Abstract
One of the main controversies with regard to depressive personality disorder (DPD) concerns the co-occurrence with the established DSM-IV personality disorders (PDs). The main aim of this study was to examine to what extent DPD and the DSM-IV PDs share genetic and environmental risk factors, using multivariate twin modeling. The DSM-IV Structured Interview for Personality was applied to 2,794 young adult twins. Paranoid PD from Cluster A, borderline PD from Cluster B, and all three PDs from Cluster C were independently and significantly associated with DPD in multiple regression analysis. The genetic correlations between DPD and the other PDs were strong (.53–.83), while the environmental correlations were moderate (.36–.40). Close to 50% of the total variance in DPD was disorder specific. However, only 5% was due to disorder-specific genetic factors, indicating that a substantial part of the genetic vulnerability to DPD also increases the vulnerability to other PDs.
Depressive personality disorder (DPD) was first formally introduced in the fourth version of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; American Psychiatric Association, 1994), where it was placed in Appendix B among other diagnostic categories in need of further studies. In the ongoing work on DSM-5, the Personality and Personality Disorders Work Group suggests that DPD is not included as a specific type, but is represented by a combination of core impairment in personality functioning and a set of prominent personality traits (www.dsm5.org).
One of the key questions originally posed by the DSM-IV personality disorder (PD) work group was to what extent DPD can be distinguished from Axis I mood disorders and from the 10 established DSM-IV PDs (Phillips, Hirschfeld, Shea, & Gunderson, 1995). Several studies addressing diagnostic overlap have been published, both with regard to mood disorders (Klein & Miller, 1993; Klein & Shih, 1998; McDermut, Zimmerman, & Chelminski, 2003; Ryder, Bagby, & Dion, 2001; Ryder, Bagby, & Schuller, 2002) and PDs (Huprich, 2004; Huprich, Zimmerman, & Chelminski, 2006; Klein & Shih, 1998; Markowitz et al., 2005; McDermut et al., 2003), where there is evidence for substantial co-occurrence between DPD and borderline PD in Cluster B and the three Cluster C PDs (for review, see Huprich, 2009).
An important criterion for diagnostic validity is familial aggregation (Robins & Guze, 1970). Familial aggregation can be the result of both common genetic or environmental factors, and twin studies can be used to distinguish these causes (Plomin, DeFries, McClearn, & McGuffin, 2001). If twin studies are extended to include multiple disorders, it is possible to examine the causes of familial co-aggregation of several disorders, that is, whether their co-occurrence is due to common genetic and/or environmental risk factors—often quantified as genetic and environmental correlation (Kendler, 2001, 2006). Knowledge about common genetic and environmental risk factors can both broaden our understanding of co-occurrence of psychiatric disorders (Kendler, 2006; Krueger & Markon, 2006) and be used to inform the development of classification systems such as DSM-5 (Andrews et al., 2009). Shared genetic and environmental risk factors are 2 of 11 “validators” listed by the Diagnostic Spectra Study Group of the DSM-5 task force (Andrews et al., 2009). For example, if the genetic correlation between DPD and one or more established PDs was to be unity or very strong, this could be applied as an argument against including DPD as a separate disorder in DSM-5. Information about shared risk factors (genetic or otherwise) must, however, be judged in context with the clinical picture of the various disorders.
The Norwegian Institute of Public Health has recently assessed Axis I and Axis II disorders, including all the DSM-IV PDs, in a population-based sample of young adult twins. This dataset has previously been used to explore common genetic and environmental risk factors for the three PD clusters (Kendler, Czajkowski, et al., 2006; Reichborn-Kjennerud, Czajkowski, Neale, et al., 2007; Torgersen et al., 2008), the relationship between selected Axis I disorders and PDs (Reichborn-Kjennerud, Czajkowski, Torgersen, et al., 2007; Reichborn-Kjennerud et al., 2010) and, most recently, the underlying structure of risk factors for all 10 DSM-IV PDs (Kendler et al., 2008).
Our assessments also included DPD. We have previously published data on the co-occurrence of DPD and Axis I and Axis II disorders (Ørstavik, Kendler, Czajkowski, Tambs, & Reichborn-Kjennerud, 2007a). With regard to Axis II disorders, associations were strongest with borderline and avoidant PD (Odds ratio [OR] 61.6 and 54.4, respectively). In a second study, we investigated the genetic and environmental contributions to the co-occurrence of DPD and major depressive disorder (MDD) (Ørstavik, Kendler, Czajkowski, Tambs, & Reichborn-Kjennerud, 2007b). The genetic and environmental correlations between the two disorders were .56 and .33, respectively.
The aim of the current study was to further develop our understanding of the relationship between DPD and the DSM-IV PDs. First, we evaluate the phenotypic correlations between dimensional representations of DPD and the 10 DSM-IV PDs. Second, we use regression models to identify which PDs are independently associated with DPD. Third, we apply multivariate twin modeling to calculate the correlations between genetic and environmental risk factors for DPD and the selected DSM-IV PDs, and to determine whether there are genetic and/or environmental risk factors unique for DPD.
methods
SAMPLE
Data for these analyses come from the Norwegian Institute of Public Health Twin Panel (NIPHTP). The twins are identified through information contained in the Norwegian Medical Birth Registry, established January 1, 1967, which receives mandatory notification of all births. NIPHTP is based on twins born between 1967 and 1979, during which period 15,370 like- and unlike-sex twins were born in Norway. Two questionnaire studies have been conducted in this sample: in 1992 (twins born 1967–1974) and in 1998 (twins born 1967–1979). Details with regard to these questionnaires are given elsewhere (Harris, Magnus, & Tambs, 2002; Tambs et al., 2009). In short, the second questionnaire was sent to 12,700 twins, and 8,045 responded after one reminder (3,334 pairs and 1,377 singletons, response rate 63%). Data for the present report derive from an interview study of Axis I and Axis II psychiatric disorders, which began in 1999. Participants were recruited from among the complete pairs who in the second questionnaire agreed to participate in the interview study, and 68 pairs who were accidentally drawn directly from NIPHTP. Of these 6,442 potential participants (3,221 pairs), 0.8% were unwilling or unable to participate, and 16.2% were excluded because only one twin of a pair agreed to the interview. After two contacts requesting participation, 38.2% did not respond. In 22 pairs where both twins initially agreed to be interviewed, one of the twins was unable or unwilling to participate in the interview. Thus the final sample consisted of 2,794 twins (44% of those initially eligible), 1,386 complete pairs and 22 singletons.
Zygosity was initially determined by questionnaire items previously shown to categorize correctly 97.5% of pairs (Harris et al., 2002). In all but 385 like-sex pairs, where one or both of the twins was either unwilling or unable to donate a blood sample, zygosity was also determined by molecular methods based on the genotyping of 24 microsatellite markers. Seventeen of these pairs with DNA information (2.5%) were found to be misclassified by the questionnaire data and were corrected. From the corrected data we estimated that in our entire sample the zygosity misclassification rate was 0.7%, which is unlikely to substantially bias results (Neale, 2003).
MEASURES
A Norwegian version of the Structured Interview for DSM-IV Personality (SIDP-IV; Pfohl, Blum, & Zimmerman, 1995) was used to assess PDs. This instrument is a comprehensive semistructured diagnostic interview for the assessment of all DSM-IV Axis II diagnoses, including DPD. The SIDP was initially developed in 1983 and has been used in a number of studies in many countries, including Norway (Helgeland, Kjelsberg, & Torgersen, 2005; Torgersen, Kringlen, & Cramer, 2001). The instrument includes nonpejorative questions organized into topical sections rather than by disorders. This allows for a more natural flow of the interview and increases the likelihood that useful information from related questions may be taken into account when rating related criteria within that section. Each DSM-IV criterion is represented by a set of questions; for example, for DPD criterion 2, “Self-concept centers around beliefs of inadequacy, worthlessness and low self-esteem,” the interviewer asks, “How would you describe your usual level of self-esteem?” and “When you compare yourself to other people, do you usually feel you are as good as others, or do you feel that they are better than you?” For avoidant criterion 2, “Is unwilling to get involved with people unless certain of being liked,” the corresponding question is “How often do you avoid getting to know people because you are worried that they may not like you.” If the answer is “often,” the interviewer goes on to ask, “Has this affected the number of friends you have?” The DSM-IV criterion associated with each set of questions is rated as: 0 = “not present or limited to rare isolated examples,” 1 = “subthreshold – some evidence of the trait, but not sufficiently pervasive to consider the criterion present,” 2 = “present – criterion is clearly present for most of the past 5 years (i.e., present at least 50% of the time), and 3 = “strongly present.” The SIDP-IV uses the “five-year rule,” meaning that the behavior, cognitions, and feelings predominating for most of the past 5 years are considered to be representative of the individual’s long-term personality functioning.
Interviewers were mostly psychology students in their final part of training to become clinical psychologists and experienced psychiatric nurses. They were trained by professionals (one psychiatrist and two psychologists) with extensive previous experience with the instrument, and closely followed up individually during the whole data collection period. The interviews were carried out between June 1999 and May 2004, and were largely conducted face-to-face. For practical reasons, 231 interviews (8.3%) were obtained by telephone. Each twin in a pair was interviewed by a different interviewer.
Interrater reliability was assessed by having two raters score 70 audiotaped interviews. The number of subjects with categorically classified PDs was too low to calculate Kappa coefficients. Intraclass correlations for the scaled PDs, using the number of endorsed criteria including subthreshold level (see below), were very high: .96 for DPD and .81–.96 for the established DSM-IV PDs. We also evaluated the internal consistencies of the PDs by calculating Cronbach’s alphas for the sum-scores based on polychoric correlations. Alphas were .87 for DPD and .72–.89 for the established DSM-IV PDs.
Approval was received from the Norwegian Data Inspectorate and the Regional Ethical Committee, and written informed consent was obtained from all participants after they were given a complete description of the study.
STATISTICS
In this population-based sample of twins, the prevalences of individual PD diagnoses in each group were too low to compute tetrachoric correlations and obtain useful analyses, as this depends on the presence of concordant pairs in all five zygosity groups. We therefore used a dimensional approach, constructing ordinal variables based on the number of endorsed criteria. To optimize statistical power, we used the number of endorsed subthreshold criteria (≥ 1) instead of criteria above threshold (≥ 2), assuming that the liability for each trait is continuous and normally distributed, that is, that the classification 0–3 represents different degrees of severity. This assumption was evaluated with multiple threshold tests for each criterion. The same procedure was used to test the assumption that the number of positive criteria for PDs represents different degrees of severity. All of the multiple threshold tests were done separately for each zygosity group, and none was significant (all p >. 05) (Kendler, Czajkowski, et al., 2006; Ørstavik et al., 2007a; Reichborn-Kjennerud, Czajkowski, Neale, et al., 2007; Torgersen et al., 2008). Few subjects endorsed all or most of the criteria for an individual PD. To avoid empty cells, we collapsed the upper categories for the summed score. The maximum number of categories, ranging from 3 to 5, was created for each disorder. These categories have been applied in all the previous publications from this sample (e.g., Kendler, Czajkowski, et al., 2006; Kendler et al., 2008; Ørstavik et al., 2007a; Reichborn-Kjennerud, Czajkowski, Neale, et al., 2007; Torgersen et al., 2008), and were, in the current study, applied in all the statistical analyses described below. For ease of expression, we refer in this article to personality disorders (PDs) in place of the more accurate term dimensional representation of PDs
The correlations between DPD and the 10 DSM-IV PDs were assessed using polychoric correlations (Mplus 5.2; Muthén & Muthén, 2007). To identify PDs that were independently related to DPD, we applied forward stepwise multiple regression analysis based on polychoric correlations with DPD as the dependent variable. Dependence within twin pairs was adjusted for by use of the “complex sample” feature of Mplus (Flora & Curran, 2004).
In the classical twin model, individual differences in liability are assumed to arise from three latent factors: additive genetic (A), that is, genetic effects that combine additively; common or shared environment (C), which includes all environmental exposures that are shared by the twins and contribute to their similarity; and individual-specific or unique environment (E), which includes all environmental factors not shared by the twins plus measurement error. Because monozygotic (MZ) twins share all their genes and dizygotic (DZ) twins share on average 50% of their segregating genes, A contributes twice as much to the resemblance in MZ compared to DZ twins for a particular trait or disorder. By definition, MZ and DZ twins share all their C factors and none of their E factors. In multivariate analyses, the twin model is extended to explore shared etiology between two or more disorders, by including possible common genetic (A) and latent environmental (C and E) factors.
In this study, our purpose was to explore the genetic and environmental correlations between DPD and the DSM-IV PDs and to examine to what extent genetic and/or environmental factors were unique to DPD. A Cholesky decomposition model is well suited for this purpose (Neale & Cardon, 1992). In a multivariate Cholesky model, three latent factors (A, B, and C) are specified for each phenotype. Each of these load on the specific phenotype to which they are assigned in addition to the phenotypes ordered after them in the model. The first set of factors (A1, C1, and E1) influences the first PD, as well as all the others. The last set in an analysis of x phenotypes (Ax, Cx, and Ex) accounts for residual influences specific to the last ordered phenotype (in our case DPD) and not shared with the other phenotypes (PDs). Based on this model, the correlation between the genetic factors (ra), shared environmental factors (rc), and individual-specific environmental factors (re) influencing the different phenotypes can be estimated.
Very large samples are required to have sufficient statistical power to detect sex-specific effects (Neale, Eaves, & Kendler, 1994). In a previous study of this sample, we found evidence for sex-specific effects for DPD (Ørstavik et al., 2007a). However, this has not been found for any of the other PDs (Kendler, Czajkowski, et al., 2006; Reichborn-Kjennerud, Czajkowski, Neale, et al., 2007; Torgersen et al., 2008), and not in a bivariate analysis of DPD and MDD (Ørstavik et al., 2007b). Thus the multivariate models used here were fitted with equal parameters for males and females. Because of significant prevalence differences for some of the phenotypes, different thresholds were used for the two sexes.
A full model, including all latent variables and paths, was tested against nested submodels with reduced numbers of parameters. The fit of the alternative models can be compared using the difference in twice the log likelihood (2lnL), which under certain regularity conditions is asymptomatically distributed as χ2 with degrees of freedom (df) equal to the difference in number of parameters (Δχ2 test). According to the principle of parsimony, models with fewer parameters are preferable if they do not result in a significant deterioration of fit. Akaike Information Criterion (AIC) is an index that combines fit and parsimony, and is calculated as Δχ2-2Δdf (Akaike, 1987). A lower AIC value indicates superior fit.
A basic assumption in twin analyses is that MZ and DZ twins are equally correlated in their exposure to trait-relevant environments. We tested the validity of this “equal environment assumption” by applying polychotomous logistic regression controlling for the correlational structure of our data using independent estimating equations as operationalized in the SAS procedure GENMOD (SAS Institute, 2005). Two variables that reflected, respectively, similarity of childhood (number of years that the twins were in the same class at school and the years the twins lived in the same residence) and adult environments (frequency of person and telephone contact during the last year and the distance between their current residences) were constructed. In same-sex pairs, we tested whether the PD score in twin 1 interacted with our measure of environmental similarity in predicting the relevant PD score in twin 2 (dependent variable). We controlled for main effects of zygosity, sex, age, and level of environmental similarity as well as shared environment effects and genetic effects. None of the analyses testing the impact of environmental similarity on twin resemblance approached significance (all p-values > .10).
results
SAMPLE CHARACTERISTICS
Our final sample consisted of 1,022 males and 1,772 females 221 monozygotic male (MZM) pairs, 116 dizygotic male (DZM) pairs, 448 monozygotic female (MZF) pairs, 261 dizygotic female (DZF) pairs, 340 dizygotic opposite sex (DZO) pairs, and 22 single responders. The mean age of participants was 28.2 years (range 19-36).
PHENOTYPIC CORRELATIONS
All the 11 PDs examined in this study were positively intercorrelated (Table 1). The phenotypic correlations between DPD and the 10 DSM-IV PDs were moderate to high, ranging from .24 (antisocial PD) to .55 (avoidant PD) (Table 1). The mean phenotypic correlation was highest for borderline PD (.43), followed by paranoid and schizotypal PD (both .42) and DPD (.40). Borderline PD and DPD were the only PDs with phenotypic correlations exceeding .50 for more than one other PD.
TABLE 1. Phenotypic Correlations Between Depressive Personality Disorder and the 10 DSM-IV Personality Disorders (PDs).
| Depressive PD |
Paranoid PD |
Schizoid PD |
Schizotypal PD |
Antisocial PD |
Borderline PD |
Histrionic PD |
Narcissistic PD |
Dependent PD |
Avoidant PD |
Obsessive-compulsive PD |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| Depressive PD | 1.00 | .46 | .27 | .42 | .24 | .51 | .31 | .31 | .51 | .55 | .37 |
| Paranoid PD | .46 | 1.00 | .32 | .58 | .29 | .49 | .43 | .42 | .36 | .37 | .40 |
| Schizoid PD | .27 | .32 | 1.00 | .48 | .20 | .30 | .17 | .29 | .27 | .41 | .26 |
| Schizotypal PD | .42 | .58 | .48 | 1.00 | .25 | .46 | .37 | .35 | .43 | .43 | .34 |
| Antisocial PD | .24 | .29 | .20 | .25 | 1.00 | .52 | .34 | .43 | .21 | .15 | .21 |
| Borderline PD | .51 | .49 | .30 | .46 | .52 | 1.00 | .50 | .42 | .47 | .39 | .34 |
| Histrionic PD | .31 | .43 | .17 | .37 | .34 | .50 | 1.00 | .52 | .35 | .12 | .36 |
| Narcissistic PD | .31 | .42 | .29 | .35 | .43 | .42 | .52 | 1.00 | .34 | .25 | .41 |
| Dependent PD | .51 | .36 | .27 | .43 | .21 | .47 | .35 | .34 | 1.00 | .56 | .24 |
| Avoidant PD | .55 | .37 | .41 | .43 | .15 | .39 | .12 | .25 | .56 | 1.00 | .23 |
| Obsessive-compulsive PD | .37 | .40 | .26 | .34 | .21 | .34 | .36 | .41 | .24 | .23 | 1.00 |
| Mean Phenotypic Correlations | .40 | .41 | .30 | .41 | .29 | .43 | .35 | .38 | .36 | .32 | .31 |
REGRESSION ANALYSIS
Results from the multiple regression analysis are displayed in Table 2. Five of the DSM-IV PDs were independently associated with DPD: paranoid PD from Cluster A, borderline PD from Cluster B, and all Cluster C PDs (avoidant, dependent, and obsessive-compulsive PD).
TABLE 2. Adjusted Associations Between Dimensional Representations of Depressive Personality Disorder and the 10 DSM-IVPersonality Disorders, Based on Polychoric Correlations.
| Personality disorder | β | 95% Confidence Interval |
|---|---|---|
| Paranoid | .13 | .06 – .19 |
| Schizoid | −.05 | −.10 – .00 |
| Schizotypal | .03 | −.04 – .09 |
| Antisocial | −.02 | −.08 – .04 |
| Borderline | .19 | .15 – .21 |
| Histrionic | .00 | −.05 – .06 |
| Narcissistic | .01 | −.06 – .04 |
| Avoidant | .32 | .26 – .37 |
| Dependent | .16 | .10 – .21 |
| Obsessive-compulsive | .15 | .11 – .20 |
MODEL FITTING
Fit statistics from the twin models are shown in Table 3. Model 1 is the full model containing A, C, and E factors. Borderline PD was ordered first in the model, followed by the three Cluster C PDs and paranoid PD. In Model 2, all pathways from the latent genetic variables were set to zero (CE model). This resulted in a substantial increase in χ2 (Δχ2 = 55.32, p < .001), indicating a significant contribution from genetic factors. In Model 3, all the shared environmental pathways were constrained to zero (AE model). This model fitted the data well as indicated by a substantial improvement in AIC (−35.67). The subsequent models are all reduced versions of Model 3. In Model 4, we set to zero the path from the specific genetic factor for DPD, resulting in an improvement in fit (AIC −.11, compared to Model 3). In the next model, we set to zero the path from paranoid PD to DPD, which fitted the data even better (AIC −1.98). Further reductions in the model (Models 6–8) did not increase the fit. In Model 9, we constrained to zero the paths from the specific latent environmental effect for paranoid PD, resulting in a significant deterioration in fit. Further reducing the model by dropping latent environmental effects from the other PDs all gave significant reduction in fit (not included in Table 3), indicating that individual-specific environmental factors shared with all the other PDs contribute to their co-occurrence with DPD.
TABLE 3. Model Fitting Results.
| Model | Δ χ 2 | Δ df | p | AIC |
|---|---|---|---|---|
| 1. ACE | ||||
| 2. CE* | 55.32 | 21 | <.001 | 13.319 |
| 3. AE* | 3.33 | 21 | .99 | −35.67 |
| 4. AE + drop specific A DPD** | 1.892 | 1 | .17 | −.11 |
| 5. AE+ drop A from paranoid PD to DPD** | .016 | 1 | .90 | −1.98 |
| 6. AE+ drop A from paranoid PD to DPD and specific A DPD** | 2.617 | 2 | .27 | −1.38 |
| 7. AE + drop A from paranoid PD and obsessive-compulsive PD to DPD** |
5.158 | 2 | .08 | 1.16 |
| 8. AE drop A from paranoid PD and obsessive-compulsive PD and spec A DPD** |
7.068 | 3 | .07 | 1.07 |
| 9. AE + drop E from paranoid PD to DPD** | 11.748 | 1 | 9.748 | <.001 |
Note. Abbreviations: A, additive genetic effects; C, shared environmental effects; E, individual-specific environmental effects; df, degrees of freedom; AIC, Akaike’s information criterion. Best-fitting model in bold.
Fit statistics calculated from full model.
Fit statistics calculated from model 3 (AE model).
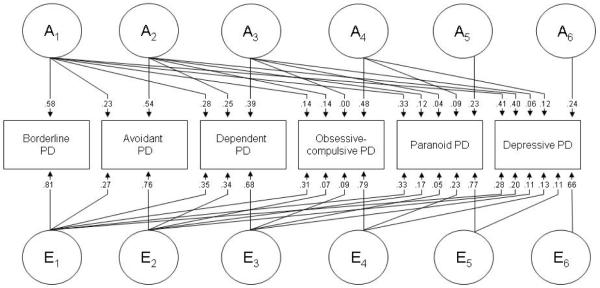
Figure 1 shows the best-fitting model (Model 5) with parameter estimates. In this model, the covariance between DPD and the other PDs are accounted for solely by additive genetic and unique environmental factors. The path coefficients to DPD show that a substantial part of the genetic contribution to DPD is accounted for by genes that influence borderline PD (path from A1), but also from genes that influence avoidant but not borderline PD (path from A2). Contributions from genes that influence the other Cluster C disorders (dependent and obsessive-compulsive PD) but not the two ordered first in the model were, on the other hand, minor, and not present for paranoid PD. A similar pattern was seen for the individual-specific environmental factors.
FIGURE 1.
Parameter estimates for the most parsimonious multivariate twin model. A indicates additive genetic factors; E individual-specific environmental factors. Subscripts refer to factors that influence the personality disorder (PD) that are shared, respectively, by all six PDs (A1 and E1), avoidant PD and the four PDs ordered subsequently in the model (A2 and E2), dependent PD and the three PDs ordered subsequently in the model (A3 and E3), obsessive-compulsive PD and the two PDs ordered subsequently in the model (A4 and E4), paranoid PD and DPD (A5 and E5) and factors that are specific to DPD (A6 and E6).
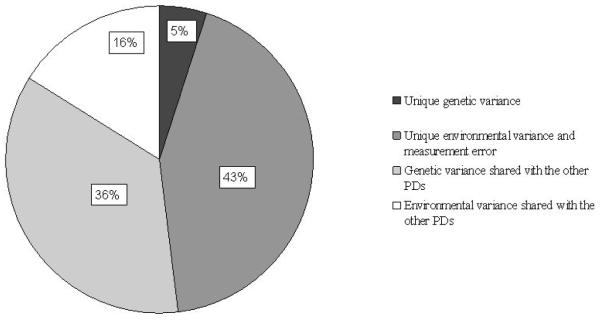
Figure 2 illustrates the proportion of variance accounted for by disorderspecific genetic and environmental factors and factors shared with the other PDs. About half of the variance in liability to DPD was disorder specific, but most of this was due to individual specific environmental factors. Only 5% of the total variance could be accounted for by genetic factors specific to DPD.
FIGURE 2.
Proportion of variance for depressive personality disorder (DPD) accounted for by common and specific genetic and environmental factors.
Table 4 displays the genetic and environmental correlations between DPD and the other PDs. The genetic correlations were strong, ranging from .53 (obsessive-compulsive PD) to .83 (avoidant PD). The environmental correlations were moderate, and differed little between the different PDs (.36–.40). In general, the genetic correlations between DPD and the DSM-IV PDs were stronger than those within the DSM-IV PDs, while the environmental correlations tended to be in a more similar range.
TABLE 4.
Genetic and Environmental Correlations Between Dimensional Representations of Borderline, Avoidant, Dependent, Obsessive-Compulsive, Paranoid, and Depressive Personality Disorder (PD). Environmental Correlations Above the Diagonal, Genetic Correlations Below.
| Borderline PD | Avoidant PD | Dependent PD | Obsessive- compulsive PD |
Paranoid PD | Depressive PD | |
|---|---|---|---|---|---|---|
| Borderline PD | — | .34 | .41 | .37 | .37 | .37 |
| Avoidant PD | .40 | — | .52 | .20 | .31 | .37 |
| Dependent PD | .51 | .63 | — | .27 | .29 | .38 |
| Obsessive- compulsive PD | .28 | .36 | .26 | — | .40 | .33 |
| Paranoid PD | .74 | .53 | .56 | .45 | — | .36 |
| Depressive PD | .65 | .83 | .70 | .53 | .69 | — |
discussion
ASSOCIATIONS BETWEEN DPD AND THE 10 DSM-IV PDS
Applying polychoric correlations of dimensional representations of DPD and the 10 DSM-IV PDs, we found that DPD correlated significantly with all the other PDs. The highest correlations (>.50) were seen with avoidant, dependent, and borderline PD. This finding is in line with several other studies (Huprich, 2004; Huprich et al., 2006; Klein & Shih,1998; Markowitz et al., 2005; McDermut et al., 2003), including one that used Pearson’s correlations (Huprich, 2004). Huprich found that DPD correlated positively and significantly with all PDs except antisocial, histrionic, and obsessive-compulsive PD. The problem of comorbidity is, however, not restricted to DPD. The highest mean correlation was seen for borderline PD.
After controlling for the interrelationships between the PDs, DPD was independently associated with paranoid, borderline, and the three Cluster C PDs. To our knowledge, this is the first study to determine the association between DPD and other PDs controlling for their comorbidity. The results are interesting in view of our previous study on the relationship between the DSM-IV PDs and MDD (Reichborn-Kjennerud et al., 2010), where paranoid, borderline, and avoidant PD were independently associated with MDD. The association was strongest with borderline PD. In the current study, however, the association was strongest between DPD and avoidant PD (Table 2), which is in accordance with previous findings of a close phenotypic relationship between DPD and avoidant PD (Huprich et al., 2006).
GENETIC AND ENVIRONMENTAL RISK FACTORS SHARED WITH OTHER PDS
To our knowledge, this is the first study of common etiological factors underlying DPD and other PDs. We found strong genetic correlations between DPD and all five of the PDs included in the model, and strongest between DPD and avoidant PD. A second important finding was that DPD has genetic risk factors in common with the Cluster C PDs that are not shared with borderline PD. This pattern differs from that found in the above-mentioned study of MDD and PDs (Reichborn-Kjennerud et al., 2010), where all genetic effects influencing MDD that were shared with any of the PDs were also shared with borderline PD. The path coefficients from dependent and obsessive-compulsive PDs were small, indicating that DPD to a very limited extent shares genetic risk factors with the other Cluster C PDs that are not also shared with avoidant PD. Thus the main difference between DPD and MDD in terms of genetic liability shared with the DSM-IV PDs seems to be the close genetic relationship between DPD and avoidant PD.
Our findings are also interesting in light of a previous study on the structure of genetic and environmental risk factors for all the DSM-IV PDs, based on the same sample as in our study, where three genetic factors were identified (Kendler et al., 2008). The first was a broad factor that had high loadings on PDs from all three clusters, including borderline PD (but not avoidant). The second had high loadings on borderline and antisocial PD, and the third had high loadings on schizoid and avoidant PD. The authors suggest that the first factor represents general genetic liability to PD pathology, the second disinhibition or impulsive aggression, and the third introversion. The results from the present study indicate that DPD shares genetic liability not only with PDs from all three DSM-IV clusters, but also with PDs from all three factors identified by Kendler et al. Our results also indicate that DPD is one of few PDs that share genetic liability with avoidant PD.
Our findings can also be viewed in light of previous studies on how PDs relate to normal personality traits. All the Five Factor Model (FFM) domains have been shown to be heritable (Bouchard & Loehlin, 2001). DPD is characterized by high levels of neuroticism and low levels of extraversion, both on the domain (Dyce & O’Connor, 1998) and facet levels (Bagby, Schuller, Marshall, & Ryder, 2004). This pattern, although to a lesser degree, has also been observed for most of the other PDs that were independently related to DPD in our study, and especially for avoidant PD (for review, see Saulsman & Page, 2004). In a recent study, Vachon, Sellbom, Ryder, Miller, and Bagby (2009) found that the FFM rating of DPD correlated with the other DSM-IV PDs in a manner that corresponded closely to the genotypic correlations found in the present study. For instance, DPD correlated .85 with avoidant PD, and about .55 with obsessive-compulsive, paranoid, and dependent PD (Vachon et al., 2009). Thus, although we have no measures of normal personality traits in this study, a plausible explanation for the genetic correlation is, in part, transmission through heritable traits that closely resemble those of the FFM.
The Personality and Personality Disorders Work Group for DSM-5 has suggested that 5 of the 10 DSM-IV PDs should be retained (as personality types) in the revised classification system, among them borderline, avoidant, and obsessive-compulsive PD. DPD will not be included as a specific type, but represented by a combination of core impairment in personality functioning and the prominent personality traits pessimism, anxiousness, depressivity, low self-esteem, guilt/shame, and anhedonia. Five of these traits are also listed as associated with the avoidant personality type, while the sixth, depressivity, is included among trait facets characterizing the borderline personality type. Given that the specific traits suggested for DSM-5 PD assessment are heritable, the strong genetic correlation between DPD and avoidant PD found in the current study is well reflected in the proposed assessments of the two PDs in DSM-5.
The environmental correlations were lower than the genetic correlations, and similar to those observed between the DSM-IV PDs (Table 4). This pattern is concordant with what has previously been found for genetic and environmental correlations between PDs and Axis I disorders, for example, in the above-mentioned study on MDD and the DSM-IV PDs (Reichborn-Kjennerud et al., 2010).
GENETIC AND ENVIRONMENTAL RISK FACTORS UNIQUE TO DPD
Our results show that even when controlling for comorbidity with other PDs, about 50% of the liability to DPD was unique to this disorder. A small part (5%) was due to genetic factors specific to DPD. This indicates that DPD has some heritable properties that are not shared with the other PDs. Previous studies have shown that DPD co-occurs substantially with mood disorders, and mood disorders are also common in subjects with other DSM-IV PDs, especially borderline PD (Hasin, Goodwin, Stinson, & Grant, 2005; Len-zenweger, Lane, Loranger, & Kessler, 2007; Skodol et al., 1999). However, while borderline PD is characterized by mood instability, dysthymic disorder is characterized by persisting depression and anhedonia, traits that are also included in the proposed DPD criteria. In their tripartite model of anxiety and depression, Watson and Clark (1992) described how this absence of positive affect is specific to depression. Mood disorders are moderately heritable (Kendler, Gatz, Gardner, & Pedersen, 2006; Sullivan, Neale, & Kendler, 2000), so the disorder-specific genetic variance might reflect gloominess and absence of positive affect.
A substantial part of the variance for DPD was due to disorder-specific unique environmental variance. In twin studies, measurement error increases the estimates of E. Our measures of interrater reliability and internal consistency of DPD indicate that measurement error accounts for at least 15% of the variance in DPD, still leaving about 30% of the variance explained by environmental risk factors not shared with the other PDs. This is in line with previous findings suggesting that comorbidity between psychiatric disorders often is a result of common genetic liabilities, while environmental factors are more important in the manifestation of specific disorders (Kendler, 2006; Kendler, Prescott, Myers, & Neale, 2003).
IMPLICATIONS
Knowledge about common genetic and environmental risk factors can, to some degree, be used to inform the development of classification systems like the DSM-5 (Andrews et al., 2009). We found that the genetic correlations between DPD and the five selected DSM-IV PDs were high and, for some of the PDs, higher than those observed between the existing DSM-IV PDs. Moreover, the genetic correlations between DPD and most of the PDs in this study were higher than the correlation between DPD and MDD (rg = .56) found in a previous study from the same sample (Ørstavik et al., 2007b). This suggests that DPD is more closely genetically related to personality disorders than to mood disorders and supports the classification of DPD on Axis II. An important limitation to this conclusion is, however, that we have not been able to include dysthymic disorder in our twin models. The very high genetic correlation we observed between DPD and avoidant PD, and the fact that DPD shares genetic liability factors with avoidant PD that is not shared with borderline PD, supports the classification of DPD among PDs currently in Cluster C. On the other hand, the close genetic relationship between DPD and the other PDs could be used to argue that DPD is a redundant diagnostic category. Genetic correlations in this range (.6 to unity) have, however, been reported both for several co-occurring Axis I internalizing disorders (Hettema, Neale, Myers, Prescott, & Kendler, 2006; Kendler, Gardner, et al., 2006; Nelson et al., 2000) and for avoidant PD and social phobia (Reichborn-Kjennerud, Czajkowski, Torgersen, et al., 2007). Thus information about common genetic etiology is not in itself a sufficient background for answering fundamental nosological questions involving cooccurring mental disorders, but must be judged in context with the relative importance of other potential validators (Kendler, 2006).
LIMITATIONS
There are five important limitations to consider when interpreting the results from this study. First, because of low prevalences, we were unable to analyze categorical PD diagnoses and instead examined dimensional representation of the DSM-IV diagnoses. We made the assumption that the two approaches reflect the same underlying liability and supported this by using multiple threshold tests (Kendler, Czajkowski, et al., 2006; Ørstavik et al., 2007a; Reichborn-Kjennerud et al., 2007a; Torgersen et al., 2008). Furthermore, many have argued that PDs are best conceptualized as dimensional rather than categorical constructs (Krueger, Skodol, Livesley, Shrout, & Huang, 2007; Morey et al., 2007). Second, because of the large number of PDs included in the multivariate twin analyses, we were not able to obtain reliable confidence intervals for our best-fitting model. Although our sample size was large, endorsement rates for many of the PD criteria included were low. Previous estimates of confidence intervals from our studies involving some of the PDs included in the present study (Ørstavik et al., 2007b; Reichborn-Kjennerud et al., 2010) indicate that our estimated parameters and correlations are given with moderate levels of precision. Third, twins were only interviewed once. Although our interrater reliability was high, the test-retest reliability is likely to be considerably lower (McGlashan et al., 2005). In twin analyses, measurement errors are included in E, which implies that a reduction in reliability would result in decreased heritability estimates. Fourth, substantial attrition was observed in this sample from the birth registry through three waves of contact. We report detailed analyses of the predictors of nonresponse across waves elsewhere, including how attrition might influence the biometric twin analyses (Tambs et al., 2010). Briefly, cooperation throughout the NIPHTP studies was strongly predicted by female sex, monozygosity, and higher educational status, but not by symptoms of psychiatric disorders. More specifically, prediction of participation from the second questionnaire of the NIPHTP (Q2) to the current study revealed no effect of the 22 mental health variables included in the analyses (Tambs et al., 2009). Fifth, the prevalence of PDs varies across studies: Estimates for the PDs included here were similar to estimates from the U.K. (Coid, Yang, Tyrer, Roberts, & Ullrich, 2006), but lower than in a recent U.S. study (Lenzenweger et al., 2007). Thus our results may not be generalized to other cultural, ethnic, and age groups.
Acknowledgments
The work was supported by grant MH-068643 (PI Kendler) and MH-65322 (PI Neale) from the National Institutes of Health. The twin program of research at the Norwegian Institute of Public Health is supported by grants from The Norwegian Research Council, The Norwegian Foundation for Health and Rehabilitation, The Borderline Personality Disorder Research Foundation, and the European Commission under the program “Quality of Life and Management of the Living Resources” of 5th Framework Program (no. QLG2-CT-2002-01254). Genotyping on the twins was performed at the Starr Genotyping Resource Centre at the Rockefeller University. We are very thankful to the twins for their participation.
Footnotes
This article was accepted under the editorship of Paul S. Links.
references
- Akaike H. Factor analysis and AIC. Psychometrica. 1987;52:317–332. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Andrews G, Goldberg DP, Krueger RF, Carpenter WT, Hyman SE, Sachdev P, et al. Exploring the feasibility of a meta-structure for DSM-V and ICD-11: Could it improve utility and validity? Psychological Medicine. 2009;39:1993–2000. doi: 10.1017/S0033291709990250. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Schuller DR, Marshall MB, Ryder AG. Depressive personality disorder: Rates of comorbidity with personality disorders and relations to the five-factor model of personality. Journal of Personality Disorders. 2004;18:542–554. doi: 10.1521/pedi.18.6.542.54796. [DOI] [PubMed] [Google Scholar]
- Bouchard TJ, Loehlin JC. Genes, evolution, and personality. Behavioral Genetics. 2001;31:243–273. doi: 10.1023/a:1012294324713. [DOI] [PubMed] [Google Scholar]
- Coid J, Yang M, Tyrer P, Roberts A, Ullrich S. Prevalence and correlates of personality disorder in Great Britain. British Journal of Psychiatry. 2006;188:423–431. doi: 10.1192/bjp.188.5.423. [DOI] [PubMed] [Google Scholar]
- Dyce JA, O’Connor BP. Personality disorders and the five-factor model: A test of facet-level predictions. Journal of Personality Disorders. 1998;12:31–45. doi: 10.1521/pedi.1998.12.1.31. [DOI] [PubMed] [Google Scholar]
- Flora DB, Curran PJ. An empirical evaluation of alternative methods of estimation for confirmatory factor analysis with ordinal data. Psychological Methods. 2004;9:466–491. doi: 10.1037/1082-989X.9.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JR, Magnus P, Tambs K. The Norwegian Institute of Public Health Twin Panel: A description of the sample and program of research. Twin Research. 2002;5:415–423. doi: 10.1375/136905202320906192. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: Results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Archives of General Psychiatry. 2005;62:1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- Helgeland MI, Kjelsberg E, Torgersen S. Continuities between emotional and disruptive behavior disorders in adolescence and personality disorders in adulthood. American Journal of Psychiatry. 2005;162:1941–1947. doi: 10.1176/appi.ajp.162.10.1941. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Myers JM, Prescott CA, Kendler KS. A population-based twin study of the relationship between neuroticism and internalizing disorders. American Journal of Psychiatry. 2006;163:857–864. doi: 10.1176/ajp.2006.163.5.857. [DOI] [PubMed] [Google Scholar]
- Huprich SK. Convergent and discriminant validity of three measures of depressive personality disorder. Journal of Personality Assessment. 2004;82:321–328. doi: 10.1207/s15327752jpa8203_08. [DOI] [PubMed] [Google Scholar]
- Huprich SK. What should become of depressive personality disorder in DSM-V? Harvard Review of Psychiatry. 2009;17:41–59. doi: 10.1080/10673220902735694. [DOI] [PubMed] [Google Scholar]
- Huprich SK, Zimmerman M, Chelminski I. Disentangling depressive personality disorder from avoidant, borderline, and obsessive-compulsive personality disorders. Comprehensive Psychiatry. 2006;47:298–306. doi: 10.1016/j.comppsych.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Twin studies of psychiatric illness: An update. Archives of General Psychiatry. 2001;58:1005–1014. doi: 10.1001/archpsyc.58.11.1005. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Reflections on the relationship between psychiatric genetics and psychiatric nosology. American Journal of Psychiatry. 2006;163:1138–1146. doi: 10.1176/ajp.2006.163.7.1138. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Czajkowski N, Roysamb E, Tambs K, Torgersen S, et al. The structure of genetic and environmental risk factors for DSM-IV personality disorders: A multivariate twin study. Archives of General Psychiatry. 2008;65:1438–1446. doi: 10.1001/archpsyc.65.12.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Czajkowski N, Tambs K, Torgersen S, Aggen SH, Neale MC, et al. Dimensional representations of DSM-IV cluster A personality disorders in a population-based sample of Norwegian twins: A multivariate study. Psychological Medicine. 2006;36:1583–1591. doi: 10.1017/S0033291706008609. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Gatz M, Pedersen NL. The sources of comorbidity between major depression and generalized anxiety disorder in a Swedish national twin sample. Psychological Medicine. 2006;36:1–10. doi: 10.1017/S0033291706009135. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. American Journal of Psychiatry. 2006;163:109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Klein DN, Miller GA. Depressive personality in nonclinical subjects. American Journal of Psychiatry. 1993;150:1718–1724. doi: 10.1176/ajp.150.11.1718. [DOI] [PubMed] [Google Scholar]
- Klein DN, Shih JH. Depressive personality: Associations with DSM-III-R mood and personality disorders and negative and positive affectivity, 30-month stability, and prediction of course of Axis I depressive disorders. Journal of Abnormal Psychology. 1998;107:319–327. doi: 10.1037//0021-843x.107.2.319. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Markon KE. Reinterpreting comorbidity: A model-based approach to understanding and classifying psychopathology. Annual Review of Clinical Psychology. 2006;2:111–133. doi: 10.1146/annurev.clinpsy.2.022305.095213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Skodol AE, Livesley WJ, Shrout PE, Huang YQ. Synthesizing dimensional and categorical approaches to personality disorders: Refining the research agenda for DSM-V Axis II. International Journal of Methods in Psychiatric Research. 2007;16:S65–S73. doi: 10.1002/mpr.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzenweger MF, Lane MC, Loranger AW, Kessler RC. DSM-IV personality disorders in the National Comorbidity Survey replication. Biological Psychiatry. 2007;62:553–564. doi: 10.1016/j.biopsych.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz JC, Skodol AE, Petkova E, Xie H, Cheng J, Hellerstein DJ, et al. Longitudinal comparison of depressive personality disorder and dysthymic disorder. Comprehensive Psychiatry. 2005;46:239–245. doi: 10.1016/j.comppsych.2004.09.003. [DOI] [PubMed] [Google Scholar]
- McDermut W, Zimmerman M, Chelminski I. The construct validity of depressive personality disorder. Journal of Abnormal Psychology. 2003;12:49–60. [PubMed] [Google Scholar]
- McGlashan TH, Grilo CM, Sanislow CA, Ralevski E, Morey LC, Gunderson JG, et al. Two-year prevalence and stability of individual DSM-IV criteria for schizotypal, borderline, avoidant, and obsessive-compulsive personality disorders: Toward a hybrid model of Axis II disorders. American Journal of Psychiatry. 2005;162:883–889. doi: 10.1176/appi.ajp.162.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey LC, Hopwood CJ, Gunderson JG, Skodol AE, Shea MT, Yen S, et al. Comparison of alternative models for personality disorders. Psychological Medicine. 2007;37:983–994. doi: 10.1017/S0033291706009482. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. Muthén & Muthén; Los Angeles: 2007. [Google Scholar]
- Neale MC. A finite mixture distribution model for data collected from twins. Twin Research and Human Genetics. 2003;6:235–239. doi: 10.1375/136905203765693898. [DOI] [PubMed] [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Klüver; Dordrecht: 1992. [Google Scholar]
- Neale MC, Eaves LJ, Kendler KS. The power of the classical twin study to resolve variation in threshold traits. Behavioral Genetics. 1994;24:239–258. doi: 10.1007/BF01067191. [DOI] [PubMed] [Google Scholar]
- Nelson EC, Grant JD, Bucholz KK, Glowinski A, Madden PAF, Reich W, et al. Social phobia in a population-based female adolescent twin sample: Co-morbidity and associated suicide-related symptoms. Psychological Medicine. 2000;30:797–804. doi: 10.1017/s0033291799002275. [DOI] [PubMed] [Google Scholar]
- Ørstavik RE, Kendler KS, Czajkowski N, Tambs K, Reichborn-Kjennerud T. Genetic and environmental contributions to depressive personality disorder in a population-based sample of Norwegian twins. Journal of Affective Disorders. 2007a;99:181–189. doi: 10.1016/j.jad.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Ørstavik RE, Kendler KS, Czajkowski N, Tambs K, Reichborn-Kjennerud T. The relationship between depressive personality disorder and major depressive disorder: A population-based twin study. American Journal of Psychiatry. 2007b;164:1866–1872. doi: 10.1176/appi.ajp.2007.07010045. [DOI] [PubMed] [Google Scholar]
- Pfohl B, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality (SIDP-IV) University of Iowa, Department of Psychiatry; Iowa City: 1995. [Google Scholar]
- Phillips KA, Hirschfeld RM, Shea MT, Gunderson JG. Depressive personality disorder. In: Livesley WJ, editor. The DSM-IV personality disorders. Guilford Press; New York: 1995. pp. 287–302. [Google Scholar]
- Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral genetics. Worth; New York: 2001. [Google Scholar]
- Reichborn-Kjennerud T, Czajkowski N, Neale MC, Ørstavik RE, Torgersen S, Tambs K, et al. Genetic and environmental influences on dimensional representations of DSM-IV cluster C personality disorders: A population-based multivariate twin study. Psychological Medicine. 2007a;37:645–653. doi: 10.1017/S0033291706009548. [DOI] [PubMed] [Google Scholar]
- Reichborn-Kjennerud T, Czajkowski N, Roysamb E, Ørstavik RE, Neale MC, Torgersen S, et al. Major depression and dimensional representations of DSM-IV personality disorders: A population-based twin study. Psychological Medicine. 2010;40:1475–84. doi: 10.1017/S0033291709991954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichborn-Kjennerud T, Czajkowski N, Torgersen S, Neale MC, Ørstavik RE, Tambs K, et al. The relationship between avoidant personality disorder and social phobia: A population-based twin study. American Journal of Psychiatry. 2007b;164:1722–1728. doi: 10.1176/appi.ajp.2007.06101764. [DOI] [PubMed] [Google Scholar]
- Robins E, Guze SB. Establishment of diagnostic validity in psychiatric illness: Its application to schizophrenia. American Journal of Psychiatry. 1970;126:983–987. doi: 10.1176/ajp.126.7.983. [DOI] [PubMed] [Google Scholar]
- Ryder AG, Bagby RM, Dion KL. Chronic, low-grade depression in a nonclinical sample: Depressive personality or dysthymia? Journal of Personality Disorders. 2001;15:84–93. doi: 10.1521/pedi.15.1.84.18641. [DOI] [PubMed] [Google Scholar]
- Ryder AG, Bagby RM, Schuller DR. The overlap of depressive personality disorder and dysthymia: A categorical problem with a dimensional solution. Harvard Review of Psychiatry. 2002;10:337–352. doi: 10.1080/10673220216230. [DOI] [PubMed] [Google Scholar]
- SAS Institute . SAS Online Doc. version 9.1.3. SAS Institute Inc; Cary, NC: 2005. [Google Scholar]
- Saulsman LM, Page AC. The five-factor model and personality disorder empirical literature: A meta-analytic review. Clinical Psychology Review. 2004;23:1055–1085. doi: 10.1016/j.cpr.2002.09.001. [DOI] [PubMed] [Google Scholar]
- Skodol AE, Stout RL, McGlashan TH, Grilo CM, Gunderson JG, Shea MT, et al. Co-occurrence of mood and personality disorders: A report from the Collaborative Longitudinal Personality Disorders Study (CLPS) Depression and Anxiety. 1999;10:175–182. [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: Review and meta-analysis. American Journal of Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Tambs K, Rønning T, Prescott CA, Kendler KS, Reichborn-Kjennerud T, Torgersen S, et al. The Norwegian Institute of Public Health twin study of mental health: Examining recruitment and attrition bias. Twin Research and Human Genetics. 2009;12:158–168. doi: 10.1375/twin.12.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgersen S, Czajkowski N, Jacobson K, Reichborn-Kjennerud T, Roysamb E, Neale MC, et al. Dimensional representations of –DSM–IV cluster B personality disorders in a population-based sample of Norwegian twins: A multivariate study. Psychological Medicine. 2008;38:1617–1625. doi: 10.1017/S0033291708002924. [DOI] [PubMed] [Google Scholar]
- Torgersen S, Kringlen E, Cramer V. The prevalence of personality disorders in a community sample. Archives of General Psychiatry. 2001;58:590–596. doi: 10.1001/archpsyc.58.6.590. [DOI] [PubMed] [Google Scholar]
- Vachon DD, Sellbom M, Ryder AG, Miller JD, Bagby RM. A five-factor model description of depressive personality disorder. Journal of Personality Disorders. 2009;23:447–465. doi: 10.1521/pedi.2009.23.5.447. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA. On traits and temperament: General and specific factors of emotional experience and their relation to the five-factor model. Journal of Personality. 1992;60:441–476. doi: 10.1111/j.1467-6494.1992.tb00980.x. [DOI] [PubMed] [Google Scholar]