Abstract
This review summarizes the epidemiology of cancer of the female reproductive system and associated lifestyle factors. It also assesses the available evidence for occupational factors associated with these cancers. Cervical, endometrial, and ovarian cancers are relatively common, and cause significant cancer morbidity and mortality worldwide, whereas vulvar, vaginal, fallopian tube cancers, and choriocarcinomas are very rare. As several lifestyle factors are known to play a major role in the etiology of these cancers, very few published studies have investigated possible relationships with occupational factors. Some occupational exposures have been associated with increased risks of these cancers, but apart from the available evidence on the relationships between asbestos fibers and ovarian cancer, and tetrachloroethylene and cervical cancer, the data is rather scarce. Given the multifactorial nature of cancers of the female reproductive system, it is of the utmost importance to conduct occupational studies that will gather detailed data on potential individual confounding factors, in particular reproductive history and other factors that influence the body's hormonal environment, together with information on socio-economic status and lifestyle factors, including physical activity from multiple sources. Studies on the mechanisms of carcinogenesis in the female reproductive organs are also needed in order to elucidate the possible role of chemical exposures in the development of these cancers.
Keywords: Uterine cervical neoplasms, Endometrial neoplasms, Ovarian neoplasms, Vaginal neoplasms, Occupational exposure
Introduction
Cancers of the female reproductive system - namely cancer of the cervix uteri (cervical cancer), cancer of the corpus uteri (which includes mostly adenocarcinomas originating in the endometrium and some other rarer cancers, such as sarcomas), ovarian, vulvar, vaginal, fallopian tube cancers, and choriocarcinoma - are an important cause of cancer morbidity and mortality worldwide. Cervical, endometrial, and ovarian cancers are relatively common (Fig. 1), whereas vulvar, vaginal, fallopian tube cancers, and choriocarcinomas are very rare.
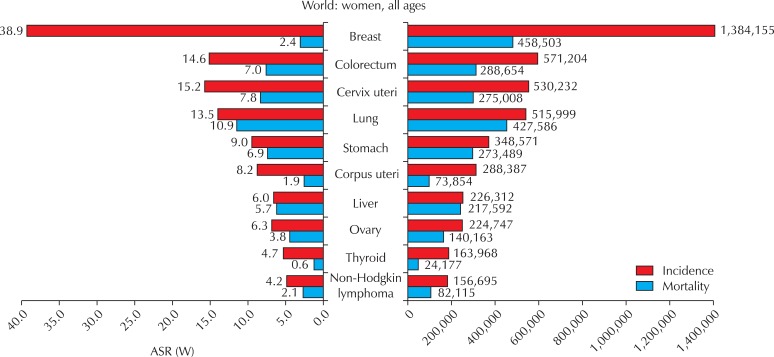
Fig. 1.
Cancer incidence and mortality among females. GLOBOCAN, 2008 [1]. ASR (W): world age-standardized incidence rate.
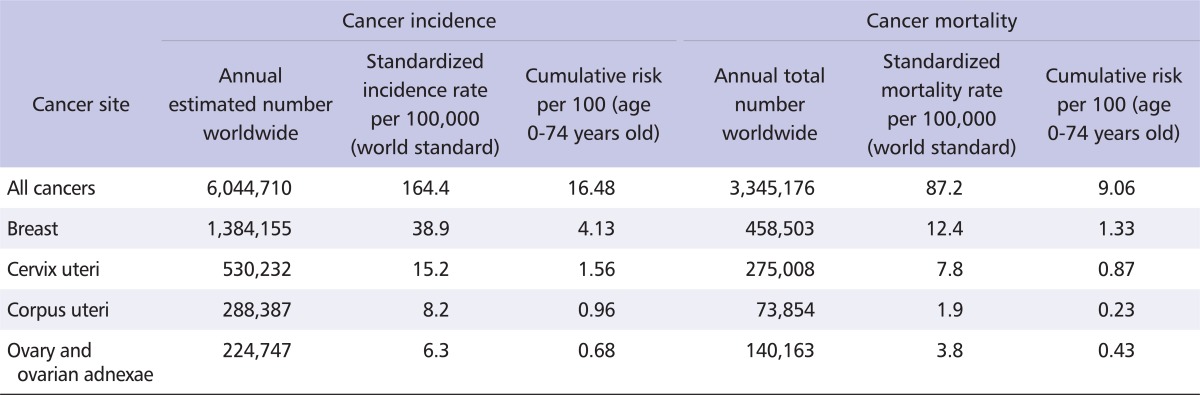
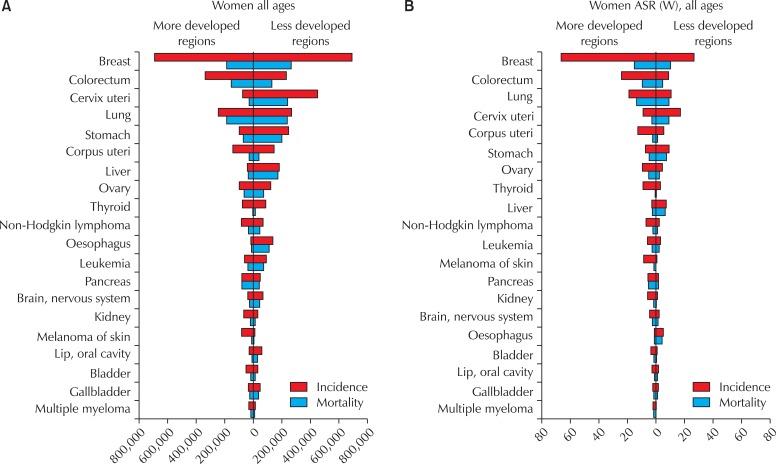
Cervical cancer is the third most common cancer in women worldwide, behind breast and colorectal cancers; it is also the seventh most common cancer overall, with an estimated 530,232 new cases in 2008 (Table 1) [1]. More than 85% of the global burden of cervical cancer occurs in less developed regions, where it accounts for 13% of all cancers in women. High standardized incidence rates (greater than 20 per 100,000 women) are found in Eastern, Western, and Southern Africa, South-Central Asia, South America, Melanesia, and Central Africa. Rates are lowest in Western Asia, North America, and Australia/New Zealand (less than 6 per 100,000 women) [1]. The overall mortality : incidence ratio of cervical cancer is 52%; it was responsible for 275,000 deaths in 2008, about 88% of which occurred in less developed regions (Fig. 2).
Table 1.
Statistics on selected cancer sites among women worldwide, GLOBOCAN, 2008 [1]
Fig. 2.
Cancer incidence and mortality among women in more and less developed regions of the world. ASR (W): world age-standardized incidence rate. GLOBOCAN, 2008 [1].
Endometrial cancer is the sixth most common cancer in women worldwide, with an estimated 288,387 new cases in 2008, and a standardized incidence rate of 8.2 per 100,000 women (Table 1). While the global burden, in terms of the number of cases, is evenly distributed between less developed and more developed regions (Fig. 2A), incidence and mortality rates are higher in more developed regions (Fig. 2B). North America and Western Europe show some of the highest standardized incidence rates (greater than 10 per 100,000 women), with the lowest rates occurring in Asia and Africa [2]. Overall, the mortality : incidence ratio of endometrial cancer is 26%, and it was responsible for 73,854 deaths in 2008.
Cancers of the ovary and ovarian adnexae, including fallopian tube cancer, constitute the eighth most common cancers among women worldwide (Fig. 1), with 224,747 incident cases (standardized incidence rate 6.3 per 100,000 women); 140,163 deaths are estimated to have occurred in 2008. Both more developed and less developed regions of the world are affected (Fig. 2), although the incidence rates are at least twice as high in Europe and North America than in Asia and Africa [1,2]. The mortality : incidence ratio is 62% (Table 1).
The worldwide number of new cancers of the female genitalia is unknown for most countries. However, it can be estimated based on the incidence rates in countries where information is available [3]. In 2002, the estimated number of new cancers of the female genitalia worldwide was 40,000. The age-standardized incidence rates of vulvar cancer worldwide are estimated to vary between 0.5 and 1.5 per 100,000, without a clear geographical pattern. The standardized incidence rates of vaginal cancer are estimated to be between 0.3 and 0.7 per 100,000 in most countries [4,5].
Choriocarcinomas constitute about 0.6% of all cancers of the female reproductive system. In 2002, there were about 5,800 cases reported worldwide, the vast majority occurring in less developed regions. Age-standardized incidence rates range from 0.04 in Southern Africa and Northern Europe to 0.43 per 100,000 women in South-East Asia [6,7]. In Vietnam, the incidence rate has been reported to be 1.98 per 100,000 women [7].
So far, the etiology of cancers of the female reproductive system has been primarily attributed to lifestyle factors. As women contribute substantially to the labor market, it is important to assess whether environmental factors are also at play. This review summarizes the epidemiology of cancers of the female reproductive system and associated lifestyle factors. It also assesses the available evidence for occupational factors associated with these cancers.
Methods
A summary of known non-occupational risk factors was prepared through a systematic review of the scientific literature published in the English language through 2011, including textbooks and scientific articles.
A list of peer-reviewed scientific literature, focusing on the occupational risk factors of cancers of the female reproductive system published between 1981 and 2011, was obtained through searches of the Medline and Embase bibliographic databases. The following keywords were used: occupation* or work*, AND neoplasms or cancers, AND female, AND cervix uteri or cervical, corpus uteri or endometri*, ovar*, vulva*, vagina*, fallopian tube, and choriocarcinoma. The reference lists of the retrieved papers were also searched for additional relevant papers. Finally, the International Agency for Research on Cancer (IARC) monographs (http://monographs.iarc.fr/) [8] were consulted for further information on carcinogenicity.
Etiology and Lifestyle-Related Risk Factors
Cervical cancer
There are 2 main histological types of cervical cancer: squamous cell carcinoma (SCC) and adenocarcinoma. As for several other cancer types, when diagnosed in early stages, the prognosis of patients with cervical cancer is good (5-year survival rate above 90%), but when diagnosed in advanced stages, the prognosis is extremely poor, even in countries with high-quality health care facilities available to all patients. The introduction of cervical cancer screening has dramatically reduced cervical cancer mortality in several countries. However, in areas where screening is not available, such as less developed regions, cervical cancer is a major cause of cancer death among women [1]. In countries where cervical cancer screening is available, mortality is concentrated among women who do not participate in screening, or those above the recommended screening age [9].
Cervical cancer is caused by persistent infection with human papilloma virus (HPV) types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, or 59; persistent infections with HPV16 and 18 cause about 70% of all cervical cancers worldwide. Persistent infection with HPV types 26, 53, 66, 67, 68, 70, 73, or 82 may also be causally related to cervical cancer. The recent introduction of mass vaccination against HPV16 and 18 in several countries is expected, in the long term, to dramatically decrease the incidence of and mortality from cervical cancer. However, the full benefit of mass HPV vaccination will not be observed for several decades. Therefore, screening will remain an essential tool in reducing cervical cancer mortality.
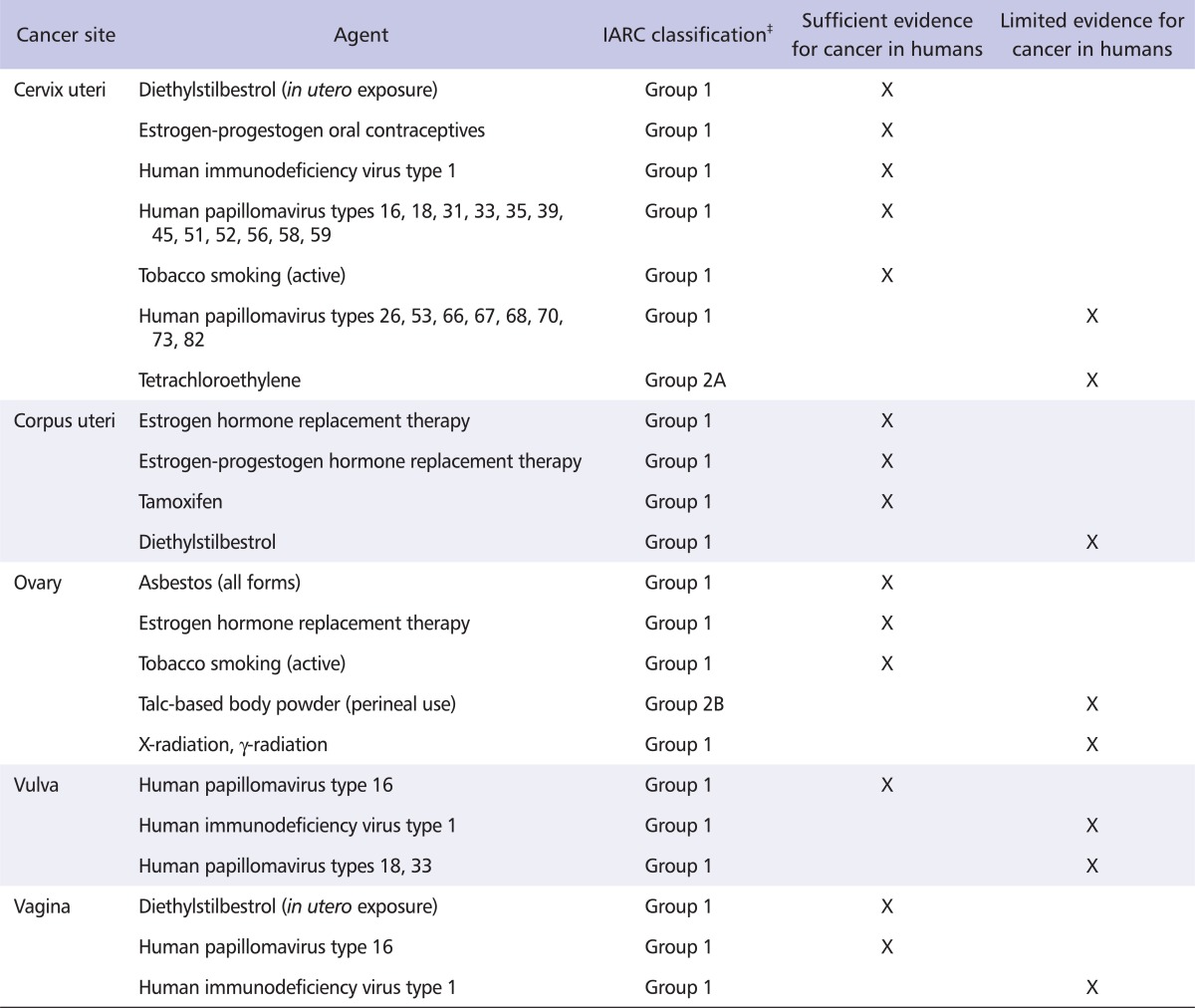
Other exposures that are considered carcinogenic to the cervix uteri are in utero exposure to diethylstilbestrol (associated with SCC of the cervix), use of combined estrogen-progestogen oral contraceptives (associated with both in situ and invasive cervical cancer), human immunodeficiency virus type 1 (HIV1) infection, and tobacco smoking [8].
Endometrial cancer
Endometrial cancer affects postmenopausal women almost exclusively. There are several histological subtypes of endometrial cancer, the most common being of epithelial origin, which can be further classified as adenocarcinomas of endometrioid type or Type I (70-80% of all endometrial cancers, including mucinous and adenosquamous tumors) or as adenocarcinomas of non-endometrioid type or Type II (20-30% of all endometrial cancers, including serous, mucinous, and clear cell histology, as well as rarer types such as carcinosarcomas and undifferentiated carcinomas) [8]. Type I endometrial cancer is usually hormone sensitive, and occurs in women exposed to estrogens unopposed by progesterone. It is well differentiated with mild to moderate nuclear pleiomorphism, and has a low potential for myometrial invasion and metastasis [10]. Type II endometrial cancer usually arises from atrophic endometrial tissues, and is poorly differentiated. It is not associated with estrogen or progestogen stimulation, has a high probability of myometrial invasion and metastasis, and a very poor prognosis [11-14]. Overall, 5-year survival for endometrial adenocarcinomas is over 90% when diagnosed in early stages (i.e., localized disease), but less than 50% when the disease is diagnosed in advanced stages (with distant metastases).
Endometrial cancer risk has been previously associated with several host factors, including high body mass index, nulliparity or low parity, early age at first birth, history of type 2 diabetes mellitus (non-insulin dependent), and family history of cancer, particularly endometrial cancer. In addition, endogenous hormone levels have been positively associated with endometrial cancer risk in several prospective cohort studies [15], while cigarette smoking has been associated with a decreased risk [16]. Although high body mass index has been associated with endometrial cancer risk, no dietary factor has been singled out as being etiologically associated with any certainty [17]. Alcohol consumption is not associated with endometrial cancer risk [18].
Both estrogen-only and combined estrogen-progestogen hormone replacement therapy are classified as recognized causes of endometrial cancer [8]. The increased risk for estrogen-induced endometrial cancer decreases with the number of days per month that progestogens are added to the regimen. Tamoxifen, a drug mainly used to prevent breast cancer recurrence or its occurrence in high-risk women, has also been linked to endometrial cancer with sufficient evidence in humans [8]. There is evidence suggesting a lack of carcinogenicity, with an inverse relationship observed between the use of combined estrogen-progestogen oral contraceptives and endometrial cancer. In addition, a positive association has been observed between exposure to diethylstilbestrol and endometrial cancer [8].
Mesenchymal tumors occurring in the corpus uteri are aggressive and rare. The main histological types are carcinosarcoma, leiomyosarcoma, endometrial stromal sarcoma, and undifferentiated endometrial sarcoma [19]. Some studies define carcinosarcomas as poorly differentiated metaplastic carcinomas [20]. Depending on the histological classification used, uterine sarcomas represent about 3 to 9% of cancers of the corpus uteri and 1% of all cancers of the female reproductive system [19,21]. The prognosis for certain histological types, such as uterine sarcoma, is quite poor; overall 5-year survival ranges from 17 to 53% [21-23]. For endometrial stromal sarcoma, the prognosis is better than for other uterine sarcomas.
Uterine sarcomas are of largely unknown etiology. Possible etiological factors include a history of pelvic radiation, obesity, long use of estrogen hormone replacement therapy or tamoxifen, and use of oral contraceptives [24-26]. The incidence of uterine sarcoma also varies between races; the age-adjusted incidence for Blacks has been reported at twice that of Whites and more than twice that of women of other races [26,27].
Ovarian cancer
The etiology of ovarian cancer is not well understood. An excellent in-depth review on this subject has recently been published [28], and we refer interested readers to this review for more detailed information. Briefly, ovarian cancers are usually classified according to the cell types they originate from: epithelial (about 90%), stromal (5%), or germ cell (less than 5%) [29]. Epithelial ovarian cancer can be further classified into the histological subtypes of serous, mucinous, endometrioid and clear cell [30].
According to the IARC, there is sufficient evidence that epithelial ovarian cancer is caused by estrogen hormone replacement therapy and tobacco smoking, and limited evidence regarding perineal use of talc-based body powder and exposure to X-radiation and gamma (γ)-radiation (for medical purposes) [8]. Besides these risk factors, having a family history of the disease increases risk, as does being a carrier of mutations in BRCA1/BRCA2 genes [31], or being affected by hereditary non-polyposis colorectal cancer syndrome. Several studies indicate that height and body weight are associated with risk, in particular among non-users of hormone replacement therapy. On the other hand, there are a few factors known to be associated with a decreased risk of ovarian cancer, such as high parity and use of oral contraceptives, and possibly breastfeeding, incomplete pregnancies, hysterectomy and tubal ligation [28].
Studies on other potential risk factors, such as obesity, sedentary lifestyle and alcohol consumption, have yielded inconsistent results [8,17].
Other cancers of the female reproductive system
The majority of vulvar cancers are SCC; 3 histological subtypes of SCC (basaloid, warty and verrucous), and the precursor lesion vulvar intraepithelial neoplasia, are associated with HPV infection [4,32]. There is sufficient evidence that infection with HPV16 causes vulvar cancer, and limited evidence regarding infection with HPV18 or 33, and with HIV1.
There are 2 main histological types of vaginal cancer, SCC and adenocarcinoma, and a rarer histological subtype, clear cell carcinoma. Most vaginal cancers are SCC, and many are preceded by vaginal intraepithelial neoplasia. There is sufficient evidence that HPV16 is causally related to vaginal cancer, and limited evidence that HIV1 is also associated with risk [8]. Diethylstilbestrol causes clear cell adenocarcinoma in the vagina of women who were exposed in utero [8,33]; simultaneous or prior cancers of the female reproductive system confer an increased risk, especially if the women have been treated with pelvic irradiation [32].
The etiology of fallopian tube cancer is not well understood, probably because of the rarity of the disease, which makes studies rather difficult. The vast majority of reported cases are serous adenocarcinomas; clinical patterns, diagnosis, treatment, and prognosis are similar to those of ovarian cancers. Parity and sterilization procedures seem to decrease this risk. Infections with Chlamydia trachomatis (which may cause salpingitis) or HPV do not seem to be associated with increased risk [34].
Most choriocarcinomas derive from the placental trophoblastic tissue. The known risk factors include maternal age (women younger than 20 or over 40 years), a previous history of hydatidiform mole (another trophoblastic disease), and possibly use of oral contraceptives [7].
Occupational Exposures and Cancers of the Female Reproductive System
The IARC and its series of monographs on carcinogenic risks to humans are recognized worldwide as dependable sources when it comes to identifying carcinogenic agents and circumstances. Carcinogenic agents are classified using a 5-category classification system: Group 1 agents are deemed carcinogenic to humans; Group 2A, agents probably carcinogenic to humans; Group 2B, agents possibly carcinogenic to humans; Group 3, agents not classifiable as to their carcinogenicity to humans; and Group 4, agents probably not carcinogenic to humans [35]. The evidence considered by the working groups in order to classify the agents comes mainly from human and animal studies. Thus, some agents may be classified as carcinogenic to humans based on sufficient evidence in humans, or limited evidence in humans, but sufficient evidence in animals. Finally, an agent can be considered carcinogenic to a certain organ, but not necessarily to another one.
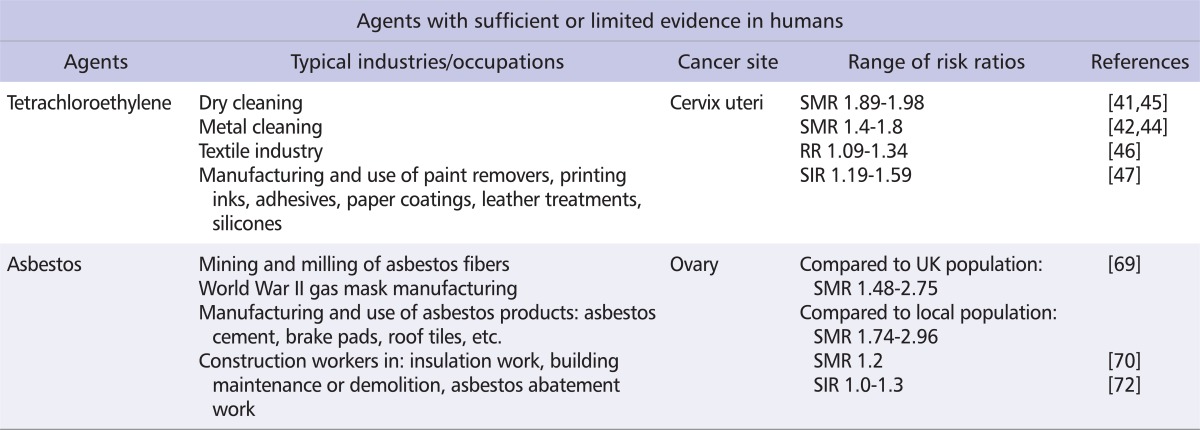
Table 2 shows the known or suspected causes of cancers of the female reproductive system, abstracted from a summary of the IARC Monographs (Table 2) [8]. Two of these agents or exposure circumstances are directly related to occupational exposures: asbestos (Group 1 agent), which is considered to be carcinogenic to the human ovary [36], and tetrachloroethylene (Group 2A agent), which is considered to be probably carcinogenic to the human cervix uteri [37] (Table 3). Exposure to other agents with sufficient evidence of carcinogenicity (Group 1 agents) to the human cervix uteri, corpus uteri, ovary, vulva, or vagina generally occurs though medical treatments (diethylstilbestrol, oral contraceptives or hormone replacement therapy, X-radiation and γ-radiation), environmental exposure (atomic bomb survivors), personal lifestyle habits (smoking, perineal use of talc-based body powder), or viral infections (HIV1, and several HPV types) [8,38].
Table 2.
Known and suspected causes of cancers of the female reproductive system*, as identified in the IARC Monographs, volumes 1-102†
*As of the end of 2011, the IARC had not classified any agent as a recognized or suspected carcinogen (Group 1, 2A or 2B) to the human fallopian tube.
†This table is adapted from Cogliano et al. [8] and does not include risk factors not covered in IARC Monographs volumes 1-102, notably reproductive and other hormonal factors, diet and nutritional factors, and genetic susceptibility traits.
‡Group 1: carcinogenic to humans, Group 2A: probably carcinogenic to humans, Group 2B: possibly carcinogenic to humans.
IARC: International Agency for Research on Cancer.
Table 3.
Known and suspected occupational causes of cancers of the female reproductive system, as identified in the International Agency for Research on Cancer Monographs
SMR: standardized mortality ratio, RR: relative risk, SIR: standardized incidence ratio.
Cervical cancer
Tetrachloroethylene
Tetrachloroethylene (or perchloroethylene) has been used as 1 of the main dry cleaning fluids around the world since the early 1950s. It is currently used as a solvent in metal cleaning and in the textile industry, but it is also a component of paint removers, printing inks, adhesives, paper coatings, and leather treatments, and is also a carrier solvent for silicones [37]. In the early 1980s, it was estimated that 688,000 workers in the US had potentially been exposed to tetrachloroethylene [39]. Approximate estimates of the number of exposed workers in the European Union were 820,000 workers in the early 1990s [40].
The available human evidence considered by the IARC Working Group to classify tetrachloroethylene as probably carcinogenic to the cervix uteri comes from 3 cohort studies. However, exposure to other chlorinated solvents in these studies could not be excluded, nor was it possible to control for potential confounding factors [37]. Two cohort studies of dry cleaners showed an excess risk of 60 to 70%, based on 8 [41] and 21 deaths [42], respectively, whereas a cohort of workers monitored for tetrachloroethylene exposure reported 2 cases of cervical cancer [43].
Since the publication of the IARC Monograph, updates of the 2 cohorts of dry cleaners confirmed the increased risk of cervical cancer with exposure to tetrachloroethylene, with excess risks of 60% (standardized mortality ratio [SMR] 1.6, 95% confidence interval [CI] 1.0-2.3, based on 27 deaths) [44] and 95% (SMR 1.95, 95% CI 1.00-3.40) [45]. A Swedish record-linkage study reported a small increased risk for women registered as dry cleaning workers at the time of either the 1960 or the 1970 censuses. However, women who were registered as working in the industry at the time of both censuses showed no such increase [46]. A recent cohort study of Swedish dry cleaners and laundry workers found a small, non-statistically significant excess risk of cervical cancer, based on 25 cases (standardized incidence ratio [SIR] 1.25, 95% CI 0.81-1.85), but the 19 cases exposed exclusively to tetrachloroethylene had an even smaller risk of 1.19 [47]. The studies published since the IARC's last evaluation in 1995 unfortunately did not take into account potential confounding factors for cervical cancer, such as HPV infection and other socio-economic factors. Therefore, they do not strengthen the evidence for an association between the dry cleaning industry, in which tetrachloroethylene is the main solvent used, and an increased risk of cervical cancer.
Other occupational exposures
Several job titles have been associated with an increased risk of cervical cancer in more than 1 study, but most of these studies were exploratory in nature and did not adjust for important potential confounders such as socio-economic status and HPV infection. Examples of these job titles are: hotel/restaurant personnel and waitresses, food preparers, machine operators, cleaners, upholsterers, dry cleaners, beverage workers, other construction workers, and drivers [41,42,44,45,48-51]. Women working in agriculture also appear to be at an increased risk [49-54]. A cohort study of professional firefighters in Florida reported a 5-fold increased risk of cervical cancer, unadjusted for lifestyle habits [55]. A Swedish registry-based cohort study found a 39% non-significant increase in risk associated with shift work (SIR 1.39, 95% CI 0.82-2.19), however, the definition of shift work used in the study was very rough, including occupations in which at least 40% of the workers reported working rotating shifts (3 shifts per day), or workers who worked at least 1 night in the week preceding the interview [56].
A Finnish record-linkage study reported excess risks of cervical cancer of about 20 to 40% with exposures to aliphatic and alicyclic, aromatic, and chlorinated hydrocarbon solvents. The authors reported similar excess risks with silica and wood dust exposures, after standardization by birth cohort, follow-up period, and socio-economic status [57]. A study using a similar design reported a 48% increased risk of cervical cancer among Swedish workers exposed to diesel exhaust fumes, with a suggestion of a dose-response relationship [58]. Certain textile workers exposed to organic dusts, solvents, and dyes have been found to have small increases in cervical cancer risk in record-linkage studies [49,57]. A cohort study of textile workers also reported an excess risk (SIR 1.82, 95% CI 1.19-2.67) that was further increased in women who had worked in the industry for 10 years or more (SIR 2.44, 95% CI 1.21-4.35). Once again, the estimates were not adjusted for potential confounding factors [59].
An exposure circumstance that had not been identified previously is also worth mentioning. A Finnish record-linkage study explored cancer risk among workers exposed to molds of agricultural and industrial origin, and to bacteria of non-human origin, attributing exposures using a job-exposure matrix. The authors reported that women in the highest category of mold and of bacterial exposure had cervical cancer relative risks (RR) of 3.1 (95% CI 1.0-9.2) and 2.6 (95% CI 1.5-4.7), respectively [60].
Regarding cervical cancer, we can conclude that, apart from occupational exposure to tetrachloroethylene, which has been classified as probably carcinogenic to the human cervix uteri (Group 2A agent), all other occupational exposures for which there is some evidence of an association require well-designed confirmatory studies with proper adjustment for potential confounding factors.
Endometrial cancer
None of the agents or circumstances classified as carcinogenic to the corpus uteri by the IARC are related to occupational exposures. Some exposures have been associated with an increased risk of endometrial cancer in a few studies, but the results were not solid enough to support their classification as carcinogenic. It is generally believed that the role of further environmental or occupational factors in the causation of endometrial cancer is unclear and probably small [57].
For example, occupations involving professional or administrative tasks, such as those of teacher, secretary, telephone operator, and musician [49,51,61-63], have all been associated with increased risks of endometrial cancer. A Swedish registry-based cohort study did not find increased risks associated with shift work, but the definition of shift work used in the study was very rough, including occupations in which at least 40% of the workers reported working rotating shifts (3 shifts per day), or workers who worked at least 1 night in the week preceding the interview [56]. A cohort study in the US reported an increased risk among nurses who worked at least 20 years in rotating shifts; the risk was larger in a subgroup of obese nurses, after adjustment for potential confounding factors (body mass index > 30 kg/m2; RR 2.09, 95% CI 1.24-3.52), and increased with the duration of shift work [64]. All of these occupations are sedentary, which is consistent with the idea that physical activity is a protective factor for endometrial cancer [65].
A case-control study among Italian agricultural communities reported an increased risk of corpus uteri cancer among women who worked in farming occupations for 10 to 19 years (odds ratio [OR] 2.4, 95% CI 1.0-5.9) [53].
A record-linkage study reported an excess endometrial cancer risk of 1.2 among Finnish women in jobs that involved exposure to animal dust, and of 1.3 for women working in sedentary jobs, after standardization by birth cohort, follow-up period, socio-economic status, mean parity, and mean age at first birth by occupation [57].
Most studies did not look at specific subtypes of corpus uteri cancers. One record-linkage study in the Nordic countries focused on the possible occupational etiology of uterine sarcomas. SIRs of leiomyosarcoma and endometrial stromal sarcoma were computed for 53 occupational categories [51]. The occupational groups with significantly increased SIRs of leiomyosarcoma were shoe and leather workers (SIR 2.59, 95% CI 1.12-5.11), farmers (SIR 1.62, 95% CI 1.18-2.17), and teachers (SIR 1.38, 95% CI 1.07-1.76). The SIR for domestic assistants was 0.64 (95% CI 0.41-0.96). No occupation had an elevated SIR of endometrial stromal sarcoma [66].
In conclusion, no particular occupational exposure appears to convey an excess risk of endometrial cancer. However, as physical activity is a modifiable lifestyle factor that affects the risk of endometrial cancer, occupations with a moderate to high intensity of physical activity may help reduce the risk by contributing to the total amount of regular physical activity.
Ovarian cancer
Asbestos
Although asbestos has been banned or restricted in several countries, it is estimated that 125 million people are still exposed to asbestos fibers in the workplace [67]. Apart from the mining and milling of asbestos fibers, occupational exposures mainly occur during the manufacturing and use of asbestos products (asbestos cement, brake pads, roof tiles, etc.), building insulation, maintenance and demolition, and asbestos abatement work [39]. With respect to lung cancer or mesothelioma risk, there appears to be differences in potency according to the type and dimension of the fibers, although the overall conclusion is that all types of asbestos fibers are carcinogenic to humans [36]. Approximate estimates of the number of exposed workers in the early 1990s were 682,000 in the US [68], and 1.2 million in the European Union [40].
The available human evidence used by the IARC to classify asbestos fibers as carcinogenic to the ovary [36] comes from cohort studies of women who manufactured gas masks during World War II [69,70], and from studies suggesting that asbestos can accumulate in the ovaries of occupationally exposed women [71]. In particular, the study of 2 cohorts of women in the United Kingdom who manufactured gas masks reported a larger mortality risk from ovarian cancer for women exposed to crocidolite and chrysotile fibers than for those exposed to chrysotile fibers only: the former group had a risk of dying from ovarian cancer 2.96 times that of the non-exposed women in the area; women exposed to chrysotile only had a risk 1.74 times that of the non-exposed women in the area [69]. A smaller cohort study of another group of United Kingdom workers also showed a borderline significant increased risk of 1.8 (95% CI 0.9-3.3) of dying from ovarian cancer [70].
A borderline increased risk of 1.3 (95% CI 0.9-1.8) was reported by a study linking census-based job titles of Finnish women with subsequent risk of incident ovarian cancer, after translating job titles into the exposure to asbestos using a Finnish job-exposure matrix (FINJEM), and adjusting for reproductive factors [72]. A Russian study also reported a significantly elevated risk of mortality among bookbinders (SMR 2.9, 95% CI 1.5-5.0) who were exposed to asbestos-contaminated talc fillers in paper [73].
X-radiation and γ-radiation
Workers are exposed to X-radiation or γ-radiation when handling radioactive materials (e.g., health care workers, nuclear energy workers, industrial radiographers, and nuclear weapons workers) or because of natural sources of radiation in the workplace (e.g., aircraft personnel exposed to cosmic radiation or miners exposed to radon) [38]. The United Nations Scientific Committee on Exposure to Atomic Radiation estimated in 2008 that about 13 million workers were exposed to natural sources of ionizing radiation, whereas another 9.8 million were exposed to artificial sources; medical workers are considered to constitute about two-thirds of the latter group of workers [74]. It appears that the annual occupational effective doses have been diminishing regularly, and in 2000-2002 they were estimated to vary between 0.1 and 1.0 millisieverts annually for exposures to artificial sources, compared to an annual average of 2.9 millisieverts for exposure to natural sources [74].
The available human evidence of a relationship between ovarian cancer and exposure to X-radiation and γ-radiation has been classified as limited (Table 2) [8], and no mention of increased ovarian cancer risk was suggested in relation to occupational exposures by the IARC Working Group [38].
Studies published since the last IARC evaluation still report inconsistent results. A death certificate study of US health care workers reported a statistically significant risk of mortality among radiologic technicians (mortality OR 1.8, 95% CI 1.2-2.8) [61]. However, a cohort study of US radiologic technologists did not report any increased risk of incidence of [75] or mortality from [76] ovarian cancer. A study of Chinese medical X-ray workers mentioned a small, non-statistically significant increased risk, but did not provide the actual risk estimates for ovarian cancer [77]. A cohort study of workers at a US uranium production facility did not show an increased risk among the workers exposed to radiation, but there was only one death from ovarian cancer and no incident cases between 1946 and 1995 [78]. A cohort study of French nuclear energy production workers reported a small increased risk of ovarian cancer and cancer of other and unspecified female genital organs (International Classification of Diseases 9th revision codes 183 and 184, SMR 1.1, 90% CI 0.76-1.56) [79]. Analyses of the Canadian National Dose Registry did not find increased risks of incident ovarian cancer in women exposed to ionizing radiation in the workplace [80,81]. A few other studies, utilizing various methods, did not find increased risks of ovarian cancer incidence or mortality with exposure to ionizing radiation or with the occupation of radiologic technician [51,72,77,82]. In summary, if occupational exposures to X-radiation or γ-radiation do confer an increased risk of ovarian cancer, their overall impact is likely to be limited compared to other risk factors.
Other occupational exposures
During the last 10 years, relatively few studies reported on occupational exposures in relation to ovarian cancer. Many of these studies were record-linkage studies from the Nordic countries; this is worth mentioning because the risks obtained with these designs are likely to be diluted toward the null value due to aggregate-level data and possible misclassification of exposures and job titles [72].
Hormones, anti-neoplastic drugs, or other pharmaceuticals
Estrogen hormone replacement therapy has been classified as carcinogenic to the human ovary [83], but occupational exposures to these pharmaceuticals were not considered by the IARC Working Group. Very little additional data is available. Hormonal effects have been reported in workers exposed to steroids (e.g., gynecomastia and loss of libido in men and menstrual problems in women) [84]. A well-designed cohort study among employees with possible exposure to chemical, pharmacological, or biological agents in a pharmaceutical company in Sweden reported 2 cases of ovarian cancer, as expected [85]. A few record-linkage studies reported small or non-existent increased risks of incident ovarian cancer in pharmacy technicians or workers in the pharmaceutical industry [51,82,86,87]. A death certificate study reported a statistically significant risk of mortality among pharmacists (mortality OR 2.4, 95% CI 1.6-3.7) [61]. Thus, there is not enough evidence to conclude that fabrication or handling of pharmaceutical drugs is associated with an increased risk of ovarian cancer.
Organic solvents, aromatic hydrocarbons, and exhaust fumes
Increased risks of ovarian cancer have been associated with occupational exposure to several organic solvents in studies of different designs. Record-linkage studies conducted in the Nordic countries showed indications of increased risks for exposure to aromatic hydrocarbon solvents (SIR 1.3, 95% CI 1.0-1.7) [72]. They also mentioned that solvent use among occupations associated with ovarian cancer suggests that aliphatic and aromatic hydrocarbons may be etiologically important [82]. The latter study reported increased risks for several job titles that are associated with solvent exposure, such as shoe worker (RR 1.82, 95% CI 1.01-3.3), graphic worker (RR 1.58, 95% CI 1.02-2.5), and worker in the machine and electronics industry (RR 1.26, 95% CI 1.01-1.6) [82]. Another record-linkage study found a small increased risk for printers [51]. A cohort study of printing industry workers reported an increased risk among bookbinders; the authors pointed out that bookbinders were exposed to solvents, glues, and paper dust [73]. Results for dry cleaners were inconsistent: one study reported no increase in the risk of ovarian cancer in Finland [72], whereas a small increased risk was found in a Swedish study [82] of similar design. In summary, although several studies have found an excess risk of ovarian cancer among women occupationally exposed to organic solvents or to aromatic hydrocarbons, the available evidence is still limited, owing to the scanty exposure information in most studies.
Two Finnish studies reported a 2- to 3-fold increased risk of ovarian cancer associated with exposure to diesel engine exhaust fumes [72,88]; the same studies also reported a 50 to 70% increased risk associated with exposure to gasoline exhaust fumes. These findings have to be replicated in other contexts and with other study designs before definite conclusions can be drawn on the effect of exposure to exhaust fumes on ovarian cancer.
Specific job titles
Several clerical and professional occupations, such as teacher, librarian, nurse, secretary, retail sales clerk, and others, have repeatedly been associated with a small excess risk of ovarian cancer incidence or mortality in different settings, often in studies based on routinely collected data [49,51,61-63,89]. A cohort study of agricultural workers in Northern Italy did not find an increased risk of mortality from ovarian cancer among women working on farms [53]. Moreover, a multi-center case-control study found similar results for cancer incidence [90]. However, a large cohort study of agricultural workers in the US recently reported an increased risk among private pesticide applicators (relative SIR 2.88, 95% CI 1.50-5.54, based on 9 cases) [91].
An IARC Working Group recently concluded that a modest excess risk of ovarian cancer appeared to be linked to the occupation of hairdresser and related occupations, but the lack of adjustment for potential confounders did not allow for confounding to be ruled out [92]. A recent meta-analysis of 10 studies, published between 1977 and 2003 on ovarian cancer among hairdressers and related occupations, concluded that there was a small excess risk of about 16% [93]. An excess risk of the same magnitude was also reported by a recent record-linkage study [51]. A large cohort study of female cosmetologists and manicurists in California did not find an increased risk of incident ovarian cancer, but the cohort was young (less than 20% of the cohort was 50 years of age or older) and there was no adjustment for reproductive factors [94].
Overall, apart from occupational exposure to asbestos fibers, which is recognized by the IARC as being carcinogenic to the human ovary, the other occupational exposures evoked so far have to be considered as hypotheses that need confirmation in carefully designed individual-level studies.
Other cancers of the female reproductive system
A recent review of the IARC Monographs did not identify any other occupational exposure that could be causally related to other cancers of the female reproductive system [8] (Table 2). Very little additional information is available on the possible role of occupational factors in the etiology of these cancers. Primary cancers of the vulva, vagina, fallopian tube, or choriocarcinoma are rare, and very few studies mention them individually, or even as a group. We found one record-linkage cohort study of Swedish hairdressers that reported no increased risk for cancers of the female reproductive system other than ovarian, cervical, and endometrial cancers [95].
A recent record-linkage study reported elevated SIRs of less than 20% for cancer of the vulva among domestic assistants and building caretakers [51]. The other available evidence linking occupational exposures to vulvar cancer relies on single studies, the findings of which have not been replicated. One case-control study reported 'moderately high' ORs among private household maids and servants, and work in laundry, cleaning, and other garment services [96].
An excess risk of 2.6 was found for vaginal cancer among chemical process workers, whereas the risk was lower for building caretakers (SIR 1.30). The authors noted that no occupational risk factors had been previously identified for these cancers and that HPV infection was a well-known risk factor that could not be adjusted for in the study [51].
Using the same study design, Riska and colleagues [97] recently reported 2- to 4-fold increased risks of fallopian tube cancer among smelting workers (based on 6 cases), artistic workers (n = 14), and hairdressers (n = 25). The authors stressed that their results have to be validated by studies with individual information on important potential confounding factors, such as socio-economic status, reproductive history, and lifestyle factors [97].
Finally, an elevated risk of choriocarcinoma has been reported among nurses (based on 4 cases) and agricultural workers (n = 2), but only in one study (a Finnish record-linkage study) [98].
Mechanisms of Carcinogenesis
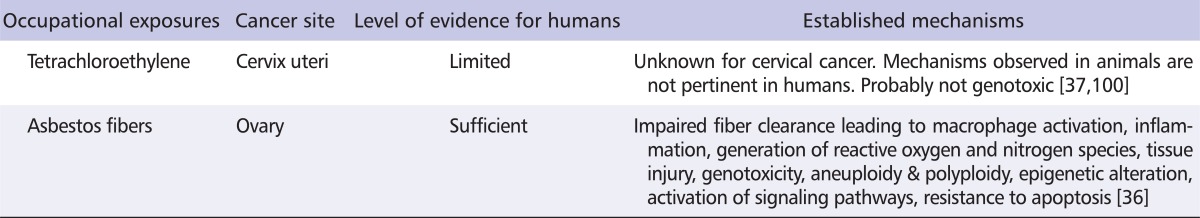
Information on the mechanisms involved in the development of any cancer varies with the biological activity of each carcinogen and covers changes in physiology, as well as functional changes at the cellular and at the molecular level [99]. The mechanisms of carcinogenesis have been described extensively for asbestos fibers, essentially in the lungs and the pleura. As translocation of fibers to the ovaries has been demonstrated [71], it can be presumed that similar mechanisms are responsible for ovarian carcinogenesis (Table 4). However, the mechanisms possibly responsible for the carcinogenicity of tetrachloroethylene have not yet been elucidated. A few pathways have been proposed for organs other than the cervix uteri based on animal models (e.g., peroxisome proliferation), but humans are relatively insensitive to those pathways [100].
Table 4.
Established mechanisms of carcinogenesis in the ovary and the cervix uteri
To date, there is no molecular marker that can be used to identify specific occupational exposures that are related to cancers of the female reproductive system.
Conclusion
In conclusion, a few studies suggest that some occupational exposures are associated with an increased risk of cancers of the female reproductive system. However, apart from the evidence for asbestos fibers and tetrachloroethylene, the data is rather scarce. As lifestyle habits are known to play a major role in the etiology of these cancers, most published studies did not gather information on occupational history. Given the multifactorial nature of cancers of the female reproductive system, it is of the utmost importance to conduct occupational studies that will gather detailed data on potential individual confounding factors, in particular reproductive history and other factors that influence the body's hormonal environment, together with information on socio-economic status and lifestyle factors, including physical activity from multiple sources.
Studies on the mechanisms of carcinogenesis in female reproductive organs are also needed in order to elucidate the possible role of chemical exposures in the development of these cancers.
Acknowledgments
The authors thank Ms Margrethe Meo for technical assistance and Ms Trudy Perdrix-Thoma for language review and editing.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2, Cancer incidence and mortality worldwide: IARC CancerBase No. 10 [Internet] Lyon (France): International Agency for Research on Cancer; 2010. [cited 2011 Aug 15]. Available from: http://globocan.iarc.fr/ [Google Scholar]
- 2.Merritt MA, Cramer DW. Molecular pathogenesis of endometrial and ovarian cancer. Cancer Biomark. 2010;9:287–305. doi: 10.3233/CBM-2011-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer incidence in five continents, Vol. VIII. IARC Scientific Publication No. 155. Lyon (France): International Agency for Research on Cancer; 2002. p. 781. [Google Scholar]
- 4.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24(Suppl 3):S3, 11–25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 5.Bosch FX, Cardis E. Cancer incidence correlations: genital, urinary and some tobacco-related cancers. Int J Cancer. 1990;46:178–184. doi: 10.1002/ijc.2910460206. [DOI] [PubMed] [Google Scholar]
- 6.Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: the size of the problem. Best Pract Res Clin Obstet Gynaecol. 2006;20:207–225. doi: 10.1016/j.bpobgyn.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Altieri A, Franceschi S, Ferlay J, Smith J, La Vecchia C. Epidemiology and aetiology of gestational trophoblastic diseases. Lancet Oncol. 2003;4:670–678. doi: 10.1016/s1470-2045(03)01245-2. [DOI] [PubMed] [Google Scholar]
- 8.Cogliano VJ, Baan R, Straif K, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Wild CP. Preventable exposures associated with human cancers. J Natl Cancer Inst. 2011;103:1827–1839. doi: 10.1093/jnci/djr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrae B, Andersson TM, Lambert PC, Kemetli L, Silfverdal L, Strander B, Ryd W, Dillner J, Törnberg S, Sparén P. Screening and cervical cancer cure: population based cohort study. BMJ. 2012;344:e900. doi: 10.1136/bmj.e900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherman ME. Theories of endometrial carcinogenesis: a multidisciplinary approach. Mod Pathol. 2000;13:295–308. doi: 10.1038/modpathol.3880051. [DOI] [PubMed] [Google Scholar]
- 11.Basil JB, Goodfellow PJ, Rader JS, Mutch DG, Herzog TJ. Clinical significance of microsatellite instability in endometrial carcinoma. Cancer. 2000;89:1758–1764. doi: 10.1002/1097-0142(20001015)89:8<1758::aid-cncr16>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 12.Faquin WC, Fitzgerald JT, Lin MC, Boynton KA, Muto MG, Mutter GL. Sporadic microsatellite instability is specific to neoplastic and preneoplastic endometrial tissues. Am J Clin Pathol. 2000;113:576–582. doi: 10.1309/4mgm-fmrc-6awk-yqy2. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton CA, Cheung MK, Osann K, Chen L, Teng NN, Longacre TA, Powell MA, Hendrickson MR, Kapp DS, Chan JK. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer. 2006;94:642–646. doi: 10.1038/sj.bjc.6603012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hendrickson M, Ross J, Eifel P, Martinez A, Kempson R. Uterine papillary serous carcinoma: a highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982;6:93–108. doi: 10.1097/00000478-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Eliassen AH, Hankinson SE. Endogenous hormone levels and risk of breast, endometrial and ovarian cancers: prospective studies. Adv Exp Med Biol. 2008;630:148–165. [PubMed] [Google Scholar]
- 16.International Agency for Research on Cancer (IARC) Monographs on the evaluation of carcinogenic risks to humans. Volume 100: A review of human carcinogens. Part E: Personal habits and indoor combustions. Lyon (France): IARC; 2012. pp. 102–105. [PMC free article] [PubMed] [Google Scholar]
- 17.World Cancer Research Fund, American Insitute for Cancer Research (AICR) Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington DC: AICR; 2007. p. 517. [Google Scholar]
- 18.Secretan B, Straif K, Baan R, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens--Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033–1034. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 19.McMeekin DS. Sarcoma of the uterus. In: DiSaia PA, Creasman WT, editors. Clinical gynecologic oncology. 7th ed. Philadelphia (PA): Mosby Elsevier; 2007. pp. 185–199. [Google Scholar]
- 20.McCluggage WG. Uterine carcinosarcomas (malignant mixed Mullerian tumors) are metaplastic carcinomas. Int J Gynecol Cancer. 2002;12:687–690. doi: 10.1136/ijgc-00009577-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Abeler VM, Røyne O, Thoresen S, Danielsen HE, Nesland JM, Kristensen GB. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology. 2009;54:355–364. doi: 10.1111/j.1365-2559.2009.03231.x. [DOI] [PubMed] [Google Scholar]
- 22.Kokawa K, Nishiyama K, Ikeuchi M, Ihara Y, Akamatsu N, Enomoto T, Ishiko O, Motoyama S, Fujii S, Umesaki N. Clinical outcomes of uterine sarcomas: results from 14 years worth of experience in the Kinki district in Japan (1990-2003) Int J Gynecol Cancer. 2006;16:1358–1363. doi: 10.1111/j.1525-1438.2006.00536.x. [DOI] [PubMed] [Google Scholar]
- 23.Koivisto-Korander R, Butzow R, Koivisto AM, Leminen A. Clinical outcome and prognostic factors in 100 cases of uterine sarcoma: experience in Helsinki University Central Hospital 1990-2001. Gynecol Oncol. 2008;111:74–81. doi: 10.1016/j.ygyno.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Wickerham DL, Fisher B, Wolmark N, Bryant J, Costantino J, Bernstein L, Runowicz CD. Association of tamoxifen and uterine sarcoma. J Clin Oncol. 2002;20:2758–2760. doi: 10.1200/JCO.2002.20.11.2758. [DOI] [PubMed] [Google Scholar]
- 25.Arenas M, Rovirosa A, Hernández V, Ordi J, Jorcano S, Mellado B, Biete A. Uterine sarcomas in breast cancer patients treated with tamoxifen. Int J Gynecol Cancer. 2006;16:861–865. doi: 10.1111/j.1525-1438.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 26.Brooks SE, Zhan M, Cote T, Baquet CR. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989-1999. Gynecol Oncol. 2004;93:204–208. doi: 10.1016/j.ygyno.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 27.Harlow BL, Weiss NS, Lofton S. The epidemiology of sarcomas of the uterus. J Natl Cancer Inst. 1986;76:399–402. [PubMed] [Google Scholar]
- 28.Permuth-Wey J, Sellers TA. Epidemiology of ovarian cancer. Methods Mol Biol. 2009;472:413–437. doi: 10.1007/978-1-60327-492-0_20. [DOI] [PubMed] [Google Scholar]
- 29.Chen VW, Ruiz B, Killeen JL, Coté TR, Wu XC, Correa CN. Pathology and classification of ovarian tumors. Cancer. 2003;97(10 Suppl):2631–2642. doi: 10.1002/cncr.11345. [DOI] [PubMed] [Google Scholar]
- 30.Averette HE, Nguyen H. Gynecologic cancer. In: Murphy GP, Lawrence L Sr, Lenhard RE, editors. American Cancer Society textbook of clinical oncology. 2nd ed. Atlanta (GA): American Cancer Society; 1995. pp. 552–579. [Google Scholar]
- 31.Iarmarcovai G, Bonassi S, Botta A, Baan RA, Orsière T. Genetic polymorphisms and micronucleus formation: a review of the literature. Mutat Res. 2008;658:215–233. doi: 10.1016/j.mrrev.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Tavassoli FA, Devilee P International Agency for Research on Cancer (IARC) IARC WHO classification of tumours series, vol. 4. Pathology and genetics of tumours of the breast and female genital organs. Lyon (France): IARC; 2003. p. 432. [Google Scholar]
- 33.Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971;284:878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- 34.Riska A, Leminen A. Determinants of incidence of primary fallopian tube carcinoma (PFTC) Methods Mol Biol. 2009;472:387–396. doi: 10.1007/978-1-60327-492-0_18. [DOI] [PubMed] [Google Scholar]
- 35.Agents classified by the International Agency for Research on Cancer (IARC) Monographs, volumes 1-102 [Internet] Lyon (France): IARC; 2011. [cited 2011 Aug 15]. Available from: http://monographs.iarc.fr/ENG/Classification/ClassificationsGroupOrder.pdf. [Google Scholar]
- 36.Straif K, Benbrahim-Tallaa L, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Guha N, Freeman C, Galichet L, Cogliano V WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens--part C: metals, arsenic, dusts, and fibres. Lancet Oncol. 2009;10:453–454. doi: 10.1016/s1470-2045(09)70134-2. [DOI] [PubMed] [Google Scholar]
- 37.International Agency for Research on Cancer (IARC) Monographs on the evaluation of carcinogenic risks to humans. Lyon (France): IARC; 1995. Volume 63: Dry cleaning, some chlorinated solvents and other industrial chemicals; p. 551. [PMC free article] [PubMed] [Google Scholar]
- 38.International Agency for Research on Cancer (IARC) Monographs on the evaluation of carcinogenic risks to humans. Lyon (France): IARC; 2000. Volume 75: Ionizing radiation. Part 1: X- and gamma (γ)-radiation, and neutrons; p. 491. [Google Scholar]
- 39.National Toxicology Program (NTP) Report on carcinogens. 12th ed. Research Triangle Park (NC): Department of Health and Human Services, Public Health Service, NTP; 2011. p. 499. [Google Scholar]
- 40.Kauppinen T, Toikkanen J, Pedersen D, Young R, Ahrens W, Boffetta P, Hansen J, Kromhout H, Maqueda Blasco J, Mirabelli D, de la Orden-Rivera V, Pannett B, Plato N, Savela A, Vincent R, Kogevinas M. Occupational exposure to carcinogens in the European Union. Occup Environ Med. 2000;57:10–18. doi: 10.1136/oem.57.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruder AM, Ward EM, Brown DP. Cancer mortality in female and male dry-cleaning workers. J Occup Med. 1994;36:867–874. [PubMed] [Google Scholar]
- 42.Blair A, Stewart PA, Tolbert PE, Grauman D, Moran FX, Vaught J, Rayner J. Cancer and other causes of death among a cohort of dry cleaners. Br J Ind Med. 1990;47:162–168. doi: 10.1136/oem.47.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anttila A, Pukkala E, Sallmén M, Hernberg S, Hemminki K. Cancer incidence among Finnish workers exposed to halogenated hydrocarbons. J Occup Environ Med. 1995;37:797–806. doi: 10.1097/00043764-199507000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Blair A, Petralia SA, Stewart PA. Extended mortality follow-up of a cohort of dry cleaners. Ann Epidemiol. 2003;13:50–56. doi: 10.1016/s1047-2797(02)00250-8. [DOI] [PubMed] [Google Scholar]
- 45.Ruder AM, Ward EM, Brown DP. Mortality in dry-cleaning workers: an update. Am J Ind Med. 2001;39:121–132. doi: 10.1002/1097-0274(200102)39:2<121::aid-ajim1000>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 46.Travier N, Gridley G, De Roos AJ, Plato N, Moradi T, Boffetta P. Cancer incidence of dry cleaning, laundry and ironing workers in Sweden. Scand J Work Environ Health. 2002;28:341–348. doi: 10.5271/sjweh.684. [DOI] [PubMed] [Google Scholar]
- 47.Seldén AI, Ahlborg G., Jr Cancer morbidity in Swedish dry-cleaners and laundry workers: historically prospective cohort study. Int Arch Occup Environ Health. 2011;84:435–443. doi: 10.1007/s00420-010-0582-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savitz DA, Andrews KW, Brinton LA. Occupation and cervical cancer. J Occup Environ Med. 1995;37:357–361. doi: 10.1097/00043764-199503000-00013. [DOI] [PubMed] [Google Scholar]
- 49.Sala M, Dosemeci M, Zahm SH. A death certificate-based study of occupation and mortality from reproductive cancers among women in 24 US states. J Occup Environ Med. 1998;40:632–639. doi: 10.1097/00043764-199807000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Carpenter L, Roman E. Cancer and occupation in women: identifying associations using routinely collected national data. Environ Health Perspect. 1999;107(Suppl 2):299–303. doi: 10.1289/ehp.99107s2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pukkala E, Martinsen JI, Lynge E, Gunnarsdottir HK, Sparén P, Tryggvadottir L, Weiderpass E, Kjaerheim K. Occupation and cancer - follow-up of 15 million people in five Nordic countries. Acta Oncol. 2009;48:646–790. doi: 10.1080/02841860902913546. [DOI] [PubMed] [Google Scholar]
- 52.Wesseling C, Ahlbom A, Antich D, Rodriguez AC, Castro R. Cancer in banana plantation workers in Costa Rica. Int J Epidemiol. 1996;25:1125–1131. doi: 10.1093/ije/25.6.1125. [DOI] [PubMed] [Google Scholar]
- 53.Settimi L, Comba P, Carrieri P, Boffetta P, Magnani C, Terracini B, Andrion A, Bosia S, Ciapini C, De Santis M, Desideri E, Fedi A, Luccoli L, Maiozzi P, Masina A, Perazzo PL, Axelson O. Cancer risk among female agricultural workers: a multi-center case-control study. Am J Ind Med. 1999;36:135–141. doi: 10.1002/(sici)1097-0274(199907)36:1<135::aid-ajim19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 54.Colt JS, Stallones L, Cameron LL, Dosemeci M, Zahm SH. Proportionate mortality among US migrant and seasonal farmworkers in twenty-four states. Am J Ind Med. 2001;40:604–611. doi: 10.1002/ajim.1126. [DOI] [PubMed] [Google Scholar]
- 55.Ma F, Fleming LE, Lee DJ, Trapido E, Gerace TA. Cancer incidence in Florida professional firefighters, 1981 to 1999. J Occup Environ Med. 2006;48:883–888. doi: 10.1097/01.jom.0000235862.12518.04. [DOI] [PubMed] [Google Scholar]
- 56.Schwartzbaum J, Ahlbom A, Feychting M. Cohort study of cancer risk among male and female shift workers. Scand J Work Environ Health. 2007;33:336–343. doi: 10.5271/sjweh.1150. [DOI] [PubMed] [Google Scholar]
- 57.Weiderpass E, Pukkala E, Vasama-Neuvonen K, Kauppinen T, Vainio H, Paakkulainen H, Boffetta P, Partanen T. Occupational exposures and cancers of the endometrium and cervix uteri in Finland. Am J Ind Med. 2001;39:572–580. doi: 10.1002/ajim.1056. [DOI] [PubMed] [Google Scholar]
- 58.Boffetta P, Dosemeci M, Gridley G, Bath H, Moradi T, Silverman D. Occupational exposure to diesel engine emissions and risk of cancer in Swedish men and women. Cancer Causes Control. 2001;12:365–374. doi: 10.1023/a:1011262105972. [DOI] [PubMed] [Google Scholar]
- 59.Kuzmickiene I, Didziapetris R, Stukonis M. Cancer incidence in the workers cohort of textile manufacturing factory in Alytus, Lithuania. J Occup Environ Med. 2004;46:147–153. doi: 10.1097/01.jom.0000111601.85534.12. [DOI] [PubMed] [Google Scholar]
- 60.Laakkonen A, Verkasalo PK, Nevalainen A, Kauppinen T, Kyyrönen P, Pukkala EI. Moulds, bacteria and cancer among Finns: an occupational cohort study. Occup Environ Med. 2008;65:489–493. doi: 10.1136/oem.2007.034017. [DOI] [PubMed] [Google Scholar]
- 61.Petralia SA, Dosemeci M, Adams EE, Zahm SH. Cancer mortality among women employed in health care occupations in 24 U.S. states, 1984-1993. Am J Ind Med. 1999;36:159–165. doi: 10.1002/(sici)1097-0274(199907)36:1<159::aid-ajim23>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 62.Bernstein L, Allen M, Anton-Culver H, Deapen D, Horn-Ross PL, Peel D, Pinder R, Reynolds P, Sullivan-Halley J, West D, Wright W, Ziogas A, Ross RK. High breast cancer incidence rates among California teachers: results from the California Teachers Study (United States) Cancer Causes Control. 2002;13:625–635. doi: 10.1023/a:1019552126105. [DOI] [PubMed] [Google Scholar]
- 63.MacArthur AC, Le ND, Abanto ZU, Gallagher RP. Occupational female breast and reproductive cancer mortality in British Columbia, Canada, 1950-94. Occup Med (Lond) 2007;57:246–253. doi: 10.1093/occmed/kqm002. [DOI] [PubMed] [Google Scholar]
- 64.Viswanathan AN, Hankinson SE, Schernhammer ES. Night shift work and the risk of endometrial cancer. Cancer Res. 2007;67:10618–10622. doi: 10.1158/0008-5472.CAN-07-2485. [DOI] [PubMed] [Google Scholar]
- 65.John EM, Koo J, Horn-Ross PL. Lifetime physical activity and risk of endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:1276–1283. doi: 10.1158/1055-9965.EPI-09-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koivisto-Korander R, Martinsen JI, Weiderpass E, Leminen A, Pukkala E. Incidence of uterine leiomyosarcoma and endometrial stromal sarcoma in Nordic countries: results from NORDCAN and NOCCA databases. Maturitas. 2012;72:56–60. doi: 10.1016/j.maturitas.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 67.Concha-Barrientos M, Nelson DI, Driscoll T, Steenland NK, Punnett L, Fingerhut MA, Prüss-Üstün A, Leigh J, Tak SW, Corvalan C. Selected occupational risk factors. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Comaprative quantification of health risks: global and regional burden of diseases attribuatable to selected major risk factors. vol. 1, 2. Geneva (Switzerland): World Health Organizatio; 2004. pp. 1651–1802. [Google Scholar]
- 68.Toxicological profile for asbestos [Internet] Atlanta (GA): Agency for Toxic Substances and Disease Registry, Centers for Disease Control and Prevention; 2001. [cited 2011 Aug 15]. Available from: http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=30&tid=4. [PubMed] [Google Scholar]
- 69.Acheson ED, Gardner MJ, Pippard EC, Grime LP. Mortality of two groups of women who manufactured gas masks from chrysotile and crocidolite asbestos: a 40-year follow-up. Br J Ind Med. 1982;39:344–348. doi: 10.1136/oem.39.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McDonald JC, Harris JM, Berry G. Sixty years on: the price of assembling military gas masks in 1940. Occup Environ Med. 2006;63:852–855. doi: 10.1136/oem.2006.028258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heller DS, Gordon RE, Westhoff C, Gerber S. Asbestos exposure and ovarian fiber burden. Am J Ind Med. 1996;29:435–439. doi: 10.1002/(SICI)1097-0274(199605)29:5<435::AID-AJIM1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 72.Vasama-Neuvonen K, Pukkala E, Paakkulainen H, Mutanen P, Weiderpass E, Boffetta P, Shen N, Kauppinen T, Vainio H, Partanen T. Ovarian cancer and occupational exposures in Finland. Am J Ind Med. 1999;36:83–89. doi: 10.1002/(sici)1097-0274(199907)36:1<83::aid-ajim12>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 73.Bulbulyan MA, Ilychova SA, Zahm SH, Astashevsky SV, Zaridze DG. Cancer mortality among women in the Russian printing industry. Am J Ind Med. 1999;36:166–171. doi: 10.1002/(sici)1097-0274(199907)36:1<166::aid-ajim24>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 74.United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) Sources and effects of ionizing radiation. UNSCEAR 2008 report to the General Assembly with scientific annexes. vol 1. New York (NY): United Nations; 2010. p. 20. [Google Scholar]
- 75.Sigurdson AJ, Doody MM, Rao RS, Freedman DM, Alexander BH, Hauptmann M, Mohan AK, Yoshinaga S, Hill DA, Tarone R, Mabuchi K, Ron E, Linet MS. Cancer incidence in the US radiologic technologists health study, 1983-1998. Cancer. 2003;97:3080–3089. doi: 10.1002/cncr.11444. [DOI] [PubMed] [Google Scholar]
- 76.Doody MM, Mandel JS, Lubin JH, Boice JD., Jr Mortality among United States radiologic technologists, 1926-90. Cancer Causes Control. 1998;9:67–75. doi: 10.1023/a:1008801404245. [DOI] [PubMed] [Google Scholar]
- 77.Wang JX, Zhang LA, Li BX, Zhao YC, Wang ZQ, Zhang JY, Aoyama T. Cancer incidence and risk estimation among medical x-ray workers in China, 1950-1995. Health Phys. 2002;82:455–466. doi: 10.1097/00004032-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 78.McGeoghegan D, Binks K. The mortality and cancer morbidity experience of workers at the Springfields uranium production facility, 1946-95. J Radiol Prot. 2000;20:111–137. doi: 10.1088/0952-4746/20/2/301. [DOI] [PubMed] [Google Scholar]
- 79.Telle-Lamberton M, Bergot D, Gagneau M, Samson E, Giraud JM, Néron MO, Hubert P. Cancer mortality among French Atomic Energy Commission workers. Am J Ind Med. 2004;45:34–44. doi: 10.1002/ajim.10306. [DOI] [PubMed] [Google Scholar]
- 80.Ashmore JP, Krewski D, Zielinski JM, Jiang H, Semenciw R, Band PR. First analysis of mortality and occupational radiation exposure based on the National Dose Registry of Canada. Am J Epidemiol. 1998;148:564–574. doi: 10.1093/oxfordjournals.aje.a009682. [DOI] [PubMed] [Google Scholar]
- 81.Sont WN, Zielinski JM, Ashmore JP, Jiang H, Krewski D, Fair ME, Band PR, Létourneau EG. First analysis of cancer incidence and occupational radiation exposure based on the National Dose Registry of Canada. Am J Epidemiol. 2001;153:309–318. doi: 10.1093/aje/153.4.309. [DOI] [PubMed] [Google Scholar]
- 82.Shields T, Gridley G, Moradi T, Adami J, Plato N, Dosemeci M. Occupational exposures and the risk of ovarian cancer in Sweden. Am J Ind Med. 2002;42:200–213. doi: 10.1002/ajim.10099. [DOI] [PubMed] [Google Scholar]
- 83.International Agency for Research on Cancer (IARC) Monographs on the evaluation of carcinogenic risks to humans. Lyon (France): IARC; 2011. Volume 100: A review of human carcinogens. Part A: Pharmaceuticals; p. 437. [Google Scholar]
- 84.Heron RJ, Pickering FC. Health effects of exposure to active pharmaceutical ingredients (APIs) Occup Med (Lond) 2003;53:357–362. doi: 10.1093/occmed/kqg115. [DOI] [PubMed] [Google Scholar]
- 85.Edling C, Friis L, Mikoczy Z, Hagmar L, Lindfors P. Cancer incidence among pharmaceutical workers. Scand J Work Environ Health. 1995;21:116–123. doi: 10.5271/sjweh.18. [DOI] [PubMed] [Google Scholar]
- 86.Hansen J, Olsen JH. Cancer morbidity among Danish female pharmacy technicians. Scand J Work Environ Health. 1994;20:22–26. doi: 10.5271/sjweh.1433. [DOI] [PubMed] [Google Scholar]
- 87.Hansen J, Olsen JH, Larsen AI. Cancer morbidity among employees in a Danish pharmaceutical plant. Int J Epidemiol. 1994;23:891–898. doi: 10.1093/ije/23.5.891. [DOI] [PubMed] [Google Scholar]
- 88.Guo J, Kauppinen T, Kyyrönen P, Heikkilä P, Lindbohm ML, Pukkala E. Risk of esophageal, ovarian, testicular, kidney and bladder cancers and leukemia among finnish workers exposed to diesel or gasoline engine exhaust. Int J Cancer. 2004;111:286–292. doi: 10.1002/ijc.20263. [DOI] [PubMed] [Google Scholar]
- 89.Robinson CF, Walker JT. Cancer mortality among women employed in fast-growing U.S. occupations. Am J Ind Med. 1999;36:186–192. doi: 10.1002/(sici)1097-0274(199907)36:1<186::aid-ajim26>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 90.Nanni O, Ravaioli A, Bucchi L, Falcini F, Ricci R, Buiatti E, Amadori D. Relative and absolute cancer mortality of women in agriculture in northern Italy. Eur J Cancer Prev. 2005;14:337–344. doi: 10.1097/00008469-200508000-00005. [DOI] [PubMed] [Google Scholar]
- 91.Koutros S, Alavanja MC, Lubin JH, Sandler DP, Hoppin JA, Lynch CF, Knott C, Blair A, Freeman LE. An update of cancer incidence in the Agricultural Health Study. J Occup Environ Med. 2010;52:1098–1105. doi: 10.1097/JOM.0b013e3181f72b7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.International Agency for Research on Cancer (IARC) Monographs on the evaluation of carcinogenic risks to humans. Volume 99: Some aromatic amines, organic dyes and related exposures. Lyon (France): IARC; 2010. p. 692. [PMC free article] [PubMed] [Google Scholar]
- 93.Takkouche B, Regueira-Méndez C, Montes-Martínez A. Risk of cancer among hairdressers and related workers: a meta-analysis. Int J Epidemiol. 2009;38:1512–1531. doi: 10.1093/ije/dyp283. [DOI] [PubMed] [Google Scholar]
- 94.Quach T, Doan-Billing PA, Layefsky M, Nelson D, Nguyen KD, Okahara L, Tran AN, Von Behren J, Reynolds P. Cancer incidence in female cosmetologists and manicurists in California, 1988-2005. Am J Epidemiol. 2010;172:691–699. doi: 10.1093/aje/kwq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Czene K, Tiikkaja S, Hemminki K. Cancer risks in hairdressers: assessment of carcinogenicity of hair dyes and gels. Int J Cancer. 2003;105:108–112. doi: 10.1002/ijc.11040. [DOI] [PubMed] [Google Scholar]
- 96.Mabuchi K, Bross DS, Kessler II. Epidemiology of cancer of the vulva. A case-control study. Cancer. 1985;55:1843–1848. doi: 10.1002/1097-0142(19850415)55:8<1843::aid-cncr2820550833>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 97.Riska A, Martinsen JI, Kjaerheim K, Lynge E, Sparen P, Tryggvadottir L, Weiderpass E, Pukkala E. Occupation and risk of primary fallopian tube carcinoma in Nordic countries. Int J Cancer. 2012;131:186–192. doi: 10.1002/ijc.26337. [DOI] [PubMed] [Google Scholar]
- 98.Loukovaara M, Pukkala E, Lehtovirta P, Leminen A. Epidemiology of choriocarcinoma in Finland, 1953 to 1999. Gynecol Oncol. 2004;92:252–255. doi: 10.1016/j.ygyno.2003.08.039. [DOI] [PubMed] [Google Scholar]
- 99.International Agency for Research on Cancer (IARC) Monographs on the evaluation of carcinogenic risks to humans. Preamble. Lyon (France): IARC; 2006. p. 25. [PMC free article] [PubMed] [Google Scholar]
- 100.Toxicological profile for tetrachloroethylene (PERC) [Internet] Atlanta (GA): Agency for Toxic Substances and Disease Registry, Centers for Disease Control and Prevention; 1997. [cited 2011 Aug 15]. Available from: http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=265&tid=48. [PubMed] [Google Scholar]