Abstract
This review discusses the various regulatory characteristics of microRNAs that are capable of generating widespread changes in gene expression via post translational repression of many mRNA targets and control self-renewal, differentiation and division of cells. It controls the stem cell functions by controlling a wide range of pathological and physiological processes, including development, differentiation, cellular proliferation, programmed cell death, oncogenesis and metastasis. Through either mRNA cleavage or translational repression, miRNAs alter the expression of their cognate target genes; thereby modulating cellular pathways that affect the normal functions of stem cells, turning them into cancer stem cells, a likely cause of relapse in cancer patients. This present review further emphasizes the recent discoveries on the functional analysis of miRNAs in cancer metastasis and implications on miRNA based therapy using miRNA replacement or anti-miRNA technologies in specific cancer stem cells that are required to establish their efficacy in controlling tumorigenic potential and safe therapeutics.
Keywords: Stem cell functions, Cancer stem cells, Cellular pathways, miRNA, oncomiR, Tumor suppressor miRNAs, miRNA based therapeutics
INTRODUCTION
Stem cells, a pool of precursor cells, exist in an undifferentiated state and have exclusive capability to self-renew over an extended period of time and undergo asymmetrical division which promotes healthy growth in normal cells due to polarity involved in cell division. One of the daughter cells retains stem cell properties while another becomes the committed progenitor called a transit amplifying cell and differentiates into a variety of cells that contribute to organ formation and function[1].
Stem cells are classified into two major classes: embryonic stem cells (ESCs) and adult stem cells. ESCs can be isolated at the blastocyst stage from the embryo, are pluripotent and induce lineage specific differentiation in cell culture. Adult stem cells are multipotent, have a tissue specific role in growth and maintenance in adult tissues and can produce only a limited number of differentiated cell types in vivo. The role of stem cells in tissue growth, homeostasis and repair in many organ systems make it an important therapeutic tool in the treatment of many human diseases[2].
The stem cell properties, including proliferation, self-renewal and differentiation, are controlled by a complex network of extrinsic and intrinsic signaling pathways. Dysfunction of these regulators can adversely affect the normal functions of stem cells and may either result in the loss of tissue homeostasis or cancer. Following genomic stress, appropriate DNA repair pathways, including mismatch repair, O6-alkylguanine DNA alkyltransferase repair, nucleotide excision repair, base excision repair, non-homologous DNA end-joining repair, and homologous recombination repair, are activated in order to maintain the genomic integrity. However, in the absence of DNA repair, cellular responses are activated to induce apoptosis and remove damaged cells from the organ as a part of a defense mechanism.
This review briefly focuses on the critical functions of microRNAs as regulators of post transcriptional gene expression that play a vital role, not only in maintaining the normal stem cell functions, but they also may modulate various signaling pathways that may turn stem cells into cancer stem cells with extensive self-renewal potential and aberrant differentiation. Recently, culture as well as in vivo studies in animal models with human cancers have shown the significance of miRNAs in modulating the expression level of responsive proteins by target mRNA cleavage and translational repression via the RNA interference (RNAi) pathway in the potential elimination of cancer stem cells.
MicroRNAs
MicroRNAs are the regulators of gene expression in many biological processes, including development, proliferation, apoptosis, stress response and fat metabolism. These newly discovered classes of molecules are 21-23 nucleotide short non coding RNA sequences, many of them are evolutionary conserved among distantly related organisms and may be expressed in a tissue-specific or developmental stage-specific manner. They are normally expressed as polycistronic transcripts and play an important role in various fundamental biological processes, such as cell cycle, cell growth and differentiation, apoptosis and embryo development, and cardiac and immune system function via regulating mRNA functions at post transcriptional as well as post translational level[3].
MicroRNAs were discovered in 1993 during a study of the gene lin-14 in Caenorhabditis elegans (C. elegans) development, where partial binding of 61 nucleotide precursor from lin-4 gene matured to a 22 nucleotide to complementary sequences in the 3’ UTR of the lin-14 and mRNA inhibited the translation of lin-14 mRNA[4]. This is followed by the characterization of second miRNA, lethal-7 (let-7), which repressed lin-41, lin-14, lin-28, lin-42 and daf-12 expression during developmental stage transitions in C. elegans in 2000[5]. Computational and experimental evidence provide a recent estimate of around 700 miRNAs hairpin sequences which are currently known to be contained in the publicly accessible miRNA database, miRBase (http://microrna.sanger.ac.uk/)[6]. More than 5300 human genes are supposed to be regulated by miRNA, which accounts for 30% of all the genes and around 60% of protein non coding genes. Many of the miRNAs are conserved between distantly related organisms, suggestive of their important roles in the biological system.
BIOGENESIS OF MicroRNAs
MiRNAs are endogenous and naturally generated within animal cells. They can inhibit the translation of mRNAs bearing the partially complementary target sequences, thus is one of the key components of RNAi within the cells. MiRNAs control various cellular, physiological and developmental processes and their aberrant expression link them with various diseases, including cancer; cardiovascular disease; schizophrenia; renal function disorders; Tourette’s syndrome; psoriasis; primary muscular disorders; Fragile-X mental retardation syndrome; chronic hepatitis; polycythemia vera; AIDS; and obesity[7-18]. To better understand the potential role of miRNA as important regulatory molecules in various cellular pathways by negatively controlling the gene and protein expression and their links with cancer, it is important to discuss the miRNA biogenesis pathway (Figure 1).
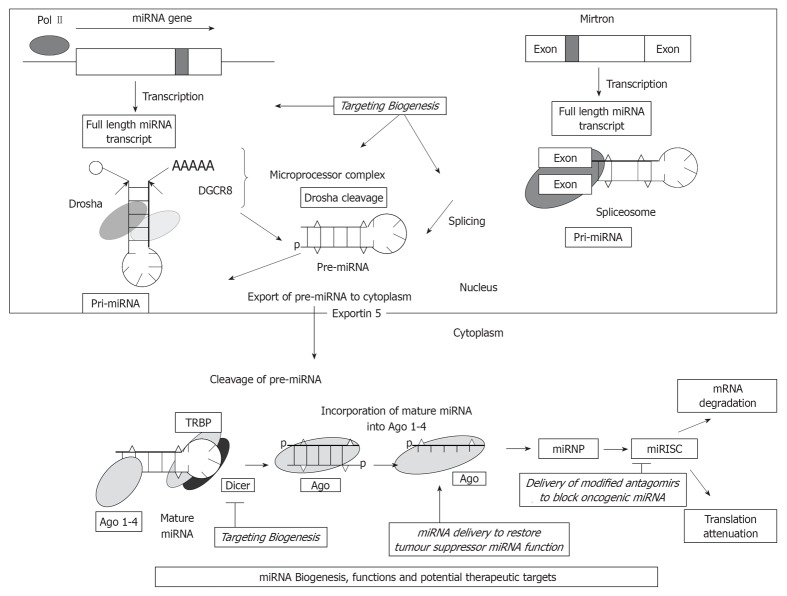
Figure 1.
MiRNA biogenesis, functions and potential therapeutic targets. miRNA transcript excised to form pri-miRNA, gets cleaved by Drosha and exported from nucleus to cytoplasm by Exportin-5. 70 n hairpin-loop precursor-miRNA (pre-miRNA) then processed by Dicer into mature RNA. The figure also explains the various potential miRNA therapeutic targets including biogenic pathways, restoring the tumor suppressor functions of miRNAs and blocking the oncogenic properties of miRNAs. miRNA mediated silencing involves either inhibition of translation or degradation of their target mRNA transcripts depending on the degree of complementarity. TRBP: Transactivating response RNA binding protein; miRISC: miRNA-induced silencing complex; miRNP: MicroRNA ribonucleoprotein complex; DGCR8: DiGeorge syndrome critical region gene 8.
The biogenesis of miRNA involves multiple processing steps, including transcription, processing, maturation and degradation. MiRNAs are randomly placed in a mammalian genome and found as isolated transcriptional units, co-transcribed as part of other transcriptional units, or clustered together and transcribed as polycistronic primary transcripts. They are either produced from their own genes or from introns. The process begins with the transcription of primary (pri) miRNA transcript, generally by RNA polymerase II, while those with upstream Alu sequences, transfer RNAs, and mammalian wide interspersed repeat promoter units by RNA polymerase III[19,20]. Primary miRNA having hundreds or thousands of nucleotides and one or more miRNA stem loops are then capped at 5’ and polyadenylated at 3’ end[21]. This is followed by the cleavage of pri-miRNA with the enzyme Drosha, RNA III endonuclease and a double stranded RNA binding protein, DiGeorge syndrome critical region gene 8 (DGCR8), together form a microprocessor complex or Pasha in invertebrates to form a resulting hairpin of around 70 nucleotides in length, known as a precursor-miRNA (pre-miRNA) which has 5’ phosphate and 2 nucleotide 3’ overhang[22]. Pre-miRNAs that are spliced directly out of introns are known as miRtrons.
Nucleocytoplasmic shuttle Exportin-5 exports processed pre-miRNA from the nucleus, by a RAs-related Nuclear protein-GTP dependent process[23]. This follows the subsequent cleavage of Pre-miRNA by another RNA III endonuclease known as Dicer in cytoplasm in partnership with its TRBP (human immunodeficiency virus transactivating response RNA binding protein), a RNA binding protein to form a final product of 21-23 nucleotide miRNA with 5’phosphates and a 2-nucleotide 3’ overhangs and generate two complementary RNA fragments. General inhibition of Drosha-mediated processing of many nuclear pri-miRNAs and Dicer-mediated processing of cytoplasmic pre-miRNA can regulate many important biological mechanisms[24,25]. One of either the strands of the duplex mature miRNAs are incorporated into the members of the argonaute (Ago) protein family, Ago 1-4, forming miRNPs (microRNA ribonucleoprotein complex) along with other proteins such as GW182 and known as miRNA-induced silencing complex. Mature miRNAs direct miRNPs to target mRNAs which share complementation with the seed region consisting of nucleotides at positions 2-8 of 5’ end of mature miRNA which result in either translational repression or more commonly mRNA degradation[26]. Targeting the regulators involved in the alternative splicing of mRNAs has been shown to upregulate the expression of mRNAs[27,28].
STEM CELLS AND miRNA FUNCTIONS
Differential gene expression under epigenetic, transcriptional, translational and posttranslational control, as well as signaling from neighboring cells, regulates normal stem cell properties. The regulatory miRNA levels are lower in stem cells but their dynamic expression profile in these cells provide evidence of their significance in maintaining the self-renewal, pluripotency and regulating differentiation of their progeny cells (Table 1). miR-15b/miR-16r, miR17-92, miR-21 and the miR-290-295 clusters are the four prominently expressed miRNA clusters in ESCs and are an integral part of their control. Many transcription factors regulated by miRNAs control the pluripotency and differentiation that are the major functions of stem cells. MiRNAs facilitate differentiation in murine ESCs with conditional knockout of Dicer1 and DGCR8 by downregulating the pluripotency markers like Oct4 and Nanog homeobox (Nanog)[29,30]. Directly targeting the transcripts of self-renewing factors, like Oct4, sex-determining region Y-box containing gene 2 (Sox2), Kruppel-like factor 4 (KLF4) with miR-145 and Nanog, liver receptor homologue 1, the positive regulators of Oct4 expression, with miR-34 in human ESCs promote differentiation. Lin-28, marker for pluripotent stem cells, forms a negative feedback loop with the let-7 family miRNAs, whereas let-7 miRNAs in differentiated stem cells target the Lin-28 miRNA[31]. MiR-290 and two other related families, including miR-370 and miR-302 cluster, showed an altered cell cycle profile and disrupt ESC transition from a self-renewing to a differentiated state[32].
Table 1.
miRNA mediated regulation in the maintenance and function of stem cells
| miRNA | Functions in stem cells | Mechanism(s) | Ref. |
| Pluripotent miRNAs | |||
| miR-290 cluster, miR-370, miR-302 | Promotes self-renewal | Regulate embryonic stem cell cycle | [32] |
| miR-141, miR-200, miR-429 | Maintenance of self-renewal in the absence of leukemia inhibitory factor | Regulated by cMyc proteins | [66] |
| miR-9 | Proliferation and promote NSC migration | Target Stmn1, which increases microtubule instability | [67] |
| Neurite outgrowth | Inhibit Cdc42 expression and altering the localization of Rac1 | ||
| miR-184 | NSC proliferation | Represses the expression of Numb-like 1 | [68] |
| miR-137 | Promotes NSC proliferation but inhibits neuronal maturation, dendritic morphogenesis, and spine development | Target Mind bomb 1, an ubiquitin ligase | [69] |
| Pro-differentiation miRNAs | |||
| miR-134, miR-145, miR-296, miR-470 | Initiate differentiation | Suppress pluripotent markers including Nanog, Oct4, Sox, Klf4 | [33] |
| Let-7 | Stabilize differentiation | Target transcripts that are regulated by the pluripotency transcription factors Oct4, Sox2, Nanog and Tcf3 | [34] |
| Promote somatic cell cycle by targeting both directly and indirectly the multiple activators of the G1-S transition including cdc25a, cdk6, cyclinD1 and cyclinD2 | [35-37] | ||
| miR-124 | NSC differentiation | Suppress Sox9 expression in adult NSCs and exhibit mutual inhibition mechanism of Ephrin-B1 | [70] |
Let-7: Lethal-7; NSC: Neuronal stem cell; Sox: Sex-determining region Y-box containing gene.
The two classes of pro-differentiation miRNAs play an important role in the differentiation process. MiRNAs, including miR-134, miR-145, miR-296 and miR-470, grouped under the first class of miRNAs and they directly suppress the self-renewal state by suppressing Nanog, Pou5f1 (also known as Oct4), KLF4 and Sox2, the markers of pluripotency[33]. The other class of miRNAs include the let-7 family of miRNAs that stabilizes the differentiated cell fate by targeting the transcripts that are regulated by the pluripotency transcription factors Oct4, Sox2, Nanog and Tcf3[34]. In addition, Let-7 also promotes the somatic cell cycle by targeting, both directly and indirectly, the multiple activators of the G1-S transition, including cdc25a, cdk6, cyclinD1 and cyclinD2, thereby making the G1 phase cells most susceptible to pro-differentiation signaling cascades, including MAPK signaling[35-37].
Studies have shown the potential role of miRNAs in different aspects of neuronal development, such as proliferation of neural stem cells (NSCs) and progenitors, neuronal differentiation, maturation and synaptogenesis[38]. Overexpression of miR-124 and miR-137 in undifferentiated NSCs result in morphological changes and expression of markers indicating neuronal differentiation[39]. Trim-NHL proteins, a new class of regulatory RNA binding proteins, act as an ESC expressed E3 ubiquitin ligase that function to degrade Ago2 protein, a component of the RISC complex, and modulate the activity of the entire miRNA pathway and are found to be associated with the differentiation of NSCs[40,41].
MiRNA expression profiles and functional studies explain their importance in stem cell biology; however, detailed investigation will be required to understand the specific role of miRNA for the maintenance and proper function of particular stem cell types.
CANCER STEM CELLS AND miRNA FUNCTIONS
Failure to repair errors in stem cells result in the accumulation of epigenetic abnormalities, initiate the signaling cascades that support tumorigenesis, allow the cells to escape the restrictions of its niche and transform them into cancer stem cells. These cells are structurally and functionally distinct from other cells within the tumor mass and are capable of self-renewing mitosis where one of the daughter cells functions as a stem cell while other becomes a progenitor cell[42]. Cancer stem cells are characterized by cell surface marker profiles, form tumorospheres and have increased resistance to chemo- and radio-therapeutic agents, a likely cause of cancer relapse in patients. Cancer stem cells have been isolated for hematological malignancies, mainly acute myelogenous leukemia, chronic myeloid leukemia, acute lymphoblastic leukemia (ALL), multiple myeloma and solid tumor organs of breast, brain, lung, prostate, testis, ovary, stomach, colon, skin, liver and pancreas[43]. Increased resistance to anti-cancer therapeutics, limitless proliferative capacity, aberrant differentiation and multidrug resistance trait associated with the overexpression of genes that code for transmembrane efflux pump proteins are the innate properties of cancer stem cells that offers a great challenge in long term remission[44].
Several profiling studies have determined potential implications of high percentage of miRNAs in cancer due to its close proximity to chromosomal breakpoints; cancer associated genomic regions and/or fragile sites and dysregulated expression levels in many malignancies. Multiple functional studies on miRNAs using various algorithms and statistical methods validate their involvement, functions, characteristics, correlations and associations with cancer through targeting proto-oncogenes or tumor suppressor genes (Table 2)[45].
Table 2.
Aberrant miRNA expression in cancer stem cell
| miRNA | Tumor type | Mechanism(s) | Ref. |
| miRNA as oncomiR | |||
| miR-17-92 polycistron | Upregulated in lung, breast, stomach, prostate, colon and pancreatic cancers | Regulate c-Myc expression | [46,47] |
| miR-21, miR-205 | Head and neck cancer | Target transcripts of tumor suppressive genes including kinesin family member 1B isoform α, hypermethylated in cancer 2, and pleomorphic adenoma gene 1 | [71] |
| miR-372, miR-373 | Testicular germ cells | Neutralize p53-mediated CDK inhibition, possibly through direct inhibition of the expression of the tumor-suppressor LATS2 | [72] |
| miR-21 | Breast cancer | Target tumor suppressor tropomyosin 1 | [73] |
| miR-126 | Gastric carcinoma | Targets SOX2, and PLAC1 | [48] |
| Let-7 | Hepatocellular carcinoma | Targets SOCS1, caspase-3 | [56] |
| miR-181 | Hepatocellular carcinoma | Targets RASSF1A, TIMP3 as well as nemo-like kinase | [56] |
| miR-495 | Breast cancer | Modulated by transcription factor E12/E47, suppresses E-cadherin expression to promote cell invasion and inhibits regulated in development and DNA damage responses 1 expression to enhance cell proliferation in hypoxia through post-transcriptional mechanism | [74] |
| miRNAs as tumor suppressors | |||
| Let-7 | Colon adenocarcinomas | Target Lin-28b which promotes cell migration, invasion and transforms immortalized colonic epithelial cells | [50] |
| miR-15 miR-16 cluster | Chronic lymphocytic leukemia | Targets the apoptotic inhibitor Bcl-2 | [47] |
| miR-29 | Cholangiocarcinoma | Regulate the anti-apoptotic protein Mcl-1 | [75] |
| miR200c | Head and neck squamous cell carcinoma | Negatively modulates the expression of BMI1 and ZEB1 | [62] |
| miR-125b | Glioma | Decreases the cell cycle regulated proteins CDK6 and CDC25A | [76] |
Let-7: Lethal-7; SOX2: Sex-determining region Y-box 2; PLAC1: Placenta-specific 1 gene.
MiRNAs differentially regulate the key properties of cancer stem cells, including cell-cycle exit and differentiation, prosurvival and antistress mechanisms (e.g., resistance to anoikis) and epithelial-mesenchymal transitions (EMT), migration and invasion, which contribute to enhanced tumor initiation and metastatic potential (Figure 2). miR-17-92 polycistron has been reported as the first onco-miR that accelerates tumor development in lung, breast, stomach, prostate, colon and pancreatic cancers by regulating c-Myc expression[46,47]. MiR-126 mediated inhibition of sex-determining region Y-box 2 (SOX2) [SOX2, a crucial transcription factor for the maintenance of ESC pluripotency and the determination of cell fate] and placenta-specific 1 gene may contribute to gastric carcinogenesis[48]. Increased expression of 2 miRNA clusters, 106a-363 and in particular 302-367 in mouse fibroblasts, positively regulate the mesenchymal-to-epithelial transition, cell cycle and epigenetic functions and could allow potent increases in induced pluripotent stem cell generation efficiency[49].
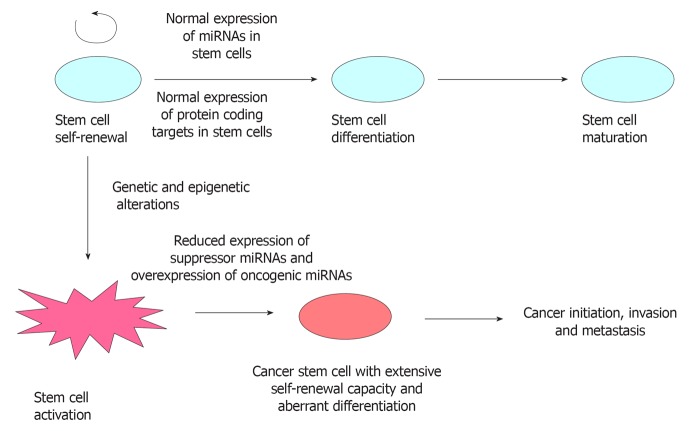
Figure 2.
Stem cells express a unique set of miRNAs that maintain self-renewal, promote differentiation and maturation through various regulatory mechanisms. Distinct small sub population of cells arises from stem cells due to accumulation of genetic and epigenetic abnormalities that might function as cancer stem cells. These cells display differential expression of miRNAs which regulate the fundamental properties that contribute to enhanced tumor initiation and metastatic potential.
The first functional evidence of tumor suppressive miRNAs was the miR-15/miR-16 cluster, located in a genomic region of chromosome 13 and often deleted in chronic lymphocytic leukemias (CLLs). These miRNAs are not expressed in CLLs but play an oncogenic role by accumulating oncogenic targets, the apoptotic inhibitor Bcl-2[47]. Lin-28 represses biogenesis of let-7 microRNAs and its overexpression has been correlated with reduced patient survival and increased probability of tumor recurrence in human colon adenocarcinomas[50].
In a systematic miRNA expression profiling analysis in human ALL patients, 77 miRNAs were up-regulated and 67 miRNAs were down-regulated in the patient group when compared to the control group with fold changes > 2.0. Among differentially expressed miRNAs, miR-9, miR-181a and miR-128 were of high significance, whereas miR-582-5p, miR-223, miR-143, miR-126, etc. displayed the least significance in patients[51]. Shimono et al[52] identified 37 differentially expressed miRNAs in CD44+CD24-/lo breast cancer stem cells (BCSCs) and among these miR-200c-141, miR-200b-200a-429 and miR-183-96-182 clusters were significantly downregulated.
Knowing the functional role of miRNAs in a specific tumor, therapies can be targeted to cancer stem cells in order to correct their aberrant expression levels. miRNA based therapeutics aim to potentially reverse the tumorigenic properties of cancer stem cells by targeting its biogenesis pathways, restoring the tumor suppressor functions of and/or blocking the oncogenic properties of miRNAs via the RNAi pathway.
THERAPEUTIC IMPLICATIONS
Dysregulated miRNAs via modulating cancer stem cell properties are highly associated with tumor initiation, tumor maintenance, metastasis and therapy resistance. Studies have shown the potential implications of miRNA based therapeutics as a novel strategy to target therapy-resistant cancer stem cells. miRNAs identified as oncogenic that promote cancer, when targeted by locally administered antagomiRs, and miRNAs recognized as tumor suppressors can be downregulated using an appropriate viral vector system could eliminate the cancer stem cells significantly. Lack of tumor specificity and low transfection efficiency associated with the in vivo systemic delivery of pharmaceutical formulations of functional miRNA and/or its antagonists to tumor cells via non viral mediated gene transfer limits their use[53,54]. Among the current approaches of gene delivery, systemic administration of miRNA using adeno associated viral vectors, not only minimizes the risk of vector-related toxicities, but also increases gene transfer efficiency, could be a successful strategy[55].
Inhibition of let-7 results in the increased chemosensitivity of hepatocellular cancer stem cells (HSCs) to sorafenib and doxorubicin, while silencing of miR-181 leads to reduction in HSCs motility and invasion by controlling the aberrant expressions of cytokine IL-6 and transcription factor Twist[56]. Induction of the tumor-suppressive miRNAs let-7a and miRNA-96 and suppression of the TGFβ-induced oncogenic miRNA-181a in BCSCs epigenetically preserve the differentiated phenotype of mammary epithelium and prevent EMT-related cancer-initiating cell self-renewal[57]. Downregulation of miR-125b-2 expression in glioblastoma multiforme (GBM) derived stem cells could allow temozolomide, a chemotherapeutic agent, to induce apoptosis by increasing the cytochrome c release from mitochondria, induction of Apaf-1, activation of caspase-3, poly-ADP-ribose polymerase and proapoptotic protein Bax while decreasing the expression of Bcl-2[58]. Specific inhibition of miR-21 by an anti-miR-21 locked nucleic acid modulates its upstream regulator activator protein-1, composed of c-Jun and c-Fos family transcription factors and tumor suppressor programmed cell death 4, and thereby increases drug sensitivity of cancer stem cells to anticancer drugs[59].
Forced expression of miR-124 and miR-137 in human derived GBM-derived stem cells leads to loss of their self-renewal and oncogenic capacity, leaving normal stem and precursor cells unharmed[39]. Overexpression of miR-128 significantly blocked glioma CSC self-renewal by directly targeting BMI-1 and caused a decrease in histone methylation [H3K27me(3)] and Akt phosphorylation, and up-regulation of p21(CIP1) levels, whereas transfection of GBM cancer stem cells with miR-34a could cause cell-cycle arrest or apoptosis, inhibit xenograft growth, and mediated by downregulation of multiple oncogenic targets, including c-MET, Notch-1/2 and CDK6[60,61]. In another study, miR145 (a tumor-suppressive miRNA) has been studied as a negative regulator of GBM tumorigenesis by targeting Oct4 and Sox2 in GBM-CD133(+). miR 145 delivery, using polyurethane-short branch polyethylenimine as a therapeutic-delivery vehicle, to GBM-CD133(+) significantly inhibited their tumorigenic and CSC-like abilities and facilitated their differentiation into CD133(-)-non-CSCs[62]. miR-34a overexpressed in bulk prostate cancer cells (CD44+) cells, when transfected with mature oligonucleotide mimics or infected with lentiviral vectors encoding pre-miR-34a, and exerted pronounced inhibitory effects on prostasphere establishment, migration and metastasis in vivo[63]. Restoration of miR-200c may be a promising therapeutic approach in head and neck squamous cell carcinoma. It could significantly inhibit the malignant CSC-like properties of ALDH1(+)/CD44(+) cells by negatively modulating the expression of BMI1 and inhibiting the metastatic capability of EMT by reducing the expression of ZEB1, Snail and N-cadherin, but up-regulating the E-cadherin expression[64]. Overexpression of miR-328 directly targets ABCG2 and MMP16, reverses drug resistance, inhibits cell invasion of side population (SP) cells from colorectal cancer, and thereby decreases invasive and strong tumor formation ability[65].
Studies on the physiological and behavioral differences between cancer stem cells and normal stem cells are required to help in the identification of specific mRNAs in cancer stem cells which may regulate oncogenesis or suppression to influence tumor development or progression that could act as a suitable drug target for safe and effective therapeutics.
CONCLUSION
miRNAs, a newly identified class of regulatory non-coding endogenous RNAs, have pivotal functions in stem cell maintenance. A small SP of cells identified in a variety of cancers harbor stem cell properties called cancer stem cells which are responsible for relapse and treatment failure in many cancer patients. These cells express miRNAs aberrantly where they can function as oncogenes or tumor suppressor genes. Identification of miRNA as a signature molecule to CSCs and their potential role make them good therapeutic targets for next-generation anti-cancer drugs and directly impact the current efforts in the safe eradication of malignancies.
Footnotes
Supported by The Department of Science and Technology, Govt. of India for providing BOYSCAST fellowship 2011-2012
Peer reviewers: Lei Liu, Professor, Department of Oral and Maxillofacial Surgery, West China Hospital of Stomatology, Sichuan University, 14, Section 3, Renminnan Road, Chengdu, Sichuan Province, 610041, China; Ivana de la Serna, PhD, Assistant Professor, University of Toledo College of Medicine, Department of Biochemistry and Cancer Biology, Toledo, OH 43614, United States
S- Editor Wang JL L- Editor Roemmele A E- Editor Zheng XM
References
- 1.Vaish M. Mismatch repair deficiencies transforming stem cells into cancer stem cells and therapeutic implications. Mol Cancer. 2007;6:26. doi: 10.1186/1476-4598-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hatfield S, Ruohola-Baker H. microRNA and stem cell function. Cell Tissue Res. 2008;331:57–66. doi: 10.1007/s00441-007-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwak PB, Iwasaki S, Tomari Y. The microRNA pathway and cancer. Cancer Sci. 2010;101:2309–2315. doi: 10.1111/j.1349-7006.2010.01683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 5.Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 6.Berezikov E, Guryev V, van de Belt J, Wienholds E, Plasterk RH, Cuppen E. Phylogenetic shadowing and computational identification of human microRNA genes. Cell. 2005;120:21–24. doi: 10.1016/j.cell.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 7.Bagasra O, Prilliman KR. RNA interference: the molecular immune system. J Mol Histol. 2004;35:545–553. doi: 10.1007/s10735-004-2192-8. [DOI] [PubMed] [Google Scholar]
- 8.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruchova H, Yoon D, Agarwal AM, Mendell J, Prchal JT. Regulated expression of microRNAs in normal and polycythemia vera erythropoiesis. Exp Hematol. 2007;35:1657–1667. doi: 10.1016/j.exphem.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiore R, Schratt G. MicroRNAs in synapse development: tiny molecules to remember. Expert Opin Biol Ther. 2007;7:1823–1831. doi: 10.1517/14712598.7.12.1823. [DOI] [PubMed] [Google Scholar]
- 11.Esau CC, Monia BP. Therapeutic potential for microRNAs. Adv Drug Deliv Rev. 2007;59:101–114. doi: 10.1016/j.addr.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Latronico MV, Catalucci D, Condorelli G. Emerging role of microRNAs in cardiovascular biology. Circ Res. 2007;101:1225–1236. doi: 10.1161/CIRCRESAHA.107.163147. [DOI] [PubMed] [Google Scholar]
- 13.Perkins DO, Jeffries CD, Jarskog LF, Thomson JM, Woods K, Newman MA, Parker JS, Jin J, Hammond SM. microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 2007;8:R27. doi: 10.1186/gb-2007-8-2-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonkoly E, Wei T, Janson PC, Sääf A, Lundeberg L, Tengvall-Linder M, Norstedt G, Alenius H, Homey B, Scheynius A, et al. MicroRNAs: novel regulators involved in the pathogenesis of psoriasis. PLoS One. 2007;2:e610. doi: 10.1371/journal.pone.0000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Cheng G, Mahato RI. RNAi for treating hepatitis B viral infection. Pharm Res. 2008;25:72–86. doi: 10.1007/s11095-007-9504-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen JF, Callis TE, Wang DZ. microRNAs and muscle disorders. J Cell Sci. 2009;122:13–20. doi: 10.1242/jcs.041723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heneghan HM, Miller N, Kerin MJ. Role of microRNAs in obesity and the metabolic syndrome. Obes Rev. 2010;11:354–361. doi: 10.1111/j.1467-789X.2009.00659.x. [DOI] [PubMed] [Google Scholar]
- 18.Karolina DS, Wintour EM, Bertram J, Jeyaseelan K. Riboregulators in kidney development and function. Biochimie. 2010;92:217–225. doi: 10.1016/j.biochi.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borchert GM, Lanier W, Davidson BL. RNA polymerase III transcribes human microRNAs. Nat Struct Mol Biol. 2006;13:1097–1101. doi: 10.1038/nsmb1167. [DOI] [PubMed] [Google Scholar]
- 21.Cai X, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10:1957–1966. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregory RI, Chendrimada TP, Shiekhattar R. MicroRNA biogenesis: isolation and characterization of the microprocessor complex. Methods Mol Biol. 2006;342:33–47. doi: 10.1385/1-59745-123-1:33. [DOI] [PubMed] [Google Scholar]
- 23.Lund E, Dahlberg JE. Substrate selectivity of exportin 5 and Dicer in the biogenesis of microRNAs. Cold Spring Harb Symp Quant Biol. 2006;71:59–66. doi: 10.1101/sqb.2006.71.050. [DOI] [PubMed] [Google Scholar]
- 24.Obernosterer G, Leuschner PJ, Alenius M, Martinez J. Post-transcriptional regulation of microRNA expression. RNA. 2006;12:1161–1167. doi: 10.1261/rna.2322506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomson JM, Newman M, Parker JS, Morin-Kensicki EM, Wright T, Hammond SM. Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes Dev. 2006;20:2202–2207. doi: 10.1101/gad.1444406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pratt AJ, MacRae IJ. The RNA-induced silencing complex: a versatile gene-silencing machine. J Biol Chem. 2009;284:17897–17901. doi: 10.1074/jbc.R900012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boutz PL, Chawla G, Stoilov P, Black DL. MicroRNAs regulate the expression of the alternative splicing factor nPTB during muscle development. Genes Dev. 2007;21:71–84. doi: 10.1101/gad.1500707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 29.Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, Livingston DM, Rajewsky K. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005;19:489–501. doi: 10.1101/gad.1248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Medvid R, Melton C, Jaenisch R, Blelloch R. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat Genet. 2007;39:380–385. doi: 10.1038/ng1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peter ME. Let-7 and miR-200 microRNAs: guardians against pluripotency and cancer progression. Cell Cycle. 2009;8:843–852. doi: 10.4161/cc.8.6.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melton C, Blelloch R. MicroRNA Regulation of Embryonic Stem Cell Self-Renewal and Differentiation. Adv Exp Med Biol. 2010;695:105–117. doi: 10.1007/978-1-4419-7037-4_8. [DOI] [PubMed] [Google Scholar]
- 33.Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137:647–658. doi: 10.1016/j.cell.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 34.Melton C, Judson RL, Blelloch R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature. 2010;463:621–626. doi: 10.1038/nature08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burdon T, Smith A, Savatier P. Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol. 2002;12:432–438. doi: 10.1016/s0962-8924(02)02352-8. [DOI] [PubMed] [Google Scholar]
- 36.Johnson CD, Esquela-Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D, Wilson M, Wang X, Shelton J, Shingara J, et al. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007;67:7713–7722. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- 37.Schultz J, Lorenz P, Gross G, Ibrahim S, Kunz M. MicroRNA let-7b targets important cell cycle molecules in malignant melanoma cells and interferes with anchorage-independent growth. Cell Res. 2008;18:549–557. doi: 10.1038/cr.2008.45. [DOI] [PubMed] [Google Scholar]
- 38.Bian S, Sun T. Functions of noncoding RNAs in neural development and neurological diseases. Mol Neurobiol. 2011;44:359–373. doi: 10.1007/s12035-011-8211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papagiannakopoulos T, Kosik KS. MicroRNAs: regulators of oncogenesis and stemness. BMC Med. 2008;6:15. doi: 10.1186/1741-7015-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rybak A, Fuchs H, Hadian K, Smirnova L, Wulczyn EA, Michel G, Nitsch R, Krappmann D, Wulczyn FG. The let-7 target gene mouse lin-41 is a stem cell specific E3 ubiquitin ligase for the miRNA pathway protein Ago2. Nat Cell Biol. 2009;11:1411–1420. doi: 10.1038/ncb1987. [DOI] [PubMed] [Google Scholar]
- 41.Schwamborn JC, Berezikov E, Knoblich JA. The TRIM-NHL protein TRIM32 activates microRNAs and prevents self-renewal in mouse neural progenitors. Cell. 2009;136:913–925. doi: 10.1016/j.cell.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124:1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 43.Garg M. Mismatch repair system: Therapeutic approaches to cancer stem cells. In: Singh SR, Mishra PK, Hou SX, editors. Stem cells: Organogenesis and cancer. Kerala: Transworld Research Network; 2010. pp. 271–291. [Google Scholar]
- 44.Garg M. Gain of antitumor functions and induction of differentiation in cancer stem cells contribute to complete cure and no relapse. Crit Rev Oncog. 2009;15:57–78. doi: 10.1615/critrevoncog.v15.i1-2.30. [DOI] [PubMed] [Google Scholar]
- 45.Garg M. MicroRNA profiling involved in human tumorigenesis using Bioinformatics tools. In: Tuteja R, editor. Bioinformatics: Genome Bioinformatics and Computational Biology. Germany: Nova Science Publishers Inc; 2011. p. In press. [Google Scholar]
- 46.O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 47.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 48.Otsubo T, Akiyama Y, Hashimoto Y, Shimada S, Goto K, Yuasa Y. MicroRNA-126 inhibits SOX2 expression and contributes to gastric carcinogenesis. PLoS One. 2011;6:e16617. doi: 10.1371/journal.pone.0016617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liao B, Bao X, Liu L, Feng S, Zovoilis A, Liu W, Xue Y, Cai J, Guo X, Qin B, et al. MicroRNA cluster 302-367 enhances somatic cell reprogramming by accelerating a mesenchymal-to-epithelial transition. J Biol Chem. 2011;286:17359–17364. doi: 10.1074/jbc.C111.235960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.King CE, Cuatrecasas M, Castells A, Sepulveda AR, Lee JS, Rustgi AK. LIN28B promotes colon cancer progression and metastasis. Cancer Res. 2011;71:4260–4268. doi: 10.1158/0008-5472.CAN-10-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H, Yang JH, Zheng YS, Zhang P, Chen X, Wu J, Xu L, Luo XQ, Ke ZY, Zhou H, et al. Genome-wide analysis of small RNA and novel MicroRNA discovery in human acute lymphoblastic leukemia based on extensive sequencing approach. PLoS One. 2009;4:e6849. doi: 10.1371/journal.pone.0006849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, Diehn M, Liu H, Panula SP, Chiao E, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pirollo KF, Xu L, Chang EH. Non-viral gene delivery for p53. Curr Opin Mol Ther. 2000;2:168–175. [PubMed] [Google Scholar]
- 54.Xu L, Pirollo KF, Chang EH. Tumor-targeted p53-gene therapy enhances the efficacy of conventional chemo/radiotherapy. J Control Release. 2001;74:115–128. doi: 10.1016/s0168-3659(01)00324-8. [DOI] [PubMed] [Google Scholar]
- 55.Kota J, Chivukula RR, O’Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meng F, Glaser SS, Francis H, DeMorrow S, Han Y, Passarini JD, Stokes A, Cleary JP, Liu X, Venter J, et al. Functional analysis of microRNAs in human hepatocellular cancer stem cells. J Cell Mol Med. 2012;16:160–173. doi: 10.1111/j.1582-4934.2011.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oliveras-Ferraros C, Cufí S, Vazquez-Martin A, Torres-Garcia VZ, Del Barco S, Martin-Castillo B, Menendez JA. Micro(mi)RNA expression profile of breast cancer epithelial cells treated with the anti-diabetic drug metformin: induction of the tumor suppressor miRNA let-7a and suppression of the TGFβ-induced oncomiR miRNA-181a. Cell Cycle. 2011;10:1144–1151. doi: 10.4161/cc.10.7.15210. [DOI] [PubMed] [Google Scholar]
- 58.Shi L, Zhang S, Feng K, Wu F, Wan Y, Wang Z, Zhang J, Wang Y, Yan W, Fu Z, et al. MicroRNA-125b-2 confers human glioblastoma stem cells resistance to temozolomide through the mitochondrial pathway of apoptosis. Int J Oncol. 2012;40:119–129. doi: 10.3892/ijo.2011.1179. [DOI] [PubMed] [Google Scholar]
- 59.Misawa A, Katayama R, Koike S, Tomida A, Watanabe T, Fujita N. AP-1-Dependent miR-21 expression contributes to chemoresistance in cancer stem cell-like SP cells. Oncol Res. 2010;19:23–33. doi: 10.3727/096504010x12828372551759. [DOI] [PubMed] [Google Scholar]
- 60.Godlewski J, Nowicki MO, Bronisz A, Williams S, Otsuki A, Nuovo G, Raychaudhury A, Newton HB, Chiocca EA, Lawler S. Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer Res. 2008;68:9125–9130. doi: 10.1158/0008-5472.CAN-08-2629. [DOI] [PubMed] [Google Scholar]
- 61.Li Y, Guessous F, Zhang Y, Dipierro C, Kefas B, Johnson E, Marcinkiewicz L, Jiang J, Yang Y, Schmittgen TD, et al. MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes. Cancer Res. 2009;69:7569–7576. doi: 10.1158/0008-5472.CAN-09-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang YP, Chien Y, Chiou GY, Cherng JY, Wang ML, Lo WL, Chang YL, Huang PI, Chen YW, Shih YH, et al. Inhibition of cancer stem cell-like properties and reduced chemoradioresistance of glioblastoma using microRNA145 with cationic polyurethane-short branch PEI. Biomaterials. 2012;33:1462–1476. doi: 10.1016/j.biomaterials.2011.10.071. [DOI] [PubMed] [Google Scholar]
- 63.Liu C, Tang DG. MicroRNA regulation of cancer stem cells. Cancer Res. 2011;71:5950–5954. doi: 10.1158/0008-5472.CAN-11-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lo WL, Yu CC, Chiou GY, Chen YW, Huang PI, Chien CS, Tseng LM, Chu PY, Lu KH, Chang KW, et al. MicroRNA-200c attenuates tumour growth and metastasis of presumptive head and neck squamous cell carcinoma stem cells. J Pathol. 2011;223:482–495. doi: 10.1002/path.2826. [DOI] [PubMed] [Google Scholar]
- 65.Xu XT, Xu Q, Tong JL, Zhu MM, Nie F, Chen X, Xiao SD, Ran ZH. MicroRNA expression profiling identifies miR-328 regulates cancer stem cell-like SP cells in colorectal cancer. Br J Cancer. 2012;106:1320–1330. doi: 10.1038/bjc.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cartwright P, McLean C, Sheppard A, Rivett D, Jones K, Dalton S. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development. 2005;132:885–896. doi: 10.1242/dev.01670. [DOI] [PubMed] [Google Scholar]
- 67.Delaloy C, Liu L, Lee JA, Su H, Shen F, Yang GY, Young WL, Ivey KN, Gao FB. MicroRNA-9 coordinates proliferation and migration of human embryonic stem cell-derived neural progenitors. Cell Stem Cell. 2010;6:323–335. doi: 10.1016/j.stem.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu C, Teng ZQ, Santistevan NJ, Szulwach KE, Guo W, Jin P, Zhao X. Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell Stem Cell. 2010;6:433–444. doi: 10.1016/j.stem.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smrt RD, Szulwach KE, Pfeiffer RL, Li X, Guo W, Pathania M, Teng ZQ, Luo Y, Peng J, Bordey A, et al. MicroRNA miR-137 regulates neuronal maturation by targeting ubiquitin ligase mind bomb-1. Stem Cells. 2010;28:1060–1070. doi: 10.1002/stem.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu JY, Chung KH, Deo M, Thompson RC, Turner DL. MicroRNA miR-124 regulates neurite outgrowth during neuronal differentiation. Exp Cell Res. 2008;314:2618–2633. doi: 10.1016/j.yexcr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tran N, McLean T, Zhang X, Zhao CJ, Thomson JM, O’Brien C, Rose B. MicroRNA expression profiles in head and neck cancer cell lines. Biochem Biophys Res Commun. 2007;358:12–17. doi: 10.1016/j.bbrc.2007.03.201. [DOI] [PubMed] [Google Scholar]
- 72.Voorhoeve PM, le Sage C, Schrier M, Gillis AJ, Stoop H, Nagel R, Liu YP, van Duijse J, Drost J, Griekspoor A, et al. A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Cell. 2006;124:1169–1181. doi: 10.1016/j.cell.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 73.Zhu S, Si ML, Wu H, Mo YY. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1) J Biol Chem. 2007;282:14328–14336. doi: 10.1074/jbc.M611393200. [DOI] [PubMed] [Google Scholar]
- 74.Hwang-Verslues WW, Chang PH, Wei PC, Yang CY, Huang CK, Kuo WH, Shew JY, Chang KJ, Lee EY, Lee WH. miR-495 is upregulated by E12/E47 in breast cancer stem cells, and promotes oncogenesis and hypoxia resistance via downregulation of E-cadherin and REDD1. Oncogene. 2011;30:2463–2474. doi: 10.1038/onc.2010.618. [DOI] [PubMed] [Google Scholar]
- 75.Mott JL, Kobayashi S, Bronk SF, Gores GJ. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007;26:6133–6140. doi: 10.1038/sj.onc.1210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi L, Zhang J, Pan T, Zhou J, Gong W, Liu N, Fu Z, You Y. MiR-125b is critical for the suppression of human U251 glioma stem cell proliferation. Brain Res. 2010;1312:120–126. doi: 10.1016/j.brainres.2009.11.056. [DOI] [PubMed] [Google Scholar]