Abstract
Background/Aims
Identification of patients at high risk for perioperative cardiac events (POCE) is clinically important. This study aimed to determine whether preoperative measurement of plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) could predict POCE, and compared its predictive value with that of conventional cardiac risk factors and stress thallium scans in patients undergoing vascular surgery.
Methods
Patients scheduled for non-cardiac vascular surgery were prospectively enrolled. Clinical risk factors were identified, and NT-proBNP levels and stress thallium scans were obtained. POCE was the composite of acute myocardial infarction, congestive heart failure including acute pulmonary edema, and primary cardiac death within 5 days after surgery. A modified Revised Cardiac Risk Index (RCRI) was proposed and compared with NT-proBNP; a positive result for ischemia and a significant perfusion defect (≥ 3 walls, moderate to severely decreased, reversible perfusion defect) on the thallium scan were added to the RCRI.
Results
A total of 365 patients (91% males) with a mean age of 67 years had a median NT-proBNP level of 105.1 pg/mL (range of quartile, 50.9 to 301.9). POCE occurred in 49 (13.4%) patients. After adjustment for confounders, an NT-proBNP level of > 302 pg/mL (odds ratio [OR], 5.7; 95% confidence interval [CI], 3.1 to 10.3; p < 0.001) and a high risk by the modified RCRI (OR, 3.9; 95% CI, 1.6 to 9.3; p = 0.002) were independent predictors for POCE. Comparison of the area under the curves for predicting POCE showed no statistical differences between NT-proBNP and RCRI.
Conclusions
Preoperative measurement of NT-proBNP provides information useful for prediction of POCE as a single parameter in high-risk patients undergoing noncardiac vascular surgery.
Keywords: Pro-B-type natriuretic peptide, Vascular surgical procedures, Postoperative complications
INTRODUCTION
Patients with vascular disease have a high incidence of atherosclerotic comorbidity and increased risk for perioperative cardiac events (POCE) of up to 24% [1-5]. Several risk stratification indices based on patient clinical history, including the Revised Cardiac Risk Index (RCRI) by Lee et al. [6], have been developed to predict postoperative cardiac outcome. Because thallium single-photon emission computed tomography (SPECT) has a high sensitivity for POCE and the risk increases in a manner proportional to the number of reversible defects [7], thallium SPECT has been widely performed to improve the predictive value of POCE after vascular surgery [8-10].
B-type natriuretic peptide (BNP) is a cardiac biomarker that is secreted mainly from the left ventricle in response to wall stress [11,12]. ProBNP is synthesized as a pro-hormone and is cleaved into the N-terminal proBNP (NT-proBNP) and bioactive BNP [13]. NT-proBNP is regarded not only as an important diagnostic and prognostic biomarker of congestive heart failure and coronary artery disease [14-17], but also as a predictive marker of adverse cardiac events in patients who undergo noncardiac surgery [18-20].
The present prospective cohort study aimed to assess the value of preoperatively measured NT-proBNP for prediction of POCE compared with that of the RCRI and thallium SPECT in patients undergoing elective major vascular surgery.
METHODS
Study population
Patients ≥ 20 years old who were referred for a cardiac consultation before elective noncardiac vascular surgery at Samsung Medical Center, a tertiary 1,400-bed teaching hospital in Seoul, Korea, were prospectively enrolled. Noncardiac major vascular surgery included abdominal aortic surgery, carotid artery surgery, infrainguinal vascular surgery, and extra-anatomic bypass surgery. Abdominal aortic surgery comprised abdominal aortic aneurysm and suprainguinal vascular surgeries.
Data collection
Clinical history, use of medications, and physical examination findings were recorded. In all patients, baseline laboratory tests were evaluated within 2 weeks before surgery, including a complete blood cell count, chemistry laboratory tests including NT-proBNP, electrocardiography, and chest X-ray. Additional tests such as transthoracic echocardiography and stress thallium scans were performed based on the joint decision of the surgeon and consulting physician.
Patients were followed up with daily electrocardiography, cardiac enzymes including serum troponin I, and chest X-ray for 5 days after surgery, and additional evaluation was undertaken in accordance with the clinical decision of the consulting physician or surgeon. Patients were followed by the consulting physician until discharge or up to 30 days in-hospital after surgery. Any abnormal signs or symptoms suggestive of heart failure or myocardial ischemia were followed by meticulous evaluation of postoperative cardiac status with repeated cardiac serum marker measurements and electrocardiography. If active heart failure or ongoing myocardial ischemia was found, the patient was transferred to the cardiovascular team and treated appropriately. In cases of mortality, the cause of death was determined based on the consensus of the surgeon, anesthesiologist, and cardiovascular consulting physician.
In accordance with the RCRI [6], the clinical history related to each of the following cardiac risk factors was recorded: history of ischemic heart disease, which included a history of myocardial infarction (MI), positive result on exercise test, use of nitrate, current symptoms of angina, or Q wave on electrocardiography; prior revascularization including percutaneous cardiac interventions and/or coronary artery bypass graft surgery; history of congestive heart failure; cerebrovascular accident or transient ischemic attack; diabetes mellitus (fasting glucose level ≥ 7.0 mmol/L or treatment with insulin); and chronic renal insufficiency (serum creatinine ≥ 2.0 mg/dL or on dialysis).
The cardiac risk score was calculated by assigning one point to each of these risk factors when present and was divided into low- (0 risk factors), intermediate- (1 to 2 risk factors), and high- (≥ 3 risk factors) risk groups [6,21]. A positive result on the stress thallium scan was defined as a perfusion defect at any segment to any degree and significant perfusion defect as a large (≥ 3 walls), moderate to severely decreased, reversible defect on the stress thallium scan. A modified RCRI was proposed to stress the predictive power of the thallium scan by adding one point if a positive result for ischemia was present on the thallium scan and another point if a significant perfusion defect was present on the RCRI. The prognostic value of NT-proBNP was compared with that of the RCRI and modified RCRI.
End-point definition
A POCE was defined by any of the following single or combined events: MI, development or aggravation of congestive heart failure, or primary cardiovascular death. MI was defined by a rise in postoperative troponin I above the upper normal value (0.78 ng/mL; Roche Diagnostics, Basel, Switzerland) of our facility. If there were any clinically relevant electrocardiographic or echocardiographic findings with a troponin level below the reference value, troponin was repeatedly measured to confirm the absence of myocardial damage. Diagnosis of new development of congestive heart failure required a formal reading of the chest X-ray by a radiologist consistent with the complication. Primary cardiovascular death was defined as sudden death that could not be explained by any other non-cardiovascular postoperative complications. All clinical events were collected by a research nurse and investigated by physicians. This study protocol was approved by the research ethnics committee of Samsung Medical Center.
Statistical analysis
Continuous data were expressed as means ± standard deviations (SD) or median (interquartile range). Student's t test, Fisher's exact test, and one-way ANOVA were used to compare continuous variables, and the chi-square test was used for dichotomous data. Correlations between cardiac events and clinical variables were examined with multivariate logistic models with backward selection. To evaluate the associations between perioperative cardiovascular risk predictors and clinical outcome, RCRI, the modified RCRI, and a biomarker were treated as continuous variables or ordered categorical variables. Receiver-operating characteristic (ROC) analysis was used to calculate sensitivity, specificity, and area under the curve. The optimal cutoff level of each predictor was determined at the point where the sum of sensitivity and specificity was maximal, which was used in subsequent analyses. The predictive power of each method was compared by the ROC curve using the technique of Hanley and McNeil [22]. A p value of < 0.05 was considered to indicate statistical significance. SPSS version 11.0 (SPSS Inc., Chicago, IL, USA) was used.
RESULTS
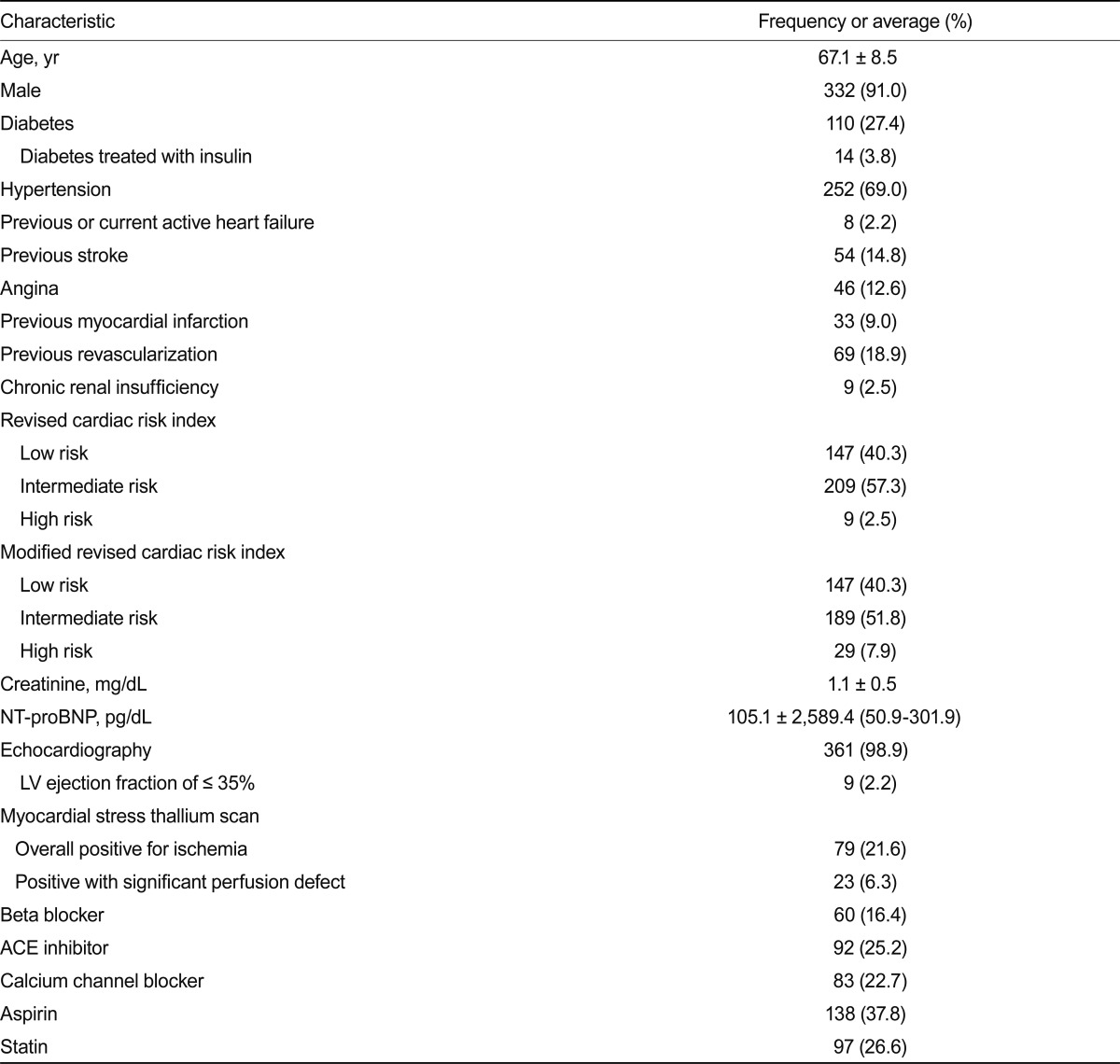
A total of 709 patients were enrolled in the study from November 2004 to January 2008. The following patients were excluded from the analysis: lack of preoperative plasma NT-proBNP measurement in 250 patients and postoperative serum troponin I measurement in 16, loss of follow-up of < 30 days in 2 patients, and no myocardial thallium SPECT in 76. The study population comprised the remaining 365 patients (91% males) with a mean age of 67.1 years (SD, 8.5).
The median NT-proBNP level was 105.1 pg/mL (range of quartile, 50.9 to 301.9) (Table 1). Positivity for myocardial ischemia on the thallium scan was observed in 79 patients (21.6%), and significant myocardial perfusion defects were present in 23 patients (6.3%). Patients were categorized as low (0 risk factors), intermediate (1 to 2 risk factors), and high (≥ 3 risk factors) risk by the modified RCRI; the majority were classified as low- to intermediate-risk group (40.3%, 51.8%, and 7.9%; low-, intermediate-, and high-risk, respectively). The median NT-proBNP level was compared among the risk groups (88.95 ± 515.36 pg/mL, 136.10 ± 1,620.59 pg/mL,and 1,132 ± 13,366.12 pg/mL; low-, intermediate-, and high-risk, respectively) and showed significant differences, with the exception of between the low- and intermediate-risk groups (p = 0.282).
Table 1.
Baseline clinical characteristics
NT-proBNP, N-terminal pro-B-type natriuretic peptide; LV, left ventricle; ACE, angiotensin-converting enzyme.
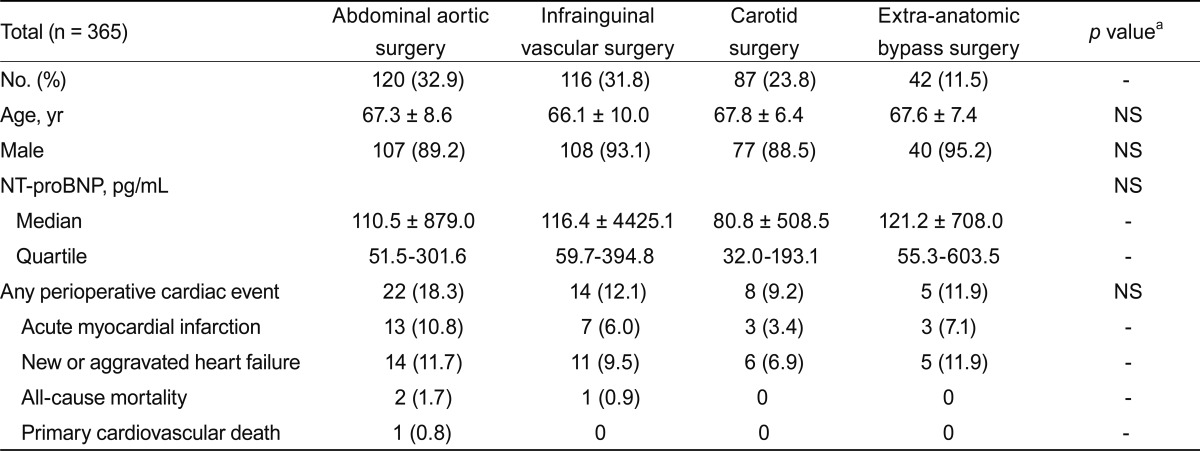
POCE occurred in 49 patients (13.4%), acute MI in 26 (7.1%), congestive heart failure in 36 (9.9%), cardiac death in 1 (0.3%), and non-cardiac death in 3 (0.8%). Individual patients may have more than one event, and all events were counted as an incidence. The median NT-proBNP did not differ significantly according to the type of surgery (Table 2). The incidence of POCE was higher in the abdominal aorta surgery group, but this difference was not statistically significant (p > 0.05).
Table 2.
Surgical procedures and clinical outcomes
NS, not significant; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
aBy one-way ANOVA or chi-square test.
Preoperative predictors of postoperative cardiac events
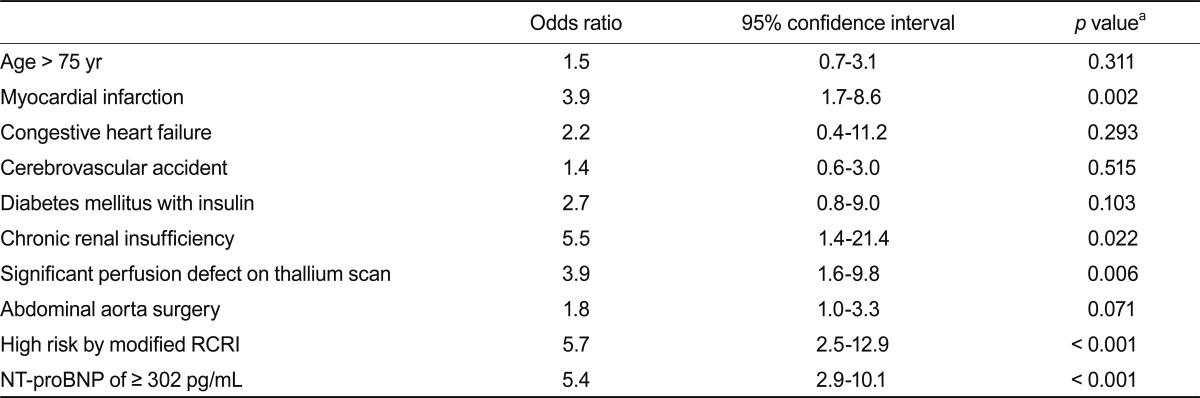
In univariate analysis (Table 3), a history of MI (odds ratio [OR], 3.9; 95% confidence interval [CI], 1.7 to 8.6; p = 0.002), chronic renal insufficiency (OR, 5.5; 95% CI, 1.4 to 21.4; p = 0.022), significant perfusion defect on thallium SPECT (OR, 3.9; 95% CI, 1.6 to 9.8; p = 0.006), high risk by modified RCRI (OR, 5.7; 95% CI, 2.5 to 12.9; p < 0.001), and NT-proBNP level of ≥ 302 pg/mL (OR, 5.4; 95% CI, 2.9 to 10.1; p < 0.001) were significantly associated with POCE. After adjusting for confounders, an NT-proBNP level of > 302 pg/mL (OR, 4.5; 95% CI, 2.3 to 8.7; p < 0.0001) and high risk by modified RCRI (OR, 3.9; 95% CI, 1.6 to 9.3; p = 0.002) were independent predictors of POCE (Table 4).
Table 3.
Univariate analysis of perioperative events
RCRI, Revised Cardiac Risk Index; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
aOn univariate analysis by chi-square test.
Table 4.
Multivariate analysis of perioperative events
RCRI, Revised Cardiac Risk Index; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
aIn a logistic regression model.
Predictive value of NT-proBNP for POCE
The predictive power of each risk predictor was investigated and compared by area under curve (AUC) of ROC analysis. The AUC of the RCRI for POCE was 0.677, and the modified RCRI was 0.676 (Fig. 1). The AUC of preoperative NT-proBNP was 0.700. There was no statistically significant difference between the AUC of NT-proBNP and the RCRI or modified RCRI (p > 0.05). When NT-proBNP (cutoff value, 302 pg/mL) was added to the RCRI, the AUC increased to 0.728, but was not significantly different than the AUC of NT-proBNP (p > 0.05). However, the predictive value of RCRI with NT-proBNP was significantly higher than that of RCRI or modified RCRI (p = 0.015). The predictive power of NT-proBNP for MI was not statistically different from that of the modified RCRI (AUC of NT-proBNP vs. modified RCRI, 0.70 vs. 0.68; p = 0.41).
Figure 1.
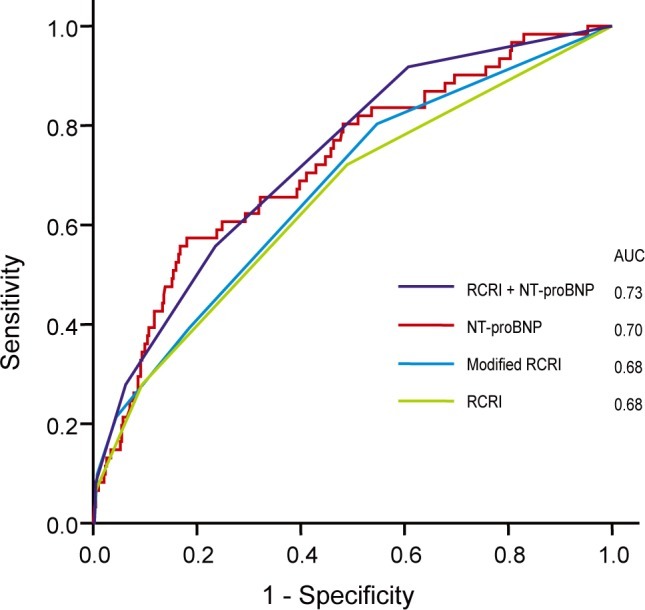
Comparison of predictive power on perioperative cardiac events. The predictive power of each risk predictors was investigated and compared each other by area under curve (AUC) of ROC analysis. For Revised Cardiac Risk Index (RCRI) and N-terminal pro-B-type natriuretic peptide (NT-proBNP), AUC = 0.73 ± 0.04 (95% confidence interval [CI], 0.65 to 0.81); for NT-proBNP, 0.70 ± 0.04 (95% CI, 0.62 to 0.78); for modified RCRI, 0.68 ± 0.04 (95% CI, 0.59 to 0.80); for RCRI, 0.68 ± 0.04 (95% CI, 0.60 to 0.76). Comparison of AUC of NT-proBNP with RCRI or modified RCRI did not show statistical difference (p > 0.05 by Hanley and McNail methods). However, the predictive value of RCRI with NT-proBNP was significantly higher than the one of RCRI or modified RCRI (p = 0.015).
ROC, receiver-operating characteristic.
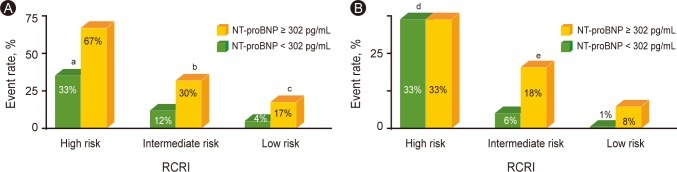
The incidence of POCE and MI was compared by means of the NT-proBNP level (302 pg/mL) and the RCRI (Fig. 2). POCE occurred significantly more frequently in patients with higher NT-proBNP (≥ 302 pg/mL); 17% of patients had a higher NT-proBNP, whereas 4% had a lower NT-proBNP (< 302 pg/mL) in the low-risk (0 risk factors) group by the RCRI (OR, 4.7; 95% CI, 1.2 to 19.1; p = 0.040), and 30% had a higher NT-proBNP whereas 12% had a lower NT-proBNP (OR, 3.2; 95% CI, 1.5 to 6.8, p = 0.004) in the intermediate-risk (1 to 2 risk factors) group. However, the incidence of POCE in the high-risk group did not differ significantly according to NT-proBNP level.
Figure 2.
Predictive value of N-terminal pro-B-type natriuretic peptide (NT-proBNP) in relation to the Revised Cardiac Risk Index (RCRI) for perioperative cardiac event (POCE)s and myocardial infarction (MI). (A) Incidence of POCE was compared by the level of NT-proBNP 302 pg/mL and the RCRI. (B) Incidence of MI was compared by the level of NT-proBNP 302 pg/mL and the RCRI.
aDiscriminative power of the RCRI in patients with NT-proBNP < 302 pg/mL, p =0.026.
bOdds ratio (OR), 3.2; 95% confidence interval (CI), 1.5 to 6.8; p = 0.004.
cOR, 4.7; 95% CI, 1.2 to 19.1; p = 0.040.
dDiscriminative power of the RCRI in patients with NT-proBNP < 302 pg/mL, p = 0.009.
eOR, 3.4; 95% CI, 1.3 to 8.7; p = 0.017.
When the incidence of POCE was compared among the risk groups categorized by the RCRI, the discriminative power of the RCRI for POCE was significant in patients with lower NT-proBNP levels (< 302 pg/mL) (p = 0.026 for POCE and p = 0.009 for perioperative MI). In patients with higher NT-proBNP levels, however, the RCRI had no statistically significant discriminative value among the groups in terms of the incidence of POCE and MI.
DISCUSSION
We found a single preoperative NT-proBNP level to be at least as reliable as the RCRI and the modified RCRI for stratification of patients undergoing vascular surgery. To our knowledge, this study is the first to compare the prognostic power of NT-proBNP with the RCRI combined with the result of myocardial stress thallium SPECT in patients undergoing vascular surgery.
Patients undergoing vascular surgery are at increased risk for cardiovascular complications [23,24], but they often have obscured symptoms because of limited functional performance. Many studies have supported the stress thallium scan as a noninvasive stress test with which to improve the predictive value of cardiac complications before non-cardiac vascular surgery [25-27]. Moderate to severe inducible ischemia on a thallium scan was reported to have a strong association with cardiac complications after major vascular surgery [28]. For this reason, the stress thallium scan has been widely performed in patients with vascular surgery. However, it is not consistently predictive of risk [29-31].
Several recent studies reported that NT-proBNP is also predictive of long-term and short-term mortality and adverse cardiac events after non-cardiac surgery [18-20,32-34]. Feringa et al. [35] reported the prognostic value of NT-proBNP in addition to clinical data and dobutamine stress echocardiography (DSE) results in 117 patients with abdominal aortic aneurysms or who underwent leg bypass surgery (cutoff value, 533 pg/mL). And they studied 335 patients and demonstrated that NT-proBNP provided better prediction than DSE, but they did not compare RCRI with DSE. Schouten et al. [33] evaluated 400 patients who underwent abdominal aortic aneurysm repair, including open repair surgery (70%) and EVAR (30%), peripheral bypass surgery, and carotid surgery and showed an additional prognostic value of NT-proBNP when combined with RCRI. The cutoff value of NT-proBNP was similar to ours at 350 pg/mL. However, despite a numerical increase in the AUC of NT-proBNP in addition to RCRI, statistical significance was not achieved in their study. A recent meta-analysis of NT-proBNP for the prediction of POCE summarized that the decision threshold used in the previous studies varied between 201 and 533 pg/mL for NT-proBNP, which includes the value in our study [36].
In our study, a single preoperative NT-proBNP measurement provided a comparable and powerful POCE predictive value compared with the clinical risk index and myocardial stress thallium scan. The thallium scan failed to demonstrate its predictive power for POCE and did not have incremental value for clinically determined risk in patients undergoing vascular surgery. Whereas the discriminative power of the RCRI for POCE was significant in patients with a lower NT-proBNP (< 302 pg/mL), it was not significant with a higher NT-proBNP (≥ 302 pg/mL). Interestingly, the incidence of POCE was significantly higher in patients with a higher NT-proBNP who had no clinical risk factors by RCRI or modified RCRI. Moreover, the predictive power of NT-proBNP was as excellent as that of the modified RCRI even for perioperative MI as a single parameter. This may confirm the role of NT-proBNP as an integrated marker preceding multiple aspects of cardiac dysfunction [37] and suggest that preoperative measurement of NT-proBNP provides reliable and practical information for the prediction of POCE not only in high-risk patients, but also in clinically low- to intermediate-risk patients undergoing vascular surgery.
ACC/AHA guidelines recommend proceeding with planned operations without noninvasive tests before vascular surgery if the patient has no risk factors and proceeding with planned operations with heart rate control in patients with fewer than two risk factors [38]. Whereas POCE occurred only in 4% of patients with no clinical risk factors by RCRI and with low NT-proBNP levels, it occurred in 17% of those with no risk factors but increased NT-proBNP in our study. Perioperative MI occurred in 6% of patients in the intermediate-risk group with a low NT-proBNP, but in 18% with increased NT-proBNP. This suggests a need for evaluation with measurement of preoperative NT-proBNP in patients with no risk factors or fewer than two risk factors before guiding perioperative medical management, which needs further study. Therefore, preoperative NT-proBNP may be a useful initial screening tool to guide whether to proceed with further comprehensive cardiac evaluation.
We were unable to observe the long-term predictive value of NT-proBNP. The clinical course of our patients was not followed for more than 30 days after discharge. However, all POCE had developed within 5 postoperative days, which reconfirms that most POCE develops in the early postoperative period [39,40]. The percentage of patients with a low to intermediate risk was higher than that in previous studies. This might in part influence the NT-proBNP cutoff value. The incidence of POCE did not differ significantly according to the NT-proBNP level in patients with more than three risk factors. This may be due in part to the small number and uneven distribution of patients in the high-risk group. The frequency of POCE was relatively high in our study. This may be due to the relatively low proportion of patients taking aspirin (38%), beta blockers (16%), and statins (27%). A selection bias may have resulted from exclusion of a significant portion of patients due to lack of measurement of NT-proBNP. We defined POCE by any of the following single or combined events: MI, development or aggravation of congestive heart failure, or primary cardiovascular death, which differs from the composite endpoint of the RCRI including MI, pulmonary edema, ventricular fibrillation or primary cardiac arrest, and complete heart block. This might result in a different statistical power in predicting POCE in our study. Further studies of preoperative NT-proBNP could help to guide perioperative medical management.
In conclusion, patients undergoing major vascular surgery have an increased risk for perioperative cardiac morbidity and mortality. A single preoperative measurement of NT-proBNP provides a noninvasive, objective, rapid, and convenient method of identifying patients at high risk, and provides physicians with additional prompt, solid information on clinical risk factors. Further studies are required to confirm our results for more widespread clinical use and to establish the NT-proBNP cutoff value for preoperative risk stratification.
Footnotes
No potential conflict of interest relevant to this article is reported.
References
- 1.Landesberg G, Shatz V, Akopnik I, et al. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol. 2003;42:1547–1554. doi: 10.1016/j.jacc.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Raby KE, Barry J, Creager MA, Cook EF, Weisberg MC, Goldman L. Detection and significance of intraoperative and postoperative myocardial ischemia in peripheral vascular surgery. JAMA. 1992;268:222–227. [PubMed] [Google Scholar]
- 3.Pasternack PF, Grossi EA, Baumann FG, et al. Silent myocardial ischemia monitoring predicts late as well as perioperative cardiac events in patients undergoing vascular surgery. J Vasc Surg. 1992;16:171–179. doi: 10.1067/mva.1992.36177. [DOI] [PubMed] [Google Scholar]
- 4.Sprung J, Abdelmalak B, Gottlieb A, et al. Analysis of risk factors for myocardial infarction and cardiac mortality after major vascular surgery. Anesthesiology. 2000;93:129–140. doi: 10.1097/00000542-200007000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Bartels C, Bechtel JF, Hossmann V, Horsch S. Cardiac risk stratification for high-risk vascular surgery. Circulation. 1997;95:2473–2475. doi: 10.1161/01.cir.95.11.2473. [DOI] [PubMed] [Google Scholar]
- 6.Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 7.Etchells E, Meade M, Tomlinson G, Cook D. Semiquantitative dipyridamole myocardial stress perfusion imaging for cardiac risk assessment before noncardiac vascular surgery: a meta-analysis. J Vasc Surg. 2002;36:534–540. doi: 10.1067/mva.2002.126563. [DOI] [PubMed] [Google Scholar]
- 8.Poldermans D, Arnese M, Fioretti PM, et al. Improved cardiac risk stratification in major vascular surgery with dobutamine-atropine stress echocardiography. J Am Coll Cardiol. 1995;26:648–653. doi: 10.1016/0735-1097(95)00240-5. [DOI] [PubMed] [Google Scholar]
- 9.Vanzetto G, Machecourt J, Blendea D, et al. Additive value of thallium single-photon emission computed tomography myocardial imaging for prediction of perioperative events in clinically selected high cardiac risk patients having abdominal aortic surgery. Am J Cardiol. 1996;77:143–148. doi: 10.1016/s0002-9149(96)90585-8. [DOI] [PubMed] [Google Scholar]
- 10.Golzar JA, Movahed A. Value of absence of a transient myocardial perfusion defect during stress myocardial perfusion study in patients undergoing major vascular surgery. Int J Cardiovasc Imaging. 2005;21:267–270. doi: 10.1007/s10554-004-6132-1. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimura M, Yasue H, Okumura K, et al. Different secretion patterns of atrial natriuretic peptide and brain natriuretic peptide in patients with congestive heart failure. Circulation. 1993;87:464–469. doi: 10.1161/01.cir.87.2.464. [DOI] [PubMed] [Google Scholar]
- 12.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 13.Hall C. Essential biochemistry and physiology of (NT-pro) BNP. Eur J Heart Fail. 2004;6:257–260. doi: 10.1016/j.ejheart.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Richards AM, Nicholls MG, Yandle TG, et al. Plasma N-terminal pro-brain natriuretic peptide and adrenomedullin: new neurohormonal predictors of left ventricular function and prognosis after myocardial infarction. Circulation. 1998;97:1921–1929. doi: 10.1161/01.cir.97.19.1921. [DOI] [PubMed] [Google Scholar]
- 15.Talwar S, Squire IB, Davies JE, Barnett DB, Ng LL. Plasma N-terminal pro-brain natriuretic peptide and the ECG in the assessment of left-ventricular systolic dysfunction in a high risk population. Eur Heart J. 1999;20:1736–1744. doi: 10.1053/euhj.1999.1694. [DOI] [PubMed] [Google Scholar]
- 16.Jernberg T, Stridsberg M, Venge P, Lindahl B. N-terminal pro brain natriuretic peptide on admission for early risk stratification of patients with chest pain and no ST-segment elevation. J Am Coll Cardiol. 2002;40:437–445. doi: 10.1016/s0735-1097(02)01986-1. [DOI] [PubMed] [Google Scholar]
- 17.Richards AM, Doughty R, Nicholls MG, et al. Plasma N-terminal pro-brain natriuretic peptide and adrenomedullin: prognostic utility and prediction of benefit from carvedilol in chronic ischemic left ventricular dysfunction: Australia-New Zealand Hear t Failure Group. J Am Coll Cardiol. 2001;37:1781–1787. doi: 10.1016/s0735-1097(01)01269-4. [DOI] [PubMed] [Google Scholar]
- 18.Yeh HM, Lau HP, Lin JM, Sun WZ, Wang MJ, Lai LP. Preoperative plasma N-terminal pro-brain natriuretic peptide as a marker of cardiac risk in patients undergoing elective non-cardiac surgery. Br J Surg. 2005;92:1041–1045. doi: 10.1002/bjs.4947. [DOI] [PubMed] [Google Scholar]
- 19.Feringa HH, Schouten O, Dunkelgrun M, et al. Plasma N-terminal pro-B-type natriuretic peptide as long-term prognostic marker after major vascular surgery. Heart. 2007;93:226–231. doi: 10.1136/hrt.2006.093716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SE, Park DG, Lee JH, Han KR, Oh DJ. Utility of B-type natriuretic peptide for predicting perioperative cardiovascular events in patients without history of cardiovascular disease undergoing major non-cardiac surgery. Korean Circ J. 2011;41:11–15. doi: 10.4070/kcj.2011.41.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldman L, Caldera DL, Nussbaum SR, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297:845–850. doi: 10.1056/NEJM197710202971601. [DOI] [PubMed] [Google Scholar]
- 22.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 23.Abraham N, Lemech L, Sandroussi C, Sullivan D, May J. A prospective study of subclinical myocardial damage in endovascular versus open repair of infrarenal abdominal aortic aneurysms. J Vasc Surg. 2005;41:377–380. doi: 10.1016/j.jvs.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 24.Le Manach Y, Perel A, Coriat P, Godet G, Bertrand M, Riou B. Early and delayed myocardial infarction after abdominal aortic surgery. Anesthesiology. 2005;102:885–891. doi: 10.1097/00000542-200505000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Kertai MD, Boersma E, Klein J, et al. Optimizing the prediction of perioperative mortality in vascular surgery by using a customized probability model. Arch Intern Med. 2005;165:898–904. doi: 10.1001/archinte.165.8.898. [DOI] [PubMed] [Google Scholar]
- 26.Chen T, Kuwabara Y, Tsutsui H, et al. The usefulness of dipyridamole thallium-201 single photon emission computed tomography for predicting perioperative cardiac events in patients undergoing non-cardiac vascular surgery. Ann Nucl Med. 2002;16:45–53. doi: 10.1007/BF02995291. [DOI] [PubMed] [Google Scholar]
- 27.Wolf YG, Landersberg G, Mosseri M, et al. Preoperative dipyridamole-thallium scanning, selective coronary revascularization and long-term survival in patients with critical lower limb ischemia. J Cardiovasc Surg (Torino) 2001;42:89–95. [PubMed] [Google Scholar]
- 28.Landesberg G, Mosseri M, Shatz V, et al. Cardiac troponin after major vascular surgery: the role of perioperative ischemia, preoperative thallium scanning, and coronary revascularization. J Am Coll Cardiol. 2004;44:569–575. doi: 10.1016/j.jacc.2004.03.073. [DOI] [PubMed] [Google Scholar]
- 29.de Virgilio C, Wall DB, Ephraim L, et al. An abnormal dipyridamole thallium/sestamibi fails to predict long-term cardiac events in vascular surgery patients. Ann Vasc Surg. 2001;15:267–271. doi: 10.1007/s100160010055. [DOI] [PubMed] [Google Scholar]
- 30.Falcone RA, Nass C, Jermyn R, et al. The value of preoperative pharmacologic stress testing before vascular surgery using ACC/AHA guidelines: a prospective, randomized trial. J Cardiothorac Vasc Anesth. 2003;17:694–698. doi: 10.1053/j.jvca.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Poldermans D, Bax JJ, Schouten O, et al. Should major vascular surgery be delayed because of preoperative cardiac testing in intermediate-risk patients receiving beta-blocker therapy with tight heart rate control? J Am Coll Cardiol. 2006;48:964–969. doi: 10.1016/j.jacc.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 32.Dernellis J, Panaretou M. Assessment of cardiac risk before non-cardiac surgery: brain natriuretic peptide in 1,590 patients. Heart. 2006;92:1645–1650. doi: 10.1136/hrt.2005.085530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schouten O, Hoeks SE, Goei D, Bax JJ, Verhagen HJ, Poldermans D. Plasma N-terminal pro-B-type natriuretic peptide as a predictor of perioperative and long-term outcome after vascular surgery. J Vasc Surg. 2009;49:435–441. doi: 10.1016/j.jvs.2008.08.063. [DOI] [PubMed] [Google Scholar]
- 34.Park SJ, Choi JH, Cho SJ, et al. Comparison of transthoracic echocardiography with N-terminal pro-brain natriuretic peptide as a tool for risk stratification of patients undergoing major noncardiac surgery. Korean Circ J. 2011;41:505–511. doi: 10.4070/kcj.2011.41.9.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feringa HH, Bax JJ, Elhendy A, et al. Association of plasma N-terminal pro-B-type natriuretic peptide with postoperative cardiac events in patients undergoing surgery for abdominal aortic aneurysm or leg bypass. Am J Cardiol. 2006;98:111–115. doi: 10.1016/j.amjcard.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 36.Karthikeyan G, Moncur RA, Levine O, et al. Is a pre-operative brain natriuretic peptide or N-terminal pro-B-type natriuretic peptide measurement an independent predictor of adverse cardiovascular outcomes within 30 days of noncardiac surgery? A systematic review and meta-analysis of observational studies. J Am Coll Cardiol. 2009;54:1599–1606. doi: 10.1016/j.jacc.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 37.Costello-Boerrigter LC, Boerrigter G, Redfield MM, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2006;47:345–353. doi: 10.1016/j.jacc.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116:e418–e499. doi: 10.1161/CIRCULATIONAHA.107.185699. [DOI] [PubMed] [Google Scholar]
- 39.Owens CD, Ridker PM, Belkin M, et al. Elevated C-reactive protein levels are associated with postoperative events in patients undergoing lower extremity vein bypass surgery. J Vasc Surg. 2007;45:2–9. doi: 10.1016/j.jvs.2006.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McFalls EO, Ward HB, Moritz TE, et al. Predictors and outcomes of a perioperative myocardial infarction following elective vascular surgery in patients with documented coronary artery disease: results of the CARP trial. Eur Heart J. 2008;29:394–401. doi: 10.1093/eurheartj/ehm620. [DOI] [PubMed] [Google Scholar]