Summary
CD8+ T cell tolerance, although essential for preventing autoimmunity, poses substantial obstacles to eliciting immune responses to tumor antigens, which are generally over-expressed normal proteins. Developing effective strategies to overcome tolerance for clinical applications would benefit from elucidation of the immunologic mechanism(s) regulating T cell tolerance to self. To examine how tolerance is maintained in vivo, we engineered dual-T cell receptor (TCR) transgenic mice in which CD8+ T cells recognize two distinct antigens: a foreign viral-protein and a tolerizing self tumor-protein. Encounter with peripheral self-antigen rendered dual-TCR T cells tolerant to self, but these cells responded normally through the virus-specific TCR. Moreover, proliferation induced by virus rescued function of tolerized self tumor-reactive TCR, restoring anti-tumor activity. These studies demonstrate peripheral CD8+ T cell tolerance to self-proteins can be regulated at the level of the self-reactive TCR complex rather than by central cellular inactivation, and suggest an alternate strategy to enhance adoptive T cell immunotherapy.
Introduction
Immune-mediated destruction of normal tissues is limited by deletion of autoreactive lymphocytes during development. However, some self-reactive T cells evade thymic deletion (Danke et al., 2004; Huseby et al., 2001; Lo et al., 1988; Morahan et al., 1989; Ohlen et al., 2002; Steinman, 2001), making peripheral tolerance induction essential to prevent autoimmunity. Unfortunately, CD8+ T cell tolerance to self-antigens can interfere with generation of effective T cell responses to tumors, as many potentially targetable tumor-associated antigens are self-proteins aberrantly expressed (Pardoll, 2003). Therefore, mechanisms that maintain tolerance have implications for both autoimmune disease and tumor immunology.
In mice and humans, T cells commonly transcribe more than the two T cell receptor (TCR) chains required for one functional TCR, with such cells constituting nearly one third of peripheral T cells (Casanova et al., 1991; Padovan et al., 1993), although the number of cells actually expressing two MHC-restricted TCR (dual-TCR) on the cell surface is predictably lower (Hardardottir et al., 1995; Niederberger et al., 2003). Nevertheless, such dual-TCR T cells could contribute to autoimmunity if a self-reactive TCR evades the thymic deletion process and enters the periphery after thymic selection of a second nonself-reactive TCR (Hardardottir et al., 1995; Heath and Miller, 1993; Padovan et al., 1993; Zal et al., 1996). Although the self-reactive TCR should still receive tolerizing signals in the periphery, it is unclear if such dual-TCR CD8+ T cells would become tolerant to all subsequent TCR signals or remain responsive to signals delivered through the other TCR. Thus, the issue is where tolerance is controlled within a tolerized T cell- are downstream cellular pathways not activated due to deficient signaling by a tolerant dysfunctional TCR complex, or is a tolerant cell programmed to not respond to signaling from a potentially competent TCR? Some insights have been provided by analyses of in vitro responses. With CD4+ T cells expressing two class II-restricted receptors, antagonism of one TCR by in vitro stimulation with altered peptide ligands (APL) inhibited proliferation to subsequent stimulation via either TCR (Dittel et al., 1999; Robertson and Evavold, 1999; Yang and Grey, 2003), implying central tolerizing mechanisms and supported by reduced phosphorylation of proximal signaling components and accumulation of negative regulatory proteins at both TCR complexes (Dittel et al., 1999). In contrast, trans-inhibition in CD8+ T cells expressing two class I-restricted receptors after stimulation with APL has been controversial, observed with proliferation but not effector functions as the readout (Daniels et al., 1999; Gascoigne and Zal, 2004; Stotz et al., 1999; Yang and Grey, 2003). As proliferative defects are the hallmark of in vivo tolerized CD8+ T cells, which often retain effector functions (Ohlen et al., 2002; Tanchot et al., 1998; Teague et al., 2006), the absence of proliferation may be the more relevant observation. Thus these in vitro systems have suggested a central mechanism regulating tolerance within the cell, but generalizing results with in vitro APL systems to in vivo tolerance of self-proteins may be misleading. The only previous analysis of dual-TCR T cells tolerized in vivo evaluated CD4+ T cells expressing one TCR restricted to a non-expressed class II allele, and one class I-restricted TCR that signaled independent of CD8, making relevance to in vivo CD8+ T cell tolerance to self-proteins unclear (Hah et al., 2005).
How distinct TCR function in dual-TCR T cells in vivo has implications not only for autoimmunity and understanding how tolerance is mechanistically maintained in a cell with a single TCR, but also for tumor immunotherapy (Gladow et al., 2004; Heemskerk et al., 2004; Hughes et al., 2005; Morgan et al., 2006; Roszkowski et al., 2005; Stanislawski et al., 2001). T cells with an introduced second TCR are being evaluated as cellular reagents for treatment of human cancer, but substantial obstacles still remain (Morgan et al., 2006). By engineering a dual-TCR transgenic murine model, we have gained new insights into the immunologic mechanisms regulating T cell tolerance, and obtained evidence that tolerization of the self-reactive TCR does not disrupt signaling or function mediated via the second receptor, suggesting tolerance can be maintained proximally at the level of the self-reactive TCR.
Results
Activation of tolerant TCR transgenic CD8+ T cells
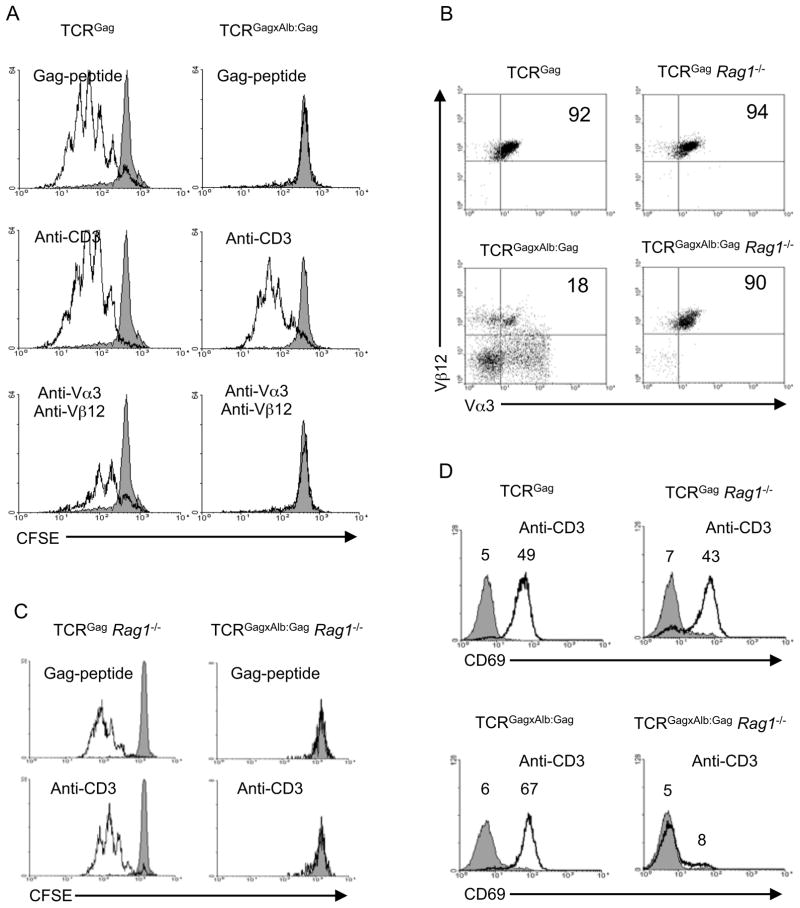
We previously described a tolerance model in which TCR transgenic (Tg) mice (TCRGag) expressing a Db-restricted Vα3Vβ12 receptor specific for the immunodominant FMuLVGag epitope were crossed with Tg mice (Alb:Gag) expressing FMuLVGag in the liver regulated by the albumin promoter (Ohlen et al., 2002). Peripheral CD8+ T cells in hybrid (TCRGagxAlb:Gag) mice exhibit attenuated TCR signaling and no proliferation in response to stimulation with Gag-antigen (Ohlen et al., 2002). This tolerant phenotype likely resulted from chronic encounter with antigen presented in the liver, as suggested by analysis of mature naive TCRGag T cells transferred into bone marrow chimeric Alb:Gag hosts, in which the cells that persisted in the periphery had become tolerant (Morimoto et al., 2007). To determine if increasing the strength of TCR signaling might overcome the defect, naïve TCRGag or tolerant TCRGagxAlb:Gag splenocytes were stimulated with not only Gag-peptide but also a potentially stronger signal with antibodies to the TCR complex. Naive T cells proliferated to Gag and anti-CD3 and not irrelevant Env-peptide, but tolerant CD8+ T cells, which failed to respond to Gag as previously reported (Ohlen et al., 2002; Teague et al., 2006), unexpectedly proliferated in response to anti-CD3 (Figure 1A). However, stimulation with Abs specific for the Vα3Vβ12 chains of the Gag-specific TCR, which induced proliferation of naïve T cells, failed to induce tolerant T cell proliferation (Figure 1A). These outcomes suggest either: i) the stimulus mediated by the Tg TCR complex via ligation with anti-CD3 is sufficiently stronger than signals generated by directly engaging the tolerant αβTCR chains to induce a response, ii) the proliferation with anti-CD3 reflects activation of a fraction of non-tolerant CD8+ T cells present in spleens of TCRGagxAlb:Gag mice expressing TCR chains other than the Tg Vα3Vβ12 (Figure 1B), or iii) anti-CD3 is triggering tolerized cells expressing Tg Vα3Vβ12 chains but is acting on endogenous non-Tg receptors also expressed by tolerant CD8+ T cells (i.e. dual-TCR). To address these possibilities, TCRGag and TCRGagxAlb:Gag mice were bred onto a Rag1−/− background (Figure 1B), preventing expression of endogenous non-Tg TCR chains which require gene rearrangement. Rag1−/− TCRGag and TCRGagxAlb:Gag T cells responded similar to Rag1+/+ counterparts after stimulation with Gag-peptide (Figure 1C), but Rag1−/− tolerant TCRGagxAlb:Gag-CD8+ T cells failed to proliferate to anti-CD3 (Figure 1C). Thus, proliferation of tolerant Rag1+/+ TCRGagxAlb:Gag T cells induced by anti-CD3 could not reflect triggering of the tolerized TCR by a more potent stimulus.
Figure 1. Activation of tolerant CD8+ T cells with anti-CD3.
(A) CFSE-dilution in CD8-gated naïve and tolerant splenocytes from TCRGag and TCRGagxAlb:Gag mice stimulated with an irrelevant Env (grey) or the Gag-peptide, anti-CD3, or antibody to Vα3 and Vβ12 (black line) for 3 days in vitro. (B) Splenocytes from Rag1+/+ and Rag1−/− TCRGag and TCRGagxAlb:Gag mice were analyzed for TCR expression by flow cytometry. Dot plots represent CD8-gated cells, and the percentage of Vα3+Vβ12+ T cells within the CD8+ population is indicated (Data representative of >10 mice from each genotype). (C) CFSE-dilution in CD8-gated Rag1−/− TCRGag and TCRGagxAlb:Gag splenocytes stimulated with Env (grey), Gag, or anti-CD3 (black line) for 3 days in vitro. (D) Staining with anti-CD69 (black line) or isotype control antibody (grey), was analyzed on CD4-depleted Vα3+Vβ12+ Rag1+/+ or Rag1−/− TCRGag and TCRGagxAlb:Gag splenocytes 5 hours after stimulation with anti-CD3. The mean fluorescence intensity (MFI) for each peak is provided and data are representative of 4 separate experiments.
To more directly determine if anti-CD3 stimulation was activating tolerant T cells via a second endogenously encoded non-tolerized receptor, CD69 expression was assessed after a 5 hour in vitro stimulation, a time point when cells could still be gated for expression of both Vα3 and Vβ12. Both Rag1+/+ and Rag1−/− naïve TCRGag T cells upregulated CD69 after stimulation with anti-CD3 (Figure 1D, top panels). Tolerant Rag1+/+ TCRGagxAlb:Gag T cells upregulated CD69 with anti-CD3 similar to naïve TCRGag T cells (Figure 1D, lower left panel), but tolerant Rag1−/− TCRGagxAlb:Gag T cells failed to respond to anti-CD3 (Figure 1D, lower right). Thus, on the Rag1+/+ background, a large fraction of tolerant self-reactive CD8+ T cells express additional endogenous TCR chains capable of transducing activation signals not delivered after ligation of the tolerogen-specific receptor, suggesting TCR complexes that have not engaged a tolerogen may remain functional in a tolerized cell.
Analysis of tolerance in CD8+ T cells expressing defined dual-TCR
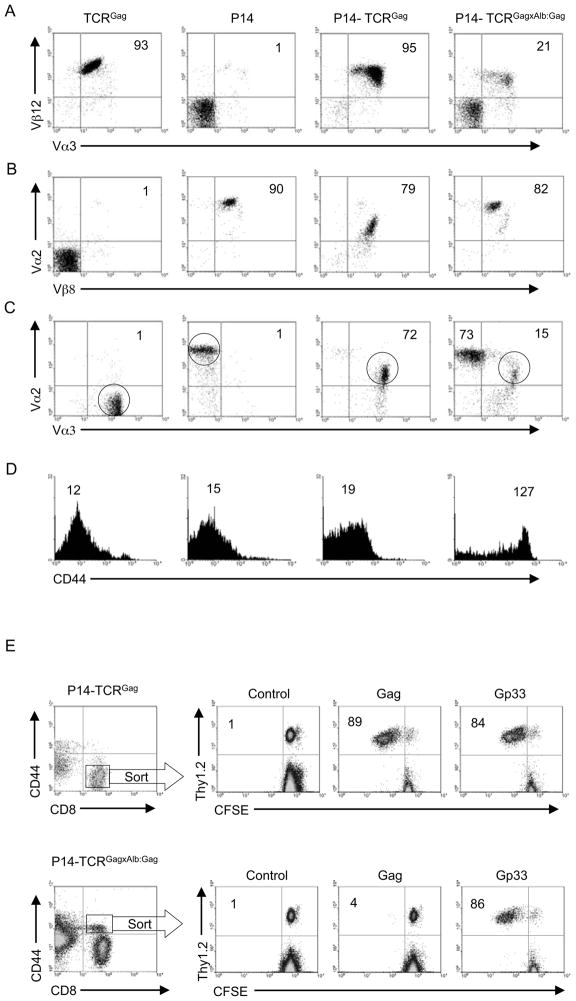
These results raised fundamental questions of how the non-responsive phenotype is being maintained in a tolerized T cell, and how previously tolerized self-reactive T cells that happen to express a second TCR might behave if a natural ligand for the second TCR is encountered in the periphery. To examine these issues further, it was necessary to develop a dual-TCR murine model in which both expressed TCR had known specificities. Therefore, TCRGag and TCRGagxAlb:Gag mice were crossed with P14 mice, which bear a TCR specific for the Db-restricted Gp33 epitope of lymphocytic choriomeningitis virus (LCMV). The resulting transgenic progeny again appeared healthy with no evidence of liver pathology, and contained similar total splenocyte numbers to the parental mice. P14-TCRGagxAlb:Gag mice had somewhat fewer total spleen CD8+ cells at 10.6±1.9×106 (±standard deviation for 3 mice per group) compared to P14-TCRGag mice at 17.4±2.3×106, likely the result of central and peripheral deletion of self-reactive CD8+ T cells as previously observed in single-receptor TCRGagxAlb:Gag mice (Ohlen et al., 2002). In mice in which both TCR were naïve (P14-TCRGag), 95% of CD8+ splenocytes expressed the Vα3Vβ12 chains of the TCRGag receptor (Figure 2A) and 79% expressed the Vα2Vβ8 chains of the P14 receptor (Figure 2B). The Gag-epitope with 3 cysteines is highly hydrophobic, and it has not proven possible to produce a tetramer stable in solution - therefore co-expression of both TCR was assessed by staining for the two Vα or Vβ chains. 72% of cells co-expressed Vα2 and Vα3 (Figure 2C), and more than 80% co-expressed Vβ8 and Vβ12 (data not shown), demonstrating the majority of cells have both specificities. These cells were CD44low, similar to naive single-receptor TCRGag and P14 T cells (Figure 2D). As described in single-receptor tolerant TCRGagxAlb:Gag mice (Ohlen et al., 2002), P14-TCRGagxAlb:Gag mice also exhibited a decreased frequency (21%) of Vα3+Vβ12+ Gag-specific CD8+ T cells compared to P14-TCRGag not expressing Gag as a self-protein (Figure 2A), attributable to partial deletion of these self-reactive cells in the thymus and/or periphery (Morimoto et al., 2007; Ohlen et al., 2002). By contrast, peripheral expression of Gag had no marked impact on the percentage of cells expressing the Vα2Vβ8 chains of the P14 receptor (Figure 2B). The decreased frequency of Vα3+Vβ12+ T cells resulted in reduced (15%) dual-TCR T cells expressing high amounts of both Vα2 and Vα3 (Figure 2C), but these cells were uniformly identifiable as a CD44hi population (Figure 2D), consistent with previous antigen encounter through the Gag-specific TCR (Ohlen et al., 2002). P14-TCRGagxAlb:Gag mice also possessed a population (73%) of apparently “single-receptor” Vα2+ T cells lacking high amounts of Vα3 (Figure 2C). Such cells were uniformly in the CD44lo compartment, expressed high levels of Vα2 Vβ8 (P14) chains and very low Vα3 Vβ12 (Figure S1), suggesting their naive phenotype resulted from failure to express sufficient Gag-reactive TCR to respond to the self-protein. The frequency (15%) of Vα2+Vα3+CD44hi dual-TCR T cells in tolerizing mice was sufficient for isolation and further analysis of the tolerant population. Sorting of cells based on CD44 and CD8 expression yielded highly pure populations of naïve and tolerant T cells (Figure S1). Thus, to avoid activation and receptor down-modulation with antibodies specific for Vα and Vβ chains during purification, tolerant cells from P14-TCRGagxAlb:Gag mice in subsequent analysis were sorted based on the CD8+CD44hi phenotype.
Figure 2. Phenotype of single and dual-TCR CD8+ T cells.
Splenocytes from TCRGag, P14 and the F1 naive (P14-TCRGag) and tolerant (P14-TCRGagxAlb:Gag) dual-TCR progeny were gated on CD8+ cells and analyzed for expression of (A) Vα3 and Vβ12 (TCRGag), or (B) Vα2 and Vβ8 (P14), or (C) both sets of TCRα chains (Vα2 and Vα3). The percent of double-positive CD8+ cells is inset. (D) Single and dual-receptor CD8+ T cells (circled in 2c) were analyzed for CD44 expression. Inset numbers represent MFI, and data are representative of more than 10 mice from each genotype. (E) Transgenic P14-TCRGag and P14-TCRGagxAlb:Gag splenocytes (Thy1.2) were FACS sorted based on CD44 and CD8 expression (within rectangle regions). Sorted T cells were combined 1:10 with congenic APC from a Thy1.1+ mouse, labeled with CFSE and stimulated with control, Gag or Gp33 peptide for 96 hours in vitro, and CFSE-dilution in Thy1.2+ T cells assessed. The percent of Thy1.2+ cells that had diluted CFSE is indicated. Data are representative of 3 experiments.
Activation of tolerant dual-TCR T cells
To investigate the function of each of these known TCR, proliferation of purified dual-TCR T cells was assessed in response to specific antigen. Naïve CD44loCD8+ P14-TCRGag T cells and tolerant CD44hiCD8+ P14-TCRGagxAlb:Gag T cells were FACS sorted, mixed at 1:10 with congenic splenocyte antigen presenting cells (APC), labeled with 5-(and-6-)-carboxyfluorescein diacetate succinimidyl ester (CFSE), and stimulated with saturating antigen concentrations (1 μg/ml) of either control, Gag, or Gp33 peptide. Naïve P14-TCRGag T cells exhibited similar proliferative responses to Gag and Gp33, with 89% and 84% of cells respectively diluting CFSE, suggesting relatively equivalent signaling from both receptors at this antigen dose (Figure 2E). Although tolerant dual-receptor P14-TCRGagxAlb:Gag T cells failed to appreciably dilute CFSE in response to Gag, suggesting a uniformly tolerant phenotype in this sorted population, robust proliferation (86%) was observed after stimulation with Gp33 (Figure 2E). Thus, the inability of a cell to respond through a tolerized TCR did not preclude activation through a second expressed receptor, suggesting tolerance is being regulated at the site of the assembled TCR complex.
Although tolerant P14-TCRGagxAlb:Gag T cells expressed the Vα3 and Vβ12 chains required for Gag recognition, mismatched TCR chain pairing could reduce expression of Gag-reactive TCR and contribute to the failure to proliferate to stimulation with Gag. Therefore, relative expression of the Gag-specific TCR in naïve and tolerant dual-TCR cells was determined by assessing TCR down-modulation after peptide stimulation, an approach previously demonstrated to induce αβTCR chain endocytosis in an antigen-specific manner (Gladow et al., 2004; Valitutti et al., 1995). Naive P14-TCRGag and tolerant P14-TCRGagxAlb:Gag T cells down-modulated Vα3 and Vβ12 chains similarly after stimulation with Gag (Figure S2), suggesting equivalent amounts of Vβ12 were appropriately paired with Vα3 on both cell types. Down-modulation of Gag-reactive TCR chains was specific, as Vα2 and Vβ8 (P14 receptor) expression was unchanged after Gag stimulation (Figure S2), but was down-modulated after Gp33 stimulation (data not shown). Thus, the tolerant phenotype does not result from lack of appropriately paired TCR chains capable of recognizing Gag.
Tolerant TCR complexes selectively exhibit proximal signaling defects
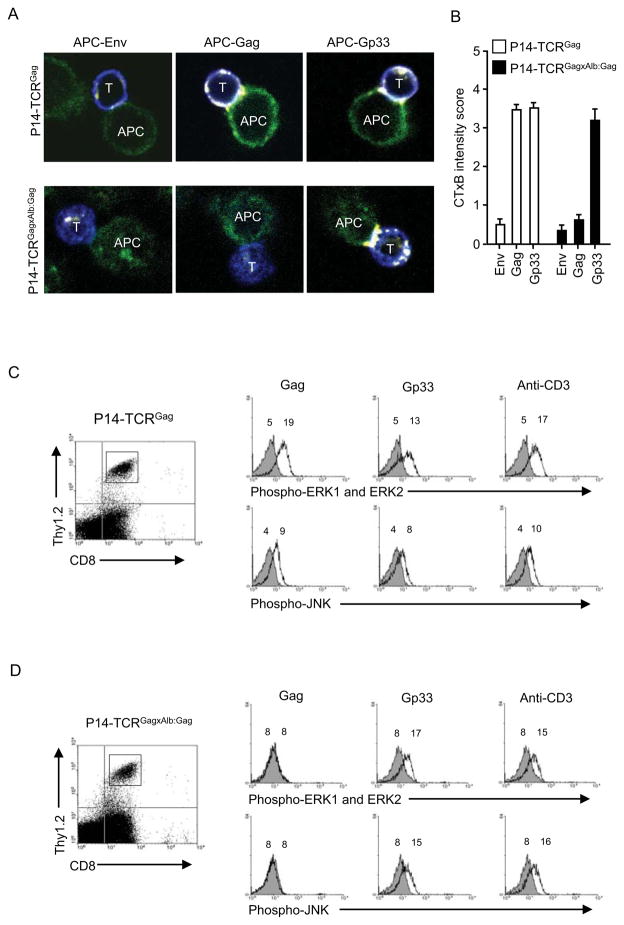
We previously demonstrated that tolerant CD8+ T cells fail to aggregate lipid rafts and form an immunologic synapse upon encounter with APC (Teague et al., 2006). To determine if this reflected an acquired global cellular defect or a property of the tolerized TCR complex, we investigated lipid raft accumulation in dual-TCR cells at the point of T cell:APC contact. FACS-sorted naive CD8+CD44lo and tolerant CD8+CD44hi dual-TCR T cells were incubated with peptide-pulsed E10 tumor cells as APC, stained with labeled Cholera Toxin B-subunit (CTxB) which specifically binds the raft marker monosialoganglioside GM1 (Janes et al., 1999; Janes et al., 2000; Rouquette-Jazdanian et al., 2005), and lipid raft aggregation visualized by confocal microscopy of T cell:APC conjugates (Figure 3A). Images of 40 conjugates from each group (240 total images) were graded for CTxB staining on a 0–5 scale, with 5 being most intense, and each set of 40 scores averaged (Figure 3B). Naïve P14-TCRGag CD8+ T cells efficiently aggregated rafts upon encountering either Gag+ or Gp33+ APC, but not APC pulsed with irrelevant Env peptide (Figure 3A). Tolerant P14-TCRGagxAlb:Gag T cells failed to aggregate lipid rafts with Gag+ APC, but did with Gp33+ APC (Figure 3A). Thus, the tolerant TCR seems selectively unable to mobilize synapse formation.
Figure 3. Distinct signaling through tolerant and naïve TCR.
(A) P14-TCRGag and P14-TCRGagxAlb:Gag splenocytes were sorted based on CD44 and CD8 expression. CD8+ T cells (blue) were stained for CTxB (yellow to white) after 30 min encounter with peptide-pulsed APC (green) and visualized by confocal microscopy, (B) and graded 0–5 for CTxB staining intensity. Average scores from 3 experiments with P14-TCRGag (open bars) and P14-TCRGagxAlb:Gag (filled bars) cells are presented with standard error of the mean. (C) Sorted naive P14-TCRGag and (D) tolerant P14-TCRGagxAlb:Gag T cells (Thy1.2) were combined 1:10 with APC form a congenic Thy1.1+ mouse, and stimulated with control (grey filled), Gag or Gp33-peptide (black lines) or anti-CD3 (black lines) in vitro. Phosphorylation of ERK and JNK in cells gated for Thy1.2 and CD8 expression (left) was assessed after 30 min by intracellular staining with phospho-specific antibodies. MFI values for each peak are inset, and data are representative of 4 separate experiments.
As antigen-specific TCR complexes appear to function independently in dual-TCR T cells, signals transduced from competent and tolerant TCR should be discernable. We previously demonstrated tolerant CD8+ T cells are defective in phosphorylation of ERK and JNK kinases after antigen stimulation (Ohlen et al., 2002). To determine if these signaling defects reflect events intimate to only tolerized TCR complexes or a more global compromise of downstream signaling components, purified naïve and tolerant T cells were stimulated with Gag or Gp33 (Figures 3C and 3D; solid lines) or an irrelevant control peptide (grey filled) presented by APC from Thy1.1 congenic mice, and responses in Thy1.2-gated cells assessed. As a control for maximal detectable responses, cells were stimulated with anti-CD3, which phosphorylated ERK and JNK by 30 minutes in both T cell subsets. Naive P14-TCRGag T cells phosphorylated ERK and JNK in response to both Gag and Gp33-antigen (Figure 3C). By contrast, tolerant P14-TCRGagxAlb:Gag T cells failed to phosphorylate either ERK or JNK in response to Gag, but did after Gp33 stimulation (Figure 3D, and pooled data from 3 separate experiments in Figure S3).
To determine if this phenomenon of TCR-specific tolerance in dual-TCR cells was unique to the Gag-reactive TCR, naive P14-TCRGag mice were alternatively tolerized in vivo through the P14-receptor by repeated injection of high doses of Gp33-peptide in incomplete Freund’s adjuvant (IFA) as previously described (Aichele et al., 1995). FACS sorted CD8+CD44hi T cells from IFA-Gp33-treated mice responded normally to Gag but exhibited blunted responsiveness to Gp33 (63% failed to dilute CFSE), suggesting the majority of cells under these tolerizing conditions had alternatively selectively tolerized the Gp33-specific TCR (Figure S4).
Rescue of tolerant TCR antigen responsiveness
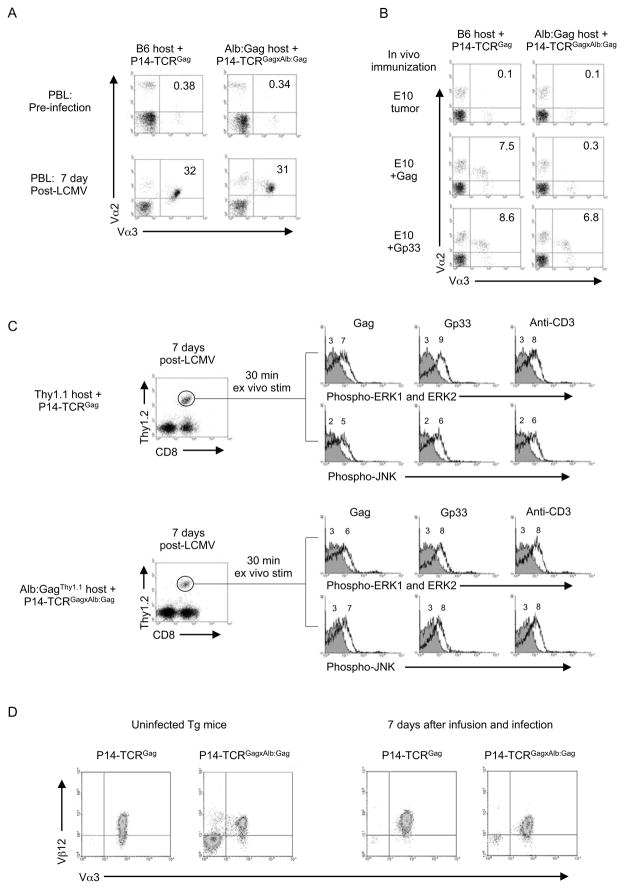
Tolerant CD8+ T cells have been rescued from the tolerant state in vitro by inducing proliferation with high doses of cytokines (Teague et al., 2006). To determine if similar rescue might occur in vivo in dual-TCR T cells if the competent non-tolerant TCR encountered its antigen, the effect of in vivo LCMV infection was examined. 1×105 sorted naive or tolerant dual-TCR T cells were transferred respectively into B6 or Alb:Gag mice and recipients infected with LCMV. Seven days after infection, similar extensive expansion of naive and tolerant Vα2+Vα3+ dual-receptor CD8+ T cells was detected in the blood of respective hosts (Figure 4A), as well as in the spleens (23.1% of 59.8±0.9×106 and 21.4% of 57.4±4.7×106 total CD8+ cells respectively with 3 mice/group). Proliferation required stimulation through the P14 receptor, because alternative immunization with Gag peptide-pulsed E10 cells failed to induce expansion of transferred tolerant dual-TCR T cells (Figure 4B), although these cells expanded to E10 cells pulsed with Gp33-peptide similar to the proliferative response of naïve T cells immunized with either Gag or Gp33-peptide. The absence of Gag responsiveness from sorted tolerant dual-TCR T cells confirmed the infused cells were uniformly tolerant, and the observed expansion to Gp33 was not the result of activation of a contaminating population of non-tolerant cells.
Figure 4. In vivo expansion and rescue of tolerant dual-TCR T cells.
Transgenic naive P14-TCRGag and tolerant P14-TCRGagxAlb:Gag splenocytes were FACS sorted based on CD44 and CD8 expression, and 1×105 sorted T cells transferred into B6 or Alb:Gag recipients. Recipient mice were immunized with either (A) LCMV or (B) peptide-pulsed irradiated E10 cells. Inset numbers are percent of total CD8+ cells from PBL at day 7 post-immunization. (C) 1×105 FACS sorted naive P14-TCRGag and tolerant P14-TCRGagxAlb:Gag T cells were transferred into Thy1.1 or Alb:GagThy1.1 recipients and infected with LCMV for 7 days. Recipient splenocytes were stimulated ex vivo with control Env (grey filled), Gag or Gp33-peptide, or anti-CD3 (black lines), and phosphorylation of ERK and JNK in cells gated for CD8 and Thy.1.2 expression (circled in dot plots on the left) assessed after 30 min. The MFI for each peak is inset. (D) Dual-TCR T cells were analyzed for relative TCR expression directly ex vivo from non-infected mice or after CD8 and CD44 FACS sorting and transfer into B6 or Alb:Gag recipients and infection with LCMV for 7 days. Splenocytes were CD4-depleted and expression of Vα3 and Vβ12 assessed on cells gated for Vα2 expression by flow cytometry. Data are representative of 3 separate experiments.
To determine if the induced in vivo proliferation of tolerant dual-receptor T cells rescued function, antigen responsiveness was analyzed ex vivo after 7-day LCMV infection. As most responding cells at this time point are entering a programmed contraction phase and poised to undergo apoptosis (Blattman et al., 2003; Grayson et al., 2002), analysis of proliferative responses ex vivo was not feasible. Therefore dual-TCR T cells were analyzed for early signaling events that distinguish a tolerant TCR– ERK and JNK phosphorylation 30 min after ex vivo stimulation. 1×105 sorted naive or tolerant dual-TCR T cells (Thy1.2) were transferred into normal B6-Thy1.1 or Alb:GagThy1.1 recipients respectively, infected with LCMV, and Thy1.2+CD8+ T cells examined ex vivo 7 days later. In contrast to tolerant P14-TCRGagxAlb:Gag T cells from non-infected mice (Figure 3D), expanded P14-TCRGagxAlb:Gag T cells phosphorylated ERK and JNK after stimulation with either Gag or Gp33, similar to transferred non-tolerant P14-TCRGag T cells (Figure 4C and Fig S3). Such rescued cells expressed similar amounts of surface Vα3 and Vβ12 as naïve and tolerant dual-TCR cells analyzed from non-infected mice (Figure 4D). Thus, restoration of Gag-responsiveness did not reflect quantitative changes in Gag-specific TCR expression.
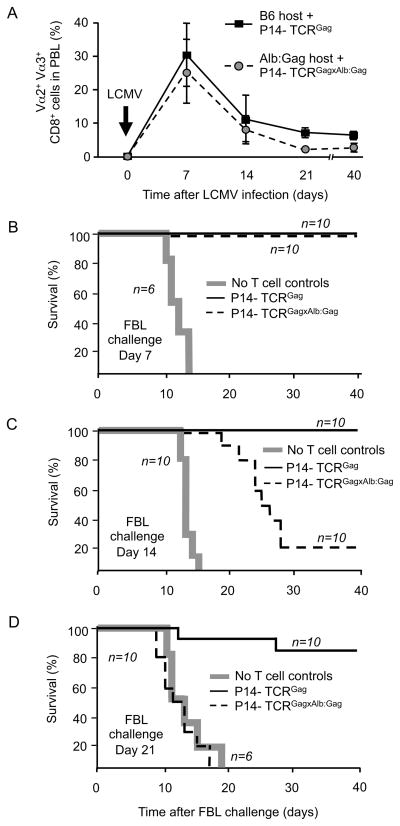
To examine if such restoration of signaling detected in vitro in previously tolerant cells also improved T cell function in vivo, 1×105 sorted naive or tolerant dual-TCR T cells were again transferred into B6 or Alb:Gag mice respectively. Recipients were infected with LCMV and challenged with 1×106 live Gag+ FBL tumor cells at 7, 14 or 21 days after infection, representing the peak, contraction, and stable memory phases of T cell activation, respectively (Antia et al., 2005) (Figure 5A). At day 7, both naive and tolerant dual-TCR T cell populations had similarly expanded, and all recipients (10/10 per group) resisted tumor challenge, surviving beyond 40 days of observation (Figure 5B), whereas control mice receiving either no T cells or T cells but not infected, developed fatal tumors by day 15 (Figure 5B and data not shown). Lytic activity of expanded naive and tolerant dual-TCR T cells was specific and nearly equivalent, as LCMV-infected mice that had received 1×105 sorted naive or tolerant cells specifically eliminated CFSE-labeled target cells pulsed with Gag peptide infused on day 7 (Figure S5). No autoimmune liver disease was detected in mice after infection and expansion of Gag-reactive T cells (data not shown), consistent with the resistance of hepatic cells to T cell-mediated damage previously observed in this model (Morimoto et al., 2007; Ohlen et al., 2002; Teague et al., 2006).
Figure 5. In vivo expansion and anti-FBL effector function by dual-TCR T cells.
1×105 sorted naive P14-TCRGag or tolerant P14-TCRGagxAlb:Gag T cells were transferred into B6 or Alb:Gag recipients respectively, and infected with LCMV. (A) At 7, 14, 21 and 40 days after infection, recipient PBL were analyzed for Vα2+Vα3+CD8+ T cells. Error bars are standard deviation of 20 mice per group from 4 separate experiments. Recipients were challenged with live FBL tumor (B) 7 days, (C) 14 days and (D) 21 days after LCMV infection. Survival was monitored for 40 days post-tumor challenge and each graph represents pooled data from the indicated number of mice (n) per group from 2 separate experiments.
At 14 days after infection, both naive and tolerant dual-TCR T cell populations had contracted comparably (Figure 5A). However, Alb:Gag recipients of tolerant P14-TCRGagxAlb:Gag T cells were less resistant to tumor challenge (2/10 survived long-term) than either B6 recipients of naive P14-TCRGag T cells (10/10) (Figure 5C), or Alb:Gag recipients that had been challenged on day 7 (10/10) (Figure 5B). Nonetheless, Alb:Gag recipients challenged on day 14 still demonstrated prolonged median survival (25 days) and 20% long-term survival, compared to control mice receiving no T cells with a median survival of 12 days and death from progressive tumor by day 16 (Figure 5C). The anti-tumor activity observed at day 7 and 14 post-LCMV cannot be explained simply by expanded cell numbers, because with the dramatic contraction of spleen size evidenced by day 14 after infection, as previously reported (Cheng and Greenberg, 2002; Lohman et al., 1996), the total number of dual-TCR T cells had declined to numbers smaller than detected in unimmunized P14-TCRGagxAlb:Gag mice (Figure 2), which had no ability to resist viable FBL tumor challenge (data not shown).
The frequency of Vα2+Vα3+ T cells at day 21 was modestly higher (~2-fold) in recipients of naive dual-TCR T cells compared to recipients of tolerant cells, but both T cell populations remained detectable at stable amounts out to at least day 40 (Figure 5A). After challenge with FBL on day 21 post-infection, 8/10 recipients of naïve P14-TCRGag T cells were protected (Figure 5D). In contrast, survival in recipients of tolerant P14-TCRGagxAlb:Gag T cells was similar to mice receiving no T cells (Figure 5D). These data suggest the activation and proliferation of tolerant dual-TCR T cells induced through the LCMV-specific TCR only transiently restored responsiveness to Gag, with the tolerant phenotype ultimately reacquired.
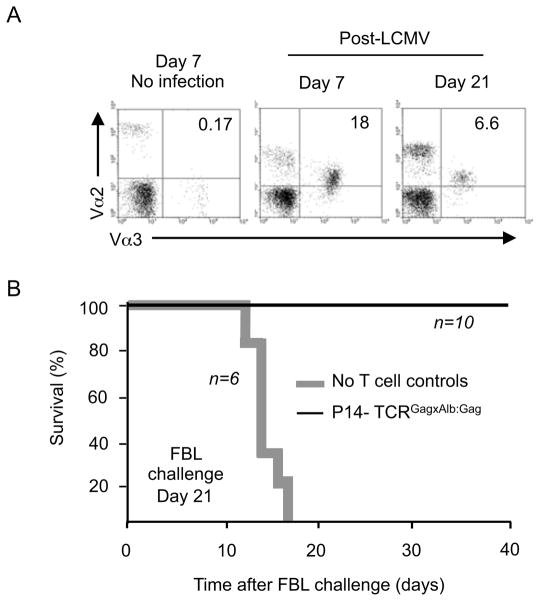
To determine if the apparent reacquisition of tolerance represented an intrinsic cellular defect or a consequence of the tolerizing Alb:Gag environment, 1×105 FACS sorted tolerant P14-TCRGagxAlb:Gag T cells were transferred into normal B6 hosts (lacking peripheral Gag-antigen) followed by LCMV infection and later challenge with FBL. Analysis at 7 and 21 days after infection revealed that Vα2+Vα3+ dual-TCR T cells had expanded and then contracted (Figure 6A). On day 21 after LCMV infection, mice were challenged with FBL tumor as previously described, and survival assessed. Control mice receiving no T cells died of progressive tumor by day 18, but all recipients of tolerant dual-TCR T cells survived (Figure 6B). Thus, the reduced anti-FBL activity of tolerant P14-TCRGagxAlb:Gag T cells observed in Alb:Gag hosts at day 14 and complete absence by day 21 after infection (Figures 5C and 5D) likely reflected reacquisition of tolerance due to recurrent encounter with tolerogen in the periphery.
Figure 6. In vivo expansion and anti-FBL effector function in the absence of tolerizing peripheral antigen.
(A) The frequency of Vα2+Vα3+CD8+ cells in PBL from B6 recipients of 1×105 FACS sorted CD44hi CD8+ tolerant P14-TCRGagxAlb:Gag T cells was assessed at days 7 and 21 after LCMV infection (or no infection control recipients). (B) B6 mice receiving 1×105 sorted tolerant P14-TCRGagxAlb:Gag T cells (black line) or no T cell controls (bold grey line) were challenged with 1×106 live FBL tumor by i.p injection 21 days after LCMV infection. Survival was monitored for 40 days and the graph represents pooled data from the indicated number of mice (n) per experimental group from 2 independent experiments.
Repeated T cell rescue after reacquisition of tolerance
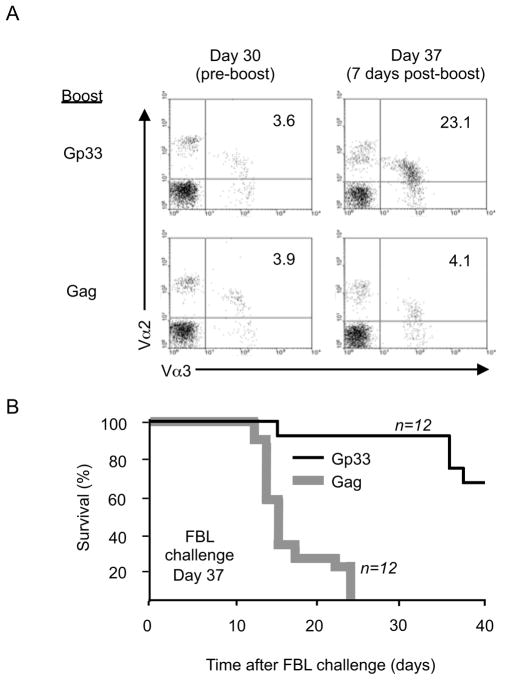
The observation that tolerant CD8+ T cells recognizing a self tumor-antigen can be activated and expanded via a second TCR to mediate anti-tumor activity, even transiently, suggests that employing dual-TCR T cells in adoptive therapy in which a tumor-reactive TCR has been intentionally introduced into a cell with known specificity might have therapeutic potential. To determine if periodically restimulating such cells in a tolerizing environment by immunization through the non-tolerized TCR might provide a means to sustain donor T cell efficacy, Alb:Gag host mice received 1×105 FACS sorted tolerant P14-TCRGagxAlb:Gag T cells followed by primary infection with LCMV. At day 30, after these cells had demonstrated reacquisition of the tolerant phenotype and no longer protected against tumor challenge (Figure 5D), mice were boosted by injection of either Gag or Gp33 peptide-pulsed B6 splenocytes. Analysis of PBL 7 days later revealed marked expansion of Vα2+Vα3+ dual-TCR CD8+ T cells if boosted with APC presenting Gp33 but not Gag (Figure 7A). If challenged with FBL tumor at day 7 after boosting, 8/12 mice immunized with Gp33 survived beyond 40 days, whereas mice immunized with Gag had a median survival of 15 days and all died from progressive tumor (Figure 7B). The cells which provided protection after proliferation induced by boosting with Gp33 expressed similar amounts of the Vα3 TCR-chain from the Gag-specific TCR as cells remaining non-responsive after boosting with Gag (Figure 7A), suggesting functional recovery reflected qualitative not quantitative alterations in the Gag-specific TCR complex.
Figure 7. Restimulation of contracted and tolerant dual-TCR T cells within the tolerizing environment.
(A) Vα2+Vα3+CD8+ cells from Alb:Gag recipients of 1×105 FACS sorted CD44hi CD8+ tolerant P14-TCRGagxAlb:Gag T cells were assessed 30 days after LCMV infection, and 7 days after secondary boost immunization (day 37) with either Gag or Gp33 peptide-pulsed B6 splenocytes. (B) At day 37 (7 days post-boost), mice were challenged with live FBL tumor and survival monitored for 40 days. The graph represents pooled data from the indicated number of mice (n) per experimental group from 3 independent experiments.
The results of these and the previous experiments suggest that the tolerant TCR complex in dual-TCR T cells must be molecularly or biochemically distinct from the functional complex. Initial efforts to dissect this have revealed that the tolerant complex is structurally less stable than functional complexes, as evidenced by a markedly reduced ability to co-precipitate CD3ε and CD3ζ with the tolerant Vβ12-containing complexes compared to the Vβ8 complexes (Figure S6). This did not reflect proportionally reduced surface expression of CD3ε or total cellular CD3ζ, as clearly demonstrated by studying tolerant Rag1−/− single-TCR T cells expressing only the tolerant TCR complex. This selective instability of tolerant TCR complexes likely contributes to the dysfunction and inability to mobilize synapse formation, but complicates efforts to definitively identify, characterize, and compare the components of the tolerant complex.
Discussion
The generation of an effective CD8+ T cell response generally requires specific CD8+ T cells expand after recognition of antigen– a capacity usually lost by tolerant T cells (Ohlen et al., 2002; Teague et al., 2006). However, how this non-responsive phenotype is maintained in tolerized T cells for subsequent encounters with cognate antigen remains unclear. Our initial analysis of tolerant self-reactive CD8+ T cells suggested that concurrently expressed endogenous TCR which are not engaged might remain functional in such tolerant cells, and our subsequent studies with dual-TCR T cells expressing two known distinct TCR complexes demonstrated that tolerant dual-TCR T cells can be induced to proliferate by triggering a competent TCR signaling cascade accessible to the nonself-reactive receptor. Such signaling was not observed after engagement of the tolerized TCR, which displayed defective phosphorylation of downstream molecules and an inability to mobilize lipid rafts to form a synapse. Compromised proximal and distal TCR signaling events have previously been assigned a role in maintenance of T cell tolerance (Chiodetti et al., 2006; Murtaza et al., 2001; Ohlen et al., 2002; Tanchot et al., 1998), particularly in CD4+ T cells tolerized to endogenous peripherally-expressed proteins with signaling deficiencies such as ERK phosphorylation implicated in tuning the activation threshold, but the defects have generally been perceived to reflect global cellular changes (Grossman and Paul, 1992; Singh and Schwartz, 2003). Such adaptive tolerance to endogenous proteins bears similarities to our model. However, in the tolerance induced in vivo by encounter with a Class I-restricted self-protein, CD8+ T cells can co-express autonomously functioning non-tolerant class I-restricted TCR complexes, suggesting such adaptive tolerance may not be cell intrinsic but regulated at the TCR. This in vivo tolerance differs from inferences provided by studying in vitro tolerogenic signals delivered by antagonist peptides, which demonstrated TCR trans-inhibition in dual TCR cells (Dittel et al., 1999; Robertson and Evavold, 1999; Yang and Grey, 2003). This disparity likely reflects fundamental differences in how tolerance has been induced or perhaps more importantly maintained long-term in a viable tolerized cell.
Consistent with tolerance being regulated by control of proximal signaling events initiated at the tolerized TCR complex, stimulating the TCR specific for a foreign protein resulted in a normal signaling cascade and proliferative response. This result implies that the assembled complexes of tolerant and functional TCR must differ, presumably as a result of selective modifications of TCR complex components and/or by the addition of inhibitory or removal of activating molecules. Analysis of tolerant TCR complexes indeed demonstrated that the integrity of these complexes was compromised, as reflected by impaired association of the critical signaling components, CD3ε and CD3ζ, with the TCR chains. Instability of tolerant TCR complexes has previously been observed in an alternative in vivo model of CD8+ T cell tolerance to a self-antigen (Guillaume et al., 2003), and our results suggest that instability can specifically and selectively target the TCR complexes that have been engaged by the tolerogen. Impaired interactions of TCR complex molecules with each other could impact the ability to cross-phosphorylate molecules, propagate signals, and engage cytoskeletal elements to form a synapse (Gil et al., 2002), but will also confound efforts to definitively characterize the molecules associated with the complex.
Rescue of function of the tolerant TCR by signaling through the functional TCR likely is a consequence of proliferation, as we and others have previously demonstrated that inducing proliferation of anergic T cells via stimulation with pharmacologic doses of cytokines can also restore antigen responsiveness (DeSilva et al., 1991; Teague et al., 2006). Thus, the modifications of tolerant TCR complexes responsible for maintaining the non-responsive phenotype are presumably reset during proliferation in which large numbers of new TCR complexes lacking the induced inhibitory changes are assembled and expressed, enabling competent TCR signaling and function upon subsequent antigen encounter. Such restoration of function appeared stable in the absence of further tolerizing signals, consistent with studies demonstrating function can be rescued by removal from the tolerizing environment (Ohlen et al., 2002; Ramsdell and Fowlkes, 1992; Rocha et al., 1993), but the observed reacquisition of tolerance in cells remaining in the tolerizing environment suggests tolerance in vivo is actively maintained by repeated encounters with the tolerogen. Although some studies have suggested reacquisition of tolerance may lead to a more pronounced state of non-responsiveness (Tanchot et al., 2001), which could conceivably limit the number of times tolerant T cells can be effectively rescued, our data suggests cells can clearly be rescued more than once.
Our observation that T cell tolerance is regulated at the level of self-reactive TCR complexes not only provides insights into the mechanisms regulating T cell tolerance but also has therapeutic implications, as the transfer of genes encoding tumor antigen-specific αβTCR into normal T cells expressing endogenous receptors is now being pursued as a strategy to generate tumor-reactive T cells for adoptive immunotherapy (Gladow et al., 2004; Heemskerk et al., 2004; Hughes et al., 2005; Morgan et al., 2006; Roszkowski et al., 2005; Stanislawski et al., 2001). Infusion of T cells expressing TCR capable of recognizing tumor antigens and mediating anti-tumor effector function does not always translate into beneficial clinical responses, and one potential obstacle is that in vivo encounter with candidate tumor antigens, which are mostly self-proteins, might be tolerogenic. Moreover, factors present in the tumor microenvironment may tolerized or anergize reactive T cells after infusion into patients (Gabrilovich, 2004; Kusmartsev et al., 2005; Pardoll, 2003). In vitro experiments have suggested that expressing a TCR specific for a tumor-associated antigen in a T cell expressing an endogenous TCR specific for a known foreign antigen such as a virus might provide a means to more efficiently expand anti-tumor responses (Heemskerk et al., 2004). Our data suggest extending this approach to periodically deliver appropriate activation signals to dual-TCR cells via a TCR not reactive with a tumor-antigen might also rescue infused T cells that have become non-responsive in vivo.
Experimental procedures
Mice
Alb:Gag, TCRGag (naive) and TCRGagxAlb:Gag (tolerant) mice have previously been described (Ohlen et al., 2002; Ohlen et al., 2001). C57BL/6 (B6), Thy1.1, and B6-Rag1−/− mice were purchased from Jackson Labs. P14 mice were a gift from Dr. Kaja Murali-Krishna (University of Washington), and dual-TCR mice were generated by crossing TCRGag and TCRAlb:Gag mice with P14. Mice were maintained under specific pathogen free conditions. All animal protocols were approved by the University of Washington Dept. of Comparative Medicine’s Animal Care Committee.
Cell Lines, Antibodies and Reagents
FBL and E10 tumor cell lines have previously been described (Ohlen et al., 2002; Teague et al., 2006). FBL-Gag peptide (CCLCLTVFL), FBL-Env peptide (EPLTSLTPRCNTAWNRLKL), and LCMV-Gp33-peptide (KAVYNFATM) were purchased from Global Peptide. CD4-depletion was performed using Dynal magnetic beads. Antibodies (Ab) to cell surface molecules were from BD Pharmingen. Phospho-specific mAb were from Cell Signaling. Cholera toxin subunit-B conjugate and 5-(and-6-)-carboxyfluorescein diacetate succinimidyl ester (CFSE) were from Molecular Probes.
FACS sorting
To avoid activation of T cells during FACS sorting by Ab specific for Vα/Vβ TCR chains, naive P14-TCRGag T cells were isolated based on CD8 and CD44low expression, whereas tolerant P14-TCRGagxAlb:Gag T cells were sorted based on CD8 and CD44hi expression.
T cell stimulation
In vitro proliferation was assessed by labeling cells with 1 μg/ml CFSE for 30 min at 37 °C, and stimulating 5×106 cells/ml with 1 μg/ml soluble peptide or Ab. In vivo T cell expansion was analyzed after i.v. transfer of 1×105 sorted T cells into B6 or Alb:Gag recipient mice and either infection with 2×105 PFU/ml LCMV or immunization with 5×106 peptide-pulsed irradiated APC. T cells were boosted in vivo by administering 5×106 peptide-pulsed syngeneic splenocytes i.p. Live tumor challenge was performed by administering 1×106 FBL cells i.p. Mice were monitored daily and euthanized upon detection of tumor-induced ascites, and are described in the text as having died from progressive tumor, which uniformly occurs within 24–48 hours of visible ascites.
Intracellular phosphorylation of ERK and JNK was assessed by stimulating cells directly ex vivo with 1 μg/ml soluble peptide or Ab for 30 min. Cells were fixed and permeabilized in Cytofix/Cytoperm buffers (BD Pharmingen), and stained with anti-phospho-ERK or anti-phospho-JNK, followed by secondary anti-rabbit-PE.
In vivo induction of T cell tolerance with peptide and IFA
Tolerance through the P14 TCR was induced by methods previously described by others (Aichele et al., 1995). Briefly, P14-TCRGag mice received 3 i.p. injections of 500 μg Gp33- peptide emulsified 1:1 in incomplete Freund’s adjuvant at three day intervals. Tolerance was assessed ex vivo 10 days following the final injection.
Confocal Microscopy
Naive and tolerant CD8+ dual-TCR T cells were purified by FACS sorting and stimulated for 30 min with peptide pulsed E10 cells (CD4+) in a 2:1 ratio, and synapse formation assessed and scored by confocal microscopy as previously described(Teague et al., 2006).
Supplementary Material
Acknowledgments
This work was supported in part by grants CA33084 and CA18029 from the US National Institutes of Health/National Cancer Institute, and by a grant from the Leukemia and Lymphoma Society. R. Teague was supported by a Ruth L. Kirschstein National Research Service Award.
References
- Aichele P, Brduscha-Riem K, Zinkernagel RM, Hengartner H, Pircher H. T cell priming versus T cell tolerance induced by synthetic peptides. J Exp Med. 1995;182:261–266. doi: 10.1084/jem.182.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antia R, Ganusov VV, Ahmed R. The role of models in understanding CD8+ T-cell memory. Nat Rev Immunol. 2005;5:101–111. doi: 10.1038/nri1550. [DOI] [PubMed] [Google Scholar]
- Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- Casanova JL, Romero P, Widmann C, Kourilsky P, Maryanski JL. T cell receptor genes in a series of class I major histocompatibility complex-restricted cytotoxic T lymphocyte clones specific for a Plasmodium berghei nonapeptide: implications for T cell allelic exclusion and antigen-specific repertoire. J Exp Med. 1991;174:1371–1383. doi: 10.1084/jem.174.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng LE, Greenberg PD. Selective delivery of augmented IL-2 receptor signals to responding CD8+ T cells increases the size of the acute antiviral response and of the resulting memory T cell pool. J Immunol. 2002;169:4990–4997. doi: 10.4049/jimmunol.169.9.4990. [DOI] [PubMed] [Google Scholar]
- Chiodetti L, Choi S, Barber DL, Schwartz RH. Adaptive tolerance and clonal anergy are distinct biochemical states. J Immunol. 2006;176:2279–2291. doi: 10.4049/jimmunol.176.4.2279. [DOI] [PubMed] [Google Scholar]
- Daniels MA, Schober SL, Hogquist KA, Jameson SC. Cutting edge: a test of the dominant negative signal model for TCR antagonism. J Immunol. 1999;162:3761–3764. [PubMed] [Google Scholar]
- Danke NA, Koelle DM, Yee C, Beheray S, Kwok WW. Autoreactive T cells in healthy individuals. J Immunol. 2004;172:5967–5972. doi: 10.4049/jimmunol.172.10.5967. [DOI] [PubMed] [Google Scholar]
- DeSilva DR, Urdahl KB, Jenkins MK. Clonal anergy is induced in vitro by T cell receptor occupancy in the absence of proliferation. J Immunol. 1991;147:3261–3267. [PubMed] [Google Scholar]
- Dittel BN, Stefanova I, Germain RN, Janeway CA., Jr Cross-antagonism of a T cell clone expressing two distinct T cell receptors. Immunity. 1999;11:289–298. doi: 10.1016/s1074-7613(00)80104-1. [DOI] [PubMed] [Google Scholar]
- Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- Gascoigne NR, Zal T. Molecular interactions at the T cell-antigen-presenting cell interface. Curr Opin Immunol. 2004;16:114–119. doi: 10.1016/j.coi.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Gil D, Schamel WW, Montoya M, Sanchez-Madrid F, Alarcon B. Recruitment of Nck by CD3 epsilon reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell. 2002;109:901–912. doi: 10.1016/s0092-8674(02)00799-7. [DOI] [PubMed] [Google Scholar]
- Gladow M, Uckert W, Blankenstein T. Dual T cell receptor T cells with two defined specificities mediate tumor suppression via both receptors. Eur J Immunol. 2004;34:1882–1891. doi: 10.1002/eji.200425041. [DOI] [PubMed] [Google Scholar]
- Grayson JM, Harrington LE, Lanier JG, Wherry EJ, Ahmed R. Differential sensitivity of naive and memory CD8+ T cells to apoptosis in vivo. J Immunol. 2002;169:3760–3770. doi: 10.4049/jimmunol.169.7.3760. [DOI] [PubMed] [Google Scholar]
- Grossman Z, Paul WE. Adaptive cellular interactions in the immune system: the tunable activation threshold and the significance of subthreshold responses. Proc Natl Acad Sci U S A. 1992;89:10365–10369. doi: 10.1073/pnas.89.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaume S, Tuosto L, Tanchot C, Di Bartolo V, Acuto O, Rocha B. Proximal changes in signal transduction that modify CD8+ T cell responsiveness in vivo. Eur J Immunol. 2003;33:2551–2556. doi: 10.1002/eji.200324196. [DOI] [PubMed] [Google Scholar]
- Hah C, Kim M, Kim K. Induction of peripheral tolerance in dual TCR T cells: an evidence for non-dominant signaling by one TCR. J Biochem Mol Biol. 2005;38:334–342. doi: 10.5483/bmbrep.2005.38.3.334. [DOI] [PubMed] [Google Scholar]
- Hardardottir F, Baron JL, Janeway CA., Jr T cells with two functional antigen-specific receptors. Proc Natl Acad Sci U S A. 1995;92:354–358. doi: 10.1073/pnas.92.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath WR, Miller JF. Expression of two alpha chains on the surface of T cells in T cell receptor transgenic mice. J Exp Med. 1993;178:1807–1811. doi: 10.1084/jem.178.5.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk MH, Hoogeboom M, Hagedoorn R, Kester MG, Willemze R, Falkenburg JH. Reprogramming of virus-specific T cells into leukemia-reactive T cells using T cell receptor gene transfer. J Exp Med. 2004;199:885–894. doi: 10.1084/jem.20031110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MS, Yu YY, Dudley ME, Zheng Z, Robbins PF, Li Y, Wunderlich J, Hawley RG, Moayeri M, Rosenberg SA, Morgan RA. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum Gene Ther. 2005;16:457–472. doi: 10.1089/hum.2005.16.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huseby ES, Liggitt D, Brabb T, Schnabel B, Ohlen C, Goverman J. A pathogenic role for myelin-specific CD8(+) T cells in a model for multiple sclerosis. J Exp Med. 2001;194:669–676. doi: 10.1084/jem.194.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes PW, Ley SC, Magee AI. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J Cell Biol. 1999;147:447–461. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes PW, Ley SC, Magee AI, Kabouridis PS. The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin Immunol. 2000;12:23–34. doi: 10.1006/smim.2000.0204. [DOI] [PubMed] [Google Scholar]
- Kusmartsev S, Nagaraj S, Gabrilovich DI. Tumor-associated CD8+ T cell tolerance induced by bone marrow-derived immature myeloid cells. J Immunol. 2005;175:4583–4592. doi: 10.4049/jimmunol.175.7.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo D, Burkly LC, Widera G, Cowing C, Flavell RA, Palmiter RD, Brinster RL. Diabetes and tolerance in transgenic mice expressing class II MHC molecules in pancreatic beta cells. Cell. 1988;53:159–168. doi: 10.1016/0092-8674(88)90497-7. [DOI] [PubMed] [Google Scholar]
- Lohman BL, Razvi ES, Welsh RM. T-lymphocyte downregulation after acute viral infection is not dependent on CD95 (Fas) receptor-ligand interactions. J Virol. 1996;70:8199–8203. doi: 10.1128/jvi.70.11.8199-8203.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morahan G, Allison J, Miller JF. Tolerance of class I histocompatibility antigens expressed extrathymically. Nature. 1989;339:622–624. doi: 10.1038/339622a0. [DOI] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto J, Tan X, Teague RM, Ohlen C, Greenberg PD. Induction of tolerance in CD8+ T cells to a transgenic autoantigen expressed in the liver does not require cross-presentation. J Immunol. 2007;178:6849–6860. doi: 10.4049/jimmunol.178.11.6849. [DOI] [PubMed] [Google Scholar]
- Murtaza A, Nugent CT, Tailor P, Asensio VC, Biggs JA, Campbell IL, Sherman LA. Altered functional and biochemical response by CD8+ T cells that remain after tolerance. Int Immunol. 2001;13:1085–1093. doi: 10.1093/intimm/13.8.1085. [DOI] [PubMed] [Google Scholar]
- Niederberger N, Holmberg K, Alam SM, Sakati W, Naramura M, Gu H, Gascoigne NR. Allelic exclusion of the TCR alpha-chain is an active process requiring TCR-mediated signaling and c-Cbl. J Immunol. 2003;170:4557–4563. doi: 10.4049/jimmunol.170.9.4557. [DOI] [PubMed] [Google Scholar]
- Ohlen C, Kalos M, Cheng LE, Shur AC, Hong DJ, Carson BD, Kokot NC, Lerner CG, Sather BD, Huseby ES, Greenberg PD. CD8(+) T cell tolerance to a tumor-associated antigen is maintained at the level of expansion rather than effector function. J Exp Med. 2002;195:1407–1418. doi: 10.1084/jem.20011063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlen C, Kalos M, Hong DJ, Shur AC, Greenberg PD. Expression of a tolerizing tumor antigen in peripheral tissue does not preclude recovery of high-affinity CD8+ T cells or CTL immunotherapy of tumors expressing the antigen. J Immunol. 2001;166:2863–2870. doi: 10.4049/jimmunol.166.4.2863. [DOI] [PubMed] [Google Scholar]
- Padovan E, Casorati G, Dellabona P, Meyer S, Brockhaus M, Lanzavecchia A. Expression of two T cell receptor alpha chains: dual receptor T cells. Science. 1993;262:422–424. doi: 10.1126/science.8211163. [DOI] [PubMed] [Google Scholar]
- Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–839. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- Ramsdell F, Fowlkes BJ. Maintenance of in vivo tolerance by persistence of antigen. Science. 1992;257:1130–1134. doi: 10.1126/science.257.5073.1130. [DOI] [PubMed] [Google Scholar]
- Robertson JM, Evavold BD. Cutting edge: dueling TCRs: peptide antagonism of CD4+ T cells with dual antigen specificities. J Immunol. 1999;163:1750–1754. [PubMed] [Google Scholar]
- Rocha B, Tanchot C, Von Boehmer H. Clonal anergy blocks in vivo growth of mature T cells and can be reversed in the absence of antigen. J Exp Med. 1993;177:1517–1521. doi: 10.1084/jem.177.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszkowski JJ, Lyons GE, Kast WM, Yee C, Van Besien K, Nishimura MI. Simultaneous generation of CD8+ and CD4+ melanoma-reactive T cells by retroviral-mediated transfer of a single T-cell receptor. Cancer Res. 2005;65:1570–1576. doi: 10.1158/0008-5472.CAN-04-2076. [DOI] [PubMed] [Google Scholar]
- Rouquette-Jazdanian AK, Foussat A, Lamy L, Pelassy C, Lagadec P, Breittmayer JP, Aussel C. Cholera toxin B-subunit prevents activation and proliferation of human CD4+ T cells by activation of a neutral sphingomyelinase in lipid rafts. J Immunol. 2005;175:5637–5648. doi: 10.4049/jimmunol.175.9.5637. [DOI] [PubMed] [Google Scholar]
- Singh NJ, Schwartz RH. The strength of persistent antigenic stimulation modulates adaptive tolerance in peripheral CD4+ T cells. J Exp Med. 2003;198:1107–1117. doi: 10.1084/jem.20030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislawski T, Voss RH, Lotz C, Sadovnikova E, Willemsen RA, Kuball J, Ruppert T, Bolhuis RL, Melief CJ, Huber C, et al. Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nat Immunol. 2001;2:962–970. doi: 10.1038/ni1001-962. [DOI] [PubMed] [Google Scholar]
- Steinman L. Myelin-specific CD8 T cells in the pathogenesis of experimental allergic encephalitis and multiple sclerosis. J Exp Med. 2001;194:F27–30. doi: 10.1084/jem.194.5.f27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotz SH, Bolliger L, Carbone FR, Palmer E. T cell receptor (TCR) antagonism without a negative signal: evidence from T cell hybridomas expressing two independent TCRs. J Exp Med. 1999;189:253–264. doi: 10.1084/jem.189.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanchot C, Barber DL, Chiodetti L, Schwartz RH. Adaptive tolerance of CD4+ T cells in vivo: multiple thresholds in response to a constant level of antigen presentation. J Immunol. 2001;167:2030–2039. doi: 10.4049/jimmunol.167.4.2030. [DOI] [PubMed] [Google Scholar]
- Tanchot C, Guillaume S, Delon J, Bourgeois C, Franzke A, Sarukhan A, Trautmann A, Rocha B. Modifications of CD8+ T cell function during in vivo memory or tolerance induction. Immunity. 1998;8:581–590. doi: 10.1016/s1074-7613(00)80563-4. [DOI] [PubMed] [Google Scholar]
- Teague RM, Sather BD, Sacks JA, Huang MZ, Dossett ML, Morimoto J, Tan X, Sutton SE, Cooke MP, Ohlen C, Greenberg PD. Interleukin-15 rescues tolerant CD8(+) T cells for use in adoptive immunotherapy of established tumors. Nat Med. 2006;12:335–341. doi: 10.1038/nm1359. [DOI] [PubMed] [Google Scholar]
- Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- Yang W, Grey HM. Study of the mechanism of TCR antagonism using dual-TCR-expressing T cells. J Immunol. 2003;170:4532–4538. doi: 10.4049/jimmunol.170.9.4532. [DOI] [PubMed] [Google Scholar]
- Zal T, Weiss S, Mellor A, Stockinger B. Expression of a second receptor rescues self-specific T cells from thymic deletion and allows activation of autoreactive effector function. Proc Natl Acad Sci U S A. 1996;93:9102–9107. doi: 10.1073/pnas.93.17.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.