Abstract
Pancreatic cancers are typically resistant to chemo and radiation therapy and are predisposed to distant metastases. Circulating tumor cells (CTCs) are tumor cells disseminated from primary and metastatic sites and can be isolated from peripheral blood. CTC may overcome the limitation of the current available tumor markers, CA19-9. As a surrogate for ‘real-time biopsy’, CTCs allow recurrent assessment of a tumor’s biological activity. We review the current methodologies for CTCs extraction and characterization including antibody-based immunological assays, PCR-based assays, and novel technologies based on the physical or biological characteristics of CTCs. CTCs also provide an accessible link to the existence of epithelial to mesenchymal transition, tumor stem cell markers, and ongoing clonal mutations and epigenetic changes in the tumor. We also explore the potential of using CTC profiling in diagnosis, selection of neoadjuvant and adjuvant therapy, detection of recurrent disease, examination of pharmacodynamic biomarkers, as well as in gene therapy and immunotherapy for pancreatic cancer. Ongoing CTC characterization not only has the potential to represent all cells shed from primary pancreatic tumor and each metastatic site, but also allows dynamic sampling at multiple time points during the clinical course to identify the subpopulations of CTCs and the specific molecules driving metastasis and chemo resistance. We predict that CTC genotyping and phenotyping will play an increasing role in personalized therapy and in identification of novel therapeutic targets as well as monitoring the course and status of the disease.
Keywords: circulating tumor cells, pancreatic cancer
Introduction
Circulating tumor cells (CTCs) are tumor cells circulate through normal vessels and capillaries, and neovessles formed by tumor-induced angiogenesis [1, 2]. Metastases result from tumor cells migrating from the primary tumor sites to distant organs, such as lung, liver, bone or brain, and are directly responsible for most cancer-related deaths. CTCs were first described in the blood of a metastatic cancer patient were in the 19th century by Thomas Ashworth [3]. Since then, isolation and characterization of CTCs in patients suffering from a variety of different cancers have been an hot topic of scientific investigations [4]. CTCs have been shown to provide predictive and prognostic information in terms of disease relapse, overall survival, and tumor response to therapy in patients with metastatic or surgically resectable colorectal [5–7], breast [8, 9], prostate [10, 11], lung [12] and ovarian cancers [13]. Identification of genetic mutation in CTCs acquired during the therapeutic course is useful to herald drug resistance in patients with cancer [12]. However, the role of CTCs in one of the most deadly cancers, pancreatic cancer, has not been extensively studied. Herein we discuss current progress in CTC detection and its role in the management of pancreatic cancer.
Pancreatic cancer
Pancreatic cancer is one of most aggressive solid tumors clinically characterized by local invasion, early metastasis, and resistance to standard chemotherapy [14]. There were an estimated 44, 030 newly diagnosed cases of pancreatic cancer in the United States in 2011; a 15% increment compared to 2008. Pancreatic cancer has one of the lowest overall survivals among all types of cancers, making it the fourth leading cause of death due to cancer in the United States [15], and the eighth and ninth leading cause of cancer death in the world in males and females independently [16]. Currently, the surgical outcome in pancreatic cancer remains unsatisfactory as many post-operative patients exhibit distant metastasis shortly after surgery and succumb to death thereafter [17, 18].
Up to date, the best tumor marker for pancreatic cancer has been carbohydrate antigen 19-9 (CA19-9), a sialylated Lewis antigen of the Mucin 1 (MUC1) protein with an overall sensitivity of 80% and specificity of 82%. Unfortunately, patients with blood type of Lewis a- and b- genotype (5–10% of the Caucasian population) are incapable of synthesizing the CA19-9 epitope [19]. In addition, CA19-9 may also be elevated in patients with non-malignant diseases such as cirrhosis, chronic pancreatitis, cholangitis, obstructive jaundice as well as in other digestive tract cancers [20]. Even among patients with resectable pancreatic cancer, CA19-9 levels are not elevated in up to 35% of the patients [21]. Better biomarkers are needed for pancreatic cancer diagnosis and prediction of therapeutic efficacy.
CTCs are a potential marker for pancreatic cancer not only because of their specificity, but also because CTCs allow repeated study of tumor genetics, proteomics and molecular biology of the cancer cells as well as pharmacodynamics throughout a patient’s clinical course. Herein, we explore the potential role of CTCs in the diagnosis, staging, prognosis, and therapy of pancreatic cancer. If CTCs are shown to correlate with the risk of distant metastasis, isolation of CTCs would also allow more accurate study of “druggable targets” in the disseminated cancer cells and thus allow a more rational selection of adjuvant therapy for localized pancreatic cancer.
Isolation and characterization of CTCs
CTCs are typically present at low concentrations in cancer patients. Numerous assays for detecting CTCs have been developed. The current common approaches for detection of CTCs in patients with pancreatic cancer include: 1) immunological assays using antibodies directed against cell surface antigen; 2) PCR-based molecular assays for tumor-derived DNA or RNA extraction from CTCs; and 3) technologies based on physical or biological properties of cancer cells (Table 1).
Table 1.
Current methods for CTC isolation in pancreatic cancer
| CTCs isolation methods | Pros | Cons | |
|---|---|---|---|
| Antibodies directed against cell surface antigen | CellSearch® [4] [22] | FDA-approved method | Low sensitivity; EpCAM antibody dependent |
| microfluidi platform (CTC-chip) [24] herringbone-chip (HB-Chip) [25] | Capable of isolating viable CTCs; more sensitive than CellSearch® | EpCAM antibody dependent | |
| PCR-based molecular assays for tumor-derived DNA or RNA [26–30] | Sensitive for the studied nucleic acids | Non-specific; the origin of the nucleic acids may not be derived from CTCs | |
| CTCs isolation based on cells physical and biological properties: Dielectrophoretic Field-Flow Fractionation [31]; fiber-optic array–scanning technology and laser-scanning cytometry scan [32] | Antibody independent; Capable of isolating viable CTCs. | Not yet widely tested in blood from patients with pancreatic cancer | |
Immunological assays
The CellSearch® system (Veridex LLC) uses antibodies directed against cell surface antigens. In this method, CTCs are first extracted in ferrofluids using epithelial cell adhesion molecule (EpCAM) antibody-coated magnetic beads, then fixed and further stained with antibodies to epithelial cytokeratin 8, 18, and 19, DAPI for nuclear staining, and CD45 for leukocyte antibody. Once mounted on a slide, the CTCs are counted to provide quantitative data. The CellSearch® system has received FDA (US Food and Drug Administration) approval to aid in monitoring patients with metastatic breast, prostate, and colon cancer [5, 6, 8–11]. In addition, the assay has also been reported to provide prognostic information in pancreatic and other cancers.
One US study using CellSearch® system examined the prevalence of CTCs in blood samples from 199 patients with nonmalignant diseases, 964 patients with metastatic carcinomas, and 145 healthy volunteers. The study included 16 patients with metastatic pancreatic cancer. Differences in vascularization of the tumors, sites of metastasis, and aggressiveness of the tumor proved to be contributing factors to the presence and the number of CTCs. The median number of CTCs from colon cancer and pancreatic cancer was relatively lower than those with prostate cancer, ovarian cancer, or breast cancer, suggesting the system was less efficient in pancreatic cancer or that fewer cells were shed into the systemic circulation possibly because of filtration of cells from the primary tumor during their transit through the hepatic portal circulation [4]. A prospective study from Japan showed that the presence of CTCs identified using the CellSearch® system in pancreatic cancer patients correlated with poorer prognosis among stage IVb patients, independent of chemotherapy administration [22]. Apart from cell counting, CTCs identified using the CellSearch® system can be further characterized [23]. However, overall, the current CellSearch® system has not shown sufficient sensitivity for localized and metastatic diseases in patients with pancreatic cancer.
Other EpCAM antibody-based methods involve use of a microfluidi platform (a CTC-chip) potentially capable of isolating viable CTCs from peripheral blood of patients with pancreatic cancer [24]. Another CTC-chip device utilizes a herringbone-chip (HB-Chip) capable of isolating clusters of CTCs containing up to 14 CTCs with highly variable cytomorphologic features. Such clusters could potentially lodge in distal capillaries in appropriate niches and initiate metastatic lesions [25]. One weakness of antibody-based immunological assays is the potential loss of any subpopulation of CTCs which do not express the specific antigens used in the capture protocol (eg: EpCAM-negative CTCs) making this method incapable of sampling the entire population of CTCs.
PCR-based molecular assays for CTCs detection
RT-PCR assays have been used following extraction of total RNA to measure target gene copies from the peripheral blood of patients with pancreatic cancer. A study of 48 patients who received pancreatectomy showed that after surgery, the copy numbers of EpCAM gene were significantly increased, however this increase was not associated with a worse prognosis [26]. Previous studies also used RT-PCR to measure the mRNA levels of CK-19 [27] CK-20 [28], carcinoembryonic antigen (CEA), epidermal growth factor receptor 1 (EGFR1) and human telomerase (hTERT) [29] in the blood of pancreatic cancer patients. One pitfall of PCR-based approaches is that the origin of the nucleic acids is not clear and the results may not represent actual CTCs as the nucleic acids could also include shedding from necrotic cells in tumor deposits, tumor-derived exosomes, cellular fragments or result from lysis of CTCs in the bloodstream. This limitation could be overcome by preforming an initial purification of nucleated cells from the circulation followed by extraction of nucleic acids from the cells prior to performing RT-PCR [30].
CTCs isolation based on the physical and biological properties of CTCs
Dielectrophoretic Field-Flow Fractionation refers to the application of dielectrophoretic (DEP) forces to differentially drive cell movement based on the differences in dielectric properties between blood cells and cancer cells that arise from differences in morphological and electrical conductivity. The CTCs isolated from this method are viable and they can be cultured in media for further characterization [31]. Other physical-biologic property technologies include the use of fiber-optic array–scanning technology and laser-scanning cytometry scans which can identify nucleated cells deposited on a glass slide according to their distinct cell-surface antigen profiles [32]. These technologies are antibody independent, thus overcoming the potential “surface antigen” limitation of immunological assays (e.g., the CellSearch® system). To the best of our knowledge, the results of these technologies have not been published using blood from patients with pancreatic cancer but have been proved to be useful in other cancers such as lung and breast cancer [31].
CTCs and tumor initiating cells
Further characterization of CTCs identified subpopulations such as tumor initiating cells (TICs). TICs, also called cancer stem cells (CSCs), are likely to be critical not only for diagnosis and treatment of early stage cancer but also for finding novel treatments for pancreatic cancer with advanced stage. Numerous reports have demonstrated that EpCAM is one of the major markers for TICs of various cancers including pancreatic cancer [33]. Li et al. identified a subpopulation of highly tumorigenic cancer cells expressing the cell surface markers CD44+, CD24+ and EpCAM+ in pancreatic cancer. These cells display stem cell-like characteristics such as self-renewal and the ability to differentiate as well as to drive continuous malignant cell expansion in an invasive and metastatic manner. As few as one hundred CD44+CD24+EpCAM+ cells were able to generate a tumor in 50% of immunocompromised mice. In contrast, cells which were negative for all three markers required at least 104 or more tumorigenic cells be implanted in order to induce tumor formation [34].
Theoretically, there are two subtypes of cancer stem cell pools within a tumor: intrinsic cancer stem cells and induced cancer stem cells. Intrinsic cancer stem cells are thought to exist within the primary tumors from the initial stage of tumorigenesis whereas the induced cancer stem cells are differentiated cancer cells which have undergone epithelial mesenchymal transition (EMT). Epithelial mesenchymal transition results from genetic and epigenetic changes of cancer cells induced by transformation signals released from their microenvironment and from stroma cells [35]. Conventionally, EMT is recognized as a pathological mechanism during the progression of various diseases including inflammation, fibrosis and cancer [36]. During the EMT process, epithelial cells undergo multiple biochemical changes involving down-regulation of epithelial markers, which confer cell-to-cell and cell-to-extracellular matrix (ECM) adhesion. There is also up-regulation of mesenchymal markers in epithelial cells during the EMT process, conferring increased production of ECM components, enhanced migratory and invasive capacity, and increased resistance to apoptosis [37, 38]. New tumor initiation at the metastatic site is now believed to originate from cells which were first transformed from an epithelial to a mesenchymal state and then migrated to the site of the metastasis. Once that cell has moved to a new location, it can undergo mesenchymal to epithelial transition (MET), the reverse procedure [39]. A recent study conducted on the CTCs from metastatic breast cancer patients showed that a major proportion of CTCs were positive for both EMT markers and stem cell characteristics, thus providing further evidence of the link between cancer stem cells and CTCs [40].
The ability of CTCs and cancer stem cells to survive and form metastasis is determined by not only their intrinsic abilities with the self-propagating potential, but also the interaction with the pre-metastatic niche, which plays a critical role in determining the cell fate and metastasis [41]. The fine balance between quiescence and proliferation/differentiation of the CTCs is controlled by the niche through both intrinsic and extrinsic signals from the environment. In this modified “seed and soil” context, the seeds are the subpopulation of CTCs which have the tumor initiating potential, and the soil is the niche microenvironment composed of many cell types which interact with the tumor cells through growth factor and cytokine networks [42–44]. This complex signaling network and the niche microenvironment regulate CTCs and their progeny and modulate CTCs phenotype [45–47]. It is critical to elucidate the interaction between CTCs and the pre-metastatic niche for pancreatic cancer prevention and treatment.
Yachida et al. sequenced the genome of primary and metastatic pancreatic cancer and reported that different metastatic sites could have or select for different clones. In addition, the clonal populations that gave rise to distant metastases could be found within the primary carcinoma. Calculating the time required for the accumulation of the mutations identified, they concluded that up to seven years are required for the development of metastatic subclones within a primary carcinoma after it has formed, and an additional 2–3 years for these clones to disseminate and cause the patient death [48]. These studies suggest there is a window opportunity for diagnosis and treatment of early stage pancreatic cancer before it spreads. On the other hand, the finding from Stanger et al. supports the notion that early spread occurred even before the primary tumor formation. Those authors reported that tagged pancreatic intraepithelial neoplasia (PanINs) cells harboring only oncogenic Kras mutations migrated away from the glandular preneoplasm into the surrounding stroma tissue, invaded and entered the bloodstream, and seeded in the liver unexpectedly early, before frank malignancy could be detected by rigorous histologic analysis. The 2.7% and 6.8% of all PanIN 2 and 3 lesions contained mesenchymal markers coexpressed proteins characteristically associated with EMT, including Zeb1, Slug, and Snail, whereas EMT was never observed in PanIN 1 lesions. The circulating PanIN cells also expressed the “cancer-initiating cell” surface proteins CD24 and CD44. Inflammation also increased the number of circulating pancreatic preneoplastic cells [49]. If EMT does play a crucial role in cancer cell spread in vivo, then detection methods that rely on cellular expression of epithelial markers alone are likely to provide an incomplete picture of metastasis.
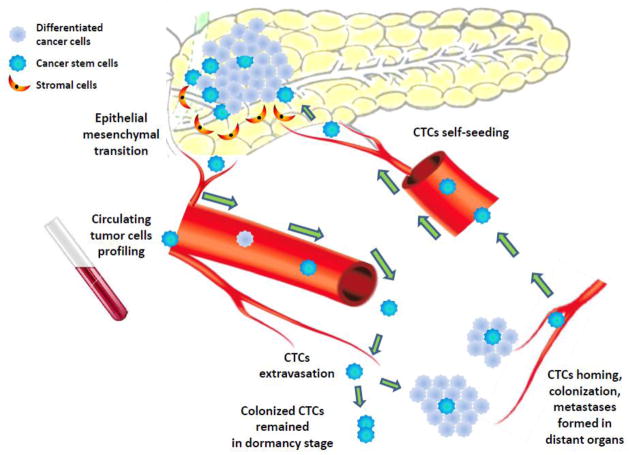
Further CTCs characterization will hopefully reveal which subgroups of CTC bearing tumor initiating cells marker are capable of seeding metastasis and are resistant to chemotherapy; and which subgroups of CTC are “helpers” but are not initiating cells as well as which subgroups of CTC are innocent bystanders (Fig. 1).
Figure 1.
Two cancer stem cell pools were found within a primary tumor of pancreas: intrinsic cancer stem cells, and induced cancer stem cells which had undergone epithelial mesenchymal transition (EMT) induced by transformation signals released from the microenvironment and from stroma cells. Circulating tumor cells (CTCs) comprise of cancer stem cells and differentiated tumor cells circulate through normal vessels and capillaries, and neovessles formed by tumor-induced angiogenesis, migrate to distant organs, extravasate, colonize and form distant metastasis clones. CTCs could also colonize their origin tumor in a process called ‘self-seeding’, and result in altering the tumors microenvironment, making it more supportive of tumor growth. CTCs profiling assays allow dynamic sampling during the clinical course to identify the subpopulations of CTCs and their specific molecules driving metastasis and chemoresistance for discovering novel targeted interventions.
CTCs and self-seeding
In 2006, Norton and his colleagues proposed a self-seeding theory to explain the increased cell density, high mitotic rate and large tumor sizes in cancer [50]. In this process, CTCs can also colonize their origin tumor in a process called ‘self-seeding’. In mice, “self-seeding” of breast cancer, colon cancer, and melanoma has since been confirmed to be preferentially mediated by aggressive CTCs, including CTCs with bone, lung or brain metastatic tropisms. They also showed that the tumor-derived cytokines interleukin-6 (IL-6) and IL-8 acted as CTC chemoattractants, whereas poor-prognosis markers such as matrix metalloproteinase (MMP1)/collagenase-1 and actin cytoskeleton component fascin-1 acted as mediators for CTCs infiltration into mammary tumors [51]. Overall “self-seeding” results in altering the tumors microenvironment making it more supportive of tumor growth including acceleration of tumor growth, induction of angiogenesis, and activation of stroma recruitment [52].
Changes in “self-seeding” could potentially affect response to therapy and prognosis. For example, in locally advanced pancreatic cancer, randomized clinical trials have shown that adding local radiation to chemotherapy may improve patients survival in comparison to chemotherapy alone [53]. One explanation for the beneficial effect of irradiation to the tumor bed could be that it results in changes of the local tumor environment resulting in a reduction of CTCs seeding back to the primary tumor site.
Use of CTCs in clinical management of pancreatic cancer
CTCs have the potential to provide a surrogate for ‘real-time biopsy’ thus allowing assessment of the tumor biological activity. As such, enumeration and characterization of CTCs in pancreatic cancer could have important role in the diagnosis, predicting risk for post-surgical recurrence, examining pharmacodynamic biomarkers, detecting treatment- resistant profiles, assisting in response measurements to therapies, and identifying prognostic and predictive molecular features (Table 2). Some potential applications of CTCs in different stages of pancreatic cancer management are discussed below.
Table 2.
Potential Use of CTCs in clinical management of pancreatic cancer
| Clinical Setting | Clinical situation | Application of CTC |
|---|---|---|
| Assistance in Diagnosis | Pancreatic cystic lesion; Borderline resectable lesion before offering neoadjuvant chemotherapy | Detected CTCs to differentiate among pre-malignancy (PanIN), benign lesion, adenocarcinoma, neuroendocrine tumor |
| Metastatic cancer of unknown primary | Gene profiling of CTC to identify cell of origin | |
| Localized Stage | Preoperative setting | Patient risk stratified by identifying CTC with metastatic potential; target CTC by using the most sensitive chemotherapy agent, monitor CTC count to drop that allow improved choice of the best timing of surgery |
| Post-operative setting | Examine pharmacodynamic biomarkers on CTCs to choose the most sensitive chemotherapy agents and assist in deciding the duration of adjuvant therapy | |
| Recurrent or metastatic disease | CTCs genotyping and phenotyping to be repeated at therapeutic decision-making points, Identify drug resistant clone, guide changes in chemotherapy agents when resistant clone is found. |
Assistance in diagnosis
Disseminated cancer cells have been reported in patients with ductal breast carcinoma in situ, a preinvasive cancer lesion [54]. Similar finding was reported in pancreatic intraepithelial neoplasia (PanIN) in a mouse model [49]. Cystic lesions of pancreas often present as a clinical dilemma as they can be adenocarcinoma, neuroendocrine tumor, pre-malignant (PanIN) or benign. Biopsy from these pancreatic lesions is associated with a high false negative rate as well as a risk of tumor seeding and many cases requires repeated biopsies over several months, or exploratory laparotomy for an accurate diagnosis. For suspicious lesion undergoing pancreatectomy, even though biopsy is not required beforehand, biopsies are required for borderline resectable lesions in order to differentiate between neuroendocrine tumor of pancreas and adenocarcinoma of the pancreas before offering pre-operative chemotherapy. In other scenarios, CTC could potentially help in the diagnosis of metastatic pancreatic cancer, especially in cases where the primary tumor was small possibly not even apparent on scans, or in cases where neither the metastatic nor the primary lesion is accessible for biopsy.
Preoperative (neoadjuvant) and postoperative (adjuvant) management in localized pancreatic cancer
CTCs can be used to identify the specific molecules driving metastasis and chemoresistance, and thus allow targeted interventions in neoadjuvant and adjuvant therapy. Many post-operative patients exhibit distant metastasis after surgery. Neoadjuvant therapy not only could potentially downstage the primary tumor to improve resectability, but also could reveal differences in cancer biology from a preoperative “window of observation” and thus potentially avoid the morbidity due to pancreaticoduodenectomy in patients who have occult micrometastatic disease which are destined to become clinically evident during preoperative therapy. Study of CTCs allows the oncology team to potentially identify the metastatic potential of the patient as CTCs likely vary in risk from innocent bystanders to those seed metastasis thus allowing identifying and targeting high risk patients as well as targeting CTCs by using the most sensitive chemotherapy agents. The tumor response to preoperative treatment might be predictable prior to surgery by noting a drop in CTC count and this allows improved choice of the best timing of surgery. After surgery, CTCs can be examined in terms of pharmacodynamic biomarkers to choose (or change to) the most sensitive chemotherapy agents and assist in deciding the duration of adjuvant therapy. Subgroup of CTCs with “organ tropism” could be potentially identified, and subsequently the susceptible organ can be targeted before overt metastasis occurs. For example, if CTCs with metastatic tropism to bone were found, bone-modulating agents can be applied to change the microenvironment of bone to make it harder for CTCs to survive in its “soil” after seeding.
Chemotherapy in recurrent or metastatic disease
CTCs can be used to identify “druggable targets” as part of personalized therapy, which has been reported mostly in breast cancer and lung cancer. Studies in pancreatic cancer CTCs have not been successful in identifying susceptible targets. We expect that like lung and breast cancer, once effective drugs are discovered for the treatment of pancreatic cancer, the ability of obtain CTCs before and after the treatment will provide more information to sub-classify patients and select the best drugs for individual cases. CTCs are of particular use for pancreatic cancer researchers by assisting in identifying potentially useful novel targeted therapies that are currently in pre-clinical phase such as gene therapy and immunotherapy. CTCs, as a bridge, can help to discover discrepancies in phenotype or genotype between metastatic tumors and the primary tumors. Possible explanations for this discrepancy include clonal heterogeneity of the primary tumor with subpopulations of cells becoming disseminated, and transformation of the genotype and phenotype of tumor cells over time. The presence of different expression profiles between CTCs and the primary tumor may well require alternations in the strategy for treatment. For example, a case report of a HER-2-positive metastatic breast cancer that recurred six years after diagnosis of Her-2 negative primary tumor demonstrated a significant response to anti-Her2 antibody, trastuzumab-containing chemotherapy [55]. Haber et al. analyzed the quantity and genotype of CTCs after the initiation of gefitinib, an epithelial growth factor receptor (EGFR) inhibitor, in four patients with lung cancer who harbored EGFR mutations [12]. The prevalence of the resistance allele T790M increased among CTCs over time thus predicting the acquisition of clinical drug resistance. One can envision the use of CTCs genotyping and phenotyping to be repeated at therapeutic decision-making points during a patient’s course of therapy in the management of pancreatic cancer to ensure that resistance has not developed and to guide changes in chemotherapy agents when resistant clone is found.
Novel targets and immunotherapy
Research in pancreatic CTCs has not yet been able to identify “druggable targets” in patients, partially due to lack of effective treatments for pancreatic cancer. In preclinical research and early phase clinical research, CTCs could potentially help to identify the susceptible gene in the individual patient and hence deliver gene therapy and immunotherapy to the susceptible patients. Molecular profiling identified multiple genes differentially regulated in human pancreatic cancer. Studies from our group found that zinc transporter ZIP4 mRNA was over-expressed in pancreatic cancer leading to tumor progression and drug resistance [56]. Silencing of ZIP4 by shRNA inhibited pancreatic cancer progression in a mouse model [57]. Recent microRNA profiling studies also indicate that pancreatic cancer has a specific microRNA signature which could be used as diagnosis and therapeutic markers. In the aggressive form of pancreatic cancer and its chemo-resistant clone, miRNA-34, miRNA-200 are down regulated (as suppressor) versus miRNA-21 and miRNA-155 which are up regulated. The detailed molecular analysis on the gene profiling and microRNA profiling of CTCs may lead to new insights and novel therapeutic strategies for pancreatic cancer.
Pancreatic cancer cells express tumor associated antigens (TAAs) such as Wilms’ tumor gene 1 (WT1) (75%), mucin 1 (MUC1) (over 85%), human telomerase reverse transcriptase (hTERT) (88%), mutated K-RAS (73%), survivin (77%), carcinoembryonic antigen (CEA) (over 90%), HER-2/neu (61.2%), or p53 (67%) as potential targets for immunotherapy. Immunotherapies aim to recruit and activate T cells that recognize tumor associated antigens (TAAs). The specific tumor associated antigens can be potentially detected in CTC, so that investigators can identify the susceptible patients bear the specific antigen and delivery specific immunotherapy to the targeted population.
Conclusions
Research on CTCs is especially promising in terms of providing new insights in the complex biology of micro-metastasis with important implications, as early identification and control of metastasis is vital to successful management of pancreatic cancer. Traditional tumor biopsy sampling can only capture part of the tumor and is unable to represent the entire tumor cell population or identify changes that occur over time. Ongoing analysis of CTCs not only has the potential to represent all cells shed from primary pancreatic tumor and each metastatic site, but allows sampling to be repeated at multiple time points (dynamic sampling) during the clinical course thus allowing early identification and characterization of newly emerging sub-clonal populations. Unlike lung cancer and other malignancies, there has not been any pivotal study using CTC to guide clinical treatment, due to delayed diagnosis and lack of effective therapeutic agent in pancreatic cancer. Pancreatic CTCs could potentially help to apply innovative technology to profile cancer cell genome, study microRNA and protein expression, detect drug resistant clone, and discover new pathways and therapeutic targets in CTCs. Moreover, pancreatic CTCs may provide biomarker target for predicting therapeutic efficacy in this malignancy which allows improved stratification of patients in the study of novel therapies. The potential role of CTCs in the diagnosis and management pancreatic cancer and understanding the biology of metastatic disease is significant.
Acknowledgments
This work was supported in part by the MacDonald General Research Fund 11RDM005 (Cen P), the Chinese Health Ministry Research Special Fund 201202007 (Ni X), the National Institutes of Health (NIH) grant R01CA138701, R21CA133604, and the William and Ella Owens Medical Research Foundation (Li M).
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Onuigbo WI. An Index of the Fate of Circulating Cancer Cells. Lancet. 1963;2:828–831. doi: 10.1016/s0140-6736(63)90518-x. [DOI] [PubMed] [Google Scholar]
- 2.Robinson KP, Mc GR, Mc GE. Circulating cancer cells in patients with lung tumors. Surgery. 1963;53:630–636. [PubMed] [Google Scholar]
- 3.Ashworth T. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Australian Medical Journal. 1869;14:146–147. [Google Scholar]
- 4.Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 5.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LW, Meropol NJ. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 6.Wong SC, Chan CM, Ma BB, Hui EP, Ng SS, Lai PB, Cheung MT, Lo ES, Chan AK, Lam MY, Au TC, Chan AT. Clinical significance of cytokeratin 20-positive circulating tumor cells detected by a refined immunomagnetic enrichment assay in colorectal cancer patients. Clin Cancer Res. 2009;15:1005–1012. doi: 10.1158/1078-0432.CCR-08-1515. [DOI] [PubMed] [Google Scholar]
- 7.Uen YH, Lu CY, Tsai HL, Yu FJ, Huang MY, Cheng TL, Lin SR, Wang JY. Persistent presence of postoperative circulating tumor cells is a poor prognostic factor for patients with stage I-III colorectal cancer after curative resection. Annals of surgical oncology. 2008;15:2120–2128. doi: 10.1245/s10434-008-9961-7. [DOI] [PubMed] [Google Scholar]
- 8.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. The New England journal of medicine. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 9.Pachmann K, Camara O, Kavallaris A, Krauspe S, Malarski N, Gajda M, Kroll T, Jorke C, Hammer U, Altendorf-Hofmann A, Rabenstein C, Pachmann U, Runnebaum I, Hoffken K. Monitoring the response of circulating epithelial tumor cells to adjuvant chemotherapy in breast cancer allows detection of patients at risk of early relapse. J Clin Oncol. 2008;26:1208–1215. doi: 10.1200/JCO.2007.13.6523. [DOI] [PubMed] [Google Scholar]
- 10.Danila DC, Heller G, Gignac GA, Gonzalez-Espinoza R, Anand A, Tanaka E, Lilja H, Schwartz L, Larson S, Fleisher M, Scher HI. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:7053–7058. doi: 10.1158/1078-0432.CCR-07-1506. [DOI] [PubMed] [Google Scholar]
- 11.Morgan TM, Lange PH, Porter MP, Lin DW, Ellis WJ, Gallaher IS, Vessella RL. Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clin Cancer Res. 2009;15:677–683. doi: 10.1158/1078-0432.CCR-08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, Inserra E, Diederichs S, Iafrate AJ, Bell DW, Digumarthy S, Muzikansky A, Irimia D, Settleman J, Tompkins RG, Lynch TJ, Toner M, Haber DA. Detection of mutations in EGFR in circulating lung-cancer cells. The New England journal of medicine. 2008;359:366–377. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan T, Zhao Q, Chen JJ, Chen WT, Pearl ML. Clinical significance of circulating tumor cells detected by an invasion assay in peripheral blood of patients with ovarian cancer. Gynecologic oncology. 2009;112:185–191. doi: 10.1016/j.ygyno.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA: a cancer journal for clinicians. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 15.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 16.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 17.Shi S, Yao W, Xu J, Long J, Liu C, Yu X. Combinational therapy: new hope for pancreatic cancer? Cancer letters. 2012;317:127–135. doi: 10.1016/j.canlet.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 18.Ni X, Yang J, Li M. Imaging-Guided Curative Surgical Resection of Pancreatic Cancer in a Xenograft Mouse Model. Cancer Lett. 2012 doi: 10.1016/j.canlet.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinberg W. The clinical utility of the CA 19-9 tumor-associated antigen. The American journal of gastroenterology. 1990;85:350–355. [PubMed] [Google Scholar]
- 20.Duffy MJ, Sturgeon C, Lamerz R, Haglund C, Holubec VL, Klapdor R, Nicolini A, Topolcan O, Heinemann V. Tumor markers in pancreatic cancer: a European Group on Tumor Markers (EGTM) status report. Ann Oncol. 2010;21:441–447. doi: 10.1093/annonc/mdp332. [DOI] [PubMed] [Google Scholar]
- 21.Goggins M. Molecular markers of early pancreatic cancer. J Clin Oncol. 2005;23:4524–4531. doi: 10.1200/JCO.2005.19.711. [DOI] [PubMed] [Google Scholar]
- 22.Kurihara T, Itoi T, Sofuni A, Itokawa F, Tsuchiya T, Tsuji S, Ishii K, Ikeuchi N, Tsuchida A, Kasuya K, Kawai T, Sakai Y, Moriyasu F. Detection of circulating tumor cells in patients with pancreatic cancer: a preliminary result. J Hepatobiliary Pancreat Surg. 2008;15:189–195. doi: 10.1007/s00534-007-1250-5. [DOI] [PubMed] [Google Scholar]
- 23.van de Stolpe A, Pantel K, Sleijfer S, Terstappen LW, den Toonder JM. Circulating tumor cell isolation and diagnostics: toward routine clinical use. Cancer research. 2011;71:5955–5960. doi: 10.1158/0008-5472.CAN-11-1254. [DOI] [PubMed] [Google Scholar]
- 24.Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, Ryan P, Balis UJ, Tompkins RG, Haber DA, Toner M. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stott SL, Hsu CH, Tsukrov DI, Yu M, Miyamoto DT, Waltman BA, Rothenberg SM, Shah AM, Smas ME, Korir GK, Floyd FP, Jr, Gilman AJ, Lord JB, Winokur D, Springer S, Irimia D, Nagrath S, Sequist LV, Lee RJ, Isselbacher KJ, Maheswaran S, Haber DA, Toner M. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18392–18397. doi: 10.1073/pnas.1012539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sergeant G, Roskams T, van Pelt J, Houtmeyers F, Aerts R, Topal B. Perioperative cancer cell dissemination detected with a real-time RT-PCR assay for EpCAM is not associated with worse prognosis in pancreatic ductal adenocarcinoma. BMC cancer. 2011;11:47. doi: 10.1186/1471-2407-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann K, Kerner C, Wilfert W, Mueller M, Thiery J, Hauss J, Witzigmann H. Detection of disseminated pancreatic cells by amplification of cytokeratin-19 with quantitative RT-PCR in blood, bone marrow and peritoneal lavage of pancreatic carcinoma patients. World J Gastroenterol. 2007;13:257–263. doi: 10.3748/wjg.v13.i2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soeth E, Grigoleit U, Moellmann B, Roder C, Schniewind B, Kremer B, Kalthoff H, Vogel I. Detection of tumor cell dissemination in pancreatic ductal carcinoma patients by CK 20 RT-PCR indicates poor survival. Journal of cancer research and clinical oncology. 2005;131:669–676. doi: 10.1007/s00432-005-0008-1. [DOI] [PubMed] [Google Scholar]
- 29.Srovnal JB, Klos A, Lovecek D, Havlik M, Radova R, Cwiertka L, Hajduch KM. The detection of circulating tumor cells is a negative prognostic factor in pancreatic cancer. AACR 102nd Annual Meeting 2011; 2011. Supplement 1 Cancer Research. [Google Scholar]
- 30.Albuquerque Ad, Kubisch I, Ernst D, Breier G, Kaul S, Fersis N. Prognostic significance of multimarker circulating tumor cell analysis in patients with advanced pancreatic cancer. Journal of Clinical Oncology; ASCO Annual Meeting Proceedings (Post-Meeting Edition); 2011. [Google Scholar]
- 31.Gascoyne PR, Noshari J, Anderson TJ, Becker FF. Isolation of rare cells from cell mixtures by dielectrophoresis. Electrophoresis. 2009;30:1388–1398. doi: 10.1002/elps.200800373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsieh HB, Marrinucci D, Bethel K, Curry DN, Humphrey M, Krivacic RT, Kroener J, Kroener L, Ladanyi A, Lazarus N, Kuhn P, Bruce RH, Nieva J. High speed detection of circulating tumor cells. Biosensors & bioelectronics. 2006;21:1893–1899. doi: 10.1016/j.bios.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 33.Deonarain MP, Kousparou CA, Epenetos AA. Antibodies targeting cancer stem cells: a new paradigm in immunotherapy? mAbs. 2009;1:12–25. doi: 10.4161/mabs.1.1.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 35.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science (New York, NY. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 36.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. The New England journal of medicine. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 37.Wallerand H, Robert G, Pasticier G, Ravaud A, Ballanger P, Reiter RE, Ferriere JM. The epithelial-mesenchymal transition-inducing factor TWIST is an attractive target in advanced and/or metastatic bladder and prostate cancers. Urologic oncology. 2010;28:473–479. doi: 10.1016/j.urolonc.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 38.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. The Journal of clinical investigation. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nature reviews. 2005;5:744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 40.Aktas B, Tewes M, Fehm T, Hauch S, Kimmig R, Kasimir-Bauer S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009;11:R46. doi: 10.1186/bcr2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- 42.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 43.Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest. 2011;121:3804–3809. doi: 10.1172/JCI57099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:R605–615. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korkaya H, Liu S, Wicha MS. Regulation of cancer stem cells by cytokine networks: attacking cancer’s inflammatory roots. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17:6125–6129. doi: 10.1158/1078-0432.CCR-10-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iinuma H, Watanabe T, Mimori K, Adachi M, Hayashi N, Tamura J, Matsuda K, Fukushima R, Okinaga K, Sasako M, Mori M. Clinical significance of circulating tumor cells, including cancer stem-like cells, in peripheral blood for recurrence and prognosis in patients with Dukes’ stage B and C colorectal cancer. J Clin Oncol. 2011;29:1547–1555. doi: 10.1200/JCO.2010.30.5151. [DOI] [PubMed] [Google Scholar]
- 47.Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F, Korkaya H, Heath A, Dutcher J, Kleer CG, Jung Y, Dontu G, Taichman R, Wicha MS. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer research. 2011;71:614–624. doi: 10.1158/0008-5472.CAN-10-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, Velculescu VE, Kinzler KW, Vogelstein B, Iacobuzio-Donahue CA. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, Leach SD, Stanger BZ. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Norton L, Massague J. Is cancer a disease of self-seeding? Nature medicine. 2006;12:875–878. doi: 10.1038/nm0806-875. [DOI] [PubMed] [Google Scholar]
- 51.Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, Massague J. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Comen E, Norton L, Massague J. Clinical implications of cancer self-seeding. Nat Rev Clin Oncol. 2011;8:369–377. doi: 10.1038/nrclinonc.2011.64. [DOI] [PubMed] [Google Scholar]
- 53.Loehrer PJ, Sr, Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, Flynn P, Ramanathan RK, Crane CH, Alberts SR, Benson AB., 3rd Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an eastern cooperative oncology group trial. J Clin Oncol. 2011;29:4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Husemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmuller G, Klein CA. Systemic spread is an early step in breast cancer. Cancer cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 55.Shigematsu H, Kadoya T, Kobayashi Y, Kajitani K, Sasada T, Emi A, Masumoto N, Haruta R, Kataoka T, Oda M, Arihiro K, Okada M. A case of HER-2-positive recurrent breast cancer showing a clinically complete response to trastuzumab-containing chemotherapy after primary treatment of triple-negative breast cancer. World journal of surgical oncology. 2011;9:146. doi: 10.1186/1477-7819-9-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li M, Zhang Y, Liu Z, Bharadwaj U, Wang H, Wang X, Zhang S, Liuzzi JP, Chang SM, Cousins RJ, Fisher WE, Brunicardi FC, Logsdon CD, Chen C, Yao Q. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci U S A. 2007;104:18636–18641. doi: 10.1073/pnas.0709307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li M, Zhang Y, Bharadwaj U, Zhai QJ, Ahern CH, Fisher WE, Brunicardi FC, Logsdon CD, Chen C, Yao Q. Down-regulation of ZIP4 by RNA interference inhibits pancreatic cancer growth and increases the survival of nude mice with pancreatic cancer xenografts. Clin Cancer Res. 2009;15:5993–6001. doi: 10.1158/1078-0432.CCR-09-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]