Abstract
Background/Aims
Chronic inflammatory status is a possible risk factor for vascular access dysfunction in hemodialysis (HD) patients, but susceptibility differences appear among individuals. Interleukin (IL)-6 is a well-known inflammatory cytokine with various polymorphisms. We examined whether IL-6 polymorphisms are associated with vascular access dysfunction in HD patients.
Methods
A total of 80 HD patients (including 42 diabetic patients) were enrolled. Polymorphisms in the IL-6 gene promoter (-634 C/G and -174 G/C) were studied using restriction length polymorphism polymerase chain reaction analysis. Vascular access patency was compared between the patient groups with respect to IL-6 polymorphisms. An additional 89 healthy individuals were enrolled in the control group. Plasma IL-6 levels were de termined by enzyme-linked immunosorbent assay.
Results
The GG genotype and G allele at position -634 in the IL-6 promoter were more frequently observed in HD patients than in controls. Furthermore, the distribution of the -634 polymorphism differed according to vascular access patency in non-diabetic HD patients. However, the G allele was not a significant risk factor for early access failure. No significant association appeared between the IL-6 -634 C/G polymorphism and plasma IL-6 levels. The C allele of the IL-6 -174 G/C polymorphism was not detected in our study population.
Conclusions
The IL-6 -634 G allele appears with greater frequently in patients with end-stage renal disease and may be associated with vascular access dysfunction in non-diabetic HD patients.
Keywords: Hemodialysis, Inflammation, Interleukin-6 polymorphism, Vascular access failure
INTRODUCTION
Vascular access dysfunction remains an important cause of morbidity and mortality in hemodialysis (HD) patients [1]. Local vascular inf lammation associated with venous neointimal hyperplasia is regarded as a hallmark of fistula stenosis. Furthermore, inflammation strongly predicts all-cause [2,3] and cardiovascular [3] mortality rates among dialysis patients [4]. The majority of patients with end-stage renal disease (ESRD) exhibit evidence of chronic inflammation [5,6]. The causes of inf lammation in ESRD patients are multifactorial and include genetic factors, such as polymorphism [5]. Interleukin (IL)-6 is one of the most extensively studied effectors in the inflammatory cascade [7]. IL-6 is a circulating multifunctional cytokine secreted in response to different types of inflammatory insults. Increased circulating levels of IL-6 have been linked to malnutrition, hypertension (HTN), left ventricular hypertrophy, atherosclerosis, and cardiovascular mortality in patients with ESRD [8-10]. IL-6 induces endothelial cell damage, stimulating intracellular adhesion molecule-1 and enhancing the attachment and diapedesis of leukocytes across endothelial cells [11]. Several studies have shown that elevated levels of IL-6 have the distinct ability to stimulate vascular smooth muscle cell growth [12], thereby enhancing endothelial synthesis of plasminogen activator inhibitor-1 [13] and contributing to progressive fistula stenosis in HD patients [1].
The human IL-6 gene is located on chromosome 7p21, and consists of 5 axons and 4 introns. IL-6 has several polymorphisms in the promoter region (-634 C/G, -174 G/C, -572 G/C, and -597 G/A) [7]. Conflicting results have been reported regarding the IL-6 -174 G/C polymorphism and diseases. Two studies have shown that subjects who are homozygous for the G allele or heterozygous for G/C at position -174 have higher plasma IL-6 levels and higher IL-6 gene transcriptional activity than individuals homozygous for the C allele [14,15]. However, another study reported that the CC genotype was associated with cardiovascular events in HD patients [16]. An IL-6 -634 C/G polymorphism has also been reported, and the -634 G allele is associated with increased secretion of IL-6 by peripheral blood mononuclear cells (PBMCs) [17,18]. The IL-6 -634 G allele has been suggested as an aggravating factor in the progression of renal disease [17,19]. Although previous studies have explored the relationship between IL-6 levels and vascular access dysfunction in HD patients [20-22], the effect of IL-6 polymorphisms on vascular access dysfunction has not been reported.
In the current study, we examined whether the IL-6 -634 C/G and -174 G/C polymorphisms are associated with the duration of patent vascular access in HD patients.
METHODS
Study population
The patient group included 80 clinically stable patients receiving maintenance HD for > 3 months in Ewha Womans University Mokdong Hospital. The duration of HD per session was 4 to 6 hours; the frequency of HD was individualized to achieve a Kt/V > 1.2. We assessed the dialysis adequacy by calculating the Kt/V for each patient. Patients were treated with synthetic membranes (polysulfone or polyamide), and dialyzers were not re-used. Heparinization during the dialysis session was done by continuous infusion using unfractionated heparin at a dose of 500 to 1,000 U/hr according to patient weight. None of the patients had a history of acute diseases, such as recent myocardial infarction, unstable angina, acute pulmonary embolism, acute neurologic disorders, malignancies, and overt systemic infections within 3 months before blood sampling for IL-6. The access patent interval was defined as the time from the access formation to the time of first vascular stenosis in each patient. Acute obstructions due to mechanical factors related to surgery within a few days of the access creation, physical compression after the end of a dialysis session, or frequent hypotension during dialysis were excluded from the definition of vascular access patency. To analyze the relationship between IL-6 polymorphisms and vascular access patency, we categorized patients into two groups: the shorter access survival group (patients with access patency < 1 year at the time of enrollment), and longer access survival group (patients with vascular access patency > 1 year). We also performed the same analysis for patients with native arteriovenous fistula (AVF) excluding patients with an arteriovenous graft (AVG). We reviewed medical records from each patient and examined medical histories of patients with diabetes mellitus (DM), HTN, and drug histories for use of renin-angiotensin system blockers (angiotensin converting enzyme inhibitors or angiotensin receptor blockers), statins, and anti-platelet agents. We assessed the nutritional status of each patient by measuring the normalized protein catabolic rate.
An additional 89 healthy individuals without medical illnesses such as DM, HTN, or renal disease were enrolled as the control group. The study was conducted in accordance with the guidelines of the Declaration of Helsinki after receiving approval from Institutional Review Board of Ewha Womans University Mokdong Hospital. Written informed consent was obtained from all subjects.
Blood sampling
Venous blood samples were taken from each patient just prior to the dialysis session and analyzed immediately. Samples were obtained from the HD needle puncture site 72 hours after the last dialysis. All patients were required to fast after midnight (> 6 hours). The standard laboratory tests included complete blood cell and platelet counts and blood chemistries, including serum albumin, protein, blood urea nitrogen, creatinine, cholesterol, and low density lipoprotein. High sensitive C-reactive protein (hsCRP) was measured via a nephelometric immunoassay (Handok Pharm Co., Seoul, Korea). IL-6 levels were determined using a commercial enzyme-linked immunosorbent assay kit (Pierce Biotechnology, Rockford, IL, USA).
Genotyping
DNA was isolated from peripheral blood leukocytes by a standard method. All subjects were genotyped for -634 C/G and -174 G/C polymorphisms using the polymerase chain reaction (PCR)-restriction fragment length polymorphism method. Genotyping for the IL-6 -634 C/G and -174 G/C polymorphisms was performed by PCR followed by BsrB I and Hsp92 II digestion, respectively. The primers for each region were as follows: forward 5'-GAGACGCCTTGAAGTAACTG-3' and reverse 5'-AACCAAAGATGTTCTGAACTGA-3' for -634 C/G; and forward 5'-TGACTTCAGCTTTACTCTTGT-3' and reverse 5'-CTGATTGGAAACCTTATTAAG-3' for -174 G/C. The final reaction volume (30 µL) contained 200 ng of genomic DNA, 10 mmol/L Tris-HCl (pH 8.3), 50 mmol/L KCl, 1.5 mmol/L MgCl2, 200 mmol/L each dNTP, 1 µmol/L of each primer, and 2 U Taq polymerase (all reagents from Fermentas GmbH, St. Leon-Rot, Germany). Initial denaturation at 94℃ for 6 minutes was followed by 35 cycles of 94℃ for 1 minute, annealing at 60℃ for 1 minute, and extension at 72℃ for 1 minute. The final extension step was at 72℃ for 1 minute. The PCR product (-634 C/G, 180 bp; -174 G/C, 191 bp) was digested with the respective restriction enzyme (BsrB I for -643 C/G; Hsp92 II for -174 G/C) and 3% agarose gel. With respect to the -634 C/G polymorphism, CC homozygotes had a 180 bp fragment, CG heterozygotes had 180 bp and 120 + 60 bp fragments, and GG homozygotes had 120 + 60 bp fragments. With respect to the -174 G/C, the G allele had 2 fragments (161 + 30 bp) and the C allele had 3 fragments (116 + 45 + 30 bp).
Statistical analysis
All values are expressed as the mean ± SD or number (%). The Hardy-Weinberg equilibrium was assessed using the χ2 test. Genotype and allele frequencies were compared between two groups using the χ2 test or Fisher's exact test (values < 5) and the odds ratio (OR) was calculated. Differences in the continuous variables between groups were assessed using ANOVA and an unpaired Student's t test. Logistic regression analysis was used to assess risk factors for vascular access failure. All data were analyzed using SPSS for Windows version 9.0 (SPSS Inc., Chicago, IL, USA). A p < 0.05 was considered to be statistically significant.
RESULTS
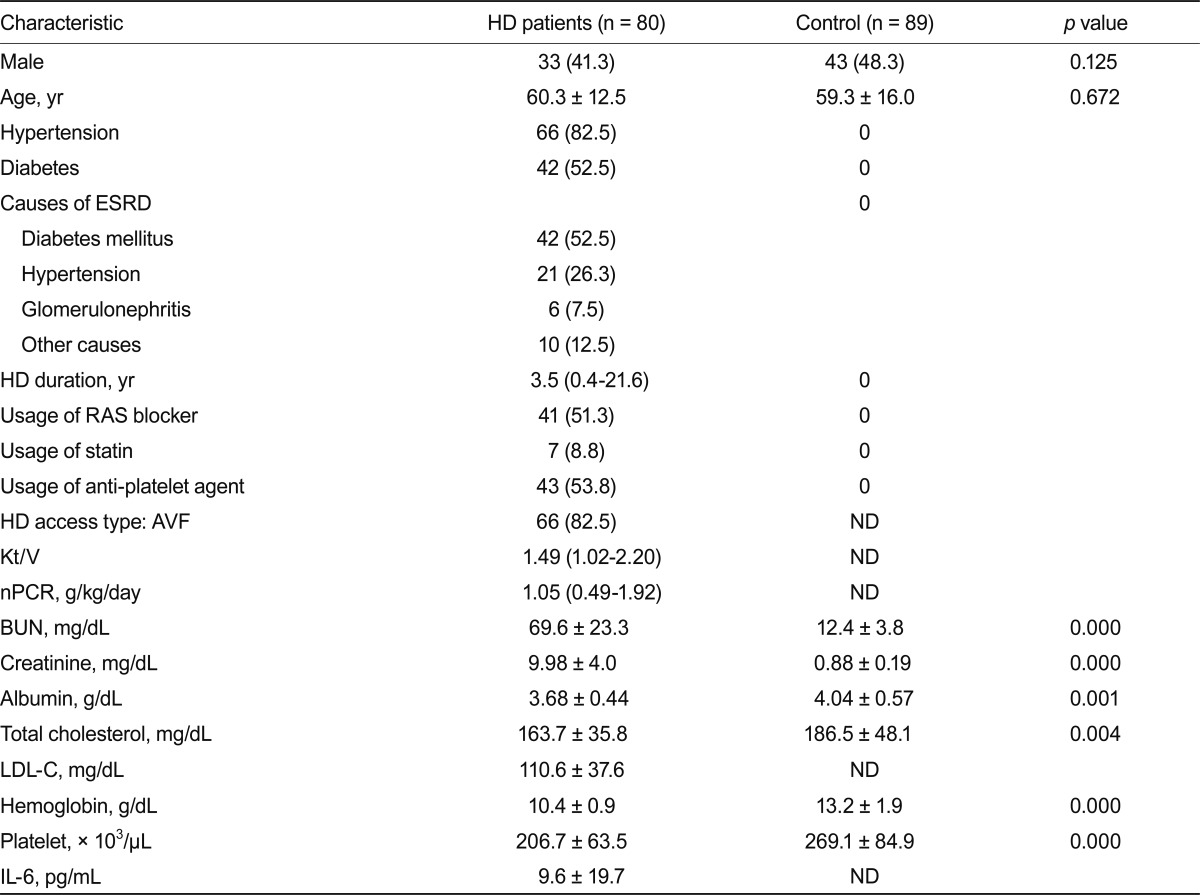
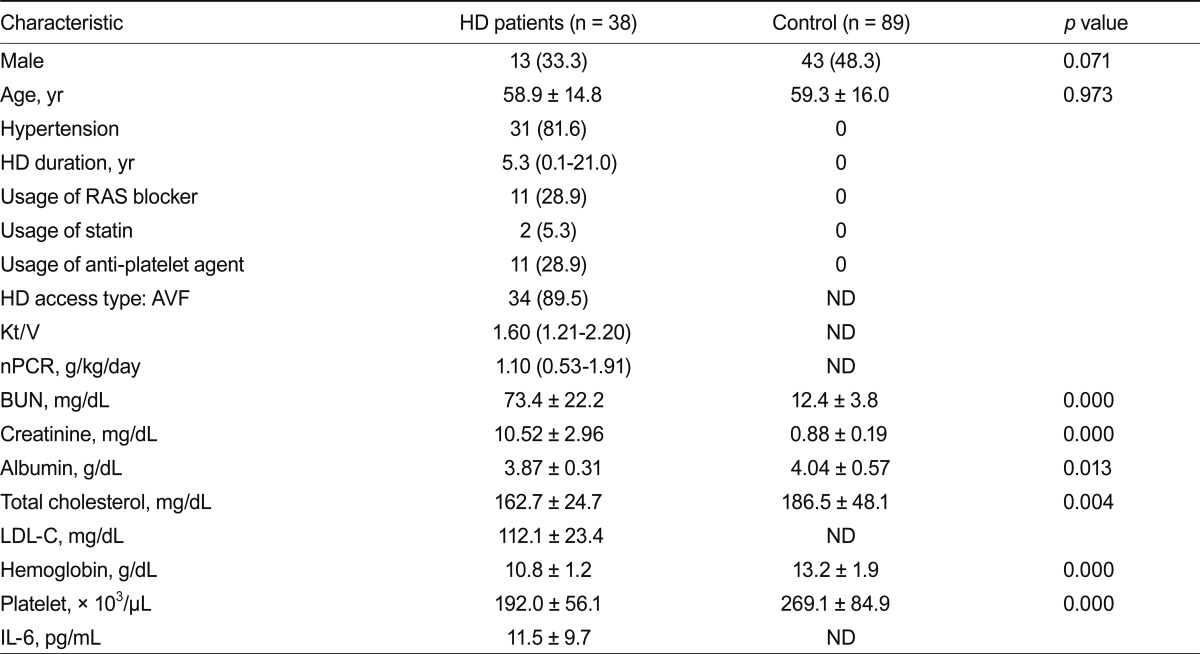
Table 1 lists the baseline clinical and laboratory characteristics of HD patients. The mean age and sex distribution of subjects did not differ significantly between the patient and control groups. Similar results were obtained after excluding diabetic patients (Table 2).
Table 1.
Baseline characteristics of subjects
Values are presented as the number (%), mean ± SD or median value with range.
HD, hemodialysis; ESRD, end-stage renal disease; RAS, renin-angiotensin system; AVF, arteriovenous fistula; ND, not determined; nPCR, normalized protein catabolic rate; BUN, blood urea nitrogen; LDL-C, low-density lipoprotein cholesterol; IL, interleukin.
Table 2.
Baseline characteristics of subjects (excluding DM patients)
Values are presented as number (%), median (range), or mean ± SD.
DM, diabetes mellitus; HD, hemodialysis; RAS, renin-angiotensin system; AVF, arteriovenous fistula; ND, not determined; nPCR, normalized protein catabolic rate; BUN, blood urea nitrogen; LDL-C, low-density lipoprotein cholesterol; IL, interleukin.
IL-6 polymorphisms
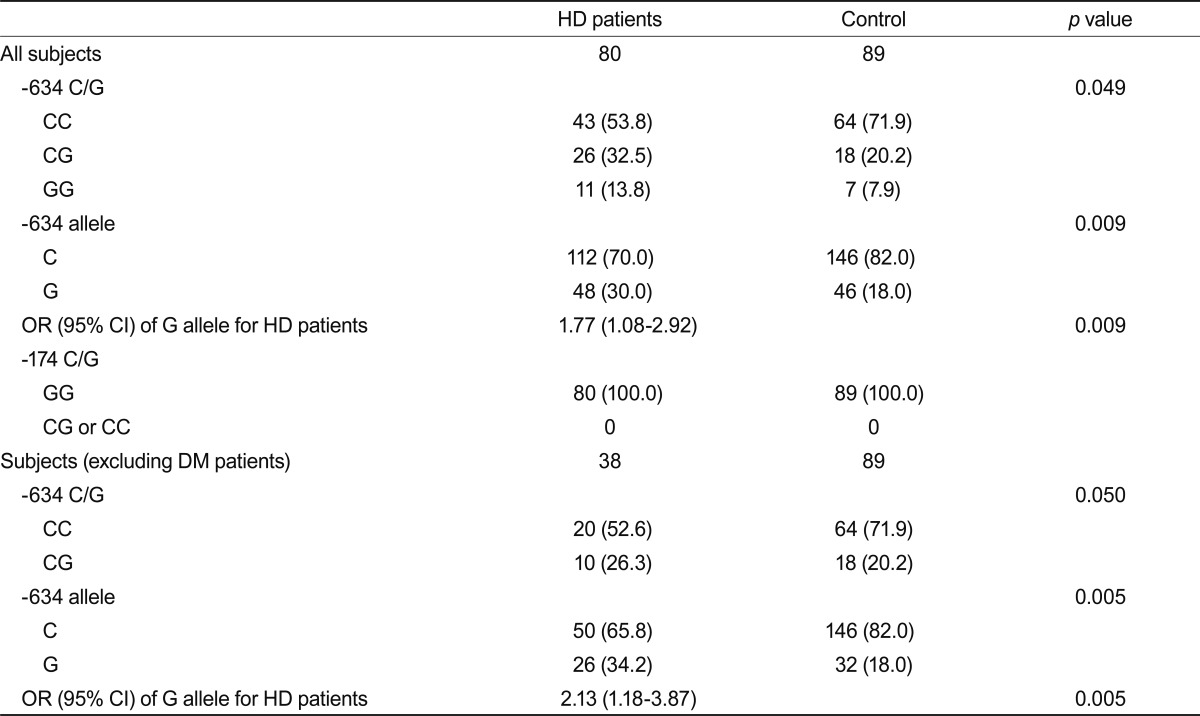
Table 3 lists the distribution of IL-6 genotypes and each allelic frequency. The distribution of the IL-6 -634 genotypes in HD patients and healthy controls was in Hardy-Weinberg equilibrium. Interestingly, the genotype distribution of the IL-6 -634 C/G polymorphisms in the HD patient group differed significantly compared with that in the control group. The GG genotype appeared more frequently in the patient group. Furthermore, the -634 G allele frequency was significantly more common in the patient group than in the control group (OR, 1.77; 95% confidence interval [CI], 1.08 to 2.92; p = 0.009). This finding was also observed after excluding diabetic patients (OR, 2.13; 95% CI, 1.18 to 3.87; p = 0.005) (Table 3).
Table 3.
Distribution of interleukin-6 -634 C/G and -174 G/C polymorphisms
Values are presented as the number (%).
HD, hemodialysis; OR, odds ratio; CI, confidence interval; DM, diabetes mellitus.
For the -174 G/C polymorphism, the -174 C allele was not detected in the patient or control group.
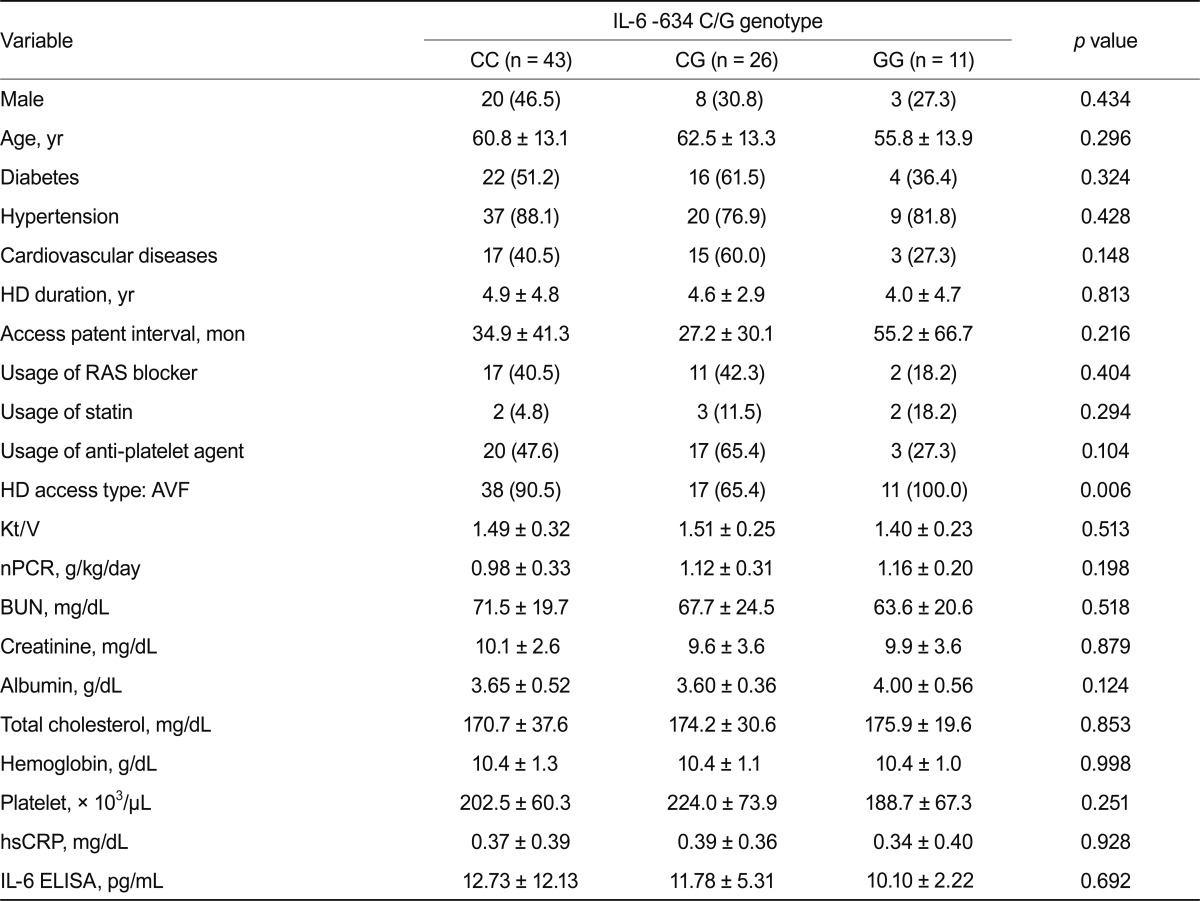
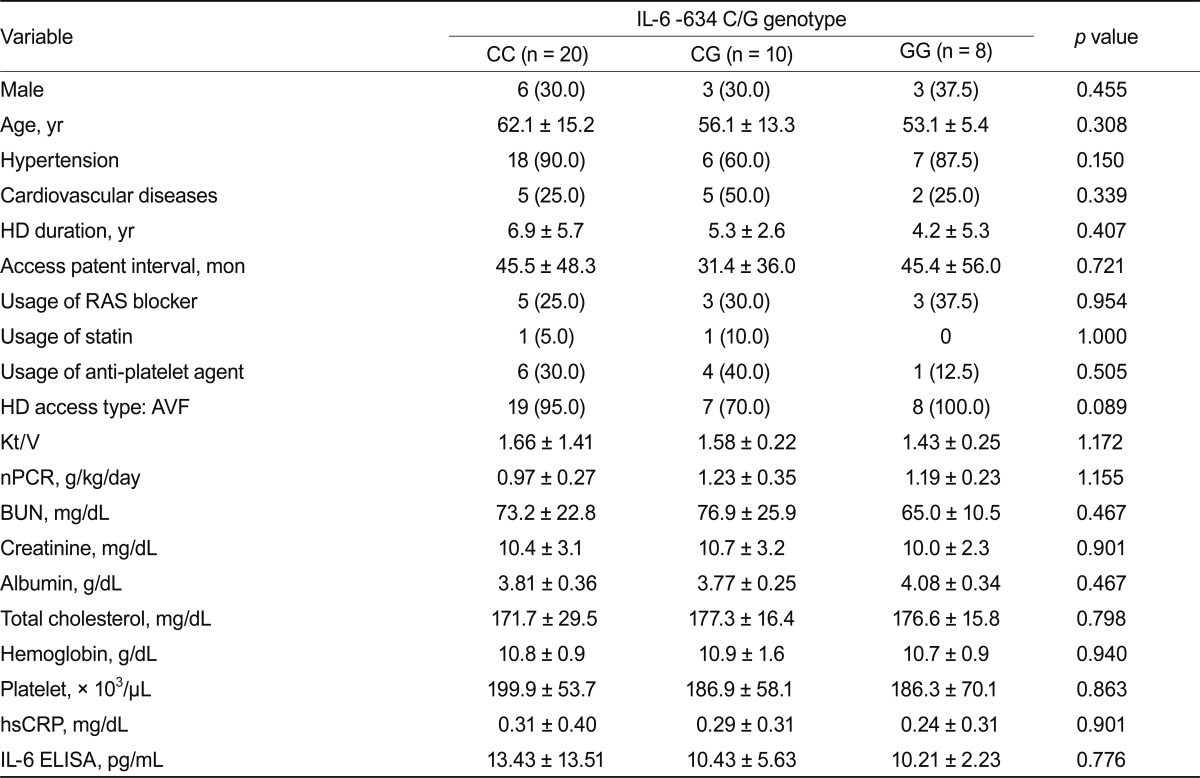
No significant differences appeared in clinical parameters based on genotypes (Table 4). Results were similar after diabetic patients were excluded (Table 5).
Table 4.
Comparison of variables based on the IL-6 -634 C/G polymorphism in HD patients
Values are presented as number (%) or mean ± SD.
IL, interleukin; HD, hemodialysis; RAS, renin-angiotensin system; AVF, arteriovenous fistula; nPCR, normalized protein catabolic rate; BUN, blood urea nitrogen; hsCRP, high sensitive c-reactive protein; ELISA, enzyme-linked immunosorbent assay.
Table 5.
Comparison of variables based on the IL-6 -634 C/G polymorphism in non-DM HD patients
Values are presented as number (%) or mean ± SD.
IL, interleukin; DM, diabetes mellitus; HD, hemodialysis; RAS, renin-angiotensin system; AVF, arteriovenous fistula; nPCR, normalized protein catabolic rate; BUN, blood urea nitrogen; hsCRP, high sensitive c-reactive protein; ELISA, enzyme-linked immunosorbent assay.
Relationship between the IL-6 -634 polymorphism and vascular access patency
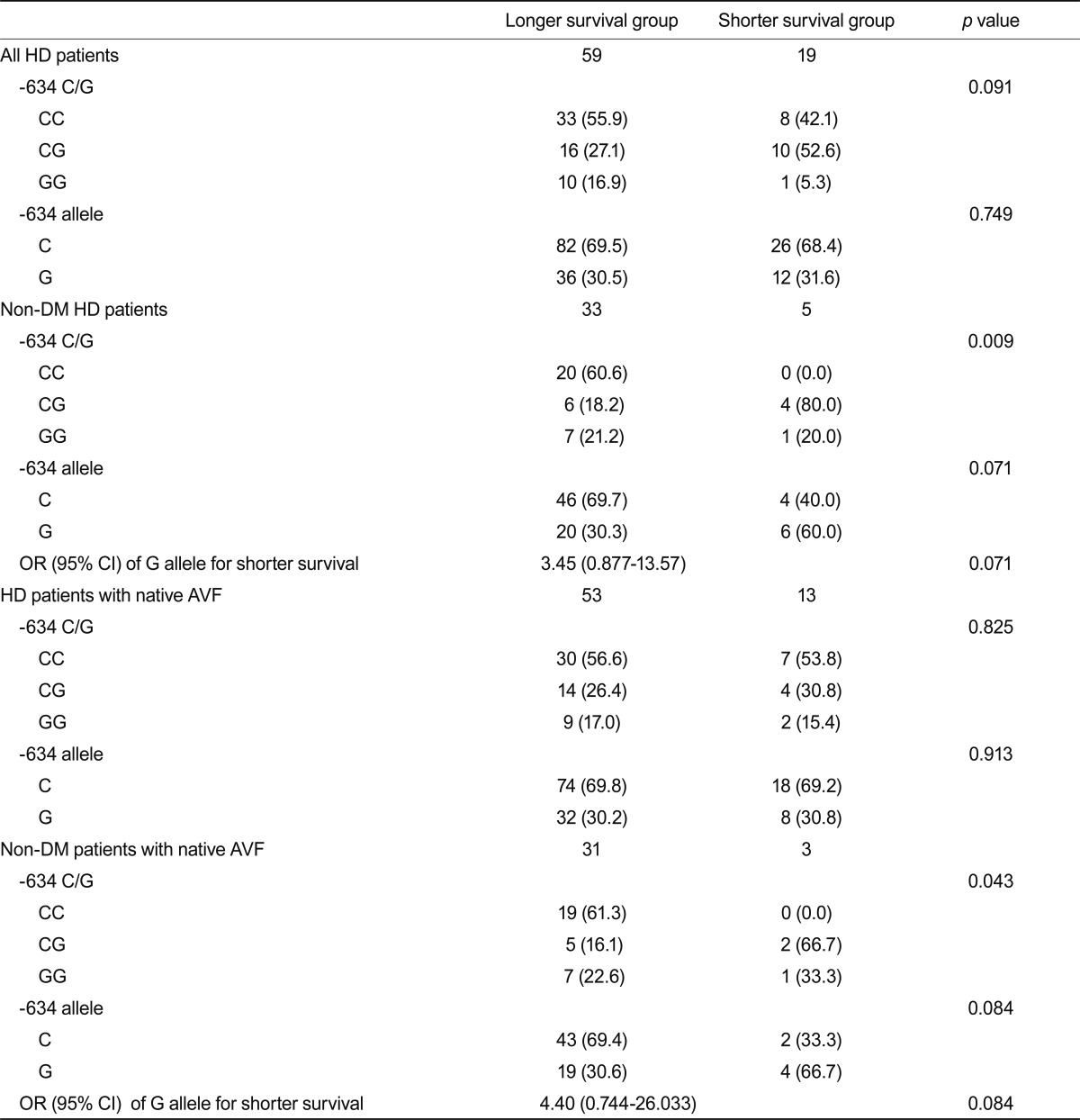
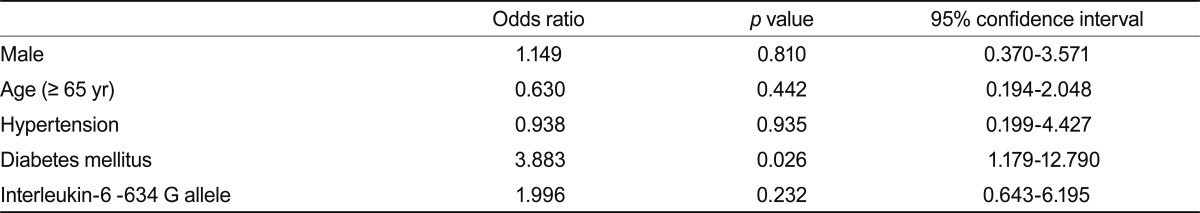
To assess the association between the IL-6 -634 polymorphism and vascular access patency, the -634 G allele frequency was examined with respect to the access patent interval (Table 6). The distribution of the -634 genotype and G allele frequency did not differ significantly based on the access patent interval. However, after excluding diabetic patients, the -634 genotype distribution differed significantly between the two groups and the G allele frequency exhibited a higher tendency in the access survival < 1 year sub-group (OR, 3.45; 95% CI, 0.88 to 13.57; p = 0.071). These findings were preserved in the analysis of patients with native AVF. However, the G allele did not reveal any statistical significance in logistic regression analysis for risk factors of early vascular access failure in either whole nondiabetic patients (Table 7) or patients with AVF (data not shown).
Table 6.
-634 C/G genotype distribution according to vascular access patency
Values are presented as the number (%).
HD, hemodialysis; DM, diabetes mellitus; OR, odds ratio; CI, confidence interval; AVF, arteriovenous fistula.
Table 7.
Logistic regression analysis for vascular access failure in hemodialysis patients
DISCUSSION
This study demonstrated a higher frequency of the IL-6 -634 GG genotype and G allele in HD patients compared with healthy controls. This tendency was preserved in non-diabetic patients. Several studies have focused on the IL-6 -634 C/G polymorphism and renal disease progression. Kitamura et al. [17] suggested that the IL-6 -634 G allele and GG genotype may be relevant predictors for progression of type 2 diabetic nephropathy and that the IL-6 -634 C/G polymorphism could be an aggravating factor rather than an initiating factor for diabetic nephropathy in type 2 diabetic patients. A recent study suggested that the -634 G allele is an independent risk factor for faster progression of chronic glomerulonephritis (GN) to ESRD [19]. Together, these data suggest that the IL-6 -634 G allele is a risk factor for renal disease progression. Because HD patients are similar to individuals with advanced renal disease, our data could be considered to support previous findings. Although important risk factors such as DM, HTN, and glomerulonephropathy affect the progression of renal failure, we cautiously suggest that the -634 G allele is another susceptibility factor in patients with ESRD.
Few studies have examined the association of IL-6 -634 C/G polymorphism and vascular diseases. Our results indicate that the genotype distribution of the -634 C/G polymorphism and the G allele was more frequent in the shorter vascular access survival sub-group (< 1 year), excluding diabetic patients. Because the mechanisms of obstruction between AVF and artificial AVG differ, we also performed an analysis excluding patients with AVG. The results did not differ from the analysis performed with all patients. However, because the shorter access survival group contained only 3 patients (excluding patients with AVG), this finding requires confirmation in a larger study. Furthermore, this result was not statistically significant in the total patient population, and the association disappeared in logistic regression analysis for risk factors in early access failure. These findings suggest that DM may be a more important factor in early access failure than the IL-6 C/G polymorphism or G allele. Although few patients were enrolled in the study and statistical significance was not achieved, the results suggest a genetic susceptibility of an association between IL-6 polymorphism and vascular access stenosis in HD patients.
We detected no -174 C alleles in our study population. Previous studies have reported considerable ethnic dif ferences in the IL-6 -174 G/C polymorphism. The C allele frequency is reportedly 0.33 to 0.55 in Caucasians, but is reportedly < 0.002, 0.000, and 0.002 in Eastern Asians, Japanese, and Southern Chinese, respectively [18,23-26]. In the Korean population, the C allele frequency is reportedly 0.006 [27]. Our results support previous findings of a very low frequency of the C allele in Eastern Asians. The reason for the rarity of the C allele in Eastern Asians has not been clarified.
Research has yielded conflicting results regarding the relationship between the IL-6 -174 G/C polymorphism and plasma IL-6 levels or other clinical inflammation-related diseases [9,14,28,29]. Most studies have suggested that G allele carriers and patients with the GG genotype produce high levels of IL-6 [14,28,30], which is possibly a predisposing factor to the development of ESRD [31]. In these studies, the CC genotype has been proposed as a possible protective factor in the development of disease. One study suggested that the GG genotype is a predisposing factor in the development of ESRD and has a synergic effect with IL-4 cytokine genes in the genetic variation of ESRD [31]. Endothelial dysfunction has also been noted in healthy individuals with the G allele [32]. However, another study showed that the -174 CC genotype was correlated with a high risk of acute post-operative cardiovascular events in patients with peripheral arterial disease (PAD) and endothelial dysfunction in these patients [33].
Different results have been reported regarding the relationship between plasma IL-6 levels and -174 genotypes. One study found that the C allele did not influence the IL-6 level [29], whereas another study found opposite results, with higher circulating IL-6 levels among C/C carriers [9]. In one study, high IL-6 levels were related with advanced PAD, but no relationship appeared between the IL-6 -174 polymorphism and PAD [34]. The authors suggested that the high IL-6 levels in patients with advanced PAD may depend more on stimuli other than the genotype of the IL-6 gene [34]. One study found that the baseline IL-6 level did not differ according to the IL-6 genotype, but peak IL-6 levels increased significantly after coronary artery bypass surgery, especially in -174 CC carriers [9]. The authors suggested the IL-6 promoter gene had a functional role in IL-6 production after acute severe injury. However, another study reported that the -174 GG type promotes PAD development in patients with type 2 DM by increasing the release of IL-6, resulting in increased plasma concentrations of fibrinogen and CRP [35]. In our study, we observed no differences in IL-6 levels with respect to genotype.
Several reasons may account for the discrepancies in results for IL-6 polymorphisms: different racial distributions, different prevalence of cardiovascular disease, random errors, and misunderstanding. Furthermore, regulation of the gene may be a complex process related to the haplotype, therefore the effect of single-nucleotide polymorphisms may be difficult to interpret [5]. Although it is known that different haplotypes (-597 G→A/-572 G→C/-174 G→C) may determine the transcriptional levels of the IL-6 gene [9], undoubtedly other factors also regulate IL-6 levels. IL-6 is not only a pro-inflammatory and anti-inflammatory cytokine, but also a multi-functional mediator. Therefore, the action of IL-6 may depend on inflammatory mechanisms in a particular disease [19]. In vitro studies have shown similar conflicting results. One study reported that human umbilical vein endothelial cell IL-6 production after pro-inflammatory stimulation, such as treatment with IL-1β or lipopolysaccharide, is not dependent on IL-6 -174 G/C genotypes [36]. Another in vitro study revealed that the -634 G allele is closely associated with increased production and secretion of IL-6 by PBMCs [17]. Because different factors affect production and secretion of IL-6 protein in vivo, further studies will be required to examine the relationship between IL-6 polymorphisms and the IL-6 level in vitro and in vivo. Larger studies consisting of different racial populations will also be required to confirm any assumptions.
This study had several limitations. First, we did not measure the IL-6 levels in the control group. Although IL-6 levels did not differ according to the -634 genotype distribution in HD patients, this finding should be studied in patients with normal renal function. Second, the control group was somewhat inappropriate because the controls included people without DM, HTN, and GN. Even if the distribution of -634 polymorphism differed between HD patients and controls, other risk factors such as DM, HTN, and GN for the morbidity of patients with ESRD might have contributed to the effect. Third, we did not measure IL-6 levels at the moment of vascular access stenosis. IL-6 is known as a dynamic cytokine sensitive to acute vascular injury. Thus, the IL-6 levels at the point of measurement may reveal a correlation between IL-6 levels and IL-6 polymorphisms. Finally, few subjects were enrolled, so the power of the genetic study was relatively low.
In summary, we demonstrated that the distribution of the IL-6 -634 polymorphism differs between HD patients and healthy controls, and the G allele appears more frequently in patients with ESRD than healthy controls. Furthermore, the distribution of the -634 genotype differs by access survival in non-diabetic patients. This finding suggests that the IL-6 -634 G allele appears more frequently in patients with ESRD and may be associated with vascular access dysfunction in non-diabetic HD patients. Finally, the IL-6 -174 G/C polymorphism is extremely rare in the Korean population and the -174 G/C polymorphism is unlikely to determine significant serum IL-6 levels in a Korean population. A prospective study with a larger population is required to confirm the genetic effects of the IL-6 gene in vascular access patency.
Footnotes
No potential conflict of interest relevant to this article is reported.
References
- 1.De Marchi S, Falleti E, Giacomello R, et al. Risk factors for vascular disease and arteriovenous fistula dysfunction in hemodialysis patients. J Am Soc Nephrol. 1996;7:1169–1177. doi: 10.1681/ASN.V781169. [DOI] [PubMed] [Google Scholar]
- 2.Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55:648–658. doi: 10.1046/j.1523-1755.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- 3.Yeun JY, Levine RA, Mantadilok V, Kaysen GA. C-Reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2000;35:469–476. doi: 10.1016/s0272-6386(00)70200-9. [DOI] [PubMed] [Google Scholar]
- 4.Kaysen GA. The microinflammatory state in uremia: causes and potential consequences. J Am Soc Nephrol. 2001;12:1549–1557. doi: 10.1681/ASN.V1271549. [DOI] [PubMed] [Google Scholar]
- 5.Stenvinkel P, Pecoits-Filho R, Lindholm B for the Dial-Gene Consortium. Gene polymorphism association studies in dialysis: the nutrition-inf lammation axis. Semin Dial. 2005;18:322–330. doi: 10.1111/j.1525-139X.2005.18317.x. [DOI] [PubMed] [Google Scholar]
- 6.Yao Q, Lindholm B, Stenvinkel P. Inflammation as a cause of malnutrition, atherosclerotic cardiovascular disease, and poor outcome in hemodialysis patients. Hemodial Int. 2004;8:118–129. doi: 10.1111/j.1492-7535.2004.01085.x. [DOI] [PubMed] [Google Scholar]
- 7.Rao M, Wong C, Kanetsky P, et al. Cytokine gene polymorphism and progression of renal and cardiovascular diseases. Kidney Int. 2007;72:549–556. doi: 10.1038/sj.ki.5002391. [DOI] [PubMed] [Google Scholar]
- 8.Rao M, Guo D, Perianayagam MC, et al. Plasma interleukin-6 predicts cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2005;45:324–333. doi: 10.1053/j.ajkd.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Brull DJ, Montgomery HE, Sanders J, et al. Interleukin-6 gene -174G>C and -572G>C promoter polymorphisms are strong predictors of plasma interleukin-6 levels after coronary artery bypass surgery. Arterioscler Thromb Vasc Biol. 2001;21:1458–1463. doi: 10.1161/hq0901.094280. [DOI] [PubMed] [Google Scholar]
- 10.Losito A, Kalidas K, Santoni S, Jeffery S. Association of interleukin-6 -174G/C promoter polymorphism with hypertension and left ventricular hypertrophy in dialysis patients. Kidney Int. 2003;64:616–622. doi: 10.1046/j.1523-1755.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- 11.Pigott R, Dillon LP, Hemingway IH, Gearing AJ. Soluble forms of E-selectin, ICAM-1 and VCAM-1 are present in the supernatants of cytokine activated cultured endothelial cells. Biochem Biophys Res Commun. 1992;187:584–589. doi: 10.1016/0006-291x(92)91234-h. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda U, Ikeda M, Oohara T, et al. Interleukin 6 stimulates growth of vascular smooth muscle cells in a PDGF-dependent manner. Am J Physiol. 1991;260(5 Pt 2):H1713–H1717. doi: 10.1152/ajpheart.1991.260.5.H1713. [DOI] [PubMed] [Google Scholar]
- 13.Juhan-Vague I, Alessi MC. Plasminogen activator inhibitor 1 and atherothrombosis. Thromb Haemost. 1993;70:138–143. [PubMed] [Google Scholar]
- 14.Fishman D, Faulds G, Jeffery R, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hulkkonen J, Pertovaara M, Antonen J, Pasternack A, Hurme M. Elevated interleukin-6 plasma levels are regulated by the promoter region polymorphism of the IL-6 gene in primary Sjogren's syndrome and correlate with the clinical manifestations of the disease. Rheumatology (Oxford) 2001;40:656–661. doi: 10.1093/rheumatology/40.6.656. [DOI] [PubMed] [Google Scholar]
- 16.Aker S, Bantis C, Reis P, et al. Influence of interleukin-6 G-174C gene polymorphism on coronary artery disease, cardiovascular complications and mortality in dialysis patients. Nephrol Dial Transplant. 2009;24:2847–2851. doi: 10.1093/ndt/gfp141. [DOI] [PubMed] [Google Scholar]
- 17.Kitamura A, Hasegawa G, Obayashi H, et al. Interleukin-6 polymorphism (-634 C/G) in the promotor region and the progression of diabetic nephropathy in type 2 diabetes. Diabet Med. 2002;19:1000–1005. doi: 10.1046/j.1464-5491.2002.00844.x. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima T, Ota N, Yoshida H, Watanabe S, Suzuki T, Emi M. Allelic variants in the interleukin-6 gene and essential hypertension in Japanese women. Genes Immun. 1999;1:115–119. doi: 10.1038/sj.gene.6363642. [DOI] [PubMed] [Google Scholar]
- 19.Buraczynska M, Jozwiak L, Ksiazek P, Borowicz E, Mierzicki P. Interleukin-6 gene polymorphism and faster progression to end-stage renal failure in chronic glomerulonephritis. Transl Res. 2007;150:101–105. doi: 10.1016/j.trsl.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Chang CJ, Ko YS, Ko PJ, et al. Thrombosed arteriovenous fistula for hemodialysis access is characterized by a marked inflammatory activity. Kidney Int. 2005;68:1312–1319. doi: 10.1111/j.1523-1755.2005.00529.x. [DOI] [PubMed] [Google Scholar]
- 21.Liu BC, Li L, Gao M, Wang YL, Yu JR. Microinflammation is involved in the dysfunction of arteriovenous fistula in patients with maintenance hemodialysis. Chin Med J (Engl) 2008;121:2157–2161. [PubMed] [Google Scholar]
- 22.Marrone D, Pertosa G, Simone S, et al. Local activation of interleukin 6 signaling is associated with arteriovenous fistula stenosis in hemodialysis patients. Am J Kidney Dis. 2007;49:664–673. doi: 10.1053/j.ajkd.2007.02.266. [DOI] [PubMed] [Google Scholar]
- 23.Zhai R, Liu G, Yang C, Huang C, Wu C, Christiani DC. The G to C polymorphism at -174 of the interleukin-6 gene is rare in a Southern Chinese population. Pharmacogenetics. 2001;11:699–701. doi: 10.1097/00008571-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Zheng C, Huang DR, Bergenbrant S, et al. Interleukin 6, tumour necrosis factor alpha, interleukin 1beta and interleukin 1 receptor antagonist promoter or coding gene polymorphisms in multiple myeloma. Br J Haematol. 2000;109:39–45. doi: 10.1046/j.1365-2141.2000.01963.x. [DOI] [PubMed] [Google Scholar]
- 25.Humphries SE, Luong LA, Ogg MS, Hawe E, Miller GJ. The interleukin-6 -174 G/C promoter polymorphism is associated with risk of coronary heart disease and systolic blood pressure in healthy men. Eur Heart J. 2001;22:2243–2252. doi: 10.1053/euhj.2001.2678. [DOI] [PubMed] [Google Scholar]
- 26.Cox ED, Hoffmann SC, DiMercurio BS, et al. Cytokine polymorphic analyses indicate ethnic differences in the allelic distribution of interleukin-2 and interleukin-6. Transplantation. 2001;72:720–726. doi: 10.1097/00007890-200108270-00027. [DOI] [PubMed] [Google Scholar]
- 27.Lim CS, Zheng S, Kim YS, et al. The-174 G to C polymorphism of interleukin-6 gene is very rare in Koreans. Cytokine. 2002;19:52–54. doi: 10.1006/cyto.2002.1951. [DOI] [PubMed] [Google Scholar]
- 28.Burzotta F, Iacoviello L, Di Castelnuovo A, et al. Relation of the -174 G/C polymorphism of interleukin-6 to interleukin-6 plasma levels and to length of hospitalization after surgical coronary revascularization. Am J Cardiol. 2001;88:1125–1128. doi: 10.1016/s0002-9149(01)02046-x. [DOI] [PubMed] [Google Scholar]
- 29.Bennet AM, Prince JA, Fei GZ, et al. Interleukin-6 serum levels and genotypes influence the risk for myocardial infarction. Atherosclerosis. 2003;171:359–367. doi: 10.1016/j.atherosclerosis.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez-Real JM, Broch M, Vendrell J, Richart C, Ricart W. Interleukin-6 gene polymorphism and lipid abnormalities in healthy subjects. J Clin Endocrinol Metab. 2000;85:1334–1339. doi: 10.1210/jcem.85.3.6555. [DOI] [PubMed] [Google Scholar]
- 31.Mittal RD, Manchanda PK. Association of interleukin (IL)-4 intron-3 and IL-6 -174 G/C gene polymorphism w ith susceptibility to end-stage renal disease. Immunogenetics. 2007;59:159–165. doi: 10.1007/s00251-006-0182-6. [DOI] [PubMed] [Google Scholar]
- 32.Brull DJ, Leeson CP, Montgomery HE, et al. The effect of the Interleukin-6-174 G > C promoter gene polymorphism on endothelial function in healthy volunteers. Eur J Clin Invest. 2002;32:153–157. doi: 10.1046/j.1365-2362.2002.00966.x. [DOI] [PubMed] [Google Scholar]
- 33.Stoica AL, Stoica E, Constantinescu I, Uscatescu V, Ginghina C. Interleukin-6 and interleukin-10 gene polymorphism, endothelial dysf unction, and postoperative prognosis in patients with peripheral arterial disease. J Vasc Surg. 2010;52:103–109. doi: 10.1016/j.jvs.2010.01.088. [DOI] [PubMed] [Google Scholar]
- 34.Danielsson P, Truedsson L, Eriksson KF, Norgren L. Inflammatory markers and IL-6 polymorphism in peripheral arterial disease with and without diabetes mellitus. Vasc Med. 2005;10:191–198. doi: 10.1191/1358863x05vm617oa. [DOI] [PubMed] [Google Scholar]
- 35.Libra M, Signorelli SS, Bevelacqua Y, et al. Analysis of G(-174)C IL-6 polymorphism and plasma concentrations of inflammatory markers in patients with type 2 diabetes and peripheral arterial disease. J Clin Pathol. 2006;59:211–215. doi: 10.1136/jcp.2004.025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiszel P, Mako V, Prohaszka Z, Cervenak L. Interleukin-6 -174 promoter polymorphism does not influence IL-6 production after LPS and IL-1 beta stimulation in human umbilical cord vein endothelial cells. Cytokine. 2007;40:17–22. doi: 10.1016/j.cyto.2007.08.001. [DOI] [PubMed] [Google Scholar]