genomic DNA using an ecotropic MuLV-specific probe. This method is useful for examining nonlymphoid as well as lymphoid neoplasms (Morse et al. 2001). Clonal chromosomal aberrations, including translocations and aneuploidy, can be revealed using spectral karyotyping of metaphase spreads (Qi et al. 2010). This approach can be applied to analyses of lymphoid and nonlymphoid neoplasms. Also, TCRβ gene rearrangements can be amplified for sequence analysis using degenerate RT-PCR (Choi et al. 2009) or for IgH using primers that detect rearrangements of diversity and joining regions from IgH genes by PCR (Xiang et al. 2011).
Summary
Reactive lesions in lymphoid tissues commonly occur with aging and under experimental conditions. The lesions follow a pattern involving specific anatomical compartments or multiple compartments of the lymph nodes, spleen, and other tissues. An interpretive diagnostic scheme is presented. Differentiation of reactive (often hyperplastic) lesions from early neoplastic lesions may be a difficult task. Patterns of tissue and cell reactions, LPD, early neoplasia, and neoplasia can be readily defined. Clonality assays can be important. This review provides information for differentiating reactive from neoplastic lesions.
FIGURE 6.
Spleen of mouse infected with P. berghei ten days earlier. The red pulp is expanded with prominent erythroid hyperplasia, and the white pulp has marked germinal center and plasma cell hyperplasia.
Acknowledgments
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: in part, by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases.
References
- Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, Palmiter RD, Brinster RL. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- Barton E, Mandal P, Speck SH. Pathogenesis and host control of gammaherpesviruses: Lessons from the mouse. Ann Rev Immunology. 2011;29:351–397. doi: 10.1146/annurev-immunol-072710-081639. [DOI] [PubMed] [Google Scholar]
- Cattoretti G, Pasqualucci L, Ballon G, Tam W, Nandula SV, Shen Q, Mo T, Murty VV, Dalla-Favera R. Deregulated BCL6 expression recapitulates the pathogenesis of human diffuse large B cell lymphomas in mice. Cancer Cell. 2005;7:445–455. doi: 10.1016/j.ccr.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Cesta MF. Normal structure, function, and histology of the spleen. Toxicol Pathol. 2006;34:455–465. doi: 10.1080/01926230600867743. [DOI] [PubMed] [Google Scholar]
- Choi CW, Chung YJ, Slape C, Aplan PD. A NUP98-HOXD13 fusion gene impairs differentiation of B and T lymphocytes and leads to expansion of thymocytes with partial TCRB gene rearrangement. J Immunol. 2009;183:6227–6235. doi: 10.4049/jimmunol.0901121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen PL, Eisenberg RA. Lpr and gld: Single gene models of systemic autoimmunity and lymphoproliferative disease. Ann Rev Immunology. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- Davidson WF, Giese T, Fredrickson TN. Spontaneous development of plasmacytoid tumors in mice with defective Fas-Fas ligand interactions. J Exp Med. 1998;187:1825–1838. doi: 10.1084/jem.187.11.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn TB. Normal and pathologic anatomy of the reticular tissue in laboratory mice, with a classification and discussion of neoplasms. J Natl Cancer I. 1954;14:1281–1433. [PubMed] [Google Scholar]
- Dunnick JK, Hardisty JF, Herbert RA, Seely JC, Furedi-Machacek EM, Foley JF, Lacks GD, Stasiewicz S, French JE. Phenolphthalein induces thymic lymphomas accompanied by loss of the p53 wild type allele in heterozygous p53-deficient (+/−) mice. Toxicol Pathol. 1997;25:533–540. doi: 10.1177/019262339702500601. [DOI] [PubMed] [Google Scholar]
- Elmore SA. Histopathology of the lymph nodes. Toxicol Pathol. 2006a;34:425–454. doi: 10.1080/01926230600964722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore SA. Enhanced histopathology of the thymus. Toxicol Pathol. 2006b;34:656–665. doi: 10.1080/01926230600865556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore SA. Enhanced histopathology of the lymph nodes. Toxicol Pathol. 2006c;34:634–647. doi: 10.1080/01926230600939997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore SA. Enhanced histopathology of the spleen. Toxicol Pathol. 2006d;34:648–655. doi: 10.1080/01926230600865523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore SA. Enhanced histopathology evaluation of lymphoid organs. Method Mol Biol. 2010;598:323–339. doi: 10.1007/978-1-60761-401-2_22. [DOI] [PubMed] [Google Scholar]
- Elmore SA. Enhanced histopathology of the immune system. Toxicol Pathol. doi: 10.1177/0192623311427571. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson TN, Tang Y, Chattopadhyay SK, Morse HC, III, Hartley JW. Retrovirus-induced lymphoproliferation as a model for developing diagnostic criteria for malignant lymphoma in mice. Toxicol Pathol. 1993;21:219–228. doi: 10.1177/019262339302100214. [DOI] [PubMed] [Google Scholar]
- Germolec DR, Kashon M, Nyska A, Kuper CF, Portier C, Kommineni C, Johnson KA, Luster MI. The accuracy of extended histopathology to detect immunotoxic chemicals. Toxicol Sci. 2004;82:504–514. doi: 10.1093/toxsci/kfh271. [DOI] [PubMed] [Google Scholar]
- Germolec DR, Nyska A, Kashon M, Kuper CF, Portier C, Kommineni C, Johnson KA, Luster MI. Extended histopathology in immunotoxicity testing: Interlaboratory validation studies. Toxicol Sci. 2004;78:107–115. doi: 10.1093/toxsci/kfh049. [DOI] [PubMed] [Google Scholar]
- Haley P, Perry R, Ennulat D, Frame S, Johnson C, Lapointe JM, Nyska A, Snyder P, Walker D, Walter G. STP position paper: Best practice guideline for the routine pathology evaluation of the immune system. Toxicol Path. 2005;33:404–407. doi: 10.1080/01926230590934304. [DOI] [PubMed] [Google Scholar]
- Hao X, Fredrickson TN, Chattopadhyay SK, Han W, Qi CF, Wang Z, Ward JM, Hartley JW, Morse HC., III The histopathologic and molecular basis for the diagnosis of histiocytic sarcoma and histiocyte-associated lymphoma of mice. Vet Pathol. 2010;47:434–445. doi: 10.1177/0300985810363705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley JW, Chattopadhyay SK, Lander MR, Taddesse-Heath L, Naghashfar Z, Morse HC, III, Fredrickson TN. Accelerated appearance of multiple B cell lymphoma types in NFS/N mice congenic for ecotropic murine leukemia viruses. Lab Invest. 2000;80:159–169. doi: 10.1038/labinvest.3780020. [DOI] [PubMed] [Google Scholar]
- Hartley JW, Fredrickson TN, Yetter RA, Makino M, Morse HC., III Retrovirus-induced murine acquired immunodeficiency syndrome: natural history of infection and differing susceptibility of inbred mouse strains. J Virol. 1989;63:1223–1231. doi: 10.1128/jvi.63.3.1223-1231.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe ES, Vardiman J, Campo E, Arber D, Harris NL. Hematopathology. St. Louis, MO: Saunders; 2011. p. 1072. [Google Scholar]
- Karupiah G, Sacks TE, Klinman DM, Fredrickson TN, Hartley JW, Chen JH, Morse HC., III Murine cytomegalovirus infection-induced polyclonal B cell activation is independent of CD4þ T cells and CD40. Virology. 1998;240:12–26. doi: 10.1006/viro.1997.8900. [DOI] [PubMed] [Google Scholar]
- Klinken SP, Fredrickson TN, Hartley JW, Yetter RA, Morse HC., III Evolution of B cell lineage lymphomas in mice with a retrovirus-induced immunodeficiency syndrome, MAIDS. J Immunol. 1988;140:1123–1131. [PubMed] [Google Scholar]
- Kogan SC, Ward JM, Anver MR, Berman JJ, Brayton C, Cardiff RD, Carter JS, de Coronado S, Downing JR, Fredrickson TN, Haines DC, Harris AW, Harris NL, Hiai H, Jaffe ES, MacLennan IC, Pandolfi PP, Pattengale PK, Perkins AS, Simpson RM, Tuttle MS, Wong JF, Morse HC., III Bethesda proposals for classification of nonlymphoid hematopoietic neoplasms in mice. Blood. 2002;100:238–245. doi: 10.1182/blood.v100.1.238. [DOI] [PubMed] [Google Scholar]
- Konishi N, Ward JM, Reynolds CW, Lijinsky W. Thymic T-cell lymphoma with the CD8þ (OX-8), CD4þ (W3/25) phenotype, induced in F344/NCr rats by nitroso-2-hydroxypropylurea. Thymus. 1988;12:225–237. [PubMed] [Google Scholar]
- Kovalchuk AL, Kim JS, Park SS, Coleman AE, Ward JM, Morse HC, III, Kishimoto T, Potter M, Janz S. IL-6 transgenic mouse model for extraosseous plasmacytoma. Proc Natl Acad Sci U S A. 2002;99:1509–1514. doi: 10.1073/pnas.022643999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunder S, Calzada-Wack J, Holzlwimmer G, Muller J, Kloss C, Howat W, Schmidt J, Hofler H, Warren M, Quintanilla-Martinez L. A comprehensive antibody panel for immunohistochemical analysis of formalin-fixed, paraffin-embedded hematopoietic neoplasms of mice: Analysis of mouse specific and human antibodies cross-reactive with murine tissue. Toxicol Pathol. 2007;35:366–375. doi: 10.1080/01926230701230296. [DOI] [PubMed] [Google Scholar]
- Kuper CF, Harleman JH, Richter-Reichelm HB, Vos JG. Histopathologic approaches to detect changes indicative of immunotoxicity. Toxicol Pathol. 2000;28:454–466. doi: 10.1177/019262330002800317. [DOI] [PubMed] [Google Scholar]
- Li Z, Wang H, Xue L, Shin DM, Roopenian D, Xu W, Qi CF, Sangster MY, Orihuela CJ, Tuomanen E, Rehg JE, Cui X, Zhang Q, Morse HC, III, Morris SW. Emu-BCL10 mice exhibit constitutive activation of both canonical and noncanonical NF-kappaB pathways generating marginal zone (MZ) B-cell expansion as a precursor to splenic MZ lymphoma. Blood. 2009;114:4158–4168. doi: 10.1182/blood-2008-12-192583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden M, Ward JM, Cherian S. Hematopoietic and Lymphoid Tissues. In: Treuting PM, Dintzis SM, editors. Comparative Anatomy and Histology: A Mouse and Human Atlas. Amsterdam, The Netherlands: Elsevier; (in press). [Google Scholar]
- Long RE, Knutsen G, Robinson M. Myeloid hyperplasia in the SENCAR mouse: Differentiation from granulocytic leukemia. Environ Health Persp. 1986;68:117–123. doi: 10.1289/ehp.8668117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebius RE, Kraal G. Structure and function of the spleen. Nature Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- Mikaelian I, Nanney LB, Parman KS, Kusewitt DF, Ward JM, Naf D, Krupke DM, Eppig JT, Bult CJ, Seymour R, Ichiki T, Sundberg JP. Antibodies that label paraffin-embedded mouse tissues: A collaborative endeavor. Toxicol Pathol. 2004;32:181–191. doi: 10.1080/01926230490274335. [DOI] [PubMed] [Google Scholar]
- Morse HC, III, Anver MR, Fredrickson TN, Haines DC, Harris AW, Harris NL, Jaffe ES, Kogan SC, MacLennan IC, Pattengale PK, Ward JM. Bethesda proposals for classification of lymphoid neoplasms in mice. Blood. 2002;100:246–258. doi: 10.1182/blood.v100.1.246. [DOI] [PubMed] [Google Scholar]
- Morse HC, III, Qi CF, Chattopadhyay SK, Hori M, Taddesse-Heath L, Ozato K, Hartley JW, Taylor BA, Ward JM, Jenkins NA, Copeland NG, Fredrickson TN. Combined histologic and molecular features reveal previously unappreciated subsets of lymphoma in AKXD recombinant inbred mice. Leukemia Res. 2001;25:719–733. doi: 10.1016/s0145-2126(01)00022-4. [DOI] [PubMed] [Google Scholar]
- Ohtani O, Ohtani Y. Structure and function of rat lymph nodes. Arch Histol Cytol. 2008;71:69–76. doi: 10.1679/aohc.71.69. [DOI] [PubMed] [Google Scholar]
- Park SS, Kim JS, Tessarollo L, Owens JD, Peng L, Han SS, Tae Chung S, Torrey TA, Cheung WC, Polakiewicz RD, McNeil N, Ried T, Mushinski JF, Morse HC, III, Janz S. Insertion of c-Myc into Igh induces B-cell and plasma-cell neoplasms in mice. Cancer Res. 2005;65:1306–1315. doi: 10.1158/0008-5472.CAN-04-0268. [DOI] [PubMed] [Google Scholar]
- Pearse G. Normal structure, function and histology of the thymus. Toxicol Pathol. 2006a;34:504–514. doi: 10.1080/01926230600865549. [DOI] [PubMed] [Google Scholar]
- Pearse G. Histopathology of the thymus. Toxicol Pathol. 2006b;34:515–547. doi: 10.1080/01926230600978458. [DOI] [PubMed] [Google Scholar]
- Pearse G, Pietersma A, Cunliffe J, Foster JR, Turton J, Derbyshire N, Randall KJ. Time-course study of the immunotoxic effects of the anticancer drug chlorambucil in the rat. Toxicol Pathol. 2009;37:887–901. doi: 10.1177/0192623309347907. [DOI] [PubMed] [Google Scholar]
- Qi CF, Shin DM, Li Z, Wang H, Feng J, Hartley JW, Fredrickson TN, Kovalchuk AL, Morse HC. III. Anaplastic plasmacytomas: Relationships to normal memory B cells and plasma cell neoplasms of immunodeficient and autoimmune mice. J Pathol. 2010;221:106–116. doi: 10.1002/path.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehg JE, Bush D, Ward JM. The utility of IHC in the identification of lymphohematopoietic cells in normal tissues, and interpretation of proliferative, inflammatory and toxic lesions. Toxicol Pathol. doi: 10.1177/0192623311430695. (in press). [DOI] [PubMed] [Google Scholar]
- Rehg JE, Sundberg JP. Utility of antiPax5 in the diagnosis of lymphoproliferative disorders and neoplasia in mice. Comp Med. 2008;58:246–252. [PMC free article] [PubMed] [Google Scholar]
- Schmidtko J, Wang R, Wu CL, Mauiyyedi S, Harris NL, Della Pelle P, Brousaides N, Zagachin L, Ferry JA, Wang F, Kawai T, Sachs DH, Cosimi BA, Colvin RB. Posttransplant lymphoproliferative disorder associated with an Epstein-Barr-related virus in cynomolgus monkeys. Transplantation. 2002;73:1431–1439. doi: 10.1097/00007890-200205150-00012. [DOI] [PubMed] [Google Scholar]
- Suttie AW. Histopathology of the spleen. Toxicol Pathol. 2006;34:466–503. doi: 10.1080/01926230600867750. [DOI] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: International Agency for Research on Cancer; 2008. p. 439. [Google Scholar]
- Tang JC, Ho FC, Chan AC, Chow EY, Srivastava G. Progression of spontaneous lymphomas in SJL mice: Monitoring in vivo clonal evolution with molecular markers in sequential splenic samples. Lab Invest. 1998;78:1459–1466. [PubMed] [Google Scholar]
- Travlos GS. Histopathology of bone marrow. Toxicol Pathol. 2006;34:566–598. doi: 10.1080/01926230600964706. [DOI] [PubMed] [Google Scholar]
- Van den Broeck W, Derore A, Simoens P. Anatomy and nomenclature of murine lymph nodes: Descriptive study and nomenclatory standardization in BALB/cAnNCrl mice. J Immunol Methods. 2006;312:12–19. doi: 10.1016/j.jim.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Waisberg M, Tarasenko T, Vickers BK, Scott BL, Willcocks LC, Molina-Cruz A, Pierce MA, Huang CY, Torres-Velez FJ, Smith KG, Barillas-Mury C, Miller LH, Pierce SK, Bolland S. Genetic susceptibility to systemic lupus erythematosus protects against cerebral malaria in mice. Proc Natl Acad Sci U S A. 2011;108:1122–1127. doi: 10.1073/pnas.1017996108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM. Classification of reactive lesions of lymph nodes. In: Jones TC, Ward JM, Mohr U, Hunt RD, editors. Hemopoetic System. Berlin, Germany: Springer-Verlag; 1990a. pp. 155–161. [Google Scholar]
- Ward JM. Classification of reactive lesions, spleen. In: Jones TC, Ward JM, Mohr U, Hunt RD, editors. Hemopoetic System. Berlin, Germany: Springer-Verlag; 1990b. pp. 220–226. [Google Scholar]
- Ward JM. Early follicular center cell lymphoma, mouse. In: Jones TC, Ward JM, Mohr U, Hunt RD, editors. Hemopoetic System. Berlin, Germany: Springer-Verlag; 1990c. pp. 212–216. [Google Scholar]
- Ward JM, Mann PC, Morishima H, Frith CH. Thymus, spleen and lymph nodes. In: Maronpot RR, editor. Pathology of the Mouse. Vienna, IL: Cache River Press; 1999. pp. 333–360. [Google Scholar]
- Ward JM. Lymphomas and leukemias in mice. Exp Toxicol Pathol. 2006;57:377–381. doi: 10.1016/j.etp.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Ward JM, Erexson CR, Faucette LJ, Foley JF, Dijkstra C, Cattoretti G. Immunohistochemical markers for the rodent immune system. Toxicol Pathol. 2006;34:616–630. doi: 10.1080/01926230600941340. [DOI] [PubMed] [Google Scholar]
- Ward JM, Tadesse-Heath L, Perkins SN, Chattopadhyay SK, Hursting SD, Morse HC., 3rd Splenic marginal zone B-cell and thymic T-cell lymphomas in p53-deficient mice. Lab Invest. 1999;79:3–14. [PubMed] [Google Scholar]
- Ward JM, Uno H, Frith CH. Immunohistochemistry and morphology of reactive lesions in lymph nodes and spleen from rats and mice. Toxicol Pathol. 1993;21:199–205. doi: 10.1177/019262339302100212. [DOI] [PubMed] [Google Scholar]
- Willard-Mack CL. Normal structure, function, and histology of lymph nodes. Toxicol Pathol. 2006;34:409–424. doi: 10.1080/01926230600867727. [DOI] [PubMed] [Google Scholar]
- Xiang H, Noonan EJ, Wang J, Duan H, Ma L, Michie S, Boxer LM. The immunoglobulin heavy chain gene 30 enhancers induce Bcl2 deregulation and lymphomagenesis in murine B cells. Leukemia. 2011;25:1484–1493. doi: 10.1038/leu.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]