Abstract
C-type lectin receptors are pattern recognition receptors that are critical for autoimmunity and the immune response. Mincle is a C-type lectin receptor expressed by a variety of antigen presenting cells including macrophages, neutrophils, dendritic cells and B cells; a variety of stimuli including stress are known to induce the expression of Mincle. Mincle is an FcRγ-associated activation receptor that senses damaged cells and upon ligation induces activated macrophages to produce inflammatory cytokines. Recently, while several studies have reported that Mincle plays an important role in macrophage responses to fungal infection its function on B cells remains to be defined. In efforts to elucidate the function of Mincle expressed by B cells, we studied the expression of Mincle on subsets of B cells and analyzed cytokines and synthesized immunoglobulin upon ligation of Mincle. The expression of Mincle on CD27−CD19+ naïve B cells is significantly higher than CD27+CD19+ memory B cells. The stimulation of TLR9 ligand induced Mincle expression on B cells. Furthermore, co-stimulation of TLR9 and Mincle ligand reduced IgG and IgA production from B cells without a significant change in the inflammatory cytokines TNFα, IL-6, IL-8 and IL-10. Our data identifies Mincle as a potentially critical player in human B cell responses.
Keywords: C-type lectin, Mincle, Toll-like receptor
Introduction
C-type lectin receptors (CLRs) are pattern recognition receptors (PRRs) expressed on the cell membrane with C-type lectin-like domains in their extracellular region. CLRs serve multiple functions through recognizing carbohydrate chains on pathogens in innate immune surveillance. Macrophage-inducible C-type lectin (Mincle), also known as Clec4e and Clecsf9, is a novel 219aa type II transmembrane protein with a highly kept C-type lectin domain [1, 2]. Mincle is expressed on myeloid cells, neutrophils, and is particularly abundant on professional antigen presenting cells including macrophages, dendritic cells and B cells [1]. Mincle is selectively associated with the Fc receptor common γ (FcR γ) chain, an immunoreceptor tyrosine-based activation motif (ITAM)-containing adaptor, and its ligation on activated macrophages has been shown to produce a variety of pro-inflammatory cytokines and chemokines [3]. Mincle expression is upregulated by various stimuli, including TLRs ligands or cytokines. The fact that Mincle-expressing cells are activated in the presence of necrotic cells led to the identification of nuclear protein SAP130 as an endogenous Mincle ligand that is released from necrotic but not from apoptotic cells.
Fungal cell walls contain a variety of complex carbohydrates which include mannose, β-glucans and chitin [4]. Dectin-1 and Dectin-2 are CLRs that are the specific receptors for β-glucans and α-mannose, respectively [5, 6]. Recently, several studies reported that Mincle has an important role in the response of macrophages to fungal infections [7–9]. Thus, one study reported that macrophages in the absence of Mincle produced markedly lower levels of TNF-α in response to Candida albicans (C. albicans) infection [8]. Another study demonstrated that bone marrow derived macrophages from normal but not Mincle deficient mice secreted significant levels of IL-6 and TNF-α in response to Malassezia species implicating a major role for Mincle in macrophage function [9]. In addition, Mincle binds to trehalose-6,6’-dimycolate (TDM) and its synthetic analog trehalose D-(+)-trehalose 6,6'-dibehenate (TDB), a key component of mycobacteria also known as cord factor [10, 11]. Mincle is essential for generation of Th1/Th17 cellular immunity to vaccination using TDB as an adjuvant [11]. However, to the best of our knowledge, the function of Mincle by B cells has not been described which is the basis of the studies reported herein.
Materials and Methods
Subjects
Heparinized (Vacutainer; BD Biosciences, Franklin Lakes, NJ) peripheral blood samples were obtained from 23 healthy subjects (13 male and 10 female, age 51.6±16.2 years old). None of them received any treatment before this experiment. The study was approved by the Institutional Review Board of the University of California at Davis (Davis, CA), and all subjects provided written, informed consent prior to enrollment. All patients were healthy volunteers who were not receiving either medical treatment or drugs.
Peripheral Blood Mononuclear Cells Isolation
Peripheral blood mononuclear cells (PBMC) from all subjects were isolated by density gradient using Histopaque-1077 (Sigma Chemical Co., St. Louis, MO) under endotoxin-free conditions. PBMCs were re-suspended in phosphate-buffered saline (PBS) (Mediatech Inc., Herndon, VA), containing 0.5% bovine serum albumin (BSA) (Fraction V, OmniPur; EMD Chemicals Inc., Gibbstown, NJ) and 0.05% sodium azide. The viability of cells was >98%, which was confirmed using trypan blue dye exclusion.
Isolate CD19+ B Cells
CD19+ B cells were isolated utilizing CD19 microbeads (Miltenyi Biotech Inc, Auburn, CA). The positively selected cells were then resuspended in PBS containing 2% fetal bovine serum (FBS). An aliquot of these microbeads isolated cells were subjected to flow cytometry and determined in all cases to be >80% CD19+.
Evaluation of Cell Phenotypes
The polychromatic phenotypic analysis of PBMC was carried out on a FACScan flow cytometer (BD Immunocytometry Systems, San Jose, CA) upgraded for the detection of five colors by Cytek Development (Fremont, CA). The staining buffer used throughout consisted of 0.2% BSA, 0.04% EDTA, and 0.05% sodium azide in PBS. The PBMC to be evaluated were dispensed into 25-uL aliquots, and incubated with anti-human Fc receptor (FcR) blocking reagent (eBioscience, San Diego, CA) for 15 minutes at 4 C. After PBS wash, a previously determined optimal concentration of the CLEC4E monoclonal antibody, clone 2D12 (Abnova Corporation, Walnut, CA) was added and the cells incubated for 30 minutes at 4 C. Purified mouse IgG2aκ (BD Biosciences, San Diego, CA) was used as a negative control. After a PBS wash, the cells were incubated with PE conjugated goat anti-mouse IgG (Biolegend, San Diego, CA) as a secondary antibody for 30 minutes at 4 C. Cells were then washed and the cells incubated with a cocktail of antibodies which included CD27 and CD19 (eBioscience), CD3 (Biolegend) and CD14 (BD Biosciences, San Jose, CA), for 30 minutes at 4°C. Cells were washed in staining buffer and fixed with 1% PFA/PBS. The data acquired were analyzed using Cellquest PRO software (BD Immunocytometry Systems).
In vitro culture of PBMC or CD19+ B cells with TDB, the oligo-di-nucleotide CpG or lipopolysaccharide (LPS)
TDB was purchased from Avanti Polar Lipids, Inc. (Alabaster, AL), E. coli lipopolysaccharides (LPS) was purchased from Sigma-Aldrich (St. Louis, MO) and CpG-B (CpG ODN 2006; TCG TCG TTT TGT CGT TTT GTC GTT) was purchased from InvivoGen (San Diego, CA). For stimulation of PBMC or B cells, TDB was dissolved in chloroform at 1 mg/ml and subsequently diluted in isopropanol and dispensed into 48-well or 96-well plates at 80 µl/well. Control plates were prepared by placing isopropyl alcohol alone. These plates were dried overnight in a biosafety cabinet. Aliquots of 1 × 106 unfractionated PBMC in a volume of 500 µL of media were seeded in 48-well flat-bottom plates and aliquots of 0.2 × 106 purified B cells in a volume of 200 µL of media were seeded in 96-well flat-bottom plates and cultured for 4 days at 37°C in a 5% CO2 humidified atmosphere. Media consisted of RPMI 1640 culture medium (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal calf serum, 100 µg/mL of streptomycin, 100 U/mL of penicillin (Invitrogen). The PBMC and purified B cell cultures were incubated with either TDB 100 ng/well, LPS 20 ng/mL or CpG-B at 0.5 or 2 µM.
Immunoglobulin assays
Levels of IgG, IgM, and IgA in the supernatant fluid were determined using a human IgG, IgM, and IgA enzyme-linked immunosorbent assay quantitation kit (Bethyl Laboratories, Montgomery, TX). Briefly, microtiter plates were coated with goat anti-human IgG, IgM, or IgA affinity-purified antibodies and incubated overnight at 4°C. Plates were washed with phosphate-buffered saline with Tween 20 (PBS-T) and blocked with 200 µL of 50 mM Tris, 0.15 M NaCl, and 1% bovine serum albumin (BSA) (pH 8.0) for 30 minutes. The plates were then washed with PBS-T, and 100 µL of test samples and standards were added to the wells and incubated for 1 hour at room temperature, followed by PBS-T washes. Next, 100 µL of a predetermined optimum dilution of horseradish peroxidase–labeled goat anti-human IgG, IgM, or IgA monoclonal antibody was added and incubated for 1 hour at room temperature. Plates were washed and developed with tetramethylbenzidine substrate for 15 minutes, and the reaction was stopped with 2N H2SO4 and read at 450 nm with a microplate reader.
Cytokine Bead Assay (CBA)
The quantitation of IL-1β, IL-6, IL-8, IL-12p70, and TNF-α in the supernatants of cultured PBMC or B cells with or without mincle ligand or TLR ligands was determined using a human Cytometry Bead Array kit (CBA; BD Biosciences, San Jose, CA). Briefly, 10 uL of each of the different bead suspensions (with beads coated with antibodies directed against each of the cytokines) were incubated with 50 uL of the supernatant fluid and 50 uL of phycoerythrin (PE)-conjugated appropriate antibody for 3 hours and then washed and re-suspended in PBS. The intensity of the fluorescence signal was then analyzed using Cellquest PRO software (BD Immunocytometry Systems). The level of the appropriate cytokine in the supernatant were then calculated based on the fluorescence readings and expressed in pg/mL. The concentration range limits for the detection using this assay is 20 to 5,000 pg/mL for each of the 6 cytokines.
Statistics
The data were expressed as the mean ± standard error of mean (SEM). The frequency of positive cell and the MFI values were compared by Fisher’s exact test. The levels of immunoglobulins and inflammatory cytokines were analyzed using Mann-Whitney nonparametric tests. The tests were statistically significant if they attained values of p< 0.05.
Results
Mincle Expression on B cells
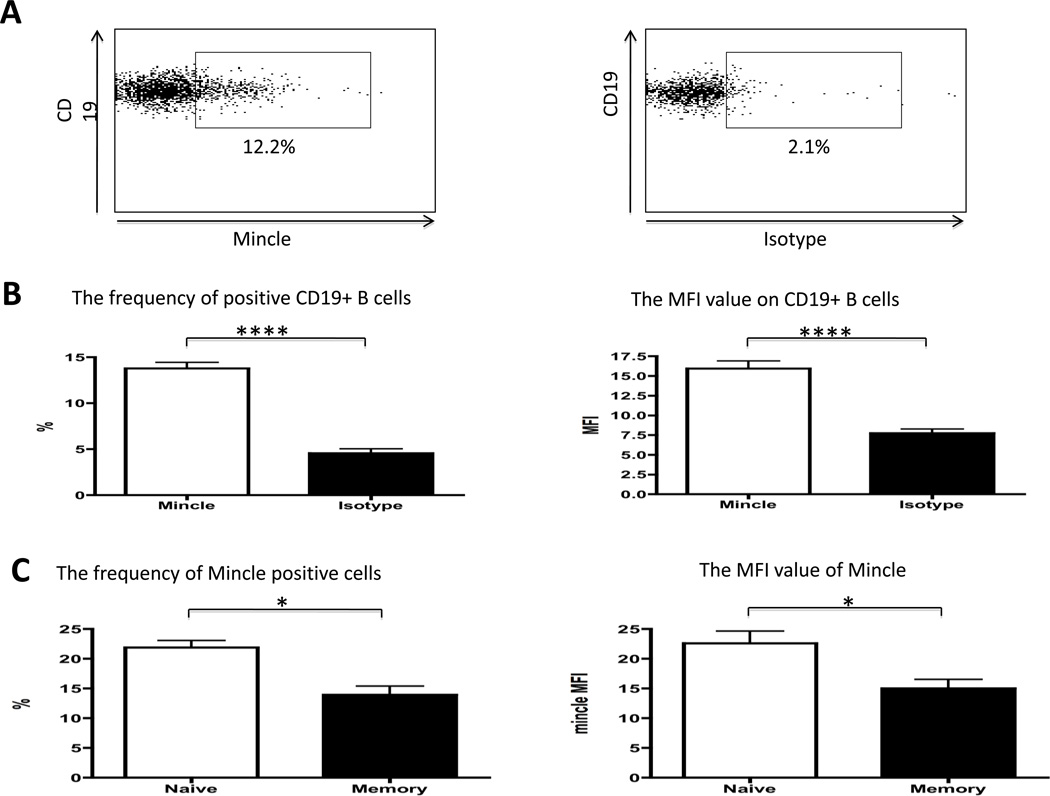
The frequency of Mincle positive cells and the relative density of expression (MFI) on B cells from a panel of normal human donors were compared anti-Mincle to isotype Ab staining using standard flow cytometric analysis. A representative profile of Mincle expression on normal human B cells is displayed in Fig. 1A. The frequency of Mincle positive CD19+ B cells from the normal donors ranged from 13.8 ± 0.7 with MFI values of 15.9 ± 1.0. The frequency of Mincle positive CD19+ B cells and the MFI was significantly higher than the isotype control (p<0.0001, respectively) (Figure 1B). When the frequency of Mincle positive cells and MFI were examined on naïve as compared to the memory B cells subsets, representative data as shown in Fig. 1C, indicates that the frequency and MFI of CD27−CD19+ naïve B cells were significantly higher than CD27+CD19+ memory B cells (p<0.05, respectively) (Figure 1C). Thus, B cells express Mincle on the cell surface and its expression lower in memory B cells compare with naïve B cells.
Figure 1.
Mincle expression on B cells was determined using standard flow cytometry. (A) Representative profiles of Mincle and isotype expression on normal human B cells were displayed. (B) Analysis of the frequency of Mincle positive cells and MFI value of Mincle on CD19+ B cells. The frequency of Mincle positive cells was significantly increased (Left panel). The MFI value of Mincle was significantly increased (Right panel). (n=23) (C) Naïve and memory B cells were determined by CD27 antibody. The frequency of Mincle positive CD27−CD19+ naïve B cells was significantly higher than CD27+CD19+ memory B cells (Left panel). The MFI value of Mincle on CD27−CD19+ naïve B cells was significantly higher than CD27+CD19+ memory B cells (Right panel). (n=8) * p<0.05, ****p<0.0001
Mincle expression on B cells in PBMC’s cultured in the presence of LPS or CpG
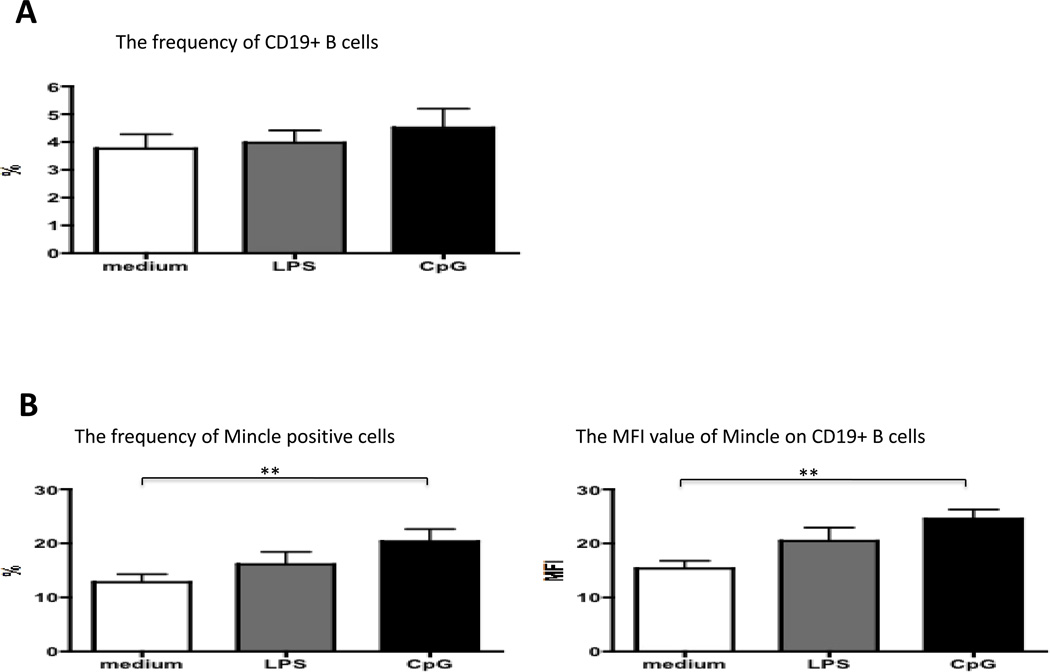
Next, we examined the role of different TLRs ligands in regulation of Mincle expression. Aliquots of PBMC (n=15) were cultured in media either containing 20 ng/mL of LPS, 2 µM of CpG-B or media for purposes of control for 4 days. The frequency of B cells and MFI values of Mincle on CD19+ B cells were subsequently tested. In vitro culture of PBMC with either LPS or CpG-B did not result in any detectable difference in the number of CD19+ B cells (Figure 2A). However, the frequency and MFI of Mincle positive CD19+ B cells in PBMC’s cultured in the presence of CpG-B, but not LPS, was significantly higher than medium control (p<0.01, respectively) (Figure 2B). These results suggest that activation of TLR9 in B cells is critical for its Mincle protein upregulation.
Figure 2.
Analysis of expression level of Mincle on CD19+ B cells in PBMC’s cultured in the presence of LPS or CpG-B for 4 days (n=15). (A) The frequencies of CD19+ B cells in culture of PBMC with either medium control, LPS or CpG were not difference. (B) The frequency of Mincle positive CD19+ B cells in PBMC’s cultured in the presence of CpG was significantly higher than medium control (Left panel). The MFI value of Mincle on CD19+ B cells in PBMC’s cultured in the presence of CpG was significantly higher than medium control (Right panel). The frequency and MFI of Mincle positive CD19+ B cells in PBMC’s cultured in medium control and the presence of LPS were not statistically difference. **p<0.01
Comparison of Mincle expression on B cells in PBMC’s cultured in the presence of TDB, CpG or CpG+TDB
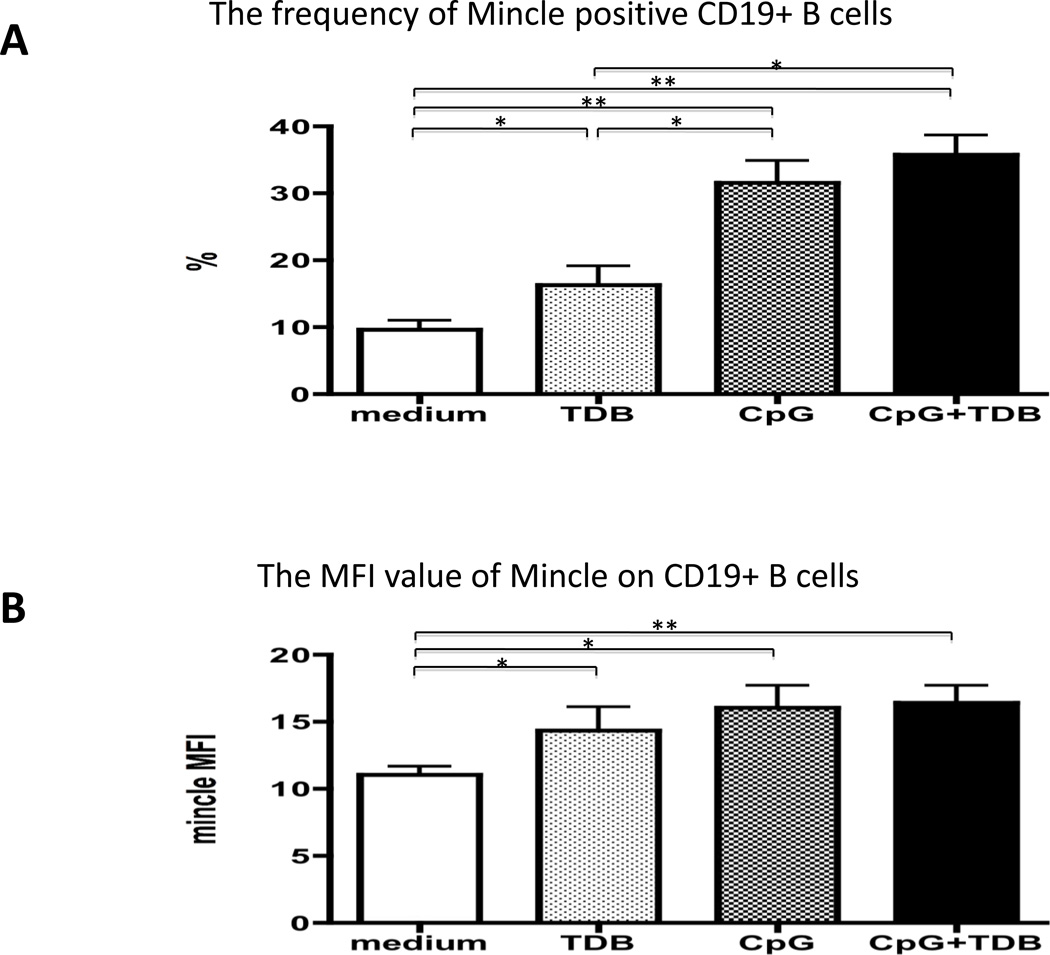
We next examined the frequencies and MFI of Mincle expression on CD19+ B cells in PBMC cultures incubated with ligand of TLR 9 or Mincle. We thus cultured aliquots of PBMC (n=8) in media containing 100 ng of TDB, 0.5 µM of CpG-B, 100 ng of TDB + 0.5 µM of CpG-B, or for purposes of control media alone for 4 days and examined the frequency and MFI of Mincle expression on CD19+ B cells. As shown in Fig. 3 A, it is clear that PBMC’s cultured in the presence of TDB, CpG-B, and CpG-B + TDB co-stimulation expressed significantly higher frequencies of Mincle positive CD19+ B cells as compared with medium control (p<0.05, p<0.01 and p<0.01, respectively) (Figure 3A). Furthermore, MFI values of Mincle on CD19+ B cell in PBMC’s cultured in the presence of TDB, CpG and CpG+TDB co-stimulation were significantly higher than medium control (p<0.05, p<0.05 and p<0.01, respectively) (Figure 3B). The frequencies of Mincle positive CD19+ B cells in PBMC’s cultured in the presence of CpG or CpG+TDB co-stimulation were significantly higher than TDB. These results indicate that recognition of Mincle ligand leads to upregulation of Mincle expression on B cells and also demonstrate that this upregulation is weaker than CpG dependent one. Furthermore, there was no additive effect on the frequency or MFI of Mincle expressing B cells in media containing both CpG+TDB co-stimulation.
Figure 3.
Comparison of expression level of Mincle on CD19+ B cells in PBMC’s cultured in the presence of 100 ng of TDB, 0.5 µM of CpG-B, 100 ng of TDB + 0.5 µM of CpG-B, or media alone for 4 days (n=8). (A) The frequencies of Mincle positive CD19+ B cells in PBMC’s cultured in the presence of TDB, CpG or CpG+TDB were significantly higher than medium control. In addition, the frequencies of Mincle positive CD19+ B cells in PBMC’s cultured in the presence of CpG or CpG+TDB were significantly higher than TDB. (B) The MFI values of Mincle positive CD19+ B cells in PBMC’s cultured in the presence of TDB, CpG or CpG+TDB were significantly higher than medium control. The MFI values of Mincle were not different between TDB, CpG-B and CpG-B+TDB co-stimulation. * p<0.05, **p<0.01
Secretion of Immunoglobulin from PBMC or B cells cultured in the presence of TDB, CpG or CpG+TDB
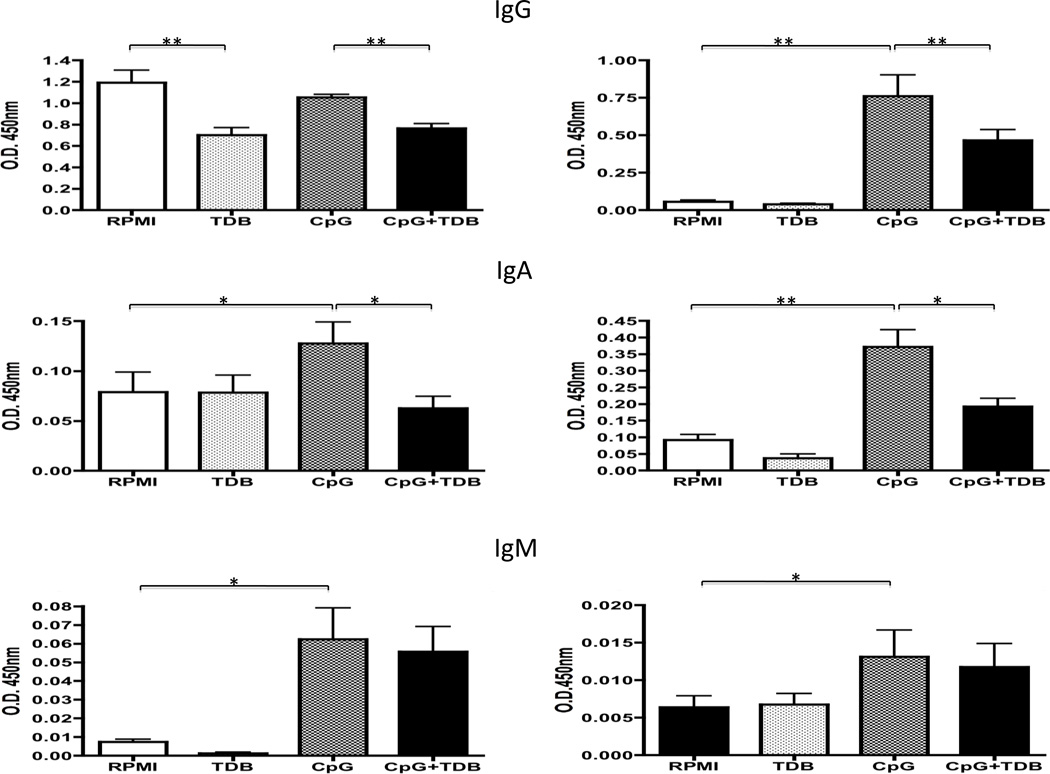
We also examined the immunostimulatory effect of Mincle ligand on B cells. Aliquots of PBMC and enriched population of CD19+ B cells (n=8) were cultured in the presence of 100 ng of TDB, 0.5 µM of CpG-B, 100 ng of TDB + 0.5 µM of CpG-B and for purposes of control media alone for 4 days. The levels of immunoglobulin in the supernatant of these cultures were measured. IgA and IgM in supernatants of PBMC’s cultured in media containing CpG was significantly higher compared to values of media alone (p<0.05, respectively). IgG, IgA and IgM in supernatants of CD19+ B cells cultured in media containing CpG was significantly higher than values for media alone (p<0.01, p<0.01 and p<0.05, respectively). IgG and IgA levels in supernatants of PBMC and CD19+ B cells cultured in media containing CpG + TDB were significantly lower than levels of cultures in media alone (p<0.05, p<0.01, respectively), although the IgM level was not different (Figure 4). The number of CD19+ B cells in PBMC’s cultured in the presence of CpG-B or CpG + TDB co-stimulation was not difference (data not shown). These results suggest that Mincle activation may inhibit IgG and IgA secretion after CpG stimulation.
Figure 4.
Analysis of immunoglobulin production in supernatant of PBMC or isolated CD19+ B cells cultured in the presence of 100 ng of TDB, 0.5 µM of CpG-B, 100 ng of TDB + 0.5 µM of CpG-B, or media alone for 4 days (n=8). Left panel is cultured PBMC, and right panel is cultured CD19+ B cell. O.D. 450nm value was determined using ELISA. IgA and IgM levels in supernatants of PBMC’s cultured in media containing CpG were significantly higher than medium control. IgG levels in supernatant of PBMC’s cultured in media containing TDB were significant lower than medium control. IgG and IgA levels in supernatants of PBMC cultured in media containing CpG + TDB were significantly lower than CpG, although IgM level was not different (Left panel). IgG, IgA and IgM levels in supernatants of CD19+ B cells cultured in media containing CpG were significantly higher than medium control. IgG and IgA levels in supernatants of CD19+ B cells cultured in media containing CpG + TDB were significantly lower than CpG, although IgM level was not different (Right panel). *p<0.05, **p<0.01
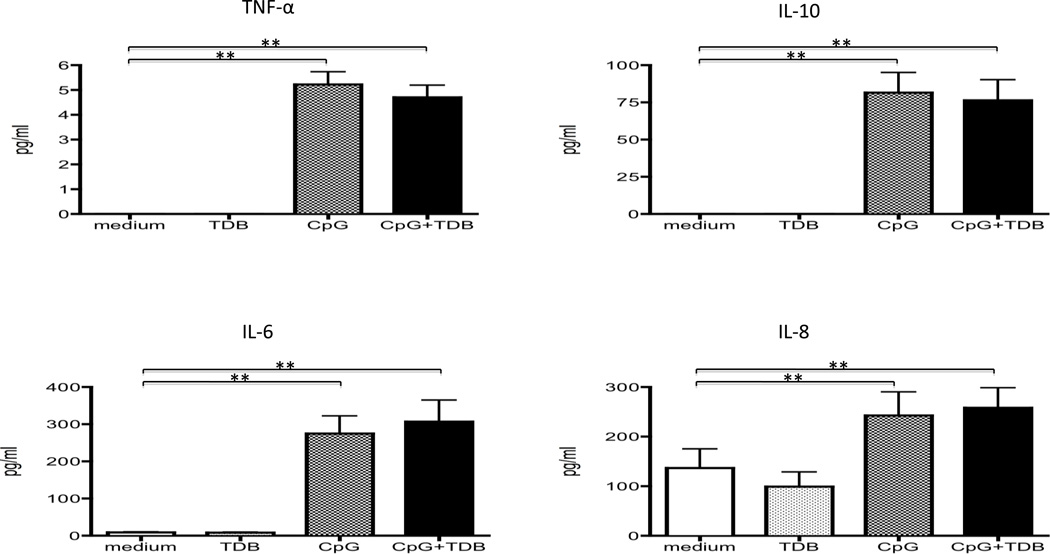
Secretion of Inflammatory cytokines in supernatant of B cells cultured in the presence of TDB, CpG or CpG+TDB
We compared the synthesis of inflammatory cytokines in the supernatant fluid of enriched population of CD19+ B cells (n=8) cultured in the presence of 100 ng of TDB, 0.5µM of CpG-B, 100 ng of TDB + 0.5µM of CpG-B and for purposes of control media alone for 4 days. Levels of TNF-α, IL-10, IL-6 and IL-8 were all increased after CpG-B stimulation (p<0.01, respectively). There was no additive effect of inflammatory cytokine production in supernatant containing both CpG-B + TDB co-stimulation (Figure 5). There were no detectable levels of IL-1β and IL-12p70 in these supernatant fluids.
Figure 5.
Analysis of inflammatory cytokine production from CD19+ B cells cultured in the presence of 100 ng of TDB, 0.5µM of CpG-B, 100 ng of TDB + 0.5µM of CpG-B and media alone for 4 days (n=8). TNF-α, IL-10, IL-6 and IL-8 are increased after CpG-B stimulation. Inflammatory cytokines are no difference between CpG-B stimulation and CpG-B +TDB co-stimulation. **p<0.01
Discussion
The CLRs are a family of Ca2+-dependent carbohydrate-binding lectins that have C-type lectinlike domain [2, 12–14]. Mincle is associated with an ITAM-coupled FcRγ that recognizes α- mannose [9] or SAP130 released by dead or dying cells [15]. The ligation of ITAM leads to a signaling cascade that begins with phosphorylation of ITAM tyrosine residues by Src-family kinases, followed by the recruitment and activation of Syk. Syk then activates a signaling cascade through CARD9 leading to NFkB and the induction of inflammatory cytokines [4, 16]. Mincle is expressed predominantly on cells of the myeloid lineage, such as macrophages, neutrophils, dendritic cells, and B cells [17], and on microglia in the brain [18]. Although this pattern of expression is similar to both Dectin-1 and Dectin-2, mouse bone marrow-derived macrophage constitutively express Mincle, not Dectin-1 or Dectin-2, and its expression is increased after Candida albicans (C. albicans) infection [8].
Mincle gene expression is strongly induced by LPS and several pro-inflammatory cytokines, including IFN-γ, IL-6, and TNF-α, using peritoneal macrophages from wild type mice [1]. In our hands, Mincle expression on monocytes from PBMC is significantly increased after LPS stimulation (data not shown). Mincle is dramatically upregulated in patients with rheumatoid arthritis [12] which suggests that its dysregulated expression might contribute to inflammation during autoimmune diseases [12]. Mincle transcription is also upregulated by various infections including S. pneumoniae, influenza A virus [13] and M. tuberculosis (31). Recently, Mincle has been implicated in anti-mycobacterial immunity due to its recognition of a cell wall component [7–9].
Several studies have demonstrated the importance of Mincle for cytokine and chemokine production from macrophages and their role in antifungal immunity. In the absence of Mincle, production of TNF-α by macrophages was reduced in response to C. albicans infection, both in vivo and in vitro [8]. Another study demonstrated that Mincle recognized Malassezia species. In addition, while neutrophil infiltration and an upregulation of both IL-6 and TNF-α was noted in the peritoneal cavity of wild type mice experimentally injected with Malassezia intraperitoneally, a similar infection of Mincle-deficient mice did not induce this cytokine response [9]. These studies are critical not only for understanding the normal immune response but, in particular, for the mechanism involved in B cell activation in autoimmune disease. Indeed, there are myriad of publications that address the role of B cells as not only antibody producing cells, but also as antigen presenting cells and as immune modulators in the pathogenesis of human and murine autoimmune disease [19–36].
SAP130 is a Mincle ligand derived from necrotic cells [3] and part of a principal autoantigen, snRNP; it interacts with SAP145, SAP155 and SAP49 to form the spliceosome complex in the U2 snRNP complex [37]. Whether this complex formation enhances the reactivity to Mincle is unclear, although SAP130 can activate Mincle expressing cells. TDM, known as cord factor, is a mycobacterial cell wall glycolipid that is the most studied immunostimulatory component of Mycobacterium tuberculosis [38]. TDB, which lacks the cyclopropane in the carbon chain, is known as a synthetic analog of TDM [39].
In this study herein, Mincle expression on B cells is strongly induced after CpG-B stimulation, not LPS stimulation. Unmethylated CpG motifs are prevalent in bacterial DNA, and ODN containing CpG motifs have been shown to activate vertebrate host defense mechanisms leading to innate and acquired immune responses [40]. A previous study reported that CpG-B is a potent stimulator for B cells [41], while CpG-A induces high levels of IFN-α in plasmacytoid dendritic cells but lacks a direct activating effect for B cells [42]. NF-IL6-deficient macrophages demonstrated a much lower level of Mincle mRNA induction after addition of inflammatory reagents [1]. The signaling mechanisms of TLR4 and TLR9 pathways are difference in B cells. TLR4 is expressed on the cell surface in complex with the MD-2 molecule, and this heterodimer participates in LPS recognition leading to intracellular signaling by the TIRAP-MyD88 pathway and the TRIF-TRAM pathway, two major pathways [43]. In contrast, TLR9 is expressed in the endoplasmic reticulum and is recruited to the endosomal/lysosomal compartments after CpG DNA stimulation, activating the MyD88 pathway without TIRAP [44]. The difference in the pathways may influence NF-IL6 activation and Mincle expression.
A previous study demonstrated that Fonsecaesa pedrosoi (F. pedrosoi), the fungus causes chromoblastomoycosis, was recognized by the Mincle receptor. Co-stimulation of F. pedrosoi with Pam3csk4 (TLR2/TLR1 Ligand), LPS (TLR4 ligand), or Imiquimod (TLR7 ligand) to human monocyte-derived macrophage or murine bone-marrow-derived dendritic cells induced significant levels of TNF as compared with respective stimulation [45]. Furthermore, β-glucan recognition by Dectin-1, requires co-stimulation of MyD88-coupled TLRs to induce robust inflammatory responses in cultures of macrophages [46]. These results suggest that a collaboration between the Syk and TLR/MyD88 pathways results in sustained degradation of the inhibitor of kB (IkB), enhancing NFkB nuclear translocation [46]. Unexpected, co-stimulation of CpG-B and TDB reduced IgG and IgA production as compared with CpG-B stimulation in our study. Although the mechanism behind these differences has not yet been elucidated, costimulation of CpG-B + TDB may induce a more effective response to CpG by B cells via the Syk and TLR/MyD88 pathway. Inflammatory cytokine production was not different between CpG stimulation and CpG + TDB co-stimulation, while immunoglobulin secretion was different. Hanten et al. demonstrated that the expression of CCL3 and CCL4 mRNA was significantly increased at 2 and 8 hours and returned to baseline at 24 hours after CpG stimulation. In contrast, the expression of IL-6 mRNA was still significantly elevated at 24 hours after CpG stimulation [47]. In this study, the supernatant fluid of cells cultured for 4 days was measured because we previously demonstrated that maximum percentages of intracellular IgM-positive B cells in patients of primary biliary cirrhosis and healthy controls were observed at 4 days after CpG stimulation [48]. Furthermore, the frequency of Mincle and MFI of CD27−CD19+ naive B cells were significantly higher than CD27+CD19+ memory B cells in this study. Since most cytokines are predominantly or exclusively produced by memory B cell, no naive B cells [49], the reason why TDB did not change cytokine production by CpG activated B cells may be due to the lower Mincle expression on memory B cells than naive B cells. Future studies should focus on culturing naive and memory cells, changing the period of culture time and TDB concentration for determining the role of Mincle on B cells including immunoglobulin and inflammatory cytokine production.
Acknowledgments
The authors thank Hajime Tanaka, Yoko M. Ambrosini, Amy Dhirapong, and Chen-yen Yang for technical support in this experiment. We also thank Ms. Nikki Phipps for support in preparing this article.
Financial support provided by National Institutes of Health grant DK39588
Abbreviations
- CLRs
C-type lectin receptors
- PRRs
pattern recognition receptors
- Mincle
Macrophage-inducible C-type lectin
- C. albicans
Candida albicans
- ITAM
immunoreceptor tyrosine-based activation motif
- PBMC
Peripheral blood mononuclear cells
- TDM
Trehalose-6,6’-dimycolate
- TDB
Trehalose D-(+)-trehalose 6,6'-dibehenate
- MARCO
macrophage receptor with collagenous structure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matsumoto M, Tanaka T, Kaisho T, Sanjo H, Copeland NG, Gilbert DJ, et al. A novel LPS-inducible C-type lectin is a transcriptional target of NF-IL6 in macrophages. J Immunol. 1999;163:5039–5048. [PubMed] [Google Scholar]
- 2.Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. FEBS J. 2005;272:6179–6217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 3.Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9:1179–1188. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]
- 4.Drummond RA, Saijo S, Iwakura Y, Brown GD. The role of Syk/CARD9 coupled C-type lectins in antifungal immunity. Eur J Immunol. 2011;41:276–281. doi: 10.1002/eji.201041252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–691. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Bugarcic A, Hitchens K, Beckhouse AG, Wells CA, Ashman RB, Blanchard H. Human and mouse macrophage-inducible C-type lectin (Mincle) bind Candida albicans. Glycobiology. 2008;18:679–685. doi: 10.1093/glycob/cwn046. [DOI] [PubMed] [Google Scholar]
- 8.Wells CA, Salvage-Jones JA, Li X, Hitchens K, Butcher S, Murray RZ, et al. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol. 2008;180:7404–7413. doi: 10.4049/jimmunol.180.11.7404. [DOI] [PubMed] [Google Scholar]
- 9.Yamasaki S, Matsumoto M, Takeuchi O, Matsuzawa T, Ishikawa E, Sakuma M, et al. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc Natl Acad Sci U S A. 2009;106:1897–1902. doi: 10.1073/pnas.0805177106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, et al. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med. 2009;206:2879–2888. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoenen H, Bodendorfer B, Hitchens K, Manzanero S, Werninghaus K, Nimmerjahn F, et al. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J Immunol. 2010;184:2756–2760. doi: 10.4049/jimmunol.0904013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura N, Shimaoka Y, Tougan T, Onda H, Okuzaki D, Zhao H, et al. Isolation and expression profiling of genes upregulated in bone marrow-derived mononuclear cells of rheumatoid arthritis patients. DNA research: an international journal for rapid publication of reports on genes and genomes. 2006;13:169–183. doi: 10.1093/dnares/dsl006. [DOI] [PubMed] [Google Scholar]
- 13.Rosseau S, Hocke A, Mollenkopf H, Schmeck B, Suttorp N, Kaufmann SH, et al. Comparative transcriptional profiling of the lung reveals shared and distinct features of Streptococcus pneumoniae and influenza A virus infection. Immunology. 2007;120:380–391. doi: 10.1111/j.1365-2567.2006.02514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strasser D, Neumann K, Bergmann H, Marakalala MJ, Guler R, Rojowska A, et al. Syk Kinase-Coupled C-type Lectin Receptors Engage Protein Kinase C-delta to Elicit Card9 Adaptor-Mediated Innate Immunity. Immunity. 2012 doi: 10.1016/j.immuni.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown GD. Sensing necrosis with Mincle. Nat Immunol. 2008;9:1099–1100. doi: 10.1038/ni1008-1099. [DOI] [PubMed] [Google Scholar]
- 16.Miyake Y, Ishikawa E, Ishikawa T, Yamasaki S. Self and nonself recognition through C-type lectin receptor, Mincle. Self Nonself. 2010;1:310–313. doi: 10.4161/self.1.4.13736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flornes LM, Bryceson YT, Spurkland A, Lorentzen JC, Dissen E, Fossum S. Identification of lectin-like receptors expressed by antigen presenting cells and neutrophils and their mapping to a novel gene complex. Immunogenetics. 2004;56:506–517. doi: 10.1007/s00251-004-0714-x. [DOI] [PubMed] [Google Scholar]
- 18.McKimmie CS, Roy D, Forster T, Fazakerley JK. Innate immune response gene expression profiles of N9 microglia are pathogen-type specific. J Neuroimmunol. 2006;175:128–141. doi: 10.1016/j.jneuroim.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Behrens M, Smart M, Luckey D, Luthra H, Taneja V. To B or not to B: role of B cells in pathogenesis of arthritis in HLA transgenic mice. Journal of autoimmunity. 2011;37:95–103. doi: 10.1016/j.jaut.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perlot T, Penninger JM. Development and function of murine B cells lacking RANK. J Immunol. 2012;188:1201–1205. doi: 10.4049/jimmunol.1102063. [DOI] [PubMed] [Google Scholar]
- 21.Seite JF, Guerrier T, Cornec D, Jamin C, Youinou P, Hillion S. TLR9 responses of B cells are repressed by intravenous immunoglobulin through the recruitment of phosphatase. Journal of autoimmunity. 2011;37:190–197. doi: 10.1016/j.jaut.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Wang YH, Yan Y, Rice JS, Volpe BT, Diamond B. Enforced expression of the apoptosis inhibitor Bcl-2 ablates tolerance induction in DNA-reactive B cells through a novel mechanism. Journal of autoimmunity. 2011;37:18–27. doi: 10.1016/j.jaut.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen T, Mingler MK, Blanchard C, Wahl B, Pabst O, Rothenberg ME. The pan-B cell marker CD22 is expressed on gastrointestinal eosinophils and negatively regulates tissue eosinophilia. J Immunol. 2012;188:1075–1082. doi: 10.4049/jimmunol.1102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aricha R, Mizrachi K, Fuchs S, Souroujon MC. Blocking of IL-6 suppresses experimental autoimmune myasthenia gravis. Journal of autoimmunity. 2011;36:135–141. doi: 10.1016/j.jaut.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Anderson AC, Chandwaskar R, Lee DH, Sullivan JM, Solomon A, Rodriguez-Manzanet R, et al. A Transgenic Model of Central Nervous System Autoimmunity Mediated by CD4+ and CD8+ T and B Cells. J Immunol. 2012;188:2084–2092. doi: 10.4049/jimmunol.1102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hickman-Brecks CL, Racz JL, Meyer DM, LaBranche TP, Allen PM. Th17 cells can provide B cell help in autoantibody induced arthritis. Journal of autoimmunity. 2011;36:65–75. doi: 10.1016/j.jaut.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teichmann LL, Kashgarian M, Weaver CT, Roers A, Muller W, Shlomchik MJ. B cell-derived IL-10 does not regulate spontaneous systemic autoimmunity in MRL.Fas(lpr) mice. J Immunol. 2012;188:678–685. doi: 10.4049/jimmunol.1102456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bayry J, Fournier EM, Maddur MS, Vani J, Wootla B, Siberil S, et al. Intravenous immunoglobulin induces proliferation and immunoglobulin synthesis from B cells of patients with common variable immunodeficiency: a mechanism underlying the beneficial effect of IVIg in primary immunodeficiencies. Journal of autoimmunity. 2011;36:9–15. doi: 10.1016/j.jaut.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Tang X, Tian L, Esteso G, Choi SC, Barrow AD, Colonna M, et al. Leukocyte-associated Ig-like receptor-1-deficient mice have an altered immune cell phenotype. J Immunol. 2012;188:548–558. doi: 10.4049/jimmunol.1102044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thurner L, Muller A, Cerutti M, Martin T, Pasquali JL, Gross WL, et al. Wegener's granuloma harbors B lymphocytes with specificities against a proinflammatory transmembrane protein and a tetraspanin. Journal of autoimmunity. 2011;36:87–90. doi: 10.1016/j.jaut.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Kim DW, Chang W, Choe J, Kim J, Park CS, et al. Wnt5a is secreted by follicular dendritic cells to protect germinal center B cells via Wnt/Ca2+/NFAT/NF-kappaB-B cell lymphoma 6 signaling. J Immunology. 2012;188:182–189. doi: 10.4049/jimmunol.1102297. [DOI] [PubMed] [Google Scholar]
- 32.Alonso R, Buors C, Le Dantec C, Hillion S, Pers JO, Saraux A, et al. Aberrant expression of CD6 on B-cell subsets from patients with Sjogren's syndrome. Journal of autoimmunity. 2010;35:336–341. doi: 10.1016/j.jaut.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Roguedas AM, Pers JO, Lemasson G, Devauchelle V, Tobon GJ, Saraux A, et al. Memory B-cell aggregates in skin biopsy are diagnostic for primary Sjogren's syndrome. Journal of autoimmunity. 2010;35:241–247. doi: 10.1016/j.jaut.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen TG, Little CB, Yenson VM, Jackson CJ, McCracken SA, Warning J, et al. Anti-IgD antibody attenuates collagen-induced arthritis by selectively depleting mature B-cells and promoting immune tolerance. Journal of autoimmunity. 2010;35:86–97. doi: 10.1016/j.jaut.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Santiago-Raber ML, Amano H, Amano E, Fossati-Jimack L, Swee LK, Rolink A, et al. Evidence that Yaa-induced loss of marginal zone B cells is a result of dendritic cell-mediated enhanced activation. Journal of autoimmunity. 2010;34:349–355. doi: 10.1016/j.jaut.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Korganow AS, Knapp AM, Nehme-Schuster H, Soulas-Sprauel P, Poindron V, Pasquali JL, et al. Peripheral B cell abnormalities in patients with systemic lupus erythematosus in quiescent phase: decreased memory B cells and membrane CD19 expression. Journal of autoimmunity. 2010;34:426–434. doi: 10.1016/j.jaut.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Das BK, Xia L, Palandjian L, Gozani O, Chyung Y, Reed R. Characterization of a protein complex containing spliceosomal proteins SAPs 49, 130, 145, and 155. Mol Cell Biol. 1999;19:6796–6802. doi: 10.1128/mcb.19.10.6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pimm MV, Baldwin RW, Polonsky J, Lederer E. Immunotherapy of an ascitic rat hepatoma with cord factor (trehalose-6, 6'-dimycolate) and synthetic analogues. Int J Cancer. 1979;24:780–785. doi: 10.1002/ijc.2910240614. [DOI] [PubMed] [Google Scholar]
- 39.Lemaire G, Tenu JP, Petit JF, Lederer E. Natural and synthetic trehalose diesters as immunomodulators. Med Res Rev. 1986;6:243–274. doi: 10.1002/med.2610060302. [DOI] [PubMed] [Google Scholar]
- 40.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 41.Hartmann G, Krieg AM. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J Immunol. 2000;164:944–953. doi: 10.4049/jimmunol.164.2.944. [DOI] [PubMed] [Google Scholar]
- 42.Krug A, Rothenfusser S, Hornung V, Jahrsdorfer B, Blackwell S, Ballas ZK, et al. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol. 2001;31:2154–2163. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 43.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 44.Peng SL. Signaling in B cells via Toll-like receptors. Curr Opin Immunol. 2005;17:230–236. doi: 10.1016/j.coi.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Sousa Mda G, Reid DM, Schweighoffer E, Tybulewicz V, Ruland J, Langhorne J, et al. Restoration of pattern recognition receptor costimulation to treat chromoblastomycosis, a chronic fungal infection of the skin. Cell Host Microbe. 2011;9:436–443. doi: 10.1016/j.chom.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dennehy KM, Ferwerda G, Faro-Trindade I, Pyz E, Willment JA, Taylor PR, et al. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur J Immunol. 2008;38:500–506. doi: 10.1002/eji.200737741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanten JA, Vasilakos JP, Riter CL, Neys L, Lipson KE, Alkan SS, et al. Comparison of human B cell activation by TLR7 and TLR9 agonists. BMC Immunol. 2008;9:39. doi: 10.1186/1471-2172-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kikuchi K, Lian ZX, Yang GX, Ansari AA, Ikehara S, Kaplan M, et al. Bacterial CpG induces hyper-IgM production in CD27(+) memory B cells in primary biliary cirrhosis. Gastroenterology. 2005;128:304–312. doi: 10.1053/j.gastro.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 49.Agrawal S, Gupta S. TLR1/2, TLR7, and TLR9 signals directly activate human peripheral blood naive and memory B cell subsets to produce cytokines, chemokines, and hematopoietic growth factors. Journal of clinical immunology. 2011;31:89–98. doi: 10.1007/s10875-010-9456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]