Abstract
Background
Previous studies examined caffeine use and caffeine dependence and risk for the symptoms, or diagnosis, of psychiatric disorders. The current study aimed to determine if generalized anxiety disorder (GAD), panic disorder, phobias, major depressive disorder (MDD), anorexia nervosa (AN), or bulimia nervosa (BN) shared common genetic or environmental factors with caffeine use, caffeine tolerance, or caffeine withdrawal.
Method
Using 2,270 women from the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders, bivariate Cholesky decomposition models were used to determine if any of the psychiatric disorders shared genetic or environmental factors with caffeine use phenotypes.
Results
GAD, phobias, and MDD shared genetic factors with caffeine use, with genetic correlations estimated to be 0.48, 0.25, and 0.38, respectively. Removal of the shared genetic and environmental parameter for phobias and caffeine use resulted in a significantly worse fitting model. MDD shared unique environmental factors (environmental correlation = 0.23) with caffeine tolerance; the genetic correlation between AN and caffeine tolerance and BN and caffeine tolerance were 0.64 and 0.49, respectively. Removal of the genetic and environmental correlation parameters resulted in significantly worse fitting models for GAD, phobias, MDD, AN, and BN, which suggested that there was significant shared liability between each of these phenotypes and caffeine tolerance. GAD had modest genetic correlations with caffeine tolerance, 0.24, and caffeine withdrawal, 0.35.
Conclusions
There was suggestive evidence of shared genetic and environmental liability between psychiatric disorders and caffeine phenotypes. This might inform us about the etiology of the comorbidity between these phenotypes.
Keywords: anxiety disorders, depression, eating disorders, caffeine, heritability
Common psychiatric disorders have consistently been shown to be comorbid with substance use disorders (Grant et al., 2005; O’Neil et al., 2011; Root et al., 2010; Salbach-Andrae et al., 2008). It has been estimated that worldwide caffeine is consumed daily by 80% of individuals (James, 1997) and can be considered a substance for potential abuse. Similar to other psychoactive substances with potential for abuse, caffeine use can be associated with symptoms of tolerance and withdrawal (Griffiths et al., 2003). The Diagnostic and Statistical Manual for Mental Disorders (DSM)-IV contains several caffeine use diagnoses (American Psychiatric Association, 1994). A genome-wide association study has suggested several genes may be associated with coffee drinking (Amin et al., 2011). Evidence suggested that caffeine dependence might be related to other substances with abuse potential. Caffeine dependence symptoms shared a common genetic factor with alcohol and nicotine dependence symptoms and a common unique environmental factor with alcohol, nicotine, cannabis, and cocaine dependence symptoms (Kendler et al., 2007). Given that there is a high comorbidity between other licit and illicit substance use and psychiatric disorders, studying the comorbidity between caffeine use, caffeine tolerance, and caffeine withdrawal and common psychiatric disorders, like anxiety disorders and depression, is of potential interest.
Thus far, some research has explored the comorbidity between caffeine use and psychiatric disorders. Caffeine use in women was associated with an increased risk of poor mental health defined as scoring four or higher on the Japanese version of the general health questionnaire (Shimbo et al., 2005). Caffeine intake, heavy caffeine use, caffeine toxicity, and symptoms of caffeine dependence were associated with an increased risk for developing major depressive disorder (MDD), generalized anxiety disorder (GAD), panic disorder, adult antisocial personality disorder, and illicit drug abuse and dependence (Kendler et al., 2006). In a separate study, some eating disorder symptoms (binge eating, concern about weight and shape, self-perception of being overweight, and expressing a fear of gaining weight) were associated with caffeine abuse (Baker et al., 2010).
Results of studies have also indicated that caffeine consumption can trigger or worsen anxiety. Caffeine has been shown to have anxiogenic effects in individuals with panic disorder (Breier et al., 1986; Charney et al., 1985; Nardi et al., 2007, 2009), performance social anxiety disorder (Nardi et al., 2009), and major depression with panic attacks (Nardi et al., 2007). Despite consuming similar amounts of caffeine as individuals without psychological disorders, those with depression expressed higher sensitivity to the anxiogenic effects of caffeine (Lee et al., 1988). Taken together, there may be shared genetic or environmental factors between psychiatric disorders and caffeine use phenotypes.
Twin studies have suggested that genetic factors play an important role in the etiology of all common psychiatric disorders that have been examined including GAD (Hettema et al., 1995; Hettema et al., 2001; Scherrer et al., 2000), phobias (Kendler et al., 2008; Kendler et al., 1999), panic (Hettema et al., 2001; Kendler et al., 2001; Middeldorp et al., 2005; Mosing et al., 2009), MDD (Bierut et al., 1999; Kendler & Prescott, 1999a; Sullivan et al., 2000), anorexia nervosa (AN; Dellava et al., 2010; Klump et al., 2001; Mazzeo et al., 2009), and bulimia nervosa (BN; Bulik et al., 2010; Kendler et al., 1991; Kortegaard et al., 2001). Fewer studies have been conducted on caffeine use phenotypes. Of the existing twin studies, the heritability of caffeine use was estimated between 0.36 and 0.58 (Kendler & Prescott, 1999b; Yang et al., 2010), caffeine tolerance at 0.40 (Kendler & Prescott, 1999b; Luciano et al., 2005), and caffeine withdrawal at 0.35 (Kendler & Prescott, 1999b). Despite the high comorbidity between psychiatric disorders and caffeine phenotypes, few twin studies have examined the nature of this comorbidity. Although no significant results were found, possibly due to sample size, in monozygotic (MZ) twins all odds ratios between caffeine use phenotypes and psychological disorders were non-significant; in the full sample including all twins, this relationship was significant. This indicated that these phenotypes likely share genetic liability (Kendler et al., 2006). The genetic correlation between caffeine abuse/dependence and BN symptom count was estimated at 0.37 (Baker et al., 2010). A genetic correlation of 0.40 was found between caffeine use and sleep disturbance (Luciano et al., 2007). Further research is needed to determine if there is shared genetic or environmental liability between specific psychiatric disorders and specific caffeine use phenotypes.
The aim of the current study was to determine the magnitude of the genetic and environmental correlations between common psychiatric disorders (GAD, panic disorder, phobias, MDD, AN-like syndrome, and BN-like syndrome) and key caffeine phenotypes (caffeine use, caffeine tolerance, and caffeine withdrawal) in women. In conducting these analyses, the possibility of the existence of consistent patterns among all the psychiatric phenotypes and caffeine phenotypes was explored. In addition, analyses could illustrate if each specific psychiatric phenotype displayed the same general relationship with all caffeine phenotypes. For all psychiatric phenotypes, broad diagnoses were used to maximize power.
Materials and Methods
Participants
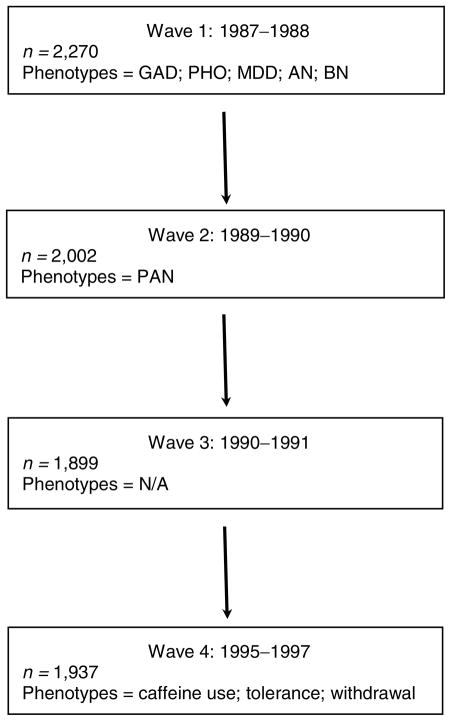
This study consisted of females who participated in the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders. This sample has previously been described (Kendler et al., 1992a, 1992b). Briefly, the female twin pair portion of this study consisted of 2,270 individuals from female–female twin pairs who completed the Wave 1 interview in person (89%) or by telephone (11%). Some of these women participated in up to three additional interview waves of data collection. Of the original 2,270 who participated in Wave 1, 1,937 participated in Wave 4 (Kendler & Prescott, 1999a). For the current study, the most comprehensive psychiatric diagnoses, with the exception of panic disorder, were conducted in Wave 1 and therefore taken from Wave 1, panic disorder was best assessed and therefore taken from Wave 2, and caffeine use was only assessed and therefore taken from Wave 4. Phobia diagnosis was adapted from the Diagnostic Interview Schedule Version III-A (Robins & Helzer, 1985) for DSM-III criteria (American Psychiatric Association, 1980); GAD, panic disorder, MDD, AN, BN, and caffeine tolerance were adapted from Structured Clinical Interview for DSM-IIIR (Spitzer et al., 1990) and are based on DSM-IIIR criteria (Spitzer et al., 1988); and caffeine withdrawal was adapted from DSM-IV (American Psychiatric Association, 1994). Figure 1 illustrates the wave from which each phenotype was obtained.
FIGURE 1.
Measurement of variables for current study.
Note: n = number; GAD = generalized anxiety disorder; PAN = panic disorder; PHO = phobia; MDD = major depressive disorder; AN = anorexia nervosa; BN = bulimia nervosa; N/A = not applicable.
Zygosity Determination
Zygosity determination, as previously been described (Kendler et al., 1992b), was based on standard questions regarding physical similarity of a twin with her co-twin (Eaves et al., 1989), photographs of the twin and her co-twin, and some DNA samples (Spence et al., 1988).
Psychiatric Phenotypes
Generalized anxiety disorder
GAD diagnosis in this sample has been well described (Kendler et al., 1992a). GAD was taken from Wave 1 and the one-month criterion was used (Kendler et al., 1992a) for the current study. The one-and six-month GAD criteria yield a sample with comparable characteristics (Breslau & Davis, 1985; Kendler et al., 1992a; Lee et al., 2009). In the current sample, 539 women (25.9% of women with non-missing data) met criterion for GAD.
Panic disorder
For the current study, panic disorder was taken from Wave 2, which has been described in detail (Kendler et al., 2001). In order to achieve adequate power, the ‘very broad’ definition of panic was used; this required a positive answer to the screen in question that was indicative of DSM-IIIR criterion A, two of the 13 symptoms listed in DSM-IIIR criterion C, and maximum panic intensity within 30 min of onset (Kendler et al., 2001). In the current sample, 235 women (13.6% of women with non-missing data) met the criterion for panic.
Phobia
Any phobia diagnosis was used in the current study and taken from Wave 1. Briefly, women were asked about 17 specific possible fears and could add in additional phobias; for phobia to be considered present, the woman had to respond affirmatively to the following: ‘the fear or its avoidance interfered with life or activities a lot’ (Kendler et al., 1995). This diagnosis has earlier been described in detail. In the current sample, 700 women (33.6% of women with non-missing data) met this criterion.
Major depressive disorder
Lifetime MDD was used for the current study taken from Wave 1. DSM-IIIR criteria were used as previously described (Kendler et al., 1995). In the current sample, 728 (35.0% of women with non-missing data) met criteria for lifetime MDD.
Anorexia nervosa like syndrome
In order to obtain sufficient power to evaluate AN, a broad definition of AN was used from Wave 1. This included women with definite, possible, or probable AN and has been described in detail (Walters & Kendler, 1995). There were 76 women (3.7% of women with non-missing data) meeting this diagnosis.
Bulimia nervosa like syndrome
Similar to AN, a broad definition of BN from Wave 1 was used. This diagnosis was derived from a computer algorithm and included definite, probable, or possible cases as previously described (Kendler et al., 1995). There were 118 women (5.7% of women with non-missing data) in the current sample meeting this definition of BN.
Caffeine Use Phenotypes
Caffeine use
In Wave 4, the women were asked to report caffeine consumption in the previous year by reporting consumption of caffeinated beverages; caffeine use was estimated based on standard amounts of caffeine in each type of beverage then divided into 10 use categories (Kendler & Prescott, 1999b): <1,150 mg/month; 1,150–2409.9 mg/month; 2,410–4,149.9 mg/month; 4,150–5,709.9 mg/month; 5,710–7,209.9 mg/month; 7,210–8,709.9 mg/month; 8,710–10,959.9 mg/month; 10,960–13,659.9 mg/month; 13,660–19,209.9 mg/month; and 19,210+ mg/month. The number of women (percent of women with non-missing data) falling into each category is as follows: 161 (8.7%); 195 (10.5%); 161 (8.7%); 207 (11.1%); 185 (9.9%); 201 (10.8%); 173 (9.3%); 199 (10.7%); 187 (10.1%); and 192 (10.3%).
Caffeine tolerance
Caffeine tolerance was considered present if the woman responded ‘yes’ to either of the following questions: ‘During the time when you were consuming caffeinated beverages the most, did you find that you needed to drink a lot more to get the desired effect than you did when you first drank them?’ and ‘ What about finding out that when you drank the same amount, it had much less effect than before?’ (Kendler & Prescott, 1999b). Using this diagnosis, 278 women (15.1% of those with non-missing data) met criteria for caffeine tolerance.
Caffeine withdrawal
Caffeine withdrawal was guided by DSM-IV (American Psychiatric Association, 1994) and considered present if headache was present along with either marked fatigue or drowsiness, marked anxiety or depression, or nausea or vomiting when caffeine was not consumed in its usual quantity (Kendler & Prescott, 1999b). In the current sample, 256 women (22.3% of those with non-missing data) met criteria for caffeine withdrawal.
Statistical Methods
Biometrical twin modeling was conducted in OpenMx and based on the properties and assumptions of the classic twin design (Neale & Cardon, 1992). Additive genetic effects (a2) were the genetic factors modeled in the current study. MZ twins are genetically identical, i.e., all genes are shared, and dizygotic (DZ) twins are no more similar than other siblings, i.e., about half of their genes are shared, identical by descent; common environmental factors (c2; e.g., living in the same household as children) are shared by twins regardless of zygosity, and unique environmental factors (e2; e.g., living in different cities as adults) are not common between any twins; the e2 parameter contained the error term. Biometrical twin modeling was used and compared different patterns of similarities and differences of MZ and DZ twins to estimate genetic, common environmental, and unique environmental parameters (Neale & Cardon, 1992; Neale & Maes, 1998).
For the current study, a Cholesky decomposition was used to estimate the unique and shared genetic and the unique and shared unique environmental components of psychiatric disorders and caffeine phenotype (Figure 2); this was repeated for each psychiatric phenotype and each caffeine phenotype. A priori, the c2 parameter was fixed to zero for both psychiatric and caffeine phenotypes. This decision was based on univariate models, which indicated that the c2 parameter was non-significant (data not shown). The Cholesky decomposition model contained a direct path from a genetic and unique environmental factor to the psychiatric phenotype and a second direct path from a genetic and unique environmental factor to the caffeine phenotype; there was then a cross path from the psychiatric phenotype to the caffeine phenotype, which allowed estimation of genetic and unique environmental correlation between phenotypes. The psychiatric phenotype was entered into the model first followed by the caffeine phenotype. The variable order had no effect on the results. Additional models were fit to determine if the genetic or environmental correlation parameters were statistically significant. This was done by fitting several submodels in which the genetic or environmental correlation parameters were fixed to zero. Model evaluation was done by likelihood ratio tests — the difference between −2 log-likelihood (−2LL) of the two models distributed as a chi-square — and Akaike’s information criterion (AIC). Given that we had low statistical power to distinguish between the models, the genetic and environmental parameters were not removed from the model.
FIGURE 2.
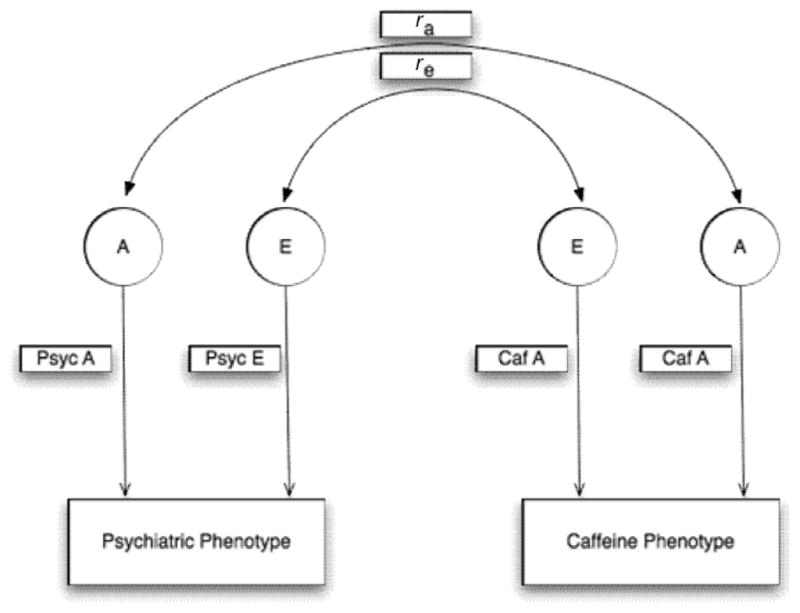
Bivariate model for psychiatric phenotypes (Psyc) and caffeine phenotypes (Caf).
Note: A =heritability component; E =unique environment component; ra = genetic correlation; re = unique environmental/error.
Raw data were analyzed using full information maximum likelihood for parameter estimates and 95% confidence intervals (CI), which handled missing data (Neale & Cardon, 1992). All statistical analyses were conducted in the R software version 2.12.2 (R Development Core Team, 2010) and all twin modeling was conducted using OpenMx version 1.0.6 (Boker et al., 2011).
Results
Model fit statistics for the full model and submodels are contained in Table 1, and broken down by caffeine phenotype and psychiatric phenotype. The submodels contained in Table 1 indicate which genetic and environmental correlation parameters were significant. Parameter estimates for psychiatric phenotype and caffeine use are contained in Table 2. The genetic correlation between GAD and caffeine use was 0.48 (significantly different from zero). The genetic correlation between phobias and caffeine use was 0.25 and MDD and caffeine use was 0.38, which were also statistically significant. For panic disorder and caffeine use, there was a negative genetic correlation, −0.22, but this was not significantly different from zero. When both the genetic and environmental correlation parameters were fixed to zero, a significant deterioration of fit resulted for GAD, phobias, and MDD (data not shown). This indicated there was significant shared liability between these psychiatric disorders and caffeine use.
TABLE 1.
Model Fit Statistics
| Caffeine phenotype | Psychiatric phenotype | −2LL | df | Δdf | Δχ2 | AIC | p-value |
|---|---|---|---|---|---|---|---|
| Caffeine use | GAD | 10716.00 | 3926 | n/a | n/a | 2864.00 | n/a |
| GAD, no rg | 10737.45 | 3927 | 1 | 21.45 | 2883.45 | .00 | |
| GAD, no re | 10716.31 | 3927 | 1 | 0.31 | 2862.31 | .58 | |
| PAN | 9821.36 | 3808 | n/a | n/a | 2205.36 | n/a | |
| PAN, no rg | 9824.26 | 3809 | 1 | 2.91 | 2206.26 | .09 | |
| PAN, no re | 9823.20 | 3809 | 1 | 1.84 | 2205.20 | .17 | |
| PHO | 11020.66 | 3926 | n/a | n/a | 3168.66 | n/a | |
| PHO, no rg | 11027.04 | 3927 | 1 | 6.38 | 3173.04 | .01 | |
| PHO, no re | 11020.67 | 3927 | 1 | 0.01 | 3166.67 | .91 | |
| MDD | 11003.75 | 3927 | n/a | n/a | 3149.75 | n/a | |
| MDD, no rg | 11022.08 | 3928 | 1 | 18.33 | 3166.08 | .00 | |
| MDD, no re | 11007.66 | 3928 | 1 | 3.90 | 3151.66 | .05 | |
| AN | 9045.16 | 3926 | n/a | n/a | 1193.16 | n/a | |
| AN, no rg | 9045.32 | 3927 | 1 | 0.16 | 1191.32 | .69 | |
| AN, no re | 9045.22 | 3927 | 1 | 0.07 | 1191.22 | .80 | |
| BN | 9283.82 | 3927 | n/a | n/a | 1429.82 | n/a | |
| BN, no rg | 9284.26 | 3928 | 1 | 0.44 | 1428.26 | .51 | |
| BN, no re | 9284.16 | 3928 | 1 | 0.34 | 1428.16 | .56 | |
| Tolerance | GAD | 3921.97 | 3917 | n/a | n/a | −3912.03 | n/a |
| GAD, no rg | 3923.75 | 3918 | 1 | 1.78 | −3912.25 | .18 | |
| GAD, no re | 3923.59 | 3918 | 1 | 1.62 | −3912.41 | .20 | |
| PAN | 2999.51 | 3799 | n/a | n/a | −4598.49 | n/a | |
| PAN, no rg | 3001.12 | 3800 | 1 | 1.61 | −4598.88 | .20 | |
| PAN, no re | 3000.22 | 3800 | 1 | 0.71 | −4599.78 | .40 | |
| PHO | 4197.50 | 3917 | n/a | n/a | −3636.50 | n/a | |
| PHO, no rg | 4198.49 | 3918 | 1 | 0.99 | −3637.51 | .32 | |
| PHO, no re | 4199.59 | 3918 | 1 | 2.09 | −3636.41 | .15 | |
| MDD | 4217.70 | 3918 | n/a | n/a | −3618.30 | n/a | |
| MDD, no rg | 4217.92 | 3919 | 1 | 0.23 | −3620.08 | .63 | |
| MDD, no re | 4223.08 | 3919 | 1 | 5.38 | −3614.92 | .02 | |
| AN | 2215.14 | 3917 | n/a | n/a | −5618.86 | n/a | |
| AN, no rg | 2220.07 | 3918 | 1 | 4.92 | −5615.93 | .03 | |
| AN, no re | 2215.58 | 3918 | 1 | 0.44 | −5620.42 | .51 | |
| BN | 2456.71 | 3918 | n/a | n/a | −5379.29 | n/a | |
| BN, no rg | 2462.58 | 3919 | 1 | 5.87 | −5375.42 | .02 | |
| BN, no re | 2459.87 | 3919 | 1 | 3.15 | −5378.13 | .08 | |
| Withdrawal | GAD | 3579.07 | 3238 | n/a | n/a | −2896.93 | n/a |
| GAD, no rg | 3581.30 | 3239 | 1 | 2.23 | −2896.70 | .14 | |
| GAD, no re | 3579.18 | 3239 | 1 | 0.10 | −2898.82 | .75 | |
| PAN | 2652.58 | 3120 | n/a | n/a | −3587.42 | n/a | |
| PAN, no rg | 2652.71 | 3121 | 1 | 0.13 | −3589.29 | .72 | |
| PAN, no re | 2652.93 | 3121 | 1 | 0.35 | −3589.07 | .55 | |
| PHO | 3857.32 | 3238 | n/a | n/a | −2618.68 | n/a | |
| PHO, no rg | 3857.56 | 3239 | 1 | 0.25 | −2620.44 | .62 | |
| PHO, no re | 3858.68 | 3239 | 1 | 1.37 | −2619.32 | .24 | |
| MDD | 3876.13 | 3239 | n/a | n/a | −2601.87 | n/a | |
| MDD, no rg | 3877.68 | 3240 | 1 | 1.55 | −2602.32 | .21 | |
| MDD, no re | 3876.69 | 3240 | 1 | 0.56 | −2603.31 | .45 | |
| AN | 1872.02 | 3238 | n/a | n/a | −4603.98 | n/a | |
| AN, no rg | 1872.50 | 3239 | 1 | 0.48 | −4605.50 | .49 | |
| AN, no re | 1872.06 | 3239 | 1 | 0.04 | −4605.94 | .84 | |
| BN | 2112.09 | 3239 | n/a | n/a | −4365.91 | n/a | |
| BN, no rg | 2113.45 | 3240 | 1 | 1.36 | −4366.55 | .24 | |
| BN, no re | 2112.12 | 3240 | 1 | 0.03 | −4367.88 | .85 |
Note: AIC = Akaike’s information criterion; GAD = generalized anxiety disorder; PAN = panic disorder; PHO = phobia; MDD = major depressive disorder; AN = anorexia nervosa; BN = bulimia nervosa; −2LL = −2 log-likelihood; n/a = not applicable; rg = genetic correlation; re = environmental correlation.
TABLE 2.
Parameter Estimates for Psychiatric Phenotype Caffeine Use
| Psychiatric disorder A | Psychiatric disorder E | Caf. A | Caf. E | Genetic correlation | Environmental correlation | |
|---|---|---|---|---|---|---|
| GAD | 0.33 (0.20–0.45) | 0.67 (0.55–0.80) | 0.49 (0.42–0.55) | 0.51 (0.45–0.58) | 0.48 (0.28–0.70) | 0.04 (−0.09–0.16) |
| PAN | 0.32 (0.14–0.49) | 0.68 (0.51–0.87) | 0.49 (0.42–0.56) | 0.51 (0.44–0.58) | −0.22 (−0.51–0.03) | 0.11 (−0.05–0.27) |
| PHO | 0.32 (0.20–0.43) | 0.68 (0.57–0.80) | 0.49 (0.42–0.56) | 0.51 (0.44–0.58) | 0.25 (0.06–0.45) | −0.01 (−0.13–0.11) |
| MDD | 0.38 (0.27–0.49) | 0.62 (0.51–0.73) | 0.49 (0.42–0.55) | 0.51 (0.45–0.58) | 0.38 (0.21–0.55) | 0.12 (0.00–0.24) |
| AN | 0.34 (0.02–0.61) | 0.66 (0.40–0.98) | 0.49 (0.42–0.56) | 0.51 (0.45–0.58) | 0.07 (−0.30–0.54) | −0.03 (−0.26–0.20) |
| BN | 0.50 (0.28–0.68) | 0.50 (0.32–0.72) | 0.49 (0.42–0.56) | 0.51 (0.46–0.58) | 0.08 (−0.15–0.33) | 0.07 (−0.16–0.28) |
Note: Common environmental parameter fixed to zero. Caf. = caffeine use; GAD = generalized anxiety disorder; PAN = panic disorder; PHO = phobia; MDD = major depressive disorder; AN = anorexia nervosa; BN = bulimia nervosa; A = genetic parameter; E = unique environmental parameter.
Parameter estimates and 95% CI for psychiatric phenotypes and tolerance are contained in Table 3. Here, MDD had an environmental correlation with caffeine tolerance of 0.23, AN had a genetic correlation of 0.64 with caffeine tolerance, and BN had a 0.49 genetic correlation with caffeine tolerance. These were statistically significant. GAD also showed modest genetic correlation with caffeine tolerance, 0.24; panic had a negative genetic correlation of −0.27 with caffeine tolerance and BN had a negative environmental correlation of −0.33 with caffeine tolerance, but these were not statistically significant. Removal of the genetic and environmental correlation parameters resulted in significantly worse fitting models for GAD, phobias, MDD, AN, and BN (data not shown), which suggested that there was significant shared liability between each of these phenotypes and caffeine tolerance.
TABLE 3.
Parameter Estimates for Psychiatric Phenotype and Caffeine Tolerance
| Psychiatric disorder A | Psychiatric disorder E | Tol. A | Tol. E | Genetic correlation | Environmental correlation | |
|---|---|---|---|---|---|---|
| GAD | 0.32 (0.19–0.44) | 0.68 (0.56–0.81) | 0.38 (0.21–0.54) | 0.62 (0.46–0.79) | 0.24 (−0.12–0.60) | 0.13 (−0.07–0.32) |
| PAN | 0.32 (0.14–0.49) | 0.68 (0.51–0.86) | 0.39 (0.22–0.54) | 0.61 (0.46–0.78) | −0.27 (−0.77–0.16) | 0.11 (−0.15–0.37) |
| PHO | 0.31 (0.19–0.42) | 0.69 (0.58–0.81) | 0.38 (0.21–0.54) | 0.62 (0.46–0.79) | 0.17 (−0.17–0.52) | 0.14 (−0.05–0.32) |
| MDD | 0.38 (0.26–0.48) | 0.62 (0.52–0.74) | 0.39 (0.22–0.54) | 0.62 (0.46–0.79) | 0.08 (−0.25–0.38) | 0.23 (0.04–0.42) |
| AN | 0.33 (0.05–0.60) | 0.67 (0.41–0.96) | 0.38 (0.21–0.53) | 0.62 (0.47–0.79) | 0.64 (0.07–1.00) | −0.12 (−0.46–0.24) |
| BN | 0.51 (0.30–0.69) | 0.49 (0.31–0.71) | 0.39 (0.22–0.55) | 0.61 (0.45–0.78) | 0.49 (0.09–0.96) | −0.33 (−0.67–0.04) |
Note: Common environmental parameter fixed to zero. Tol. = tolerance; GAD = generalized anxiety disorder; PAN = panic disorder; PHO = phobia; MDD = major depressive disorder; AN = anorexia nervosa; BN = bulimia nervosa; A = genetic parameter; E = unique environmental parameter.
Psychiatric phenotypes and caffeine withdrawal parameter estimates are presented in Table 4. Although there were no statistically significant genetic or environmental correlation between psychiatric disorders and caffeine withdrawal, the genetic correlation between GAD and caffeine withdrawal was 0.35, with very little environmental correlation. MDD, AN, and BN also showed some evidence of genetic correlation with caffeine withdrawal; here, the parameter estimates were 0.26, 0.27, and 0.32, respectively. Further, removal of both the genetic and environmental correlation parameters resulted in a significantly worse fitting model for MDD (data not shown).
TABLE 4.
Parameter Estimates for Psychiatric Phenotype and Caffeine Withdrawal
| Psychiatric disorder A | Psychiatric disorder E | With. A | With. E | Genetic correlation | Environmental correlation | |
|---|---|---|---|---|---|---|
| GAD | 0.32 (0.19–0.44) | 0.68 (0.56–0.81) | 0.28 (0.06–0.49) | 0.72 (0.52–0.94) | 0.35 (−0.12–0.99) | 0.04 (−0.18–0.25) |
| PAN | 0.32 (0.14–0.49) | 0.68 (0.51–0.86) | 0.27 (0.05–0.48) | 0.73 (0.52–0.95) | 0.10 (−0.51–0.83) | −0.09 (−0.37–0.20) |
| PHO | 0.31 (0.20–0.43) | 0.69 (0.57–0.81) | 0.29 (0.07–0.49) | 0.71 (0.51–0.93) | −0.11 (−0.65–0.36) | 0.13 (−0.09–0.34) |
| MDD | 0.38 (0.26–0.48) | 0.62 (0.52–0.74) | 0.27 (0.05–0.48) | 0.73 (0.52–0.95) | 0.26 (−0.16–0.85) | 0.08 (−0.13–0.30) |
| AN | 0.34 (0.02–0.60) | 0.66 (0.40–0.99) | 0.29 (0.07–0.49) | 0.72 (0.51–0.94) | 0.27 (−0.63–1.00) | 0.04 (−0.37–0.46) |
| BN | 0.50 (0.28–0.68) | 0.50 (0.32–0.72) | 0.28 (0.06–0.48) | 0.72 (0.52–0.94) | 0.32 (−0.24–1.00) | −0.04 (−0.41–0.34) |
Note: Common environmental parameter fixed to zero. With. = withdrawal; GAD = generalized anxiety disorder; PAN = panic disorder; PHO = phobia; MDD = major depressive disorder; AN = anorexia nervosa; BN = bulimia nervosa; A = genetic parameter; E = unique environmental parameter.
Discussion
It was well known and accepted that caffeine has anxiogenic properties. Further, caffeine use has been associated with higher depression scores on the Beck Depression Inventory and higher anxiety scores on the Beck Anxiety Inventory (Dosh et al., 2010). This study explored the nature of the relationship between common psychiatric disorders, including a variety of anxiety disorders and depression, and caffeine use. Results suggested there might be a common etiology between some common psychiatric disorders and caffeine phenotypes. It did not seem that there was a consistent, universal pattern between all common psychiatric disorders and caffeine phenotypes. However, within some psychiatric disorders, fairly consistent patterns across caffeine phenotypes emerged.
Arguably, some of the strongest evidence for shared liability between psychiatric phenotypes and caffeine use phenotypes came from GAD. It was known that individuals with GAD were highly sensitive to the effects of caffeine, and caffeine caused a greater physiological and anxiogenic response in those with GAD relative to healthy controls (Bruce et al., 1992). Comorbidity between GAD and caffeine use phenotypes has also been established (Kendler et al., 2006). In the current study, the genetic correlation between GAD and caffeine use, caffeine tolerance, and caffeine withdrawal were consistently among the highest correlations and exceeded 0.20 in all cases, with very little evidence of environmental correlations. This suggested that much of the shared liability between these phenotypes resulted from a common genetic composition. It is plausible that high caffeine consumption in women with GAD, coupled with symptoms of caffeine tolerance and withdrawal, contributed negatively to GAD.
Much of the previous research, which has been conducted on psychiatric disorders and caffeine use, has focused on panic disorder. It has been shown that individuals with panic disorder were more sensitive to the anxiogenic effects of caffeine (Breier et al., 1986; Charney et al., 1985; Nardi et al., 2007, 2009). In addition, both individuals with panic disorder and their first-degree relatives (without panic disorder) showed increased sensitivity to panic attack symptoms after a caffeine challenge test relative to control participants (Nardi et al., 2008). This suggested the relationship between caffeine sensitivity and panic disorder symptoms was genetic in nature. In the current study, there was evidence of a negative genetic correlation between panic disorder and caffeine use and panic disorder and caffeine tolerance, with little indication of positive or negative environmental correlation. If individuals with panic disorder were truly more sensitive to the effects of caffeine, it is plausible and perhaps probable that these individuals would avoid high caffeine consumption and this could be genetically programmed.
Similar to individuals with anxiety disorders, those with MDD were also more sensitive to the anxiogenic effects of caffeine (Lee et al., 1988; Nardi et al., 2007). In a psychiatric population, depressive symptoms overall have been associated with high caffeine use (Greden et al., 1978). Although all associations between MDD and caffeine use phenotypes are in the same direction, results from the current study were a bit complex. There was fairly consistent evidence suggesting that MDD and caffeine phenotypes shared liability with estimates exceeding 0.20 in all models. However, for caffeine use and caffeine withdrawal the greatest evidence for shared liability was contained in the genetic parameter, and for caffeine tolerance the greatest evidence for shared liability was in the environmental parameter. This suggested that there was a complex relationship between these phenotypes. Several possible explanations exist. It is possible that genetic and environmental parameters were important for different components of the shared liability. It is also plausible that different endophenotypes of depression may have been more likely to share comorbidity with a specific caffeine phenotype and this different pattern of comorbidity resulted in either higher genetic or environmental correlation. An alternate possibility is that the current study had poor power to discriminate the sources of phenotypic correlation due to relatively small sample sizes.
In addition, there seemed to be some association between eating disorder phenotypes (AN and BN) and caffeine phenotypes. Inpatients with eating disorders had higher caffeine use than those with anxiety disorders (Ciapparelli et al., 2010). Clinicians have reported very high caffeine consumption within individuals with AN (Sours, 1983), and there was some evidence to suggest higher caffeine intake in women with AN (Striegel-Moore et al., 2006) than in women without AN. Although there was little shared liability between AN or BN and caffeine use, there was some evidence for shared liability between AN and BN and caffeine tolerance and caffeine withdrawal. Interestingly, for AN, shared genetic and shared environmental parameters are in opposite directions. For AN and BN, both caffeine tolerance and caffeine withdrawal had genetic correlations above 0.20. A high comorbidity between AN and anxiety was previously established (Dellava et al., 2011; Godart et al., 2003; Raney et al., 2008; Thornton et al., 2011); it has been estimated that two-thirds of women with AN, BN, or both also suffer from an anxiety disorder (Kaye et al., 2004). Further, AN shared substantial genetic liability with GAD (Dellava et al., 2011) and BN also shared a common genetic factor with some anxiety disorders (Kendler et al., 1995). It is therefore possible that there is a relationship among eating disorder, anxiety disorder, and caffeine phenotypes. Alternatively, it is possible that the genetic correlation between eating disorder and caffeine phenotypes increases anxiety.
Limitations
Several limitations should be kept in mind when considering study results. First, for some phenotypes although diagnoses were similar to DSM-IIIR, the criteria used might not have been precise enough to obtain full clinical diagnoses and therefore may represent partial syndromal diagnoses. However, in other reports multiple threshold models have been tested and show that these are milder phenotypes on the same continuum of liability as fully syndromal cases (Aggen et al., 2005; Kendler et al., 1992a; Kendler et al., 2001; Neale et al., 1994; Walters & Kendler, 1995). Second, this study consisted only of Caucasian twin women born between 1934 and 1971 in mid-Atlantic states and may not generalize to other groups. Third, this study was underpowered. As a consequence of the study being underpowered, the 95% CI were quite large; as a result, some of the parameter estimates in which the 95% CIs crossed zero may become statistically significant in a larger sample. Many of the results that were not significant may become significant with larger sample sizes. In addition, the collection of the relevant variables over four waves may have attenuated the true underlying correlations. Fourth, we acknowledge that prevalence for some of the psychiatric phenotypes was higher than reported in other samples; however, a strength of this study was its use of clinically trained interviewers to arrive at diagnoses. This makes it more unlikely that these disorders were over diagnosed and suggests that the prevalences noted are accurate.
Conclusion
Results of the current study suggested that GAD and caffeine phenotypes likely have shared substantial genetic liability. There was evidence for shared liability between other common psychiatric disorders and caffeine phenotypes. Overall, these results might help provide better understanding of the etiology of the comorbidity between common psychiatric disorders and caffeine use, tolerance, and withdrawal.
Acknowledgments
This study was supported in part by grants MH-068643 and MH-49492 from the National Institute of Health (NIH). Dr Bergin was supported by NIMH T32MH20030 (PI: Michael C. Neale) and 5R37DA018673-07 (PI: Michael C. Neale). Linda Corey, PhD, provided assistance with the ascertainment of twins from the Virginia Twin Registry, now part of the Mid-Atlantic Twin Registry (MATR). The MATR, now directed by Judy Silberg, PhD, has received support from the NIH, the Carman Trust, and the W. M. Keck, John Templeton, and Robert Wood Johnson Foundations. We thank all the participants who made this research possible.
References
- Aggen SH, Neale MC, Kendler KS. DSM criteria for major depression: Evaluating symptom patterns using latent-trait item response models. Psychological Medicine. 2005;35:475–487. doi: 10.1017/s0033291704003563. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual for psychiatric disorders. 4. Washington, DC: American Psychiatric Press; 1994. DSM-IV. [Google Scholar]
- Amin N, Byrne E, Johnson J, Chenevix-Trench G, Walter S, Nolte IM, Vink JM, Rawal R, Mangino M, Teumer A, Keers JC, Verwoert G, Baumeister S, Biffar R, Petersmann A, Dahmen N, Doering A, Isaacs A, Broer L, Wray NR, Montgomery GW, Levy D, Psaty BM, Gudnason V, Chakravarti A, Sulem P, Gudbjartsson DF, Kiemeney LA, Thorsteinsdottir U, Stefansson K, van Rooij FJ, Aulchenko YS, Hottenga JJ, Rivadeneira FR, Hofman A, Uitterlinden AG, Hammond CJ, Shin SY, Ikram A, Witteman JC, Janssens AC, Snieder H, Tiemeier H, Wolfenbuttel BH, Oostra BA, Heath AC, Wichmann E, Spector TD, Grabe HJ, Boomsma DI, Martin NG, van Duijn CM. Genome-wide association analysis of coffee drinking suggests association with CYP1A1/CYP1A2 and NRCAM. Moleular Psychiatry. 2011 doi: 10.1038/mp.2011.101. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JH, Mitchell KS, Neale MC, Kendler KS. Eating disorder symptomatology and substance use disorders: Prevalence and shared risk in a population based twin sample. International Journal of Eating Disorders. 2010;43:648–658. doi: 10.1002/eat.20856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Heath AC, Bucholz KK, Dinwiddie SH, Madden PA, Statham DJ, Dunne MP, Martin NG. Major depressive disorder in a community-based twin sample: Are there different genetic and environmental contributions for men and women? Archives of General Psychiatry. 1999;56:557–563. doi: 10.1001/archpsyc.56.6.557. [DOI] [PubMed] [Google Scholar]
- Boker S, Neale MC, Maes HH, Wilde M, Spiegel M, Brick T, Spies J, Estabrook R, Kenny S, Bates T, Mehta P, Fox J. OpenMx: An open source extended structural equation modeling framework. Psychometrika. 2011;76:306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier A, Charney DS, Heninger GR. Agoraphobia with panic attacks: Development, diagnostic stability, and course of illness. Archives of General Psychiatry. 1986;43:1029–1036. doi: 10.1001/archpsyc.1986.01800110015003. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC. DSM-III generalized anxiety disorder: An empirical investigation of more stringent criteria. Psychiatry Research. 1985;15:231–238. doi: 10.1016/0165-1781(85)90080-0. [DOI] [PubMed] [Google Scholar]
- Bruce M, Scott N, Shine P, Lader M. Anxiogenic effects of caffeine in patients with anxiety disorders. Archives of General Psychiatry. 1992;49:867–869. doi: 10.1001/archpsyc.1992.01820110031004. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Thornton LM, Root TL, Pisetsky EM, Lichtenstein P, Pedersen NL. Understanding the relation between anorexia nervosa and bulimia nervosa in a Swedish national twin sample. Biological Psychiatry. 2010;67:71–77. doi: 10.1016/j.biopsych.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS, Heninger GR, Jatlow PI. Increased anxiogenic effects of caffeine in panic disorders. Archives of General Psychiatry. 1985;42:233–243. doi: 10.1001/archpsyc.1985.01790260027003. [DOI] [PubMed] [Google Scholar]
- Ciapparelli A, Paggini R, Carmassi C, Taponecco C, Consoli G, Ciampa G, Ramacciotti CE, Marazziti D, Dell’Osso L. Patterns of caffeine consumption in psychiatric patients: An Italian study. European Journal of Psychiatry. 2010;25:230–235. doi: 10.1016/j.eurpsy.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Dellava JE, Kendler KS, Neale MC. Generalized anxiety disorder and anorexia nervosa: Evidence of shared genetic variation. Depression and Anxiety. 2011;28:728–733. doi: 10.1002/da.20834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellava JE, Thornton LM, Lichtenstein P, Pedersen NL, Bulik CM. Impact of broadening definitions of anorexia nervosa on sample characteristics. Journal of Psychiatric Research. 2010;45:691–698. doi: 10.1016/j.jpsychires.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosh T, Helmbrecht T, Anestis J, Guenthner G, Kelly TH, Martin CA. A comparison of the associations of caffeine and cigarette use with depressive and ADHD symptoms in a sample of young adult smokers. Journal of Addiction Medicine. 2010;4:52–54. doi: 10.1097/ADM.0b013e3181b508ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves LJ, Eysenck HJ, Martin NG. Genes, culture and personality: An empirical approach. London: Oxford University Press; 1989. [Google Scholar]
- Godart NT, Flament MF, Curt F, Perdereau F, Lang F, Venisse JL, Halfon O, Bizouard P, Loas G, Corcos M, Jeammet P, Fermanian J. Anxiety disorders in subjects seeking treatment for eating disorders: A DSM-IV controlled study. Psychiatry Research. 2003;117:245–258. doi: 10.1016/s0165-1781(03)00038-6. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Stinson FS, Dawson DA, June Ruan W, Goldstein RB, Smith SM, Saha TD, Huang B. Prevalence, correlates, co-morbidity, and comparative disability of DSM-IV generalized anxiety disorder in the USA: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychological Medicine. 2005;35:1747–1759. doi: 10.1017/S0033291705006069. [DOI] [PubMed] [Google Scholar]
- Greden JF, Fontaine P, Lubetsky M, Chamberlin K. Anxiety and depression associated with caffeinism among psychiatric inpatients. American Journal of Psychiatry. 1978;135:963–966. doi: 10.1176/ajp.135.8.963. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Juliano LM, Chausmet AL. Caffeine: Pharmacology and clinical effects. Chevy Chase, MD: American Society of Addiction Medicine; 2003. [Google Scholar]
- Hettema J, Neale M, Kendler K. Physical similarity and the equal-environment assumption in twin studies of psychiatric disorders. Behavior Genetics. 1995;25:327–335. doi: 10.1007/BF02197281. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. American Journal of Psychiatry. 2001;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Prescott CA, Kendler KS. A population-based twin study of generalized anxiety disorder in men and women. Journal of Nervous and Mental Disorders. 2001;189:413–420. doi: 10.1097/00005053-200107000-00001. [DOI] [PubMed] [Google Scholar]
- James K. Understanding caffeine: A biobehavioral analysis. Thousand Oaks, CA: Sage; 1997. [Google Scholar]
- Kaye WH, Bulik CM, Thornton L, Barbarich N, Masters K. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. American Journal of Psychiatry. 2004;161:2215–2221. doi: 10.1176/appi.ajp.161.12.2215. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. A population-based twin study of lifetime major depression in men and women. Archives of General Psychiatry. 1999a;56:39–44. doi: 10.1001/archpsyc.56.1.39. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Caffeine intake, tolerance, and withdrawal in women: A population-based twin study. American Journal of Psychiatry. 1999b;156:223–228. doi: 10.1176/ajp.156.2.223. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Annas P, Neale MC, Eaves LJ, Lichtenstein P. A longitudinal twin study of fears from middle childhood to early adulthood: Evidence for a developmentally dynamic genome. Archives of General Psychiatry. 2008;65:421–429. doi: 10.1001/archpsyc.65.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA. Panic syndromes in a population-based sample of male and female twins. Psychological Medicine. 2001;31:989–1000. doi: 10.1017/s0033291701004226. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Fears and phobias: Reliability and heritability. Psychological Medicine. 1999;29:539–553. doi: 10.1017/s0033291799008429. [DOI] [PubMed] [Google Scholar]
- Kendler KS, MacLean C, Neale MC, Kessler RC, Heath AC, Eaves LJ. The genetic epidemiology of bulimia nervosa. American Journal of Psychiatry. 1991;148:1627–1637. doi: 10.1176/ajp.148.12.1627. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J, O’Gardner C. Caffeine intake, toxicity and dependence and lifetime risk for psychiatric and substance use disorders: An epidemiologic and co-twin control analysis. Psychological Medicine. 2006;36:1717–1725. doi: 10.1017/S0033291706008622. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Prescott CA. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Archives of General Psychiatry. 2007;64:1313–1320. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Generalized anxiety disorder in women. Archives of General Psychiatry. 1992a;49:267–272. doi: 10.1001/archpsyc.1992.01820040019002. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A population-based twin study of major depression in women: The impact of varying definitions of illness. Archives of General Psychiatry. 1992b;49:257–266. doi: 10.1001/archpsyc.1992.01820040009001. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Walters EE, Neale MC, Kessler RC, Heath AC, Eaves LJ. The structure of the genetic and environmental risk factors for six major psychiatric disorders in women: Phobia, generalized anxiety disorder, panic disorder, bulimia, major depression and alcoholism. Archives of General Psychiatry. 1995;52:374–383. doi: 10.1001/archpsyc.1995.03950170048007. [DOI] [PubMed] [Google Scholar]
- Klump KL, Miller KB, Keel PK, McGue M, Iacono WG. Genetic and environmental influences on anorexia nervosa syndromes in a population-based twin sample. Psychological Medicine. 2001;31:737–740. doi: 10.1017/s0033291701003725. [DOI] [PubMed] [Google Scholar]
- Kortegaard LS, Hoerder K, Joergensen J, Gillberg C, Kyvik KO. A preliminary population-based twin study of self-reported eating disorder. Psychological Medicine. 2001;31:361–365. doi: 10.1017/s0033291701003087. [DOI] [PubMed] [Google Scholar]
- Lee MA, Flegel P, Greden JF, Cameron OG. Anxiogenic effects of caffeine on panic and depressed patients. American Journal of Psychiatry. 1988;145:632–635. doi: 10.1176/ajp.145.5.632. [DOI] [PubMed] [Google Scholar]
- Lee S, Tsang A, Ruscio AM, Haro JM, Stein DJ, Alonso J, Angermeyer MC, Bromet EJ, Demyttenaere K, de Girolamo G, de Graaf R, Gureje O, Iwata N, Karam EG, Lepine JP, Levinson D, Medina-Mora ME, Oakley Browne MA, Posada-Villa J, Kessler RC. Implications of modifying the duration requirement of generalized anxiety disorder in developed and developing countries. Psychological Medicine. 2009;39:1163–1176. doi: 10.1017/S0033291708004807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano M, Kirk KM, Heath AC, Martin NG. The genetics of tea and coffee drinking and preference for source of caffeine in a large community sample of Australian twins. Addiction. 2005;100:1510–1517. doi: 10.1111/j.1360-0443.2005.01223.x. [DOI] [PubMed] [Google Scholar]
- Luciano M, Zhu G, Kirk KM, Gordon SD, Heath AC, Montgomery GW, Martin NG. ‘No thanks, it keeps me awake’: The genetics of coffee-attributed sleep disturbance. Sleep. 2007;30:1378–1386. doi: 10.1093/sleep/30.10.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzeo SE, Mitchell KS, Bulik CM, Reichborn-Kjennerud T, Kendler KS, Neale MC. Assessing the heritability of anorexia nervosa symptoms using a marginal maximal likelihood approach. Psychological Medicine. 2009;39:463–473. doi: 10.1017/S0033291708003310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middeldorp CM, Birley AJ, Cath DC, Gillespie NA, Willemsen G, Statham DJ, de Geus EJ, Andrews JG, van Dyck R, Beem AL, Sullivan PF, Martin NG, Boomsma DI. Familial clustering of major depression and anxiety disorders in Australian and Dutch twins and siblings. Twin Research and Human Genetics. 2005;8:609–615. doi: 10.1375/183242705774860123. [DOI] [PubMed] [Google Scholar]
- Mosing MA, Gordon SD, Medland SE, Statham DJ, Nelson EC, Heath AC, Martin NG, Wray NR. Genetic and environmental influences on the co-morbidity between depression, panic disorder, agoraphobia, and social phobia: A twin study. Depress Anxiety. 2009;26:1004–1011. doi: 10.1002/da.20611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardi AE, Lopes FL, Freire RC, Veras AB, Nascimento I, Valenca AM, de-Melo-Neto VL, Soares-Filho GL, King AL, Araújo DM, Mezzasalma MA, Rassi A, Zin WA. Panic disorder and social anxiety disorder subtypes in a caffeine challenge test. Psychiatry Research. 2009;169:149–153. doi: 10.1016/j.psychres.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Nardi AE, Lopes FL, Valenca AM, Freire RC, Veras AB, de-Melo-Neto VL, Nascimento I, King AL, Mezzasalma MA, Soares-Filho GL, Zin WA. Caffeine challenge test in panic disorder and depression with panic attacks. Comprehensive Psychiatry. 2007;48:257–263. doi: 10.1016/j.comppsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Nardi AE, Valenca AM, Nascimento I, Freire RC, Veras AB, de-Melo-Neto VL, Lopes FL, King AL, Soares-Filho GL, Mezzasalma MA, Rassi A, Zin WA. A caffeine challenge test in panic disorder patients, their healthy first-degree relatives, and healthy controls. Depression and Anxiety. 2008;25:847–853. doi: 10.1002/da.20354. [DOI] [PubMed] [Google Scholar]
- Neale M, Cardon L. Methodology for genetic studies of twins and families. Dordrecht, The Netherlands: Kluwer Academic; 1992. [Google Scholar]
- Neale M, Maes H. Methodology for genetic studies of twins and families. Dordrecht, The Netherlands: Kluwer Academic; 1998. [Google Scholar]
- Neale MC, Walters EE, Eaves LJ, Kessler RC, Heath AC, Kendler KS. Genetics of blood-injury fears and phobias: A population-based twin study. American Journal of Medical Genetics. 1994;54:326–334. doi: 10.1002/ajmg.1320540411. [DOI] [PubMed] [Google Scholar]
- O’Neil KA, Conner BT, Kendall PC. Internalizing disorders and substance use disorders in youth: Comorbidity, risk, temporal order, and implications for intervention. Clinical Psychology Review. 2011;31:104–112. doi: 10.1016/j.cpr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing (Version 2.11.1) Vienna: R Foundation for Statistical Computing; 2010. [Google Scholar]
- Raney TJ, Thornton LM, Berrettini W, Brandt H, Crawford S, Fichter MM, Halmi KA, Johnson C, Kaplan AS, LaVia M, Mitchell J, Rotondo A, Strober M, Woodside DB, Kaye WH, Bulik CM. Influence of overanxious disorder of childhood on the expression of anorexia nervosa. International Journal of Eating Disorders. 2008;41:326–332. doi: 10.1002/eat.20508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Helzer J. Diagnostic Interview Schedule (DIS): Version III-A. St. Louis, MO: Washington University School of Medicine; 1985. [Google Scholar]
- Root TL, Pisetsky EM, Thornton L, Lichtenstein P, Pedersen NL, Bulik CM. Patterns of co-morbidity of eating disorders and substance use in Swedish females. Psychological Medicine. 2010;40:105–115. doi: 10.1017/S0033291709005662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salbach-Andrae H, Lenz K, Simmendinger N, Klinkowski N, Lehmkuhl U, Pfeiffer E. Psychiatric comorbidities among female adolescents with anorexia nervosa. Child Psychiatry and Human Development. 2008;39:261–272. doi: 10.1007/s10578-007-0086-1. [DOI] [PubMed] [Google Scholar]
- Scherrer JF, True WR, Xian H, Lyons MJ, Eisen SA, Goldberg J, Lin N, Tsuang MT. Evidence for genetic influences common and specific to symptoms of generalized anxiety and panic. Journal of Affective Disorders. 2000;57:25–35. doi: 10.1016/s0165-0327(99)00031-2. [DOI] [PubMed] [Google Scholar]
- Shimbo M, Nakamura K, Jing Shi H, Kizuki M, Seino K, Inose T, Takano T. Green tea consumption in everyday life and mental health. Public Health Nutrition. 2005;8:1300–1306. doi: 10.1079/phn2005752. [DOI] [PubMed] [Google Scholar]
- Sours JA. Case reports of anorexia nervosa and caffeinism. American Journal of Psychiatry. 1983;140:235–236. doi: 10.1176/ajp.140.2.235. [DOI] [PubMed] [Google Scholar]
- Spence JE, Corey LA, Nance WE, Marazita ML, Kendler KS, Schieken RM. Molecular analysis of twin zygosity using VNTR DNA probes. American Journal of Human Genetics. 1988;43:A159. [Google Scholar]
- Spitzer R, Williams J, Gibbon M, First M. Structured clinical interview for DSM-III-R: Patient version (SCID-P) New York: Biometrics Research Department, State Psychiatric Institute; 1988. [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First M. Structured clinical interview for DSM-III-R: Patient version. Washington, DC: American Psychiatric Press; 1990. [Google Scholar]
- Striegel-Moore RH, Franko DL, Thompson D, Barton B, Schreiber GB, Daniels SR. Caffeine intake in eating disorders. International Journal of Eating Disorders. 2006;39:162–165. doi: 10.1002/eat.20216. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: Review and meta-analysis. American Journal of Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Thornton LM, Dellava JE, Root TL, Lichtenstein P, Bulik CM. Anorexia nervosa and generalized anxiety disorder: Further explorations of the relation between anxiety and body mass index. Journal of Anxiety Disorders. 2011;25:727–730. doi: 10.1016/j.janxdis.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters EE, Kendler KS. Anorexia nervosa and anorexic-like syndromes in a population-based female twin sample. American Journal of Psychiatry. 1995;152:64–71. doi: 10.1176/ajp.152.1.64. [DOI] [PubMed] [Google Scholar]
- Yang A, Palmer AA, de Wit H. Genetics of caffeine consumption and responses to caffeine. Psychopharmacology. 2010;211:245–257. doi: 10.1007/s00213-010-1900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]