Abstract
We recently showed that HeLa cell lamin B is modified by a mevalonic acid derivative. Here we identified the modified amino acid, determined its mode of link-age to the mevalonic acid derivative, and established the derivative’s structure. A cysteine residue is modified because experiments with lamin B that had been biosynthetically labeled with [3H] mevalonic acid or [35S] cysteine and then extensively digested with proteases yielded 3H- or 35S-labeled products that co-chromatographed in five successive systems. A thioether linkage rather than a thioester linkage is involved because the mevalonic acid derivative could be released from the 3H-labeled products in a pentane-extractable form by treatment with Raney nickel but not with methanolic KOH. The derivative is a farnesyl moiety because the Raney nickel-released material was identified as 2,6,10-trimethyl-2,6,10-dodecatriene by a combination of gas chromatography and mass spectrometry. The thioether-modified cysteine residue appears to be located near the carboxyl end of lamin B because treatment of 3H-labeled lamin B with cyanogen bromide yielded a single labeled polypeptide that mapped toward this end of the cDNA-inferred sequence of human lamin B.
Mevalonic acid (MVA),1 a precursor of sterols, dolichol, ubiquinone, and other isoprene derivatives (1), also has a role in the post-translational modification of cellular proteins (2). The addition of radiolabeled MVA to a variety of mammalian cells growing in culture results in the covalent attachment of MVA-derived label to a number of specific proteins (2–4). One of these modified proteins has recently been identified as lamin B, a structural component of the nuclear lamina (5, 6). Although preliminary evidence indicated that the modification is some form of isoprene (2), the exact nature of the modification and its site of attachment to protein have not been determined.
The only well-characterized examples of isoprenylated polypeptides in eukaryotes are the mating hormones secreted by the jelly fungi Tremella mesenterica (tremerogen A) (7), Tremella brasiliensis (tremerogen A-9291-1) (8), and Rhodos-poridium toruloides (rhodotorucine) (9) and a factor, the mating hormone from Saccharomyces cerevisiae (10). All of these peptide hormones have a 15-carbon farnesyl group, derived from MVA, that is directly attached to a carboxyl-terminal cysteine through a thioether linkage. In the case of tremerogen A, the farnesyl chain is further modified with a hydroxyl group (7). The isoprenylation of a cysteine residue is clearly a common theme for organisms of this type and might serve as a model for isoprenylation in higher organisms.
It was previously reported that p21ras proteins are modified by palmitoylation on the COOH-terminal cysteine residue (12). This result was recently challenged by Hancock et al. (13) who demonstrated that the COOH-terminal cysteine of p21ras is modified by a lipid derived from MVA. The exact structure of this ligand remains to be established. For some p21ras proteins, palmitoylation occurs as well, but on cysteine residues immediately upstream of the COOH-terminal cysteine (13). Isoprenylation of p21ras is essential for biological activity since transformation of cell lines expressing oncogenic forms of Ras proteins can be blocked by drugs, such as mevinolin, that inhibit the biosynthesis of MVA (13, 14). Interestingly, the p21ras proteins and the yeast a factor share a similar carboxyl-terminal amino acid sequence as predicted from the cDNA (15, 16). This carboxyl-terminal motif, Cys-A-A-X, includes an invariant cysteine residue, followed by two aliphatic residues and a terminal amino acid (15). Both a factor and the p21ras proteins undergo a processing event which cleaves the three terminal amino acids to expose the cysteine residue (10, 17). This residue is the site of isoprenylation of a factor and of p21ras. In addition, this cysteine is further modified by a carboxyl methyl ester group (10, 15). These observations suggest that this conserved sequence motif may serve as a recognition site for modification by an isoprene group. Mammalian lamin B shares this same carboxyl-terminal amino acid sequence including the invariant cysteine residue (18), which raises the possibility that this cysteine may be modified. We present evidence below that a cysteine residue in lamin B contains a thioether-linked farnesyl group. Preliminary evidence suggests that the modified cysteine may be located near the carboxyl terminus of the protein.
EXPERIMENTAL PROCEDURES
Materials
Except as noted, all radioisotopes were obtained from Du Pont-New England Nuclear, electrophoresis reagents were from Bio-Rad, and all the enzymes and the Raney nickel-activated catalyst (W2) were from Sigma. Chromatography solvents were from J. T. Baker Chemical Co. Tissue culture reagents were obtained from GIBCO. Mevinolin was the gift of Dr. A. Alberts (Merck, Sharp & Dohme Research Laboratories) and was converted to its sodium salt prior to use (19). Farnesane (2,6,10-trimethyldodecane)was from Wiley Organics.
Cell Culture
Human carcinoma (HeLa) cells were grown and harvested as previously described (5). For the [3H] MVA labeling, HeLa cells were incubated for 36 h with 30 μM mevinolin, 35 μM unlabeled MVA, and 100 μCi/ml (RS)-[5-3H]MVA lactone (30 Ci/mmol). For the [35S]cysteine labeling, the standard growth medium was replaced with cysteine-deficient RPMI-1640 medium and the cells incubated for 24 h in the presence of 200 μCi/ml L-[35S] cysteine (1022 Ci/mmol).
Purification of Lamin Proteins
Unless noted otherwise, all manipulations were done at 4 °C. Nuclei from labeled HeLa cells were prepared as previously described (5). The nuclei from 1.5 × 108 cells were resuspended in 10 ml of 10 mM Tris-HCl, pH 7.4, and 1 mM phenylmethanesulfonyl fluoride (buffer A) containing 200 units/ml DNase, 100 μg/ml RNase, 1 μg/ml aprotonin, 0.5 μg/ml leupeptin, and 0.7 μg/ml pepstatin. The samples were incubated for 30 min on ice and pelleted by centrifugation at 12,000 × g for 15 min. The nuclease treatment was repeated and the pellet washed with buffer. Subsequent extraction steps were essentially as described by Aebi et al. (20). The nuclei were washed with 1 M KCl, followed by two washes with buffer A. The pellet was then resuspended for 30 min in 2% Triton X-100,20 mM MOPS/KOH, pH 6.0, 2 mM EDTA, and 1 mM dithiothreitol. After centrifugation for 15 min at 12,000 × g, the pellet was resuspended in 2% Triton X-100, 500 mM KCl, 1 mM EDTA, 1 mM dithiothreitol, and 20 mM Tris-HCl, pH 9.0, and incubated for 30 min prior to centrifugation at 100,000 × g for 1 h. The lamins were further purified by fractionating the supernatant proteins on an 8%, denaturing, polyacrylamide gel (5). The gels were stained for 5 min with Coomassie Brilliant Blue R-250, destained for 3 h, and specific bands corresponding to lamins B and C were excised and electroeluted from the gel slices (21). The identification of the lamins was based on Western blot analysis with antibodies against lamins B and C, (5) and on the distribution of [3H]MVA-derived radioactivity. Sodium dodecyl sulfate (SDS) and Coomassie Blue were removed from the samples by lyophilizing the electroeluted material, then precipitating the protein with acetone/triethylamine/acetic acid/water in the ratio of 85:5:5:2.5 (22).
Proteolytic Digestion
Purified lamin B was resolubilized in 2 ml of a solution containing 3 mM CaCl2 0.025% SDS, and 10 mM Tris, pH 7.8, then digested with 0.75 mg/ml type VIII protease at 37 °C. An additional 0.2 mg/ml protease was added at 24 and 48 h. At 72 h, EDTA and SDS were added to a final concentration of 50 mM and 0.25%, respectively, and the pH was adjusted to 8.2 with NaOH. Protease K (type XI) was added to a concentration of 1.0 mg/ml, and the sample was incubated at 45 °C for 8 h before adding an additional 0.5 mg/ml of protease K and continuing the incubation for 16 h. The pH was readjusted to 5.3 with acetic acid, and the digestion continued for 24 h with 1.25 mg/ml carboxypeptidase Y at 37 °C. An additional 0.6 mg/ml of this protease was then added, and the incubation continued for 48 h. Samples were frozen (−20 °C) prior to being purified on DEAE. An acetic acid-equilibrated, DEAE-Sephacel column (3 × 40-mm bed volume, Pharmacia LKB Biotechnology Inc.) was washed with 10 ml of water. Then the digest was loaded onto the column, and the latter was washed with 2 ml of water and eluted with 6 ml of formic acid/ethanol/water (2:8:1). Some of the radioactivity (30–45%) was not absorbed on the column and came off during the sample loading and water wash steps. This material was then reapplied to a second, duplicate column and the procedure repeated. The pooled eluate was dried using a Speed Vac Concentrator (Savant) and the residue resuspended in 0.2 ml of formic acid/ethanol (1:4) prior to size exclusion chromatography.
Chromatography
Throughout the course of chromatographic separations the 3H- and 35S-labeled samples were analyzed separately. Size exclusion chromatography was performed in formic acid/ethanol (1:4) at a flow rate of 0.25 ml/min on a Sephadex LH-20 column (10 × 450-mm bed volume, Pharmacia LKB Biotechnology Inc.). Fractions (0.5 ml) were collected and 5% of each was used for radiometric analysis. Formic acid/ethanol was initially chosen because the proteolysate exhibited very limited solubility in either aqueous or organic solvents but was readily soluble in acidified polar organic solvents. After exclusion chromatography the pooled fractions from each peak were further purified over short silica guard columns (3 × 15 mm, Brownlee). The columns were preconditioned at 34 °C with 100 bed volumes of 2% formic acid in ethanol and then equilibrated with the running solvent, a mixture that contained 84% solvent A (hexane/isopropanol, 98.8:1.2), 16% solvent B (isopropanol/water, 93:6), and 0.1% trifluoroacetic acid (Aldrich) (23). The radioactive material was then chromatographed at a flow rate of 1.0 ml/min at 34 °C. The radioactivity that eluted in the void volume was subsequently analyzed by reverse-phase high performance liquid chromatography (HPLC). For HPLC analyses all samples were chromatographed sequentially on the same day using a single column and one freshly prepared batch of solvent. The samples were chromatographed with internal standards, tryptophan methyl ester and tryptophan octyl ester, on an Ultrasphere C18-IP column (Beckman/Altex) in 0.1% in trifluoroacetic acid in methanol at a flow rate of 1 ml/min. Selected fractions were further purified by thin layer chromatography on prescored Silica Gel H plates (40 × 170 × 0.25 mm, Analabs) that had been previously conditioned for 1 h at 110 °C. Equivalent amounts of 3H- and 35S-label were separately applied to two adjacent, analytical lanes. Then the remaining 3H- and 35S-label were separately applied to two additional lanes on the same plate, and the plate was developed for 50 min in chloroform/methanol/acetic acid/water (25:15:4:2) (24). After development of the plate, 1-cm fractions from each analytical lane were scraped into scintillation vials and the radioactivity determined. These data were then plotted and served as a template for the harvesting of selected fractions of the remaining lanes. These fractions were scraped into screw-capped tubes and extracted three times with 1-ml aliquots of 0.1% trifluoroacetic acid in methanol. The extract was filtered and frozen for subsequent analysis. Standards were visualized with iodine vapor.
Cleavage of 3H-Labeled Ligand and Synthesis of S-Farnesyl Cysteine
Raney nickel-activated catalyst was rinsed thoroughly with water and ethanol prior to use as recommended by the supplier (Sigma). In initial experiments, 3H-labeled samples of intact lamin B were suspended in 400 μl of methanol/water (1:1) in screw-capped tubes. Raney nickel (10 mg)was then added, the tubes were tightly sealed, and heated at 100 °C for 15 h. The solution was cooled at −20 °C for 1 h and then extracted twice with 1-ml aliquots of pentane. Using this method, ~50% of the radioactivity was converted to a pentane-extractable form. 3H-Labeled proteolysis products corresponding to the major peak obtained by chromatography on Sephadex LH-20 were prepared in a similar manner but with higher recoveries of radioactivity (~75%). In later experiments, samples of lamin B were treated with Raney nickel in the presence of guanidine-HCl which helped to solubilize the protein and resulted in an improved yield of released radioactivity (~80%). In this case, samples of lamin B were solubilized in 400 μl of 8 M guanidine HCl, 0.2 M sodium phosphate, pH 7.0 (25). Raney nickel (500 mg) was added, the tubes were briefly flushed with argon, tightly capped, and incubated for 15 h at 100 °C. Control samples received no catalyst. Samples were pentane-extracted as described above. Some of the samples were hydrogenated over platinum (Adams’ catalyst). The platinum (2–3 mg) was added, and the solutions were stirred under a hydrogen atmosphere for 2 h at room temperature. For mild base hydrolysis, 3H-labeled material from the major Sephadex LH-20 peak was treated with 0.1 M methanolic KOH for 1 h at 23 °C, acidified with an equal volume of 0.2 M HCl on ice, and extracted with pentane (26). S-Farnesyl cysteine was synthesized as described by Kamiya et al. (8), and its structure verified by NMR and fast atom bombardment mass spectrometry.
Bulb-to-Bulb Volatilization, Gas Chromatography, and Mass Spectrometry
Raney nickel-treated, 3H-labeled samples in either methanol/water or pentane were placed into one finger of a double fingered bulb-to-bulb tube. The solution was frozen in liquid nitrogen and the tube was then sealed under vacuum. The finger without sample was then cooled in liquid nitrogen. Volatile material moved from the sample finger at 21 °C and condensed in the cooled chamber. The distribution of ligand-associated radiolabel was determined by radiometric analysis of aliquots of the condensate and residual material. Gas-liquid chromatography (GLC) was done with a 15-meter, DB-5 megabore column (J & W Scientific) using a temperature program which started at an initial temperature of 80 °C for 2 min, then increased 2 °/min up to 220°C. The flow rate was 12 ml/min. The pentane extracts of Raney nickel cleaved samples were prepared for radiometric GLC by concentrating the samples to a small volume (~6 μl) with a stream of nitrogen while cooling in a salt-water ice bath. Peak mass was detected by flame ionization and radioactivity was detected with a Radiomatic Flow-One Beta Detector which was equipped with a 10-cc gas conductivity cell (Radiomatic Instrument Co., Tampa) and a 1:5 split ratio. O-[3H]Acetyl geraniol was used as a calibration standard and was prepared by the derivatization of 2.2 mg of geraniol with [3H]acetic anhydride (100 μl) and anhydrous pyridine (50 μl) for 60 min at 70 °C (27). Gas chromatography/mass spectrometry (GC/MS) was performed on a Hewlett Packard (Model 5710A) gas chromatograph directly coupled to a VG Analytical (Model 7070H) mass spectrometer. Analyses were performed using a 30-m DB-5 capillary column with a 5 psi head pressure of He and the same temperature program described above. Mass spectra were obtained in the electron ionization mode at 70 eV using a multiplier setting of 2200 V. Control chromatograms with pentane were made directly prior to the chromatography of lamin B samples to ensure the absence of trace contamination with standards. Spectra were enhanced by plotting the square root of the relative abundances of the ions detected.
Cyanogen Bromide Cleavage and Partial Amino Acid Sequencing
Purified lamin B (~15 μg, 19,000 cpm) was treated with cyanogen bromide (~100 μg, Eastman) in 500 μl of 70% formic acid, 0.1% SDS, gassed with argon, and incubated for 24 h in the dark. The sample was diluted with 5 ml of distilled water and lyophilized. For sequencing, the CNBr fragments were separated on a 12.5% denaturing polyacrylamide gel and then transferred to a polyvinylidene difluoride membrane and stained according to the procedure of Matsudaira (28). A Coomassie Blue-stained 17-kDa radiolabeled fragment containing the majority of the radiolabel was excised and sequenced using an automated sequenator.
RESULTS
Evidence That a Cysteine Residue in Lamin B Is Modified by MVA-derived Material
We used two different approaches to investigate the possibility that a cysteine residue in lamin B might be modified by a derivative of MVA. We subfractionated proteolytic hydrolysates of lamin B to identify low molecular weight fragments that might contain both cysteine and MVA-derived material, and we treated these fragments or intact lamin B under conditions that cleave thioester or thioether bonds. For the first approach we prepared lamin B from HeLa cells that had been prelabeled with [35S]cysteine or [3H]MVA, then used a procedure that had been developed earlier in this laboratory (2) to extensively hydrolyze lamin B with proteases, concentrate proteolytic hydrolysis products that contained MVA-derived material, and subfractionate these products by size exclusion chromatography on Sephadex LH-20. When used earlier to investigate total protein of [3H] MVA-labeled Swiss 3T3 cells, this procedure yielded a major peak, “peak A,” that had an apparent mass of 1000 Da, and a minor peak, “peak B,” that had an apparent mass of 500 Da (2). In the present investigation preliminary experiments with the total proteins of [3H]MVA-labeled HeLa cells yielded similar results (not shown). However, when we used the procedure to analyze a purified preparation of 3H-labeled lamin B, most of the radioactivity in the proteolytic hydrolysate emerged from the Sephadex LH-20 column in a position comparable with that of peak B (Fig. 1A). An analysis of 35S-labeled lamin B also yielded a peak of hydrolysate radioactivity that emerged in this position (Fig. 1A), although the peak was superimposed on a broad envelope of 35S-labeled material. Interestingly, upon being chromatographed on Sephadex LH-20, an authentic standard of S-farnesyl cysteine also emerged in the position of peak B (not shown). In contrast, a proteolytic hydrolysate of lamin C, which does not contain a bound isoprene, showed only a broad envelope of label without a distinct peak B (Fig. 1B).
Fig. 1. Chromatography of proteolytic hydrolysates of lamins B and C on Sephadex LH-20.
HeLa cells were labeled with [3H]MVA or [35S]cysteine, lamins B and C were isolated, solubilized in SDS, and extensively hydrolyzed with a combination of proteases, and the hydrolysates were concentrated on DEAE-Sephacel. 65% of the radioactivity in the hydrolysate of 3H-labeled lamin B adsorbed onto DEAE and could subsequently be eluted with formic acid/ethanol/water (2:8:1). In contrast, only 13 and 11%, respectively, of the radioactivity from the hydrolysates of 35S-labeled lamin B and lamin C behaved similarly. The figure shows chromatograms that were obtained when the DEAE-eluted, 3H- and 35S-labeled materials were each passed separately through Sephadex LH-20 in 20% formic acid in ethanol. Panel A, filled circles, 3H-labeled material from hydrolysate of lamin B (145,000 cpm loaded); open circles, corresponding, 35S-labeled material (100,000 cpm loaded). Panel B, 35S-labeled material from lamin C (80,000 cpm loaded). Note that an authentic standard of S-farnesyl cysteine coeluted with the major peak of 3H- label, shown in panel A, while cysteine and cystine emerged in the position indicated by the arrow. Hydrolysates of labeled lamin B yielded results like these in three different experiments, although the small peak of 3H-labeled material, that emerged at an elution volume of 20 ml, contained variable proportions (5–20%) of the total radioactivity. Hydrolysates of the [3H]MVA-labeled, minor nuclear protein of 66 kDa, described previously (5) also yielded results that were similar to those shown in panel A.
These results suggested that a low molecular weight proteolysis product of lamin B might contain both [35S]cysteine and [3H]MVA-derived material but did not evaluate the possibility that the two labels might have been present in separate hydrolysis products. We therefore studied the behavior of the peak B material from the Sephadex LH-20 columns in other chromatographic systems. We passed the material through a short “guard” column of silica to remove polar UV absorbing material (not shown) and subsequently through a reverse-phase column to remove residual SDS from the proteolysis step (Fig. 2A). Then, we successively chromatographed the labeled material on silica gel thin layer plates (Fig. 2B) and on a second reverse-phase column (Fig. 2C). Throughout the course of this chromatography, much of the 3H- and 35S- labeled material from the lamin B hydrolysates continued to coelute, although some of the 3H-labeled material migrated separately (Fig. 2B, Fraction 13). The basis for this heterogeneity is not known, but it may be related to the acidic conditions required for the chromatography.
Fig. 2. Reverse-phase and thin layer chromatography of 3H- and 35S-labeled proteolysis products of lamin B.
Panel A, material corresponding to the major peak of radioactivity from each of the two chromatograms shown in Fig. 1A was passed through silica guard columns (not shown), then the 3H- and 35S-labeled samples were chromatographed separately by reverse-phase HPLC in methanol 0.1% trifluoroacetic acid (see “Experimental Procedures”). In the experiments shown 19,000 cpm of the 3H- and 5,300 cpm of the 35S-label were chromatographed separately to yield 10,000 and 2,000 cpm, respectively, in fractions 16 through 22. Note that pretreatment with the guard column removed 40% of the 35S radioactivity but only 20% of the 3H radioactivity, and that the guard column did not adsorb S-farnesyl cysteine. However, the guard column adsorbed more than 85% of the 35S radioactivity from the corresponding fraction of a lamin C hydrolysate, leaving insufficient radioactivity to be detected by reverse-phase HPLC. Panel B, fractions 16 through 22 from each of the chromatograms shown in panel A were pooled separately, concentrated, and further chromatographed by thin layer chromatography. In this experiment, 570 cpm of 3H- and 570 cpm of 35S-label was applied separately on adjacent lanes of a prescored thin layer chromatography plate and developed (see “Experimental Procedures”). The distribution of radioactivity in these two lanes is shown and was used to locate fractions of interest in two adjacent lanes, one loaded with 1,100 cpm of the 3H-label and the other with 1,100 cpm of the 35S-label. Fractions 2 and 17, respectively, corresponded to the origin and solvent front. Panel C, material corresponding to fractions 9 through 11 from the experiment shown in panel B was separately eluted from each of the two adjacent lanes described above, pooled, concentrated, and chromatographed by reverse-phase HPLC in methanol, 0.1% trifluoroacetic acid. In this experiment 340 cpm of the pooled 3H material and 500 cpm of the pooled 35S material were chromatographed separately to yield 220 and 370 cpm, respectively, in fractions 16 through 22. This represents 80 and 75%, respectively, of the total radioactivity actually recovered from the column.
The fact that most of the 3H- and 35S-labeled material from the lamin B hydrolysates coeluted during the course of several successive chromatographic steps provided additional evidence that this material was present in the same proteolytic fragment(s) but did not prove that the cysteine itself was modified. We therefore used a different approach to examine the possibility that MVA-derived material might be directly linked to cysteine via a thioester or thioether bond. We found that treatment of the 3H-labeled peak B material from the Sephadex LH-20 column with methanolic KOH, under conditions that readily hydrolyzed a model thioester, led to a negligible release of 3H-radiolabel (Table I). However, treatment of the material with Raney nickel-activated catalyst, under conditions that cleaved S-farnesyl cysteine into alanine and a pentane-extractable compound, converted nearly 75% of the 3H-radiolabel into a pentane-soluble form (Table I). Importantly, a similar result was obtained with 3H-labeled lamin B that had been solubilized in 8 M guanidine HCl (Table I). It seems unlikely that anything other than a thioether bond could have been cleaved under these relatively mild conditions because little or no catalytic hydrogenation of the farnesyl double bonds in S-farnesyl cysteine occurred (see below).
Table I. Raney nickel-catalyzed release of [3H]MVA-derived material from lamin B and its proteolysis products.
Material corresponding to the major peak of 3H radioactivity shown in Fig. 1A (~1,800 cpm) was solubilized in 50% methanol/water and pre-extracted with pentane before being treated with 10 mg of Raney nickel for 2 h at 100 °C. Then the released material was extracted into pentane and its content of 3H radioactivity determined. Control samples were treated similarly but in the absence of Raney nickel. Other samples of the peak B material were treated with 0.1 M methanolic KOH for 1 h at 23 °C, acidified with 0.2 M HCl, and extracted into pentane. Intact lamin B (~15,000 cpm) was solubilized in 8 M guanidine-HCl, and 500 mg of Raney nickel was added. The solution was heated for 15 h at 100 °C under argon gas, cooled at −20 °C for 1 h, whereupon the released material was extracted into pentane. Values in parentheses represent the number of times each experiment was done.
| Sample | Percent radioactivity released into pentane
|
|
|---|---|---|
| Before treatment | After treatment | |
| Peak B material | ||
| +Raney nickel (7) | 2.0 ± 0.1 | 75 ± 2 |
| −Raney nickel (7) | 2.0 ± 0.1 | 5 ± 2 |
| +Methanolic KOH (2) | 5 ± 1 | |
| −Methanolic KOH (2) | <1.0 | |
| Lamin B | ||
| +Raney nickel (2) | 1.5 | 80 ± 2 |
| −Raney nickel (1) | 12 | |
Evidence That the MVA-derived Material That Is Linked to Cysteine Is a Farnesyl Group
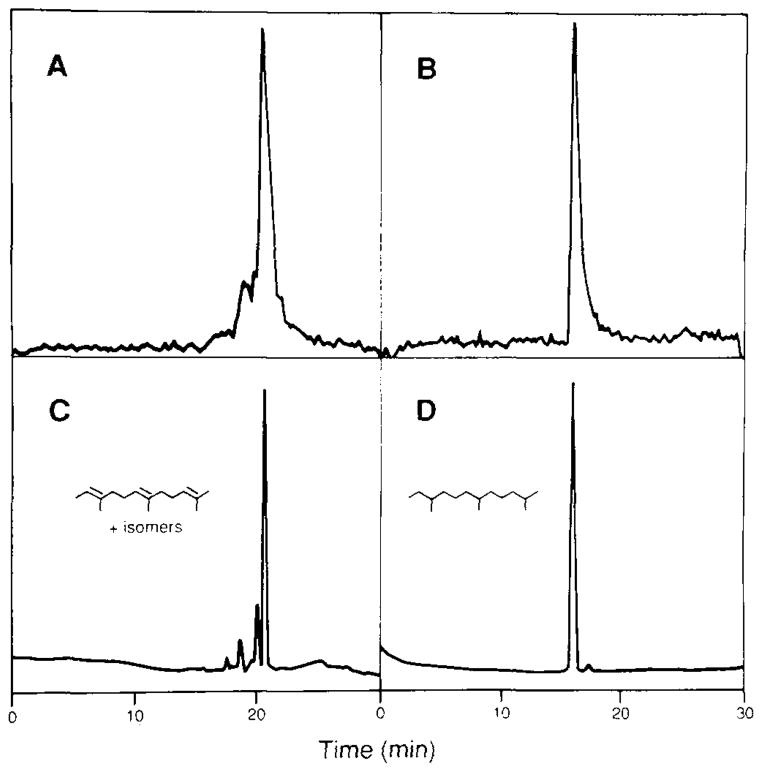
We used the Raney nickel-catalyzed cleavage technique to obtain additional information about the MVA-derived material. A bulb-to-bulb transfer experiment (“Experimental Procedures”) demonstrated that nearly 80% of the pentane-extractable 3H radiolabel from Raney nickel-treated peak B could be volatilized at room temperature (not shown). This suggested that further analysis by gas-liquid chromatography might be informative. We used 3H-labeled material that had been cleaved from intact lamin B for this analysis, rather than the corresponding material from the Sephadex LH-20 chromatography peak B, to avoid potential artifacts that might have been caused by the acidic chromatography conditions. The material from intact lamin B showed one major and several minor peaks of radioactivity (Fig. 3A). However, a parallel sample of the cleaved material that had been hydrogenated over platinum yielded only a single peak of radioactivity (Fig. 3B). Similar results were obtained with material that had been cleaved from authentic S-farnesyl cysteine (Fig. 3, C and D).
Fig. 3. Gas-liquid chromatographic analysis of [3H]MVA-derived material released upon treating lamin B with Raney nickel.
3H-Labeled lamin B was solubilized with 8 M guanidine HCl, 0.2 M sodium phosphate, p H 7.0, and treated with Raney nickel. The released material was then extracted into pentane and analyzed by GLC before or after hydrogenation over platinum. Panel A, nonhydrogenated, 3H-labeled material released from lamin B by Raney nickel. Panel B, 3H-labeled material released from lamin B by Raney nickel that was subsequently hydrogenated over platinum. Similar results were obtained upon analyzing 3H-labeled material that had been released from the minor [3H]MVA-labeled protein of 66 kDa (5). Panel C, Raney nickel-released material from S-farnesyl cysteine. Panel D, material that had been similarly released from S-farnesyl cysteine, then hydrogenated over platinum before being analyzed. Authentic 2,6,10-trimethyldodecane (farnesane) co-chromatographed with this hydrogenated sample (not shown). Note that 58% of the 3H-labeled material that was released from lamin B was recovered from the column.
When the cleavage products of S-farnesyl cysteine were analyzed by GC/MS, the mass spectra of all of the peaks were nearly identical and all contained a parent ion peak at 206. This indicates that a mixture of isomeric trimethyldodecatrienes was formed. 1H NMR analysis of the mixture indicated that the major product was 2,6,10-trimethyl-2,6,10-dodecatriene. When this mixture was hydrogenated over platinum, a single product was obtained which had a retention time and mass spectrum identical to the retention time and mass spectrum of authentic farnesane (2,6,10-trimethyldodecane). Thus, treatment with Raney nickel under our conditions led to cleavage of the thioether bond without hydrogenation of the double bonds. In addition, the nickel caused double bond isomerizations without skeletal rearrangements.
In order to establish the structure of the isoprene compound liberated upon Raney nickel catalysis, lamin B was purified and cleaved as previously described, and the pentane extract was concentrated and analyzed by GC/MS. A peak in the total ion current chromatogram corresponding to the major peak of 3H radioactivity shown in Fig. 3A was then further analyzed by mass spectrometry. Its fragmentation pattern was nearly identical to that of the hydrocarbon derived from Raney nickel cleavage of authentic S-farnesyl cysteine (Fig. 4, A and C). A companion sample of lamin B was treated with Raney nickel, hydrogenated over platinum, and analyzed by GC/MS. A peak was seen that had a retention time and fragmentation pattern that were nearly identical to the retention time and fragmentation pattern of authentic farnesane (Fig. 4, B and D). Based on these results, we conclude that lamin B is modified with a farnesyl group.
Fig. 4. Enhanced electron ionization spectra of MVA-derived material released upon treating lamin B with Raney nickel.
Lamin B from 1.2 ×109 cells was solubilized with 8 M guanidine HCl, 0.2 M sodium phosphate, pH 7.0, then treated with Raney nickel. The released material was extracted into pentane and analyzed by GC/MS before or after hydrogenation. S-farnesyl cysteine was treated in a similar manner. Panel A, spectrum of a nonhydrogenated peak from the lamin B chromatogram which corresponded to the major peak of radioactivity in Fig. 3A. The tR of this spectrum was 18.93 min. The aliquot analyzed represents lamin B from 0.1 ×109 cells. Panel B, spectrum of a hydrogenated peak from the lamin B chromatogram which corresponded to the peak of radioactivity in Fig. 3B. The tR of this spectrum was 14.43 min. The aliquot analyzed represents lamin B from 0.1× 109 cells. Similar results were obtained upon the analysis of material that had been released from the minor protein of 66 kDa (5). Panel C, spectrum of the major peak from the Raney nickel-treated S-farnesyl cysteine chromatogram. The tR of this spectrum was 19.12 min. Panel D, spectrum of authentic 2,6,10-trimethyldodecane (farnesane). The tR of this spectrum was 14.52 min. All spectra shown have been enhanced to highlight ions of low abundance (see “Experimental Procedures”).
Sequencing a Labeled Cyanogen Bromide Fragment
A final series of experiments was done to investigate the location of the modified cysteine in the lamin B sequence. It was of particular interest to determine whether the farnesyl group is associated with the conserved Cys-A-A-X motif at the carboxyl terminus, analogous to the isoprenylation of a factor and p21ras. To address this question, purified 3H-labeled lamin B was treated with cyanogen bromide, and the resulting fragments were separated on a one-dimensional polyacrylamide gel. Several major and minor peptides were revealed when the gel was stained with Coomassie Blue (Fig. 5, lane 1). However, subsequent fluorography of the same gel revealed only one predominant band of radioactivity with an apparent molecular mass of 17 kDa (Fig. 5, lane 2). Furthermore, all of the Coomassie Blue-stained bands seen in the gel were individually excised and examined for radioactivity by scintillation counting. A Coomassie-stained band of 17 kDa contained 83% of the background-corrected radiolabel (Fig. 4, arrow). The remainder was located in two very faint bands of ~30 kDa, presumably the products of incomplete digestion.
Fig. 5. Cyanogen bromide cleavage products of 3H-labeled lamin B.
Purified 3H-labeled lamin B (~15 μg, 19,000 cpm) was treated with cyanogen bromide for 24 h in 70% formic acid. The cleavage products were separated on a 12.5% polyacrylamide gel, stained with Coomassie Blue (lane 1), and subsequently fluorographed (lane 2). The arrow indicates the labeled Coomassie band that was excised for amino acid sequence analysis. This 17-kDa peptide represented 50–100 pmol of material and contained 83% of the radioactivity recovered from the gel by direct radiometric analysis of excised gel slices after correction for background.
To locate the radiolabeled fragment of 17 kDa within the sequence of human lamin B, the peptides that had been generated by cyanogen bromide cleavage were electrophoretically transferred to a polyvinylidene difluoride membrane and stained with Coomassie Blue. Then the 17-kDa band containing the [3H]MVA-derived radiolabel was excised and partially sequenced by automated stepwise Edman degradation. It was possible to sequence 18 amino acid residues into the labeled 17-kDa peptide. In each sequencing cycle, a major amino acid peak was seen indicating that the excised cyanogen bromide fragment was nearly pure. When the sequence was compared with the cDNA-inferred amino acid sequence of human lamin B (Fig. 6), it mapped with near identity to a portion of the predicted human lamin B sequence (30), beginning 117 amino acids from the carboxyl terminus. It also mapped to a similar region in lamin B-like proteins from the mouse (18), Xenopus (31) and Drosophila (32). The labeled peptide appeared to contain the carboxyl terminus of the protein because the corresponding portion of the cDNA-inferred sequence of human lamin B contains no additional methionine residues. Lastly, and most importantly there is only a single cysteine residue in this entire predicted stretch of amino acids, the cysteine in the highly conserved Cys-A-A-X motif. Therefore, these results combined with those from the chromatography and Raney nickel experiments strongly suggest that lamin B contains a farnesyl group that is linked by a thioether bond to a cysteine residue near the carboxyl end of the protein.
Fig. 6. Alignment of the partial sequence of the labeled 17-kDa, cyanogen bromide cleavage product with the predicted carboxyl-terminal amino acid sequence of human lamin B.
The 17-kDa labeled fragment (50–100 pmol) shown in Fig. 5, was transferred to a polyvinylidene difluoride membrane and partially sequenced. The resulting sequence of 18 amino acids (CNRr) was compared with the inferred sequence of human lamin B (HLb). Only the carboxyl 118 amino acids of lamin B are shown for comparison. The partial sequence mapped with near identity to the human lamin B sequence beginning at amino acid 482. The sequence mapped to this same position in the Xenopus LI sequence with an alignment score of 5.3 standard deviations. The conserved carboxyl Cys-A-A-X motif is underlined.
DISCUSSION
Lamin B was recently shown to be modified by a derivative of MVA (5). The results of the present study provide evidence that this derivative is linked to cysteine through a thioether bond, that it is identical to a farnesyl group, and that it is located at or near the carboxyl terminus of the protein. Two types of evidence suggest that the derivative is linked to cysteine. First, when proteolytic hydrolysates of [3H]MVA- and [35S]cysteine-labeled lamin B were successively chromatographed in five different systems, they consistently yielded comigrating peaks of hydrophobic, radioactive material. Second, Raney nickel-catalyzed hydrogenolysis of the MVA-labeled material from these hydrolysates or of intact, MVA-labeled lamin B effectively released the bound radioactivity into a pentane-soluble form. In addition, a similar hydrogenolysis procedure effectively released a farnesyl moiety from an authentic standard of S-farnesyl cysteine. However, a mild base hydrolysis procedure did not have these effects. Therefore, the radioactive MVA-labeled material in lamin B is most probably linked to a cysteine residue through a thioether linkage.
When pentane-soluble material that had been released from intact, MVA-labeled lamin B was analyzed by gas-liquid chromatography, a major peak of radioactivity was observed. The retention time of this peak was similar to that of a peak of pentane-soluble material that had been released from the S-farnesyl cysteine standard. When the pentane-soluble material from lamin B was hydrogenated over platinum and then analyzed by gas-liquid chromatography, a single peak of radioactivity was seen with a retention time nearly identical to that of farnesane. Mass spectral analysis of material prepared from much larger amounts of lamin B showed that the released ligand was identical to the triene released from authentic S-farnesyl cysteine.
Preliminary evidence suggests that the thioether-modified cysteine in lamin B is located at or near the carboxyl terminus. First, treatment of MVA-labeled lamin B with cyanogen bromide generated a single, major, radiolabeled fragment in high yield. A comparison of the partial amino acid sequence of this labeled fragment with the cDNA-inferred sequence of human lamin B indicated that the fragment contained the protein’s carboxyl-terminal domain. Second, based on this comparison, the labeled fragment would be predicted to contain only a single cysteine residue, viz. the invariant cysteine that is associated with the carboxyl-terminal Cys-A-A-X sequence motif. We therefore propose that this cysteine residue is the attachment site for the MVA-derived modification, analogous to the modification of similarly located cysteine residues in yeast a factor and p21ras.
Several key questions remain to be answered regarding the precise structure of the modified carboxyl-terminal domain of lamin B. One question concerns the point of attachment of the cysteine sulfur atom to the farnesyl group. Although it is likely that the cysteine is attached to the terminal carbon of the isoprene, this has not been conclusively determined in the present study. A number of different structures in which the sulfur is attached at different locations on the trimethyldodecatriene skeleton would yield an identical fragment after cleavage with Raney-nickel. A second question relates to the exact location of the thioether-modified cysteine within the carboxyl-terminal domain. The cDNA-inferred sequence of human lamin B ends with -RSCAIM, but the carboxyl-terminal sequence of the mature protein has yet to be determined. The modified cysteine in lamin B might conceivably be the carboxyl-terminal amino acid because this is the case for yeast a factor and p21ras (10, 15) which have the same predicted carboxyl-terminal Cys-A-A-X motif. If cysteine is in fact the carboxyl-terminal amino acid of lamin B, a third question would arise concerning its carboxyl group. The corresponding carboxyl groups of yeast a factor and p21ras have been shown to be methylated (10, 15). Therefore, this possibility would have to be considered for a carboxyl-terminal cysteine in lamin B. Indeed, lamin B has already been shown to be methylated in a cell cycle-dependent manner (33). Although the site or sites of methylation of lamin B have yet to be established, one obvious possibility is that the carboxyl group of a carboxyl-terminal cysteine might be involved. Experiments are underway to isolate a small farnesylated peptide from the COOH terminus of lamin B so that these remaining structural questions can be addressed.
These questions warrant special attention in view of the possibility that the carboxyl-terminal domain of lamin B might have a special functional role. Thus, preliminary experiments by other investigators have suggested that the carboxyl-terminal region of lamin B may be required for assembly of the nuclear lamina and in particular that one or more of the last six predicted amino acid residues at the carboxyl terminus might be of critical importance (34). In addition, a putative receptor for lamin B has recently been identified in nuclear membrane preparations (35, 36). It is therefore conceivable that a cysteine residue modified with a farnesyl group at the carboxyl terminus of lamin B might mediate receptor binding on the inner nuclear membrane. Once the complete structure of the carboxyl terminusis known, this possibility should be relatively easy to test.
Acknowledgments
We are pleased to acknowledge Drs. Ken Walsh and Harry Charbonneau for helpful discussion and for sequencing the 17-kDa fragment, Dr. Larry Gerace for his gift of the anti-lamin antibodies, Dr. William N. Howald and the Mass Spectrometry Facility of the Department of Medicinal Chemistry, University of Washington, for help with the mass spectral analyses, Dr. K. Michael Pollard for sharing his human lamin B cDNA sequence prior to publication, and H.-K. Lin for synthesis of the isomeric trienes.
Footnotes
This work was supported by the Howard Hughes Medical Institute.
Note Added in Proof-We have synthesized all four possible cis/trans-isomers of 2,6,10-trimethyl-2,6,10-dodecatriene by a Wittig reaction between geranyl acetone or neryl acetone and the ylide derived from (ethyl)triphenylphosphorium bromide. In addition, the all-trans-isomer was prepared by treatment of all-trans-farnesyl bromide with super hydride. The structures of all compounds were confirmed by 1H and 13C NMR in comparison with the published spectral values (Nishino, C., and Bowers, W. S. (1976) Tetrahedron 32, 2875). Furthermore, all four isomeric trienes can be well separated by GC, and the comigration of the material from lamin B with the all-trans-isomer establishes an all-trans-structure for the farnesyl group attached to the protein.
The abbreviations used are: MVA, mevalonic acid; GLC, gas-liquid chromatography; GC/MS, gas-liquid chromatography-coupled mass spectrometry; HPLC, high performance liquid chromatography; SDS, sodium dodecyl sulfate; MOPS, 4-morpholineethanesulfonic acid.
References
- 1.Schroepfer GJ. Annu Reu Biochem. 1981;50:585–621. doi: 10.1146/annurev.bi.50.070181.003101. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt RA, Schneider CJ, Glomset J. J Biol Chem. 1984;259:10175–10180. [PubMed] [Google Scholar]
- 3.Sinensky M, Logel J. Proc Natl Acad Sci U S A. 1985;82:3257–3261. doi: 10.1073/pnas.82.10.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maltese WA, Sheridan KM. J Cell Physiol. 1987;133:471–481. doi: 10.1002/jcp.1041330307. [DOI] [PubMed] [Google Scholar]
- 5.Wolda SL, Glomset JA. J Biol Chem. 1988;263:5997–6000. [PubMed] [Google Scholar]
- 6.Beck LA, Hosick TJ, Sinensky M. J Cell Biol. 1988;107:1307–1316. doi: 10.1083/jcb.107.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakagami Y, Yoshida M, Isogai A, Suzuki A. Science. 1981;212:1525–1526. doi: 10.1126/science.212.4502.1525. [DOI] [PubMed] [Google Scholar]
- 8.Kamiya Y, Sakura A, Tamura S, Takahash N, Tsuchiya E, Abe K, Fukui S. Agric Biol Chem. 1979;43:363–369. [Google Scholar]
- 9.Fujino M, Kitada C, Sakagami Y, Isogai A, Tamura S, Suzuki A. Naturwissenschaften. 1980;67:406–408. [Google Scholar]
- 10.Anderegg RJ, Betz R, Carr SA, Crabb JW, Duntze W. J Biol Chem. 1988;263:18236–18240. [PubMed] [Google Scholar]
- 11.Towler DA, Gordon JI, Adams SP, Glaser L. Annu Rev Biochem. 1988;57:69–99. doi: 10.1146/annurev.bi.57.070188.000441. [DOI] [PubMed] [Google Scholar]
- 12.Chen ZQ, Ulsh LS, DuBois G, Shih T. J Virol. 1985;56:607–612. doi: 10.1128/jvi.56.2.607-612.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hancock JF, Magee AI, Childs JE, Marshall CJ. Cell. 1989;57:1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- 14.Schafer WR, Kim R, Sterne R, Thorner J, Kim SH, Rine J. Science. 1989;245:379–385. doi: 10.1126/science.2569235. [DOI] [PubMed] [Google Scholar]
- 15.Clarke S, Vogel JP, Deschenes RJ, Stock J. Proc Natl Acad Sci U S A. 1988;85:4643–4677. doi: 10.1073/pnas.85.13.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Betz R, Crabb JW, Meyer HE, Wittig R, Duntze W. J Biol Chem. 1987;262:546–548. [PubMed] [Google Scholar]
- 17.Gutierrez L, Magee AI, Marshall CJ, Hancock JF. EMBO J. 1989;8:1093–1098. doi: 10.1002/j.1460-2075.1989.tb03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoger TH, Krohne G, Franke WW. Eur J Cell Biol. 1988;47:283–290. [PubMed] [Google Scholar]
- 19.Kaneko I, Hazama-Shimada Y, Endo A. Eur J Biochem. 1978;87:313–321. doi: 10.1111/j.1432-1033.1978.tb12380.x. [DOI] [PubMed] [Google Scholar]
- 20.Aebi U, Cohn J, Buhle L, Gerace L. Nature. 1986;323:463–468. [Google Scholar]
- 21.Braatz JA, McIntire KR. Prep Biochem. 1977;7:495–509. doi: 10.1080/00327487708065516. [DOI] [PubMed] [Google Scholar]
- 22.Konigsberg WH, Henderson L. Methods Enzymol. 1983;91:254–259. doi: 10.1016/s0076-6879(83)91022-4. [DOI] [PubMed] [Google Scholar]
- 23.Dugan LL, Demediuk P, Pendley CE, Horrocks LA. J Chromatgr. 1986;378:317–327. doi: 10.1016/s0378-4347(00)80728-8. [DOI] [PubMed] [Google Scholar]
- 24.Skipski VP, Peterson RF, Barclay M. Biochem J. 1964;90:374–379. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaffer MH, Stark GR. Biochem Biophys Res Commun. 1976;71:1040–1047. doi: 10.1016/0006-291x(76)90759-2. [DOI] [PubMed] [Google Scholar]
- 26.Olson EN, Towler DA, Glaser L. J Biol Chem. 1985;260:3784–3790. [PubMed] [Google Scholar]
- 27.Knapp DR. Handbook of Analytical Derivatization Reactions. John Wiley & Sons; New York: 1979. p. 30. [Google Scholar]
- 28.Matsudaira P. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 29.Hemming FW. MTP Int Reu Sci Series One Biochem. 1974;4:39–97. [Google Scholar]
- 30.Pollard KM, Chan EK, Sullivan KF, Glass C, Tan EM. J Cell Biol. 1988;107:249. doi: 10.1128/mcb.10.5.2164. (abstr.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krohne G, Wolin SL, McKeon FD, Franke WW, Kirschner MW. EMBO J. 1987;6:3801–3808. doi: 10.1002/j.1460-2075.1987.tb02716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gruenbaum Y, Landesman Y, Drees B, Bare JW, Saumweber H, Paddy MR, Sedat JW, Smith DE, Benton BM, Fisher PA. J Cell Biol. 1988;106:585–596. doi: 10.1083/jcb.106.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chelsky D, Olson JF, Koshland DE., Jr J Biol Chem. 1987;262:4303–4309. [PubMed] [Google Scholar]
- 34.Hoger T, Waizenegger I, Krohne G. Biochem Cell Biol. 1988;66:s32.3. (abstr.) [Google Scholar]
- 35.Worman HJ, Yuan J, Blobel G, Georgatos SD. Proc Natl Acad Sci US A. 1988;85:8531–8534. doi: 10.1073/pnas.85.22.8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Senior A, Gerace L. J Cell Biol. 1988;107:2029–2036. doi: 10.1083/jcb.107.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]