Abstract
Background
The occurrence of oropharyngeal candidiasis (OPC) in combination with HIV disease progression is a very common phenomenon. However, not all HIV-infected patients develop OPC, even when they progress to low CD4+ T cell counts. Because T-cell immunity is defective in AIDS, the innate defence mechanisms are likely to have a central role in antifungal immunity in these patients. We investigated whether genetic variations in the innate immune genes DECTIN-1, TLR2, TLR4, TIRAP and CASPASE-12 are associated with the presence of OPC in HIV-infected subjects from East-Africa.
Methods
A total of 225 HIV patients were genotyped for several single nucleotide polymorphisms (SNP) and this was correlated with the occurrence of OPC in these patients. In addition, primary immune cells obtained from individuals with different genotypes were stimulated with C.albicans and cytokine production was measured.
Results
The analysis revealed that no significant differences in the polymorphism frequencies could be observed, although a tendency towards a protective effect on OPC of the DECTIN-1 I223S SNP was apparent. Furthermore, IFNγ production capacity was markedly lower in cells bearing the DECTIN-1 SNP I223S. It could also be demonstrated that the 223S mutated form of the DECTIN-1 gene exhibits a lower capacity to bind zymosan.
Conclusion
These data demonstrate that common polymorphisms of TLR2, TLR4, TIRAP and CASPASE-12 do not influence susceptibility to OPC in HIV-infected patients in East-Africa but suggest an immunomodulatory effect of the I223S SNP on dectin-1 function and possibly the susceptibility to OPC in HIV patients.
Keywords: HIV, oropharyngeal candidiasis, dectin-1, TLR2, TLR4, Mal/TIRAP, caspase-12
Introduction
Candida albicans is an ubiquitous dimorphic fungal microorganism, often colonizing the gastrointestinal and reproductive tracts. The commensal carriage of C.albicans elicits and sustains an acquired immune response, including the production of C.albicans antigen specific IgA and IgG antibodies 1. Infection by Candida spp. in the mouth and upper digestive tract, designated as oropharyngeal candidiasis (OPC), is a very common mucosal infection in individuals that are infected with human immunodeficiency virus (HIV). OPC is an opportunistic infection, almost entirely caused by C.albicans and occurs in 50 to 95% of HIV-seropositive patients at least once during their progression to AIDS 2-4. In many patients, OPC is often the first clinical sign of HIV-seropositivity and is included in the clinical staging system of HIV-infection from the World Health Organization (WHO). Recently, a significant improvement in morbidity rates was observed after the introduction of highly active antiretroviral therapy (HAART) 5-7. OPC remains however the most frequent HIV-associated oral disease in Sub-Saharan Africa where access to HAART is still limited.
It is well established that low CD4+ T cell counts are a major determinant for the occurrence of OPC in HIV-infected subjects 4. A critical threshold is 200 cells/μl. This knowledge is supported by the observation that an effective immune response towards C.albicans is Th1 dependent, although recently also a role for Th17 cells in mucosal fungal host defence has emerged 8. Furthermore, in vitro peripheral blood mononucleated cells (PBMCs) stimulated with C.albicans antigens are known to respond with the production of Th1 and Th17 cytokines 9-11. However, not all patients with low CD4+ T cell counts display OPC, while others with relatively high T cell counts do suffer from OPC. These observations suggest that the susceptibility to OPC in HIV-infected patients cannot be fully ascribed to the impaired acquired immune response (i.e. low CD4+ T cell counts) and point towards an important role for the innate immune cells that reside in the mucosal layers of the upper digestive tract.
Recognition of C.albicans by the innate immune system and subsequent induction of pro-inflammatory cytokines is mediated by a broad panel of pattern recognition receptors (PRR), recognizing conserved bacterial and fungal motifs called pathogen-associated molecular patterns (PAMPs). These PRR include Toll-like receptors (TLRs), such as TLR2, -4 and -6, and C-type lectin receptors (CLRs) like dectin-1 and the mannose receptor (reviewed in 12). Intracellular signalling of TLRs is mediated by several adaptor molecules, such as MyD88 (Myeloid Differentiation primary response gene 88) and Mal (MyD88 Adapter-Like, also known as TIRAP) that positively regulate transcription factor activation and are crucial for downstream signalling (reviewed in 13).
Single nucleotide polymorphisms (SNPs) in TLRs and their adaptor molecules have been reported to influence the susceptibility towards infectious diseases. For instance, the TLR2 R677W SNP has been demonstrated to increase susceptibility to lepromatous leprosy 14 and tuberculosis 15 and another SNP in TLR2, R753Q, has been proposed to predispose to Candida sepsis 16. Similarly, TLR4 polymorphisms D299G alone or in co-segregation with T399I are reported to account for higher susceptibility to Gram-negative osteomyelitis 17, disseminated candidiasis 18, pulmonary aspergillosis 19 and tuberculosis 20. Recently a common polymorphism has been described in DECTIN-1, which leads to an early stopcodon and is associated with mucocutaneous candidiasis 21 . In addition, the HapMap database also displays an additional polymorphism of DECTIN-1 in African populations, namely I223S. Furthermore, the TIRAP variant S180L is correlated with resistance to bacterial infections and malaria 22. Another well characterized SNP, in CASPASE-12, that leads to an inactive truncated protein, has been suggested to modulate proinflammatory cytokine responses, and protects against mortality due to sepsis 23. The ancestral variant is still present in African populations, of which 20-30% express the active variant of caspase-12.
Whether genetic variants of DECTIN-1, TLR2, TLR4, TIRAP and CASPASE-12 modulate the host defence against C.albicans is largely unknown. Moreover, the role of the polymorphisms in these genes in determining the susceptibility of HIV-seropositive patients towards OPC has not been studied to date. In the present study we assessed whether single nucleotide polymorphisms (SNPs) in DECTIN-1 (I223S, rs16910527 and Y238X, rs16910526), TLR2 (R753Q, rs5743708; P631H, rs5743704 and R677W, rs unknown), TLR4 (D299G, rs4986790 and T399I, rs4986791), TIRAP (S180L, rs8177374) and/or CASPASE-12 (T/C, nucleotide 125) contribute to the susceptibility of HIV-seropositive patients towards oropharyngeal candidiasis in an HIV/AIDS condition. We have also investigated whether these polymorphisms are correlated with differential cytokine production capacity induced after in vitro stimulation of whole blood with C.albicans.
Patients, materials and methods
Study design, patients and setting
The role of the various polymorphisms in the susceptibility to OPC was assessed in a group of 225 HIV-seropositive patients. An independent physician, who categorized them in accordance with the WHO clinical staging criteria 24, performed clinical examination of all study patients. A standard oral examination method recommended by WHO was used 25. The intraoral tissues were examined for changes in size, colour and shape of anatomical areas as well as for clinical signs of OPC. Specimens were obtained by firmly swabbing the lesion site with a sterile cotton wool swab. The swabs were sent immediately to the laboratory for microbiological confirmation using 10% KOH. Among them, 117 patients had OPC and 108 patients never have developed OPC. Participants were recruited at the Muhimbili National Hospital (MNH) HIV-clinic in Dar es Salaam, Tanzania, from April 2007 until August 2008. Clinical manifestations of OPC in these 117 patients were pseudomembranous candidiasis only (65%), or a combination of pseudomembranous and erythematous, hyperplastic or angular cheilitis. In one subject only angular cheilitis was observed. OPC clinical score indicated that 95% had moderate to severe infections. Other clinical characteristics of the patients are presented in Table 1. The functional consequences on the effect of the various SNPs on the capacity of cytokine production were investigated in a group of 48 African healthy volunteers who were not infected with HIV.
Table 1.
Clinical characteristics of the 225 Tanzanian HIV-seropositive patients. The HIV treatment consisted of a HAART of different reverse transcriptase inhibitors, in most cases stavudine, lamivudine and nevirapine, received by 86% of treated patients. Antibacterial drugs, if prescribed, was cotrimoxazole.
| Patient subgroup | OPC positive (n=117) | OPC negative (n=108) | P-value |
|---|---|---|---|
| Age (mean(range)) | 35 (18-61) | 35 (18-63) | 0.78 |
| Gender (male- female) | 29-88 | 25-83 | 0.74 |
| HAART use (number (%)) | 48 (41%) | 56 (52%) | 0.16 |
| Duration of HAART use (months(range)) | 12 (1-40) | 13 (2-29) | 0.79 |
| CD4+ T cell counts (mean±SEM) | 126 ± 9 | 217 ± 16 | <0.0001 |
| Smoking | 5 (4.3%) | 4 (3.7%) | 0.82 |
| Antifungal drug use | 77 (66%) | 7 (6%) | <0.0001 |
| Antibacterial drug use | 55 (47%) | 29 (27%) | 0.002 |
Blood collection and CD4+ T cell counts
Five ml blood samples were collected from patients and transferred to EDTA tubes. The same day cell pellets were separated and enumeration of CD4+ T cells was done using a flow cytometry count machine after staining patients’ blood with anti-CD4 monoclonal antibodies 26.
DNA isolation and SNP detection
DNA was isolated from whole blood using the isolation kit Puregene (Gentra Sytems, Minneapolis, MN, USA), according to the manufacturers’ protocol. Detection of the SNPs in TLR4, CASPASE-12 and TIRAP was performed as described earlier 18;22;23. The presence of TLR2 P631H was assessed by applying a predesigned TaqMan SNP assay C__25607736_10 on the 7300 ABI Real-Time PCR system (both from Applied Biosystems, Foster City, CA, USA). Detection of the TLR2 SNPs R753Q and R677W and of the DECTIN-1 SNPs, conventional PCR and sequence analysis were performed. For the TLR2 R753Q SNP the forward primer 5′-TTCAAGTTGTGTCTTCATAAGCG-3′ and the reverse primer 5′-CAGATTTACCCAAAATCCTTCC-3′ were used. For the detection of TLR2 R677W these were forward 5′-ATTTGTTTCTTACAGTGAGCGGG-3′ and reverse 5′-GGAAATGGGAGAAGTCCAGTTC-3′. For detection of the two DECTIN-1 SNPs one single primer pair was used, with forward 5′-AATCACAGCCTCTCCCTTCA-3′ and reverse 5′-GATTTAAGCCTCCTTTTCCAA-3′. Conditions were equal for these PCR reactions, except for the annealing temperature, which was 56°C for TLR2 R753Q, 58°C for TLR2 R677W and 60°C for the DECTIN-1 SNPs.
Cloning and transfection of wild-type and 223S mutated dectin-1
DECTIN-1 isoforms A and B were amplified with the following primers; reverse 5′-CCCTTCCTCGAGCATTGAAAACTTC-3′, containing the Xho-1 site for the inframing cloning of an HA tag, and the forward primer 5′-AAAGGATCCAGGGGCTCTCAAGAACAATG-3′ has a BamHI site, which allows cloning into pFB-neo vectors containing the HA tag. For the construction of the mutant dectin-1 protein the following overlapping primers were used to introduce the I to S mutation (ATT to AGT): forward 5′-TGTGTATGGAGTCACGTGTCAG-3′ and reverse 5′-AAATGACTGACACGTGACTCCATAC-3′. These primers were used together with the above mentioned forward and reverse primers to introduce the SNP and the fragments were cloned into the HA-tagged pFB-neo vector. Surface expression of the various dectin-1 constructs was assessed using anti-HA antibody (Covance, Princeton, NJ, USA) and anti-mouse-RPE (Jackson ImmunoResearch, West Grove, PA, USA). NIH3T3 cells were transduced and FITC-zymosan (Sigma, St. Louis, MO) (25 particles/cell) binding assays for 1hour at 37°C, with or without laminarin (Sigma, St. Louis, MO, USA) pretreatment (100 μg/ml, 20 min, 4°C) were performed as previously described 27.
In vitro whole blood stimulation
Blood was collected from 48 healthy volunteers by venous puncture and directly stimulated with 106/ml heat-killed C.albicans for 24 hours at 37°C. C.albicans had previously been incubated for 30 min. at 56°C to heat-kill. After stimulation, concentrations of the cytokines TNFα, IL-1β, IFNγ and IL-10 were measured by ELISA (R&D Systems, MN, USA), using the manufacturer’s protocol.
Ethical issues
The ethics committee of the Muhimbili University of Health and Allied Sciences (MUHAS) and Muhimbili National Hospital, Dar es salaam, Tanzania approved the study protocol. All participants gave informed written consent. The following information was provided to ensure that patients had the information necessary to make an informed choice: a complete description of the aim of the study, potential benefits and risks, blood collection procedures and assurance of confidentiality of any information given as well as test results. Study personnel provided any additional information required by the patients. All patients seen in this study received appropriate care and treatment according to national guidelines on care and treatment of HIV-infected individuals. All patient information and test results were confidentially kept.
Statistical analysis
Statistics on the CD4+ T cell counts, clinical characteristics and on the cytokine data were performed by using the SPSS program (Rel. 14.0.2, 2006; SPSS, Chicago, IL), and differences were tested by the χ2 test or Mann–Whitney U test, when appropriate. Differences in genotype frequencies were statistically tested by using the χ2 test. P ≤ 0.05 was considered to represent a statistically significant difference.
Results
The influence of DECTIN-1, TLR2, TLR4, TIRAP and CASPASE-12 polymorphisms on OPC
In general, the OPC negative group had a significantly higher average CD4+ T cell count, as expected (see Table 1). As a consequence, antifungal and antibacterial drugs were more frequently prescribed in the OPC positive group due to higher prevalence of infectious complications. As shown in Table 2, the frequency of TLR4 D299G and the TIRAP S180L was similar between patients with and without OPC. The mentioned TLR2 SNPs and the TLR4 T399I were all absent in the cohort. No differences were detected regarding CASPASE-12 SNP frequencies (15.0% versus 14.4%) in HIV-seropositive patients with and without OPC. The studied polymorphisms in DECTIN-1; the Y238X allele was present in a very low, but equal frequency in both groups. For the I223S polymorphism a marked difference in allele frequency was observed (7.4% in the group without OPC compared to 4.3% in the group with OPC, RR=0.58), although the difference did not reach statistical significance (p> 0.05). The comparison of the genetic distribution of the genotyped SNPs between OPC positive and OPC negative HIV patients stratified for CD4+ T cell count intervals revealed similar findings (Table 3), with the difference in DECTIN-1 I223S frequency reaching statistical significance in the subgroup with CD4+ T cell counts <100 (P=0.048).
Table 2.
SNP frequencies in TLR4, TIRAP, CASPASE-12 and DECTIN-1 in a cohort of HIV-infected Tanzanian patients.
| Polymorphism | OPC status | Total | Homozygous wild-type |
Heterozygous | Homozygous mutant |
Allele frequencies | |
|---|---|---|---|---|---|---|---|
| Wild-type | Mutant | ||||||
| TLR4 D299G | OPC | 117 | 107 | 10 | 0 | 95.6% | 4.3% |
| No OPC | 108 | 99 | 9 | 0 | 95.7% | 4.2% | |
| TIRAP S180L | OPC | 117 | 113 | 4 | 0 | 98.3% | 1.7% |
| No OPC | 108 | 104 | 3 | 1 | 97.7% | 2.3% | |
| CASPASE-12 S/L | OPC | 117 | 83 | 33 | 1 | 85.0% | 15.0% |
| No OPC | 108 | 79 | 27 | 2 | 85.6% | 14.4% | |
| DECTIN-1 Y238X | OPC | 117 | 115 | 2 | 0 | 99.1% | 0.9% |
| No OPC | 108 | 106 | 2 | 0 | 99.1% | 0.9% | |
| DECTIN-1 I223S | OPC | 117 | 108 | 8 | 1 | 95.7% | 4.3% |
| No OPC | 108 | 94 | 12 | 2 | 92.6% | 7.4% | |
Table 3.
Genetic distribution of SNPs in TLR4, TIRAP, CASPASE-12 and DECTIN-1 stratified for CD4+ T cell count intervals.
| CD4+ T cell count intervals and minor allele frequency of the SNPs (%) OPC positive – OPC negative | ||||
|---|---|---|---|---|
| Polymorphism | < 100 (N=67) | 100–200 (N=94) | 200–300 (N=38) | >300 (N=26) |
| TLR4 D299G | 2% – 4% | 7% – 5% | 4% – 0% | 8% – 6% |
| TIRAP S180L | 1% – 0% | 3% – 3% | 0% – 0% | 0% – 3% |
| CASPASE-12 S/L | 20% – 15% | 14% – 15% | 11% – 14% | 0% – 21% |
| DECTIN-1 Y238X | 0% – 0% | 2% – 1% | 0% – 0% | 0% – 3% |
| DECTIN-1 I223S | 2% – 14% * | 5% – 5% | 11% – 9% | 8% – 9% |
Indicates statistically significant difference.
The functional effects of TLR4, CASPASE-12 and DECTIN-1 polymorphisms on cytokine production
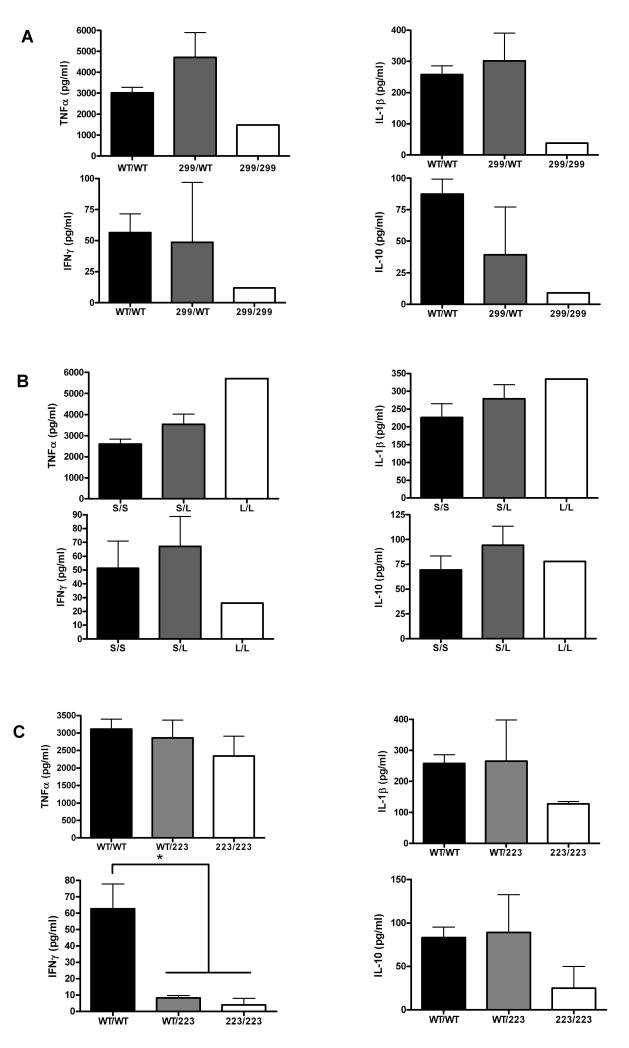
In order to link these genotype data to the function of these receptors in mediating cytokine production, in vitro stimulations with heat killed C.albicans in whole blood were performed in a group of 48 healthy volunteers. No significant differences in cytokine production could be detected after comparing stimulation of cells from volunteers homozygous for the wild-type genotype with stimulation of cells from volunteers either heterozygous or homozygous for the variants of TLR4 (Figure 1A), CASPASE-12 (Figure 1B) or TIRAP (not shown). In contrast, the IFNγ production capacity was lower in cells from individuals heterozygous and homozygous for the DECTIN-1 223S SNP compared to cells from wild-type individuals. The production of the other cytokines was, in contrast, comparable between the different DECTIN-1 genotypes (Figure 1C). Similar to the HIV-infected individuals, the TLR4 T399I SNP and the studied TLR2 SNPs were completely absent in the healthy volunteers as well.
Figure 1.
Cytokine concentrations measured after 24 hours at 37°C of whole blood stimulation with 106/ml heat-killed C.albicans in blood collected from 48 volunteers, corrected for background. Cytokines were detected by ELISA. Results are stratified by (A) TLR4 D299G genotype (44 homozygous for the wild-type allele, 3 heterozygous and 1 homozygous for 299G), (B) CASPASE-12 genotype (26 homozygous for the short variant S, 21 heterozygous and 1 homozygous for the long variant L) and (C) DECTIN-1 I223S genotype (43 homozygous for the wild-type allele, 3 heterozygous and 2 homozygous for 223S). Values are mean ± SD. * P ≤ 0.05.
We have identified one subject who was homozygous for the TLR4 299G allele and displayed lower cytokine production for all four cytokines tested compared to wild-type and heterozygous individuals (Figure 1A). In contrast, the one individual homozygous for the long form of caspase-12 produced normal amounts of cytokines upon stimulation with heat-killed C.albicans (Figure 1B).
Functional consequences of DECTIN-1 SNP I223S
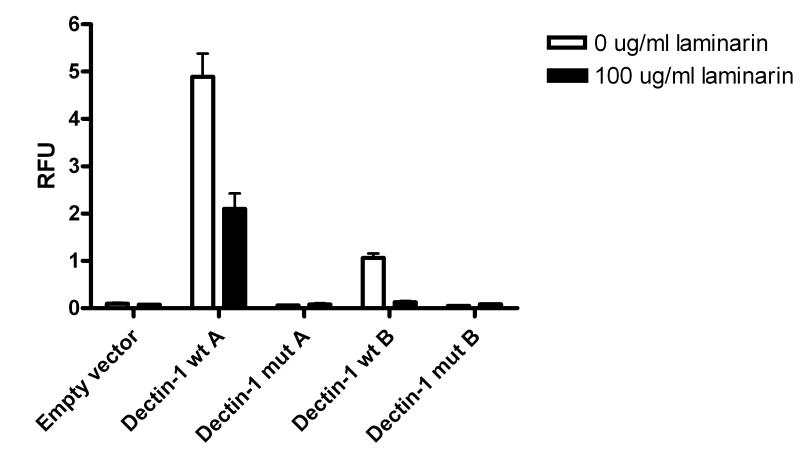
In order to assess whether the mutated dectin-1 protein, bearing 223S, is indeed functionally different from the wild-type receptor, both the wild-type and the mutated form of DECTIN-1 were cloned and transfected into NIH3T3 cells. The binding to fluorescently labelled zymosan was measured in the cells expressing the wild-type or the 223S mutated dectin-1 protein. The binding of zymosan by the mutated dectin-1 was diminished compared to the wild-type dectin-1 on isoforms A and B, both known to be expressed on the cell membrane and able to bind β-glucan. The dectin-1 antagonist laminarin blocked the binding to zymosan to both isoforms A and B of the wild-type dectin-1, demonstrating the specificity of β-glucan by dectin-1 binding in this assay (Figure 2A). Furthermore, protein expression of the 223S mutated dectin-1 was largely absent on the membrane of NIH3T3 cells, whereas the wild-type dectin-1 protein was clearly present on the cell membrane (Figure 2B).
Figure 2.
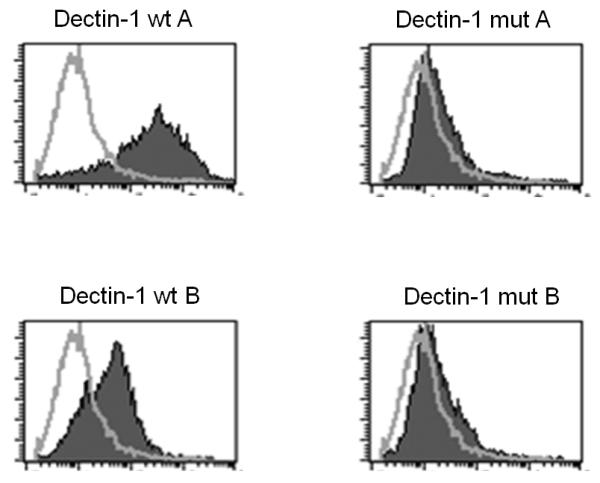
(A) Binding assay of fluorescently labelled zymosan to the wild-type (wt) and the 223S mutated (mut) form of dectin-1 cloned and transfected with an hemagglutinin tag in NIH3T3 cells, including competitive blocking by laminarin, on the dectin-1 isoforms A and B. Values are relative fluorescent units (RFU). (B) Evaluation of membrane expression of the cloned dectin-1 constructs, wild-type (wt) and 223S mutant (mut) of both isoforms A and B, transfected with an hemagglutinin tag in NIH3T3 cells and measured by using HA-antibodies and flow cytometry.
Discussion
In the present study we have investigated the role of genetic variants of DECTIN-1, TLR2, TLR4, TIRAP and CASPASE-12 for the susceptibility to OPC in East-African HIV-seropositive patients. Host defence to C.albicans in general, and OPC in particular, is believed to be dependent on a combination of innate and acquired immune responses. The importance of the latter has become apparent during the AIDS pandemics, as OPC is one of the most common opportunistic infections in HIV-infected patients. However, the variations in the susceptibility to OPC between individuals with low CD4+ T cell counts suggest an additional important role for innate immune responses. This is supported by the fact that the C.albicans infection remains restricted to the mucosal layers and that disseminated candidiasis in HIV-seropositive patients is rare. Finally, oral burdens of C.albicans have been shown to be augmented in HIV-infected patients even prior to the first episode of OPC 28;29, and before a decline in CD4+ T cell count could be observed.
Fungal pathogens in general, and C.albicans in particular, are recognized by pattern recognition receptors such as TLRs and C-type lectins, that subsequently activate antifungal inflammatory reactions. SNPs in DECTIN-1, TLR2, TLR4, TIRAP and CASPASE-12 are demonstrated to influence the susceptibility to different infectious diseases and we have assessed their role for the susceptibility to OPC in HIV-infected patients 17-20;22;23;30;31. In the present study, no association could be established between heterozygosity for TLR4 299G or CASPASE-12 long variant and susceptibility to OPC. The studied TLR2 SNPs were all absent in this Tanzanian cohort, which is in accordance with a similar study in a West-African population 32. Furthermore, TLR4 T399I, TIRAP S180L and DECTIN-1 Y238X were not detected or present in low frequencies in the cohort studied. In contrast, the frequency of the DECTIN-1 I223S SNP was lower in the group of patients with HIV and OPC (4.3% vs. 7.4%), which may suggest a protective effect of this polymorphism against the occurrence of OPC in HIV patients. After stratifying for intervals of CD4+ T cell counts, this difference reached statistical significance in the subgroup with CD4+ T cell counts below 100. The allele frequency of this SNP was demonstrated to be 6.6% in a group of 91 healthy Tanzanian subjects, which was similar to the HIV-infected patients that did not develop OPC.
We performed functional cytokine assays with cells from healthy volunteers to accompany the genetic studies. Although blood from healthy volunteers contains higher numbers of CD4+ T cells than HIV patients, it is reasonable that the potential effect of the studied SNPs on cytokine responses would display similar trends. The epidemiological data are supported by the cytokine studies, because no significant differences were observed between individuals homozygous for wild-type TLR4 or CASPASE-12, and individuals bearing polymorphisms in these genes. Interestingly, the release of cytokines, IL-1β in particular, is not different between individuals with different CASPASE-12 genotypes. This difference in regulation of IL-1β release between different CASPASE-12 genotypes was previously suggested by Saleh et al. 23. In contrast, our findings bring into question whether human caspase-12 is involved in modulating IL-1β processing. However, it should be noted that clinical outcome (i.e. sepsis versus mucosal candidiasis) and origin of the populations (African-American (Saleh et al.) versus East-African in our study) was different.
We have identified one individual homozygous for the TLR4 299G allele, and his cells produced significantly lower amounts of cytokines compared to wild-type individuals (Figure 1A). It remains to be demonstrated whether this tendency is reproducible in other individuals homozygous for the TLR4 299G polymorphism. In contrast, heterozygous individuals for this SNP exhibited a slightly higher TNFα production capacity, which is consistent with previous findings 33.
The presence of the DECTIN-1 I223S polymorphism was accompanied by a lower IFNγ production capacity upon stimulation with heat-killed C.albicans. In contrast, the production of the other cytokines measured appear not be affected by this DECTIN-1 genotype, although dectin-1 is known to be involved in these cytokine responses. The release of IL-12p40 and IL-18 cytokines, known to be involved in IFNγ induction, were not markedly different between the different DECTIN-1 genotypes (data not shown). This suggests that a different mechanism accounts for the differences observed in the IFNγ response. The lower IFNγ response in the individuals bearing the DECTIN-1 223S allele has been supported by molecular studies. For both isoforms A and B an altered binding capacity of the 223S mutated form of dectin-1 to zymosan was demonstrated by cloning the receptor and subsequent transfection studies. Furthermore, after transfection, the cell surface expression of the dectin-1 223S mutated form was reduced compared to the wild-type dectin-1, which was true for both isoforms A and B. This finding is in contrast with a study from Adachi et al. 34, in which the equivalent amino acid in murine dectin-1 (I222) was mutated into an alanine, which did not affect β-glucan binding. However, in this same study mutating the adjacent amino acids (W121 and H223) into alanine diminished the capacity of β-glucan binding tremendously. This implies that this protein region consisting of these three amino acids is important for β-glucan recognition. Both the differences between murine and human dectin-1 regarding their protein sequence and the different amino acid alterations that were studied (from isoleucine to serine instead of alanine) could explain the discrepancy between the studies.
Less efficient binding of zymosan to dectin-1 would imply a less effective recognition of C.albicans by innate immune cells in the mucosa and consequently a less profound immune response. This finding seems contradictory to the certain degree of protection against OPC offered by this SNP. The precise mechanism mediating this protection remains elusive. However, one could speculate that the lower IFNγ responses that are observed as a consequence of the presence of the DECTIN-1 I223S SNP could lead to less T-cell activation and therefore less clonal expansion. This could result in a reduced spread of HIV virus particles, which might be beneficial for mucosal anti-Candida responses. This seems true specifically in case CD4+ T cell counts are very low, which is indicative for a predominant role of the innate immune system in resistance to OPC when CD4+ T cell responses are severely impaired. On the other hand, the differences of the prevalence of the DECTIN-1 polymorphism was borderline statistically significant between patients with or without OPC, and one may question the importance of this polymorphism for the susceptibility to OPC.
The TLR4 polymorphism studied in the present study has been previously reported to be associated with disseminated candidiasis 18. However, two important differences with the present study are important in this respect. Firstly, disseminated candidiasis has a different pathogenesis than OPC, with innate responses likely to be more important in systemic candidiasis. Secondly, the distribution of the TLR4 SNPs D299G and T399I is different between populations in Europe and Africa. In Caucasian populations, from which a cohort has been analysed in the previous study, the D299G and T399I SNPs are in complete linkage, determining a different TLR4 haplotype than that in African populations in which the T399I SNP is largely absent. The difference in TLR4 haplotypes (299/399 co-segregation in Europe versus 299 alone in Africa) was also demonstrated to have functional consequences for TLR4 signalling, and therefore a comparison between the TLR4 polymorphisms between these two studies cannot be made 33.
Also HIV viral load is a predictive factor of the occurrence of OPC in HIV patients 35. Unfortunately, no data were available on HIV viral load of the patients in our cohort which would have allowed us to correct for this potential confounding factor.
In conclusion, no differences have been observed in the frequency of the studied SNPs in the genes encoding TLR2, TLR4, Mal/TIRAP and caspase-12 in Tanzanian HIV-seropositive patients with and without OPC. In addition, these different genotypes also did not have an effect on cytokine responses after an in vitro challenge with heat-killed C.albicans. One possible exception may be constituted by individuals homozygous for TLR4 299G, which seems to result in a reduced cytokine production capacity after C.albicans stimulation. However, the low frequency of homozygous individuals bearing this SNP in the study population precludes us from any conclusions regarding a putative increase in the susceptibility to OPC. The frequency of the DECTIN-1 polymorphism I223S that has the tendency towards an association with protection from OPC in this cohort of HIV-infected patients, was associated with lower IFNγ responses and a decreased capacity to bind zymosan. However, further investigation in larger cohorts is warranted to determine whether this DECTIN-1 SNP has indeed a protective effect against OPC in HIV-seropositive patients.
Acknowledgements
We thank Matthew B. B. McCall for the experiments performed with the healthy volunteers, including the cytokine measurements. M.G.N. was supported by a Vici grant of the Netherlands Organization for Scientific Research. J.A.W. and G.D.B were supported by a Wellcome Trust Programme Grant.
Footnotes
Conflict of interest No conflicts of interest have been reported by the authors.
Reference List
- 1.Wozniak KL, Leigh JE, Hager S, et al. A comprehensive study of Candida-specific antibodies in the saliva of human immunodeficiency virus-positive individuals with oropharyngeal candidiasis. J Infect Dis. 2002;185:1269–76. doi: 10.1086/339886. [DOI] [PubMed] [Google Scholar]
- 2.Samaranayake LP. Oral mycoses in HIV infection. Oral Surg Oral Med Oral Pathol. 1992;73:171–80. doi: 10.1016/0030-4220(92)90191-r. [DOI] [PubMed] [Google Scholar]
- 3.Samaranayake LP, Holmstrup P. Oral candidiasis and human immunodeficiency virus infection. J Oral Pathol Med. 1989;18:554–64. doi: 10.1111/j.1600-0714.1989.tb01552.x. [DOI] [PubMed] [Google Scholar]
- 4.Rabeneck L, Crane MM, Risser JM, et al. A simple clinical staging system that predicts progression to AIDS using CD4 count, oral thrush, and night sweats. J Gen Intern Med. 1993;8:5–9. doi: 10.1007/BF02600284. [DOI] [PubMed] [Google Scholar]
- 5.Martins MD, Lozano-Chiu M, Rex JH. Declining rates of oropharyngeal candidiasis and carriage of Candida albicans associated with trends toward reduced rates of carriage of fluconazole-resistant C. albicans in human immunodeficiency virus-infected patients. Clin Infect Dis. 1998;27:1291–94. doi: 10.1086/515006. [DOI] [PubMed] [Google Scholar]
- 6.Arribas JR, Hernandez-Albujar S, Gonzalez-Garcia JJ, et al. Impact of protease inhibitor therapy on HIV-related oropharyngeal candidiasis. AIDS. 2000;14:979–85. doi: 10.1097/00002030-200005260-00009. [DOI] [PubMed] [Google Scholar]
- 7.Cauda R, Tacconelli E, Tumbarello M, et al. Role of protease inhibitors in preventing recurrent oral candidosis in patients with HIV infection: a prospective case-control study. J Acquir Immune Defic Syndr. 1999;21:20–25. doi: 10.1097/00126334-199905010-00003. [DOI] [PubMed] [Google Scholar]
- 8.Conti HR, Shen F, Nayyar N, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van der Graaf CA, Netea MG, Verschueren I, et al. Differential cytokine production and Toll-like receptor signaling pathways by Candida albicans blastoconidia and hyphae. Infect Immun. 2005;73:7458–64. doi: 10.1128/IAI.73.11.7458-7464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellocchio S, Montagnoli C, Bozza S, et al. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J Immunol. 2004;172:3059–69. doi: 10.4049/jimmunol.172.5.3059. [DOI] [PubMed] [Google Scholar]
- 11.van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe. 2009;5:329–40. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Netea MG, Brown GD, Kullberg BJ, et al. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6:67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- 13.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–64. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 14.Bochud PY, Hawn TR, Aderem A. Cutting edge: a Toll-like receptor 2 polymorphism that is associated with lepromatous leprosy is unable to mediate mycobacterial signaling. J Immunol. 2003;170:3451–54. doi: 10.4049/jimmunol.170.7.3451. [DOI] [PubMed] [Google Scholar]
- 15.Ben-Ali M, Barbouche MR, Bousnina S, et al. Toll-like receptor 2 Arg677Trp polymorphism is associated with susceptibility to tuberculosis in Tunisian patients. Clin Diagn Lab Immunol. 2004;11:625–26. doi: 10.1128/CDLI.11.3.625-626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woehrle T, Du W, Goetz A, et al. Pathogen specific cytokine release reveals an effect of TLR2 Arg753Gln during Candida sepsis in humans. Cytokine. 2008;41:322–29. doi: 10.1016/j.cyto.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Montes AH, Asensi V, Alvarez V, et al. The Toll-like receptor 4 (Asp299Gly) polymorphism is a risk factor for Gram-negative and haematogenous osteomyelitis. Clin Exp Immunol. 2006;143:404–13. doi: 10.1111/j.1365-2249.2005.03002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van der Graaf CA, Netea MG, Morre SA, et al. Toll-like receptor 4 Asp299Gly/Thr399Ile polymorphisms are a risk factor for Candida bloodstream infection. Eur Cytokine Netw. 2006;17:29–34. [PubMed] [Google Scholar]
- 19.Carvalho A, Pasqualotto AC, Pitzurra L, et al. Polymorphisms in Toll-Like Receptor Genes and Susceptibility to Pulmonary Aspergillosis. J Infect Dis. 2008;197:618–21. doi: 10.1086/526500. [DOI] [PubMed] [Google Scholar]
- 20.Ferwerda B, Kibiki GS, Netea MG, et al. The toll-like receptor 4 Asp299Gly variant and tuberculosis susceptibility in HIV-infected patients in Tanzania. AIDS. 2007;21:1375–77. doi: 10.1097/QAD.0b013e32814e6b2d. [DOI] [PubMed] [Google Scholar]
- 21.Ferwerda B, Ferwerda G, Plantinga TS, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361:1760–1767. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khor CC, Chapman SJ, Vannberg FO, et al. A Mal functional variant is associated with protection against invasive pneumococcal disease, bacteremia, malaria and tuberculosis. Nat Genet. 2007;39:523–28. doi: 10.1038/ng1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saleh M, Vaillancourt JP, Graham RK, et al. Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature. 2004;429:75–79. doi: 10.1038/nature02451. [DOI] [PubMed] [Google Scholar]
- 24.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 25.Kramer IR, Pindborg JJ, Bezroukov V, et al. Guide to epidemiology and diagnosis of oral mucosal diseases and conditions. Community Dent Oral Epidemiol. 1980;8:1–26. doi: 10.1111/j.1600-0528.1980.tb01249.x. World Health Organization. [DOI] [PubMed] [Google Scholar]
- 26.Landay A, Ohlsson-Wilhelm B, Giorgi JV. Application of flow cytometry to the study of HIV infection. AIDS. 1990;4:479–97. doi: 10.1097/00002030-199006000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Willment JA, Gordon S, Brown GD. Characterization of the human beta -glucan receptor and its alternatively spliced isoforms. J Biol Chem. 2001;276:43818–23. doi: 10.1074/jbc.M107715200. [DOI] [PubMed] [Google Scholar]
- 28.Tylenda CA, Larsen J, Yeh CK, et al. High levels of oral yeasts in early HIV-1 infection. J Oral Pathol Med. 1989;18:520–524. doi: 10.1111/j.1600-0714.1989.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 29.Vargas KG, Joly S. Carriage frequency, intensity of carriage, and strains of oral yeast species vary in the progression to oral candidiasis in human immunodeficiency virus-positive individuals. J Clin Microbiol. 2002;40:341–50. doi: 10.1128/JCM.40.2.341-350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue Y, Daly A, Yngvadottir B, et al. Spread of an inactive form of caspase-12 in humans is due to recent positive selection. Am J Hum Genet. 2006;78:659–70. doi: 10.1086/503116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schroder NW, Diterich I, Zinke A, et al. Heterozygous Arg753Gln polymorphism of human TLR-2 impairs immune activation by Borrelia burgdorferi and protects from late stage Lyme disease. J Immunol. 2005;175:2534–40. doi: 10.4049/jimmunol.175.4.2534. [DOI] [PubMed] [Google Scholar]
- 32.Mockenhaupt FP, Cramer JP, Hamann L, et al. Toll-like receptor (TLR) polymorphisms in African children: Common TLR-4 variants predispose to severe malaria. Proc Natl Acad Sci U S A. 2006;103:177–82. doi: 10.1073/pnas.0506803102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferwerda B, McCall MB, Alonso S, et al. TLR4 polymorphisms, infectious diseases, and evolutionary pressure during migration of modern humans. Proc Natl Acad Sci U S A. 2007;104:16645–50. doi: 10.1073/pnas.0704828104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adachi Y, Ishii T, Ikeda Y, et al. Characterization of beta-glucan recognition site on C-type lectin, dectin 1. Infect Immun. 2004;72:4159–71. doi: 10.1128/IAI.72.7.4159-4171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mercante DE, Leigh JE, Lilly EA, et al. Assessment of the association between HIV viral load and CD4 cell count on the occurrence of oropharyngeal candidiasis in HIV-infected patients. J Acquir Immune Defic Syndr. 2006;42:578–83. doi: 10.1097/01.qai.0000225011.76439.99. [DOI] [PubMed] [Google Scholar]